Abstract
Nicotine-containing electronic cigarette liquid (e-liquid) is prohibited in many countries, creating requirements for rapid detection approaches for on-site inspection or screening for large amounts of samples. Here, we demonstrate a simple way to identify nicotine using surface-enhanced Raman scattering (SERS) with substrates made of silver nanoparticle arrays imbedded in anodic aluminum oxide nanochannels (Ag/AAO). Compared with the reported colloidal nanoparticle-based SERS, that required serial dilutions to enable colloid aggregation in the viscous e-liquid, a small amount of undiluted e-liquid sample can be directly added onto our solid-phase Ag/AAO substrate without any pre-treatment. The sensitivity of our SERS measurements is 2–3 orders of magnitude higher than that required for identification of nicotine in e-liquid, which is typically around 1000–18,000 ppm. Using such nanoparticle array-based SERS, we have tested 22 commercially available e-liquid products, using the corresponding gas chromatography-mass spectrometry (GC–MS) reports as the reference. The SERS measurements were done within one hour and successfully identified 20 samples. Only 2 samples showed SERS interference from ingredients that were not suitable for SERS analysis.
Keywords: Electronic cigarette liquid, Nicotine, SERS
1. Introduction
Electronic cigarettes are battery-powered devices that heat electronic cigarette liquid (e-liquid) to generate aerosol for inhalation. Various kinds of e-liquids are made from a mixture of vegetable glycerin (VG) and propylene glycol (PG) with additional flavoring or coloring additives. Commercial e-liquid products can be nicotine-free or contain nicotine, usually ranging from 1 to 18 mg mL−1, or 1000–18,000 ppm. However, inaccurate labeling is frequently reported and nicotine has even been detected in nicotine-free e-liquids [1–4], causing unwitting inhalation of nicotine. Nicotine-containing e-liquid is prohibited in many countries or regions, including Taiwan [5]. The current standard protocols for detecting e-liquid, such as gas chromatographymass spectrometry (GC–MS) applied in Taiwan, are typically performed in the laboratory setting [6]. The low throughput and cumbersome procedures prohibit the use of such technique for onsite inspection.
The surface-enhanced Raman scattering (SERS) platform has been widely used for rapid detection of a variety of chemicals. It relies on the unique SERS spectrum of the analyte, enhanced by a suitable SERS-active substrate. Previous studies have demonstrated that SERS could detect nicotine in aqueous solution at low ppm levels [7,8], whereas e-liquid usually contains thousands to tens of thousands ppm of nicotine. Nevertheless, a few challenges exist when applying SERS for e-liquid analysis. Firstly, the viscous property of e-liquid may hinder aggregation of colloid particles and, thereby, abolish colloid-based SERS signals. Secondly, the additives of e-liquid may either interfere or give rise to additional signals or fluorescence backgrounds that obscure signals of the target compound. A previous study overcame these obstacles by serial dilution of the e-liquid nearly 4000-fold before SERS detection with colloid gold nanoparticles [9]. This high dilution reduced the interference from additives and revealed the target nicotine SERS spectrum, which was undetectable on their system without dilution. The dilution procedure, however, hindered the throughput of the examination.
This study aimed to contrive a simple nicotine-detecting platform that is suitable for on-site inspection or rapid screening for large amounts of samples without cumbersome sample preparation procedures or sophisticated laboratory settings. We developed a SERS-based platform using substrates made of silver nanoparticle arrays imbedded in porous anodic aluminum oxide (Ag/ AAO) [10] for this purpose.
2. Materials and methods
2.1. Reagents
Glycerol (#49767), propylene glycol (#W294004), and nicotine (#N3876) were purchased from Sigma–Aldrich (St. Louis, MO, USA). To mimic e-liquid, glycerol and propylene glycol at 3:7, 5:5, or 7:3 (w/w) ratios were first mixed thoroughly with hours of agitation on the seesaw rocker. Nicotine was prepared at 2%, 0.2%, or 0.02% (w/w) with double-distilled water (ddH2O). Simulated e-liquids containing 1,000, 100, or 10 ppm of nicotine were prepared by adding 5 μl of 2%, 0.2%, or 0.02% nicotine, respectively, into 95 μl of each glycerol/ propylene glycol mixture and then mixed by several cycles of vortexing and spin-down.
2.2. E-liquid samples
Commercial e-liquid samples and their nicotine concentration determined by GC–MS were provided by Department of Health, New Taipei City Government (New Taipei City, Taiwan). To spike in 100 ppm of nicotine, 95 μl of e-liquid was added into 5 μl of 0.2% nicotine aqueous solution and mixed thoroughly by pipetting.
2.3. SERS-active substrates
The fabrication of SERS-active substrates was reported previously [10]. Briefly, a ~150 nm aluminum film was coated on glass slides and then anodized and etched to generate arrays of nanochannels with a pore diameter of ~50–60 nm. Silver nanoparticles were grown into the nanochannels via electrochemical plating. The substrates were cleaned by rinsing with ddH2O and vacuum-sealed in plastic bags. Each substrate was freshly used immediately after the bag was unsealed. Quality control tests of the substrates were performed right before use by measuring SERS spectra of the 10−4 M adenine solution, dripped near four corners of the effective area. Scanning electron microscopy (SEM) images of the substrate were acquired with a JEOL JSM-6700F scanning electron microscope (Tokyo, Japan).
2.4. SERS measurements
Measurements were performed with a Horiba LabRAM HR800 Raman microscope (Kyoto, Japan) with a HeNe laser emitting at 632.8 nm. One microliter of sample was dripped on the SERS-active substrate and covered with a low fluorescence optical glass. Laser beam was focused with a 20X objective lens to the bottom surface of the droplet and the backward scattered radiation collected by the same lens was dispersed with a 80 cm spectrograph. Signal accumulation time was 1 s and detected with a liquid nitrogen-cooled charge-coupled device. The spectral accuracy and precision were calibrated to be <7 and 0.1 cm−1, respectively. Each SERS spectrum was consisted of 8 measurements from different positions of the same droplet. After removing occasionally occurred spectra that exhibited dissimilar spectral patterns, the remaining spectra were processed using a custom-made baseline removal program based on a sensitive nonlinear iterative peak clipping algorithm [11] and averaged to acquire the mean spectrum.
3. Results and discussion
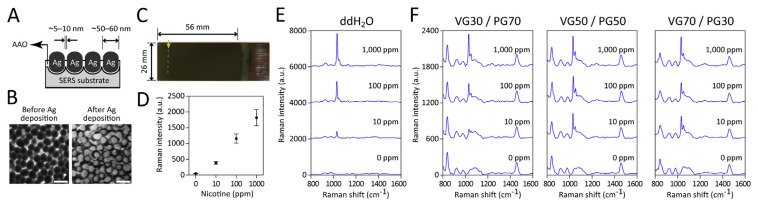
To achieve optimal SERS signals, the pore diameter and inter-channel gap of AAO nanochannels were adjusted to ~50–60 nm and ~5–10 nm, respectively (Fig. 1A, B). One microliter of sample could be applied directly, and around 40–50 samples could be parallel processed on a 26 mm × 56 mm Ag/AAO substrate (Fig. 1C). Quality control tests of every substrate were performed right before use to eliminate substrates showing high signal variation or low signal intensity. The signal reproducibility within the substrate was demonstrated by measuring SERS spectra of the nicotine solution, dripped in four different positions on the same substrate. As shown in Fig. 1D and Table S1, the ratio of the standard deviation to the mean (RSD) of nicotine peak intensity within the substrate was 2.4–15.4%. The use of Ag/AAO SERS-active substrates, with very high and extremely uniform Raman enhancement factors, avoided the need of colloid aggregation required for colloid-based SERS enhancement [9] and the large Raman signal fluctuation caused by the intrinsically random aggregation process.
Fig. 1.
(A) Schematic diagram showing the cross-sectional view of the Ag/AAO substrate. (B) Top-view SEM images of the Ag/AAO substrate before and after silver deposition (scale bars, 100 nm). (C) Photograph demonstrating 5 e-liquid samples dripped in a row (arrow) on the Ag/AAO substrate. The effective area is around 26 mm × 56 mm, which easily handles 40–50 samples. (D) The intensity of the peak at 1030 cm−1 of different concentrations of nicotine prepared in ddH2O. Each sample was dripped onto four different positions on the same Ag/AAO substrate. Data shown in mean ± SD. SERS spectra of different concentrations of nicotine prepared in (E) ddH2O or (F) VG/PG mixtures at 30:70, 50:50, or 70:30 ratios, respectively. SERS measurements using Ag/AAO substrates were done with a Raman microscope equipped with a HeNe laser emitting at 632.8 nm. The spectra shown are the averages of 5–8 individual spectra.
We first examined the sensitivity and specificity of our platform. Nicotine solutions prepared at 10–1000 ppm in ddH2O were dripped onto the Ag/ AAO substrates and their SERS spectra were recorded. As shown in Fig. 1D and E, 10 ppm of nicotine solution exhibited a dominant peak at 1030 cm−1, a shoulder at 1053 cm−1, and other smaller peaks at 928 and 980 cm−1. Compared with the normal Raman spectrum of nicotine, the peaks at 1030 and 1053 cm−1 are tentatively assigned to the symmetrical breathing and trigonal ring deformation of pyridine moiety. These two peaks are also evident in the previous works [7–9,12]. There is no apparent assignment of both the smaller peaks at 928 and 980 cm−1. At 100 and 1000 ppm, we observed a dose-response increase in intensity of the peak at 1030 cm−1 by around 1120 artificial units (a.u.) and 1790 a.u., respectively.
We then examined nicotine SERS spectra in VG/ PG mixtures at ratios of 30/70, 50/50, or 70/30. As shown in Fig. 1F, VG/PG mixtures possess background signals at 840, 922, 980, and 1462 cm−1, and a broad spectrum at 1055–1135 cm−1. A peak at 1030 cm−1 appeared when adding nicotine to the VG/PG mixtures. At 100 and 1000 ppm, an increase in intensity of around 270–430 a.u. and 360–490 a.u., respectively, of the peak at 1030 cm−1 was detected with different VG/PG ratios. Such a nicotine spectrum was weaker in VG/PG mixtures than in ddH2O, but it still increased in a dose-response manner. Since commercial e-liquid usually contains nicotine at 1000–18,000 ppm, the sensitivity of the Ag/AAO substrate-based SERS employed here is 2–3 orders of magnitude higher than that required for detecting nicotine content in e-liquids.
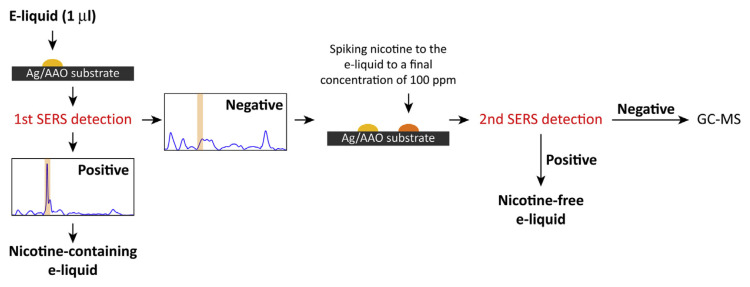
To examine the commercial e-liquid, we developed a two-step protocol, as shown in Fig. 2. The samples dripped onto the Ag/AAO substrates were first subjected to SERS detection. The samples exhibiting the featured 1030 cm−1 signal were considered nicotine-containing e-liquids. For the sample not showing the 1030 cm−1 signal, exogenous nicotine was added to the sample at a final concentration of 100 ppm, and the nicotine spiked sample was subjected to another round of SERS detection. Samples that were positive for nicotine indicated that the original e-liquid did not contain ingredients that might interfere with nicotine detection and the sample was, therefore, reported as nicotine-free. The samples that failed to detect the spiked nicotine suggested that the SERS detection was inactive and that the samples need to be examined by other methods, such as GC–MS.
Fig. 2.
Schematic illustration of the SERS detection protocol. The sample having the featured peak at 1030 cm−1 was considered positive for nicotine. Those samples that failed to exhibit a positive signal in the first-round SERS detection were subjected to a second round of measurements after adding 100 ppm exogenous nicotine to the samples. If the spiked nicotine could be revealed by the second round of SERS detection, the initial negative response revealed by the first round of SERS detection was correct and the original e-liquid was considered nicotine-free. On the other hand, if the second round of detection failed to reveal the spiked nicotine, the sample might have contained ingredients that interfered with SERS detection, and in such cases, other methods, such as GC–MS, should be employed.
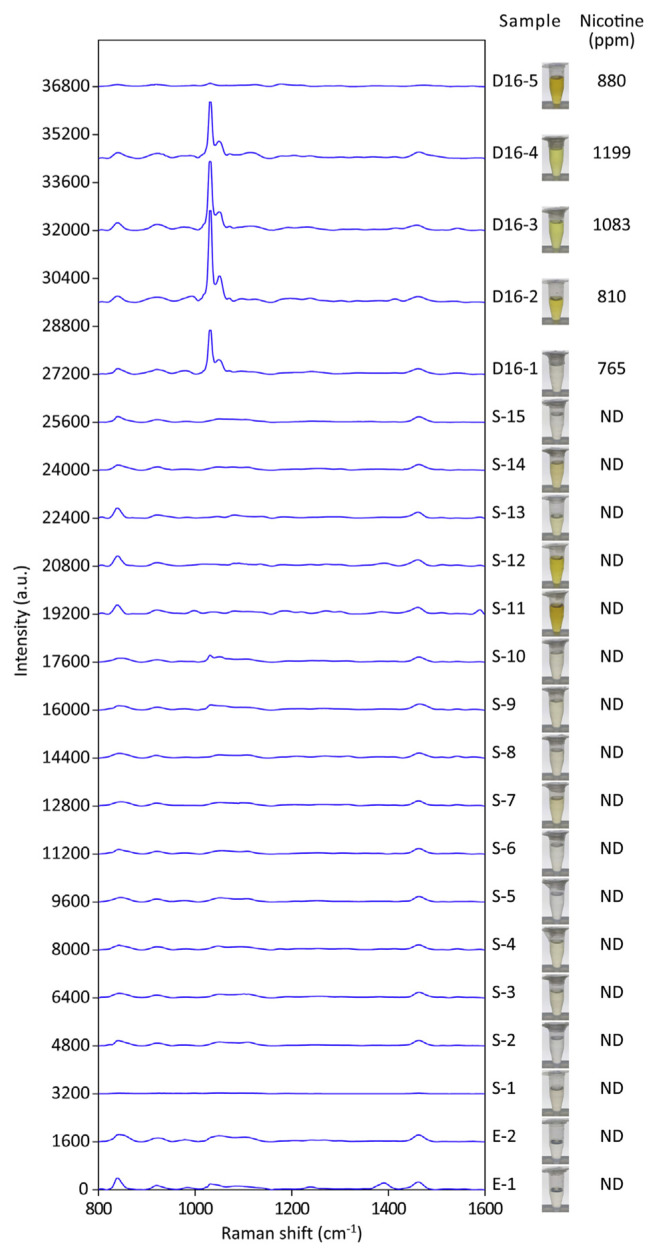
SERS and GC–MS analyses on 22 commercial e-liquid products were conducted in single-blind trials (Figs. 3 and S1). The gross appearances of these e-liquid products were different under white-light illumination due to different additives and coloring added to the solution. The first round of SERS tests revealed 4 samples, D16-1 to D16-4, each with a dominant 1030 cm−1 peak (Figs. 3 and S1). The intensity of the 1030 cm−1 signal readouts ranged from 1470 a.u. to 3050 a.u., much larger than 360–490 a.u. attributed by 1000 ppm nicotine in simulated e-liquids (Fig. 1F). The GC–MS results showed that all four samples positive for the peak at 1030 cm−1 contained nicotine, ranging from 765 to 1199 ppm.
Fig. 3.
SERS spectra of the 22 commercially available e-liquid products in the first round of the SERS test. All spectra shown are the averages of 5–8 readouts. The appearances of individual e-liquid products are demonstrated under while-light illumination. The nicotine content of the samples was also determined by the GC–MS methodology. Nicotine was non-detectable (ND), except for samples D16-1 to D16-5.
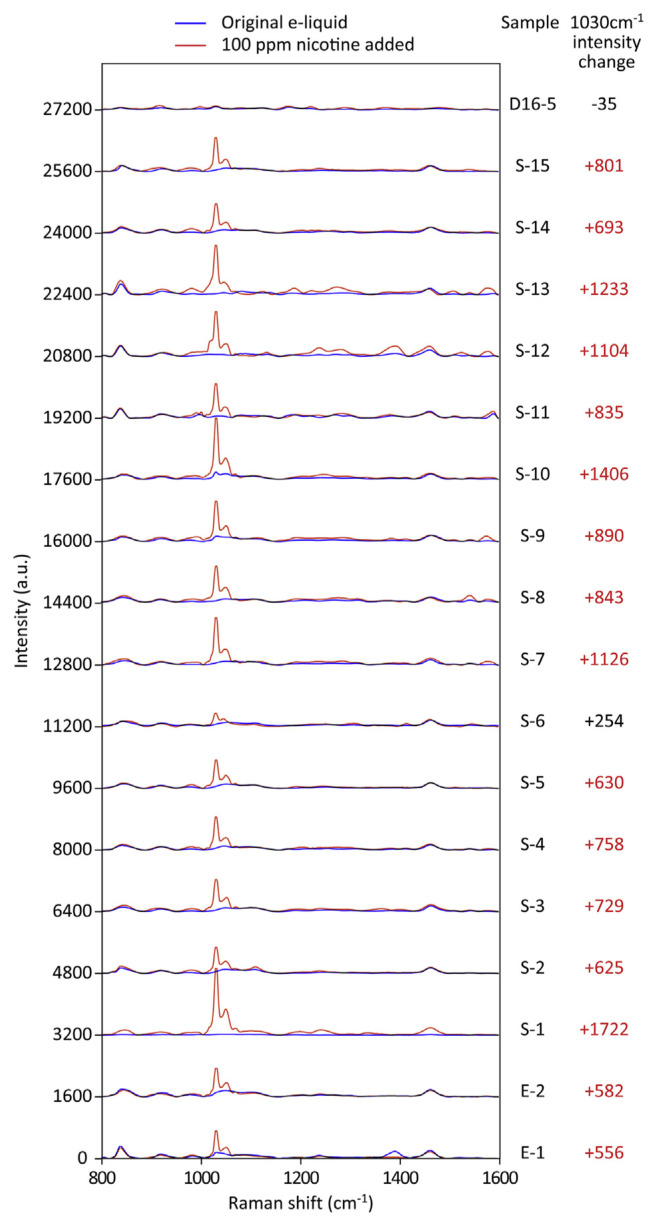
The first round of SERS tests showed that 18 of the 22 tested samples were negative for the peak at 1030 cm−1 in the nicotine spectra. These samples were subjected to the second round of SERS tests (Fig. 4). Simulated e-liquids containing 100 ppm nicotine increased the intensity by around 270–430 a.u at 1030 cm−1 (Fig. 1F and data not shown) on the same batch of SERS substrates. Therefore, we expected at least this level of increase if the sample is true-negative and does not interfere with SERS detection. After addition of 100 ppm nicotine to the samples, we found that 16 of the 18 samples exhibited positive nicotine signals, possessing an increased intensity of at least 550 a.u. at 1030 cm−1 (Fig. 4). These results indicated that the e-liquid products contained no additives or coloring that might interfere with SERS detection and that the first round of readouts indicating no nicotine were indeed correct; these findings were consistent with the corresponding non-detectable results of GC–MS.
Fig. 4.
SERS spectra of the 18 commercially available e-liquid products that did not show the featured nicotine signal at 1030 cm−1 in the first round of the SERS test. Exogenous nicotine was added at a final concentration of 100 ppm to the e-liquid samples. SERS spectra before (blue traces) and after (red traces) spiking with nicotine are shown. Significant increases in intensity at 1030 cm−1 are marked by red numbers. All spectra shown are the averages of 5–8 readouts.
Sample D16-5 showed almost no change on the SERS spectrum after spiking with nicotine (Fig. 4), indicating that certain ingredients within the e-liquid might have interfered with nicotine detection using SERS. GC–MS analyses showed that sample D16-5 contained 880 ppm of nicotine (Fig. 3). The scenario of sample S-6, which showed a smaller increase (Fig. 4), was a little different. Its additive might also partially interfere with SERS detection; nonetheless, if sample S-6 contained regular levels of nicotine (i.e. more than 1000 ppm), the addition of 100 ppm nicotine should have little effect on its SERS spectrum, rather than an increase by 254 a.u. at 1030 cm−1. Therefore, sample S-6 could be nicotine-free or at least not containing nicotine as high as thousands ppm. GC–MS analyses showed that sample S-6 was nicotine-free (Fig. 3). Taken together, only 2 samples out of 22 tested were not suitable for SERS analysis.
We also tested whether dilutions with ddH2O helped to reveal the nicotine signal from sample D16-5, which possessed the most severe SERS interference. After 100-fold dilution, the featured 1030 cm−1 peak still did not appear in our system, although the overall SERS intensity in the raw spectrum decreased (data not shown). These results indicated that the unknown additives within the e-liquid might still compete with nicotine absorption to the substrate surface and prevent the detection of nicotine. Switching to a longer excitation wavelength could be an option; however, using 785 nm instead of 633 nm did not improve nicotine detection by SERS in these experiments (data not shown).
Our results showed that the positive SERS spectrum, especially the 1030 cm−1 peak, is indicative of nicotine-containing e-liquids; there was no false-positive readout using GC–MS detection as the reference. The interpretation of negative SERS spectra, however, needs further consideration. Most, if not all, commercial e-liquid products encompass a large variety of additives and coloring materials, which could obscure SERS signals, causing a false-negative readout. Dark coloring or strong flavoring did not always produce more interference in SERS detection than colorless samples. For example, samples S-11 and S-12 were browner than others, but they did not obscure the spiked nicotine signal, whereas the transparent sample S-6 was noted to dim the 1030 cm−1 peak of the spiked nicotine (Figs. 3 and 4).
To avoid the false-negative readouts, we designed a SERS detection protocol involving two rounds. Only those samples that exhibited a negative nicotine spectrum in the first round of detection and a positive readout after spiking with nicotine in the second round of detection were considered nicotine-free e-liquid products. To better evaluate how much increase should be observed after adding 100 ppm nicotine, we suggest that simulated e-liquids with or without 100 ppm nicotine could be included on the same substrate as controls. Meanwhile, the original e-liquid and its 100 ppm nicotine added pair could be dripped at the nearby positions on the same substrate to reduce systematic variation. The samples showing a negative nicotine signal for both the first round and second round of SERS measurement suggested the presences of SERS interference from ingredients of the e-liquid; such samples are not suitable for SERS analysis. Around 10% of the samples randomly collected from the market belong to this category. Interestingly, the spiked nicotine caused some unexpectedly high increases in SERS intensity at 1030 cm−1, such as in samples S1 and S-10. A possible reason for such unanticipated enhancements could be additives of the e-liquid that resulted in a high-degree of amplifications.
Another concern about array-based SERS is the reproducibility of different spots and substrates. We performed quality tests of every substrate to eliminate substrates showing high signal variation or low sensitivity. Table S1 showed a typical RSD of signal intensity on the same substrate which was 2.4–15.4%. We assume that this is low enough in this study, considering the difference of nicotine peak intensity between negative and positive samples. The reproducibility among different substrates is, to some extent, subject to batch effects. We used four substrates from the same batch in this study (excluding substrates #2 and #3 in Table S1 which were intended to show batch effects) and their signal intensity was 2364 ± 796 a.u. (mean ± SD) in quality control tests. Nonetheless, we argue that the reproducibility among different substrates is not a serious issue in this approach, because substrates are first subject to quality control tests and simulated e-liquids with or without 100 ppm nicotine could be included as controls on the same substrate. It is not necessary to compare spectra obtained from different substrates.
Nicotine detection by SERS is mainly qualitative, or at best, semiquantitative. The reason behind the lack of linearity for quantification may be due to the interferences from diverse additives of e-liquids that may either reduce or increase the nicotine SERS spectra to different extents. SERS-based quantification is possible using deuterium-labeled nicotine (d4-nicotine), whose peak is down-shifted to 994 cm−1 [9], as a standard for calculations. However, e-liquid additives may still unequally interfere with nicotine and d4-nicotine signals and affect quantification accuracy.
4. Conclusions
We report here a simple nicotine-detecting approach using Ag/AAO substrate-based SERS. The sensitivity is 2–3 orders of magnitude higher than that required for detecting nicotine content in commercial e-liquid products. Compared with colloidal nanoparticle-based SERS that requires serial dilutions to enable colloid aggregation in the viscous e-liquid [9], a miniscule amount (1 μl) of undiluted e-liquid can be directly dripped onto the solid-phase Ag/AAO substrate for SERS measurements. Each e-liquid sample could be tested within 3 min without cumbersome sample preparation procedures or sophisticated laboratory settings. Such features make on-site inspection possible using portable Raman detectors to screen large amounts of e-liquid products, a situation that governing authorities typically encounter in the field. At its current stage, SERS measurements cannot compete with GC–MS in terms of diagnostic sensitivity and false-negative rate, but the latter takes around 20–25 min to examine each e-liquid sample. Our simple and quick procedure could significantly reduce samples that need to be analyzed by GC–MS.
Acknowledgments
This study was supported by Ministry of Science and Technology, Taiwan (grand number MOST 107-2745-M-001-004-ASP and MOST 108-2319-B-010-001).
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.38212/2224-6614.1064.
Funding Statement
This study was supported by Ministry of Science and Technology, Taiwan (grand number MOST 107-2745-M-001-004-ASP and MOST 108-2319-B-010-001).
References
- 1. Davis B, Dang M, Kim J, Talbot P. Nicotine concentrations in electronic cigarette refill and do-it-yourself fluids. Nicotine Tob Res. 2015;17:134–41. doi: 10.1093/ntr/ntu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goniewicz ML, Gupta R, Lee YH, Reinhardt S, Kim S, Kim B, et al. Nicotine levels in electronic cigarette refill solutions: a comparative analysis of products from the U.S., Korea, and Poland. Int J Drug Pol. 2015;26:583–8. doi: 10.1016/j.drugpo.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Raymond BH, Collette-Merrill K, Harrison RG, Jarvis S, Rasmussen RJ. The nicotine content of a sample of e-cigarette liquid manufactured in the United States. J Addict Med. 2018;12:127–31. doi: 10.1097/ADM.0000000000000376. [DOI] [PubMed] [Google Scholar]
- 4. Chivers E, Janka M, Franklin P, Mullins B, Larcombe A. Nicotine and other potentially harmful compounds in “nicotine-free” e-cigarette liquids in Australia. Med J Aust. 2019;210:127–8. doi: 10.5694/mja2.12059. [DOI] [PubMed] [Google Scholar]
- 5.Institute for Global Tobacco Control, Johns Hopkins Bloomberg School of Public Health. Country laws regulating e-cigarettes: a policy scan. [Accessed 10 September 2019]. Available at: https://www.globaltobaccocontrol.org/e-igarette_policyscan.
- 6. Gholap VV, Kosmider L, Halquist MS. A standardized approach to quantitative analysis of nicotine in e-liquids based on peak purity criteria using high-performance liquid chromatography. J Anal Methods Chem. 2018;2018:1720375. doi: 10.1155/2018/1720375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barber TE, List MS, Haas JW, III, Wachter EA. Determination of nicotine by surface-enhanced Raman scattering (SERS) Appl Spectrosc. 1994;48:1423–7. [Google Scholar]
- 8. Bell SE, Sirimuthu NM. Rapid, quantitative analysis of ppm/ ppb nicotine using surface-enhanced Raman scattering from polymer-encapsulated Ag nanoparticles (gel-colls) Analyst. 2004;129:1032–6. doi: 10.1039/b408775e. [DOI] [PubMed] [Google Scholar]
- 9. Itoh N, Bell SE. High dilution surface-enhanced Raman spectroscopy for rapid determination of nicotine in e-liquids for electronic cigarettes. Analyst. 2017;142:994–8. doi: 10.1039/c6an02286c. [DOI] [PubMed] [Google Scholar]
- 10. Wang HH, Liu CY, Wu SB, Liu NW, Peng CY, Chan TH, et al. Highly Raman-enhancing substrates based on silver nanoparticle arrays with tunable sub-10nm gaps. Adv Mater. 2006;18:491–5. [Google Scholar]
- 11. Morhác M, Matousek V. Peak clipping algorithms for background estimation in spectroscopic data. Appl Spectrosc. 2008;62:91–106. doi: 10.1366/000370208783412762. [DOI] [PubMed] [Google Scholar]
- 12. Alharbi O, Xu Y, Goodacre R. Simultaneous multiplexed quantification of nicotine and its metabolites using surface enhanced Raman scattering. Analyst. 2014;139:4820–7. doi: 10.1039/c4an00879k. [DOI] [PubMed] [Google Scholar]