Abstract
Aim
The aim of the study was to examine the relationship between coffee, tea, caffeine consumption and risk of all-cause death and cardiovascular disease (CVD) death in CVD population.
Methods
This cohort study included 626 CVD participants aged ≥18 years old who derived from the National Health and Nutrition Examination Surveys (NHANES) database 2003–2006. The end time of follow-up was 2015, and with a median follow-up time of 113.5 (63, 133) months. CVD death was defined as a death caused by congestive heart failure (CHF), coronary heart disease (CHD), angina pectoris, heart attack or stroke. Cox model and competitive-risk model were used to explore the relationship of coffee, tea, caffeine, decaffeinated coffee/tea on the risk of the all-cause death and CVD death for CVD population, respectively. Additionally, we explored the effect of urinary caffeine and caffeine metabolites on all-cause death.
Results
All patients were divided into survival group (n = 304), non-CVD death group (n = 223), and CVD death group (n = 99). The incidence of all-cause death and CVD death was ~51.44 and 15.81% in the study. After adjusting age, body mass index (BMI), cancer, estimated glomerular filtration rate (eGFR), energy, the history of CVD medications, carbohydrate and family income to poverty ratio (PIR), the results suggested coffee, caffeine, iced tea and hot tea consumption (≥4 cups per day) were associated with an increased risk of the all-cause death in CVD patients; while hot tea (1–3 cups per day), decaffeinated coffee/iced tea/hot tea could reduce the risk of the all-cause death. Likewise, coffee, caffeine, iced tea (≥4 cups per day), hot tea, decaffeinated iced tea/ hot tea (Always) could enhance the risk of the CVD death in CVD population. We also found that 1-methylxanthine showed a significant positive association on the risk of all-cause death in CVD population.
Conclusion
Our study indicated that higher consumption of coffee, tea and caffeine could increase the risk of all-cause and CVD death for CVD patients.
Keywords: all-cause mortality, CVD, caffeine, decaffeinated coffee/tea, coffee and tea, specific mortality
Introduction
Cardiovascular disease (CVD) has been recognized as a frequently diagnosed chronic disease in the world, and remains the major cause of premature death and disability in human beings (1, 2). Recent evidence suggests that the prevalence rate of CVD was over 500 million people and ~20 million people died from CVD per year (3), which seriously reduced quality of life and increased the burden of disease for many families. Previous studies have been observed that dietary factors might play an important role in the development of CVD mortality (4–6).
It is reported that coffee and tea are the most widely consumed beverages worldwide after water, and also regarded as the principal source of caffeine (7). Traditionally, people have been advised to reduce their coffee, tea and caffeine intake because it may increase some indicators which were harmful to physical health, such as blood pressure, total cholesterol, and triglycerides (8). However, several studies have found that chronic coffee consumption, tea consumption and caffeine intake could reduce the risk of CVD death through anti-inflammatory, anti-oxidant, lower blood sugar and fat functions (9–11), but these studies excluded the patients with CVD in their population selection. To the best of our knowledge, few studies in recent years have focused on the relationship of coffee, tea, caffeine intake and death in patients diagnosed with CVD, and these results remained controversial. de Vreede-Swagemakers et al. reported that heavy coffee consumption was associated with an increased risk of sudden cardiac death in patients who suffered sudden cardiac arrest and had a history of coronary artery disease (12). Conversely, in the study of Ribeiro et al., they pointed out that coffee consumption was related to lower risk of cardiovascular mortality and all-cause mortality in patients with myocardial infarction (13). Also, caffeine intake was thought to reduce cerebral blood flow for patients with ischemic stroke, which brought adverse clinical outcomes for patients (14). For a study of US women with CVD, there was no relationship between long-term consumption of filtered caffeinated coffee and all-cause or CVD death (15).
Herein, for patients with CVD, we conducted a follow-up study to investigate the relationship between coffee consumption, tea consumption, caffeine intake, decaffeinated coffee, decaffeinated tea on the risk of all-cause death and CVD death based on the National Health and Nutrition Examination Surveys (NHANES) database, and explore the effect of caffeine metabolites on all-cause death.
Methods
Study Design and Participants
All information of participants in this cohort study were obtained from NHANES database (16), which was a program of studies to evaluate the health and nutritional status of adults and children in the United States. National Center for Health Statistics (NCHS) has administered NHANES, a nationally representative survey consisting of about 5,000 persons from 15 different counties each year, continuously since 1999. The survey combines interviews and physical examinations, including demographic, socioeconomic, dietary, and health-related questions, medical, dental, and physiological measurements and laboratory tests (17) (https://www.cdc.gov/nchs/nhanes/index.htm).
Our study collected data of 20,470 participants between 2003 and 2006 from NHANES database. Included criteria: patients who were aged ≥18 years old and diagnosed as CVD in the present analysis. Simultaneously, patients who met any of the following criteria need to be excluded, (1) participants without CVD; (2) participants with incomplete information of coffee and tea consumption; (3) participants with extreme total energy intakes of <500 or >5,000 kcal/day for women and <500 or >8,000 kcal/day for men. All data of this study came from a publicly available database and ethical approval was obtained from the institutional review board at the NCHS Ethics Review Board (17).
Data Collection
Baseline data were collected, including age, gender, race, body mass index (BMI), education, family income to poverty ratio (PIR), smoking status, alcohol (gm), cancer, hypertension, diabetes, the history of CVD medications (18), cholesterol, protein (gm), total fat (gm), carbohydrate (gm), estimated glomerular filtration rate (eGFR, mL/min/m2), energy (10 kcal), coffee intake (cup/day), caffeine (100 mg), iced tea intake (cup/day), hot tea intake (cup/day), decaffeinated coffee, decaffeinated iced tea, decaffeinated hot tea. The NHANES asked all participants to provide some responses that had beverage consumption over the past 12 months (19); the survey question was “Did you drink coffee?” if your answer was yes, a follow-up question was asked “Did you drink how many cups of coffee, caffeinated or decaffeinated?” response options included <1 cup per day, 1–3 cups per day, ≥4 cups per day; then, they were asked “Did you drink decaffeinated, and how often do you drink decaffeinated coffee?” the choices in this question were, almost never, about a quarter to three quarters of the time, or always. The same question structures that contained frequency, decaffeinated vs. caffeinated types applied to iced tea and hot tea.
In addition, accurately determining caffeine dose from coffee and tea might be challenging (20, 21). Herein, urinary caffeine and caffeine metabolite levels have been proposed as an effective indicator of assessing caffeine intake. All NHANES participants were required to provide urine samples in a mobile examination center (MEC), and used ultra-high performance liquid chromatography-electrospray ionization-tandem quadrupole mass spectrometry to analyze the urine samples for caffeine and caffeine metabolites (21), including 1-methyluric acid (1U), 3-methyluric acid (3U), 7-methyluric acid (7U), 1,3-dimethyluric acid (13U), 1,7-dimethyluric acid (17U), 3,7-dimethyluric acid (37U), 1,3,7-trimethyluric acid (137U), 1-methylxanthine (1X), 3-methylxanthine (3X), 7-methylxanthine (7X), theophylline (1,3-dimethylxanthine, 13X), paraxanthine (1,7-dimethylxanthine, 17X), theobromine (3,7-dimethylxanthine, 37X), caffeine (1,3,7-trimethylxanthine, 137X), and 5-acetylamino-6-amino-3-methyluracil (AAMU).
Definition of CVD
Participants were identified as CVD patients if the answer was “yes” to any of the following question (22), “Has a doctor or other health professional ever told you that you have congestive heart failure (CHF)/coronary heart disease (CHD)/angina pectoris/heart attack/stroke?”
Definition of eGFR
eGFR was calculated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) (23): = 141 × min (Scr/κ,1) α × max (Scr/κ, 1) −1.029 × 0.993 age × 1.108 (if female) × 1.159 (if black), κ is 0.7 for females and 0.9 for males, α is −0.329 for females and −0.411 for males, min indicates the minimum of Scr/κ or 1, and max indicates the maximum of Scr/κ or 1. Scr stands for serum creatinine (mg/dL).
Outcome Variables and Follow-Up
Outcomes were recorded. CVD death was defined as a death caused by CHF, CHD, angina pectoris, heart attack or stroke. All-cause death was defined as all deaths. The end time of follow-up was 2015 with a median follow-up time of 113.5 (63, 133) months. The follow-up was terminated once patients occurred death in this study.
Statistical Analysis
In the present study, the data of normally distributed was represented by mean ± standard deviation (Mean ± SD), and intergroup comparison adopted t-test. The data of non-normally distributed were described by the median with interquartile spacing [M (Q1, Q3)], Mann-Whitney U rank sum test was used to perform the comparison between groups. The number of cases and composition ratio N (%) was used to express the categorical variables, Chi-square or Fisher's exact test was applied for intergroup comparison.
We employed the Cox model to explore the relationship of coffee, tea, caffeine, decaffeinated coffee/tea intake, and urinary caffeine and caffeine metabolite on the risk of all-cause death in CVD population. Considering that the competitive-risk model could better reflect the real death risk of related to CVD, we established a competitive-risk model to investigate the association between coffee, tea, caffeine, decaffeinated coffee/tea intake and the risk of CVD specific-death in CVD population. In the current study, relevant confounders were adjusted in three models: Model 1 was unadjusted, Model 2 adjusted for age and BMI. Model 3 adjusted age, BMI, cancer, eGFR, energy, the history of CVD medications, carbohydrate and family PIR. Hazard ratios (HR) and 95% confidence interval (CI) were computed in the study. The missing values were interpolated by SAS (Supplementary Table 1). All statistical analyses were performed by SAS and Python, and P < 0.05 was regarded as statistically significant for all tests.
Results
Baseline Characteristics
After excluding some patients who were not CVD (n = 19,221), had incomplete information of coffee and tea consumption (n = 344), and had extreme total energy intakes (n = 279), a total of 626 eligible patients were enrolled in the study ultimately. These patients were divided into survival group (n = 304), non-CVD death group (n = 223), and CVD death group (n = 99). The incidence of all-cause death and CVD death was ~51.44 and 15.81% in the study. As shown in Table 1, the mean age was 66.85 years and the mean BMI was 29.73 kg/m2 in this population. In addition, most of CVD patients (64.04%) had coffee intake with 1–3 cups per day, 84.43% CVD patients had iced tea intake with <1 cup per day, and 90.71% CVD patients had hot tea intake with <1 cup per day. Detailed baseline information was given in Table 1.
Table 1.
Baseline characteristics of all included participants.
| Variables | Total (n = 626) | Survival group (n = 304) | Non-CVD death group (n = 223) | CVD death group (n = 99) | P |
|---|---|---|---|---|---|
| Gender, n (%) | 0.312 | ||||
| Male | 358 (53.07) | 157 (49.95) | 134 (57.02) | 67 (56.75) | |
| Female | 268 (46.93) | 147 (50.05) | 89 (42.98) | 32 (43.25) | |
| Age, year, Mean (S.E) | 66.85 (0.81) | 62.18 (0.94) | 72.24 (1.03) | 73.59 (1.48) | <0.001 |
| BMI, kg/m2, Mean (S.E) | 29.73 (0.31) | 30.22 (0.47) | 28.93 (0.39) | 29.57 (1.03) | 0.101 |
| Race, n (%) | 0.340 | ||||
| Mexican American | 78 (3.07) | 43 (3.60) | 25 (2.44) | 10 (2.31) | |
| Other Hispanic | 6 (0.74) | 4 (0.82) | 1 (0.47) | 1 (1.07) | |
| Non-Hispanic White | 426 (83.64) | 179 (80.91) | 171 (86.42) | 76 (88.43) | |
| Non-Hispanic Black | 96 (8.43) | 69 (11.08) | 18 (5.26) | 9 (4.92) | |
| Other Race | 20 (4.12) | 9 (3.60) | 8 (5.42) | 3 (3.26) | |
| Education, n (%) | 0.072 | ||||
| At least high school | 415 (74.57) | 209 (78.64) | 140 (68.91) | 66 (70.89) | |
| Less than high school | 210 (25.43) | 95 (21.36) | 82 (31.09) | 33 (29.11) | |
| Family income to poverty ratio (PIR), Mean (S.E) | 2.72 (0.08) | 2.87 (0.12) | 2.46 (0.11) | 2.69 (0.17) | 0.040 |
| Smoking status, n (%) | 0.794 | ||||
| Yes | 330 (53.54) | 166 (54.74) | 113 (52.59) | 51 (50.80) | |
| No | 296 (46.46) | 138 (45.26) | 110 (47.41) | 48 (49.20) | |
| Alcohol, gm, Mean (S.E) | 6.89 (1.03) | 7.49 (1.58) | 5.68 (1.36) | 7.21 (2.10) | 0.634 |
| Protein, gm, Mean (S.E) | 71.83 (1.86) | 73.70 (2.53) | 71.42 (3.31) | 65.10 (3.29) | 0.123 |
| Cholesterol, n (%) | 0.111 | ||||
| Yes | 580 (93.31) | 277 (91.71) | 207 (94.25) | 96 (97.67) | |
| No | 46 (6.69) | 27 (8.29) | 16 (5.75) | 3 (2.33) | |
| Total fat, gm, Mean (S.E) | 72.53 (2.05) | 75.17 (3.27) | 71.60 (3.78) | 63.88 (4.25) | 0.072 |
| Carbohydrate, gm, Mean (S.E) | 227.91 (6.03) | 236.20 (7.52) | 224.39 (9.21) | 202.02 (9.36) | 0.008 |
| eGFR, mL/min/m2, Mean (S.E) | 68.34 (0.94) | 74.85 (1.34) | 60.35 (1.87) | 60.08 (2.64) | <0.001 |
| Energy, 10 kcal, Mean (S.E) | 183.93 (4.01) | 188.62 (5.55) | 182.28 (6.22) | 168.53 (6.85) | 0.015 |
| Hypertension, n (%) | 0.340 | ||||
| Yes | 446 (70.69) | 217 (68.40) | 68 (73.48) | 161 (73.78) | |
| No | 180 (29.31) | 87 (31.60) | 31 (26.52) | 61 (26.22) | |
| The history of CVD medications, n (%) | <0.001 | ||||
| Yes | 525 (82.07) | 235 (75.30) | 201 (89.79) | 89 (92.05) | |
| No | 101 (17.93) | 69 (24.70) | 22 (10.21) | 10 (7.95) | |
| Diabetes | 0.223 | ||||
| Yes | 11 (1.59) | 7 (2.12) | 2 (0.81) | 2 (1.20) | |
| No | 174 (31.81) | 99 (35.72) | 48 (26.29) | 27 (28.50) | |
| Unknown | 441 (66.60) | 198 (62.16) | 173 (72.90) | 70 (70.30) | |
| Cancer, n (%) | <0.001 | ||||
| Yes | 114 (19.07) | 41 (13.91) | 51 (26.67) | 22 (22.71) | |
| No | 512 (80.93) | 263 (86.09) | 172 (73.33) | 77 (77.29) | |
| Coffee, n (%) | 0.191 | ||||
| <1 cup per day | 137 (21.80) | 75 (25.48) | 43 (17.66) | 19 (16.23) | |
| 1–3 cups per day | 412 (64.04) | 192 (60.51) | 153 (68.98) | 67 (67.12) | |
| ≥4 cups per day | 77 (14.16) | 37 (14.01) | 27 (13.36) | 13 (16.66) | |
| Caffeine, 100 mg, Mean (S.E) | 2.05 (0.11) | 2.03 (0.18) | 2.14 (0.19) | 1.91 (0.22) | 0.794 |
| Iced tea, n (%) | 0.773 | ||||
| <1 cup per day | 531 (84.43) | 264 (86.23) | 183 (81.14) | 84 (84.65) | |
| 1–3 cups per day | 78 (11.47) | 34 (9.89) | 31 (14.38) | 13 (11.26) | |
| ≥4 cups per day | 17 (4.09) | 6 (3.88) | 9 (4.48) | 2 (4.09) | |
| Hot tea, n (%) | 0.773 | ||||
| <1 cup per day | 570 (90.71) | 279 (90.28) | 207 (92.81) | 84 (87.68) | |
| 1–3 cups per day | 49 (7.54) | 21 (7.62) | 13 (5.33) | 15 (12.32) | |
| ≥4 cups per day | 7 (1.74) | 4 (2.10) | 3 (1.86) | 0 (0.00) | |
| Decaffeinated coffee, n (%) | 0.547 | ||||
| Almost never | 335 (55.33) | 153 (52.85) | 127 (59.32) | 55 (56.30) | |
| About a quarter to three quarters of the time | 101 (16.16) | 58 (18.31) | 30 (14.03) | 13 (12.28) | |
| Always | 190 (28.51) | 93 (28.84) | 66 (26.65) | 31 (31.42) | |
| Decaffeinated iced tea, n (%) | <0.001 | ||||
| Almost never | 447 (71.75) | 199 (65.04) | 177 (82.95) | 71 (73.50) | |
| About a quarter to three quarters of the time | 103 (16.63) | 68 (22.14) | 24 (9.40) | 11 (10.69) | |
| Always | 76 (11.62) | 37 (12.82) | 22 (7.65) | 17 (15.81) | |
| Decaffeinated hot tea, n (%) | <0.001 | ||||
| Almost never | 447 (72.22) | 204 (67.73) | 178 (82.49) | 65 (66.93) | |
| About a quarter to three quarters of the time | 85 (13.75) | 56 (17.75) | 17 (6.97) | 12 (12.97) | |
| Always | 94 (14.04) | 44 (14.53) | 28 (10.54) | 22 (20.10) | |
| Follow-up time, months, Mean (S.E) | 104.07 (2.56) | 131.71 (2.01) | 70.70 (2.64) | 67.64 (5.06) | <0.001 |
BMI, body mass index; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate.
The Associated Factors With the Risk of the All-Cause Death in CVD Population
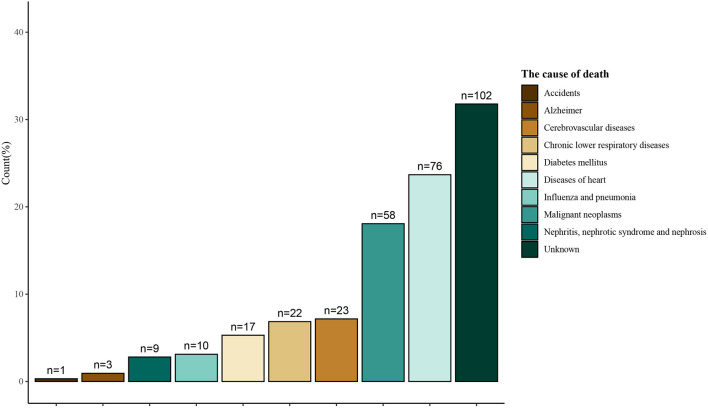
Table 2 shows the relationship of coffee consumption, caffeine consumption, iced tea consumption, hot tea consumption, and decaffeinated coffee/iced tea/hot tea on the risk of the all-cause death among CVD population. After adjusting for relevant confounders (P < 0.05), the results displayed that coffee consumption, caffeine consumption, iced tea consumption, hot tea consumption (≥4 cups per day) were associated with an increased risk of the all-cause death in CVD patients (Model 3). Nevertheless, hot tea consumption (1–3 cups per day), decaffeinated coffee, decaffeinated iced tea, and decaffeinated hot tea might reduce the risk of the all-cause death among CVD population. Additionally, Figure 1 also reveals the causes death of CVD patients.
Table 2.
The related factors with the risk of the all-cause death in CVD population.
| Variables | Model 1 | P | Model 2 | P | Model 3 | P |
|---|---|---|---|---|---|---|
| HR (95%CI) | HR (95%CI) | HR (95%CI) | ||||
| Coffee | ||||||
| <1 cup per day | Ref | Ref | Ref | |||
| 1–3 cups per day | 1.425 (1.422–1.428) | <0.001 | 1.064 (1.062–1.066) | <0.001 | 1.093 (1.091–1.095) | <0.001 |
| ≥4 cups per day | 1.261 (1.258–1.264) | <0.001 | 1.271 (1.268–1.274) | <0.001 | 1.389 (1.385–1.393) | <0.001 |
| Caffeine | 0.982 (0.982–0.983) | <0.001 | 1.103 (1.102–1.103) | <0.001 | 1.106 (1.105–1.106) | <0.001 |
| Iced tea | ||||||
| <1 cup per day | Ref | Ref | Ref | |||
| 1–3 cups per day | 1.248 (1.246–1.251) | <0.001 | 1.211 (1.208–1.213) | <0.001 | 1.279 (1.276–1.282) | <0.001 |
| ≥4 cups per day | 1.086 (1.083–1.090) | <0.001 | 1.795 (1.789–1.801) | <0.001 | 1.639 (1.632–1.645) | <0.001 |
| Hot tea | ||||||
| <1 cup per day | Ref | Ref | Ref | |||
| 1–3 cups per day | 1.054 (1.052–1.057) | <0.001 | 0.838 (0.836–0.840) | <0.001 | 0.944 (0.941-0.946) | <0.001 |
| ≥4 cups per day | 1.011 (1.005–1.017) | <0.001 | 1.543 (1.534–1.552) | <0.001 | 1.529 (1.520–1.538) | <0.001 |
| Decaffeinated coffee | ||||||
| Almost never | Ref | Ref | Ref | |||
| About a quarter to three quarters of the time | 0.761 (0.760–0.763) | <0.001 | 0.841 (0.839–0.842) | <0.001 | 0.869 (0.867–0.871) | <0.001 |
| Always | 0.909 (0.907–0.910) | <0.001 | 0.765 (0.764–0.767) | <0.001 | 0.787 (0.786–0.789) | <0.001 |
| Decaffeinated iced tea | ||||||
| Almost never | Ref | Ref | Ref | |||
| About a quarter to three quarters of the time | 0.422 (0.421–0.423) | <0.001 | 0.644 (0.634–0.646) | <0.001 | 0.697 (0.695–0.698) | <0.001 |
| Always | 0.760 (0.758–0.761) | <0.001 | 0.774 (0.773–0.776) | <0.001 | 0.772 (0.771–0.774) | <0.001 |
| Decaffeinated hot tea | ||||||
| Almost never | Ref | Ref | Ref | |||
| About a quarter to three quarters of the time | 0.503 (0.502–0.504) | <0.001 | 0.600 (0.598–0.601) | <0.001 | 0.611 (0.609–0.612) | <0.001 |
| Always | 0.934 (0.932–0.936) | <0.001 | 0.905 (0.903–0.906) | <0.001 | 0.943 (0.941–0.945) | <0.001 |
HR, hazard ratios; CI, confidence interval.
Model 1: unadjusted.
Model 2: adjusted for age and BMI.
Model 3: adjusted for age, BMI, cancer, eGFR, energy, the history of CVD medications, carbohydrate and family PIR.
Figure 1.
The causes of the all-cause death of CVD patients.
Also, Table 3 shows the estimated association of urinary caffeine and caffeine metabolites and the risk of the all-cause death in CVD population by using Cox model. After adjusting for age, BMI, cancer, eGFR, energy, the history of CVD medications, carbohydrate and family PIR (Model 3), 1X (HR = 1.003, 95%CI: 1.000–1.006) was significantly associated with higher risk of all-cause death in CVD population.
Table 3.
The association of urinary caffeine metabolites and the risk of the all-cause death in CVD population.
| Variables | Model 1 | P | Model 2 | P | Model 3 | P |
|---|---|---|---|---|---|---|
| HR (95%CI) | HR (95%CI) | HR (95%CI) | ||||
| 1-methyluric acid, umol/L | 1.002 (1.000–1.003) | 0.006 | 1.002 (1.000–1.003) | 0.004 | 1.001 (1.000–1.003) | 0.142 |
| 3-methyluric acid, umol/L | 1.052 (1.019–1.084) | 0.002 | 1.055 (1.017–1.093) | 0.006 | 1.037 (0.982–1.091) | 0.198 |
| 7-methyluric acid, umol/L | 1.002 (0.999–1.004) | 0.139 | 1.002 (1.000–1.005) | 0.097 | 1.002 (0.996–1.007) | 0.551 |
| 1,3-dimethyluric acid, umol/L | 1.004 (0.999–1.009) | 0.095 | 1.004 (0.999–1.008) | 0.133 | 1.003 (0.996–1.011) | 0.383 |
| 1,7-dimethyluric acid, umol/L | 1.003 (1.000–1.005) | 0.032 | 1.002 (1.000–1.004) | 0.055 | 1.002 (0.998–1.005) | 0.310 |
| 3,7-dimethyluric acid, umol/L | 1.016 (0.952–1.080) | 0.624 | 1.029 (0.971–1.087) | 0.334 | 1.033 (0.959–1.107) | 0.391 |
| 1,3,7-trimethyluric acid, umol/L | 1.015 (0.985–1.045) | 0.334 | 1.014 (0.987–1.042) | 0.314 | 1.017 (0.978–1.057) | 0.398 |
| 1-methylxanthine, umol/L | 1.002 (0.999–1.005) | 0.188 | 1.003 (1.000–1.006) | 0.020 | 1.005 (1.000–1.009) | 0.035 |
| 3-methylxanthine, umol/L | 1.002 (1.000–1.004) | 0.059 | 1.002 (1.000–1.004) | 0.079 | 1.001 (0.997–1.005) | 0.662 |
| 7-methylxanthine, umol/L | 1.001 (0.999–1.002) | 0.323 | 1.001 (1.000–1.003) | 0.150 | 1.001 (0.999–1.004) | 0.274 |
| 1,3-dimethylxanthine (theophylline), umol/L | 0.978 (0.900–1.057) | 0.586 | 0.994 (0.927–1.061) | 0.865 | 1.007 (0.926–1.087) | 0.869 |
| 1,7-dimethylxanthine (paraxanthine), umol/L | 0.997 (0.987–1.007) | 0.589 | 1.003 (0.993–1.014) | 0.529 | 1.007 (0.990–1.025) | 0.414 |
| 3,7-dimethylxanthine (theobromine), umol/L | 0.999 (0.992–1.006) | 0.698 | 0.999 (0.992–1.005) | 0.719 | 0.995 (0.980–1.010) | 0.524 |
| 1,3,7-trimethylxanthine (caffeine), umol/L | 1.010 (0.993–1.027) | 0.261 | 1.010 (0.994–1.027) | 0.233 | 1.014 (0.990–1.039) | 0.243 |
| AAMU, umol/L | 1.001 (1.000–1.002) | 0.020 | 1.002 (1.000–1.003) | 0.005 | 1.001 (1.000–1.003) | 0.095 |
HR, hazard ratios; CI, confidence interval.
Model 1: unadjusted.
Model 2: adjusted for age and BMI.
Model 3: adjusted for age, BMI, cancer, eGFR, energy, the history of CVD medications, carbohydrate and family PIR.
The Associated Factors With Risk of the CVD Death in CVD Population
Likewise, we also assessed the association between coffee consumption, caffeine consumption, iced tea consumption, hot tea consumption, decaffeinated coffee/iced tea/hot tea and the risk of the CVD death among CVD patients. As shown in Table 4, the results of Model 3 described that coffee consumption, caffeine consumption, iced tea consumption (≥4 cups per day, HR = 1.773, 95%CI: 1.763–1.784), hot tea consumption, decaffeinated iced tea (always, HR = 1.434, 95%CI: 1.430–1.439), decaffeinated hot tea (HR = 1.725, 95%CI: 1.719–1.731) could enhance the risk of the CVD death in CVD population during the follow-up period. Conversely, iced tea consumption (1–3 cups per day, HR = 0.870, 95%CI: 0.866–0.873), decaffeinated coffee, decaffeinated iced tea (about a quarter to three quarters of the time, HR = 0.728, 95%CI: 0.725–0.730) were related to the decreased risk of the CVD death in CVD patients.
Table 4.
The related factors with the risk of the CVD death in CVD population.
| Variables | Model 1 | P | Model 2 | P | Model 3 | P |
|---|---|---|---|---|---|---|
| HR (95%CI) | HR (95%CI) | HR (95%CI) | ||||
| Coffee | ||||||
| <1 cup per day | Ref | Ref | Ref | |||
| 1–3 cups per day | 1.398 (1.394–1.402) | <0.001 | 1.125 (1.122–1.129) | <0.001 | 1.266 (1.262–1.271) | <0.001 |
| ≥4 cups per day | 1.545 (1.539–1.551) | <0.001 | 1.483 (1.477–1.489) | <0.001 | 2.027 (2.018–2.036) | <0.001 |
| Caffeine | 0.949 (0.948–0.949) | <0.001 | 1.082 (1.081–1.083) | <0.001 | 1.126 (1.125–1.127) | <0.001 |
| Iced tea | ||||||
| <1 cup per day | Ref | Ref | Ref | |||
| 1–3 cups per day | 0.936 (0.932–0.939) | <0.001 | 0.863 (0.859–0.866) | <0.001 | 0.870 (0.866–0.873) | <0.001 |
| ≥4 cups per day | 0.902 (0.898–0.907) | <0.001 | 1.717 (1.707–1.727) | <0.001 | 1.773 (1.763–1.784) | <0.001 |
| Hot tea | ||||||
| <1 cup per day | Ref | Ref | Ref | |||
| 1–3 cups per day | 2.318 (2.310–2.326) | <0.001 | 1.911 (1.905–1.918) | <0.001 | 2.005 (1.998–2.012) | <0.001 |
| ≥4 cups per day* | – | <0.001 | – | <0.001 | – | <0.001 |
| Decaffeinated coffee | ||||||
| Almost never | Ref | Ref | Ref | |||
| About a quarter to three quarters of the time | 0.772 (0.769–0.775) | <0.001 | 0.826 (0.822–0.829) | <0.001 | 0.901 (0.897–0.904) | <0.001 |
| Always | 0.982 (0.979–0.984) | <0.001 | 0.777 (0.775–0.780) | <0.001 | 0.724 (0.722–0.726) | <0.001 |
| Decaffeinated iced tea | ||||||
| Almost never | Ref | Ref | Ref | |||
| About a quarter to three quarters of the time | 0.588 (0.586–0.591) | <0.001 | 0.708 (0.705–0.710) | <0.001 | 0.728 (0.725–0.730) | <0.001 |
| Always | 1.397 (1.392–1.401) | <0.001 | 1.497 (1.492–1.502) | <0.001 | 1.434 (1.430–1.439) | <0.001 |
| Decaffeinated hot tea | ||||||
| Almost never | Ref | Ref | Ref | |||
| About a quarter to three quarters of the time | 0.892 (0.889–0.895) | <0.001 | 1.081 (1.077–1.084) | <0.001 | 0.955 (0.952–0.959) | <0.001 |
| Always | 1.957 (1.950–1.963) | <0.001 | 1.859 (1.853–1.865) | <0.001 | 1.725 (1.719–1.731) | <0.001 |
HR, hazard ratios; CI, confidence interval.
Model 1: unadjusted.
Model 2: adjusted for age and BMI.
Model 3: adjusted for age, BMI, cancer, eGFR, energy, the history of CVD medications, carbohydrate and family PIR.
The symbol * indicates that the sample size was 0 and no calculation was performed.
Discussion
In this retrospective cohort study of 626 patients were diagnosed with CVD, we observed that the high consumption of coffee, tea, and caffeine was associated with an increased risk of all-cause and specific-death for CVD patients. Our study also found that decaffeinated coffee/tea could decrease the all-cause death risk in CVD patients. Decaffeinated coffee could affect the lower risk of CVD death with respect to the CVD patients. Additionally, we also found that urinary caffeine metabolites level (1X) showed a significant positive association on the risk of all-cause death in CVD population.
In the present study, the findings showed that higher consumption of coffee, tea and caffeine enhanced the risk of all-cause and CVD death for CVD patients, which suggested that CVD patients should drink moderate amount of coffee, tea and as well as caffeine intake. Caffeine is a methylxanthine alkaloids and stimulant alkaloid of central nervous system, and commonly found in the coffee, tea, soft drinks, and chocolate (24). Currently, caffeine is thought to affect the cardiovascular system mainly through anti-adenosine receptors, inhibiting phosphodiesterase activity, promoting the release of catecholamines from adrenal glands, activating sympathetic nerves and renin-angiotensin system (25, 26). Our findings showed the fact that drinking 1–3 cups of iced tea per day was associated with a reduced risk of CVD death for CVD patients, the possible reason may be related to the solubility of active components. It is well-known that the solubility of caffeine decreases with increasing temperature (27), which also suggested that caffeine might have a less solubility and bioavailability in iced tea. We speculated when patients with CVD drank moderate iced tea, the bioavailability of caffeine reduce, which may decrease the risk of hyperactive sympathetic system by caffeine and be beneficial to the health of CVD patients (28, 29). However, interestingly, more than 4 cups of iced tea per day was considered as a risk factor in the CVD specific-death, this may be related to temperature or population selection, more research needs to be explored in detail. Notedly, among urinary caffeine metabolites, 1X was related to an increased risk of death for CVD patients. Hence, our findings also indicated that 1X exerts a more pronounced effect than other metabolites, leading to a rise in the likelihood of death of CVD patients.
Torres-Collado et al. pointed out that there was not significant association between the decaffeinated coffee and risk of CVD death in a representative sample of an adult population in Valencia and Spain (30), which was inconsistent with our results. In our study, decaffeinated coffee/tea was significantly related to the decreased risk for the CVD all-cause death, which indicated that decaffeinated coffee/tea might play a protective effect for death for CVD patients. Coffee and tea contain a variety of other chemicals besides caffeine. For instance, coffee contains polyphenols, such as chlorogenic acids, alkaloids, phenolic compounds, trace elements and so on (31); tea is also rich in tea polyphenols, catechin polymers, tea polysaccharides and other functional components as well as other natural products (32). Chlorogenic acids, alkaloids, phenolic compounds, trace elements, tea polyphenols, catechin polymers and tea polysaccharides have been proved to play vital roles in the anti-oxidant, anti-inflammatory, and anti-thrombosis, improving endothelial function, inhibiting the aggregation of platelet, reducing blood sugar and blood lipid, regulating metabolism and improving intestinal microbiome (32–34). This might be the underlying mechanism that decaffeinated coffee/tea decreased the death risk for CVD patients.
With respect to the coffee and tea, this study showed that higher coffee and tea consumption increased the death risk for CVD patients, and while decaffeinated coffee/tea was considered to play a protective effect for death for CVD patients. Some studies have shown that coffee and caffeine may increase the sympathetic nervous system of coffee drinkers, leading to autonomic imbalances (28, 29); for CVD patients, autonomic imbalance might cause the poor prognosis (29, 35). The findings suggest that caffeinated beverages should be consumed in moderation for patients with CVD. More studies are required to further confirm this association and give more evidences about mechanism.
The current study has several limitations. Firstly, the study had a relatively small sample size, which might have limited the statistical power, but the follow-up period of the study was long enough to investigate the association between coffee, tea, caffeine, decaffeinated coffee/tea consumption and risk of all-cause death and CVD death in CVD population. Secondly, laboratory measures such as caffeine, were measured only twice in 2 days and are not necessarily representative. Lastly, because all information in this retrospective cohort study was derived from the NHANES database, we did not collect the information about the consumption of medications prescribed for patients with CVD, the types of coffee or tea consumption, duration of brewing, the method of coffee or tea preparation and beverage cup sizes, more researches are needed to explore this association.
Conclusion
In conclusion, our study indicated that for CVD patients, higher consumption of coffee, tea and caffeine could increase the risk of all-cause and CVD death. More studies are needed to further elucidate the association.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/index.htm.
Ethics Statement
Ethical approval was not provided for this study on human participants because due to the data from NHANES database were publicly available, this study did not require institutional review board approval. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
HZ, FL, and PZ designed the study and wrote the manuscript. HZ, FL, and NX collected and analyzed the data. HZ and LY contributed to literature search. PZ and FL critically reviewed and improved the drafts of the manuscript. All authors have read and approved the final manuscript.
Funding
This research was supported by Major project of Fujian Science and Technology Program (2016YZ0001-1) and Excellent Young Doctor Training Program in Fujian Provincial Health System (2013-ZQN-ZD-4).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are grateful for the support from Fujian Provincial Center for Geriatrics and Fujian Provincial Institute of Clinical Geriatrics. We would like to thank the technical assistance of the Institute of Fujian Provincial Key Laboratory of Geriatrics.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.842856/full#supplementary-material
References
- 1.Mensah GA, Roth GA, Fuster V. The global burden of cardiovascular diseases and risk factors: 2020 and beyond. J Am Coll Cardiol. (2019) 74:2529–32. 10.1016/j.jacc.2019.10.009 [DOI] [PubMed] [Google Scholar]
- 2.Flora GD, Nayak MK. A brief review of cardiovascular diseases, associated risk factors and current treatment regimes. Curr Pharm Des. (2019) 25:4063–84. 10.2174/1381612825666190925163827 [DOI] [PubMed] [Google Scholar]
- 3.Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. (2020) 76:2982–3021. 10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodríguez-Artalejo F, López-García E. Coffee consumption and cardiovascular disease: a condensed review of epidemiological evidence and mechanisms. J Agric Food Chem. (2018) 66:5257–63. 10.1021/acs.jafc.7b04506 [DOI] [PubMed] [Google Scholar]
- 5.Kim Y, Je Y, Giovannucci E. Coffee consumption and all-cause and cause-specific mortality: a meta-analysis by potential modifiers. Eur J Epidemiol. (2019) 34:731–52. 10.1007/s10654-019-00524-3 [DOI] [PubMed] [Google Scholar]
- 6.Shin S, Lee JE, Loftfield E, Shu XO, Abe SK, Rahman MS, et al. Coffee and tea consumption and mortality from all causes, cardiovascular disease and cancer: a pooled analysis of prospective studies from the Asia Cohort Consortium. Int J Epidemiol. (2021) 51:626–40. 10.1093/ije/dyab161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sen A, Papadimitriou N, Lagiou P, Perez-Cornago A, Travis RC, Key TJ, et al. Coffee and tea consumption and risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. (2019) 144:240–50. 10.1002/ijc.31634 [DOI] [PubMed] [Google Scholar]
- 8.Heckman MA, Weil J, Gonzalez de.Mejia E. Caffeine (1, 3, 7-trimethylxanthine) in foods: a comprehensive review on consumption, functionality, safety, regulatory matters. J Food Sci. (2010) 75:77–87. 10.1111/j.1750-3841.2010.01561.x [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Liu S, Zhou H, Hanson T, Yang L, Chen Z, et al. Association of green tea consumption with mortality from all-cause, cardiovascular disease and cancer in a Chinese cohort of 165,000 adult men. Eur J Epidemiol. (2016) 31:853–65. 10.1007/s10654-016-0173-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim SA, Tan LJ, Shin S. Coffee consumption and the risk of all-cause and cause-specific mortality in the Korean population. J Acad Nutr Diet. (2021) 121:2221–32.e4. 10.1016/j.jand.2021.03.014 [DOI] [PubMed] [Google Scholar]
- 11.Mineharu Y, Koizumi A, Wada Y, Iso H, Watanabe Y, Date C, et al. Coffee, green tea, black tea and oolong tea consumption and risk of mortality from cardiovascular disease in Japanese men and women. J Epidemiol Community Health. (2011) 65:230–40. 10.1136/jech.2009.097311 [DOI] [PubMed] [Google Scholar]
- 12.de Vreede-Swagemakers JJ, Gorgels AP, Weijenberg MP, Dubois-Arbouw WI, Golombeck B, van Ree JW, et al. Risk indicators for out-of-hospital cardiac arrest in patients with coronary artery disease. J Clin Epidemiol. (1999) 52:601–7. 10.1016/S0895-4356(99)00044-X [DOI] [PubMed] [Google Scholar]
- 13.Ribeiro EM, Alves M, Costa J, Ferreira JJ, Pinto FJ, Caldeira D. Safety of coffee consumption after myocardial infarction: a systematic review and meta-analysis. Nutr Metab Cardiovasc Dis. (2020) 30:2146–58. 10.1016/j.numecd.2020.07.016 [DOI] [PubMed] [Google Scholar]
- 14.Ragab S, Lunt M, Birch A, Thomas P, Jenkinson DF. Caffeine reduces cerebral blood flow in patients recovering from an ischaemic stroke. Age Ageing. (2004) 33:299–303. 10.1093/ageing/afh091 [DOI] [PubMed] [Google Scholar]
- 15.Lopez-Garcia E, Rodriguez-Artalejo F, Li TY, Mukamal KJ, Hu FB, van Dam RM. Coffee consumption and mortality in women with cardiovascular disease. Am J Clin Nutr. (2011) 94:218–24. 10.3945/ajcn.110.010249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ALHarthi SSY, Natto ZS, Midle JB, Gyurko R, O'Neill R, Steffensen B. Association between time since quitting smoking and periodontitis in former smokers in the National Health and Nutrition Examination Surveys (NHANES) 2009 to 2012. J Periodontol. (2019) 90:16–25. 10.1002/JPER.18-0183 [DOI] [PubMed] [Google Scholar]
- 17.Iranpour S, Sabour S. Inverse association between caffeine intake and depressive symptoms in US adults: data from National Health and Nutrition Examination Survey (NHANES) 2005-2006. Psychiatry Res. (2019) 271:732–9. 10.1016/j.psychres.2018.11.004 [DOI] [PubMed] [Google Scholar]
- 18.Ghavami HS, Khoshtinat M, Sadeghi-Farah S, Kalimani AB, Ferrie S, Faraji H. The relationship of coffee consumption and CVD risk factors in elderly patients with T2DM. BMC Cardiovasc Disord. (2021) 21:241. 10.1186/s12872-021-02058-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu CM, Wu AM, Tseng VL, Yu F, Coleman AL. Frequency of a diagnosis of glaucoma in individuals who consume coffee, tea and/or soft drinks. Br J Ophthalmol. (2018) 102:1127–33. 10.1136/bjophthalmol-2017-310924 [DOI] [PubMed] [Google Scholar]
- 20.Bracken MB, Triche E, Grosso L, Hellenbrand K, Belanger K, Leaderer BP. Heterogeneity in assessing self-reports of caffeine exposure: implications for studies of health effects. Epidemiology. (2002) 13:165–71. 10.1097/00001648-200203000-00011 [DOI] [PubMed] [Google Scholar]
- 21.Long L, Tang Y. Urine caffeine metabolites and hearing threshold shifts in US adults: a cross-sectional study. Sci Rep. (2021) 11:21631. 10.1038/s41598-021-01094-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dinh A, Miertschin S, Young A, Mohanty SD. A data-driven approach to predicting diabetes and cardiovascular disease with machine learning. BMC Med Inform Decis Mak. (2019) 19:211. 10.1186/s12911-019-0918-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duggal V, Thomas IC, Montez-Rath ME, Chertow GM, Kurella Tamura M. National estimates of CKD prevalence and potential impact of estimating glomerular filtration rate without race. J Am Soc Nephrol. (2021) 32:1454–63. 10.1681/ASN.2020121780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Voskoboinik A, Kalman JM, Kistler PM. Caffeine and arrhythmias: time to grind the data. JACC Clin Electrophysiol. (2018) 4:425–32. 10.1016/j.jacep.2018.01.012 [DOI] [PubMed] [Google Scholar]
- 25.Cappelletti S, Piacentino D, Sani G, Aromatario M. Caffeine: cognitive and physical performance enhancer or psychoactive drug? Curr Neuropharmacol. (2015) 13:71–88. 10.2174/1570159X13666141210215655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Voskoboinik A, Koh Y, Kistler PM. Cardiovascular effects of caffeinated beverages. Trends Cardiovasc Med. (2019) 29:345–50. 10.1016/j.tcm.2018.09.019 [DOI] [PubMed] [Google Scholar]
- 27.Jeliński T, Kubsik M, Cysewski P. Application of the solute-solvent intermolecular interactions as indicator of caffeine solubility in aqueous binary aprotic and proton acceptor solvents: measurements and quantum chemistry computations. Materials. (2022) 15:2472. 10.3390/ma15072472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corti R, Binggeli C, Sudano I, Spieker L, Hänseler E, Ruschitzka F, et al. Coffee acutely increases sympathetic nerve activity and blood pressure independently of caffeine content: role of habitual versus nonhabitual drinking. Circulation. (2002) 106:2935–940. 10.1161/01.CIR.0000046228.97025.3A [DOI] [PubMed] [Google Scholar]
- 29.Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol. (2010) 141:122–31. 10.1016/j.ijcard.2009.09.543 [DOI] [PubMed] [Google Scholar]
- 30.Torres-Collado L, Compañ-Gabucio LM, González-Palacios S, Notario-Barandiaran L, Oncina-Cánovas A, Vioque J, et al. Coffee consumption and all-cause, cardiovascular, and cancer mortality in an adult mediterranean population. Nutrients. (2021) 13:1241. 10.3390/nu13041241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farias-Pereira R, Park CS, Park Y. Mechanisms of action of coffee bioactive components on lipid metabolism. Food Sci Biotechnol. (2019) 28:1287–96. 10.1007/s10068-019-00662-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan N, Mukhtar H. Tea polyphenols in promotion of human health. Nutrients. (2018) 11:39. 10.3390/nu11010039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turnbull D, Rodricks JV, Mariano GF, Chowdhury F. Caffeine and cardiovascular health. Regul Toxicol Pharmacol. (2017) 89:165–85. 10.1016/j.yrtph.2017.07.025 [DOI] [PubMed] [Google Scholar]
- 34.Li L, Su C, Chen X, Wang Q, Jiao W, Luo H, et al. Chlorogenic acids in cardiovascular disease: a review of dietary consumption, pharmacology, and pharmacokinetics. J Agric Food Chem. (2020) 68:6464–84. 10.1021/acs.jafc.0c01554 [DOI] [PubMed] [Google Scholar]
- 35.Weinstein G, Davis-Plourde K, Beiser AS, Seshadri S. Autonomic imbalance and risk of dementia and stroke: the Framingham study. Stroke. (2021) 52:2068–76. 10.1161/STROKEAHA.120.030601 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/index.htm.