Abstract
Triple-negative breast cancers (TNBCs) lack specific targeted therapy options and have evolved into highly chemo-resistant tumors that metastasize to multiple organs. The present study demonstrated that the proline dehydrogenase (PRODH) mRNA level in paired (tumor vs. normal) human breast tissue samples (n=234) was 6.6-fold greater than normal cells (*p=0.021). We established stable PRODH-overexpressing TNBC (HS578T) cells, and the malignant phenotypes were evaluated using soft agar colony formation and Transwell migration assays. The results demonstrated that PRODH induced epithelial-mesenchymal transition in cancer cells and increased cell proliferation. The present study found that the tea polyphenol epigallocatechin-3-gallate (EGCG) significantly inhibited PRODH and its regulated proteins, such as alpha-smooth muscle actin (alpha-SMA) expression in TNBC cells. These findings support the targeting of the PRODH signaling pathway as a potential therapeutic strategy in preventing cancer cell metastasis. The patient-derived xenograft (PDX) mouse model is highly relevant to real human tumor growth. We established a TNBC-PDX (F4, n=4 in each group)mouse model. The PDX mice were treated with EGCG (50 mg/kg), and the results indicated that EGCG significantly inhibited PDX tumor growth (*p = 0.013). These experiments provide additional evidence to evaluate the antitumor effects of EGCG-induced PRODH inhibition for clinical therapeutic application, especially in TNBC patients.
Keywords: Triple-negative breast cancer, Proline dehydrogenase, Epigallocatechin-3-gallate, Patient-derived xenograft
1. Introduction
Breast cancer is the most common diagnosis, and it is the leading cause of cancer death in women. More than 1.3 million cases are diagnosed, and over 450,000 women die from breast cancer annually [1]. Despite the decrease in mortality and incidence, breast cancer remains a major cause of cancer mortality and accounts for 5% of all cancer deaths in women in the US [1]. Breast cancer tumors are routinely classified as hormone receptor- (e.g., estrogen receptor (ER) and progesterone receptor (PR)), and HER2/Neu-amplified tumors. The distinct subtypes have prognostic and therapeutic implications. Tumors that express ER and PR are treated with targeting agents to block hormone production or increase the degradation of receptors [2]. HER2/Neu-amplified tumors are treated with agents that inhibit the HER2/Neu receptor [3, 4]. The last subtype of breast cancer is referred to as triple-negative breast cancer (TNBC) because it lacks of all these markers. TNBC represents approximately 10–15% of all breast cancer patients, and it exhibits poor therapeutic outcomes compared to the other subtypes of breast cancer. TNBC patients are treated with predominantly chemotherapy [5, 6].
Cancer cell lines are useful for preclinical investigations. The most successful example is the anti-HER2/Neu mouse monoclonal antibody, which was humanized to create trastuzumab®, and exhibited significant antitumor effects in HER2/ Neu-amplified cells but not in cells lacking amplified HER2/Neu [7, 8]. These results formed the basis of the predicted clinical trial outcomes of trastuzmab. However, two previous studies demonstrated that dasatinib, an orally available tyrosine kinase inhibitor (TKI) that inhibits cancer cell growth at subnanomolar levels, had the greatest response against TNBC [9, 10]. However, the clinical trial data from a phase II study showed a partial response [11]. These data are disappointing because the preclinical data in cell lines did not successfully translate into clinical therapeutic applications. TNBC is a heterogeneous group of tumors, and it is necessary to identify whether a tumor subset may benefit from a particular therapy, such as EGFR-targeted agents. Although EGFR expression is a marker of TNBC cells, it is not necessarily a pathogenic driver of the transformation of TNBC cells [12]. Further preclinical studies demonstrated that TNBC cell lines were relatively resistant to a single EGFR targeting agent [13, 14].
The use of TNBC cell lines for animal xenograft studies is the most direct method to assess the efficacy of antitumor agents. Molecularly targeted agents were evaluated in xenografts to confirm their in vitro effects in an in vivo model. However, an important issue is that in vitro cultured systems lack the proper microenvironment for the interaction of tumor cells and surrounding stromal tissues. Therefore, patient-derived xenograft (PDX) models of cancer were developed via the implantation of tissue from a patient’s tumor into immunodeficient mice. These PDX models are used to create an environment that allows tumor growth to be monitored.
Previous studies demonstrated that phytochemicals provided an easy approach with fewer side effects are valuable options and effective chemo-preventive agents for TNBC [15, 16]. The tea polyphenol EGCG effectively inhibited the development of breast cancer, including TNBC [17]. Our previous study demonstrated that EGCG induced cell apoptosis and contributed to breast tumor regression via downregulating the expression of the estrogen receptor in breast cancer cells [18]. Our previous study also showed that downregulation of α9-nicotinic receptor expression correlated with the anti-proliferative effects of EGCG in breast cancer cells [18, 19]. Despite promising preclinical findings, the applicability of tea as a human cancer therapy is limited due to a lack of efficient systemic studies. Notably, our studies showed that EGCG inhibition of the α9-nicotinic receptor blocked many different types of smoking-induced diseases, including cancer [20–23].
An increased activity of amino acid transporters was observed in breast cancer [24, 25]. Metabolic signaling and amino acid metabolism are significantly regulated in breast neoplasms [24]. A previous study demonstrated that a nutrient mixture containing high concentrations of lysine, proline, ascorbic acid, and epigallocatechin gallate significantly suppressed the tumor growth of breast cancer cells in female athymic nude mice and significantly inhibited MMP expression, angiogenesis, and invasion in breast cancer cells in vitro, which offered promise for therapeutic use in the treatment of breast cancer [26]. The study demonstrated that amino acid metabolism genes played crucial roles in tumor development and may serve as prospective drug targets or biomarkers for breast cancer [26]. From prokaryotes to the highest eukaryotes, proline is catabolized by a unique and structurally conserved flavoprotein, proline dehydrogenase (PRODH) [27, 28]. A recent study demonstrated that high mRNA expression levels of PRODH correlated with good prognoses [29]. The present study found that PRODH mRNA overexpression was detected preferentially in human tumors. PRODH supports breast cancer cell growth and survival by consuming proline for anaplerotic glutamate production and bypasses glutaminase (GLS1) to fuel oxidative phosphorylation and sustain ATP levels [30]. PRODH enzymatic inhibition using proline competitive inhibitors, such as L-tetrahydro-2-furoic acid (L-THFA) or (S)-5-oxo-2-tetrahydrofurancarboxylic acid (5-oxo), induced breast cancer cell apoptosis (cleaved PARP) and reduced viable cell growth within 48 hrs. Combined PRODH knock-down or enzymatic inhibition with a p53wt restoring drug (e.g., MI-63 or nutlin-3a) or a clinical GLS1 inhibitor (CB-839 or Calithera) synergistically induced apoptosis and growth inhibition against malignant (e.g., MCF7, ZR-75-1, and DU4475), but not normal (MCF10 A), breast epithelial cells [31]. This study first demonstrated that the tea polyphenol EGCG markedly reduced tumor growth via attenuation of PRODH protein levels in a TNBCPDX mouse model. These experiments allowed investigators to evaluate the function of tumor growth driving genes and therapeutic strategies to disrupt these pathways.
2. Material and methods
2.1. Cell culture
BT-549 (ATCC® HTB-122™), Hs 587T (ATCC® HTB-126™), MDA-MB-436 (ATCC® HTB-130™), MDA-MB-468 (ATCC® HTB-132™), MDA-MB-231 (ATCC® HTB-26™), MDA-MB-453 (ATCC® HTB- 131™), HCC1395 (ATCC® SC-CRL-2324™), HCC38 (ATCC® CRL-2314™), MCF 10A (ATCC® CRL-10317™), AU565 (ATCC® CRL-2341™), SK-BR-3 (ATCC® HTB-30™), BT-474 (ATCC® HTB-20™), HCC1419 (ATCC® CRL-2326™), HCC1954 (ATCC® CRL-2338™), T-47D (ATCC® HTB-133™), MCF7 (ATCC® HTB-22™) and ZR-75-1 (ATCC® CRL-1500™) cells were purchased from American Type Culture Collection (ATCC, Manassas, Virginia, USA). The cells were cultured in Dulbecco’s modified Eagle medium/nutrient mixture F-12 (DMEM/ F12, Gibco, California, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco, California, USA) and 50 U/mL penicillin/ streptomycin/neomycin (Invitrogen, California, USA) in a humidified (5% CO2, 37°C) incubator. All human breast tumor samples (n = 234) were obtained as specimens from anonymous donors from Taipei Medical University Hospital, Taipei, Taiwan, as approved by the Institutional Review Board (IRB) and ethics committee of the institution (P102025). Histological inspection confirmed that all patient samples consisted of greater than 80% tumor tissue. All samples (each paired tumor tissue vs. normal tissue) were collected and categorized according to clinical characteristics, such as age.
2.2. Cell proliferation assay
Cell proliferation was evaluated using the cell-counting method. Cells (2 × 104 per well) were seeded in 12-well plates and allowed to grow for different times. The growth rate was determined by the cell number, which was counted in a hemocytometer in triplicate every day of culture.
2.3. Immunohistochemistry (IHC) analysis
PRODH protein expression in PDX tumor tissues was detected using IHC. Paraffin-embedded PDX tumor tissues were cut into 8-μm sections. The sections were preincubated in 3% H2O2 and 0.3% Triton X-100 before microwaving for antigen retrieval. For PRODH immunostaining, sections were microwaved in Tris buffer (pH 6) for 30 minutes. The antigenicity of the tumor cells in sections was blocked in 5% horse serum (Chemicon, Temecula, CA) for 30 minutes and incubated with a diluted (1:100) PRODH (NBP1-92288, Novus, Massachusetts, USA)-specific antibody for 2 hrs at room temperature. Staining was developed using the streptavidin-biotin-peroxidase method and an LSAB 2 kit purchased from Dako (Carpinteria, CA). Briefly, sections were washed in phosphate-buffered saline (PBS) and incubated with a biotinylated anti-rabbit secondary antibody. The samples were washed again in the same buffer and incubated with a streptavidin-biotin-peroxidase complex. Staining was completed after incubation with substrate-chromogen solution. The duration of incubation in solution with 3,3′-diaminobenzidine was determined using low-power microscopic inspection. Slides were washed, dehydrated, and coverslipped using a mixture of distyrene, plasticizer, and xylene (DPX mounting medium) (44581, Sigma-Aldrich, St. Louis, MO, USA). Adjacent sections on the same slides were counterstained with hematoxylin for general histological orientation.
2.4. In vitro cell migration (Transwell) assay
An in vitro cell migration assay was performed using 24-well Transwell inserts (8 μm) (Corning, New York, USA). Briefly, Transwell inserts were seeded with 2 × 104 Hs 587T cells, and the lower chambers were filled with HA (NAT-172, New York, USA) at different concentrations. The assay was performed at 37°C for 4 hrs. Cells were fixed and stained with 0.5% crystal violet (Sigma-Aldrich, Missouri, USA). The migrated cells in the Transwell inserts in all fields were calculated using microscopy (DMI 4000B, Leica, Wetzlar, Germany), and the calculation was repeated in three independent experiments.
2.5. RNA extraction and quantitative reverse transcription PCR
Total RNA was isolated from human cell lines and breast tumor tissue samples from patients using TRIzol (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. A LightCycler thermocycler (LC 2.0, Roche Molecular Biochemicals, Mannheim, Germany) was used for real-time quantitative PCR. PRODH mRNA fluorescence intensity was measured and normalized to β-glucuronidase expression using built-in software (Roche LightCycler version 4). The following primer sequences were used: PRODH primer sense sequence: 5′-GGATGCCTATGACAATG-3′ and PRODH primer antisense sequence: 5′-CCTTGGCGTTGTGCTTC-3′.
2.6. Protein extraction and western blotting
For protein extraction, cells were washed twice with ice-cold PBS and lysed on ice in Golden lysis buffer (20 mM Tris-HCl (pH 8.0), 137 mM NaCl, 5.95 mM EDTA, 5 mM EGTA, 10 mM NaF, 1% Triton X-100, and 10% glycerol) supplemented with protease inhibitors (Roche, Indianapolis, USA) and phosphatase inhibitors (Sigma-Aldrich, St. Louis, USA). The proteins were separated using 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride membranes. Specific antibodies against PRODH (NBP1-92288, Novus, Massachusetts, USA), cyclin A (GT29756, GeneTex, California, USA), cyclin B (GT100911, GeneTex, California, USA), cyclin D (GT108624, GeneTex, California, USA), cyclin E (GT23927, GeneTex, California, USA), p27 (GT114, GeneTex, California, USA), α-SMA (GTX100034, GeneTex, California, USA), fibronectin (GTX112794, GeneTex, California, USA), E-cadherin (GTX100443, GeneTex, California, USA), N-cadherin (GTX127345, GeneTex, California, USA), β-actin (GTX109639, GeneTex, California, USA) and GAPDH (sc-47724, Santa Cruz Biotechnology, California, USA) were diluted 1:2000 in Tris-buffered saline/Tween 20, and the membranes were incubated for 2 hrs at room temperature. Horseradish peroxidase-conjugated anti-mouse IgG (sc-2344, Santa Cruz Biotechnology, California, USA) and anti-rabbit IgG (sc-2004, Santa Cruz Biotechnology, California, USA) secondary antibodies were diluted 1:4000 and incubated with the membranes for 1 hour at room temperature.
2.7. In vivo breast cancer patient-derived TNBC tumor xenograft mouse model
Nonobese diabetic–severe combined immunodeficiency–IL2R gamma-null (NSG) mice and patient-derived xenograft (PDX) tumor-bearing mice (J000100674) were obtained from breeding pairs originally purchased (JAX#4659679) from Jackson Laboratories (005557, Bar Harbor, ME, USA). NSG mice were bred in a pathogen-free unit and maintained in sterile cages. Mice were handled and cared for with strict adherence to the guidelines established by the Animal Resource Center and following study protocols approved by the Laboratory Animal Center and Use Committee at Taipei Medical University (IACUC protocol LAC-2018-0343). PDX tumor specimens from PDX tumor-bearing mice (J000100674) were transplanted into female NSG mice. The animals were monitored for engraftment via routine palpation, and the tumors were harvested when they reached a volume of 0.5 cm3. PDX tumor-bearing mice were randomly divided into three groups (n = 4 each group). One group was injected subcutaneously with EGCG (50 mg/kg) over the course of 5 injections every other day. Another group was injected with lipodox (1.5 mg/ kg) via the tail vein over the course of 2 injections once weekly. PBS-treated mice served as the treatment control. The tumor volume was measured and monitored every two days post injection for up to 11 days. The tumor volume was calculated as (Length × width2)/2.
2.8. Statistical analysis
All data are expressed as the means with 95% confidence intervals (CIs) of at least three determinations unless stated otherwise. A paired t-test was performed to compare PRODH mRNA expression in paired normal tissues vs. tumor tissues dissected from breast cancer patients. The difference in PRODH mRNA expression detected in tumor samples vs. normal samples was analyzed using the Scheffe test. All statistical comparisons were performed using SigmaPlot graphing software (San Jose, CA) and Statistical Package for the Social Sciences version 21.0.0 (SPSS, Chicago, IL). All statistical tests were two-sided. A P value of 0.05 or less indicated statistical significance.
3. Results
3.1. PRODH overexpression was preferentially detected in human breast tumor cells
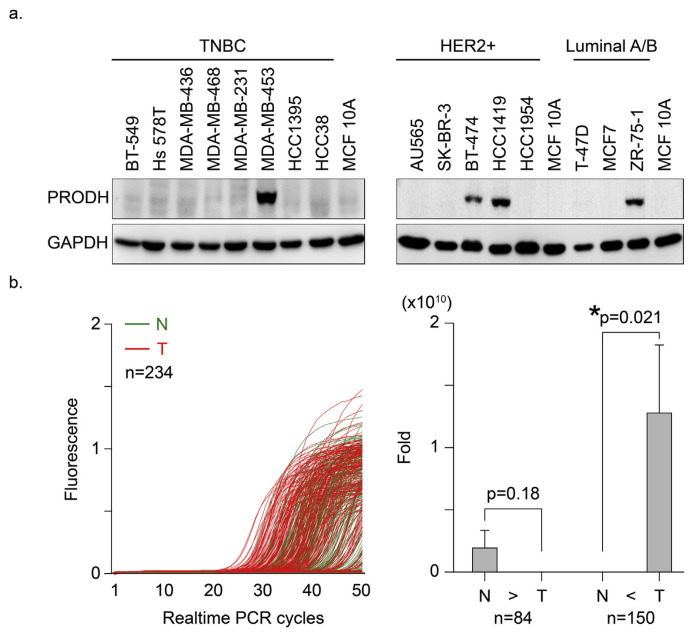
The present study found that PRODH protein was detected at a higher level in various types of human breast cancer cell lines than normal breast epithelial (MCF-10A) cells (Fig. 1a). This result suggested that PRODH was expressed in tumor cells with unique clinical characteristics in association with the clinical diagnosis classification. To test this hypothesis, PRODH mRNA levels were detected using real-time (RT)-PCR in paired (tumor vs. normal) human breast tissue samples (n = 234). The PCR amplification curves were “left-shifted” in the tumor tissues (Fig. 1b, red lines) relative to the profiles of normal tissues (green lines), which indicated that the tumor samples contained greater quantities of PRODH mRNA overall. The results from Fig. 1b were calculated and demographic evaluation of clinical criteria and PRODH mRNA expression fold changes between tumor vs normal paired samples were performed in Table 1. The data were divided into two groups (normal higher than tumor, N > T, n = 84, vs. tumor higher than normal, T > N, n = 150) according to PRODH mRNA expression patterns. PRODH expression in tumor cells was 6.6-fold (*p = 0.021) greater than normal cells in the T > N group (copy number for normal cells = 2112 vs. tumor cells = 23838, difference = 21726, 95% CI = −34403 to −9047, *p=0.001) (Fig. 1b, right panel).
Fig. 1.
PRODH overexpression was preferentially detected in human breast tumor cells. (a). Human breast cancer cell lines were subdivided into three groups (TNBC, HER2+ and Lumina A/B). The cells were cultured according to standard protocols. Normal human breast epithelial cells (MCF 10A) were used as a normal control. Protein lysates were harvested, and the expression levels of the PRODH protein were detected using Western blotting. GAPDH protein was assessed and used as a control. (b). The mRNA expression level of PRODH in normal and malignant human breast tissues. Left, the HNMT mRNA expression profiles of paired human breast tumor (red lines) and normal (green lines) tissues (n = 234) were detected using real-time PCR. PRODH mRNA expression levels in 150 patient samples in which expression was higher in the tumor tissue than normal tissue (T > N) vs. 84 samples in which expression was higher in normal tissue than tumor tissue (N > T). The results are presented as fold changes ± SEMs; error bars indicate 95% confidence intervals. Normal vs. tumor tissue in group 1 (T > N), *p = 0.021; normal vs. tumor tissue in group 2 (N > T), p < 0.018. Data were analyzed using the Wilcoxon signed ranks test, and the P-values presented are two-sided.
Table 1.
Demographic evaluation of clinical criteria and PRODH mRNA expression fold changes between tumor vs normal paired samples.
| Characteristic | Total No. of Patients | PRODH (N > T) | PRODH (N < T) | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| No. | Mean fold change | P | No. | Mean fold change | P | ||
| Age | 0.17 | 0.07 | |||||
| <45 y | 43 | 20 | 69,000,000 | 23 | 520,000,000 | ||
| ≥45y | 191 | 64 | 2,100,000,000 | 127 | 15,000,000,000 | ||
| Nodal status | 0.74 | 0.85 | |||||
| N0 | 111 | 41 | 3,300,000,000 | 70 | 17,000,000,000 | ||
| N1 | 63 | 24 | 65,000,000 | 39 | 14,000,000,000 | ||
| N2 | 27 | 10 | 34,000,000 | 17 | 7,400,000,000 | ||
| N3 | 25 | 6 | 6,200,000 | 19 | 25,000,000,000 | ||
| Stage | 0.29 | 0.3 | |||||
| 0 | 2 | 2 | 4.5 | 0 | |||
| I | 50 | 18 | 7,200,000,000 | 32 | 350,000,000 | ||
| II | 119 | 47 | 670,000,000 | 72 | 23,000,000,000 | ||
| III | 60 | 16 | 23,000,000 | 44 | 4,200,000,000 | ||
| IV | 1 | 0 | 1 | 22,000,000,000 | |||
| ER status | 0.62 | 0.74 | |||||
| Negative | 48 | 18 | 560,000,000 | 30 | 16,000,000,000 | ||
| Positive | 186 | 66 | 2,300,000,000 | 120 | 12,000,000,000 | ||
| PR status | 0.37 | 0.62 | |||||
| Negative | 88 | 32 | 310,000,000 | 56 | 9,200,000,000 | ||
| Positive | 145 | 51 | 2,700,000,000 | 94 | 150,000,000,000 | ||
| Her-2 status | 0.84 | 0.09 | |||||
| Negative | 196 | 75 | 2,000,000,000 | 121 | 8,200,000,000 | ||
| Positive | 38 | 9 | 1,100,000,000 | 29 | 32,000,000,000 | ||
| Path | 0.99 | 0.96 | |||||
| DCIS | 12 | 3 | 1,200 | 0 | |||
| Infiltration ductal carcinoma | 289 | 70 | 2,300,000,000 | 132 | 14,000,000,000 | ||
| Mucinous carcinoma | 8 | 4 | 14.3 | 4 | 35,000,000 | ||
| ILC | 13 | 3 | 2.7 | 7 | 110,000,000 | ||
| IPC | 1 | 1 | 2.3 | 0 | |||
| Mix | 8 | 2 | 5,600 | 6 | 35,000,000,000 | ||
| other | 1 | 1 | 1,900,000,000 | 0 | |||
3.2. PRODH induced TNBC tumor cell malignant phenotypes
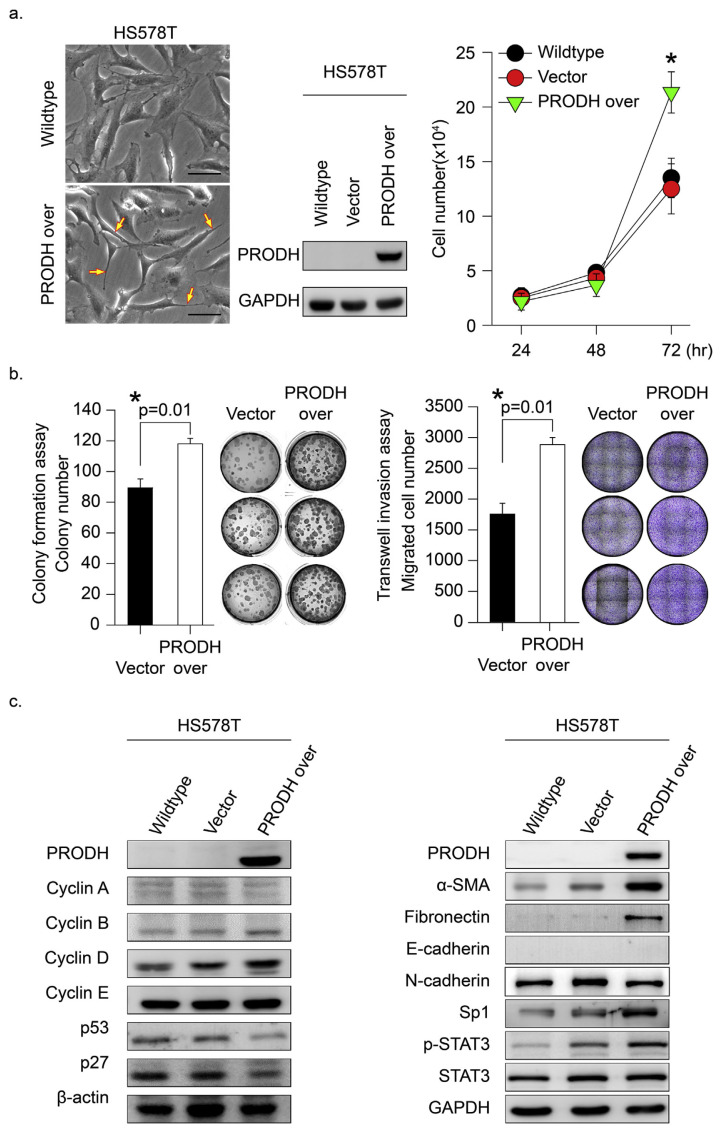
TNBC does not express estrogen or progesterone receptors and does not contain much HER2 protein [32]. Because TNBC cancer cells lack these molecular targets, treatment options for TNBC are limited. Therefore, the identification of a specific molecular marker with carcinogenic-driving effects is urgently needed in TNBC. To identify a potential marker, TNBC (HS578T) cells with low PRODH protein expression (Fig. 1a) were selected as a cell research model. We established stable PRODH-over-expressing HS578T cells, and the malignant phenotypes were evaluated (Fig. 2a). The gross cell morphology in the HS578T cells was significantly changed to a spindle shape with highly malignant phenotypes (Fig. 2a, left yellow arrowheads indicated). The in vitro results indicated that PRODH overexpression significantly enhanced HS578T tumor cell proliferation (Fig. 2a, *p = 0.006). Malignant phenotypes, such as soft agar colony formation and Transwell migration assays, in PRODH-overexpressing HS578T cells were evaluated. The results indicated that PRODH enhanced tumor growth migration and soft agar colony formation (Fig. 2b, *p = 0.01). Notably, we found that forced overexpression of PRODH in TNBC (HS578T) cells significantly induced cell cycle regulatory protein (cyclin D and B) activation. P53 and p27 were significantly downregulated in PRODH-overexpressing cells (Fig. 2c, left panel). PRODH-induced metastasis-related signal proteins were determined, and α-SMA and fibronectin were significantly upregulated in HS578T cancer cells (Fig. 2c, right panel). These results suggest that PRODH-enhanced tumor growth malignancy was mediated via activation of the cell growth cycle and migration-regulated signals.
Fig. 2.
PRODH expression induced malignant phenotype changes in HS578T cells. (a) We established stable PRODH-overexpressing HS578T cells using the pcDNA5-TO system as described in the Materials and Methods. Middle panel, PRODH protein expression levels in wild-type, PRODH-overexpressing, and vector control HS578T cells are shown. Left, the established cell gross morphology is shown. Right panel, cell growth and proliferation were determined. (b) The malignant phenotypes of soft agar colony formation and migration were evaluated in HS578T cells stably expressing PRODH using the pcDNA5-TO system and vector control cells. Left, the soft agar colonies in the entire area of each plate were counted. Comparisons were performed for vector vs. PRODH-overexpression groups (*p = 0.01). Right, invasive capacity measured at 24 hrs using a Transwell assay. Quantification of the cell number was performed using ImageJ software. Comparisons were performed for HS578T-vector vs. HS578Y-PRODH overexpression (*p = 0.01). Data represent the means of three samples in each group; error bars are 95% confidence intervals. Data were analyzed using Student’s t-test; all P-values are two-sided. (c) HS578T cells with or without PRODH overexpression were established, and protein markers related to tumor cell growth proliferation (left) and tumor metastasis (right) were detected using Western blotting. The proteins β-actin and GAPDH served as a control.
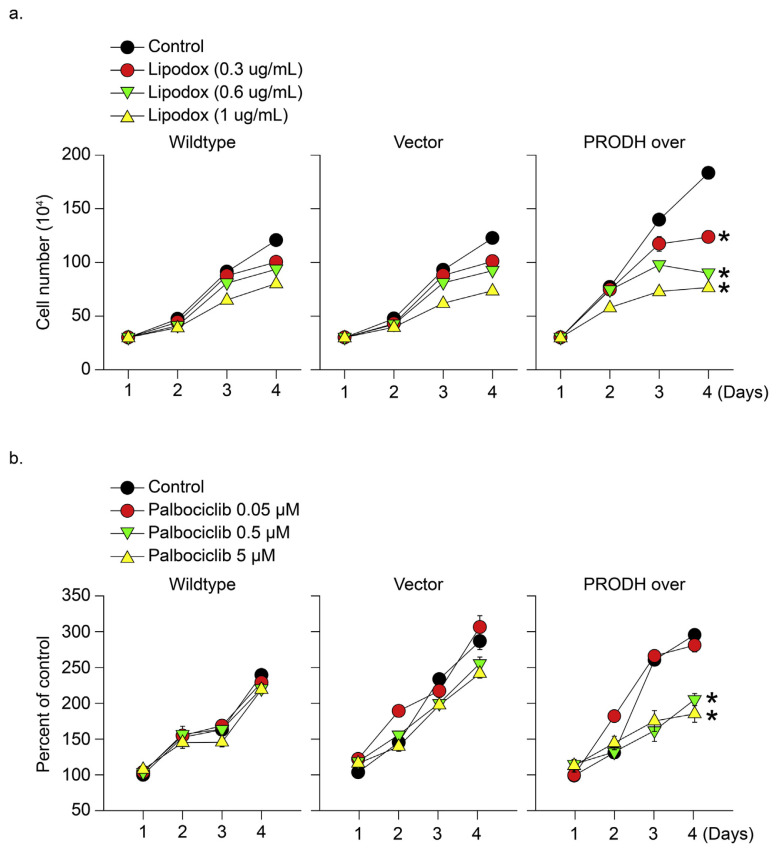
3.3. PRODH overexpression sensitized TNBC tumor cells to clinical therapeutic drugs
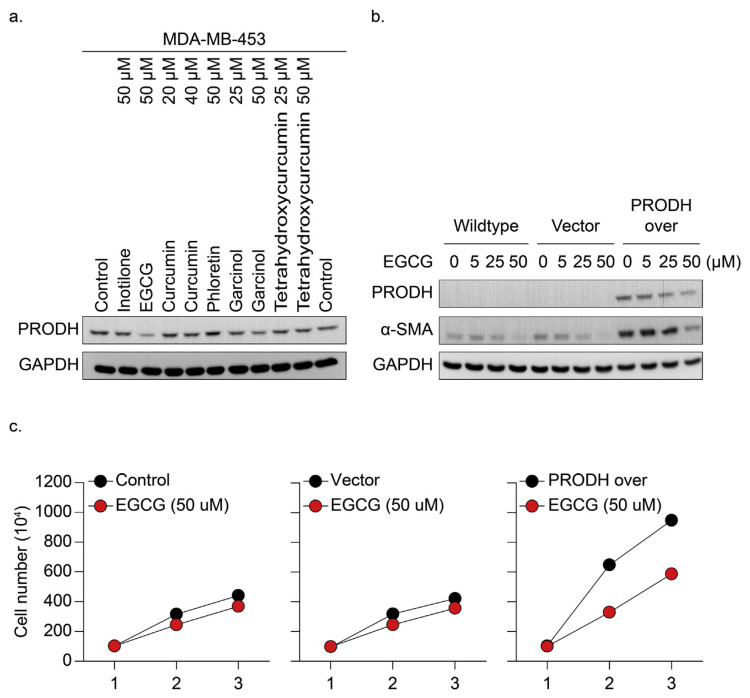
To examine whether the expression of PRODH in TNBC is a valid marker for clinical therapeutic sensitivity, PRODH-overexpressing and vector control cells were treated with clinically used TNBC therapeutic drugs (lipodox and palbociclib) for 24–48 hrs. Cell growth inhibition was detected predominantly in PRODH-overexpressing TNBC cells compared to the control groups (Fig. 3a and b). These results suggest that PRODH overexpression enhanced drug sensitivity in TNBC cells compared to the control group (*p < 0.05). We further tested compounds isolated from natural products for 24 hrs for PRODH protein inhibition in MDA-MB-453 cells overexpressing PRODH protein (Fig. 1a). The cells were collected, and the protein lysates were harvested for detection of PRODH formation (Fig. 4a). Our data indicated that PRODH protein levels were significantly inhibited in tea polyphenol EGCG (50 μM)-treated HS578T cells. Dose-dependent experiments were further performed. HS578T cells were treated with EGCG (5–50 μM) for 24 hrs, and the PRODH protein level was determined using immunoblotting analysis in PRODH-overexpressing TNBC, vector, and wild-type control cells (Fig. 4b). The results further demonstrated that EGCG effectively downregulated PRODH protein expression in TNBC-overexpressing cells. However, the inhibition of PRODH-induced α-SMA expression was only detected in cells treated with higher concentrations of EGCG (>50 μM). The PRODH overexpression and vector control cells were treated with EGCG (50 μM) for 24–48 hrs. Cell growth inhibition was detected predominantly in PRODH-overexpressing TNBC cells compared to the control groups (Fig. 4c).
Fig. 3.
PRODH overexpression sensitized TNBC tumor cells to clinical therapeutic drugs. (a) Wild-type HS578T, PRODH-overexpressing, and vector control cells were treated with clinical therapeutic agents, including (a) lipodox (0–1 μg/mL) and (b) palbociclib (0.05–5 μM) for 4 days. The cell growth number was calculated, and the results revealed that inhibition of cell viability was only observed in the PRODH-overexpressing cells.
Fig. 4.
EGCG inhibited PRODH-overexpressing cells via downregulation of PRODH protein expression. (a) Wild-type TNBC (MDA-MB-453) cells, which express PRODH at a higher level (Fig. 1a), were treated with different compounds isolated from natural products for 24 hrs. The protein lysates were isolated. Western blotting was performed, and the protein expression of PRODH was detected via immunoblotting analysis. GAPDH protein expression served as a control. (b) Wild-type, PRODH-overexpressing, and vector control HS578T cells were treated with EGCG (50 μM) for 24–48 hrs, and the results revealed that EGCG-induced cell growth inhibition was only observed in the PRODH-overexpressing cells. (c) Wild-type, PRODH-overexpressing, and vector control HS578T cells were treated with different concentrations of EGCG (5–50 μM) for 24–48 hrs. The protein lysates were isolated. Western blotting was performed, and the protein expression of PRODH and α-SMA was detected using immunoblotting analysis. GAPDH protein expression served as a control.
3.4. EGCG and lipodox induced TNBC-PDX tumor growth inhibition via downregulation of PRODH protein expression
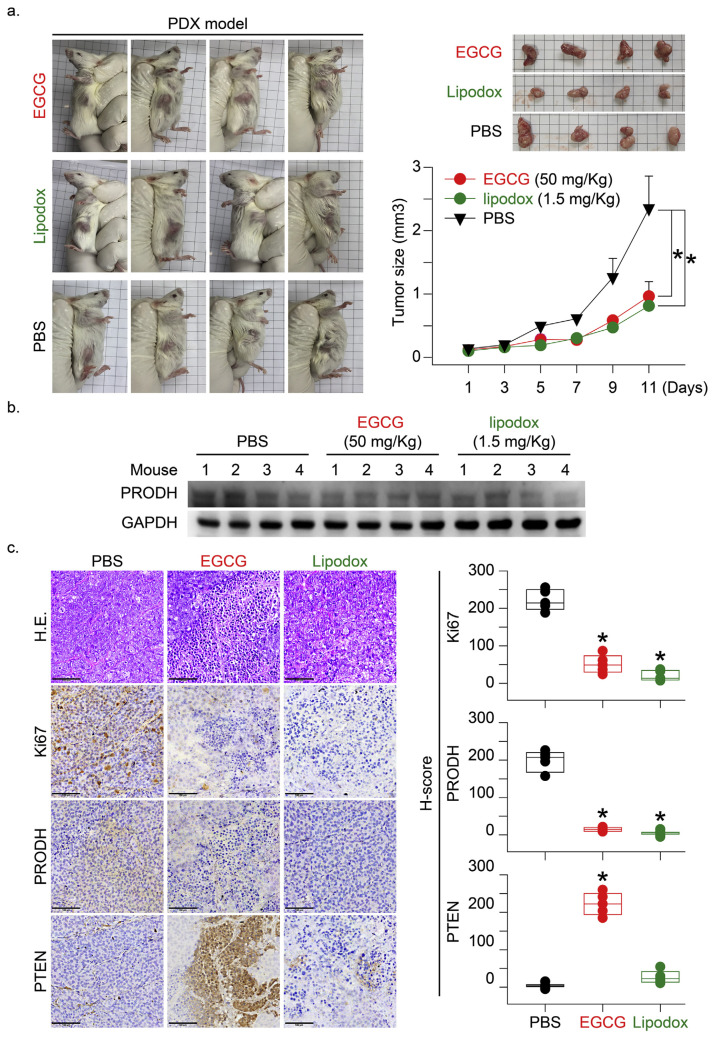
To test whether PRODH expression in tumor tissue directly influenced tumor growth, we established a TNBC-PDX (F4, n = 4 in each group) mouse model. Tumor chunks from representative cases were implanted in the fat pad area near the mammary gland tissue in mice (Fig. 5a). The in vitro study results indicated that EGCG and doxorubicin inhibited PRODH protein expression and cell growth proliferation. We suggest that inhibition of PRODH would be valuable as a molecular target for designing novel anti-tumor agents. The PDX mice were treated with EGCG (50 mg/kg), and the clinical therapeutic drug (lipodox, 1.5 mg/kg) was used as a positive control. The results indicated that both agents significantly inhibited PDX tumor growth (Fig. 5a, *p = 0.013 and 0.026, respectively). Protein expression of PRODH in PDX tumors was determined in the drug-treated mice. PRODH protein expression in EGCG- and lipodox-treated PDX tumors was significantly inhibited, as evaluated using immunoblotting or immunohistochemical (IHC) staining (Fig. 5b and c). The Ki-67 protein (also known as MKI67) is a cellular marker for proliferation and it is used in IHC analyses. It is strictly associated with cell proliferation. Phosphatase and tensin homolog (PTEN) acts as a tumor suppressor gene via the action of its phosphatase protein product. This phosphatase is involved in the regulation of the cell cycle and prevents cells from growing and dividing too rapidly. It is a target for the evaluation of anticancer drugs [33]. The IHC staining results indicated that Ki-67 protein expression was inhibited, and PTEN protein expression was induced in the EGCG- and lipodox-treated mice. These experiments provide additional evidence to evaluate the application of PRODH as a clinical therapeutic, especially in TNBC patients. The mechanism of PRODH was evaluated in vivo to elucidate the function of tumor growth-driving genes.
Fig. 5.
EGCG induced TNBC-PDX tumor growth inhibition via downregulation of PRODH protein expression. (a) To test whether PRODH expression in tumor tissue directly influenced tumor growth, the TNBC-PDX (F4, n = 4 in each group) mouse model was established. The PDX mice were treated with EGCG (50 mg/kg, subcutaneous injection, 5 injections every other day), and lipodox (1.5 mg/kg, subcutaneous injection, 2 injections once a week) was used as a positive therapeutic control. PBS-treated mice served as the negative treatment control. At the end of the experiment, the mice were sacrificed, and the xenograft tumors were dissected to determine the levels of PRODH protein expression in TNBC-PDX tumor tissues. (b) The protein lysates were harvested from tumor tissues, and the expression levels of the PRODH protein were detected using Western blotting (n = 4). GAPDH protein was assessed and served as a control. (c) The TNBC-PDX tumors were dissected from mice at the end of the experiment. IHC staining was performed using antibody-specific targets of PRODH, Ki67, and PTEN according to the Materials and Methods. Protein immunolocalization was visualized in individual tumor tissues. Scale bar = 15 μm.
4. Discussion
The carcinogenic characteristics of TNBC are a more aggressive phenotype that is similar to stem cell-like cancer cells (cancer stem cells, CSCs) [34]. TNBC cancer cells are heterogeneous, and CSCs are primarily responsible for tumor relapse, treatment resistance and metastasis. Therefore, the targeted treatment of TNBC remains a major challenge as a clinically unmet needs. Daily diets are associated with tumor formation in many types of cancer. Modification of daily diets in cancer patients improves outcomes [35, 36]. For example, improved outcomes in colon cancer and patients on immunotherapy were found with high-fiber diets [37]. Food-derived polyphenols also attenuate the formation and virulence of CSCs [38], which suggests that these compounds and their analogs are promising agents for preventing breast cancer. The tea polyphenol EGCG regulated the PI3K/AKT signaling pathway in human breast cancer [18]. The clinical therapeutic agents doxorubicin and EGCG exhibited anti-carcinogenic effects in breast tumor formation when used alone via the silencing of STAT3 and Notch-1 genes [17, 39]. Our previous studies demonstrated that EGCG induced TNBC breast cancer cell proliferation via inhibition of the α9-nicotinic receptor-mediated signaling pathways [18]. However, the exact mechanism of the EGCG-induced anti-tumor effect remains controversial.
Treatment with EGCG profoundly inhibited PRODH protein overexpression in TNBC (MDA-MB-231) cancer cells (Fig. 5d, bars 2 versus 1). An in vivo study also demonstrated that PRODH protein expression in TNBC-PDX tumor xenografts was significantly downregulated in the EGCG-treated mice. We found that overexpression of PRODH in TNBC cells significantly upregulated cell growth cycle protein (cyclins D and B) expression. Notably, p53 and p27 were significantly downregulated in the PRODH-overexpressing cells in this study (Fig. 2c). This observation was similar to a previous study that indicated that PRODH was one of the most strongly upregulated genes by p53, and it was simply referred to as a “p53-induced gene” in human cancer cells [40]. The results suggest that EGCG-induced PRODH downregulation exhibits potent cell growth cycle protein inhibition effects in TNBC cancer cells. Further study demonstrated that EGCG inhibited CSCs in different types of cancers [17, 41, 42]. The present study demonstrated that overexpression of PRODH induced epithelial-mesenchymal transition (EMT) changes with an upregulation of fibronectin, alpha-smooth muscle actin (alpha-SMA), and epithelial-mesenchymal transition transcription factors, such as SP1 and STAT3, which were aberrantly expressed in TNBC cells (Fig. 2c). Increased tumor cell migration was also observed in PRODH-overexpressing cells (Fig. 2b). EGCG-inhibited TNBC formation occurred via various mechanisms, such as the promotion of apoptosis and inhibition of tumor growth proliferation34–35. Cotreatment with EGCG and curcumin blocked STAT3 phosphorylation, prevented STAT3-mediated inflammatory signaling and suppressed CD44 expression in breast cancer CSCs [43]. A previous study demonstrated that EGCG-induced targeting of ERα36 was an effective method to eradicate breast cancer CSCs [44]. ER-α36 is a variant of ERα that is highly expressed in ER-negative breast cancer cells [45], and it is a novel molecular target to eradicate stem cells in ER-negative and TNBC cells [46, 47]. Our previous study demonstrated that EGCG inhibited ER-mediated signaling pathways in ER-positive breast cancer cells [19]. These results suggest that another important carcinogenic molecule (such as PRODH, Fig. 1) is expressed in all types of breast cancer cells and should be an important target for EGCG. EGCG also inhibited Wnt signaling via the targeting of HMG-box protein 1, a suppressor of Wnt signaling, which decreased the tumorigenicity and invasiveness of mammary cancer cells [48, 49]. These results suggested that EGCG inhibited the self-renewal of BCSCs in TNBC.
PRODH is involved in proline catabolism [50]. Proline is synthesized from glutamate or ornithine via L-glutamate-gamma-semialdehyde (GSAL)/ Delta (1)-pyrroline-5-carboxylate (P5C), which is reduced to proline by P5C reductase (PYCR) in an NAD(P)H-dependent reaction. Proline metabolism is involved in cancer cell growth proliferation and promotes cancer metastasis [50]. Inhibitors that specifically target PRODH and PYCR isoforms are tools for studying proline metabolism and the functions of the proline-P5C cycle in cancer [51]. The in vivo administration of a PRODH competitive inhibitor, L-tetrahydro-2-furoic acid (L-THFA, 60 mg/kg), into mice was reported [52]. The investigators observed excellent host tolerance to this competitive PRODH inhibitor, and it reduced pulmonary metastasis formation by 50% after 16–18 days of sequential treatment without any significant impact on primary tumor growth [52]. TNBC commonly develops resistance to chemotherapy, but markers predictive of chemoresistance in this disease are lacking. These findings reveal that targeting the PRODH signaling pathway is a potential therapeutic strategy in preventing cancer cell metastasis (Fig. 6). The PDX mouse model is highly relevant to real human tumor growth, and the tumors maintained their original molecular characteristics and heterogeneity. TNBC PDX mice were sensitized to doxorubicin and EGCG in the present study. PRODH protein in TNBC tumors was inhibited in the EGCG-treated mice. Therefore, our results have predictive power for translating the study results from the bench to bedside (Fig. 6). The synergistic enhanced anti-tumor effect of doxorubicin and EGCG provides a safe and effective strategy for the treatment of TNBC.
Fig. 6.
Mechanisms of EGCG-induced antitumor effects via inhibition of PROHD in the TNBC-PDX model.
Acknowledgments
This study was supported by the Health and welfare surcharge of tobacco products grant (MOHW109-TDU-B-212-134014); Ministry of Science and Technology, Taiwan (MOST108-2320-B-038-033-MY3 and MOST109-2320-B-038-028 awarded to Dr. Ho, MOST109-2314-B-038-033-MY3 awarded to Dr. Tu, MOST109-2320-B-038-017 awarded to Dr. Chen.
Funding Statement
This study was supported by the Health and welfare surcharge of tobacco products grant (MOHW109-TDU-B-212-134014); Ministry of Science and Technology, Taiwan (MOST108-2320-B-038-033-MY3 and MOST109-2320-B-038-028
Footnotes
Conflict of interest
The authors declare that they have no competing interests.
Reference
- 1. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2. Chlebowski RT, Anderson GL, Aragaki AK, Manson JE, Stefanick ML, Pan K, Barrington W, Kuller LH, Simon MS, Lane D, Johnson KC, Rohan TE, Gass MLS, Cauley JA, Paskett ED, Sattari M, Prentice RL. Association of Menopausal Hormone Therapy With Breast Cancer Incidence and Mortality During Long-term Follow-up of the Women’s Health Initiative Randomized Clinical Trials. JAMA. 2020;324:369–80. doi: 10.1001/jama.2020.9482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Oshi M, Takahashi H, Tokumaru Y, Yan L, Rashid OM, Nagahashi M, Matsuyama R, Endo I, Takabe K. The E2F Pathway Score as a Predictive Biomarker of Response to Neoadjuvant Therapy in ER+/HER2−. Breast Cancer Cells. 2020:9. doi: 10.3390/cells9071643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dekker TJA. Therapy for HER2-Positive Metastatic Breast Cancer. N Engl J Med. 2020;382:e98. doi: 10.1056/NEJMc2004854. [DOI] [PubMed] [Google Scholar]
- 5. Saatci O, Kaymak A, Raza U, Ersan PG, Akbulut O, Banister CE, Sikirzhytski V, Tokat UM, Aykut G, Ansari SA, Dogan HT, Dogan M, Jandaghi P, Isik A, Gundogdu F, Kosemehmetoglu K, Dizdar O, Aksoy S, Akyol A, Uner A, Buckhaults PJ, Riazalhosseini Y, Sahin O. Targeting lysyl oxidase (LOX) overcomes chemotherapy resistance in triple negative breast cancer. Nat Commun. 2020;11:2416. doi: 10.1038/s41467-020-16199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Radovich M, Jiang G, Hancock BA, Chitambar C, Nanda R, Falkson C, Lynce FC, Gallagher C, Isaacs C, Blaya M, Paplomata E, Walling R, Daily K, Mahtani R, Thompson MA, Graham R, Cooper ME, Pavlick DC, Albacker LA, Gregg J, Solzak JP, Chen YH, Bales CL, Cantor E, Shen F, Storniolo AMV, Badve S, Ballinger TJ, Chang CL, Zhong Y, Savran C, Miller KD, Schneider BP. Association of Circulating Tumor DNA and Circulating Tumor Cells After Neoadjuvant Chemotherapy With Disease Recurrence in Patients With Triple-Negative Breast Cancer: Preplanned Secondary Analysis of the BRE12-158 Randomized Clinical Trial. JAMA Oncol. 2020 doi: 10.1001/jamaoncol.2020.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hudziak RM, Lewis GD, Winget M, Fendly BM, Shepard HM, Ullrich A. p185HER2 monoclonal antibody has anti-proliferative effects in vitro and sensitizes human breast tumor cells to tumor necrosis factor. Mol Cell Biol. 1989;9:1165–72. doi: 10.1128/mcb.9.3.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pegram M, Hsu S, Lewis G, Pietras R, Beryt M, Sliwkowski M, Coombs D, Baly D, Kabbinavar F, Slamon D. Inhibitory effects of combinations of HER-2/neu antibody and chemotherapeutic agents used for treatment of human breast cancers. Oncogene. 1999;18:2241–51. doi: 10.1038/sj.onc.1202526. [DOI] [PubMed] [Google Scholar]
- 9. Huang F, Reeves K, Han X, Fairchild C, Platero S, Wong TW, Lee F, Shaw P, Clark E. Identification of candidate molecular markers predicting sensitivity in solid tumors to dasatinib: rationale for patient selection. Cancer Res. 2007;67:2226–38. doi: 10.1158/0008-5472.CAN-06-3633. [DOI] [PubMed] [Google Scholar]
- 10. Finn RS, Dering J, Ginther C, Wilson CA, Glaspy P, Tchekmedyian N, Slamon DJ. Dasatinib, an orally active small molecule inhibitor of both the src and abl kinases, selectively inhibits growth of basal-type/triple-negative” breast cancer cell lines growing in vitro. Breast Cancer Res Treat. 2007;105:319–26. doi: 10.1007/s10549-006-9463-x. [DOI] [PubMed] [Google Scholar]
- 11. Herold CI, Chadaram V, Peterson BL, Marcom PK, Hopkins J, Kimmick GG, Favaro J, Hamilton E, Welch RA, Bacus S, Blackwell KL. Phase II trial of dasatinib in patients with metastatic breast cancer using real-time pharmacodynamic tissue biomarkers of Src inhibition to escalate dosing. Clin Cancer Res. 2011;17:6061–70. doi: 10.1158/1078-0432.CCR-11-1071. [DOI] [PubMed] [Google Scholar]
- 12. Zakaria Z, Zulkifle MF, Hasan Wan, Wan, Azhari AK, Abdul Raub SH, Eswaran J, Soundararajan M, Syed Husain SNA. Epidermal growth factor receptor (EGFR) gene alteration and protein overexpression in Malaysian triple-negative breast cancer (TNBC) cohort. Onco Targets Ther. 2019;12:7749–56. doi: 10.2147/OTT.S214611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zecchin D, Moore C, Michailidis F, Horswell S, Rana S, Howell M, Downward J. Combined targeting of G proteincoupled receptor and EGF receptor signaling overcomes resistance to PI3K pathway inhibitors in PTEN-null triple negative breast cancer. EMBO Mol Med. 2020;12:e11987. doi: 10.15252/emmm.202011987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu Y, Zhou Y, Huang KH, Fang X, Li Y, Wang F, An L, Chen Q, Zhang Y, Shi A, Yu S, Zhang J. Targeting epidermal growth factor-overexpressing triple-negative breast cancer by natural killer cells expressing a specific chimeric antigen receptor. Cell Prolif. 2020;53:e12858. doi: 10.1111/cpr.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sanchez-Valdeolivar CA, Alvarez-Fitz P, Zacapala-Gomez AE, Acevedo-Quiroz M, Cayetano-Salazar L, Olea-Flores M, Castillo-Reyes JU, Navarro-Tito N, Ortuno-Pineda C, Leyva-Vazquez MA, Ortiz-Ortiz J, Castro-Coronel Y, Mendoza-Catalan MA. Phytochemical profile and antiproliferative effect of Ficus crocata extracts on triple-negative breast cancer cells. BMC Complement Med Ther. 2020;20:191. doi: 10.1186/s12906-020-02993-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kushwaha PP, Vardhan PS, Kapewangolo P, Shuaib M, Prajapati SK, Singh AK, Kumar S. Bulbine frutescens phytochemical inhibits notch signaling pathway and induces apoptosis in triple negative and luminal breast cancer cells. Life Sci. 2019;234:116783. doi: 10.1016/j.lfs.2019.116783. [DOI] [PubMed] [Google Scholar]
- 17. Steed KL, Jordan HR, Tollefsbol TO. SAHA and EGCG Promote Apoptosis in Triple-negative Breast Cancer Cells, Possibly Through the Modulation of cIAP2. Anticancer Res. 2020;40:9–26. doi: 10.21873/anticanres.13922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tu SH, Ku CY, Ho CT, Chen CS, Huang CS, Lee CH, Chen LC, Pan MH, Chang HW, Chang CH, Chang YJ, Wei PL, Wu CH, Ho YS. Tea polyphenol (−)-epigallocatechin-3-gallate inhibits nicotine- and estrogen-induced alpha9-nicotinic acetylcholine receptor upregulation in human breast cancer cells. Mol Nutr Food Res. 2011;55:455–66. doi: 10.1002/mnfr.201000254. [DOI] [PubMed] [Google Scholar]
- 19. Wu CH, Lee CH, Ho YS. Nicotinic acetylcholine receptor-based blockade: applications of molecular targets for cancer therapy. Clin Cancer Res. 2011;17:3533–41. doi: 10.1158/1078-0432.CCR-10-2434. [DOI] [PubMed] [Google Scholar]
- 20. Tu SH, Chen MY, Chen LC, Mao YT, Ho CH, Lee WJ, Lin YK, Pan MH, Lo CY, Chen CL, Yen Y, Whang-Peng J, Ho CT, Wu CH, Ho YS. Pu-erh tea extract attenuates nicotine-induced foam cell formation in primary cultured monocytes: an in vitro mechanistic study. J Agr Food Chem. 2016;64:3186–95. doi: 10.1021/acs.jafc.6b00624. [In English] [DOI] [PubMed] [Google Scholar]
- 21. Chen LC, Chen MY, Tu SH, Pan MH, Lo CY, Ho CT, Wu CH, Ho YS. Pu-erh tea attenuates smoking-induced foam cell formation through inhibition of the alpha 9-nicotinic-acetylcholine receptor expression in monocytes: An ex vivo study. J Funct Foods. 2016;22:132–44. [In English] [Google Scholar]
- 22. Tu SH, Ku CY, Ho CT, Chen CS, Huang CS, Lee CH, Chen LC, Pan MH, Chang HW, Chang CH, Chang YJ, Wei PL, Wu CH, Ho YS. Tea polyphenol (−)-epigallocatechin-3-gallate inhibits nicotine- and estrogen-induced alpha 9-nicotinic acetylcholine receptor upregulation in human breast cancer cells. Molecular Nutrition & Food Research. 2011;55:455–66. doi: 10.1002/mnfr.201000254. [In English] [DOI] [PubMed] [Google Scholar]
- 23. Weng MS, Ho CT, Ho YS, Lin JK. Theanaphthoquinone inhibits fatty acid synthase expression in EGF-stimulated human breast cancer cells via the regulation of EGFR/ErbB-2 signaling. Toxicol Appl Pharm. 2007;218:107–18. doi: 10.1016/j.taap.2006.10.021. [In English] [DOI] [PubMed] [Google Scholar]
- 24. Ansari RE, Craze ML, Althobiti M, Alfarsi L, Ellis IO, Rakha EA, Green AR. Enhanced glutamine uptake influences composition of immune cell infiltrates in breast cancer. Br J Cancer. 2020;122:94–101. doi: 10.1038/s41416-019-0626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saito Y, Li L, Coyaud E, Luna A, Sander C, Raught B, Asara JM, Brown M, Muthuswamy SK. LLGL2 rescues nutrient stress by promoting leucine uptake in ER(+) breast cancer. Nature. 2019;569:275–9. doi: 10.1038/s41586-019-1126-2. [DOI] [PubMed] [Google Scholar]
- 26. Roomi MW, Ivanov V, Kalinovsky T, Niedzwiecki A, Rath M. In vitro and in vivo antitumorigenic activity of a mixture of lysine, proline, ascorbic acid, and green tea extract on human breast cancer lines MDA-MB-231 and MCF-7. Med Oncol. 2005;22:129–38. doi: 10.1385/MO:22:2:129. [DOI] [PubMed] [Google Scholar]
- 27. Lee YH, Nadaraia S, Gu D, Becker DF, Tanner JJ. Structure of the proline dehydrogenase domain of the multifunctional PutA flavoprotein. Nat Struct Biol. 2003;10:109–14. doi: 10.1038/nsb885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Servet C, Ghelis T, Richard L, Zilberstein A, Savoure A. Proline dehydrogenase: a key enzyme in controlling cellular homeostasis. Front Biosci (Landmark Ed) 2012;17:607–20. doi: 10.2741/3947. [DOI] [PubMed] [Google Scholar]
- 29. Wang CY, Chiao CC, Phan NN, Li CY, Sun ZD, Jiang JZ, Hung JH, Chen YL, Yen MC, Weng TY, Chen WC, Hsu HP, Lai MD. Gene signatures and potential therapeutic targets of amino acid metabolism in estrogen receptor-positive breast cancer. Am J Cancer Res. 2020;10:95–113. [PMC free article] [PubMed] [Google Scholar]
- 30. Huynh TYL, Zareba I, Baszanowska W, Lewoniewska S, Palka J. Understanding the role of key amino acids in regulation of proline dehydrogenase/proline oxidase (prodh/ pox)-dependent apoptosis/autophagy as an approach to targeted cancer therapy. Mol Cell Biochem. 2020;466:35–44. doi: 10.1007/s11010-020-03685-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Scott GK, Yau C, Becker BC, Khateeb S, Mahoney S, Jensen MB, Hann B, Cowen BJ, Pegan SD, Benz CC. Targeting mitochondrial proline dehydrogenase with a suicide inhibitor to exploit synthetic lethal interactions with p53 upregulation and glutaminase inhibition. Mol Cancer Ther. 2019;18:1374–85. doi: 10.1158/1535-7163.MCT-18-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wahba HA, El-Hadaad HA. Current approaches in treatment of triple-negative breast cancer. Cancer Biol Med. 2015;12:106–16. doi: 10.7497/j.issn.2095-3941.2015.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee YR, Chen M, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor: new modes and prospects. Nat Rev Mol Cell Biol. 2018;19:547–62. doi: 10.1038/s41580-018-0015-0. [DOI] [PubMed] [Google Scholar]
- 34. Lee KL, Kuo YC, Ho YS, Huang YH. Triple-negative breast cancer: current understanding and future therapeutic breakthrough targeting cancer stemness. Cancers (Basel) 2019:11. doi: 10.3390/cancers11091334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Escrich R, Vela E, Solanas M, Moral R. Effects of diets high in corn oil or in extra virgin olive oil on oxidative stress in an experimental model of breast cancer. Mol Biol Rep. 2020;47:4923–32. doi: 10.1007/s11033-020-05492-6. [DOI] [PubMed] [Google Scholar]
- 36. Langlais CS, Chan JM. Opportunities and challenges for research on low-carbohydrate diets in prostate cancer. Nat Rev Urol. 2020;17:437–8. doi: 10.1038/s41585-020-0326-8. [DOI] [PubMed] [Google Scholar]
- 37. Mann SD, Sidhu MD, Gowin KD. Understanding the mechanisms of diet and outcomes in colon, prostate, and breast cancer; malignant gliomas; and cancer patients on immunotherapy. Nutrients. 2020:12. doi: 10.3390/nu12082226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ko YS, Jung EJ, Go SI, Jeong BK, Kim GS, Jung JM, Hong SC, Kim CW, Kim HJ, Lee WS. Polyphenols Extracted from Artemisia annua L. Exhibit Anti-Cancer Effects on Radio-Resistant MDA-MB-231 Human Breast Cancer Cells by Suppressing Stem Cell Phenotype, beta-Catenin, and MMP-9. Molecules. 2020:25. doi: 10.3390/molecules25081916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Alkaraki A, Alshaer W, Wehaibi S, Gharaibeh L, Abuarqoub D, Alqudah DA, Al-Azzawi H, Zureigat H, Souleiman M, Awidi A. Enhancing chemosensitivity of wild-type and drug-resistant MDA-MB-231 triple-negative breast cancer cell line to doxorubicin by silencing of STAT 3, Notch-1, and beta-catenin genes. Breast Cancer. 2020;27:989–98. doi: 10.1007/s12282-020-01098-9. [DOI] [PubMed] [Google Scholar]
- 40. Polyak K, Xia Y, Zweier JL, Kinzler KW, Vogelstein B. A model for p53-induced apoptosis. Nature. 1997;389:300–5. doi: 10.1038/38525. [DOI] [PubMed] [Google Scholar]
- 41. Fujiki H, Sueoka E, Rawangkan A, Suganuma M. Human cancer stem cells are a target for cancer prevention using (−)-epigallocatechin gallate. J Cancer Res Clin Oncol. 2017;143:2401–12. doi: 10.1007/s00432-017-2515-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bimonte S, Cascella M, Barbieri A, Arra C, Cuomo A. Current shreds of evidence on the anticancer role of EGCG in triple negative breast cancer: an update of the current state of knowledge. Infect Agent Cancer. 2020;15:2. doi: 10.1186/s13027-020-0270-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chung SS, Vadgama JV. Curcumin and epigallocatechin gallate inhibit the cancer stem cell phenotype via downregulation of STAT3-NFkappaB signaling. Anticancer Res. 2015;35:39–46. [PMC free article] [PubMed] [Google Scholar]
- 44. Pan X, Zhao B, Song Z, Han S, Wang M. Estrogen receptor-alpha 36 is involved in epigallocatechin-3-gallate induced growth inhibition of ER-negative breast cancer stem/progenitor cells. J Pharmacol Sci. 2016;130:85–93. doi: 10.1016/j.jphs.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 45. Zhang XT, Kang LG, Ding L, Vranic S, Gatalica Z, Wang ZY. A positive feedback loop of ER-alpha 36/EGFR promotes malignant growth of ER-negative breast cancer cells. Oncogene. 2011;30:770–80. doi: 10.1038/onc.2010.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Guo M, Wang M, Deng H, Zhang X, Wang ZY. A novel anticancer agent Broussoflavonol B downregulates estrogen receptor (ER)-alpha 36 expression and inhibits growth of ER-negative breast cancer MDA-MB-231 cells. Eur J Pharmacol. 2013;714:56–64. doi: 10.1016/j.ejphar.2013.05.047. [DOI] [PubMed] [Google Scholar]
- 47. Guo M, Wang M, Zhang X, Deng H, Wang ZY. Broussoflavonol B restricts growth of ER-negative breast cancer stem-like cells. Anticancer Res. 2013;33:1873–9. [PubMed] [Google Scholar]
- 48. Kim J, Zhang X, Rieger-Christ KM, Summerhayes IC, Wazer DE, Paulson KE, Yee AS. Suppression of Wnt signaling by the green tea compound (−)-epigallocatechin 3-gallate (EGCG) in invasive breast cancer cells. Requirement of the transcriptional repressor HBP1. J Biol Chem. 2006;281:10865–75. doi: 10.1074/jbc.M513378200. [DOI] [PubMed] [Google Scholar]
- 49. Luo X, Guo L, Zhang L, Hu Y, Shang D, Ji D. Bioinformatics analysis of microarray profiling identifies the mechanism of focal adhesion kinase signalling pathway in proliferation and apoptosis of breast cancer cells modulated by green tea polyphenol epigallocatechin 3-gallate. J Pharm Pharmacol. 2018;70:1606–18. doi: 10.1111/jphp.13010. [DOI] [PubMed] [Google Scholar]
- 50. Tanner JJ, Fendt SM, Becker DF. The proline cycle as a potential cancer therapy target. Biochemistry. 2018;57:3433–44. doi: 10.1021/acs.biochem.8b00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang M, White TA, Schuermann JP, Baban BA, Becker DF, Tanner JJ. Structures of the Escherichia coli PutA proline dehydrogenase domain in complex with competitive inhibitors. Biochemistry. 2004;43:12539–48. doi: 10.1021/bi048737e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Elia I, Broekaert D, Christen S, Boon R, Radaelli E, Orth MF, Verfaillie C, Grunewald TGP, Fendt SM. Proline metabolism supports metastasis formation and could be inhibited to selectively target metastasizing cancer cells. Nat Commun. 2017;8:15267. doi: 10.1038/ncomms15267. [DOI] [PMC free article] [PubMed] [Google Scholar]