Abstract
Glycidyl esters (GEs) and 3-chloroprapane-1,2-diol esters (3-MCPDEs) are processing contaminants in refined edible oils that have raised concerns globally owing to their potentially carcinogenic properties. Official analytical methods for GEs and 3-MCPDEs, such as AOCS Cd 29a-13 and AOCS Cd 29b-13, require up to 16 h for chemical hydrolysis. Also, parallel experiments should be conducted to correct for the conversion of analytes during hydrolysis in AOCS Cd 29b-13. For AOCS Cd 29c-13 with the shortest operating time, the reaction time (3.5–5.5 min) and temperature of alkaline hydrolysis should be carefully controlled, implying the accuracy may be influenced by human errors. Here, we propose a novel method based on Candida rugosa lipase hydrolysis and direct detection of free form GEs, glycidol, which was achieved by sample preparation with modified QuEChERS, to prevent side reactions in previous approaches, and also to shorten the overall sample preparation time. Glycidol was directly analyzed without halogenation and derivatization, whereas 3-MCPD required derivatization for analysis by GC-MS. Our method showed good accuracy and precision in terms of repeatability, intermediate precision, and reproducibility (inter-laboratory precision). The limit of detection (LOD) and limit of quantification (LOQ) for glycidol were 0.02 and 0.1 mg/kg, which is sufficient for practical applications. The proposed method was further compared with AOCS Cd 29c-13 by determination of GEs content in commercial oil samples and spiked samples. Our method with a streamlined procedure seems to possess potential advantage of reduced errors from operational factors. This proposed method based on direct detection of glycidol may serve as a simplified alternative for routine analysis of GEs and 3-MCPDEs in edible oils.
Keywords: 3-chloro-1, 2-propanediol, 3-chloro-1, 2-propanediol esters, Glycidol, Glycidyl esters, Modified QuEChERS
1. Introduction
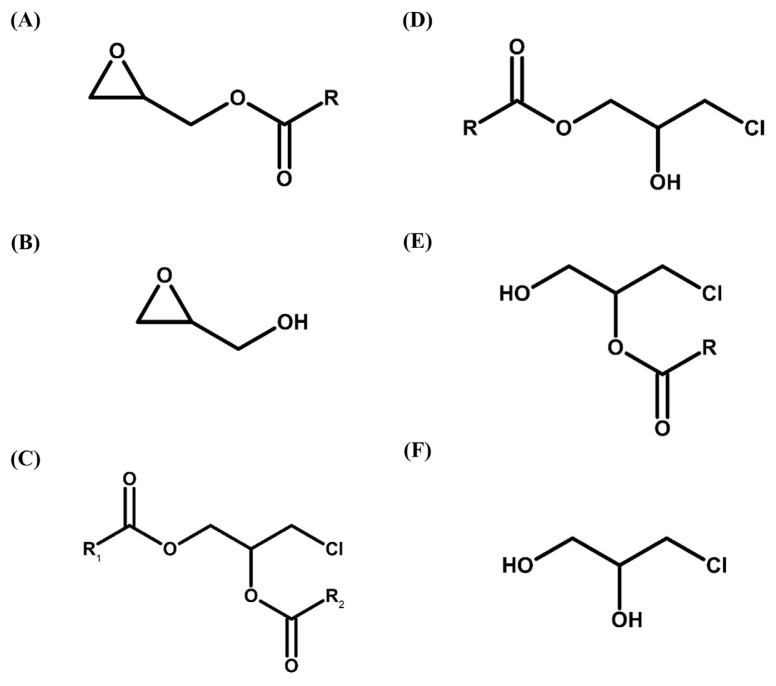
Glycidyl esters (GEs) and 3-chloro-1,2-propanediol esters (3-MCPDEs) are heat-induced processing contaminants mainly generated during the deodorization (>200°C) of edible oil refining [1–3]. Because of the potential health risks of GEs and 3-MCPDEs for humans, European Union regulation 2018/290 set maximum limits for GEs content in vegetable oils and fats, infant formula and other related foodstuffs in February 2018. GEs and 3-MCPDEs were reported to be hydrolyzed in vivo into glycidol and 3-chloro-1,2-propanediol (3-MCPD), classified as group 2A (probable human carcinogens) and 2B (possible human carcinogens) carcinogens respectively by the International Agency for Research on Cancer [4–6]. Therefore, public concern has been raised in recent years, which has led to the development of various quantitative analytical methods. Chemical structures of GEs, glycidol, 3-MCPDEs, including 3-MCPD diesters and 3-MCPD monoesters, and 3-MCPD are shown in Fig. 1.
Fig. 1.
Chemical structures of (A) glycidyl esters (GEs), (B) glycidol (free form of GEs), (C) 3-MCPD diesters, (D) 3-MCPD monoesters with fatty acid ester group at sn-1 position, and (E) 3-MCPD monoesters with fatty acid ester group at sn-2 position, and (F) 3-MCPD (free form of 3-MCPDEs). R: fatty-acyl group; R1: fatty-acyl group on sn-1; R2: fatty-acyl group on sn-2.
Analytical methods for GEs and 3-MCPDEs can be classified as direct and indirect. Direct methods refer to the direct detection and quantification of intact GEs or 3-MCPDEs without any chemical modification in sample preparation. Generally, GEs and 3-MCPDEs are separated from acylglycerols by gel permeation or double solid-phase extractions, or the sample should be diluted to reduce the interference from acylglycerols in oil matrix [1, 7–10]. By using direct methods, the composition and levels of each species of GEs and 3-MCPDEs with different fatty acyl groups can be obtained without interference from potential side reactions. However, expensive reference standards are required for analysis of every possible species of GEs and 3-MCPDEs. Also, coelution of 2-MCPDEs and 3-MCPDEs limits the use of direct methods for routine analysis [11, 12].
Indirect methods are based on the conversion of GEs and 3-MCPDEs into their free forms, glycidol and 3-MCPD, by chemical (alkaline or acidic) or enzymatic hydrolysis. The released glycidol and 3-MCPD generally undergoes an additional halogenation step to convert glycidol into 3-MCPD or 3-bromo-1,2-propanediol (3-MBPD) for derivatization by phenylboronic acid (PBA). Quantification is achieved by determining PBA derivatives of 3-MCPD (from 3-MCPDEs) or 3-MBPD (from GEs) by gas chromatography-mass spectrometry (GC-MS). The content of GEs and 3-MCPDEs determined by indirect methods is usually expressed as the content or equivalent of glycidol and 3-MCPD (free form of GEs and 3-MCPDEs), respectively. Indirect methods require much fewer reference standards than direct methods. Therefore, indirect methods are preferred for routine analysis.
The followings are commonly used official methods from the American Oil Chemists’ Society (AOCS) for determining GEs and 3-MCPDEs content in edible oils, AOCS Cd 29a-13 (acidic hydrolysis), Cd 29b-13 (alkaline hydrolysis at −22°C to −25°C), Cd 29c-13 (alkaline hydrolysis at room temperature), Cd 29d-19 (enzymatic hydrolysis using Candida rugosa lipase at room temperature) [13–16]. AOCS Cd 29a-13 and Cd 29b-13 are both time-consuming, requiring about 16 h for hydrolysis [13, 14]. Also, AOCS Cd 29a-13 (acidic hydrolysis) may lead to overestimation of GEs content in samples containing partial acylglycerols [13, 17]. AOCS Cd 29c-13 requires only 3.5–5.5 min for hydrolysis; however, reaction time and temperature of alkaline transesterification should be precisely controlled during alkaline hydrolysis to allow correction for partial conversion of 3-MCPD into glycidol [18]. Joint JOCS/AOCS Cd 29d-19 uses C. rugosa lipase for hydrolysis of GEs and 3-MCPDEs under mild conditions, requiring only 30 min for hydrolysis and reducing the bidirectional conversion of glycidol and 3-MCPD under alkaline or acidic conditions [16, 17, 19] However, owing to the lack of suitable derivatization reagent, analysis of glycidol by GC-MS requires halogenation to transform glycidol into 3-MCPD or 3-MBPD for derivatization by PBA in almost every analytical method reported to date. Overall, the complexity of the conversion between glycidol and 3-MCPD under alkaline or acidic conditions and other unintended side reactions from sample preparation in indirect methods complicate the determination of GEs and 3-MCPDEs contents, especially for GEs whose sample pretreatment requires an additional halogenation step.
Here, we propose a method combining enzymatic hydrolysis and direct determination of glycidol to prevent side reactions from alkaline/acidic hydrolysis or halogenation. Direct detection of glycidol, first reported in this work, was used to prevent potential side reactions from halogenation and simplify the sample preparation. The main challenge is that glycidol and 3-MCPD are both compounds with high polarity. Therefore, they are difficult to be extracted from lipase reaction mixture (aqueous layer) into the organic layer subjected to the analysis by GC-MS. Also, glycidol is considered unstable in acidic aqueous solution [20, 21]. These conditions may explain why direct detection of glycidol has never been adopted in previous approaches.
This study applied QuEChERS, a sample preparation method widely used to extract high-polarity compounds by using acetonitrile (ACN) with salts, such as anhydrous magnesium sulfate, sodium chloride, or sodium acetate, to achieve efficient extraction of glycidol and 3-MCPD [22]. Effects of the composition of salts on extraction efficiency were evaluated. Chemical stability of glycidol as well as recovery of glycidol and 3-MCPD in each step of sample preparation was investigated to evaluate the applicability of direct determination of glycidol and to verify that our proposed method is applicable for both GEs and 3-MCPDEs. Also, validation of the analytical procedure was conducted to evaluate the performance of the method. Furthermore, our proposed method was compared with AOCS Cd 29c-13 by measuring the same oil samples spiked with known amounts of GEs.
2. Materials and methods
2.1. Materials
2.1.1. Standards and chemicals
Standards, 1,2-dipalmitoyl-3-chloropropanediol (3-MCPD-PP), 1-palmitoyl-3-chloropropanediol (3-MCPD-1-P), 2-palmitoyl-3-chloropropanediol (3-MCPD-2-P), glycidyl oleate, and 3-chloro-1,2-propanediol-d5 (3-MCPD-d5) were from Toronto Research Chemicals (North York, Canada). Glycidol standard was from Wako Pure Chemicals Industries (Osaka, Japan). 3-Chloro-1,2-propanediol (3-MCPD) and furfuryl alcohol standard were from Sigma-Aldrich (St. Louis, MO, USA). Lipase (from Candida rugosa, free form, > 700 U/mg) for hydrolysis of GEs and 3-MCPDEs was from Sigma-Aldrich (St. Louis, MO, USA). Solvents used in this study including acetonitrile (ACN) and n-hexane were from Merck (Darmstadt, Germany). Magnesium sulfate anhydrous and sodium formate for QuEChERS were from Alfa Aesar Chemicals (Seoul, Korea). Potassium phosphate dibasic and citric acid monohydrate were from J.T. Baker Chemicals (Phillipsburg, NJ, USA). Phenylboronic acid (PBA) was from Sigma-Aldrich (St. Louis, MO, USA). QuEChERS extraction kits (original: 6.0 g magnesium sulfate and 1.5 g sodium chloride; EN 15662: 4.0 g magnesium sulfate, 1.0 g sodium chloride and 1.0 g sodium citrate, and 0.5 g disodium citrate sesquihydrate, and AOAC 2007.01: 6.0 g magnesium sulfate and 1.5 g sodium acetate), QuEChERS Dispersive 15-mL Universal kit (Agilent 5982-0029CH: 400 mg primary secondary amine (PSA), 400 mg C18, 45 mg graphitized carbon black (GCB), and 1200 mg magnesium sulfate), and ceramic homogenizer were from Agilent Technologies, Inc. (Santa Clara, CA, USA). All chemicals and reagents were of reagent or analytical grade.
2.1.2. Purified extra-virgin olive oil
Purified extra-virgin olive oil was used as oil matrix. Purified extra-virgin olive oil was prepared by extracting 100 mL extra-virgin olive oil with 200 mL ACN twice with the upper layer discarded to remove trace amounts of GEs or 3-MCPDEs.
2.2. Sample preparation
2.2.1. Lipase hydrolysis
A 2 g amount of oil sample was weighed in a 50-mL centrifuge tube. A 10 mL Mcllvaine buffer (pH 7, 0.1 M citric acid and 0.2 M disodium hydrogen phosphate) with 100 mg C. rugosa lipase (>700 U/mg) and a ceramic homogenizer were added to the same 50-mL centrifuge tube. The sample mixture was shaken vertically on a Geno Grinder mechanical shaker (Geno/Grinder P2010, SPEX SamplePrep, Metuchen, NJ, USA) at 1000 strokes/min for 30 min at room temperature for C. rugosa lipase hydrolysis of GEs and 3-MCPDEs. A 10 mL amount of n-hexane was added into the sample mixture (aqueous phase), shaken vigorously for 1 min and centrifuged (AllegraTM 25R, Beckman Coulter, Miami, FL, USA) at 5000 rpm for 5 min. This step was repeated twice with another 10 mL of n-hexane to remove free fatty acids (organic phase) released during the lipase hydrolysis of oil samples.
2.2.2. Modified QuEChERS—extraction and clean-up
After the lipase hydrolysis, 10 mL of ACN and salts (6.0 g magnesium sulfate and 3.0 g sodium formate) were added into the sample mixture and shaken vertically on the Geno Grinder mechanical shaker at 1000 strokes/min for 1 min at room temperature to extract the released glycidol and 3-MCPD from the aqueous phase. Sample mixture was centrifuged at 5000 rpm for 5 min. A 10 μL internal standard solution (furfuryl alcohol in ACN for glycidol; 3-MCPD-d5 in ACN for 3-MCPD) was added into the sample mixture with final levels of 1.0 (furfuryl alcohol) and 0.1 (3-MCPD-d5) mg/L and centrifuged again at 5000 rpm for 1 min. A 5 mL amount of organic phase was transferred to a 15-mL centrifuge tube containing 400 mg PSA, 400 mg C18, 45 mg GCB, and 1200 mg magnesium sulfate for clean-up. The tube was shaken vertically on the Geno Grinder mechanical shaker at 1000 strokes/min for 1 min at room temperature. Sample mixture was centrifuged at 5000 rpm for 5 min. A 1 mL amount of supernatant containing glycidol and 3-MCPD was carefully concentrated to nearly dryness under gentle nitrogen stream, and re-dissolved with 200 μL ACN for analysis of glycidol. For 3-MCPD, a 1 mL amount of supernatant was transferred to another sample vial for derivatization.
2.2.3. Derivatization
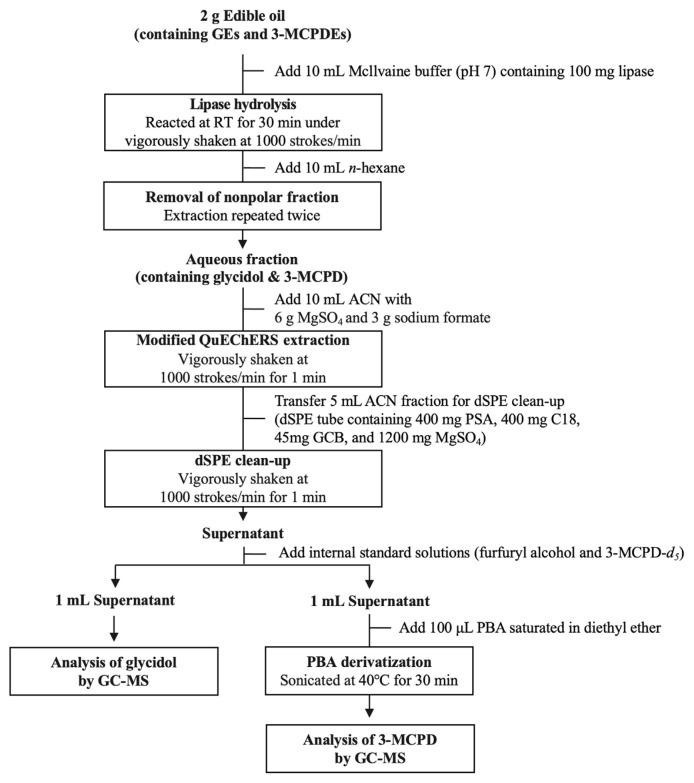
Derivatization was carried out by adding 100 μL PBA solution (saturated in diethyl ether) to the vial containing 1 mL supernatant, which was then sonicated for 30 min at 40°C. The reaction mixture was dried to nearly dryness under gentle nitrogen stream. The residue was re-dissolved in 1 mL n-hexane and analyzed by GC-MS. The overall sample preparation process for the proposed method shows in Fig. 2.
Fig. 2.
Sample preparation process of the proposed method. dSPE, dispersive solid-phase extraction; PSA, primary secondary amine; GCB, graphitized carbon black.
2.3. Analysis of glycidol and PBA derivatives of 3-MCPD by GC-MS
Agilent 7890B GC system with Agilent 5977B mass selective detector was used for analysis of glycidol and 3-MCPD. Mass selective detector was operated in the electron impact ionization (EI) mode with electron energy set at 70 eV. The temperature of transfer line and ion source was set at 250 and 230°C, respectively. Separation involved using DB-WAX (60 m × 0.25 mm ID, 0.25 μm) and DB-5 (30 m × 0.25 mm ID, 0.25 μm) capillary columns for glycidol and PBA derivatives of 3-MCPD, respectively. The carrier gas was helium with a constant flow of 1.4 mL/min. The temperature of the injector was set at 250°C. A 1 μL amount of sample was injected under splitless mode.
The temperature program for glycidol analysis was as follows: 70°C (held for 2 min) to 170°C at 3°C/min, 170°C to 235°C (held for 5 min) at 35°C/min. The temperature program for 3-MCPD analysis was as follows: 50°C (held for 1 min) to 145°C (held for 5 min) at 40°C/min, 145°C to 160°C at 2°C/min, 160°C to 320°C (held for 5 min) at 40°C/min. The quantification of GEs/3-MCPDEs was based on the response ratio of quantifiers of glycidol/3-MCPD and their internal standards, furfuryl alcohol/3-MCPD-d5 respectively in selective ion monitoring (SIM) mode. The following ions m/z 31, 44 (for glycidol), 53, 81, 98 (for furfuryl alcohol), 146, 147, 196 (for 3-MCPD), 149, 150, and 201 (for 3-MCPD-d5) were chosen for monitoring in SIM mode. Ions with m/z 44, 98, 147, and 150 were selected as quantifiers for glycidol, furfuryl alcohol, 3-MCPD, and 3-MCPD-d5, respectively, while other ions were used as qualifiers. The contents of GEs (glycidol equivalent, mg/kg) and 3-MCPDEs (3-MCPD equivalent, mg/kg) were calculated as follows (eq (1) and eq (2)).
| eq1 |
| eq2 |
where CGlycidol is the concentration of glycidol in samples (mg/kg); CIS1 is the concentration of internal standard 1 (furfuryl alcohol) in samples (mg/kg); RGlycidol is the quantifier (m/z 44) response of glycidol; RIS1 is the quantifier (m/z 98) response of internal standard 1 (furfuryl alcohol); RRF is the relative response factor for glycidol and internal standard 1; C3-MCPD is the concentration of 3-MCPD in samples (mg/kg); CIS2 is the concentration of internal standard 2 (3-MCPD-d5) in samples (mg/kg); R3-MCPD is the quantifier (m/z 147) response of 3-MCPD; RIS2 is the quantifier (m/z 150) response of internal standard 2 (3-MCPD-d5).
2.4. Data analysis
Data acquired from GC-MS were analyzed by using MassHunter Workstation Software (Quantitative Analysis vB.07.01 SP2 for GC-MS, Agilent Technologies, Santa Clara, CA, USA). Recovery of glycidol by QuEChERS with or without sodium chloride was compared by Student t test. Recoveries were considered significantly different at p < 0.05. Data are represented as mean ± SD.
3. Results and discussion
This study aims to simplify the sample pretreatment and to prevent undesirable side reactions especially for analysis of GEs content, which needs halogenation and derivatization in sample preparation. To solve these problems, we propose a method based on lipase hydrolysis of GEs and 3-MCPDEs and direct determination of glycidol by GC-MS. Direct determination of glycidol was first reported in this study, so each step in the sample pretreatment was optimized to achieve the highest recovery of glycidol. After optimizing the method for sufficient recovery of glycidol, the recovery of 3-MCPD was determined to evaluate the applicability of our proposed method for analysis of 3-MCPDEs content.
3.1. Method development
3.1.1. Modified QuEChERS-extraction and clean-up
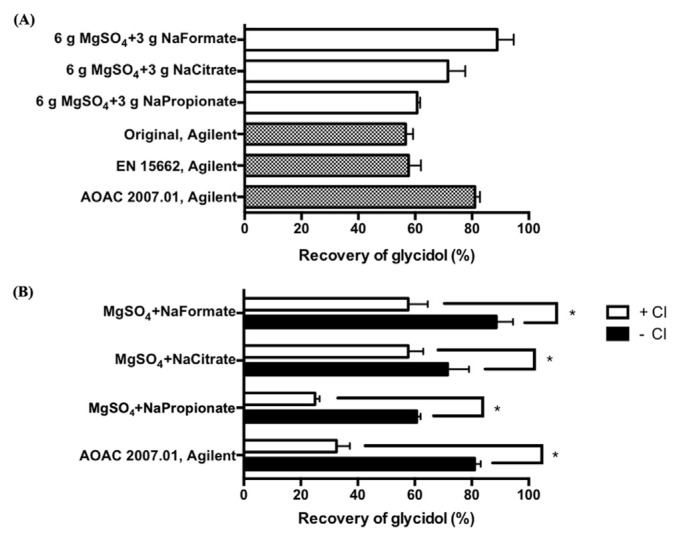
The compounds, glycidol and 3-MCPD, both have high polarity, so the most difficult part for direct determination of glycidol without halogenation and derivatization is to efficiently extract the released glycidol from the lipase reaction mixture (aqueous phase) into organic phase for direct analysis by GC-MS. Extraction efficiency of glycidol and 3-MCPD by conventional extraction with different solvents (i.e., diethyl ether, ethyl acetate, and ACN with sodium chloride) was preliminarily evaluated. However, all recoveries of glycidol and 3-MCPD were <30% owing to their high polarity. To enhance the extraction efficiency, we applied sample preparation with QuEChERS, a generally used sample preparation technique to extract polar pesticides for pesticide multiresidue analysis [22]. Because glycidol is more polar than 3-MCPD according to their log P, we first evaluated the recovery of glycidol by QuEChERS with different types of salts for ACN extraction to ensure that released glycidol from GEs could be extracted efficiently from the lipase reaction mixture to the organic phase. The recovery of glycidol with three commercial QuEChERS extraction kits —original, EN 15662, and AOAC 2007.01— showed poor recovery except for AOAC 2007.01 (Fig. 3a). However, sodium acetate in AOAC 2007.01 showed interference owing to the slight overlapping of peaks represented as acetic acid and glycidol. Thus, other salts with different molecular weights, such as sodium formate and sodium propionate, were chosen to replace sodium acetate. The highest recovery of glycidol was shown in extraction with 6.0 g magnesium sulfate and 3.0 g sodium formate (88.9 ± 5.8%) which was even higher than that with commercial QuEChERS extraction kits AOAC 2007.01 (Fig. 3a). The recovery of 3-MCPD under the same extraction condition was 83.5 ± 0.8%, so glycidol as well as 3-MCPD could be extracted. Thus, 6.0 g magnesium sulfate and 3.0 g sodium formate were chosen for extraction of glycidol and 3-MCPD.
Fig. 3.
Extraction recovery of glycidol by (A) QuEChERS with different salts or (B) recovery of glycidol by QuEChERS with or without 1.5 g sodium chloride. Data are triplicate determinations. Data with * are significantly different at p < 0.05. A 10 mL amount of Mcllvaine buffer (pH 7) spiked with glycidol at 1.0 ppm was extracted with 10 mL ACN with different salts. Component of commercial QuEChERS kits: Original: 6.0 g magnesium sulfate and 1.5 g sodium chloride; EN15662: 6.0 g magnesium sulfate, 1.5 g sodium chloride, 1.0 g sodium citrate, and 0.5 g disodium citrate sesquihydrate; AOAC 2007.01: 6.0 g magnesium sulfate and 1.5 g sodium acetate.
We found the extraction recoveries of glycidol were low when the QuEChERS extraction kits containing sodium chloride (original and EN 15662) were used (Fig. 3a), which implies that glycidol might turn into 3-MCPD in the presence of sodium chloride during the extraction. To further investigate the effect of sodium chloride on extraction, the recovery of glycidol was measured by using salts with or without sodium chloride for extraction at pH 7. The recoveries of glycidol were all lower in the presence of sodium chloride (Fig. 3b), so glycidol might be transformed to 3-MCPD during the extraction under neutral conditions. Generally, glycidol is prone to convert to 3-MCPD under acidic conditions [19]. However, glycidol was partially converted into 3-MCPD in the presence of sodium chloride even in a neutral buffering system (Fig. 3b). Because sodium chloride is a strong chloride donor, glycidol might turn into 3-MCPD under neutral conditions with excessive chloride in the lipase buffer. Also, QuEChERS extraction was reported to release heat because of the exothermic hydration of magnesium sulfate [22]. The released heat during QuEChERS extraction could improve the extraction efficiency but may also accelerate the nucleophilic attack of chloride on glycidol to generate 3-MCPD during the extraction. Therefore, excessive chloride should be avoided during sample preparation. Other salts that could also increase the ionic strength of buffer, such as sodium formate in our case, are more suitable than sodium chloride to be used for extraction of glycidol and 3-MCPD.
3.1.2. Lipase hydrolysis
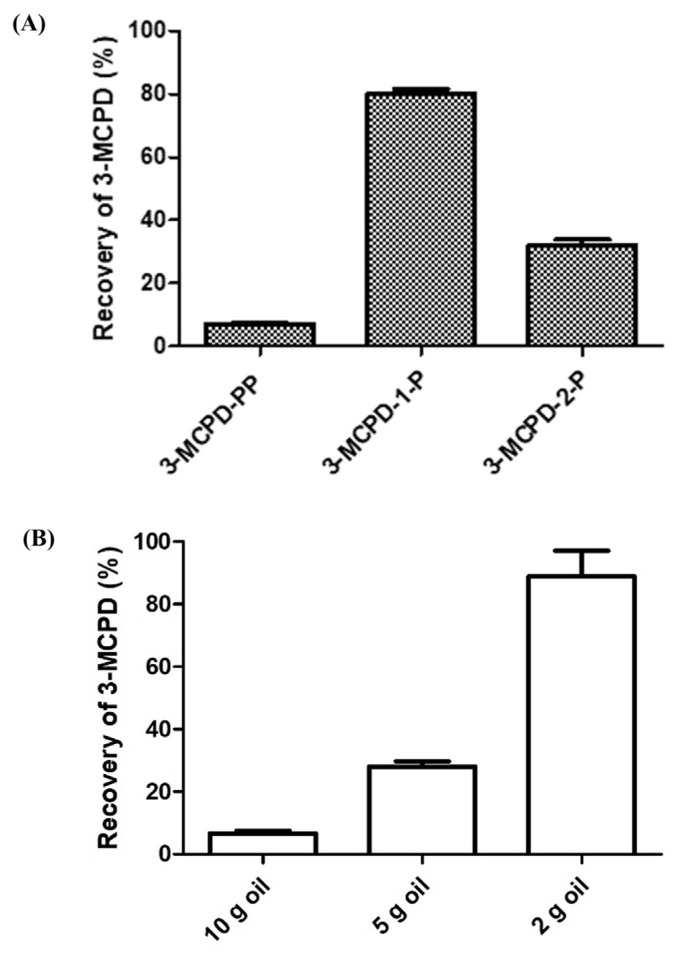
The epoxide ring of glycidol was reported to open in acidic aqueous solution [20, 21], so we investigated the chemical stability of glycidol in lipase reaction mixture at neutral pH but under vigorous shaking on the Geno Grinder mechanical shaker at 1000 strokes/min for 0–2 h. We found no significant decrease in recovery of glycidol, so glycidol was stable under the lipase reaction conditions (Fig. S1). To ensure that C. rugosa lipase could hydrolyze GEs and 3-MCPDEs in oils efficiently, glycidyl oleate, 3-MCPD-PP (diester), 3-MCPD-1-P (monoester), or 3-MCPD-2-P (monoester) was spiked in 10 g purified extra-virgin olive oil at 1.0 mg/kg glycidol or 3-MCPD equivalent and reacted with different amount of C. rugosa lipase at pH 7 for 30 min to examine the recoveries of glycidol and 3-MCPD. The recovery of glycidol with 100 mg lipase was 87.6 ± 2.7%, higher than that with 10 mg and 1 g lipase (68.9 ± 7.6% and 56.2 ± 0.1%). A 100 mg amount of C. rugosa lipase was sufficient for hydrolysis of GEs in 10 g oil. However, recoveries of 3-MCPD for 3-MCPD-PP and 3-MCPD-2-P under the same conditions were only 6.64 ± 0.74% and 31.9 ± 1.2%, whereas recovery for 3-MCPD-1-P was 80.1 ± 1.1% (Fig. 4a).
Fig. 4.
Recovery of 3-MCPD from (A) different 3-MCPDEs (3-MCPDPP, 3-MCPD-1-P-, 3-MCPD-2-P) in 10 g purified extra-virgin olive oil and from (B) 3-MCPD-PP in 2, 5, 10 g purified extra-virgin olive oil with 100 mg C. rugosa lipase. Data are triplicate determinations. Amounts of 2, 5, or 10 g purified extra-virgin olive oil spiked with 3-MCPDEs at 1.0 mg/kg were reacted with 100 mg C. rugosa lipase (>700 U/mg) in 10 mL Mcllvaine buffer (pH 7) shaken vigorously on the Geno Grinder mechanical shaker at 1000 strokes/min for 30 min at room temperature. 3-MCPD-PP: 1,2-dipalmitoyl-3-chloropropanediol; 3-MCPD-1-P: 1-palmitoyl-3-chloropropanediol; 3-MCPD-2-P: 2-palmitoyl-3-chloropropanediol.
The poor recovery of 3-MCPD-PP and 3-MCPD-2-P might due to the steric hindrance and preference for C. rugosa lipase to hydrolyze on the sn-1,3-position [23].
Since 3-MCPD diesters are the major form of 3-MCPDEs in most edible oils [11], sufficient hydrolysis of 3-MCPD diesters is necessary. Therefore, we chose 3-MCPD-PP as the target to ensure the complete hydrolysis of all species of 3-MCPDEs. As the relative ratio of lipase and oil is an important factor for sufficient hydrolysis, we investigated the recovery of 3-MCPD from 3-MCPD-PP with reduced amount of oil sample to improve the hydrolysis of 3-MCPD-PP. The recovery of 3-MCPD from 3-MCPD-PP could increase from 6.64 ± 0.74% (10 g oil) to 84.2 ± 6.7% when the amount of oil was reduced to 2 g (Fig. 4b). To achieve sufficient hydrolysis for both GEs and 3-MCPDEs, we selected 2 g oil samples with 100 mg C. rugosa lipase.
3.2. Method validation
After we ensured that glycidol and 3-MCPD were efficiently recovered from GEs and 3-MCPD during each step in the sample preparation, known amounts of GEs and 3-MCPDEs were spiked in the oil matrix (purified extra-virgin olive oil) for validation. The analytical method for glycidol was validated in terms of linearity, limit of detection (LOD), limit of quantification (LOQ), accuracy, and precision (repeatability, intermediate precision, and reproducibility), but only linearity, accuracy, and repeatability were evaluated for 3-MCPD because the analysis of 3-MCPD via PBA derivatization was similar with previous approaches. Validation of the analytical procedure was performed to follow criteria set by Taiwan FDA.
3.2.1. LOD, LOQ, and linearity
LOD and LOQ for glycidol in oil matrix was determined as 0.02 and 0.1 mg/kg based on S/N > 3 and >10, respectively. SIM chromatograms of glycidol in oil matrix at LOD and LOQ levels (0.02 and 0.1 mg/kg) were shown in Fig. S2. Linearity of glycidol was evaluated by solvent calibration because no significant matrix effect was observed by comparing the slopes of calibration curves in solvent and in oil matrix. We found good linearity for both glycidol and 3-MCPD with R2 > 0.999 in the working range of concentrations, 0.1–5.0 ppm for glycidol in ACN with 1.0 ppm internal standard and 0.02–2.0 ppm for 3-MCPD derivatives in n-hexane with 0.1 ppm internal standard (Fig. S3). Mass spectra and SIM chromatograms of glycidol, furfuryl alcohol (internal standard for glycidol), PBA derivative of 3-MCPD, and PBA derivative of 3-MCPD-d5 (internal standard for 3-MCPD) in solvent were shown in Fig. S4 and S5.
3.2.2. Accuracy
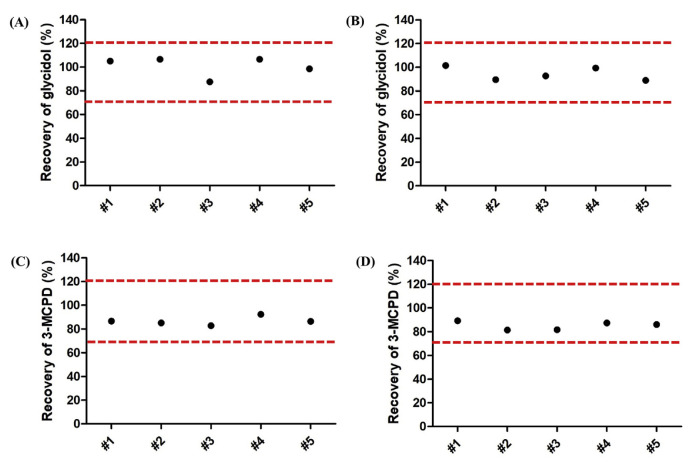
Accuracy was evaluated by determining recoveries of glycidol and 3-MCPD in oil matrix spiked with glycidyl oleate and 3-MCPD-PP at two levels, 0.5 and 1.0 mg/kg (glycidol and 3-MCPD equivalent). All recoveries of glycidol and 3-MCPD were in the ranges of 87.5% to 106.5% and 81.4% to 92.4% (Fig. 5), which meets the criteria set by Taiwan FDA for evaluating accuracy (recovery 70–120% for 0.10–1.0 mg/kg concentration range). SIM chromatograms for glycidol and 3-MCPD in oil matrix at levels of 0.5 and 1.0 mg/kg are shown in Fig. S6.
Fig. 5.
Method validation for accuracy (intra-day analysis). Recovery of glycidol from oil matrix (2 g purified extra-virgin olive oil) spiked with (A) glycidyl oleate and 3-MCPD-PP at 0.5 mg/kg glycidol or 3-MCPD equivalent, or (B) glycidyl oleate and 3-MCPD-PP at 1.0 mg/kg glycidol or 3-MCPD equivalent. Recovery of 3-MCPD from oil matrix spiked with (C) glycidyl oleate and 3-MCPD-PP at 0.5 mg/kg glycidol or 3-MCPD equivalent or (D) glycidyl oleate and 3-MCPD-PP at 1.0 mg/kg glycidol or 3-MCPD equivalent. Data are five determinations. Broken lines represent the criteria set by Taiwan FDA for evaluating accuracy (recovery 70–120% for 0.1–1.0 mg/kg concentration range).
3.2.3. Precision-repeatability (intra-day analysis)
Repeatability was evaluated by determining the relative standard deviation (RSD) of five determinations of spiked samples, oil matrix spiked with glycidyl oleate and 3-MCPD-PP at two levels, 0.5 and 1.0 mg/kg glycidol and 3-MCPD equivalent in the same day. The RSD of the five determinations of glycidol at two levels, 0.5 and 1.0 mg/kg, was 7.2% and 5.4% (Table 1). The RSD of five determinations of 3-MCPD equivalent at two levels, 0.5 and 1.0 mg/kg, was 3.6% and 3.7% (Table 1). We found good repeatability for the analysis of glycidol as well as 3-MCPD.
Table 1.
Evaluation for repeatability, intermediate precision, and reproducibility of the proposed method.
| Repeatability (intra-day) | ||||
|---|---|---|---|---|
| Spiked glycidyl oleate level (glycidol equivalent, mg/kg) | Recovery (%) | RSD (%) | Relative ion intensity, Q1a (%) | |
|
| ||||
| 0.5 | 100.8 ± 7.3 | 7.2 | 39.6 ± 0.7 | |
| 1.0 | 94.4 ± 5.1 | 5.4 | 38.4 ± 0.4 | |
|
| ||||
| Spiked 3-MCPD-PP level (3-MCPD equivalent, mg/kg) | Recovery (%) | RSD (%) | Relative ion intensity, q1b (%) | Relative ion intensity, q2c (%) |
|
| ||||
| 0.5 | 86.7 ± 3.2 | 3.6 | 24.6 ± 1.9 | 22.8 ± 0.6 |
| 1.0 | 85.1 ± 3.1 | 3.7 | 24.5 ± 1.6 | 22.2 ± 1.0 |
|
| ||||
| Intermediate precision (inter-day) | ||||
|
| ||||
| Spiked glycidyl oleate level (glycidol equivalent, mg/kg) | RSD (%) | |||
|
| ||||
| 0.5 | 4.3 | |||
| 1.0 | 3.9 | |||
|
| ||||
| Reproducibility (inter-lab) | ||||
|
| ||||
| Determined GEs content (glycidol equivalent, mg/kg) | ||||
|
|
||||
| This study | Lab A | RSD (%) | ||
|
| ||||
| Sample A | 0.88 | 0.98 | 11.4 | |
| Sample B | 2.89 | 3.06 | 5.73 | |
| Sample C | 5.01 | 4.79 | 4.55 | |
| Sample D | 4.32 | 4.40 | 1.77 | |
| Sample E | 8.90 | 7.98 | 10.3 | |
RSD, relative standard deviation. Repeatability data are mean ± SD (n = 5). Evaluation for intermediate precision was performed by different analysts working on three different days with three determinations per day. A 2 g amount of purified extra-virgin olive oil was spiked with glycidyl oleate at two levels, 0.5 and 1.0 mg/kg glycidol equivalent, for repeatability and intermediate precision experiments. Blind samples for reproducibility analysis were sent to a third-party lab represented as Lab A and analyzed by using the same method. Sample A: purified extra virgin olive oil spiked with glycidyl oleate at 1.0 mg/kg (glycidol equivalent); sample B: commercial rice bran oil; sample C: sample B spiked with additional 2.0 mg/kg (glycidol equivalent) glycidyl oleate; sample D: commercial palm oil; sample E: sample D spiked with additional 5.0 mg/kg (glycidol equivalent) glycidyl oleate. 3-MCPD-PP: 1,2-dipalmitoyl-3-chloropropanediol.
Relative ion intensity, Q1 (%), is defined as response ratio of qualifier ion (m/z 31) to quantifier ion (m/z 44) of glycidol.
Relative ion intensity, q1 (%), is defined as response ratio of qualifier ion (m/z 146) to quantifier ion (m/z 147) of 3-MCPD.
Relative ion intensity, q2 (%), is defined as response ratio of qualifier ion (m/z 196) to quantifier ion (m/z 147) of 3-MCPD.
3.2.4. Precision — intermediate precision (inter-day analysis)
Intermediate precision was evaluated by determining the RSD of triplicate determinations of spiked samples, oil matrix spiked with glycidyl oleate at two levels, 0.5 and 1.0 mg/kg (glycidol equivalent), in three different days. The RSD for glycidol at two levels, 0.5 and 1.0 mg/kg, was 4.3% and 3.9%, showing good intermediate precision (Table 1).
3.2.5. Precision — reproducibility (inter-laboratory analysis)
Reproducibility of the proposed method was evaluated by sending five blind samples, samples A–E, to a third-party laboratory, Lab A, which conducted the same sample preparation process as our proposed method. The RSD for GEs content for samples A–E obtained by Lab A and us was in the range of 1.77% to 11.4%, showing good reproducibility (Table 1).
3.3. Determination of GEs content in oil samples by the proposed method and AOCS Cd 29c-13
We further compared our method with one of the commonly used official methods with the shortest operation time, AOCS Cd 29c-13 (alkaline hydrolysis at room temperature for 3.5–5.5 min), by sending five commercial edible oil or spiked oil samples, samples A–E, to an accredited third-party laboratory, Lab B. We found considerable deviation of GEs content from true values when using AOCS Cd 29c-13, especially for samples D and E, represented as palm oil and palm oil spiked with an additional 5.0 mg/kg (glycidol equivalent) glycidyl oleate (Table 2). Significant overestimation of GEs content was observed in commercial and spiked palm oil samples (sample D and E) which contained higher content of 3-MCPDEs than other samples (Table 2). In contrast, samples A–C showed significant underestimation (Table 2). AOCS Cd 29c-13 was proved to be comparable with other official methods, AOCS Cd 29a-13 and AOCS Cd 29b-13 via comprehensive validation [15]. However, literatures also reported the necessity of precisely controlled temperature and time of alkaline transesterification in AOCS Cd 29c-13 to obtain reliable results [15, 18]. Although partial conversion from 3-MCPD to glycidol could be taken into account via 13C correction [18, 24], the correction still relies on well-trained analysts to conduct parallel experiments. Our streamlined procedure may possess potential advantage of reduced influences from operational factors. By combining mild enzymatic hydrolysis with direct determination of glycidol, our proposed method with simplified sample pretreatment was shown to be reliable.
Table 2.
Determination of GEs content in oil samples by the proposed method and AOCS Cd 29c-13.
| Determined GEs content (glycidol equivalent, mg/kg) | Theoretical GEs content (glycidol equivalent, mg/kg) | Δdetermined-theoretical a(%) | |||
|---|---|---|---|---|---|
|
|
|
||||
| This study | AOCS Cd 29c-13 |
This study | AOCS Cd 29c-13 |
||
| Sample A | 0.88 | 0.67 | 1.0 | −12.0 | −33.0 |
| Sample B | 2.89 | 2.10 | ΔSample C–B = 2.0 | 6.00 | −45.0 |
| Sample C | 5.01 | 3.20 | |||
| Sample D | 4.32 | 8.10 | ΔSample E–D = 5.0 | −8.40 | 38.0 |
| Sample E | 8.90 | 15.0 | |||
The same blind samples A to E were analyzed by our proposed method (this study) and AOCS Cd 29c-13 (conducted by an accredited third-party lab, Lab B), respectively. Sample A: purified extra virgin olive oil spiked with glycidyl oleate at 1.0 mg/kg (glycidol equivalent); sample B: commercial rice bran oil; sample C: sample B spiked with additional 2.0 mg/kg (glycidol equivalent) glycidyl oleate; sample D: commercial palm oil; sample E: sample D spiked with additional 5.0 mg/kg (glycidol equivalent) glycidyl oleate.
Δ determined-theoretical a(%) is defined as .
4. Conclusion
This study provides a different insight into the analytical approach for determining GEs and 3-MCPDEs content in edible oils. We develop a method based on direct detection of glycidol, and achieve efficient extraction of highly polar glycidol and 3-MCPD by sample preparation with modified QuEChERS. Our method was proved to be accurate and precise by method validation as well as comparison with one of the commonly used official methods, AOCS Cd 29c-13. We provide a simple and reliable analytical approach for determining GEs and 3-MCPDEs content in edible oils. Also, furfuryl alcohol rather than expensive isotope-labeled internal standards was used for glycidol quantification. Unintended side reactions from alkaline, acidic hydrolysis, and halogenation may be prevented by combining enzymatic hydrolysis and direct detection of glycidol. Furthermore, the simplified procedure may possess potential advantage of reduced errors from operational factors. Complicated and time-consuming sample preparation has long been an unsolved problem for analysis of GEs and 3-MCPDEs content, which becomes an obstacle for self-management in the edible oil industry. This method may offer industries and governments a rapid and simple alternative for routine analysis of GEs and 3-MCPDEs content in edible oils.
Supplementary Information
Acknowledgment
This work was a part of the research project supported by the National Health Research Institutes, Taiwan [Grant no.: NHRI-107A1-EMCO-01181810].
Abbreviations
- ACN
acetonitrile
- AOCS
American Oil Chemists’ Society
- 3-MBPD
3-bromo-1,2-propanediol
- 3-MCPD
3-chloro-1,2-propanediol
- 3-MCPD-d5
3-chloro-1,2-propanediol-d5
- 3-MCPDEs
3-chloro-1,2-propanediol esters
- 3-MCPD-PP
1,2-dipalmitoyl-3-chloropropanediol
- dSPE
dispersive solid-phase extraction
- EI
electron impact
- GC-MS
gas chromatography-mass spectrometry
- GEs
glycidyl esters
- GCB
graphitized carbon black
- LOD
limit of detection
- LOQ
limit of quantitation
- 3-MCPD-1-P
1-palmitoyl-3-chloropropanediol
- 3-MCPD-2-P
2-palmitoyl-3-chloropropanediol
- PSA
primary secondary amine
- RSD
relative standard deviation
- SIM
selective ion monitoring
Appendix A. Supplementary material
Chemical stability of glycidol in lipase reaction mixture during different time (0–2 h). Data are triplicate determinations. 10 mL Mcllvaine buffer (pH 7) spiked with glycidol at 1 mg/kg was shaken vigorously at 1000 strokes/min at room temperature for 0–2 h.
Selective ion monitoring (SIM) chromatograms of (A) quantifier (m/z 44), and (B) qualifier (m/z 31) of glycidol and (C) imposed SIM chromatograms of quantifier and qualifier of glycidol at 0.02 mg/kg (LOD). SIM chromatograms of (D) quantifier, and (E) qualifier of glycidol and (C) imposed SIM chromatograms of quantifier and qualifier of glycidol at 0.1 mg/kg (LOQ) in oil matrix.
Calibration curves for glycidol and 3-MCPD in solvent.
Selective ion monitoring (SIM) chromatograms of (A) glycidol (10 ppm) and (B) furfuryl alcohol (internal standard, IS) (1 ppm) in solvent. Mass spectra of (C) glycidol and (D) furfuryl alcohol in solvent.
Selective ion monitoring (SIM) chromatograms of (A) PBA derivatives of 3-MCPD (1 ppm) and (B) PBA derivatives of 3-MCPD-d5 (internal standard, IS) (100 ppb) in solvent. Mass spectra of (C) PBA derivatives of 3-MCPD and (D) PBA derivatives of 3-MCPD-d5 in solvent.
Selective ion monitoring (SIM) chromatograms of (A) glycidol (0.5 mg/kg) and IS (1.0 mg/kg), (B) glycidol (1.0 mg/kg) and IS (1.0 mg/kg), (C) 3-MCPD (0.5 mg/kg) and IS (0.1 mg/kg), (D) 3-MCPD (1.0 mg/kg) and IS (0.1 mg/kg) in oil matrix (2 g purified extra-virgin olive oil).
Funding Statement
This work was a part of the research project supported by the National Health Research Institutes, Taiwan [Grant no.: NHRI-107A1-EMCO-01181810].
Footnotes
Conflicts of interest
The authors have no conflicts of interest.
References
- 1. Weißhaar R, Perz R. Fatty acid esters of glycidol in refined fats and oils. Eur J Lipid Sci Technol. 2010;112:158–65. [Google Scholar]
- 2. Pudel F, Benecke P, Fehling P, Freudenstein A, Matthäus B, Schwaf A. On the necessity of edible oil refining and possible sources of 3-MCPD and glycidyl esters. Eur J Lipid Sci Technol. 2011;113:368–73. [Google Scholar]
- 3. Hrncirik K, van Duijn G. An initial study on the formation of 3-MCPD esters during oil refining. Eur J Lipid Sci Technol. 2011;113:374–9. [Google Scholar]
- 4. Liu M, Gao BY, Qin F, Wu PP, Shi HM, Luo W, et al. Acute oral toxicity of 3-MCPD mono- and di-palmitic esters in Swiss mice and their cytotoxicity in NRK-52E rat kidney cells. Food Chem Toxicol. 2012;50:3785–91. doi: 10.1016/j.fct.2012.07.038. [DOI] [PubMed] [Google Scholar]
- 5. Abraham K, Appel KE, Berger-Preiss E, Apel E, Gerling S, Mielke H, et al. Relative oral bioavailability of 3-MCPD from 3-MCPD fatty acid esters in rats. Arch Toxicol. 2013;87:649–59. doi: 10.1007/s00204-012-0970-8. [DOI] [PubMed] [Google Scholar]
- 6. CONTAM. Risks for human health related to the presence of 3- and 2-monochloropropanediol (MCPD), and their fatty acid esters, and glycidyl fatty acid esters in food. EFSA J. 2016;14:e04426. [Google Scholar]
- 7. Masukawa Y, Shiro H, Nakamura S, Kondo N, Jin N, Suzuki N, et al. A new analytical method for the quantification of glycidol fatty acid esters in edible oils. J Oleo Sci. 2010;59:81–8. doi: 10.5650/jos.59.81. [DOI] [PubMed] [Google Scholar]
- 8. Haines TD, Adlaf KJ, Pierceall RM, Lee I, Venkitasubramanian P, Collison MW. Direct Determination of MCPD Fatty Acid Esters and Glycidyl Fatty Acid Esters in Vegetable Oils by LC-TOFMS. J Am Oil Chem Soc. 2011;88:1–14. doi: 10.1007/s11746-010-1732-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Blumhorst MR, Venkitasubramanian P, Collison MW. Direct determination of glycidyl esters of fatty acids in vegetable oils by LC–MS. J Am Oil Chem Soc. 2011;88:1275–83. doi: 10.1007/s11746-011-1873-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Am. Oil Chem. Soc, editor. AOCS official method. p. Cd 28-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. MacMahon S, Begley TH, Diachenko GW. Occurrence of 3-MCPD and glycidyl esters in edible oils in the United States. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2013;30:2081–92. doi: 10.1080/19440049.2013.840805. [DOI] [PubMed] [Google Scholar]
- 12. MacMahon S, Begley TH, Diachenko GW. Analysis of processing contaminants in edible oils. Part 2. Liquid chromatography–tandem mass spectrometry method for the direct detection of 3-monochloropropanediol and 2-monochloropropanediol diesters. J Agric Food Chem. 2013;61:4748–57. doi: 10.1021/jf400581g. [DOI] [PubMed] [Google Scholar]
- 13.Am. Oil Chem. Soc, editor. AOCS Official Method. p. Cd 29a-13. [Google Scholar]
- 14.Am. Oil Chem. Soc, editor. AOCS Official Method. p. Cd 29b-13. [Google Scholar]
- 15.Am. Oil Chem. Soc, editor. AOCS Official Method. p. Cd 29c-13. [Google Scholar]
- 16.Am. Oil Chem. Soc, editor. Joint JOCS/AOCS Official Method. p. Cd 29d-19. [Google Scholar]
- 17. Zelinkova Z, Giri A, Wenzl T. Assessment of critical steps of a GC/MS based indirect analytical method for the determination of fatty acid esters of monochloropropanediols (MCPDEs) and of glycidol (GEs) Food control. 2017;77:65–75. doi: 10.1016/j.foodcont.2017.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zwagerman R, Overman P. A novel method for the automatic sample preparation and analysis of 3-MCPD-, 2-MCPD-, and glycidylesters in edible oils and fats. Eur J Lipid Sci Technol. 2016;118:997–1006. [Google Scholar]
- 19. Kaze N, Sato H, Yamamoto H, Watanabe Y. Bidirectional conversion between 3-monochloro-1, 2-propanediol and glycidol in course of the procedure of DGF standard methods. J Am Oil Chem Soc. 2011;88:1143–51. [Google Scholar]
- 20. Brönsted J, Kilpatrick M, Kilpatrick M. Kinetic studies on ethylene oxides. J Am Oil Chem Soc. 1929;51:428–61. [Google Scholar]
- 21. Parker R-E, Isaacs N. Mechanisms of epoxide reactions. Chem Rev. 1959;59:737–99. [Google Scholar]
- 22. Anastassiades M, Lehotay SJ, Štajnbaher D, Schenck FJ. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J AOAC Int. 2003;86:412–31. [PubMed] [Google Scholar]
- 23. Stadler P, Kovac A, Haalck L, Spener F, Paltauf F. Stereo-selectivity of Microbial Lipases: The Substitution at Position sn-2 of Triacylglycerol Analogs Influences the Stereo-selectivity of Different Microbial Lipases. Eur J Biochem. 1995;227:335–43. doi: 10.1111/j.1432-1033.1995.tb20394.x. [DOI] [PubMed] [Google Scholar]
- 24. Zwagerman R, Overman P. Optimized Analysis of MCPD-and Glycidyl Esters in Edible Oils and Fats Using Fast Alkaline Transesterification and 13C-Correction for Glycidol Overestimation: Validation Including Interlaboratory Comparison. Eur J Lipid Sci Technol. 2019;121:1800395. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Chemical stability of glycidol in lipase reaction mixture during different time (0–2 h). Data are triplicate determinations. 10 mL Mcllvaine buffer (pH 7) spiked with glycidol at 1 mg/kg was shaken vigorously at 1000 strokes/min at room temperature for 0–2 h.
Selective ion monitoring (SIM) chromatograms of (A) quantifier (m/z 44), and (B) qualifier (m/z 31) of glycidol and (C) imposed SIM chromatograms of quantifier and qualifier of glycidol at 0.02 mg/kg (LOD). SIM chromatograms of (D) quantifier, and (E) qualifier of glycidol and (C) imposed SIM chromatograms of quantifier and qualifier of glycidol at 0.1 mg/kg (LOQ) in oil matrix.
Calibration curves for glycidol and 3-MCPD in solvent.
Selective ion monitoring (SIM) chromatograms of (A) glycidol (10 ppm) and (B) furfuryl alcohol (internal standard, IS) (1 ppm) in solvent. Mass spectra of (C) glycidol and (D) furfuryl alcohol in solvent.
Selective ion monitoring (SIM) chromatograms of (A) PBA derivatives of 3-MCPD (1 ppm) and (B) PBA derivatives of 3-MCPD-d5 (internal standard, IS) (100 ppb) in solvent. Mass spectra of (C) PBA derivatives of 3-MCPD and (D) PBA derivatives of 3-MCPD-d5 in solvent.
Selective ion monitoring (SIM) chromatograms of (A) glycidol (0.5 mg/kg) and IS (1.0 mg/kg), (B) glycidol (1.0 mg/kg) and IS (1.0 mg/kg), (C) 3-MCPD (0.5 mg/kg) and IS (0.1 mg/kg), (D) 3-MCPD (1.0 mg/kg) and IS (0.1 mg/kg) in oil matrix (2 g purified extra-virgin olive oil).