Executive Summary
The Stanford-Lancet Commission on the North American Opioid Crisis was formed in response to the soaring opioid-related morbidity and mortality that the United States (USA) and Canada have experienced over the past 25 years. The Commission is supported by Stanford University and brings together diverse Stanford scholars with other leading experts around the USA and Canada with the goal of understanding the opioid crisis and proposing solutions to it domestically while attempting to stop its spread internationally.
Unlike some other Lancet Commissions, this one focuses on a long-entrenched problem that has already been well-characterized, including in multiple National Academies of Science, Engineering, and Medicine reviews.1–3 The Commission therefore focused on developing a coherent, empirically grounded analysis of the causes of and solutions to the opioid crisis.
The North American crisis emerged when insufficient regulation of the pharmaceutical and health care industries facilitated a profit-driven quadrupling of opioid prescribing.4–7 This involved a departure from long-established practice norms, particularly in the expanded prescribing of extremely potent opioids for a broad range of chronic non-cancer pain conditions.8–10 Hundreds of thousands of individuals fatally overdosed on prescription opioids, and millions more became addicted or were harmed in other ways, including disability, family breakdown, crime, unemployment, and bereavement.11–14 In response to the large pool of individuals who were addicted to prescription opioids, heroin markets expanded, increasing morbidity and mortality further.15,16 As heroin markets became saturated with illicit synthetic opioids such as fentanyl, an already horrific situation became a public health catastrophe that has only worsened since the onset of the COVID-19 pandemic.17–21 Since 1999, more than 600,000 people in the USA and Canada have died from an opioid overdose, and the rate of mortality in each country exceeds that of the worst of the HIV/AIDS epidemic.14,21–24
The first wave of the opioid crisis began in the 1990s when the long-acting opioid OxyContin and other high potency opioids were employed for an extremely wide array of patients.4 The first wave inflicted the most harm on white and indigenous people in both the USA and Canada.25–27 An unusually high number of middle-class people and people living in selected rural areas (e.g., Appalachia in the USA, The Yukon in Canada) were affected relative to prior epidemics of opioid addiction and overdose.26,28 The second wave, as heroin markets became resurgent in response to demand from individuals addicted to prescription opioids, began around 2010 and led to rapidly rising mortality among African Americans in the USA, and more generally in urban areas in the USA and Canada.29,30 These demographic shifts persisted into the third wave of the crisis, which began around 2014.14,26,30 This wave was characterized by rising addiction and fatal overdoses involving synthetic opioids such as fentanyl.17,31 2020 was the worst year on record in both countries: Canada saw a 62% increase in fatal opioid overdoses since 2019 (to 6214 deaths) and preliminary data from the USA suggests a 33% increase (to 69,710 deaths).21,32 Each wave added to rather than replaced the prior waves, with addiction and overdoses continuing among individuals using any or all of prescription opioids, heroin, and synthetic opioids such as fentanyl.14,21
In both the USA and Canada, fatal opioid overdoses are concentrated among men and young to middle-aged people.14,21 The mortality rate among African Americans in the USA has grown rapidly and is now on par with that of whites and American Indians and Alaska Natives.14 People experiencing homelessness and those recently released from incarceration have been particularly hard hit throughout the crisis and continue to face shockingly high overdose mortality rates.33–36 Overdoses involving both stimulants and opioids is common and seems to be increasing in both the USA and Canada.21,37 A significant number of opioid overdoses also involve concurrent use of benzodiazepines.38
The Commission’s analysis of the crisis focused on seven domains: (1) the North American opioid crisis as a case study in multi-system regulatory failure; (2) opioids’ dual nature as a benefit and a risk to health; (3) building integrated, well-supported and enduring systems for the care for people with substance use disorders; (4) maximizing the benefit and minimizing the adverse effects of criminal justice system involvement of people who are addicted to opioids; (5) creating healthy environments that can yield long-term declines in the incidence of addiction; (6) stimulating greater innovation in the response to the opioid crisis; and (7) preventing the North American opioid crisis from spreading globally. In each area, the Commission recommends evidence-informed policies that are responsive to identified challenges.
Domain 1.
The Commission concludes that the initial wave of the opioid crisis arose from weak laws and regulations as well as poor implementation of these laws and regulations.5 This included failures at the U.S. Food and Drug Administration which approved OxyContin with what was later shown to be a fraudulent description of the drug as less addictive.4 Further problems arose from overly cozy relationships between opioid manufacturers with universities, professional societies, patient advocacy groups, and lawmakers; and aggressive product promotion to prescribers and, to a lesser extent, the general public.39–42 These problems were compounded by the limited tools regulators have post-approval, particularly given that the law in the USA makes government largely dependent on the pharmaceutical industry to conduct adequate post-approval surveillance and to provide risk management education to prescribers, which the industry does poorly.43,44 The Commission therefore recommends curtailing pharmaceutical product promotion, insulating medical education from pharmaceutical industry influence, closing the “revolving door” between regulators and industry, making post-approval drug monitoring and risk mitigation a function of government, and firewalling bodies with formal power over prescribing from industry influence. To lessen the often overwhelming political clout of the industry, it also recommends exposing “astroturf” advocacy groups funded by industry and restoring limits on corporate donations to political campaigns.
Domain 2.
The Commission is cognizant that perceptions of opioid medication are currently polarized and over-simplified, when the reality is that these drugs are in some cases of great benefit and in others very harmful.45 Regulators must hold this dual nature of opioids in mind rather than “throwing the switch” one way or the other toward overly lax or overly restrictive prescribing policies, both of which have significant potential for harm. The drug approval process would be improved by considering the risk of a medication being diverted (e.g., for sale and for misuse by someone other than the patient) and also by conducting more long-term, pragmatic clinical trials on opioids’ risks and benefits. Improving the management of pain is critical, and could be facilitated in the USA by re-energizing the National Pain Strategy that was prepared at the close of the Obama Administration.46 The medical profession should promote opioid stewardship both for its own value and also to help restore trust in medicine among policymakers and the public, which the opioid crisis has damaged.47 Methods for fostering opioid stewardship include prescription drug monitoring programs, “nudges” toward safer prescribing, and expanding access to opioid agonist therapy for addiction that still maintaining adequate controls on it.48–51
Domain 3.
The Commission noted the lack of accessible, high-quality, non-stigmatizing, and integrated health and social care services for people experiencing opioid use disorder in the USA and to a lesser but still significant extent in Canada.52 This situation could be improved by financing such care through the mechanisms that support the rest of the health care system. The Commission recommends ending this situation by reforming public and private health insurance systems, including cutting off funding for care that is likely harmful. The Commission suggests that care systems should follow established models of chronic disease management to promote many pathways to recovery from addiction.53 It also calls for a setting aside of longrunning disputes between factions in the field, urging them to unify under the banner of public health. Finally, a major investment in workforce development is recommended, specifically increasing the number of addiction specialists and increasing the addiction-related knowledge and skills of general practitioners.54
Domain 4.
Although some advocates believe that the criminal justice system should have no role in responding to addiction, some role is inevitable given the public safety harms of intoxicated conduct and the fact that many arrests of people who are addicted involve nondrug crimes (e.g., domestic violence).55 The Commission therefore focused on ways of maximizing the good the justice system could do while minimizing the damage it can inflict. The former include providing addiction treatment and other health services during incarceration;56 the latter include forgoing incarceration for possession of illicit opioids for personal use, repealing collateral penalties for drug-related crimes, and ending punishment for opioid use during pregnancy.57,58
Domain 5.
Epidemics of disease are never resolved through the provision of services to identified cases; rather, prevention of new cases is essential. One practical method for achieving this in the USA is to adopt the methods used in other countries to facilitate disposal of the billions of excess opioid pills in households.59 Because most risk factors for developing drug problems are generic (e.g., chaotic, unrewarding environments, unremitting stress, social exclusion, violence and other trauma, sexual assault, parental abuse and neglect, and individual risk factors such as having difficulty managing emotions, coping with challenges, and exercising behavioral self-control), another important tactic is to support “horizontal” prevention programs for youth that strengthen core capacities that reduce risk not only for drug use, but for many other problems such as depression, anxiety, school failure, and obesity.60 Restrictions on youth-targeted advertising of addictive drugs (e.g., alcohol, tobacco, cannabis, pharmaceuticals) is another example of a valuable prevention effort that keeps the environment in mind. Finally, the Commission notes the evidence that enriching the environment more broadly, particularly for children and adolescents in economically struggling, high-stress regions, can plausibly lower the incidence of addiction over the long term.
Domain 6.
In surveying the terrain, the Commission was dismayed to note the slow pace of innovation in society’s response to drug problems, whether in law enforcement, health care, data science, new drug development, or technology. Many programs and policies worthy of endorsement today are variants of approaches that could have been recommended 20 years ago. The Commission therefore recommends implementing public policies that correct for failures in patent law and market incentives, prioritizing opioid molecule redesign and non-opioid medication development, and weighing international data more heavily in medication approval decisions.61,62 It also suggests deploying innovative strategies to disrupt fentanyl transactions (e.g., “spoofing” internet-based drug markets) as well as tasking a federal agency to conduct “out of the box” demonstration projects (e.g., delivery of substance use disorder prevention and treatment programs in unconventional settings, development of a device for automated naloxone administration, or application of machine learning methods to predict response to pain and risk of addiction in patients for whom an opioid prescription is being considered).
Domain 7.
Finally, the Commission warns that pharmaceutical companies based in the USA are actively expanding opioid prescribing outside North America, including using fraudulent and corrupting tactics that have now been banned domestically.63,64 This raises risks of a repeat of the tobacco experience, in which an addiction-promoting industry adapted to tighter regulation in wealthy countries by expanding its business in developing nations.65,66 The Commission urges regulators in the USA to stop pharmaceutical producers from exporting fraudulent opioid promotion practices abroad. In order to give poor nations an alternative to partnering with for-profit multinational corporations, the Commission recommends that the World Health Organization and donor nations coordinate provision of free, generic morphine for analgesia to hospitals and hospices in low-income nations.
Because the opioid crisis developed over decades, reversing it in North America and preventing it from spreading abroad will not be easy. Even perfect attainment of all the recommendations here will not eliminate the opioid crisis: tragically, many future deaths are inevitable at this point. But implementing the Commission’s recommendations has substantial potential to save lives and reduce suffering from the crisis both in the USA and Canada, and around the world. The gains of such polices will be long lasting if they curtail the power of health care systems to cause addiction and maximize their ability to treat it.
Introduction
Over the past quarter century, the United States and Canada have experienced an increasingly devastating opioid crisis which has cost those nations more lives than World War I and II combined.67 Although COVID-19 has seized the attention of policymakers and the public, the epidemic of addiction and overdose that preceded it remains unabated, and indeed appears to have been worsened by the consequences of COVID-19.68 This deepening disaster led Stanford University School of Medicine and The Lancet to assemble a Commission on the North American Opioid Crisis. This paper presents the findings and analysis of the Commission and the recommendations that follow from them.
A brief review of the evolution and status of the North American opioid crisis provides the context for the Commission’s recommendations. One aspect of the crisis is not new: opioid addiction was a prevalent reality for more than a century before the crisis began. Beginning in the late 19th century when chemistry and capitalism combined to dramatically expand population exposure to tobacco, stimulants (e.g., cocaine, amphetamines), sedatives (e.g., benzodiazepines), and opioids (e.g., morphine and heroin), addiction became a much more prevalent public health problem in North America and in many other societies as well. But nothing in the history of either the USA or Canada regarding opioids was ever remotely on the scale of the past quarter century.
The approval of Purdue Pharma’s long-acting opioid medication OxyContin in 1995 is as reasonable a point as any to date the beginning of the modern opioid crisis.69 OxyContin was fraudulently marketed as less addictive than other opioids and hence more acceptable to use for a broad range of indications and at high doses. But when crushed to immediately release all the contents, OxyContin and other long-acting opioids that followed were more potent than any formulation that had preceded them. The widespread availability of pharmaceutical opioids also has no historical parallel. Backed by the most aggressive marketing campaign in the history of the pharmaceutical industry, OxyContin became the most well-known of a number of opioid medications (both extended release and immediate release) whose prescription rate exploded in the USA and Canada.4 Regulators failed to step in, for reasons ranging from industry cooptation to incompetence to a sincere but mistaken belief that they were ushering in a new era of improved patient care.70
Departing from decades of medical tradition that employed opioids mainly for cancer, surgery, and palliative care, North American regulators, physicians, and dentists expanded opioid prescribing to a broad range of non-cancer pain conditions from lower back pain to headaches to sprained ankles.8–10,71 Per capita opioid prescribing in morphine milligram equivalents roughly quadrupled in the next 15 years, to the point that the Canadian and USA health care systems were writing as many opioid prescriptions annually as there are adults in those two nations.26 This level of opioid exposure had no parallel in their national histories nor worldwide.72,73 The United Nations gathers data converting different types of opioids into a “standard daily dose”, which allows comparison across countries. These data (figure 1) show that the USA and Canada exceeded developed country norms by a significant multiple,74 which is particularly notable for the USA’s case because the comparator nations in the chart mostly have older populations and all have universal health care access, both of which would be expected to increase prescribing.
Figure 1:
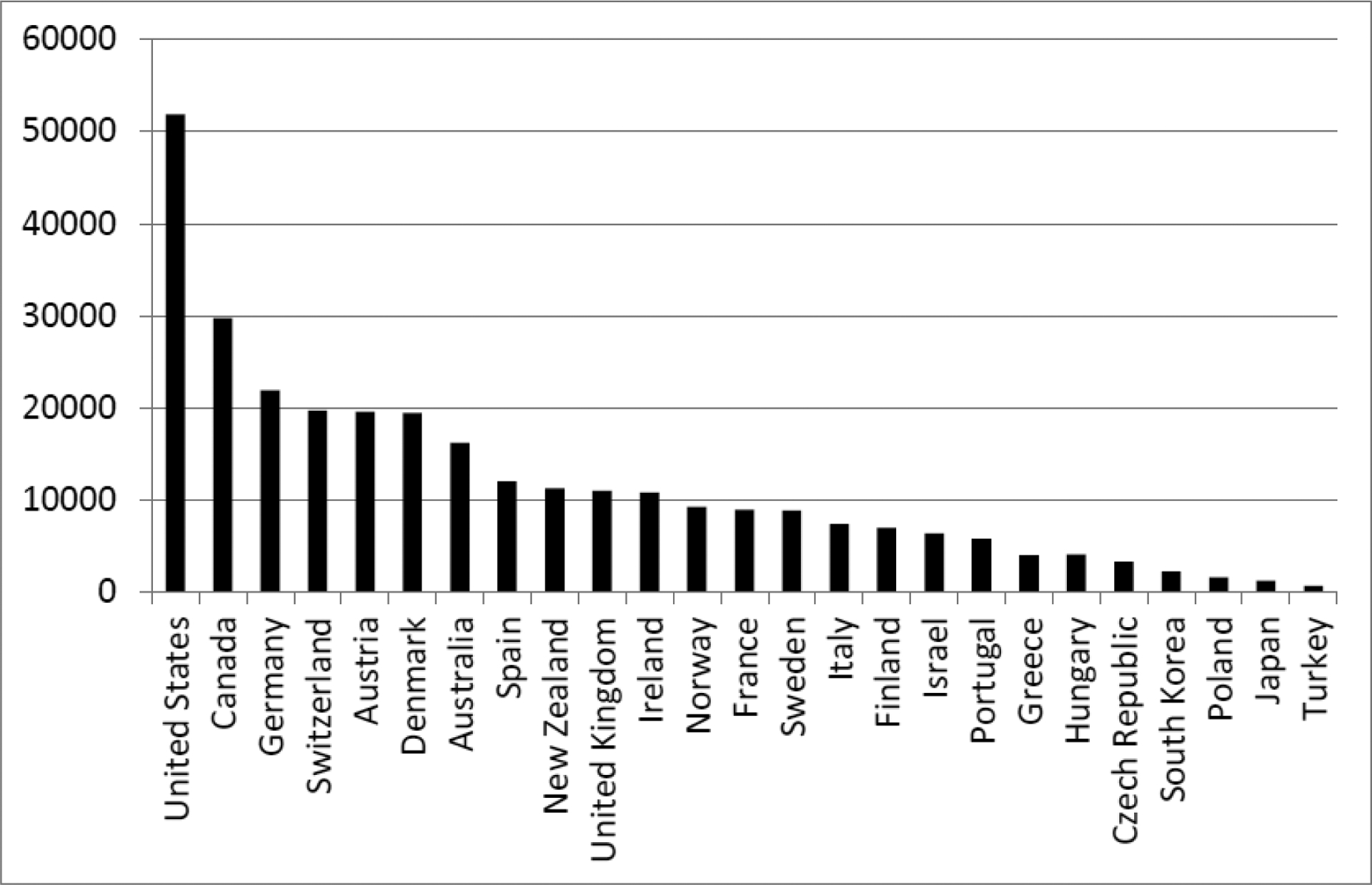
National per capita prescription opioid consumption, in standard daily doses/million inhabitants, during North American peak (years 2010–2012)
The political and cultural environment at the time the crisis emerged was not conducive to an early response; indeed complacency allowed it to worsen. To attain respectability, trust, and influence throughout the world, opioid manufacturers strategically donated a small share of their profits to prominent institutions, including hospitals, medical and dental schools, universities, museums, art galleries, and sporting events.70,13,75 This secured good will, increasing the credibility of the industry’s message that it was a selfless healer pushing back against cruel anti-opioid prejudices. Also, in the wake of the aggressive response to the USA’s crack cocaine epidemic, a backlash against any form of drug supply control was ascendant, and some prominent cultural commentators characterized any concerns about opioid overprescribing as a war on drugs-style crackdown,76 reinforcing messages of the corporations that were profiting from the epidemic.
Some patients in pain may have benefited from increased opioid prescribing, but the overall impact was catastrophic. The health-related consequences were prescription opioid-linked morbidity (e.g., addiction, depression, hormonal dysregulation) and mortality (e.g., from overdoses and accidents) rising roughly in parallel with prescribing (figure 2). The damage also went beyond health to include increased unemployment, disability, crime, truancy, and family disintegration.13 The U.S. Centers for Disease Control and Prevention (CDC) estimated the annual cost of the epidemic at a trillion dollars in 2017, equal to a staggering 5% of gross domestic product.13 Despite Canada and the USA differing on many dimensions that are sometimes assumed to limit the spread of drug-related morbidity and mortality, e.g., universalized health care, level of inequality, and availability of addiction-focused health services, the worst hotspots in the two countries experienced a similar opioid overdose death rate.77 The opioid crisis showed that in the absence of adequate supply control over any addictive drug, damage to human health and well-being is unavoidable, a lesson we will return to later when we discuss the prospects of the crisis spreading beyond North America.
Figure 2:
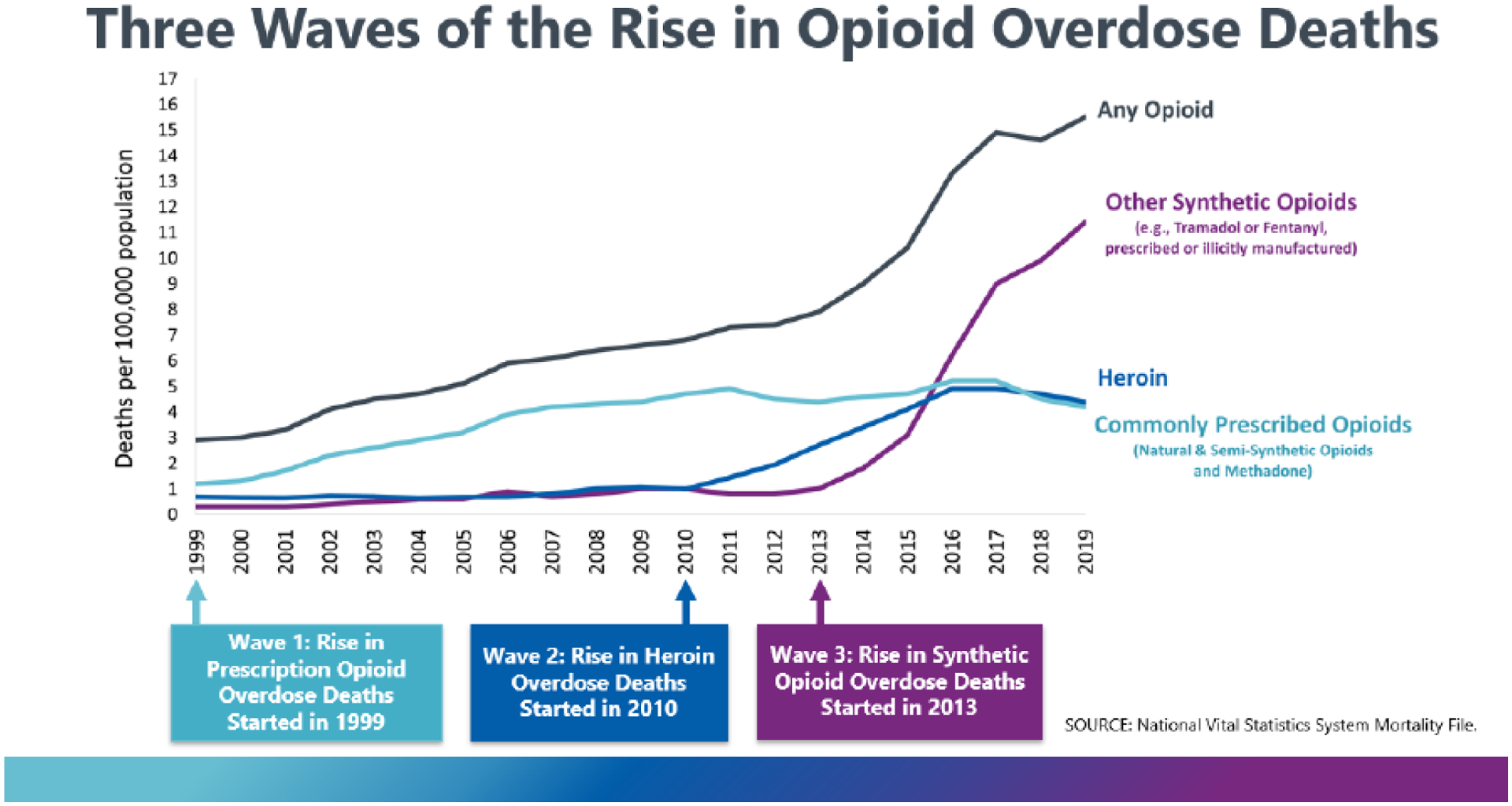
The three waves of fatal opioid overdoses in the United States
The first wave of the current crisis involved prescription opioids and occurred at a time when illicit markets in heroin were isolated and stable in much of Canada and the USA. The second wave, which began around 2010, was fueled by the first. It was catalyzed when drug traffickers recognized that individuals addicted to prescription opioids were a fertile market for heroin.15 As traffickers expanded heroin markets, including in small cities and towns where they had never existed before,78 many prescription opioid-addicted individuals were drawn in by the comparatively low price of heroin.67 Approximately 80% of Americans who initiated heroin use started with prescription opioids,79 even before any significant controls on prescribing were introduced. Once efforts began to stop the rise in prescriptions and to reduce their diversion, some prescription opioid-addicted individuals began shifting to heroin more rapidly than they otherwise would have.80,81
The third wave of the opioid crisis began around 2014 as illicit producers began adding extraordinarily powerful synthetic opioids (e.g., fentanyl) to counterfeit pharmaceutical pills, heroin, and stimulants.31,82 This wave brought unprecedented lethality on top of - rather than instead of - the prior waves, both of which continue today. Large numbers of North Americans are still becoming addicted to prescription opioids each year, and most of those who die from heroin and fentanyl are previous or current consumers of prescription opioids.83,84
Each wave of the opioid crisis did not crash with equal force on all shores of North American life. In the USA, the first wave had a greater adverse impact on white Americans than African-Americans, in part because the former are more likely to have health insurance and hence ready access to prescription opioids. Racist beliefs among some providers that African Americans have unusually higher tolerance for pain or are particularly prone to divert medication may also have reduced opioid prescribing to African Americans relative to whites.85,86
In both the USA and Canada, indigenous peoples had extremely high overdose rates during the first wave of the epidemic.25,27 In the USA, the volume of opioid prescriptions in Native lands rivalled that of Appalachia.87 Canadian First People’s also had high rates of prescribing and opioid-related harm,25 which can be partially traced to “fly-in” physicians on short-term contracts – who administer much of the health care for many of Canada’s indigenous communities – favoring quicker pharmaceutical fixes over more time-consuming pain treatments (e.g., physical therapy).88
Many middle class, employed people experienced prescription opioid use disorder (OUD), but the problem was more prevalent among unemployed individuals28 and those living in economically distressed geographic areas in the USA and Canada. People experiencing homelessness and unstably housed people are at high risk for overdose, though few jurisdictions collect data on housing status as part of routine surveillance.26,33–35 People who have recently been released from incarceration have faced very high overdose risk.35,36 Unlike prior opioid epidemics in North America, some of the regions with highest mortality were predominantly rural (e.g., West Virginia and Maine in the USA; The Yukon in Canada). Other rural areas had lower than average mortality. National USA survey data gathered in 2015 data showed that rates of prescription OUD eventually became similar across rural, suburban, and urban areas.28
The second and third waves hit urban areas and some minority populations harder than did the first wave.89 In the USA, African-Americans now suffer from the fastest growing overdose death rate.90 Some of these deaths are among stimulant users and long-term heroin users who were likely unaware that the drugs they consumed were laced with fentanyl analogs.
No group was immune from any of the three waves of the opioid crisis, even though each wave hit different communities differently. The combined effect has harmed almost every subpopulation in the USA or Canada, with enormous human cost (see panel 1). In the U.S., the current mortality rate for opioid toxicity is over 20 per 100,000 population, while in Canada the rate is over 17 per 100,000. Both of these exceed the mortality rate at their respective nation’s peak of the HIV/AIDS epidemic.22–24
Panel 1: Voices of individuals and families facing opioid addiction.
“I’ve got terrible pain, but I’m also addicted to painkillers, and right now my addiction is worse than my pain.” Patient in recovery from alcohol use disorder for 10 years who became addicted to prescribed morphine”.40
“I started seeing a lot of pills around 15 years old and I told myself I was never going to do them. But kids were selling Oxys at school for $3 a pill. By the time I was 19, I was looking in every medicine cabinet and bathroom.”
-Jonathan Whitt, Minford, Ohio91
“Those people you keep hearing about on television who they find passed out in parking lots? That was me…I wasn’t homeless or in trouble. I was just bankrupt inside. I was empty. There wasn’t another use left in me.”
-Nina Zakas, Charleston, West Virginia92
“I don’t want Nick to be only a statistic or thought of as a throwaway person who didn’t matter. People always said how positive, polite and well-mannered he was. But I don’t want people to think that that should be the criteria for not dying of a fentanyl overdose.”
-Patricia O’Connor (mother), Vancouver, British Columbia93
“After the surgeries, when I got back home… at that point I was lost. I was in a different world, on deep, deep, deep medications, different types then I started finding myself calling more, and then at some point your mind turns to the only thing that really makes any difference is to get pain medication. It was kind of an irrational thing, that this is supposed to help me get up and move around, but it’s keeping me down and destroying me.” Patient addicted to prescribed meperidine.94
Current status of the North American opioid crisis
In the USA and Canada, 2020 was the worst year on record for fatal opioid overdoses both in terms of number of deaths and percent increase over the previous year. Opioid toxicity deaths in Canada increased 63% to 6,214 from 3,830 the prior year, bringing the total of such deaths in Canada to 21,174 between 2016–2020, the period for which national data are available.21 Provisional USA data from 2020 indicate that fatal opioid overdoses rose 37% from 50,963 to 69,710, bringing the total of such deaths since 1999 to over 583,000 people.32 Although the 2020 spikes may be partially attributed to the effects of the COVID-19 pandemic, a rising trajectory of fatalities was evident in the USA throughout 2019 and in both countries during the first quarter of 2020, before the pandemic took hold in either nation.19,20,95,96
The COVID-19 pandemic has affected virtually every aspect of life and society, and substance use disorder and overdose are no exception. Leading up to the pandemic, fentanyl and other synthetic opioids had already begun spreading to the entire USA after being rare in illicit drug markets west of the Mississippi River and common in western Canada.19 Disruptions to drug supply following shelter-in-place restrictions may have further favored synthetic opioids, which are cheaper by weight compared to lower-potency drugs and are largely distributed by mail.96,97 A study of five of the U.S. Drug Enforcement Administration’s 33 High Intensity Drug Trafficking Areas found that between March and September 2020, the number (but not aggregate weight) of seizures of fentanyl and methamphetamine increased significantly compared to the same period in 2019, while there was no significant change in count or weight for heroin or cocaine seizures.98 OUD treatment policy also changed in several key ways discuss in the Commission’s report, including an expansion of telehealth in both countries, greater provision of take-home methadone in the USA, and suspension and eventual removal of the X-waiver requirement to prescribe buprenorphine for OUD treatment to up to 30 patients in the USA.99–101 The implementation of these measures varied greatly, in ways that are still being evaluated, but evidence from some states suggests that the number of people receiving medications for OUD increased after regulations were relaxed.102–104 Changes to incarceration policy and practices may also have influenced overdose rates and treatment for OUD as some jails and prisons reduced populations or adapted corrections-based treatment for OUD in response to the pandemic.105–107 Importantly, the social effects of living through a pandemic – including isolation, unemployment, lack of familiar structure to daily life and well-founded worry in uncertain times – have likely contributed to rising overdoses and worse outcomes for people living with OUD.108
This epidemiological overview draws on the most recently available national mortality data from the U.S. (CDC Wide-ranging Online Data for Epidemiologic Research), Canada (Public Health Agency of Canada’s Special Advisory Committee on the Epidemic of Opioid Overdoses), and various subnational jurisdictions with more recent data available.14,21 Fatal overdoses are only one dimension of the opioid crisis, but such data are more recent and of higher quality than data in other areas, and also, overdose fatalities are arguably a proxy for other harms. Therefore, we conducted basic analyses on most recently available national data from each country to describe the current landscape of opioid overdose mortality in terms of geographic, demographic, and social factors. State/province-level statistics rely entirely on data from 2020 – provisional data from the United States and final data from Canada. USA provisional data do not include information on age, race/ethnicity, or sex; therefore, analyses of U.S. demographics use the 2019 data from the CDC as it is the most recent available as of July 2021.
Though much of the data are directly comparable, the CDC and Public Health Agency of Canada categorize opioid toxicity deaths differently in two important ways: origin and category. The most recent Public Health Agency of Canada report dichotomizes between pharmaceutical and non-pharmaceutical opioids and separately categorizes drugs as fentanyl, fentanyl analogs, or non-fentanyl opioids. The CDC does not classify by pharmaceutical origin and uses the categories heroin, natural and semi-synthetic opioids (includes most prescription opioids such as oxycodone, hydrocodone, codeine), methadone, other synthetic narcotics (this includes synthetic opioids like fentanyl and fentanyl analogs, fentanyl precursor chemicals, tramadol, meperidine, and novel psychoactive substances like isotonitazine or U-47700109), and opium. The CDC data do not distinguish pharmaceutical from illicitly manufactured fentanyl, though the former accounts for a vanishingly small proportion of deaths in both countries.31
For CDC data analyses undertaken for this report, deaths occuring in January to December 2019 were included using International Classification of Diseases Code 10th edition (ICD-10) codes. Deaths were included if they had one of the following as underlying cause of death: accidental poisoning (X.40–X44), intentional self-poisoning (X60–64), assault by drugs (X85), or poisoning of undetermined intent (Y10–14); and T40.0–T40.5 as contributing cause of death. In total this includes 49,126 fatal opioid toxicity deaths, many involving multiple categories of opioids or other substances. This number is slightly lower than the 50,963 in the provisional 2019 statistics mentioned above because it does not include deaths classified under ICD-10 code T40.6, “Poisoning by, adverse effect of and underdosing of other and unspecified narcotics” but not ICD-10 codes T40.0–40.5. By non-exclusive category, these include other synthetic narcotics (n=36,359, 74%), heroin (n=14,019, 29%), natural and semi-synthetic opioids (n=11,886, 24%), methadone (n=2,740, 6%), and opium (n=2, 0.004%). Detailed USA mortality data with specific drugs involved were downloaded from public online datasets (Connecticut, Cook County, Illinois; Dallas-Fort Worth area of Texas; San Diego County, California), or provided to commission authors via public records requests (Milwaukee County, Wisconsin; Jefferson County, Alabama; Los Angeles County, California).
The report from Public Health Agency of Canada’s Special Advisory Committee on the Epidemic of Opioid Overdose documented 6,214 opioid overdose deaths in 2020. During this period, 80% of deaths involved fentanyl, 10% involved fentanyl analogues, and 31% involved non-fentanyl opioids, though as in the USA, many deaths involved substances across two or more of these categories. Overall, 77% of fatal overdoses involved only non-pharmaceutical opioids, 12% involved only pharmaceutical opioids, and 7% involved both.21
In both countries, the mortality rate varied substantially between states and provinces (figure 3), with the highest rates in western Canada (British Columbia and Alberta), Appalachia (particularly West Virginia and Ohio), and the northeastern seaboard of the USA. Canada’s 2020 age-adjusted rate was 17.2 per 100,000 population (a 67% increase over the 2019 rate of 10.3). Although 2020 age-adjusted rates are not yet available for the USA, the number of opioid-involved deaths are estimated to have increased 37% from 2019 to 2020.32
Figure 3.
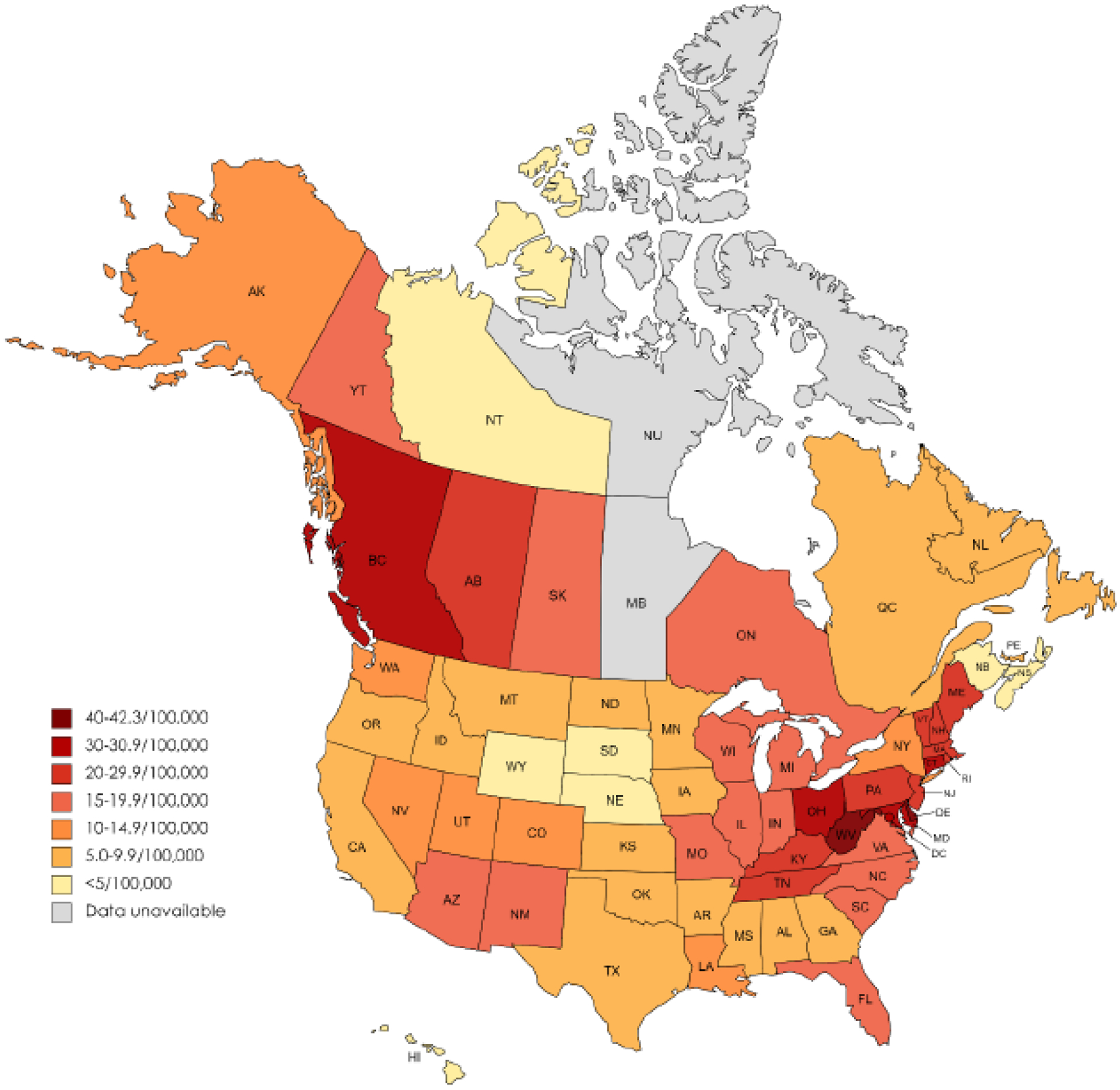
Age-adjusted per capita opioid overdose mortality, Jan-Dee 2019 (United States), and Jan-Sept 2020 (Canada)
Canadian data from Public Health Agency of Canada. U.S. data from U.S. Centers for Disease Control and Prevention Wide-Ranging Online Data for Epidemiologic Research
In the USA and Canada, the majority of fatal opioid overdoses occur among males, with a greater sex disparity in Canada.14,21 In 2019, 70% of fatal opioid overdoses in the USA occurred among males, who had a population- and age-adjusted death rate 2.4 times that of females. In 2020, males in Canada died from opioid overdose at 3.3 times the rate of females, with males composing 77% of opioid overdose deaths during this period.21 In Canada, the largest sex disparity was in British Columbia, where males died from opioid toxicity at a rate 5.5 times higher that of females during this period. The province of New Brunswick had the smallest sex disparity, with the opioid toxicity mortality rate among males being 1.9 times that of females. In the USA, the 2019 rate ratio for age-adjusted opioid overdose mortality among males versus females was greatest in Connecticut (3.3), Massachusetts (3.2), and the District of Columbia (3.1) and smallest in Nebraska (1.2), Idaho (1.3), and Utah (1.3).
Types of opioids involved in fatal overdose also differ by sex. In the most recently available data, opioid-related deaths among females were about twice as likely to involve a prescription (pharmaceutical) opioid (30% in Canada, 33% in the USA) compared to males (16% in Canada, 14% in the USA).21 Nearly 80% of fatal fentanyl overdoses in Canada in 2020 occurred among males, as did 82% of fatal overdoses involving fentanyl analogs; similarly, males accounted for 75% of fatal heroin overdoses and 72% of synthetic narcotic overdoses in the USA in 2019.
As of 2019, U.S. fatal opioid overdose rates were still highest among non-Hispanic white Americans although the rate among American Indians and Alaskan Natives has been almost as high throughout the crisis. Since 2011, the mortality rate has grown fastest among non-Hispanic African Americans approaching those of both these groups (figure 4).90 Although comprehensive national data on racial and ethnic distribution of deaths during 2020 were not available as of July 2021, an analysis of 2020 emergency medical services data found that fatal overdose-associated cardiac arrests (not disaggregated by opioid-related versus non-opioid-related) among Black and Hispanic Americans grew disproportionately over the previous year.95 In 2019, the age-adjusted rates were highest among non-Hispanic whites (18.7/100,000), non-Hispanic American Indian or Alaska Natives (17.2/100,000), and non-Hispanic Black or African Americans (16.9/100,000). Despite similar rates nationally, substantial racial disparities emerge at the state level.
Figure 4.
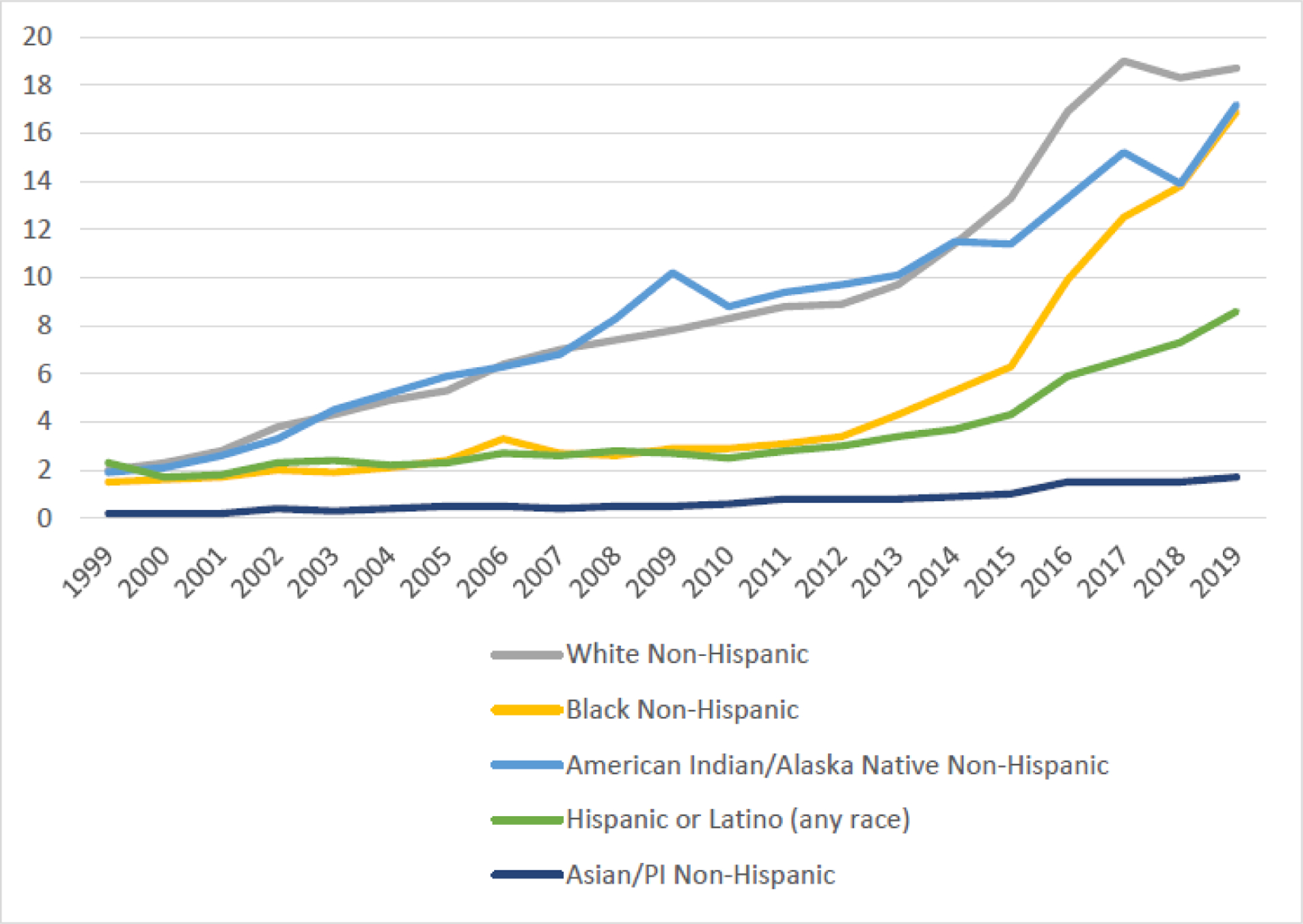
U.S. age-adiusted opioid-involved mortality rate per 100.000 population, by race and ethnicity, 1999–2019
Data from U.S. Centers for Disease Control and Prevention Wide-Ranging Online Data for Epidemiologic Research
Of the 33 states with sufficient race and ethnicity data to evaluate the mortality rate ratio between non-Hispanic whites and non-Hispanic Black or African Americans, only six have age-adjusted mortality rates for these two groups that are within 10% of each other. Compared to white non-Hispanics, the age-adjusted mortality rate for Black non-Hispanics was substantially higher in 10 states, including five Midwestern states where it was more than double that of non-Hispanic whites (Iowa, Minnesota, Missouri, Wisconsin, Illinois). Seven states in the Southern USA had age-adjusted opioid overdose mortality rates among white non-Hispanics that were more than double those of non-Hispanic Black or African Americans (Mississippi, Florida, South Carolina, Louisiana, Georgia, Alabama, North Carolina). Only 10 states had sample sizes large enough to calculate rate ratios comparing age-adjusted mortality of American Indian or Alaska Natives and non-Hispanic whites. Notably, six of these states had higher age-adjusted mortality among American Indian or Alaska Natives, including Minnesota (over 10 times that of non-Hispanic whites), Wisconsin (more than triple), Washington (more than double), Alaska (85% higher), North Carolina (45% higher), and California (29% higher).Though mortality rates were generally lower among Hispanics compared to the general population, the age-adjusted opioid overdose mortality rate was higher among Hispanics of any race compared to non-Hispanics in New Mexico (68% higher), Pennsylvania (17% higher), Colorado (15% higher), and New York (11% higher).
In the most recently available data from both countries, about 88% of overdose deaths occurred among people between the ages of 20–59.14,21 Fatal overdoses involving synthetic narcotics -- including fentanyl or fentanyl analogs -- were most common among individuals aged 30–39, with about a third occurring in this age group.14,21 In Canada, deaths involving non-fentanyl opioids skewed older, with 37% of non-fentanyl opioid deaths occurring among individuals 50 and over.21 Similarly, mortality rates in the USA for natural and semi-synthetic opioids (the category most closely matching prescription opioids) peaked in the 55–64 age group in 2019.14
Polysubstance overdoses, particularly co-involvement with stimulants, is common in fatal overdoses in the USA and Canada but varies substantially within both countries. Mortality data unfortunately does not distinguish between intentional co-use of opioids and stimulants versus unintentional contamination. That said, in Canada, 51% of fatal opioid overdoses in 2020 also involved stimulants.21 USA provisional data indicates that from 2019 to 2020, fatal overdoses involving “psychostimulants with abuse potential” (a category that includes methamphetamine as well as other stimulants) increased by 46% and fatal overdoses involving cocaine increased by 21%. Though it is not possible to determine overlap with opioids using the USA provisional data, estimates from the 24 states and District of Columbia participating in the State Unintentional Drug Overdose Reporting System showed that 33% of overdoses in 2019 involved both stimulants and opioids.37 Overlap between stimulants and opioids varies substantially in the U.S. jurisdictions that make mortality data available more quickly than the CDC (figure 5).
Figure 5.
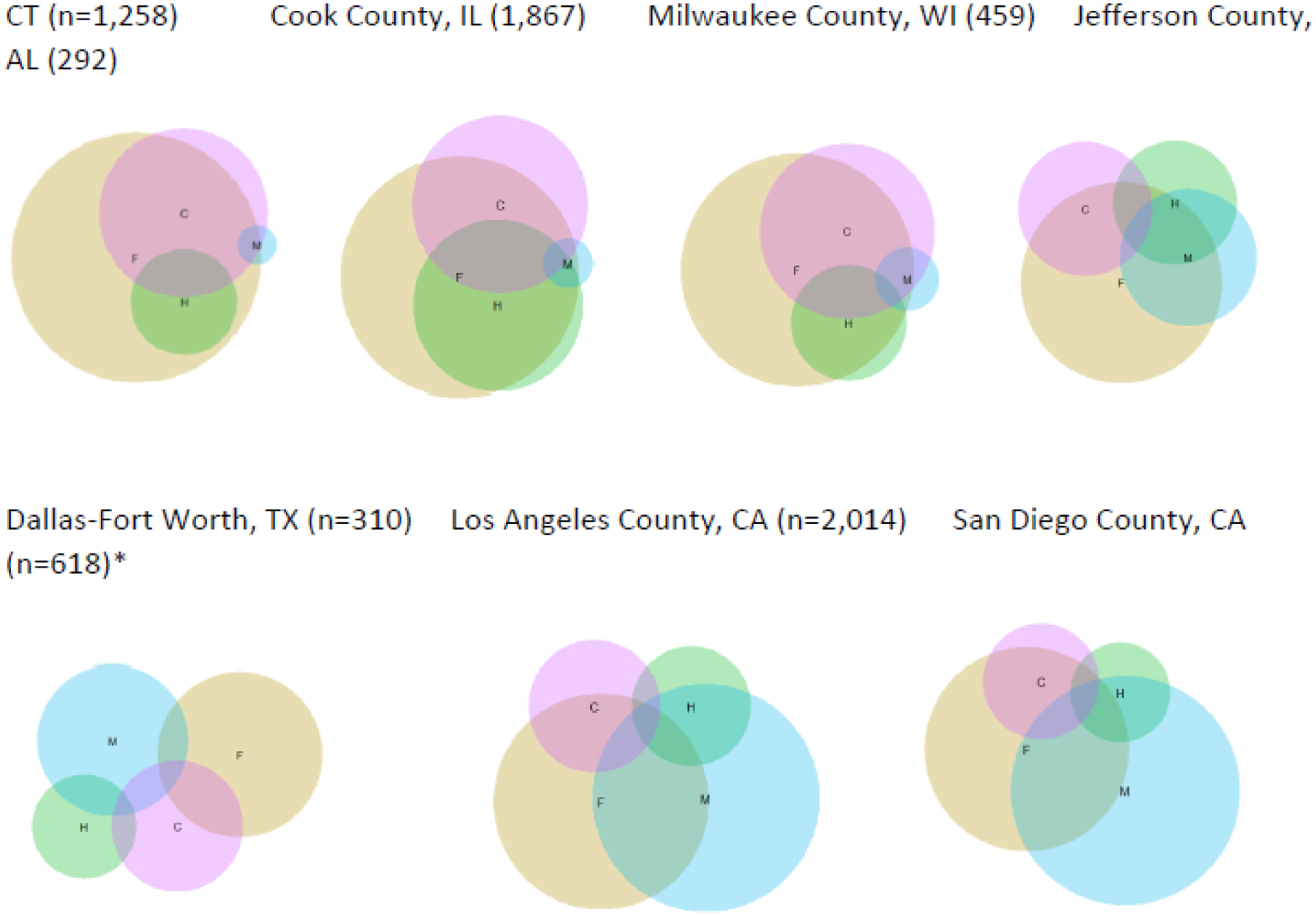
Drug combinations involved in fatal overdoses from U.S. jurisdictions with detailed medical examiner data, 2020
F: Fentanyl and fentanyl analogs H: Heroin M: Methamphetamine C: Cocaine
*Includes data from January – September, 2020
Differences in drugs involved in fatal overdose can be striking even across proximal geographic areas. In British Columbia in 2020, 86% of fatal overdoses involved fentanyl, with a similar proportion in the first four months of 2021.110 A few hours’ drive south across the USA-Canada border in King County, Washington, fentanyl was involved in 33% of overdose deaths in 2020.111 From January to September 2020, 73% of all fatal overdoses in Canada involved fentanyl and 60% of opioid-involved deaths also involved stimulants.21 In USA jurisdictions with detailed medical examiner data that becomes available well in advance of CDC mortality data, it is possible to see marked differences in substances involved in fatal overdoses.19,109 For example, in the eastern and mid-western USA (i.e., Connecticut, Chicago, Milwaukee), there is near total overlap between heroin and fentanyl in fatal overdose, and cocaine is the more common stimulant. Conversely, methamphetamine is more common in the southern and western jurisdictions with these data available, and a substantial proportion of heroin deaths do not involve fentanyl.
The Stanford-Lancet Commission on the North American Opioid Crisis
The Commission was created in the fall of 2019 at the invitation of The Lancet editors to Keith Humphreys. Stanford University School of Medicine subsequently agreed to partner with The Lancet and to provide all funding for the Commission’s work. Ten Stanford-affiliated individuals already working on some aspect of the opioid crisis were invited by join by Humphreys as were eight leading experts from around the USA and Canada. Three additional scholars were asked to join in the special role of reviewer.
Commissioners were drawn from the fields of addiction, biochemistry, emergency medicine, epidemiology, health economics, internal medicine, law, pain medicine, policy analysis, psychiatry, pharmacology, and public health. The Commission included clinicians, researchers, educators, public policymakers, and individuals with lived experience of addiction and chronic pain. All Commissioners were based in the USA or Canada, reflecting the fact that the opioid crisis is at this writing concentrated in those two countries.
After an initial meeting of Stanford-based Commissioners in January, 2020 to begin charting the project’s timeline and goals, all Commissioners (including those in the reviewer role) and The Lancet’s Americas Editor convened for two days at Stanford University in February 2020 for a series of discussions of various aspects of the epidemic. Each discussion section was facilitated by a different Commissioner, and generated lists of key analytic themes, critical data, and potential policy actions. After this meeting, the reviewing Commissioners provided initial feedback and then, to avoid groupthink, absented themselves from all deliberations for the ensuing ten months.
Unlike some other Lancet Commissions, this one focuses on a long-entrenched problem that has already been well-characterized, including in multiple National Academies of Sciences, Engineering, and Medicine reviews (on which several Commissioners had served).1–3 The Commission therefore did not conduct another comprehensive literature review, but instead focused on developing a coherent, empirically-grounded analysis of the causes of and solutions to the opioid crisis. Some epidemic modelling work was done to support this process, and is described in detail elsewhere.112
The Commission moved its deliberations online with the onset of the COVID-19 pandemic. Subgroups with special expertise investigated and debated individual issues over email chains before presenting them to the full Commission for further discussion. Any major substantive conclusion or recommendation that did not command at least 90% support from Commissioners was modified until it achieved such support or was dropped if it could not.
Every Commissioner reviewed, contributed to, and approved an initial full report, which was then critiqued by the three reviewing Commissioners who had attended the first meeting as well as other reviewers and editors picked by The Lancet in early 2021. This is the revised report of the Commission.
Although more limited in geographic scope than other Lancet commissions in being largely focused on two countries, the Commission draws on evidence from beyond them to discuss ways to prevent the international spread of the opioid crisis. The term “North American” is a linguistic convenience referring to the continent where the two countries are based, and does not imply that every country (e.g., Mexico113,114) and territory (e.g., Greenland) on the continent is experiencing an opioid crisis.
The Commission members are scholars rather than elected officials with a democratic mandate, and thus tightly tied its analysis and recommendations to science rather than recommending actions on the basis of purely political and philosophical rationales. All Commission recommendations therefore had to be grounded in evidence of likely benefit to public health, public safety, or both.
The Commission took a population public health perspective, emphasizing general principles and policies for responding to the crisis. It therefore did not attempt to delineate clinical issues such as how to manage the care of individual types of patients with OUD or what precise human service elements each individual health care organization should offer such patients.
The Commission’s model of the opioid crisis estimates that in the absence of any intervention, the USA will experience a staggering 547,000 opioid overdoses from 2020 to 2024.112 The Commission therefore proposes bold responses (summary in table 1) which the USA and Canada can adopt to better meet this enormous public health challenge. The remainder of this report analyzes the challenges created or illuminated by the opioid crisis in seven key domains and presents recommendations that are responsive to each of them.
Table 1:
Abbreviated list of Commission recommendations
|
Domain 1: The North American opioid crisis as a case study in multi-system regulatory failure Curbing industry influence on prescribers
Curbing industry influence on regulators
Curbing industry influence on the political process
|
|
Domain 2: Opioids dual nature as a benefit and a risk to health Recognizing the risks and benefits of opioids in the drug approval process
|
Domain 3: Building integrated, well-supported, enduring systems for the care of substance use disorders
|
Domain 4: Maximizing the benefit and minimizing the adverse effects of_criminal justice system involvement with people who are addicted to opioids
|
Domain 5: Creating healthy environments that can yield long-term declines in the incidence of addiction
|
Domain 6: Stimulating innovation in the response to addiction
|
Domain 7: Preventing opioid crises beyond North America
|
Domain 1: The North American opioid crisis as a case study in multi-system regulatory failure
“The opioid crisis is, among other things, a parable about the awesome capability of private industry to subvert public institutions”
--Patrick Radden Keefe5, Empire of Pain, p.364
The current opioid crisis resembles prior drug crises (e.g., the rise of heroin addiction in North American cities in the 1960s and 1970s) in some respects but differs in others. Most particularly, the origins of the current crisis reflect dramatic failures within the corporate sector, regulatory and legislative bodies, the medical profession, and the health care system. Because the epidemic of opioid addiction and overdose emerged from and is still to some extent being fueled by legally prescribed opioids, policy responses must be uniquely tailored to that reality. Illuminating regulatory failures is also essential for helping the USA and Canada avoid similar mistakes with other prescription medications, and for informing other nations about how to avoid opioid crises of their own.
Perhaps the most important fact to remember about the North American opioid crisis was that for some people, it brought not suffering but enormous wealth. OxyContin alone is estimated to have generated revenues of over $35 billion for Purdue Pharma and its owners, the Sackler family.115 John Kapoor’s shares in the pharmaceutical company he founded, Insys Therapeutics, were worth $650 million before he was imprisoned for having his sales representatives bribe doctors to prescribe a fentanyl spray and training other staff to defraud insurers who asked for justification for the prescriptions.116 Johnson & Johnson, Endo, Teva, and other opioid manufacturers also reaped substantial revenue from soaring prescription rates. Many pharmaceutical distributors also profited handsomely while knowingly making astonishingly large shipments of pills which they were required to report to regulators but did not.117,118 Profit-seeking was not a phenomenon entirely external to the health care system: some hospitals, clinics, pharmacies, professional societies, and individual health care professionals also enriched themselves, as did some individuals who “doctor shopped” to obtain many prescriptions they could resell.4,40,118
Public health professionals have long advocated that manufacturers, distributors and retailers of addictive drugs in explicitly recreational markets (e.g., alcohol and tobacco) be tightly regulated to prevent them from maximizing profit at the expense of public health and safety. The North American opioid crisis makes it agonizingly clear that the same lesson applies within ostensibly well-regulated medical systems. These risks are not limited to opioids: barbiturate overprescribing generated harm in the past,119 excessive benzodiazepine120,121 and stimulant122 prescribing is causing harm currently, and the future could bring new crises involving other prescription drugs. Assignment of blame, punishment, and restitution for the past is a matter currently under consideration in multiple courts of law. A key question for the future is how to regulate industries – including the health care industry – to prevent the profit motive from fomenting oversupply and overprescribing of pharmaceuticals with addictive potential.
Opioid manufacturers directed their efforts to dramatically expand the market for their products toward three main targets: prescribers, regulators, and policymakers. We now analyze these areas in turn.
Pharmaceutical industry influence on the practice and education of prescribers
In 2016, the pharmaceutical industry spent USA$20.3 billion marketing its products directly to prescribers. This form of marketing comprises in-person office visits, large and small gifts (e.g., branded office supplies, meals and receptions at conferences, travel expenses), and direct financial payments for endorsing industry products in lectures and case conferences. Industry engages in these practices because they are effective at increasing prescribing of their products.123,124 OxyContin was the subject of the most lavishly funded promotion campaign in the history of medicine,4,125 which was highly successful at generating revenue for Purdue Pharma and helped ignite an epidemic of addiction and overdose in North America. Counties in the USA that were targeted with higher levels of physician-focused marketing had higher rates of opioid prescribing and overdose mortality one year later.126
Some opioid manufacturers also promoted their products by changing the design of prescription modules within electronic medical record systems. In January 2020, an electronic medical record vendor was fined $145 million by the United States government for accepting kickbacks from Purdue Pharma in exchange for co-designing software that promoted OxyContin prescription for patients for whom the drug was not appropriate.127,128
Promoting opioids directly to patients is less of a concern than other prescription drugs because of legal restrictions, which should of course remain in place. But the role of direct-to-consumer advertising in opioid overprescribing nonetheless bears mention. New Zealand and the United States are the only countries which allow direct-to-consumer marketing that makes claims about pharmaceutical products.129 From 1997 to 2016, the pharmaceutical industry in the USA increased spending on such advertising from USA$1.3 billion to USA$6 billion,130 which exceeded the entire 2016 budget (USA$4.9 billion) of the Food and Drug Administration (FDA) that year.131
Opioid manufacturers have multiple ways of using direct-to-consumer advertising to promote opioids even while abiding by the letter of the law forbidding mention of them. This includes indirect promotion, such as buying a USA$10 million dollar Super Bowl television advertisement for a non-controlled drug that makes long-term opioid use more tolerable by reducing constipation.132 The industry also sponsors “unbranded” campaigns to change the public perception of illness and broadly expand the market for medications, as opioid manufacturers did to normalize use of opioids for “non-cancer chronic pain”.133 The bombardment of USA citizens with pharmaceutical advertising increases prescribing even for medicines not mentioned in the ads,134 perhaps because direct-to-consumer advertising changes public expectations about the responsibilities, role, and power of physicians. Making individuals who have medical disorders aware of effective pharmacotherapies is valuable, but direct-to-consumer advertising can also be a form of public health miseducation (see panel 2). Among other problems, it can foster the false impression that if pressured enough by patients,41 physicians can and should provide medicines that eliminate every source of human suffering.
Panel 2: Another industry whose consumer-targeted advertising worsens the opioid crisis: medical cannabis.
The cannabis industry began marketing cannabis legalization as a solution to opioid overdoses after a study found that between 1999 and 2010 states with medical cannabis programs had lower than expected opioid overdose mortality.140 The association between these two population-level indicators was vulnerable to the ecological fallacy, wherein individual-level relationships may differ from the aggregate relationship. Moreover, when 7 additional years of data were added to the time series, the pattern of results reversed: States with medical cannabis laws had higher than expected opioid overdose mortality from 1999 to 2017, even after controlling for more and less restrictive laws (i.e., medical versus recreational versus low potency only).141
Nevertheless, the cannabis industry promoted the initial study findings on billboards and in advertising campaigns (figure 6). Further, several states unwisely added OUD as a qualifying condition for medical cannabis based on the initial ecological correlation, a level of evidence that would be considered unacceptable anywhere else in medicine.142 In states where OUD was a qualifying condition, more medical cannabis dispensaries advertised cannabis as a replacement for FDA-approved medications for OUD.143 These dangerous practices continue despite being based on study findings that did not survive replication.
Prescription drug manufacturers also attempt to influence prescribing by influencing education provided by universities, hospitals, and professional societies. Collaboration between universities and private companies can spur innovation, and every academically-based member of the Stanford-Lancet Opioid Commission works at an institution that has received outside donations to support scholarly and educational activities. These realities co-exist with evidence that corruption of the educational process by opioid manufacturers has been present during the opioid crisis.
For example, in explaining its 2019 decision to strip the Sackler name from its School of Graduate Biomedical Sciences, Center for Medical Education and other entities to which the family had donated, Tufts University acknowledged how educational decisions at the institution were inappropriately shaped in a fashion that served Purdue Pharma’s interests (e.g., suppressing a book documenting the opioid crisis, using corporate materials in teaching), and how the company made use of its Tufts connections, including at one point having a Tufts faculty member appear in an advertisement for the company’s products.39,135 Similar influence processes have been documented at other universities.136
The pharmaceutical industry also attempts to shape education in academic medical centers through on-campus representatives. Some evidence of the impact of these activities comes from evaluations of academic medical centers that restrict them, which often though not always experience decreases in prescriptions for marketed drugs.137 For example, in a study of 85 medical centers, restrictions on receiving gifts, limits on accepting paid speaking and consulting engagements, requirements to disclose industry ties, and bans on sales representatives were associated with 8.8% lower volume of opioids prescribed.138
Professional societies, which are leading providers of education both for clinicians and the public, are another potential site of unacknowledged industry influence. To take a recent case in point, five leading pain specialists publicly resigned from the taskforce on the 2021 Global Year Against Back Pain139 established by the International Association for the Study of Pain and the European Pain Federation. The scientists resigned to protest undisclosed links between the task force and the opioid manufacturer Grünenthal, bringing the education campaign into public disgrace even before it was launched.
Recommendations for reducing pharmaceutical industry influence on the practice and education of prescribers
Recommendation 1a: Curtail pharmaceutical product promotion
The simplest way to curtail prescriber-focused marketing is for lawmakers to ban it outright. Some individual states in the USA and health care systems have restrictions of this form, but they are not national in scope.144,145 In contrast, in Germany, professional traditions and laws generally forbid the provision of gifts or benefits to physicians that could influence future prescribing decisions or could be considered a reward for past prescribing decisions.146 In 2018, Canada’s Health Minister finally asked opioid manufacturers to voluntarily cease marketing to physicians, and in 2019 Health Canada announced its intent to put formalized rules in place to legally restrict the content of such promotion.147
The Commission recommends that the USA make comparable national policy moves immediately. Because of its ability to mislead patients, including to the point where they pressure prescribers to make suboptimal care decisions,41 the Commission also recommends that the USA join the rest of the world in banning direct-to-consumer advertising of pharmaceuticals that makes therapeutic claims.
Because the current members of the U.S. Supreme Court have ruled that corporations have the same speech rights as individuals, a ban on pharmaceutical advertising is unlikely in the near term. A less potent but still valuable interim alternative is to remove the ability of pharmaceutical companies to deduct the costs of advertising from their income when filing annual tax returns. This policy, which has been proposed both by Republican and Democratic U.S. Senators and by President Joe Biden would raise the cost of advertising relative to other investments the industry might make, for example in research and development.148,149
Last but not least, the Commission recommends that pharmaceutical industry involvement in the design and implementation of electronic prescribing systems be forbidden. Such systems should be designed solely to improve patient care and should not be exploited as a commercial platform.
Recommendation 1b: Decouple pharmaceutical industry donations to universities and professional associations from control over the content of medical education.
Any for-profit industry given the power to shape educational programming that could increase sales of its products is very likely to take advantage of the opportunity. Universities and professional societies should therefore only accept educational funding that is donated into a common pool over which the pharmaceutical industry has no input of any kind. The nature and content of courses on prescribing should be established by scientists, clinicians, and educators free of industry ties. These principles have gathered increasing support across medicine over the last decade and are embraced in the Council of Medical Specialty Societies’ code for interactions with industry150 and in the Accreditation Council of Continuing Medical Education standards for continuing medical education.151 These principles should also be supported by accreditors of medical, nursing, dental, and pharmacy schools. Finally, it should go without saying that concealing pharmaceutical industry support of clinician or public education efforts, including conferences given by professional associations and patient advocacy organizations, is never acceptable.
Industry influence over the regulation of addictive pharmaceuticals
OxyContin is a highly potent extended release opioid that was approved for wide use by the FDA under the fraudulent premise that it was less addictive than other opioids, a mistake that stained the FDA’s reputation. But the FDA was not the only pliant regulator. Between 1994 to 2015, the quota of oxycodone that the Drug Enforcement Administration permitted to be legally manufactured was raised over 20 times from 3.85 million grams in 1994 to a high of 153.75 million grams in 2013.5
Drug approval is intended to be one step in a process that is modifiable or even reversible if problems arise, but further regulatory failures prevented such corrections from happening for years in the opioid crisis. Had post-marketing studies of the many approved opioid medications been promptly conducted, the risks of addiction would have come to light more quickly. Had effective risk evaluation strategies been rolled out to prescribers, opioids might have been prescribed more safely. And had the second wave of regulators who should have been activated after a drug was approved (e.g., medical boards, accreditation organizations) acted more quickly, lives might have been saved. Understanding the role of industry influence thus must go beyond drug approval to what happens afterwards, and must go beyond illegal conduct to conduct that is within the bounds of defective laws.
Industry clearly often succeeds at “regulatory capture”, i.e., having corporate interest prioritized over the public interest. A common method of doing this is luring experienced individuals out of the regulatory world with lucrative salaries. This revolving door not only deprives regulators of talent, but also communicates to current official holders and regulatory agency staff that their future earnings could be shaped by whether the decisions they make today please the industries they oversee.152
Former U.S. Congressman Billy Tauzin, for example led the crafting of Medicare legislation that dramatically expanded government purchasing of pharmaceutical products while simultaneously forbidding the government to bargain for lower drug prices. The day after his term ended, Tauzin became a leading pharmaceutical industry lobbyist at more than ten times his Congressional salary.153
Most cases are less dramatic. When drug distribution firms oversupplied opioids and violated laws requiring that they report such suspicious shipments, they were investigated by the Drug Enforcement Administration. One of the tactics the industry used to fight these charges was to hire away key Drug Enforcement Administration employees to work for their side.154 Multiple federal prosecutors in the USA who had initially been openly critical of Purdue Pharma recanted when they subsequently were hired by the company.5 The FDA can be subject to similar pressures.152 The FDA official who oversaw the agency’s approval of OxyContin, subsequently began working for Purdue Pharma at a salary which federal prosecutors allege was triple his government pay.155 Similar concerns have been raised across the FDA’s portfolio.156
Under the law in the USA, once a risky drug is approved, monitoring those risks and educating prescribers about them is substantially at the pharmaceutical industry’s discretion. To protect public health, any post-approval risks or harms of medicines should be monitored, and prescribers should be equipped to mitigate such risks and harms if they arise. Rather than have the FDA do such studies itself, the law empowers it only to mandate that manufacturers conduct them. Many of these “mandated” surveillance studies have not even begun years after an approved drug is in use, others have been completed late, and still others have not been conducted at all.44,157 Those studies have been conducted often have not analyzed or revealed their data43 or were designed in a fashion that made detecting adverse effects very unlikely.158 This is not surprising given that identifying risks to approved drugs is of benefit to the public but by definition can reduce sales and profits for drug manufacturers.
Similarly, when the FDA has mandated that manufacturers create and evaluate risk evaluation and mitigation strategies to help physicians prescribe opioids more safely, compliance has been grudging and there is no evidence that patients have significantly benefitted.159,160 Target numbers for training physicians are often not met,159,161 the training materials themselves are often of questionable utility,162,163 mandated evaluations have often not been conducted,159 those evaluations conducted by industry are rarely methodologically rigorous,84–88 and when evaluations do provide data, industry has rarely implemented changes to risk evaluation and mitigation strategies in response.164
Theoretically, the FDA has the power to respond to such industry non-compliance by pulling a medication from the market, but rarely exercises it. National medical leaders have advanced different explanations for this. Dr. Marcia Angell, former editor of The New England Journal of Medicine suggests that the FDA is reticent because it sees its institutional purposes and incentives as aligned with increasing the number of drugs on the market.165 Indeed, in recent decades, successive Congresses and Presidential administrations have made changes to the FDA’s authorizing legislation specifically intended to have it approve medications more quickly.44,166
Dr. Drummond Rennie, former deputy editor of JAMA, argues that the FDA is wary of offending pharmaceutical manufacturers because user fees provide part of its budget, which causes the agency to see industry rather than the public as its client.44 Whether or not this is correct, the FDA may make some decisions out of rational fears of the pharmaceutical industry’s considerable influence in Congress, which the Commission discusses elsewhere in this report. In any event, the FDA cannot be blamed for following a law which gives more control over post-approval surveillance and risk evaluation and mitigation strategies to the pharmaceutical industry than to the government.
Once a medication is approved, another layer of regulators comes into play. This includes governmental agencies (e.g., state and provincial medical boards) as well as non-governmental organizations which are formally ceded regulatory powers by government (e.g., accreditation bodies specifically recognized in legislation, such as The Joint Commission). Industry connections to such bodies can be extensive. In the USA, the Joint Commission accredits hospitals and other health care organizations and its accreditation is formally recognized in the law of many states. A U.S. Government Accountability Office investigation found that the Joint Commission’s pain management education programs were funded and co-authored by opioid manufacturers, and that the partnership with Purdue Pharma “may have facilitated its access to hospitals to promote OxyContin.”167 The Joint Commission also promulgated in its accreditation standards the concept that pain is the “fifth vital sign”, putting pressure on health care organizations to increase opioid prescribing.168 The Joint Commission began de-emphasizing the term in 2002, later clarified that the “fifth vital sign” concept was intended to raise awareness of pain, and also acknowledged that it had been misinterpreted to mean that pain should be assessed at every patient contact, a practice which tended to fuel opioid prescribing.168 Clinical practice guidelines written by individuals with ties to opioid manufacturers have echoed inaccurate promotional messages, including two such guidelines that have been retracted by the World Health Organization and led it to strengthen its conflict of interest policies.169
Provincial and state medical boards also have power through adjudicating of patient complaints against prescribers, guiding practice norms in the field, and advising legislators and regulators. Industry is also involved at this level. For example, the U.S. Federation of State Medical Board’s guidelines on opioid prescribing were developed with the aid of individuals with extensive industry ties, and in 2003 distribution of the guidelines was funded by Purdue Pharma.170
Even were it possible for medical regulators to have extensive industry ties but be in no way affected by them in their professional judgements, public perception of potential corruption still matters. Any regulatory standard for opioid prescribing or pain care -- even one involving some individuals who have the highest of motives -- risks significant loss of credibility if funded by companies that have been criminally convicted of knowingly misrepresenting the risks and benefits of opioids.171
Recommendations for limiting industry influence over the regulation of addictive pharmaceuticals
Recommendation 1c: Close the “revolving door” of officials overseeing the pharmaceutical industry leaving government to work on the industry’s behalf.
Transfers of knowledge and skills between the public and private sector are not necessarily harmful to the public good and indeed may sometimes benefit it.172 But when such transfers occur for the purpose of promoting industry capture of regulators, society suffers not only in terms of public health but also in terms of increased cynicism and political alienation. The public good would be served by extending the length of “cooling off” periods in state and federal law constraining lobbying on behalf of an industry that an elected or appointed official used to oversee (e.g., mandating a two-year period, as envisioned in one proposed piece of legislation).173–175 Positive incentives should also be considered. For example, civil servants working in regulatory agencies could be paid higher salaries, with added retention incentives for senior officials with particularly deep knowledge of regulatory processes.
Recommendation 1d: Post-FDA approval data collection on adverse effects of medications and provider education on risk mitigation should be made the responsibility of government.
Gathering data on post-approval drug safety and on how to mitigate identified risk are essential for reducing drug-related morbidity and providing quality health care more generally. Current law entrusts the conduct of these activities, which are vital to public health, to a for-profit industry whose revenue would be threatened by prompt, competent, and transparent assessment of and education about the risks of approved medications. That so much of the industry’s work in this area is slow, low quality, or in some cases even non-existent is not surprising. The Commission recommends a fundamental change in approach: direct governmental control over post-approval drug surveillance and of the development, implementation, and evaluation of risk evaluation and mitigation strategies is needed.
Congress must decide where in government these activities are based, but to avoid conflicts of institutional interest they should not be overseen by FDA’s Office of New Drugs, which generally sees its charge as bringing more medications to market. The funding and authority to monitor and mitigate post-approval drug risks -- including the power to pull an approved drug from the market if warranted -- could be given to drug safety officials within the FDA, or, as some have proposed, to an independent agency outside of the FDA.44
Recommendation 1e: Bodies that have legal or regulatory power to shape prescribing should accept no funding from industry and include no individuals with direct financial ties to industry.
We have already discussed the need to insulate from industry influence organizations that have some ability to persuade prescribers (e.g., medical schools). The need for firewalls is even stronger in areas where an organization has formal legal or regulatory power to shape prescribing. The Federation of State Medical Board’s eventual decision to stop accepting funding from the pharmaceutical and medical device industry was a positive step and should be uniformly adopted by USA state and Canadian provincial medical boards.
Prohibitions against industry influence in this arena are justifiable entirely because of concerns about protecting patients. But it bears considering that such rules also protect prescribers who practice ethically and compassionately. Just like patients, physicians have a right to expect that the rules under which prescribing is conducted were set based on scientific evidence and intended solely to benefit patients, not to enrich industry.
Finally, the Commission notes that the spate of multi-billion dollar lawsuits surrounding the opioid crisis in the United States can also create conflicts of interest. The restrictions on regulatory bodies proposed in this recommendation should apply not only to material connections to the pharmaceutical industry, but also to law firms suing some element of the industry and individuals hired as expert witnesses by those firms.
Industry influence over the political process
Election campaigns in the USA are expensive, and office holders are attuned to raising sufficient funds to compete in them. Corporations and their employees have always been significant donors to political campaigns, but changes in campaign financing laws, most notably the U.S. Supreme Court’s 2010 decision in Citizens United vs. Federal Election Commission,176 have removed almost all limits on their political campaign contributions (Canada in contrast has maintained caps on donation amounts). Discussing the impact of this decision across all areas of corporate influence is beyond the Commission’s scope and expertise. We instead make the more focused observation that in the specific case of the pharmaceutical industry, the removal of donation limits plausibly worsened the opioid crisis and increased the risk of subsequent crisis involving prescribed medications.
The power that lobbying and unconstrained political donations give the pharmaceutical industry is hard to overstate. Over a 10-year period, groups attempting to place some limits on opioid prescribing (e.g., activist groups of people who had lost loved ones to overdose) spent $4 million on lobbying and campaign contributions in USA state legislatures. Over the same period, the pharmaceutical industry spent $880 million to persuade state legislators to serve their business interests.177 Even under the conservative assumptions that only a minority of that money was spent on opioids and that political donations from law firms suing the opioid industry have recently entered the political equation as a partially countering force, the opioid industry’s lobbying power is clearly enormous.
The financial power of the pharmaceutical industry at the federal level is equally undeniable. For example, when the Drug Enforcement Administration caught opioid distribution companies breaking the law by not reporting massive, suspicious, shipments of opioids to particular communities, the companies asked Congress to pass a law curtailing the agency’s power to conduct such investigations. The industry had contributed $1.5 million to the campaigns of 23 lawmakers who sponsored the new law, including US$100,000 to Representative Tom Marino, who led the law’s passage in the House of Representatives.154 Soon afterward, President Trump nominated Marino to become the Director of the White House Office of National Drug Control Policy.67
In addition to influencing policymakers with donations, opioid manufacturers have also followed the lead of other industries (tobacco, fossil fuels) by engaging in “astroturfing”, i.e., creating or infiltrating putatively grassroots groups which are covertly funded by industry and carry its messages. A notable example in Canada was a coalition of industry-funded “patient advocacy organizations” arguing against a government effort to reduce drug prices.42 A prominent USA example is the American Pain Foundation which publicly presented itself as an independent voice of pain patients while echoing opioid manufacturers’ messages about the ample benefits and minimal risks of opioids.178 When it came to the attention of investigative journalists and the Congress that 88% of the American Pain Foundation’s annual budget was provided by opioid manufacturers and medical device makers, and that it closely coordinated its public messaging with industry representatives, the organization was dissolved.179,180 Other non-profit organizations in the opioid arena (e.g., Pain UK) have been criticized by regulators for failing to disclose links with opioid manufacturers.181
Surveys of patient advocacy groups across all areas of health estimate that between 67–83% receive funding from for-profit entities (e.g., pharmaceutical and/or medical device industries).182,183 One study of advocacy groups reported that 88% publish lists of donors in annual reports or on a website, but only 2% explicitly state that all corporate donors are listed, and 43% do not report any information on amount of donations received.182 Extensive, rising, and underreported financial support of patient advocacy groups by the pharmaceutical industry has also been documented in other nations.184
Recommendations for countering industry influence over the political process
Recommendation 1f: The USA should restore limits on the political campaign donations of pharmaceutical companies.
The incentives in the USA;s system of campaign finance are well-aligned with the interests of the for-profit pharmaceutical industry and poorly aligned with public health. Pleas for individual virtue and courage by politicians are welcome, but insufficient. The current composition of the U.S. Supreme Court would appear to make immediate reform in this area unlikely. But in the long term, reinstating restrictions on corporate donations to political campaigns would help prevent the regulatory capture that augments risk of epidemics to addictive pharmaceutical products.
Recommendation 1g: Prevent the pharmaceutical industry from covertly funding “astroturf” advocacy organizations.
Patient advocacy is part of a healthy democratic society, and grassroots organizations are of course welcome to accept donations and to advocate. However, when such organizations are financed by a for-profit industry, they should not be allowed in the public square to represent industry messages as if they were independently derived grassroots opinion. Just as drug packaging must be labelled to identify its active ingredient, the same principle should apply to drug-related advocacy.
In the USA, corporations enjoy rights to free speech comparable to individuals, but they do not have a right to purchase deceptive speech. For example, the Federal Trade Commission has sued companies for purchasing positive online reviews of their products from third parties.185 Scott186 has suggested productive regulatory changes in the USA context. First, to protect consumers, the Uniform Deceptive Trade Practice Act should be modernized to define astroturfing as a deceptive business practice and to mandate disclosure of material connections between “grassroots” groups that endorse a company’s products and the company in question. Second, because astroturfing can also represent a form of fraud against investors by conveying that a company’s products are more popular with the public than they are in reality, the Securities and Exchange Commission should exercise its authority to require full disclosure in annual corporate filings of all funding of advocacy groups in which corporations engage.
Legislative bodies and advisory boards at all levels of government should also discourage astroturfing in hearings by adopting a public disclosure norm. Specifically, immediately after witnesses are sworn in and are therefore legally required to tell the truth, the committee chair could direct that each witness publicly state whether they or their organization have any financial connections to the industry whose products and practices are the subject of the hearing.
Journalism has responsibilities in this area as well because mass media is one of the most common routes through which astroturf groups disseminate pro-industry messages. Journalists who consider quoting members of putatively grassroots advocacy organizations should adopt as standard practice asking whether the organization is funded by the industry and including that information in any reported coverage of the organization.
Domain 2: Opioids’ dual nature as a benefit and a risk to health
The second of the seven domains addressed by The Commission is opioids’ “dual nature”. Opioids are both essential to modern medical practice and at the same time potentially dangerous. Their dual nature stems from the fact that they simultaneously activate brain pathways that reduce pain, slow breathing, and produce euphoria that can lead to addictive use. A further complexity is that opioid use can lead to OUD, yet the provision of opioid agonist therapies (e.g., buprenorphine) often benefits people with the disorder.
Opioids can be prescribed in ways that have significant negative spillovers as happened extensively in North America but can also be prescribed in ways with fewer adverse consequences (as in Germany, where prescribing is extensive but OUD and overdose are not).45 Unrestrained opioid prescribing cannot reduce the population burden of pain (e.g., through analgesic prescribing) nor of opioid addiction and overdose (e.g., through buprenorphine or methadone provision) without significant collateral damage. At the same time, blanket downscaling of opioid prescribing can also do significant damage.
Many prescribers who contributed to the quadrupling of opioid prescribing sincerely believed that they were contributing to resolving the crisis of pain in North America. Many equally well-meaning people today believe that throwing the switch the other way will resolve the opioid crisis or, ironically, that flooding the addiction treatment system with opioid agonists such as buprenorphine and methadone will do so. The human brain has an inbuilt tendency toward affectively simple judgements, preferring to categorize things as good or bad rather than good and bad.187 The human tendency toward black and white judgements is more pronounced when emotions run high,188 as is often the case when opioids are debated. Rising above those instincts to deal directly with the dual nature of opioids is essential for medication approval decisions, the care of pain patients, and opioid stewardship.
Recognizing the risks and benefits of opioids in the drug approval process
During the opioid crisis, the healthcare system supplied billions of dollars of dangerous, addictive drugs that were diverted to illegal markets. In addition to doing damage directly, the massive expansion of prescription opioids also indirectly made illicit drug markets more deadly by creating an opportunity for heroin traffickers to expand their business.15 Risks that a medicine will be diverted, and that it may exacerbate the damage of illegal drugs markets, were historically not considered in FDA’s approval process. In 2018, the then-head of the FDA, Dr. Scott Gottlieb, ventured that the agency should evaluate applications for opioid medication approval in light of diversion risks and potential interplay with other drugs already available and in use in the health care system.189
Certain general risk factors for diversion are obvious, including the drug having recreational or performance-enhancing rewards for users and having indicated conditions that are hard to verify objectively. Other risk factors will require careful assessment on a case by case basis. The same points hold when anticipating interplay of the medication’s supply with illegal markets, which will require careful assessment of which illegal drugs may be a complement or substitute for the medication in question.
Another weakness in the approval process – which is not unique to opioids -- is the reliance on short-term studies to assess safety and efficacy. In order to bring a product to market, pharmaceutical manufacturers have to meet the evidentiary standards of regulatory agencies (e.g., the FDA, Health Canada, the European Medicines Agency). This includes proving efficacy, typically in a short-term trial (e.g., 12 weeks). Manufacturers rarely extend the study longer because this would raise costs and risk revealing longer-term adverse effects that could lessen market share. In the case of opioids, this can lead to underdetection of longer-term physical dependence, OUD, and overdose, as well as fading ability to reduce pain over time.
Clinical trials of opioids for chronic non-cancer pain average only 5 weeks in length.190 Such short-term trials tend to show superior pain control from opioids versus placebo or non-opioid treatments, and few side effects. However, to cite one well-known exception, the SPACE trial191 found that for back, knee, and hip pain, opioids produced more side effects and were poorer at reducing pain intensity than non-opioid medications (e.g. ibuprofen) over a one year period among Veterans Health Administration patients. The regulatory environment and the profit motives of industry create structural barriers to funding longer-term studies of opioid medication.
Additionally, across all areas of medication development, regulatory guidelines allow manufacturers to exclude broad swathes of the patient population from clinical trials but do not restrict drugs approved with such evidence from being prescribed to the patients who were excluded. If, for example, a manufacturer expects that a common comorbidity among pain patients (e.g., depression) raises the risk that a new opioid will result in addiction, they can exclude depressed patients from the trial secure in the knowledge that they will still profit from sales to depressed patients post-approval. Many studies of exclusion criteria across diseases have shown that clinical trials tend to exclude the most vulnerable individuals, including people with serious comorbidities, the elderly, and pregnant and lactating women.192 Such individuals will receive the treatment within the health care system anyway after approval, thereby “enrolling in an experiment”, only without the usual informed consent or monitoring that would attend a scientific study.
Recommendations for recognizing the risks and benefits of opioids in the drug approval process
Recommendation 2a: Drug approval agencies should more heavily weigh concerns about diversion of medications to illicit markets, and, the potential interplay of the medication’s supply with other legal and illegal drugs.
The Commission endorses former FDA head Gottlieb’s call for drug approval processes to encompass considerations beyond the clinical effect of a drug on the individual to whom it is prescribed. We also recommended broadening Gottlieb’s proposal in two ways. First, intentional and unintentional diversion risk should be considered when national regulatory agencies contemplate approval of all substances with addictive liability (e.g., stimulants, benzodiazepines) not just opioids. Second, such agencies should weigh how introducing a new drug could have interplay not only with approved medicines, but also with drugs available in illegal markets. Regulatory agencies should be provided added funding to conduct such assessments, which will require them to research illicit drug markets and to employ staff with the relevant expertise to analyze the data.
In calling for greater consideration of the aforementioned risks that extend beyond the patient, the Commission does not suggest that they be the only consideration in drug approval. A desperately needed medicine could still be approved even if it had significant diversion risk. In such cases, regulators might advise that its use be limited to within health care facilities. Cocaine is an FDA-approved Schedule II drug with almost no diversion because it is used for surgery and administered by the clinician at a medical site, such as a hospital. Likewise, Germany has a per capita opioid prescribing rate close to Canada’s, but no evident opioid crisis because only in Canada are opioids frequently provided in prescriptions to ambulatory patients rather than employed mainly in supervised settings.45 Policymakers thus have options between approving unrestricted use and denying approval in cases where a medication has unique therapeutic value but also poses risk.
Recommendation 2b: Governments should invest in the type of studies of opioids and their impact on pain, function, and addiction that are specifically discouraged by current medication approval regulations: long-term clinical trials enrolling broadly representative samples of patients.
Over-reliance on short-term trials with selected patients is built into the approval process, creating risks that medications will be approved with no consideration of their longer-term harms. This can only be rectified by a sustained public commitment of resources to longer-term trials, or, by changes to regulations to make drug approval contingent on longer-term trials. The Commission recommends expanded support for pragmatic trials of medications that enroll all individuals who are likely to receive the medication in practice.193 In addition to having a greater chance of detecting adverse effects, because of their heterogeneous samples, such trials also have more power to identify subgroups of patients who benefit particularly from medications. Such trials do not necessarily require public funding; manufacturers could be required to provide the funding to non-industry investigators to conduct them. Importantly, the findings of such longer-term trials should be consistently reviewed by drug approval agencies so that they can make informed judgements on whether medications approved on the basis of short-term results should be restricted or pulled from the market because of their longer-term harms. The close monitoring of potential long-term effects of COVID-19 vaccines is a model worth applying to opioids.
The care of chronic pain in an opioid crisis
As populations age, chronic pain conditions become increasingly pervasive causes of functional impairment, reduced quality of life, and morbidity (e.g., depression).194 For example, low back pain is a leading cause of disability globally from adolescence through late life and ranks ninth in terms of overall disease burden.195,196 Low back pain was also one of the ten leading contributors to global decreases in disability-adjusted life years from 1990 to 2019.196 In the USA, 8% of adults report experiencing “high-impact” chronic pain, defined as pain that limits life and work activities on most days in the past 6 months.197 Although not a direct cause of death, pain can contribute indirectly to mortality by raising the risk of suicide and opioid overdose, for example. Pain is often poorly managed by existing health care systems and is usually an orphan in public policy circles and research funding organizations. The lack of investment in basic and clinical science research may contribute to the high costs of chronic pain. Lower back and neck pain account for more spending than any other condition in the USA’s health care system, and much of this spending supports interventions of debatable effectiveness.198,199 Despite its health care impact, back pain was not tracked by the National Institutes of Health as a research condition and disease funding category until 2016,200 during which only US$23 million in research funding was devoted to it.
Most prescribers, advocates, and health care organizations were responding to a genuine problem when they increased opioid prescribing: patients in pain, sometimes excruciating, long-lasting pain. Debate continues about the proper role of opioids in acute and chronic pain management. Our purpose here is not to review that debate, only to suggest that as long as pain is prevalent and poorly managed, overuse of opioids and attendant harms are more likely.46
Debates about the proper level of opioid prescribing are sometimes bitter and unproductive because participants do not attend to the diversity within the relevant population.201 Patient subpopulations that are affected by prescribing policies include -- but are not limited to -- individuals not currently on opioids, individuals receiving short-term opioids for acute pain, chronic pain patients on long-term opioid analgesics, addicted individuals receiving medications for OUD, patients experiencing both OUD and chronic pain, individuals with untreated addiction, and various combinations thereof (see panel 3).
Panel 3: Voices of patients and physicians about decisions on whether to prescribe opioids.
“I constantly struggle on wanting desperately to believe the patients about their pain, but having that fear that it’s being diverted. Medications are being diverted or not used appropriately all the time. So, the subjectivity of it I find I struggle with all the time. And, again when I graduated residency it was everyone is innocent until proven guilty type thing. But, I feel in our high-risk clinic almost it’s guilty until proven innocent, and that saddens me as a physician.”
– Family physician, Ontario, Canada202
“[Buprenorphine] can diffuse within the [addiction treatment departments], but to get it to diffuse beyond that, that’s the challenge. And that’s where it really will have its benefit…Because, there are more people with the problem than can be handled [in addiction treatment settings]…I think primary care clinicians need to take ownership and responsibility for helping their patients with addictions.”
– Physician, USA203
“When something stresses me out maybe they used to do every 2 weeks of getting my medication. Now it’s down to once a month or they [the physician] may say instead of third of the pills [30 pills], we’re only going to give you 15. So, it [the opioid epidemic] has definitely affected me personally and I hate that.”
– Patient with sickle cell anemia, USA204
“(The law) affects people like me (who are employed) because they won’t give (opioids) to you unless, you know, you go (…) to the special clinic, the classes, to get them. Well, I knew that I couldn’t get (medications) until I went to the classes. I had to go to the classes in the winter. I had to hop out and catch the bus and go out west to go to the (pain) clinic to see the doctor.”
– Patient with chronic pain, Indiana, USA
“I don’t think people in chronic pain think about long term. We are basically, how do I get through today? I just gotta get through today.”
– Patient tapering opioids, Colorado, USA
Opioid prescribing policy should also recognize that within and across cultures, there are substantial differences in perceptions of the proper role of doctors, the appropriate level of patient autonomy in care decisions, the meaning and tolerability of pain, the acceptability of risk, and the degree to which addiction is stigmatized, among many other factors that shape how opioids’ dual nature must be balanced (see panel 4). These cultural forces merit attention not only because they affect patient expectations, but also because they influence the conduct and outlook of health care professionals and policymakers.
Panel 4: Opioid prescribing in Francophone regions.
France and the regions it has influenced have distinct patterns of opioid prescribing for reasons that are not well understood. The Swiss cantons in which higher potency opioids are the most heavily prescribed tend to be German-speaking, whereas those where lower potency opioids predominate are in the Francophone region.205 Among Canadian provinces, Quebec has the lowest rate of high-dose oxycodone prescribing; neighboring Ontario exceeds it almost 8-fold.6 And perhaps most remarkably, despite the proportion of their populations reporting pain being similar, USA opioid prescribing rates in 2012–2013 were more than 5-fold those in France.206
Advertisers have always appreciated the role of culture in driving product use, which is why for example they attempt to brand addictive products as culturally essential, e.g., the Marlboro cowboy or Newcastle Brown Ale. Policymakers need to be equally aware that responding to the opioid crisis may require efforts to shift cultural attitudes in ways that support compassionate care of pain and addiction and towards judicious opioid prescribing (e.g., media campaigns, public education).
One positive sign that medicine is now grappling more effectively with the dual nature of opioid medications is the rise of the concept of “opioid stewardship” which is defined by Canada’s Institute for Safe Medicine Practices as “as coordinated interventions designed to improve, monitor, and evaluate the use of opioids in order to support and protect human health”.47 Efforts to promote opioid stewardship explicitly recognize that opioids are essential for medical care and at the same time carry risks that must be carefully managed at the individual and health care system level. Sensible opioid stewardship programs recognize that that patients can be harmed by clinical decision to prescribe or not to prescribe opioids. They also incorporate ameliorative strategies to protect patient subpopulations who face particular risks when a health care organization alters its prescribing policy.
Recommendations for the care of chronic pain in an opioid crisis
Recommendation 2c: Nations should implement comprehensive strategies for the prevention and management of pain, of which opioid prescribing is but one part
Pain patients are more likely to receive better care – whether with opioids or not – if the care of pain is embraced as an urgent priority and organized in a rational fashion. The Commission therefore recommends that all nations develop a comprehensive pain strategy that embraces an interdisciplinary approach, is based on scientific evidence, addresses both prevention and treatment, and is insulated from pharmaceutical and medical device industry influence.207 Also critical are a commitment to ensuring health equity across racial and ethnic groups, and a spirit of compassion towards and willingness to listen to individuals in pain, individuals experiencing addiction, and their families. The USA’s National Pain Strategy (see table 2), upon which multiple members of the Commission worked, was released near the end of the Obama Administration but was not funded or sufficiently implemented.46 The Commission calls on the Biden Administration to revive it.
Table 2:
Priority areas and objectives of the National Pain Strategy46
Priority Area 1: Population Research
|
Priority Area 2: Prevention and Care
|
Priority Area 3: Disparities
|
Priority Area 4: Service Delivery and Reimbursement
|
Priority Area 5: Professional Education and Training
|
Priority Area 6: Public Education and Communication
|
Recommendation 2d: Policies restricting opioids should be sensitive to the needs of current and future pain patients.
Responding to system-wide overprescribing by throwing the switch suddenly in the other direction can have negative consequences for current and future pain patients. Opioid stewardship initiatives and guidance documents in the USA and U.K. emphasize that expanding effective non-opioid alternatives for pain increases the likelihood that less frequent prescription of opioids will be a net benefit rather than a net harm to pain patients.208,209 Relatedly, progressively tapering the opioid doses of existing patients should be an individually-tailored activity which is done carefully and slowly,208 in which prescribers are specifically trained and for which they are compensated. Canada’s de-prescribing network is a promising effort to develop norms of practice in this area.210 The U.S. Department of Health and Human Services guideline deals well with the complexity of tapering opioid dosage in clinical settings, including shared decision-making to develop a collaborative approach with patients.211
Promoting opioid stewardship in medicine
Proper opioid stewardship balances the benefits and risks of opioids in the care of patients. One underappreciated ingredient in such stewardship is trust.
From the earliest days of the opioid crisis, some individual physicians (e.g., Art Van Zee)36 sounded the alarm about the conduct of opioid manufacturers and the mounting death toll. Other physicians, professional associations, and health care organizations joined the ranks calling for change in the ensuing decades. Yet other individual physicians and physician-dominated organizations impeded efforts to rein in the industry’s misconduct. The motives behind this resistance varied, but the stance was harmful to the public. Most practicing physicians were not on the front lines of what became a civil war in medicine but were still affected by it, and at times made prescribing decisions with the best of intentions that they later came to regret.
Overprescribing and efforts to resist a return to judicious prescribing damaged public health. It also damaged public trust. Health professionals being sent to prison for running pill mills,212 defrauding Medicaid,6 and accepting illegal kickbacks from opioid manufacturers damaged the standing of medicine with the public.213 Physicians who promoted opioids while being covertly paid by opioid manufacturers betrayed the trust not just of their patients, but of their colleagues and students as well. Even the many well-intended but harmful opioid prescribing decisions made by ethical prescribers over the past 25 years may have damaged trust between the public and their doctors. Americans’ confidence in medical leaders has been falling for over 40 years,214 and the opioid crisis certainly has done nothing to reverse that trend.
Policymakers have also lost confidence in medicine, as witnessed by many governors and state legislators restricting the length of new prescriptions to a month, a week, or even less.215 Some physicians regard such laws with horror both because of the intrusion on autonomy they represent and because such rules may harm patients. The former is certainly true, the latter may or may not be; evaluations are in process.216 Regardless, the passage of these laws should be understood as a reflection of a loss of faith in medicine’s ability to self-regulate. Even if such restrictive prescribing laws prove harmful to patients, they may well persist or even expand if policymakers and the public do not trust physicians to practice safely without tight supervision. This may feel particularly unfair to physicians who have always prescribed carefully and it is: policymakers, like the public, sometimes make global judgements that are insensitive to nuance.
Trust is a precious commodity between individual patients and doctors, between the public and medicine as a profession, and between policymakers and health care system leaders. The COVID-19 crisis, in which many health professionals performed heroically, increased trust of physicians in many quarters in the USA.217 An excellent way to rebuild such trust around prescription opioids is for every medical provider and health care organization to become actively engaged in implementing a culture of safer prescribing through the many strategies described in this document, whether they are currently under external pressure to do so or not.218
To cite one example, the U.S. Veterans Health Administration employed 300 pharmacists to proactively provide evidence-based, in-person, “detailing” about prescription medications to medical staff. Individual and medical center-level opioid prescribing was monitored, and clinicians and managers were equipped with computerized tools and skills to monitor patients’ prescription drug use history and risk profile.219 Significant resource investments were simultaneously made to increase capacity to manage pain without opioids. Over a five-year period during which more than two million patients with incident chronic pain were treated, the proportions receiving physical/occupational therapy and specialty pain clinic care increased by 10–20%.220 Prescriptions for most non-opioid medications also became more common for pain220 and the number of patients receiving the risky combination of opioids and benzodiazepines declined by 47%. Contrary to fears that safer prescribing initiatives need to rely on forcing long-term patients to taper opioids, more than 90% of the reduction in long-term prescription opioid use resulted from reducing the number of new long-term patients.221
Electronic medical records and associated prescribing systems present two avenues for improved opioid stewardship. The first is prescription drug monitoring programs (PDMPs), which track patient prescriptions across providers and pharmacies in both the U.S. and Canada.48,222 One key purpose of PDMPs is to prevent risky drug combinations (e.g., to alert a primary care physician considering an opioid prescription that the patient is already taking a benzodiazepine prescribed by a psychiatrist). PDMPs also serve to identify patients who covertly receive more prescriptions from more prescribers than could be justifiable for health reasons. This group is small but of significant consequence: One national study in the USA documented that in 2008, 0.7% of patients averaged 32 opioid prescriptions from 10 prescribers, accounting for 4% of all opioid dispensing.223 Such individuals could be addicted to opioids or could be faking a serious pain condition in order to supply illegal markets. Proactive investigation of anomalous prescribing data (e.g., to detect “pill mills”) is another function of PDMPs. PDMPs with some law enforcement involvement appear more likely to reduce fatal opioid overdoses than those without.49
PDMPs are only as good as the information entered into them, and their value is undermined if they are hard to use, if prescribers and pharmacists receive no training in how to use them, do not use them consistently, or do not even enroll to use them at all. In recent years, higher-quality PDMPs with mandatory enrollment and checking have been shown to reduce opioid-related harms.50 It is also important that the design and monitoring of PDMP data have input from experienced medical professionals, who can for example advise on situations in which statistically unusual levels of opioid prescribing is appropriate (e.g. palliative care).
Prescribing “nudges” are another opioid stewardship strategy enabled by electronic medical records. Nudge is a behavioral economic term which refers to non-coercive ways of influencing decisions, for example by changing which choice is the default. In a study of 2910 surgery patients, changing the default number of post-surgical opioid pills from 30 to 12 reduced prescribing by more than 15% without any indication of harm to patients.51 Findings of this sort have been independently replicated.224 Nudges in no way undermine physician autonomy because prescribers retain power to easily change the number of pills provided.
Just as with pain, the dual nature of opioids must also be recognized in the care of addiction. Opioid agonist therapy, particularly methadone maintenance, is probably the most extensively and rigorously evaluated treatment in the addiction field.225 Across a range of patients, settings, and countries, methadone and other opioid agonist therapies have been shown in clinical trials and observational studies to reduce morbidity and mortality in patients with OUD, to reduce criminal behavior and infectious disease transmission in the community, and to be cost effective.226–228 These medications, along with approved antagonist medications (e.g., extended release naltrexone) thus clearly have benefit for many patients with OUD.3
Yet for a number of reasons, unconstrained opioid agonist therapy for OUD cannot solve the opioid crisis. First, many patients with OUD do not want to be on opioid agonist therapy. Second, many patients on such therapy have poor outcomes, ranging from rapid dropout229 up to and including fatal overdose and increased consumption of other drugs (e.g., cocaine230 and alcohol231). Third, international experience (e.g., Denmark232 and the United Kingdom233) shows that when controls on methadone maintenance are loosened too far, the increase in population deaths from methadone cancels out the drop in heroin deaths that comes from easier access. Fourth, the UK system, which has gone far in this direction, provides opioid agonist therapy patients an average of only a few hours per year of evidence-based psychosocial services.234
Recommendations for promoting opioid stewardship in medicine
Recommendation 2e: To rebuild trust in medicine while helping patients at the same time, individual prescribers, health care organizations, and professional associations should actively implement safer opioid prescribing initiatives, whether they are under external pressure to do so or not.
Programs like the VA Opioid Safety Initiative should be actively spread by prescribing clinicians. The primary reason to implement systematic opioid safety programs is to benefit patients. Such programs could also have the important benefit of restoring trust in medicine if physicians actively, willingly, and universally implement such efforts themselves rather than waiting until an outside regulator loses patience and imposes controls which may or may not be sensible. Many prescribers have already made steps in this direction, but these should be expanded throughout the health care system.
Recommendation 2f: Opioid stewardship initiatives should embrace mandatory prescription drug monitoring programs and prescribing “nudges”.
The Commission recommends that PDMP enrollment mandates be universal in the USA and Canadian health care systems, with additional requirements to check the system when initiating patients onto controlled substances such as opioids. Prescribers should be compensated for the costs of PMDP participation and to make the process easier, technical improvements in PDMPs should be a high priority. These include integrating PDMPs into or linking them with widely used electronic medical record systems. For electronic prescribing, the ideal system would automatically conduct the PDMP check, alert the prescriber and pharmacist of any suspected doctor shopping or potentially dangerous drug interactions, and then upload the patient data to the PDMP database if the prescription were approved. PDMPs should also build on the recent trend (e.g., in the U.S. Department of Veterans Affairs PMDP) of sharing data across states and provinces.235 Further, PDMPs should include dispensing from methadone maintenance clinics and “medical cannabis” dispensaries in order to create a more complete list of controlled substances.
“Nudges” within electronic prescribing systems also merit expansion. Implementing nudges at scale within electronic prescribing systems is a low-cost, minimally intrusive method to promote judicious prescribing.
Recommendation 2g: Availability of medications for OUD should be expanded. Even while doing so, addiction care providers should recognize that prescribing opioid agonist therapies as extensively as possible with as few constraints as possible will no more resolve the addiction crisis than it did the pain crisis.
The Commission recommends that opioid agonist therapy should be offered to every OUD patient where not medically contraindicated (e.g., by a medical comorbidity or potential drug-drug interaction). This should include patients who do not wish to participate in psychosocial services, as research does not clearly establish that such services are consistently necessary for patients to benefit from opioid agonist therapy. 229,236,237 Formal regulatory expansion of access to these medications should be considered. The COVID-19 epidemic has led federal regulators in the USA to relax some requirements currently in place around medications for OUD. These include allowing more methadone take-home doses and waiving requirements that initial buprenorphine dosing be observed in person. Such loosening of requirements is necessary in a public health emergency. When the worst of COVID-19 has passed, governments should evaluate whether the balance of benefits and risks is favorable for routinizing these emergency measures to make care more accessible.238,239
At the same time, recent history demonstrates clearly the folly of assuming that population health inherently improves when health care systems provide as many opioids as possible with as few possible regulatory constraints as possible. Policies which should attract skepticism include dispensing hydromorphone from vending machines to create a “safe supply” of opioids and eliminating supervision of methadone patients, i.e., converting the system to unmonitored, long-term prescriptions on a take-home basis. Although expressed from a public health viewpoint, these messages echo the opioid manufacturers in presuming that unrestricted opioid provision can only improve public health. The faith of some advocates that opioids are a “safe supply” as long they do not derive from illicit markets (e.g., heroin contaminated with fentanyl) is impossible to square with the hundreds of thousands of overdose deaths from legal, pharmaceutical grade opioids which preceded the introduction of fentanyl into North American heroin markets.240
Care providers should also consider that many patients with OUD have serious, unaddressed psychiatric, medical, family, employment, and housing challenges that a medication will not solve.229 Solely providing medication has generated significant resentment among some addiction recovery activists for being managed rather than treated.241 Opioid medications can be powerful and effective in the treatment of OUD, but should not be employed as an informal system of pharmacological sedation of poverty.
Domain 3: Building integrated, well-supported, enduring systems for the care of substance use disorders
Health care systems and policymakers often react to a surge in some form of addiction as if it presented a transient, novel challenge. The attention of the North American public, media, and policymakers was transfixed by heroin in the late 1960s and 1970s, cocaine in the 1980s, methamphetamine in 1990s, and opioids in this century. Use of those individual drugs indeed spiked in those periods, and some of the challenges each presented was unique. Yet while these particular drugs seized public attention, tens of millions of North Americans used many other drugs (including licit drugs like alcohol and tobacco) and experienced addiction to them as well. Even at the individual level, addiction rarely involves one drug at a time. For example, 30% of “opioid” overdoses involve concomitant use of a benzodiazepine,242 and many others involve concurrent consumption of alcohol, cocaine, or methamphetamine.243
At any given historical moment, it may appear that addiction is a newly prevalent phenomenon involving a single drug, but addiction has been prevalent in modern societies since the 19th century when innovations in chemistry and global commerce and travel combined to dramatically expand access to addictive drugs. The purpose of health and social care systems is to benefit humanity, and addiction will always be part of human experience because our species has a brain which evolved to be highly drawn to and influenced by particular molecules that are available in the modern world at a level beyond anything for which evolution prepared us.244 Nothing illustrates this better than drug-related deaths in the USA having gone up every year for decades despite different drugs coming in and out of fashion.245
From this observation it follows that health and social care systems must permanently be equipped to respond to OUD and other substance use disorders, not just the opioid problems that are currently ascendant, but all the other drugs that harm health now and in the future. Yet substance use disorder-related services have never been made a permanent, integrated part of health and social care. This problem stems from two inter-related factors: stigma and financing.
In many societies, certainly including the USA and Canada, addiction has long been stigmatized as moral failing meriting punishment rather than a disorder meriting treatment.246 Stigma is expressed and reinforced through many mechanisms, including the use of derogatory terms for people who are addicted, such as “junkie” and “pillhead”, unwillingness of insurers to cover care for addiction, and overly pessimistic beliefs about the ability of people to recover from addiction.247,248 This stigma is intensified when an addiction crisis disproportionately affects oppressed racial groups (as did crack cocaine in the 1980s) and/or low income groups (as did methamphetamine in the 1990s). One highly consequential consequence of stigma is government underinvestment in substance use disorder care, which reflects in part sentiments among some policymakers and the public that the population in need does not deserve quality treatment or cannot benefit from it.249
The USA for example spends below the point at which return on investment turns negative (i.e., even if human welfare concerns were set aside, it would be cheaper to increase financial investment).52 In other words, even budget-minded officials not moved by the humanitarian case for addiction treatment would in many cases be more responsible stewards of the public purse if they spent more, rather than less, on such care.
But the amount of money alone is not the only important factor: the form of financing heavily influences the form of a society’s health and social care services. For example, the USA’s federal government provides a “Substance Abuse Prevention and Treatment block grant” detached from all other federal health care financing, which goes to substance use-specific state agencies that are rarely embedded in mainstream health care administrative units. Predictably, this produces a system of care that is poorly integrated with the rest of health care, thereby stigmatizing it, reducing its quality and accessibility, and making it harder for patients and providers to secure the range of services many patients need. As one stark example of the substance use disorder treatment system’s lack of integration with mainstream medicine, studies conducted over the past two decades reveal that fewer than half of treatment programs in the USA have a full-time physician or nurse on staff.250,251 Because this treatment system relies heavily on these annual lump sum block grant payments from the federal government to support its services, the availability of services for all Americans who need them is not guaranteed. Rather than automatically receiving more resources from insurers when demand increases like the rest of the health care system, a block grant program simply runs out of money in such situations. This creates wait lists for essential services such as residential treatment and methadone maintenance.252,253
The U.S. federal government has responded to the opioid crisis mainly by providing more fixed amount, short-term grants to states. Grants can be useful for one-time investments (e.g., building a new clinic), but as a source of treatment financing, they perpetuate the separation of addiction-focused services from mainstream health care. This funding approach also creates systemic instability because the potential employers, employees, and patients are hesitant to rely on an addiction treatment system supported by a 24- or 36-month grant. Relative to areas of medical care with enduring, stable, financial commitments, the precariousness of substance use disorder care financing reduces accessibility, increases disparities in access, lowers organizational stability, and increases stigma.
Low and inconsistent financing also reduces the willingness of clinicians to specialize in the care of substance use disorder, and of educational institutions to provide training in this area. Substance use accounts for 1 in 6 deaths among adults globally,254 but fewer than 1% of North American physicians specialize in addiction. Addiction specialization is rare as well among nurses, social workers, and psychologists. Just as importantly, the amount of training about substance use disorder that non-specialist health and social care professionals receive is minimal.54 Medical students may receive perhaps a few hours of training devoted to a disorder that they will encounter almost every day in their practicing career (whether the official reason for care or not), and which they not only have to manage but also need to ensure not to inadvertently create. Not incidentally, training in pain management is also minimal.194
Recommendations for building integrated, well-supported care systems
Recommendation 3a: Health and social care systems should make an enduring commitment to provide services for people with substance use disorders. These services should be fully integrated with mainstream healthcare systems, be equally accessible to all people in need, and should target a range of outcomes, including but not limited to eliminating illicit drug consumption.
In one sense, nothing new is needed in the design of substance use disorder care systems, as comprehensive models of population health management are already in use for other serious chronic health problems.53 Chronic care systems include population and clinically-based early detection approaches, offer less extensive treatments for early stage disorder, and provide more involved treatments for serious cases. In such systems, primary care physicians and other generalists work individually or in interdisciplinary teams to manage cases to the limits of their expertise. When those limits are reached, generalists call on specialist support for collaborative care. Interventions that improve function and reduce morbidity and mortality are considered valuable even if they do not restore the individual to perfect health. The patient’s family is educated about the nature of the disorder and its management, and their own needs are cared for as well. Further services are provided for other problems patients may have (e.g., homelessness, joblessness, parenting challenges), whether they are causally related to the core health problem or not. Long-term recovery support services are provided to ensure that early gains are routinized and spread to broader areas of the individual’s life. This basic lesson can be applied in the design of accessible and effective, care systems for substance use disorders (figure 7).
Figure 7.
Vermont’s hub and spoke model of addiction treatment
The “hub and spoke” model,255,256 which integrates regional specialty addiction treatment centers (hubs) and geographically dispersed healthcare settings (spokes) that can provide ongoing, community-based care, is a promising method for providing such care. Certified Opioid Treatment Programs staffed by addiction specialists form the hubs, providing methadone maintenance, buprenorphine induction, and naltrexone as indicated. Spokes – which include primary care, mental health care settings, outpatient addiction treatment, and clinics specializing in chronic pain management – provide maintenance medications for OUD and links to other social services. Vermont257 and California258 are among the states that have greatly increased buprenorphine access with this model, which is also being rolled out in at least a dozen other states.259
The specific elements that substance use disorders care systems should comprise have been elaborated elsewhere and need not be reiterated in detail here. Broad categories of care include emergency interventions for managing acute crises (e.g., naloxone and emergency care for overdose, detoxification and stabilization units), case-finding in the community and in medical settings (e.g., addiction consult-liaison services in the emergency department and medical wards), outpatient and residential settings providing behavioral and pharmacological addiction treatments, mutual help groups and long-term recovery support services (e.g., peer coaching, recovery housing), and efforts to prevent and/or treat common medical comorbidities (e.g., syringe exchange, hepatitis B vaccination). Care also includes mental health services responsive to the psychiatric disorders (e.g., depression, post-traumatic stress disorder) and adverse experiences (e.g., child abuse, sexual assault, violence exposure) that are prevalent in the population.260,261 The system should assist affected people at all stages of the “Cascade of Care”,262 an organizing concept pioneered in the HIV field. Building a cascade of care requires increasing the proportion of affected individuals being identified and diagnosed, the proportion of those diagnosed individuals who are linked to care, the proportion linked to care who receive effective services, the proportion who receive services who are retained for at least six months, and the proportion of those retained who transition to long-term recovery.263 Among the useful guides for the elements of such systems and the evidence behind them are the American Society of Addiction Medicine Levels of Care,264 and the U.S. Surgeon General’s Report on Alcohol, Drugs and Health.265
Because in many areas of health care, removal of illness is (often appropriately) considered the highest success of treatment, it bears mentioning that people who experience addiction often aspire to more, namely, recovery.265 Although each individual defines recovery from addiction in their own way, common themes are the building or rebuilding of relationships with other people; contributing to the well-being of one’s family, friends, and community; being esteemed and valued by others; adopting productive roles, and having a sense of purpose in life (see panel 5). High-quality care systems help individuals achieve these goals, very commonly by linking individuals to recovery-supporting organizations (e.g., mutual help groups) and support services.266,267
Panel 5: Voices of people in recovery from addiction.
“For me, recovery wasn’t an overnight process — it was a series of events dating back to my active using days but my journey started at the needle exchange. The very first person I met who had successfully kicked heroin and stayed off for many years was a staff person at the exchange. By talking with us, encouraging us, and simply being there, the staff and volunteers reinforced that all drug users are human beings, deserving of compassion.”
-Tracey Helton Mitchell, The Big Fix: Hope After Heroin268
“I am one of the lucky ones. And I know my continuing sobriety is not the result of my actions alone. I have a loving family and an extensive support network. I have 12-step and the guidance of my sponsors. I have good health insurance. I have the money, time and resources to help me save myself.”
-Nikki Sixx, Mötley Crüe bassist269
“During the ten years of my life I was using opioids, I never had a real friend. But once I put the drugs down, I started to find my people. That’s how it is in recovery. We make friends quickly. We know what it’s like out there. We’ve all survived the same nightmare.”
- Ryan Hampton, American Fix270
“I got tired of being a junkie, and I got tired of being a patient. I help take care of my Grandma now. She has Alzheimer’s, and I do a lot of things for her, just like taking care of a little baby. My mom says I take even better care of her [Grandma] than she does…I want to be well, and hold onto my dignity as long as I can. I can think again, and I’m doing art again, and that feels really good.”
- Diana (quoted in Drug Dealer, MD)40
“I started Homecomings: From Prison to Positivity. It’s for people who’ve been to prison, come home, and tried to keep their recovery. I know the struggles. I know the anxieties. We started meeting every Tuesday from eleven to twelve, and this room got so packed that I had to add another day…We focus on getting better, whatever we’re recovering from.”
- Tarah Dorsey (quoted in The Rooms Project)271
“When we started MARS in 2006, I would talk to groups of patients [who were receiving pharmacotherapy for substance use disorders] and ask who is in recovery? Rarely more than a few would raise their hands. They had been conditioned to believe that recovery was something that happened after they were off medication,” Now thanks in part to [our] trainings around the US, it is much higher.”
– Walter Ginter272
To enhance the coordination of and the culture within which services are provided, the Commission also recommends that health workers in the field unify under the well-established and deservedly respected label of public health. This would require abandoning factional, internecine debates over whether one form of recovery is better than another, or whether use reduction or harm reduction is a better goal. Politics are inherently and justifiably a part of how health policy is made,273 but the costs and benefits of individual service options can still be evaluated based on scientific evidence rather than ideology (see panel 6). And in any event, the needs, problems, strengths and goals of people with substance use disorders vary, and responsive care systems will make space for many paths to better health. Moreover, the alleged contradictions between different philosophies are more apparent than real. For example, interventions that are putatively about reducing harm and not drug use (e.g., needle exchange) often lead to reduced drug use,274 and interventions putatively focused on abstinence rather than harm reduction often lead people to continue using drugs with less functional impairment.275 Further, individuals in their own lives often integrate components of allegedly opposing approaches into a healthier life. For example, individuals on opioid agonist therapy who attend abstinence-focused 12-step mutual help organizations have better outcomes than those who do not.276–278 If those who access services can integrate diverse helping models peaceably, those who provide such services can do so too.
Panel 6: The controversy over supervised drug consumption sites.
Three decades after the first “supervised drug consumption site” opened, a modest number exist (fewer than 200) across Australia, Canada, and Europe. Sites allow people to use drugs they procure themselves in the presence of health professionals who can administer aid in the event of overdose, teach safer injection practices, and provide health information, including about the availability of other services. Critics have attacked such sites for allegedly increasing drug use and crime,279 and for imposing costs on neighboring residents and businesses. Research on sites is methodologically weak, but generally suggests that the risk of death from overdose is lower in a site than outside of it. However, there is no evidence that accessing a site lowers an individual’s risk of fatal overdose over time or that sites lower community overdose rates.280 Rigorous research on supervised consumption sites would be useful. Because of the high cost of maintaining brick and mortar sites and the limited number of people who use drugs who access sites when they do exist, the supervised consumption concept may have more potential to affect population health if it employs technology (e.g., smartphones) to offer monitoring to individuals using drugs in any location.
Recommendation 3b: Funding mechanisms that promote marginalized and unstable substance use disorders care services should be replaced with a core, enduring commitment within mainstream public and private financing mechanisms. This commitment should be advanced through public insurance programs as well as through the regulation of private insurance.
Funding for OUD and other substance use disorders care should be expanded within the enduring financing mechanisms that support the rest of the health care system. There are several mechanisms for doing this in the USA. First, the public Medicaid insurance program has become an increasingly important part of substance use disorder treatment financing in those states that expanded Medicaid in some form under the provisions of the 2010 Affordable Care Act.281–283 Yet a number of states, including some with quite serious opioid-related problems, refused to expand Medicaid to cover more of the population. Those states would improve the care of OUD as well as related conditions (e.g., pain, depression) if they expanded Medicaid.284,285 Medicaid expansion has been linked to higher rates of substance use disorder treatment receipt and fewer overdose deaths.286–288
Second, the federal government should require coverage for the full continuum of substance use disorder care in Medicaid and Medicare, its two largest public health insurance programs. Despite improvements in coverage in recent years, many state Medicaid programs do not cover all of the substance use disorder treatment services considered essential by the American Society for Addiction Medicine.289 For example, Medicare and many state Medicaid programs do not cover residential treatment or recovery support services.290 State Medicaid programs that contract with managed care entities should explicitly stipulate the terms of coverage for substance use disorder care. Given that Medicaid is the largest payer of substance use disorder care in the USA, ensuring coverage for the full continuum of treatment in this program has the potential to improve access for as many as one million individuals with OUD.
Third, qualified health plans need better guidance regarding what constitutes “coverage” for substance use disorder care as specified in the Essential Health Benefit. The Affordable Care Act and subsequent final rules issued by the Centers for Medicare and Medicaid Services give states substantial discretion to define the scope of substance use disorder care within their state benchmark minimum requirements for coverage, including for insurance plans operating within the state exchanges (“marketplace” plans).291 Consequently, some states required plans to cover the full continuum of substance use disorder treatment services and medications recommended by the American Society of Addiction Medicine, whereas others required coverage for only the most basic outpatient services.292 The Commission therefore recommends that states provide more specific guidance regarding what services and medications must be covered to ensure adequate access to substance use disorder care.
Fourth, in their benefit design, most private insurance companies are now required by federal and state parity laws to not impose utilization management policies (e.g., prior authorization, quantity limits, cost sharing) on substance use disorder care that are more stringent that those applied to coverage of other medical and surgical services. Some insurers have not complied with the law, depriving individuals in need of care. Individual states have successfully brought suit against insurers who have violated parity,293 but routine oversight and enforcement should be systematic across all 50 states as well as the federal government. Within the USA’s profit-driven health care system, substance use disorder is one of the few conditions financed mainly by public sources, which reduces access to care and the presence of highly trained providers. Shepherding more private dollars into the system by enforcing the parity law should thus be a major priority.
Mainstreaming the financing of substance use disorder care would have the added benefit of simultaneously imposing the workforce and regulatory standards of the rest of health care on substance use disorders providers. Reflecting its underfunding and segregation from the rest of health care, the substance use disorder treatment system suffers from extremely uneven quality of care.294 Low quality of care is bad for current patients and also reduces the willingness of payors to purchase services in the future, creating a reinforcing, negative cycle. Investment in services coupled with quality standards and related improvement efforts creates a reverse, positive cycle.295,296
Recommendation 3c: Public and private payors and regulators should curtail provision of addiction-focused health care services that have significant potential for harm.
One of the tragedies of the opioid epidemic is that even though treatment funding is in short supply, it is sometimes expended on approaches that likely make patients worse off. This includes treatment programs that actively discourage patients from using approved medications or offer bogus medications (e.g., cannabis as a cure for heroin addiction). It also includes detoxification-only services with no follow-up, which may actually increase harm by lowering tolerance and thereby increasing overdose risk. Disappointingly, treatment programs accredited by external auditors are as likely to offer ineffective services as those accredited.294
The most potent route to curtailing harmful services is to stop purchasing them. The Commission recommends that government insurance programs like Medicare and Medicaid, treatment block grants, and drug court funding no longer reimburse such services, and encourage private insurers to follow the same course. It also recommends that public and private accreditation bodies prioritize elimination of services that have significant potential for harming patients.
Recommendation 3d: Health care policymakers and educators should make a major investment in addiction-related training of specialist and generalist health professionals.
Many addiction-focused curricula have been developed by educators, researchers, clinicians, and professional societies. But at the undergraduate, graduate, medical, and residency levels, such training is infrequently provided.297 Years of exhortation on this point have not had significant impact, so the Commission believes it is now time for such training to be required. Bodies responsible for the education of health professionals, most notably schools of medicine, nursing, and dentistry, as well as professional societies that provide continuing education and certify professional training programs (e.g., medical, dental, nursing, and psychological associations in the USA and Canada) should agree on minimum standards for substance use disorder-related instruction that must be met for accreditation across the curriculum. Much of the training can be directed at generalists and professionals focused on other disorders, for example training on how to manage alcohol use disorder in cardiology care, how to detect substance use disorders in family medicine clinics, how to concurrently treat substance use disorder in patients receiving psychotherapy for depression, how to detect and manage OUD in pain clinics, and how to respond to OUD presentations in the emergency department.
The USA does require additional addiction-focused continuing education for physicians who wish to prescribe buprenorphine for OUD to more than 30 patients, but the Commission prefers a broader approach. Specifically, education in managing addiction and on the risks of addiction to prescribed medication should be required before any health professional is granted a license to prescribe controlled substances.
Specialty training programs should also be significantly expanded to meet the enormous need for addiction treatment. Among specialties, psychiatry has historically done the most to treat addiction, but addiction medicine should not be regarded as only a psychiatric subspecialty. Indeed, one of the most positive developments of recent years is the 2015 recognition of addiction medicine as a medical specialty, paving the way for a diverse set of physicians to receive additional training in addiction medicine under the auspices of the U.S. Accreditation Council of Graduate Medical Education.190 Addiction medicine and addiction psychiatry fellowships provide advanced fellowship training to a diversity of specialists (family medicine, internal medicine, psychiatry, emergency medicine) to increase the work force targeting substance use disorders. Student loan repayment incentives should be expanded to encourage professionals to specialize in the addiction field.
Domain 4: Maximizing the benefit and minimizing the adverse effects of criminal justice system involvement with people who are addicted to opioids
The criminal justice system is the fourth of the seven domains analyzed by the Commission. The mantra that “we can’t arrest our way out of drug problems” is correct yet also implies something that is untrue, namely that there will or should ever be a time when the criminal justice system is not involved with addicted people.55 Contrary to some popular narratives, contact between the criminal justice system and people who use addictive and intoxicating substances will be prevalent whether drugs are legal or illegal. Alcohol, which is legal, is involved in more arrests, violence, and incarceration than any other drug.298 The criminal justice system will always have a role in responding to drug use because intoxicated and addicted people disproportionately engage in harmful conduct, including but not limited to physical violence. A famous conceptualization in the field299 characterized addiction as a chronic disorder akin to asthma, Type II diabetes, or hypertension. This is accurate in terms of addiction having genetic and behavioral risk factors, a chronic course, and meriting quality health care, and everyone working the criminal justice system should recognize these realities.55 But it is not accurate when it comes to negative externalities: people with asthma, diabetes, or hypertension do not have disproportionate rates of violent and other crimes, and hence the criminal justice system is less relevant to them than it is to people experiencing addiction.
The question therefore becomes how the criminal justice system can increase its beneficial activities regarding OUD and decrease its harmful activities, while still protecting crime victims. Because addiction is possibly the most common health problem among people who are incarcerated,300 offering addiction care tailored to individual need in all correctional health care systems is the most prominent example of the former.301 Incarceration is intended as a punishment for the individual concerned and a deterrent to others who might engage in the same crime, but for both humanitarian and utilitarian reasons, it is simultaneously an opportunity for rehabilitation.
Some correctional officials worry that pharmacotherapies (e.g., methadone) may be diverted by patients and become part of black market economies in prison. This risk is typically manageable, for example by implementing observed dosing for oral medications and by offering injectable extended-release formulations.302 It should also be noted that not making pharmacotherapies available can also create management problems (e.g., smuggling of opioids into prisons, protracted opioid withdrawal leading some incarcerated people to be combative).
Transition services extending beyond release from incarceration are of paramount importance in OUD treatment. Contrary to popular lore, obtaining a regular supply of illicit opioids while incarcerated is in fact difficult.303 As a result, most incarcerated people with OUD go through partial or complete withdrawal. Individuals who have not used opioids for an extended period lose tolerance, making their “usual dose” potentially deadly. The risk of death from opioid overdose in the immediate release period is appalling.36 Even individuals who have been receiving medications for OUD while incarcerated may be at risk if there is not a continuation of such services immediately after release, as well as provision of naloxone for overdose emergencies. Correctional facilities that have created smooth transition services have generated sizable public health benefits (see panel 7).
Panel 7: Expanding OUD treatment in correctional facilities.
Starting in July 2016, the Rhode Island Department of Corrections enacted several changes to improve OUD treatment during incarceration and reduce post-incarceration overdose deaths.56 Executive and legislative leadership were key in the success of the initiative. The Governor requested $2 million for the program that the General Assembly approved, and then the Department of Corrections implemented. Jails and prisons began screening for OUD on admission, offering to induct individuals onto their pharmacotherapy of choice, maintaining an individual’s treatment throughout incarceration, and partnering with community-based providers to prevent post-release disruptions of care. At the same time, 12 Centers of Excellence focused on substance use disorder treatment were established across the state. These served as additional linkage locations for people released from incarceration to maintain pharmacotherapy for OUD after incarceration. Early evaluations of these programs found high uptake and satisfaction with treatment during incarceration, substantial rates of continued treatment engagement post release, and 60% fewer overdose deaths post release after program implementation compared to a period prior to implementation.56,311,312
Community supervision systems (e.g., probation and parole) are another opportunity to deliver OUD treatment within the criminal justice system. One model for doing so are drug courts, which can be effective presuming they allow use of all evidence-based pharmacotherapies.274,304 Contingency management approaches combined with regular drug testing (sometimes termed “swift, certain, and fair” monitoring) also have encouraging evidence in community-based supervision settings (e.g., parole and probation) of reducing substance use, crime, and likelihood of incarceration.305
The above positive opportunities for the criminal justice system should lead no one to overlook its possible harms. Particularly in the USA where the system is so large and powerful, it has frightening potential to make the opioid crisis worse, most notably for low-income individuals and for African-Americans. Three specific policies are particularly destructive.
First, even though incarceration in a prison for possession of a personal supply of illicit opioids (or of syringes) virtually never happens in North America, some arrested individuals spend some time in local jails. The common result is withdrawal (dangerous in itself) and loss of tolerance (more dangerous because it increases risk of overdose on release).306
Second, during the height of the USA’s “war on drugs”, many states and the federal government passed laws applying long-term, sometimes permanent “collateral penalties” for individuals convicted of drug related crimes. These penalties were often applied as supplements rather than alternatives to criminal penalties (e.g., arrest and incarceration), and extended the term of punishment beyond that typically applied for more serious offenses, up to and including an individual’s lifetime. Collateral penalties include bans on public assistance, exclusion from public housing, denials of student loans, and bars to certain types of employment.57
Third, a number of states in the USA have policies that are punitive towards use of alcohol and other drugs, including opioids, during pregnancy.58 Such policies comprise laws that consider substance use during pregnancy to be criminal child abuse, policies that allow civil commitment (forced inpatient treatment) of expectant mothers on the basis of protecting the fetus from substance use, and clinician mandatory reporting laws. In some cases, courts have even viewed a mother’s use of opioid agonist therapy for OUD negatively in child welfare cases.
Recommendation 4a: Addiction-related health services, including all approved medications, should be available to all incarcerated individuals who have opioid use disorder, including during the high-risk period surrounding discharge.
Rehabilitation is one of the core missions of correctional systems. This includes a responsibility to treat health conditions such as addiction. Indeed, in the Plata decision in 2010, the U.S. Supreme Court held that providing inadequate health care in prison violated the 8th Amendment’s injunction against cruel and unusual punishment.307 Even were it not a legal requirement and an ethical imperative, there are additional practical reasons to treat OUD and other substance use disorders in prison: the marginal costs of providing addiction care to people who are incarcerated is small relative to the potential public health and safety benefits of such care.
Because prison-based addiction treatment without continuing services after release has less impact (indeed some studies find it has none)274 and because post-release is such a high-risk period, the Commission also recommends that community re-entry services after release should also be universally provided and adequately resourced. In addition to addiction treatment, incarceration should also be seen as an opportunity to attend to other health needs of the addicted population, including offering pre-natal care, providing hepatitis B vaccines,308 treating sexually transmitted infections, caring for psychiatric disorders, and offering overdose education and naloxone distribution (which could have radiating benefits to non-prisoners upon release309).
In the USA, the Commission recommends making addiction-related services available in correctional facilities by passing the Medicaid Re-Entry Act310 being considered in the current Congress. Currently, Medicaid does not generally cover services provided in correctional facilities. This hampers both in-facility service provision as well as re-entry services because once Medicaid is shut off upon incarceration, there can be paperwork hassles and delays before benefits are reactivated in the vulnerable post-release period. The Medicaid Re-Entry Act reactivates Medicaid coverage to cover addiction treatment provided in the final month of an individual’s incarceration.310 This could allow prison staff to provide the care themselves, but in most cases the likely division of responsibility will be Medicaid-funded contracts to community health care providers to care for incarcerated people both prior to and after release.
Recommendation 4b: Policies of incarcerating individuals for illicit possession of opioids or drug-related equipment (e.g., syringes) intended for personal use should be abandoned because they present significant public health risks without offsetting public health or public safety gains.
Incarceration of people who have OUD raises the risk of overdose death.306 Reducing incarceration for illicit possession of small amounts of illicit opioids (e.g., defelonization in California) has no evidence of adversely affected public health or safety.313 Some might argue that incarcerating people for illicit opioid possession has an offsetting public health benefit of deterring use by others. There are some deterrent effects of legal sanctions on drug use, but there is no evidence that they are unique to incarceration.313,314 Moving to penalties other than incarceration or to therapeutic diversion programs is very unlikely to increase population opioid use.55,315 It also could benefit the health of people with OUD.316 Although not a health harm per se, trust in the criminal justice system is not advanced when small-time heroin dealers are punished more severely than the Purdue Pharma executives who in 2007 pleaded guilty to knowingly helping trigger the opioid crisis, none of whom – shamefully and shockingly - spent even a day behind bars. The Commission therefore recommends an end to incarceration for illicit possession of opioids or drug use equipment intended for personal use.
Recommendation 4c: “Collateral penalties” for addicted individuals who have committed drug-related crimes should be abandoned because they hamper the ability of individuals to achieve and maintain recovery from addiction.
Collateral penalties do not distinguish individuals who continue to engage in illegal behavior (e.g., using and dealing heroin) after incarceration from those who do not (e.g., someone who enters recovery and leaves involvement in the drug trade behind them). The Commission considers this unjust as well as foolish: punishing people for engaging in desired behavior benefits neither the individual nor society. Further, these laws create barriers for individuals to enter and remain in recovery, for example by making it difficult for them to pursue education, employment, and housing.
Recommendation 4d: State and federal officials in the USA should abandon policies that punish opioid use, opioid use disorder, or opioid agonist therapy during pregnancy.
Pregnancy-focused punishments create barriers to disclosing illicit opioid or other substance use or entering treatment. Penalizing opioid agonist therapy for addiction during pregnancy on the theory that it harms the developing fetus has no medical basis.317 The Commission recommends that states pursuing such policies abandon them and instead focus on establishing priority access pathways to high-quality services in both the pregnancy and post-delivery period.
Domain 5: Creating healthy environments that can yield long-term declines in the incidence of addiction
Addictive drugs provide intense, albeit short-term, rewards, be it an increase in positive experience (e.g., intense pleasure) or a decrease in negative experience (e.g., escape from anxiety or withdrawal). Neuroscience and behavioral economics teach that all such rewards are relative, i.e., their value is judged depending on what other positive rewards are available in the environment, and, how much the environment produces states that are desirable to avoid (e.g., fear, alienation, a sense of worthlessness). Very broadly speaking, one would expect that when a supply of drugs is present, they would be consumed more by individuals with more environmental stressors and fewer alternative rewards, and, that among drug consumers, those in such environments would be more likely to develop addiction. This is not an easy proposition to test in an ecologically valid and ethical fashion in humans. However, experimental research with other primates has supported the concept by showing that the strains of being at the bottom of a social hierarchy increases consumption of available drugs,318 while the rewards of being on the top of hierarchy seems to make addictive drug use relatively less attractive.319
The characteristics of environments which increase risk of addiction vary, but have historically included political and economic upheaval, racial and ethnic persecution, and chronic exposure to violence and disorder. When an addiction crisis lasts for more than a generation, as has the North American opioid crisis, high-risk environments can also include large numbers of children growing up with addicted parents in communities where many other families are in the same situation, contributing to child abuse, neglect, and abandonment. In one of the most influential analyses of the roots of North American opioid epidemic, Anne Case and Angus Deaton document that it originally took hold in regions beset by de-industrialization and sustained loss of living wage employment.320 This account of the origins of “deaths of despair” is sometimes misread and often invoked in public debate to dismiss the influence of any causal factor other than poverty, which is a disservice both to reality and to the nuanced analysis Case and Deaton offer. As Case and Deaton highlight, the explosion of opioid prescribing began increasing opioid overdose deaths in an era of declining national inequality (the 1990s) and the death rate was unmoved by the 2008 financial crisis.
When matters are complex and high-stakes, many people see within murky evidence a validation of their political preferences. A widely-cited socialist account of human health321 argues that the evidence proves clearly that poverty and inequality cause addiction, from which it may seem to follow that reducing inequality would reverse the opioid crisis. These propositions are probably untrue, for five reasons. First, the degree of prescription opioid marketing and supply explains the geographic distribution of overdose deaths better than does the degree of economic privation.322,323 Second, religiously or culturally rooted abstinence from all substance use is more common in lower income groups,324 and abstinent people cannot become addicted. Third, even for the same drug and society, addiction often moves up and down the income scale (e.g., tobacco and cocaine in the USA both went from being drugs of upscale urban sophisticates to stigmatized drugs of the underclass).325,326 Fourth, when poorer nations experience rising wealth, their population’s consumption of addictive substances tends to rise sharply. Fifth, even if we knew that an economic shock has increased the prevalence of addiction, causes do not necessarily become solutions when reversed: knowing that an egg is broken because it fell to the ground does not imply that hurling its fragments skyward will reassemble it. More concretely, someone whose opioid addiction started ten years ago when the local coal mine shut down does not become unaddicted if the mine re-opens.
More generally, as Case and Deaton note “Many of the things people care about are not reducible to money or measurable in monetary terms.”320 Domain 3 of this report discussed how for many individuals, recovery from addiction involves more than a change in substance use, comprising as well strengthened connections to other people, the filling of responsible roles, respect in the eyes of others, and a subjective sense of meaning and purpose in life. Although it could never be tested in a randomized trial, it is reasonable to speculate that the presence of these same factors could lower the likelihood that individuals would become addicted in the first place.
Policymakers should attempt to alleviate poverty and inequality because of the human misery they cause. But they should not put forward the false promise that macroeconomic policy is a powerful or specific lever for reducing the prevalence of addiction. In the USA, drug-related deaths have been rising for at least 40 years through a series of diverse macroeconomic policies and economic situations.245 Nevertheless, policymakers have at their disposal evidence-informed strategies for dramatically improving human environments that have long-term potential to reduce addiction.
The highest priority investments in this domain focus on children and adolescents. Neuroscience, developmental, and epidemiological research all point strongly to youth as the time when incidence of substance use disorders is most concentrated.244 It is also the time of life when the acquisition of skills and capacities is most easily facilitated and when doing so has the largest impact on an individual’s life course.
The availability of specific substances in the environment, and attitudes and beliefs individuals hold about particular drugs, are risk factors that prevention programs should address. Indeed, the Commission’s preceding recommendations on promoting safer opioid prescribing address risks particular to that specific class of drug. But most risk factors for developing drug problems and indeed an enormous range of other problems are generic.327–329 They include chaotic, unrewarding environments, unremitting stress, social exclusion, violence and other trauma, sexual assault, parental abuse and neglect, and individual risk factors such as having difficulty managing emotions, coping with challenges, and exercising behavioral self-control.
Prevention programs can target these generic risk factors rather than focus on any single drug or indeed drugs in general. Examples include the COMBINE project in Australia330 and Communities that Care in the USA.331 both of which show evidence of affecting multiple youth outcomes including use of multiple substances as well as mental health and academic performance. The experience of Iceland (see panel 8) illustrates one unusually energetic effort to improve young people’s environments as an addiction prevention strategy, and also shows the range of settings (beyond the traditional site, i.e., schools) in which preventive efforts can be implemented.
Panel 8: Iceland’s experiment with community-wide prevention.
From 1995 to 2015, the number of tenth graders in Iceland who had ever used alcohol decreased from nearly 80% to under 35%.341 The proportion who had used cannabis or smoked cigarettes dropped as well in this period. These precipitous declines occurred during the implementation of the Icelandic Prevention Model.342 This model supports a national investment in adolescent well-being grounded in sociology and criminology theories that view problem behavior as emerging from environment features rather than individual characteristics.343 The approach is also consistent with neuroscience and behavioral economic conceptualizations of substance use rates in part reflecting the richness of alternative rewards available in the same environment. Main features include laws – strict laws limiting purchase of alcohol and tobacco to young people, restricting advertising of these projects, curfew for 13 to 16-year-olds – strengthening ties between schools and parents, emphasis on quantity of parental time with children, and increased state funding for organized sports, art, and music classes for youth.344
Because other Nordic countries also experienced declines in youth drinking during these years,345 it would be premature to attribute Iceland’s declining youth substance use solely to its prevention model. Iceland’s results are nonetheless worthy of evaluation in other settings. As of 2020, 111 communities in 32 countries have implemented components of the Iceland’s approach.346 As data from these diverse settings become available, results can shed light on which aspects of the model have replicable effects on youth substance use.
Risk and protective factors exist within children, within their environments, and within the interaction between the two. The most effective substance use prevention programs will not just focus on one of these, but embrace all of them. The Good Behavior Game, which has some long-term evidence of reducing substance use, instills emotional self-regulation in children and also changes the classroom environment.265 Restrictions on youth-targeted advertising of addictive drugs (e.g., alcohol, tobacco, cannabis, pharmaceuticals)332 is another example of valuable prevention efforts that keep the environment in mind.
Prevention programming is generally directed at children of school age. Infants, toddlers, and preschoolers obviously do not have drug problems, so it makes no sense to offer them informational or persuasive programming on the risks of drugs. However, programs that aim to strengthen health, well-being, and school readiness of children at this developmental period, as well as to improve the interactions between them and their parents, can have remarkable long-term effects (extending, at least in the case of Head Start, even into the next generation).333 These effects may include lower risk for addiction. Such programs should thus be appreciated and supported in this light, even though that is not their primary purpose.
The Nurse-Family Partnership provides home visits to first-time mothers during pregnancy and infancy.334 Nurses teach positive health behavior and effective parenting skills, and support the health and personal development of the mother. Most recipients are low-income, unmarried teenagers. The most replicated effects of Nurse-Family Partnerships are reduced rates of child abuse/neglect and second teenage pregnancy, and increased child educational attainment.334 These effects were evident in 4 of 5 randomized clinical trials, the exception being a British study in which the control condition participants received home visitation via the National Health Service (which could be taken as a sign of the value of making the program universal rather than a critique of Nurse-Family Partnerships). Some but not all trials find that the infants who were assigned to the program, relative to controls, have lower rates of substance use and related characteristics (e.g., poor impulse control, criminal justice system involvement) in adolescence and adulthood.334
The Perry Preschool Project provided high-quality educational instruction to low-income, 3 to 4-year old African-American children. Children randomized to the program had less than one-third the rate of being arrested for drug-related offenses by age 27 than controls.335,336 Similar findings were present in the Abecedarian Early Childhood Project.337 Both studies have methodological weaknesses, including attrition over the decades from samples that were never large (each enrolled fewer than 125 participants), but their findings at least remain consistent with the hypothesis that generic investments in young children’s well-being can have longer-term protective effects in the substance use domain.
As a final comment on building healthy environments, one should not overlook the obvious fact that the smaller the amount of opioids readily available, the less likely people are to initiate using them. Although many parents worry that their children could be offered opioids by a drug dealer or a friend, opioids are often more accessible in the child’s home environment. More than a sixth of Canadian adults and a third of American adults receive an opioid prescription each year,28,338 and the typical recipient takes only some of the pills dispensed (one study reported that 73% of opioid pills dispensed after surgery are not used by the patient).339 A simple way to create healthier environments that reduce the likelihood of opioid-related problems in youth as well as adults is to drain off the enormous reservoir of billions of excess opioid pills from homes.340
Recommendation 5a: Policymakers in the USA should implement more effective procedures to reduce the supply of excess opioid pills.
In most developed countries, governments require that opioid manufacturers and distributors fund widely available medication disposal programs at convenient locations, such as community pharmacies.59,347 The USA in contrast tightly regulates drop off procedures and does not ask the private sector to absorb the costs its products generate. For the first 15 years of the opioid crisis, this meant that efforts to reduce the prevalence of unused pills were limited to “prescription drug take back days” operated once or twice a year by law enforcement. These efforts are valuable but insufficient as a national policy response because opioids constitute only a tiny proportion of what is returned during such events.348,349 In 2010, the U.S. Congress expanded the number and types of organizations (e.g., pharmacies, hospitals) that can be licensed to collect and destroy unused controlled drugs. But seven years later, only 2.5% of eligible sites operated such take-back programs.350
The Commission recommends that the USA follow the example of countries (including Canada) that operate more effective drug takeback programs by mandating that accepting unused medications be a required activity for hospital-based and community pharmacies. As in other countries, the cost of these programs should be borne by pharmaceutical manufacturers and distributors. As with the early days of glass recycling, a financial incentive may initially be needed for the public to adopt the habit of returning unused medications until the behavior becomes widespread and routinized. Policymakers should consider experimenting with requiring opioid manufacturers to fund a program that would reward pharmacy customers returning the unused portion of controlled-substance prescriptions, for example a discount coupon for in-store purchases.
Recommendation 5b: Substance use prevention efforts should be “horizontal”,60 building healthy environments and strengthening individual capacities that protect against a broad range of difficulties. This includes use of licit and illicit drugs, as well as unhealthy eating, depression and anxiety, social isolation, school failure and dropout, risky sexual behavior, bullying and other antisocial behavior, and suicidality.
Narrowly targeted prevention programs are wasted on children who are not destined to develop the specific problem targeted by the program. For efficiency and impact, the Commission recommends that prevention initiatives be combined rather than implementing, say, a program discouraging smoking, another separate program promoting healthy diets and exercise, and yet another focused on making classrooms more socially supportive.
Moving to a horizontal prevention model will require significant changes in funding, management, and accountability in a field where efforts are often balkanized. For example, “alcohol prevention people” sometimes see themselves as doing something fundamentally different than “bullying prevention people.” However, the benefits to children of making substance use only one of a range of outcomes expected of prevention efforts more than justifies the dissolution of such bureaucratic boundaries and the creation of a horizontal prevention funding streams.
Implementing horizontal prevention programs on a broad scale now will not cause the current opioid epidemic to dissipate. Prevention is a long-term investment that societies should make in youth today for benefits a decade or more down the line when the most acute drug epidemic of the day could concern alcohol, stimulants, opioids, psychedelics, or some other drug that no one has heard of yet. Indeed, the crisis we may avert may not even be in the drug domain; the benefits of the investment in prevention might be less self-harm, depression, obesity, violence, or other adverse outcome that we should rejoice for the next generation to avoid no matter what its specific nature.
Recommendation 5c: Early childhood enrichment programs for low-income families should be expanded as a long-term strategy for reducing addiction, amongst many other benefits.
As mentioned, evaluations of early childhood enrichment programs such as the Nurse-Family Partnership, the Perry Preschool Project, and the Abecedarian Early Childhood Project, suggest long-term developmental benefits in a range of areas. None of these programs has any substance-specific content, nor would their chief benefit necessarily be in that realm. Yet the Commission believes that these types of programs deserve more attention in discussions of long-term preventive strategies for reducing population substance use. Because the incentives in politics are typically to focus on short-term effects, advocacy for these programs is particularly important because their benefits accrue over a long term.
Domain 6: Stimulating innovation in the response to addiction
The range and effectiveness of treatments for many chronic health problems (e.g., depression, asthma, hypertension, sleep apnea, cardiovascular disease) have improved significantly in recent decades. Sadly, this is not true of OUD nor of addiction more generally. The dominant psychological and behavioral treatments used in front-line care of addiction have changed very little in the 21st century. Since the FDA approved methadone maintenance as an OUD therapy in 1972, only two other medications (buprenorphine and naltrexone) have made it to market in the USA and they are also specifically focused on the brain’s opioid system. No approved pharmacotherapies for stimulant use disorder or cannabis use disorder exist. The treatment of addiction is in dire need of innovation. Creating new treatments, while critical, will not solve the well-documented lack of access289,351 to existing OUD treatments.352 Innovating in implementation353 – that is, finding new ways to get effective treatments to people who need them -- is also crucial.
Innovation is also needed in pain management, particularly in effective medications that do not carry risk of addiction. The main effort of industry in this regard are prescription opioids that are “tamper-resistant” (sometimes called “abuse deterrent”, a stigmatizing term the Commission believes should be abandoned). Some have been a complete failure: OxyContin itself was touted as hard to misuse because of its long-acting formulation, but it was easily and widely crushed for injection and insufflation. Subsequent tamper-resistant opioid formulations, including that which replaced OxyContin, have modest benefits in terms of reducing long-term population harm.354 An Australian study found that introduction of tamper-resistant formulations was associated with population-level drops in sales of controlled-release oxycodone but not with population-level changes in overdose indicators or treatment seeking.355 Our Commission’s modelling suggests that tamper-resistant medications modestly reduce mortality in the long term by lowering the rate of misuse initiation, but their impact is negative in the short-term because they drive some pill users to switch to illicit drugs.81,112 Also, no tamper-resistant formulation can entirely prevent opioid misuse because individuals can always simply orally consume more than the recommended dose.356 Tamper-resistant opioids formulations are thus at most a modest innovation with modest impact in an arena where greater strides are needed in safe, effective, pain care.
Data systems intended to monitor opioid use, OUD, and their consequences, are also in dire need of innovation. For example, epidemiological surveillance data relies primarily on self-report surveys of individuals despite their well-demonstrated validity limits.357 Governments lack credible estimates of how many people use heroin and/or fentanyl, how many are addicted to these drugs, how much these drugs are bought and sold for, and how users acquire them.358 In Canada, though current quarterly surveillance reports on overdose mortality are comprehensive, national opioid-related mortality data were not collected prior to 2016.12,21 Epidemiologic data on some problems prevalent among people with OUD, such as alcohol use disorder and suicide, also have validity problems.359,360 Decades into the worst drug epidemic in its history, this situation is scandalous. The National Drug Early Warning System, just renewed in July 2020, and including some novel monitoring methods, is a good step in this direction.361 Wastewater analysis is a widely used technology in Europe that with a few exceptions has been underexploited in North America.362,363 This method provides unique opportunities to rapidly monitor population-wide use patterns without the missing data and privacy concerns inherent in self-report surveys.
Law enforcement strategies for reducing the supply and use of illicit opioids have also evolved little at the national level, despite promising pilots of alternative models.314,364 The field’s lack of innovation has already been tragic enough in terms of opportunity costs, i.e., lives that could have been saved but were not. Lack of innovation has more recently become positively disastrous in the face of the rising availability of synthetic drugs such as fentanyl, which because of their high potency and lack of dependence on agricultural production pose fundamentally different challenges to public health and safety that current policies cannot meet.31
Although the Commission calls for many individual innovations throughout this document, it also connects the dots to observe that lack of innovation is a more general problem for the field. This suggests the need for specific policies that foster an innovation-friendly environment.
Recommendation 6a: Public policymakers should implement pro-innovation policies that correct for failures in patent law and market incentives.
The USA’s innovation climate is set up to reward goods that can be patented (e.g., pharmaceuticals) and incentivizes companies to create and then increase demand for habit-forming patented products. Patent law can also create barriers to access for some OUD treatments, for example by keeping the price of a medication high due to lack of competition. But other innovation policies can be used to reduce harms of medication exclusivity.61 Policymakers should consider eliminating patent-related access barriers by purchasing products from patent owners and distributing them at low cost to patients or by purchasing patent rights and allowing generic production. For example, if the U.S. government bought out Evzio’s patents on the naloxone auto-injector, it could be made publicly available at cost.61
Public policy should also encourage innovation in pursuits unlikely to lead to significant market rewards. Non-pharmacological treatments – whether for addiction or pain – do not fit into the usual system of patents or promised profits. Similarly, most public health interventions cannot be patented. Greater government funding through grants and prizes could help drive innovation in areas where patents are not suitable incentives.61 Public funding could also be more focused on projects that are unlikely to attract private sector investment. For example, government grants could have a section for applications to explain why the project would not otherwise be funded.
Pro-innovation policies work in concert with the health care provision, training, and financing policies that the Commission recommended in Domain 3. As the number of individuals with insurance who seek care for opioid-related conditions increases, the reimbursement for those services and the number of professionals providing them increase as well. This creates demand-side pressure for innovation, thereby making it more likely that supply-side efforts will meet with success.
Recommendation 6b: Government research agencies and private industry should prioritize the development of non-opioid analgesics and medications targeting addiction as well as the redesign of opioids to separate their effects (e.g., analgesia, euphoria, respiratory suppression).
As mentioned, tamper-resistant opioids are not entirely without merit, but may have at most a mild impact on making pain pharmacotherapy safer. A more consequential innovation would be to design or discover medications that do not carry risk of addiction, overdose, and other adverse effects of opioids. One route is to design opioid molecules with “biased agonism”,62 meaning that they relieve pain with less respiratory suppression and less activation of brain reward circuity underlying the acquisition of addiction.365 This approach has produced some promising preclinical findings366, but these have not been replicated or rigorously tested in clinical studies as yet.367 A non-competing alternative approach is to develop non-opioid medications and interventions (e.g., virtual reality and nerve stimulation devices) that have significant ability to relieve pain, to ameliorate addiction, or both.
The rapid development of effective vaccines for COVID-19 shows what is possible when governments make a massive, urgent commitment in the face of an epidemic. The same commitment is needed for the opioid crisis. Expanding National Institutes of Health initiatives such as the Blueprint Neurotherapeutics Network for Small Molecule Drug Discovery and Development for Disorders of the Nervous System and charging them with focusing more work on opioids, could be productive. Private industry could be incentivized to carry out similar work through tax credits for research and development (A prize-based competition makes less sense as the developer of any such molecule would likely reap enormous profits). Privately and publicly funded animal research is more likely to lead to life-saving innovations if guided by translational scientific models.
Recommendation 6c: To promote rapid adoption of treatments for opioid use disorder, regulatory agencies should increase their willingness to approve medications using data from trials conducted abroad rather than re-inventing the wheel.
In developed countries collectively, the range of medications used to treat OUD is broad, including oral methadone, injectable methadone, oral buprenorphine, injectable extended release buprenorphine, implanted buprenorphine, slow release oral morphine, injectable hydromorphone, injectable diacetylmorphine, inhaled/smoked diacetylmorphine, injectable extended release naltrexone, and naltrexone implants. Yet in any given country, only a subset of these medications is approved and available, reducing opportunities to expand the appeal of treatment options to a broader population and to tailor treatment to individual needs.
Regulatory agencies (e.g., the U.S. FDA) often consider international evidence to a limited extent, but still require extensive in-country data collection before drug approval, including new safety and dosage studies for drugs that have been used for many years in other developed countries. Given the exigency of the opioid epidemic, relaxing these requirements legislatively and administratively could bring more medications to patients with OUD more quickly.
Recommendation 6d: Law enforcement agencies should develop and implement innovative strategies to disrupt illicit fentanyl (and other novel synthetic opioids) transactions both physically and financially.
Fentanyl and fentanyl precursors in the USA and Canada are sourced via online transactions with producers in China either directly or via traffickers in Mexico.31 Due to the volume and variety of consumer goods exported from China, universal screening of either packages or financial transactions based on country of origin is unlikely to be productive. Targeting specific actors may be of short-lived utility, as chemical companies that produce fentanyl and its analogues rapidly change company names and tweak opioid molecules to avoid penalties. Up-to-date knowledge of how labs that produce fentanyl, its precursors, and novel psychoactive substances sell their wares is key to enacting any kind of strategy that will not rapidly become obsolete. A major challenge inherent in detecting fentanyl-related financial transactions is that even when such transactions raise flags, they do not obviously differ from other, potentially lower enforcement priority money-laundering activities.368 Furthermore, fentanyl, fentanyl precursors, and novel synthetic opioids are often purchased in small quantities which are easily hidden inside other consumer goods – or in small transactions – that may escape notice.97
Governments should incentivize technical solutions to these detection and interdiction challenges. One area where prizes have been used to drive innovation is fentanyl detection in mail, where the 2019 Opioid Detection Challenge awarded US$1.5 million in prizes across eight teams.369 The winning team developed a 3D computerized tomography scanning system with automated detection algorithms, similar to that used in airport baggage scanning. The runners up developed a quadrupole resonance technology that uses radio-frequency signals to search for specific materials, triggering an alarm when an illicit substance is detected. Larger prizes and efforts to pilot and scale up potential solutions deserve public investment.
Recommendation 6e: The Defense Advanced Research Projects Agency should be tasked with leading “out of the box” demonstration projects focused on the opioid crisis.
The U.S. Defense Advanced Research Projects Agency was founded in response to the Sputnik launch, with the express purpose of leading transformational change within and outside of government, with a focus on national defense. Its achievements are many, most notably having a key role in developing the World Wide Web. The Commission recommends that it be tasked to expand to its focus to the opioid crisis. Alternatively, recently proposed by President Biden, is to create a spinoff of the agency within the National Institutes of Health.370
The range of projects that could be attempted is limitless. The Commission offers a non-exhaustive, illustrative list of ideas (see table 3). These have not been tried, so we do not know how well they would work, but many would be relatively inexpensive and genuinely innovative.
Table 3:
A sampler of possible demonstration projects
| 1. | Deliver substance-focused prevention or treatment services in unconventional settings. A randomized controlled trial that found a hypertension intervention delivered in barbershops increased uptake of blood pressure checks,385 with effects still evident a year later.386 Barbershops may also be a good setting for substance-focused programs in some communities, while others may have better results in bowling alleys, dental offices, chat rooms, gaming clubs, or faith communities. |
| 2. | Interfere with online drug sales using inexpensive tricks developed by hackers. For example, IP “spoofing” (using a false IP address to impersonate a trusted computing system), can be automated and scaled to overwhelm a website in a denial of service attack. A potential way to collapse online drug transaction websites offline would be to create many impersonation IP addresses to access the site until it crashes. |
| 3. | Automate naloxone administration. Opioid users who overdose when alone cannot benefit from naloxone. A possible solution is a wearable device that automatically triggers a naloxone injection based on respiration rate, much as an insulin pump administers medication in the event of acute need. At the users’ option, the device could also be set to contact emergency medical services in the event of overdose. |
| 4. | Mount creative and accurate public messaging campaigns to reduce drug-related risks, e.g., Promoting the role of designated rescuer akin to designated driver; or campaigns informing people who use stimulants or pressed pills that fentanyl is not just an issue with heroin. |
| 5. | Use technology to limit diversion. Smart pill bottles have not been shown to be particularly helpful for improving adherence in HIV treatment,387 but they may be repurposed to reduce medicine-cabinet diversion of prescription opioids. |
| 6. | Monitor places where people who use drugs publicly discuss drugs to learn of emerging risks. Novel drugs like brorphine have shown up on r/opiates on Reddit months before their existence was widely reported. Screen-scraping these and similar fora, including on the dark web, can provide behavioral data to complement toxicology. |
| 7. | Remove technical and legal barriers to providing telehealth care across state and provincial lines and across international borders as well. For example, crisis counseling could be more available during relevant periods – i.e., dawn to dusk – if counselors working in other countries could take these shifts during their daytime hours. |
| 8. | Develop machine learning algorithms that predict response to pain, risk of addiction and overdose in patients for whom opioids are being considered to inform decisions about medication choice, dosing, and co-prescription of naloxone if an opioid is prescribed. |
| 9. | Develop and test assessments of the incidence and prevalence of opioid use, addiction, and overdose that do not involve surveying individuals. These could include a combination of wastewater analysis, scraping of social media and Internet search engine data, medical examiner data243, and natural language processing of journalistic reports and chat room dialogue from around the world. |
Domain 7: Preventing opioid crises beyond North America
The seventh and final domain analyzed by the Commission was the risk of the North American opioid crisis spreading to other nations. Whether out of fear or complacency, many people outside of the USA have convinced themselves that the opioid crisis is something that could only happen the context of the unique political and economic arrangements of the USA. As this report makes clear, this is already untrue: Canada had a comparable explosion of opioid prescribing and now has an epidemic of OUD and overdose.26
Multiple countries outside North America show sharp increases in opioid prescribing (figure 8). The Netherlands per capita opioid consumption nearly doubled over the decade ending 2017371; opioid-related hospital admissions and deaths tripled over the same period. The latest United Nations per capita prescription opioid prescription data showed that Iceland’s consumption increased by 96% in the past 7 years; opioid overdose deaths are also up sharply and now lead the Nordic countries.372 Between 1998 and 2016 in England,373 the per-capita morphine equivalent dosage dispensed increased 127%. In just a six-year period (2009 to 2015), opioid prescriptions in Brazil increased 435%.374 In Australia, between 1992 and 2012, opioid dispensing episodes increased 15-fold;375 prescription opioid-related hospitalizations more than doubled and now outpace those for heroin. In Mexico, opioid dispensing increased an average of 13% per quarter from 2015 through 2019, though the highest overall rate was roughly 150 times smaller than that of the USA at that time.114,376,377 The proportion of the Finnish population receiving opioid prescriptions rose from less than 1% in 1995 to 7% in 2016.378
Figure 8.
Example countries with rising opioid consumption, in standard daily doses per million inhabitants
“We’ve won the war on smoking” is a common expression invoked by public health officials and politicians in developed countries. But this would only be true if exporting morbidity and mortality to the rest of the world while keeping the profits at home could be considered a victory.65,66 There is risk of a similar “victory” being declared regarding prescription opioids.
Investigative journalists have documented that the Sackler family is expanding opioid markets through Mundipharma using the same tactics as they employed in the USA. Some of this expansion has been in developed countries. In an ongoing criminal investigation in Italy for example, two Mundipharma executives have been sentenced for involvement with a leading physician who promoted opioids and allegedly laundered large cash payments from Mundipharma and another opioid manufacturer in exchange.64
More of the expansion efforts are targeted at developing countries. Among the countries where Mundipharma is attempting to promote OxyContin, according to a Los Angeles Times investigation, are Brazil, China, Colombia, Egypt, Mexico, and The Philippines.379 Other investigative journalists have documented that Mundipharma is one of many Western companies promoting opioids in India380,381 and China using tactics pioneered in North America, including some that are now illegal there.382 Quite disturbingly, Furlan and colleagues document that in multiple developing countries, opioid manufacturers have significant financial and personal involvement in the production of guidelines for opioid prescribing.383
None of this is to deny the urgent need for better pain care in developing countries. That the United States and Canada have suffered from a glut of prescription opioids should not blind policymakers to the fact that in many low-income nations, a lack of these drugs causes untold misery. The Lancet Commission on Palliative Care and Pain Relief estimates that 25.5 million people die annually experiencing “serious health-related suffering”, over 80% of whom are in developing countries where adequate palliative care is lacking.376 Developing countries should not be forced to choose between letting their citizens suffer needlessly or giving in to corporate predation.
To increase the likelihood that opioid prescribing policies are geared toward maximizing population health, the Commission urges nations outside North America to consider the recommendations heretofore listed, particularly those designed to reduce regulatory corruption (e.g., those under Domain 1). This should be supplemented by the following recommendations, which could help protect nations outside of North America from experiencing opioid crises of their own.
Recommendation 7a: To avoid repeating the experience with the tobacco industry responding to increased regulation in developed countries by finding new markets overseas, the USA and other developed nations in which opioid manufacturers are based should extend restrictions and legal sanctions on companies and owners to their global operations.
Government entities in the USA have won significant civil cases against U.S. based manufacturers and distributors.4 In addition to securing damage settlements, these cases have curtailed some deceptive practices that helped trigger the epidemic, such as misleading prescribers by overstating the benefits and understating the risks of prescription opioids, making secret payments to key opinion leaders who promote their products, and engaging in false advertising.4
However, these court decisions and legal agreements do not prevent companies or their owners from engaging in the same fraudulent conduct outside the USA. A vivid case in point concerns the activities of the Sackler family, which owns both the USA-based company Purdue Pharma and a sister company, Mundipharma, which is active internationally. Purdue Pharma executives were found criminally and civilly responsible for its destructive and fraudulent tactics promoting OxyContin in the USA in 20074, and the company itself was found criminally and civilly liable in another major case in 2020.384 Purdue Pharma will go out of existence as a result of the most recent case, but this is no barrier to the Sackler family carrying on the same activities international through another company.
Political officials are fundament responsible for the well-being of their own citizens, but still have an ethical imperative is to protect people in other nations as well. They should therefore insist on legal settlements with the opioid industry in which fraudulent and dangerous practices are banned not only for the domestic market but for the international market as well, including through subsidiaries or other companies with the same owners. Otherwise epidemics of prescription opioid use disorder and overdose could become pandemic. This concern has particular urgency given the latest federal and state prosecutions against Purdue Pharma and the Sackler family, in which forcing the family to give up foreign sister companies like Mundipharma (as well as Napp Pharmaceuticals) is being considered. Preventing the family only from continuing fraudulent OxyContin promotion domestically while allowing them to do so overseas through a different company would be a terrible failure of leadership.
Recommendation 7b: To respond to pain relief and palliative care needs in low-income nations as well as to prevent such countries from being exploited by for-profit opioid manufacturers, international agencies should coordinate distribution of free, generic morphine to hospitals and hospices.
Faced with the humanitarian tragedy of untreated pain, lower income countries may be tempted to turn over the regulatory keys to the pharmaceutical manufacturers whose profit-seeking has been destructive in other nations. The international community has a moral responsibility to not force lower income nations to choose between relieving needless suffering and risking an opioid addiction epidemic brought on by multinational corporations.
Accordingly, the World Health Organization, with support of donor organizations, should coordinate delivery of generic morphine to hospitals and hospices in low income countries. This will require the support of the International Narcotics Control Board, which oversees the United Nations conventions on narcotics drugs and licenses and regulates licit opioid production.
Without the influence of the profit motive, this model has more likelihood of relieving suffering without overpromotion and overprescription of opioids. The cost of implementing such a model is far from prohibitive. The cost of providing morphine-equivalent pain treatment to every child experiencing serious health suffering in low-income countries is only US$1 million a year.376
Conclusion
Even in the era of COVID-19, the opioid crisis stands out as one of the most devastating public health disasters of this century for the USA and Canada; indeed, at this writing, the death totals of the two crises are roughly equal. The Commission’s conclusions about the crisis are in one respect simple: unrestrained profit-seeking and multi-level, multi-system regulatory failure instigated the opioid crisis and can produce further epidemics of addiction in the future. Although the present crisis is concentrated in Canada and the USA, similar crises could emerge in any nation. The public health case for the Commission’s proposed reforms of pharmaceutical and medical regulation is thus strong, and the need urgent.
The Commission is unequivocal in its view that addiction is an enduring feature of population health, though in the future the drug that takes center stage may not be opioids. For this reason, provision of addiction-related services must be a permanent feature of health and social care systems, financed and organized as a core commitment.
In other respects, the Commission pleads for attention to nuance in an era characterized by simplistic viewpoints. Opioids are neither good nor bad in any absolute sense. Rather, they are a class of drug that is simultaneously essential to medical practice and fraught with serious risks. Some regions, particularly low-income countries, lack sufficient opioids and should be supplied them through non-profit, public sector initiatives. Other regions, notably North America, have a surfeit of opioids and population health suffers as a result even though individuals within it simultaneously avoid needless pain they would experience in a low-income country. Implementing restrictions on opioid prescriptions can avert cases of addiction but at the potential cost of harm to patients who are in pain and/or are dependent on prescription opioids. Prescribing policy should be sensitive to the diverse and indeed sometimes opposing needs of different subpopulations.
Nuance is also needed regarding the criminal justice system. Law enforcement officials cannot crush the opioid crisis through brute force, and trying to do so would destroy many lives. At the same time, the use of addictive drugs changes people’s behavior, including in ways that lead to victimization of other people who understandably will seek protection from the criminal justice system. Engagement of the criminal justice system regarding drugs is thus inevitable, irrespective of whether drugs are legal or not. The goal should thus be to maximize the benefits and minimize the costs of that engagement, for the individuals concerned, their families, and for the community around them.
Nuanced thinking is also needed regarding poverty, inequality, and addiction. Alleviating poverty is a worthy goal for many reasons, but no simple promises should be made that reduced addiction will necessarily be the result. Human well-being is not a simple function of economics but it can be augmented by programs and policies that increase access to safe and rewarding environments. Cultivating health-promoting environments is a structural strategy that can translate into reduced addiction in future years, as well as the prevention of other individual and social ills.
None of what the Commission has proposed is easy, though it will be easier to achieve if we nurture a culture of innovation and resolve long-standing structural gaps in our knowledge about the epidemic and about drugs more generally. Even perfect attainment of all the recommendations here will not eliminate the opioid crisis: tragically, many future deaths are inevitable at this point.81,112 Nevertheless, significant gains in quality of life and reductions in loss of life are clearly attainable, given the resources and political will to pursue the bold policies set out here.
It took more than a generation of mistakes to create the North American opioid crisis. It may take a generation of wiser policies to resolve it. The gains of such polices will be long lasting if they curtail the power of health care systems to cause addiction and maximize their ability to treat it.
Figure 6.

Examples of cannabis industry marketing claims related to opioid use disorder and overdose
Key Messages.
The profit motives of actors within and without of the health care system will repeatedly generate harmful over-provision of addictive pharmaceuticals unless regulatory systems are fundamentally reformed.
Opioids have a dual nature as a benefit and a risk to health, function, and well-being; this dual nature needs to be taken into account in drug regulation, prescribing, and opioid stewardship.
Integrated, evidence-based, enduring systems for the care of substance use disorders should be built and supported financially on a permanent basis.
Policies are available that maximize the benefit and minimize the adverse effects of criminal justice system involvement with people who are addicted to opioids.
Fostering healthier environments (e.g., through programs for safe disposal of opioid pills, substance use prevention, and childhood enrichment) may yield long-term declines in the incidence of addiction.
Innovation – in biomedical research on pain relievers and medications for opioid use disorder treatment, supply control strategy, and delivery of substance use disorder treatment – is urgently needed in response to the opioid crisis.
Developed nations have a responsibility to prevent their opioid manufacturers from fomenting opioid overprescribing in other countries, and all nations should consider how to strengthen regulatory systems to prevent domestically-driven opioid crises.
Acknowledgements
The work of the Commission was funded entirely by Stanford University School of Medicine. The views expressed in this paper are those of the authors and do not necessarily reflect the official position of Stanford University School of Medicine or of any governmental or non-governmental entity for which they work or advise.
Declaration of Interests
The Commissioners declared the following interests over the past three years:
Keith Humphreys has been supported by the Esther Ting Memorial Professorship at Stanford University School of Medicine and research grants from the Veterans Administration Health Services Research and Development Service (#RCS 04-141-3, #HX-12-001, and #HX002714-01A2), the National Institute on Drug Abuse (#3UG1 DA015815-17S4 and #2UG1DA015815-19), the Food and Drug Administration, Wu Tsai Neurosciences Institute, The Silicon Valley Community Foundation, The County of Santa Clara California, and the American Board of Family Medicine. He has received speaking honoraria and travel expenses from the American College of Medical Toxicology, Arizona State University, Barclay’s Bank, Caron Foundation, The University of Florida, The New York Museum of Modern Art, Syracuse University, the State of Washington Physician Health Program, and The West Virginia Medical Professionals Health Program. He has received writing honorarium or royalties from the Association of Psychological Science, American Academy of Political and Social Science, Brookings Institution, Cambridge University Press, and Washington Monthly Magazine. He has been a paid consultant to AELIS Pharma Inc., The Arnold Foundation, Harvard Medical School, Harvard University Press, IQVIA, RAND Corporation, and the State of Pennsylvania Department of Health.
Chelsea L. Shover has been employed by Stanford University; the University of California, Los Angeles (UCLA); the Los Angeles County Department of Public Health; Heluna Health; and the Los Angeles LGBT Center. Additionally, she has received research funding or stipend from the National Institute on Drug Abuse (K01DA050771; T32DA035165), RAND OPTIC (Opioid Policy Tools and Information Center), Wu Tsai Neurosciences Institute, UCLA David Geffen School of Medicine. She has received speaking honoraria or travel expenses from: Emory University; New York University; UCLA; University of North Carolina at Chapel Hill; University of Southern California; University of Pittsburgh; University of Chicago; The University of Western Ontario; Nevada State Medical Association; RAND; University of California, Irvine; College on Problems of Drug Dependence; Conference on Retroviruses and Opportunistic Infections; HIV Research for Prevention; Addiction Health Services Research; American Psychopathological Association.
Christina M. Andrews has been supported by the Arnold School of Public Health and the College of Social Work at the University of South Carolina, and through research grants from the National Institute on Drug Abuse (R01DA034634, K01DA041628, U2CDA050097, and R01DA049776) and the South Carolina Department of Alcohol and Other Drug Abuse Services. She has been a paid consultant to the Robert Wood Johnson Foundation, RTI International, the Medical University of South Carolina, and the State of Pennsylvania Department of Public Health.
Amy S.B. Bohnert has been supported by grants from the National Institutes for Health (R01 DA045705), the Veterans Health Administration (IIR 13-322), the Centers for Disease Control and Prevention (U01CE002780), Blue Cross Blue Shield of Michigan, the Department of Defense, Patient-Centered Outcomes Research Institute, the Michigan Health Endowment Fund, and the Substance Abuse and Mental Health Services Administration via sub-contracts from the Michigan Department of Health and Human Services. She has received speaker honoraria or travel expenses from the Illinois Health and Hospital Association, the International Summit on Suicide Research, the Washington State Department of Labor & Industries, the American Psychopathological Association, and the High Intensity Drug Trafficking Area program. She has been paid as a consultant by New York University. She has received products from Fitbit at a reduced cost and Headpace Inc. for free for research.
Margaret L. Brandeau has been supported by salary from Stanford University as well as a research grant from the Department of Veterans Affairs, a research grant from the National Institute on Drug Abuse (R37-DA15612), and a Koret Foundation gift for Smart Cities and Digital Living. She has received travel expenses from the University of Maryland, the University of Auckland (New Zealand), the Massachusetts Institute of Technology, the European Working Group on Stochastic Modeling, INSEAD (Fontainebleau, France), the University of Michigan, and the University of Oklahoma. She has been a paid consultant to Compass Lexecon.
Jonathan P. Caulkins has been primarily supported by Carnegie Mellon University as the H. Guyford Stever University Professor of Operations Research and Public Policy at the Heinz College of Information Systems and Public Policy, including a National Science Foundation EAGER Grant on Detecting and Disrupting Illicit Supply Networks via Traffic Distribution Systems, and is adjunct staff for the RAND Corporation’s Drug Policy Research Center. He has consulted with or received honoraria from the Actis – Norwegian Policy Network on Alcohol and Drugs, Arnold Foundation, Bank of Montreal, Boston University, Foreign Affairs, Justice Research and Statistics Association, Lisbon Addictions Conference, MIT, National Affairs, National Institute of Justice, Oxford University Press, Pew, PIRE, Russell Sage Foundation, Springer Verlag, Stanford University, State Department, Texas Research Society on Alcoholism, Veteran’s Administration, Washington Monthly, and the WT Grant Foundation.
Jonathan H. Chen is employed by Stanford University. His recent research support includes: NIH/National Library of Medicine via Award R56LM013365; Gordon and Betty Moore Foundation through Grant GBMF8040; National Science Foundation SPO181514; Google, Inc SPO13604; Stanford Clinical Excellence Research Center (CERC); Stanford Departments of Medicine and Pathology. He is the co-founder of Reaction Explorer LLC that develops and licenses organic chemistry education software. He has been paid consulting or speaker fees from National Institute of Drug Abuse Clinical Trials Network, Tuolc Inc., Roche Inc., and Younker Hyde MacFarlane PLLC.
Tom Coderre has been employed by the Rhode Island Governor’s Office, (01/2018-11/2019); Substance Abuse and Mental Health Services Administration (11/2019-Present). He received honoraria for the Gaudenzia Professional Development Seminar “Trends in Drugs and the Opioid Crisis,” Inn at Villanova University, Radnor, PA
Mariano-Florentino Cuéllar has served as a Justice of the Supreme Court of California, the Herman Phleger Professor at Stanford Law School, Distinguished Visiting Jurist at NYU School of Law, the Castle Distinguished Lecturer in Ethics, Politics, and Economics at Yale University, a member of the President & Fellows of Harvard College (the Harvard Corporation), a member of the Board of Directors of the William and Flora Hewlett Foundation, a member of the Council of the American Law Institute, Chair of the Board of Directors of the Center for Advanced Study in the Behavioral Sciences, Chair of the Advisory Board of the AI Now Institute at NYU, Chair of the Advisory Board of the Seed Initiative at the Stanford Graduate School of Business, and member of the Advisory Board at the Stanford Human-Centered Artificial Intelligence Institute. His work has been supported by Stanford Law School and by a grant from the Stanford Human-Centered Artificial Intelligence Institute.
Yasmin Hurd has been supported by research grants from the National Institute on Drug Abuse (NIDA; DA050323, DA048613, DA008227, DA043247, DA030359, DA037317, and DA15446) as well as research funding from GW Pharmaceuticals. She has received speaking honoraria and travel expenses from the University of North Carolina and the American Society for the Advancement of Science (AAAS), as well as the Society for Neuroscience Public Education & Communication Committee in Washington, DC. Dr. Hurd has also received honoraria and travel expenses from Washington University in St. Louis, Temple University, Tufts School of Medicine, Indiana University, and the American College of Neuropsychopharmacology (ACNP). She received honoraria and travel expenses from the Iowa Neuroscience Institute, the Franklin Institute, SUNY Upstate Medical University, the Canadian Consortium for the Investigation of Cannabinoids, the University of Michigan, Cold Spring Harbor Laboratory, the National Institutes of Health, and Penn State Hershey College of Medicine. Dr. Hurd received travel expenses from the National Academy of Medicine (NAM), and the Mediterranean Neuroscience Society (Marrakech, Morocco). She also received partial travel funds for the GRC Plenary Lecture (Barcelona, Spain), the Mini meeting on Developmental Cannabis (Palermo, Italy), the Symposium on Addiction Neuroscience (Minnesota), the SOBP Psychiatry Conference Keynote Lecture (New York, NY), the Cannabiz Expo (Canada), the CINP Conference (Vienna, Austria), the FENS Forum of Neuro (Berlin, Germany), the CSR Advisory Meeting (Washington, DC), and the Society for Neuroscience Annual Meeting (San Diego, CA).
David N. Juurlink has received research grants from the Canadian Institutes for Health Research and the Ontario Ministry of Health. He has received financial support from the Departments of Medicine at both the University of Toronto and Sunnybrook Health Sciences Centre. He has received travel expenses and/or speaking honoraria from Dalhousie University, The University of Ottawa, the University of Saskatchewan, The University of Calgary, The Bloomberg Johns Hopkins School of Public Health, the American College of Physicians, the University of Alabama at Birmingham, the American Society of Nephrology, the Canadian Society of Internal Medicine, the Canadian Anesthesiologists’ Society, The Canadian Society of Obstetricians and Gynecologists, the Western Canada Addiction Forum, and the Canadian House of Commons Standing Committee on Health. He has received payment for expert witness testimony and has been retained by a generic drug manufacturer and distributor to provide advice related to an ongoing Canadian class action. He is a volunteer member of Physicians for Responsible Opioid Prescribing.
Howard K. Koh is a professor at Harvard University. He has been supported by grants from the Robert Wood Johnson Foundation (Awards 77667; 74275; 73359); John Templeton Foundation (Award 52125); JPB Foundation (Awards 1085; 439); Association of State and Territorial Health Officers (Award 1584). He has received honoraria from Jefferson University and Jefferson Health; Perelman School of Medicine, University of Pennsylvania; MaineHealth Center for Tobacco Independence; Harvard University Memorial Church; Association of State and Territorial Health Officials; University of Wisconsin Medical School; Wake Forest Baptist Health in Partnership with Shaw University; Robert Wood Johnson Foundation Advisory Committee; American College of Gastroenterology; Tufts University School of Medicine. He has been a strategic consultant to the Commonwealth Fund. Dr. Koh is a member of the following boards: Community COVID Coalition Advisory Group; Phillips Academy Public Health Expert Advisory Panel; Honorary Advisory Council Project 22; Policy Advisory Group; Bipartisan Policy Center; Palliative and Advanced Illness Research Center External Advisory Board (Perelman School of Medicine, University of Pennsylvania); American Cancer Society Eastern New England Area Council of Advisors; American University of Beirut International Advisory Council; National Advisory Board; Culture of Health Year in Review Advisory Committee and Culture of Health as a Business Imperative Initiative of the Robert Wood Johnson Foundation; editorial board of Journal of American Medical Association; New England Donor Services board of trustees; Josiah Macy Jr. Foundation board of directors; The Lancet - O’Neill Institute, Georgetown University Commission on Global Health and the Law.
Erin E. Krebs has been supported by research funding from United States Department of Veterans Affairs Health Services Research and Development (COR 19-489, 1I01HX003063-01A1, 5I01HX001752-05, 5I01HX002737-02, 5I01HX001288-05, 5I01HX000911-06), the Patient Centered Outcomes Research Institute (OPD-1511-33052), the National Center for Complementary and Integrative Health (5R01AT008387-04, 5UH3AT009761-04/5UG3AT009761-02, 4UH3AT009765-03/5UG3AT009765-02), and the National Institute of Diabetes and Digestive and Kidney Diseases (1U01DK123816-01). She received travel expense reimbursement from the law firm representing the State of Oklahoma to serve as an expert witness in support of the State’s litigation against opioid manufacturers. She also received travel expenses paid by the following non-profit and governmental entities: American Society of Health-System Pharmacists, Association of Academic Physiatrists, Australian Pain Society, Cleveland VA Medical Research and Education Foundation, Duke-Margolis Center for Health Policy, Foundation for Medical Excellence, Foundation for Opioid Response Efforts, Friends of VA Medical Care and Health Research, Hennepin Healthcare Research Institute, Indiana Institute for Medical Research, National Academies of Medicine, National Center for Complementary and Integrative Health, National Governors Association, North American Spine Society, Patient Centered Outcomes Research Institute, Stanford University, US Food and Drug Administration, Washington State Department of Labor & Industries.
Anna Lembke is employed as a professor at Stanford University. She has received consulting fees for her work as a medical expert witness in federal and state litigation against opioid manufacturers, distributors, and pharmacies. She has received royalties for her book “Drug Dealer, MD” from Johns Hopkins University Press. She has received speaking honoraria and/or travel expenses from the Vanderbilt University School of Medicine, the Ohio State University School of Medicine, the Alpha Omega Alpha Visiting Professorship at the University of Kansas School of Medicine, the Oregon Pain Guidance, the Indiana Prosecuting Attorneys Council (IPAC), the Perrin’s Opioid Litigation Conference, the Public Funds Forum, the Baton Rouge Health District, the Montrose Colorado Annual CME Conference, the PerformRX Pharmacy Benefits Manager Annual Conference, the American Academy of Psychiatry and the Law (AAPL), the Psych Congress, the 69th Annual Canadian Refresher Course for Family Physicians, the Ohio State University Inter-Professional Summit, the University of Texas, the Geminus Community Partners Annual Conference of Indiana, the National Council on Alcoholism and Drug Abuse (NCADA), the Stanford Sierra Camp Womens’ Alumni Wellness Retreat, the American Psychiatric Association, the Association for Medical Education and Research in Substance Abuse, and the Southwestern Gynecologic Assembly.
Sean Mackey has been supported by the Redlich Professorship and the Rosekrans Pain Research Endowment Fund at Stanford University School of Medicine and research grants awarded to Stanford University from the National Institutes of Health (R61NS118651, R03HD094577, R01DA045027, R01NS109450, R01AT008561, K24DA02926207, R01DA035484, and P01AT00665105), Patient Centered Outcomes Research Institute (PCORI OPD-1610-370707), and the Stanford Wu Tsai Neurosciences Institute. He has received speaking honoraria or travel expenses from Walter Reed, Harvard University, American Academy of Pain Medicine (travel), Washington University, FDA, NIH, University of Washington, George Washington University, New York University, Weill Cornell Medical College, Duke University, University of Utah, and World Institute of Pain (travel). He has received paid consulting or testimony (none related to opioids) from American Society of Anesthesiology; Favros Law; Fain Anderson VanDerhoef Rosendahl O’Halloran Spillane, PLLC; Cox, Wootton, Lerner, Griffin & Hansen LLP; Lewis Brisbois Bisgaard & Smith LLP; Muro & Lampe, Inc; and McCormick Barstow, LLP.
Lisa Larrimore Ouellette is employed as a professor at Stanford University. She has received travel expenses or honoraria from Cardozo Law School, Claremont McKenna College, Duke University, ETH Zürich, Georgetown University, Harvard University, the Los Angeles Intellectual Property Law Association, Michigan State University, New York University, Northwestern University, the University of Chicago, the University of Houston, the University of Kansas, the University of Nebraska, the University of San Diego, the University of Texas, the University of Villanova, and Yale University. She received a writing honoraria from the Brookings Institution to write a policy proposal for the Hamilton Project. She is a paid consultant to The MITRE Corporation to assist with evaluations of the U.S. Patent and Trademark Office requested by the Department of Commerce.
Brian Suffoletto is employed as a professor at Stanford University. He has received research support from the National Institute on Alcohol Abuse and Alcoholism (R01 AA023650; K23 AA023284), the National Institute of Drug Abuse (R21 DA043181), the National Institute of Mental Health (P50MH115838), and the National Highway Transportation Safety Authority. He has received royalties from a software license to healthStratica Inc. Dr. Suffoletto has several invention disclosures with the University of Pittsburgh for digital behavioral interventions.
Christine Timko has been supported by the Department of Veterans Affairs, and her research has been supported by the Department of Veterans Affairs (VA HSR&D IIR 15-298, IIR 18-253, and PPO 16-337) and the National Institutes of Health (NIAAA 1R01AA024136-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Academies of Sciences E, and Medicine,. Advancing Therapeutic Development for Pain and Opioid Use Disorders Through Public-Private Partnerships: Proceedings of a Workshop. Washington, DC: National Academies Press (US); 2018. [PubMed] [Google Scholar]
- 2.National Academies of Sciences E, Medicine, Health, Medicine. Pain Management and the Opioid Epidemic: Balancing Societal and Individual Benefits and Risks of Prescription Opioid Use. Washington (DC): National Academies Press (US), 2017. [PubMed] [Google Scholar]
- 3.National Academies of Sciences Engineering and Medicine. Medications for Opioid Use Disorder Save Lives, 2019. [PubMed] [Google Scholar]
- 4.Meier B Pain Killer: An Empire of Deceit and the Origins of America’s Opioid Epidemic. New York, NY: Random House; 2018. [Google Scholar]
- 5.Keefe PR. Empire of Pain: The Secret History of the Sackler Dynasty Hardcover: Doubleday: New York, NY; 2021. [Google Scholar]
- 6.Gomes T, Mamdani MM, Paterson JM, Dhalla IA, Juurlink DN. Trends in high-dose opioid prescribing in Canada. Can Fam Physician 2014; 60(9): 826–32. [PMC free article] [PubMed] [Google Scholar]
- 7.Dhalla IA, Mamdani MM, Sivilotti ML, Kopp A, Qureshi O, Juurlink DN. Prescribing of opioid analgesics and related mortality before and after the introduction of long-acting oxycodone. CMAJ 2009; 181(12): 891–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delgado MK, Huang Y, Meisel Z, et al. National Variation in Opioid Prescribing and Risk of Prolonged Use for Opioid-Naive Patients Treated in the Emergency Department for Ankle Sprains. Ann Emerg Med 2018; 72(4): 389–400.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeVries A, Koch T, Wall E, Getchius T, Chi W, Rosenberg A. Opioid Use Among Adolescent Patients Treated for Headache. Journal of Adolescent Health 2014; 55(1): 128–33. [DOI] [PubMed] [Google Scholar]
- 10.Suda KJ, Zhou J, Rowan SA, et al. Overprescribing of Opioids to Adults by Dentists in the U.S., 2011–2015. American Journal of Preventive Medicine 2020; 58(4): 473–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paulozzi LJ, Jones CM, Mack KA, Rudd RA. Vital signs: overdoses of prescription opioid pain relievers---United States, 1999–2008. MMWR Morb Mortal Wkly Rep 2011; 60(43): 1487–92. [PubMed] [Google Scholar]
- 12.Gladstone E, Smolina K, Morgan SG, Fernandes KA, Martins D, Gomes T. Sensitivity and specificity of administrative mortality data for identifying prescription opioid-related deaths. CMAJ 2016; 188(4): E67–E72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo F, Li M, Florence C. State-Level Economic Costs of Opioid Use Disorder and Fatal Opioid Overdose - United States, 2017. MMWR Morb Mortal Wkly Rep 2021; 70(15): 541–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Multiple Cause of Death. In: Statistics NCfH, editor.; 2021. [Google Scholar]
- 15.Humphreys K How legal drug companies helped revive the heroin trade. The Washington Post, June 15, 2018, 2018. https://www.washingtonpost.com/news/wonk/wp/2018/06/15/how-legal-drug-companies-helped-revive-the-heroin-trade/ (accessed. [Google Scholar]
- 16.Rudd RA, Paulozzi LJ, Bauer MJ, et al. Increases in heroin overdose deaths - 28 States, 2010 to 2012. MMWR Morb Mortal Wkly Rep 2014; 63(39): 849–54. [PMC free article] [PubMed] [Google Scholar]
- 17.Mattson CL, Tanz LJ, Quinn K, Kariisa M, Patel P, Davis NL. Trends and Geographic Patterns in Drug and Synthetic Opioid Overdose Deaths - United States, 2013–2019. MMWR Morb Mortal Wkly Rep 2021; 70(6): 202–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gladden RM, Martinez P, Seth P. Fentanyl Law Enforcement Submissions and Increases in Synthetic Opioid-Involved Overdose Deaths - 27 States, 2013–2014. MMWR Morb Mortal Wkly Rep 2016; 65(33): 837–43. [DOI] [PubMed] [Google Scholar]
- 19.Shover CL, Falasinnu TO, Dwyer CL, et al. Steep increases in fentanyl-related mortality west of the Mississippi River: Recent evidence from county and state surveillance. Drug Alcohol Depend 2020; 216: 108314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Medical Association. Issue brief: Drug overdose epidemic worsened during COVID pandemic, 2021.
- 21.Special Advisory Committee on the Epidemic of Opioid Overdoses. Opioids and Stimulant-related Harms in Canada. Ottawa: Public Health Agency of Canada, 2021. [Google Scholar]
- 22.Centers for Disease Control and Prevention. HIV surveillance--United States, 1981–2008. MMWR Morb Mortal Wkly Rep 2011; 60(21): 689–93. [PubMed] [Google Scholar]
- 23.U.S. Census Bureau. U.S. and World Population Clock. 2021. https://www.census.gov/popclock/world/ca.
- 24.Jonah L, Bourgeois AC, Edmunds M, Awan A, Varsaneux O, Siu W. AIDS in Canada-Surveillance Report, 2016. Can Commun Dis Rep 2017; 43(12): 257–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alberta Health. Opioids and Substances of Misuse among First Nations People in Alberta. 2017. https://open.alberta.ca/dataset/cb00bdd1-5d55-485a-9953-724832f373c3/resource/31c4f309-26d4-46cf-b8b2-3a990510077c/download/Opioids-Substances-Misuse-Report-FirstNations-2017.pdf (accessed December 4, 2020).
- 26.Belzak L, Halverson J. The opioid crisis in Canada: a national perspective. Health Promot Chronic Dis Prev Can 2018; 38(6): 224–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGranahan D, Parker T. The Opioid Epidemic: A Geography in Two Phases: United States Department of Agriculture, 2021. [Google Scholar]
- 28.Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM. Prescription Opioid Use, Misuse, and Use Disorders in U.S. Adults: 2015 National Survey on Drug Use and Health. Annals of internal medicine 2017; 167(5): 293–301. [DOI] [PubMed] [Google Scholar]
- 29.Alexander MJ, Kiang MV, Barbieri M. Trends in Black and White Opioid Mortality in the United States, 1979–2015. Epidemiology 2018; 29(5): 707–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hedegaard H, Spencer MR. Urban–Rural Differences in Drug Overdose Death Rates, 1999–2019: Centers for Disease Control and Prevention, 2021. [PubMed] [Google Scholar]
- 31.Pardo B, Taylor J, Caulkins JP, Kilmer B, Reuter P, Stein BD. The Future of Fentanyl and Other Synthetic Opioids. Santa Monica, CA: RAND Corporation; 2019. [Google Scholar]
- 32.Centers for Disease Control and Prevention. Vital Statistics Rapid Release: Provisional Drug Overdose Death Counts. June 9, 2021 ed; 2021.
- 33.Baggett TP, Hwang SW, O’Connell JJ, et al. Mortality among homeless adults in Boston: shifts in causes of death over a 15-year period. JAMA Intern Med 2013; 173(3): 189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamamoto A, Needleman J, Gelberg L, Kominski G, Shoptaw S, Tsugawa Y. Association between homelessness and opioid overdose and opioid-related hospital admissions/emergency department visits. Soc Sci Med 2019; 242: 112585-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martins SS, Sampson L, Cerdá M, Galea S. Worldwide Prevalence and Trends in Unintentional Drug Overdose: A Systematic Review of the Literature. American journal of public health 2015; 105(11): e29–e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Merrall ELC, Kariminia A, Binswanger IA, et al. Meta-analysis of drug-related deaths soon after release from prison. Addiction (Abingdon, England) 2010; 105(9): 1545–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Donnell J, Gladden RM, Mattson CL, Hunter CT, Davis NL. Vital Signs: Characteristics of Drug Overdose Deaths Involving Opioids and Stimulants - 24 States and the District of Columbia, January-June 2019. MMWR Morb Mortal Wkly Rep 2020; 69(35): 1189–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gladden RM, O’Donnell J, Mattson CL, Seth P. Changes in Opioid-Involved Overdose Deaths by Opioid Type and Presence of Benzodiazepines, Cocaine, and Methamphetamine - 25 States, July-December 2017 to January-June 2018. MMWR Morb Mortal Wkly Rep 2019; 68(34): 737–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yurko Salvesen, Remz PC. Report and Recommendations Concerning the Relationship of the Sackler Family and Purdue Pharma with Tufts University, 2019.
- 40.Lembke A Drug Dealer, MD: How Doctors Were Duped, Patients Got Hooked, and Why It’s So Hard to Stop. Baltimore, MD: Johns Hopkins University Press; 2016. [Google Scholar]
- 41.Fain KM, Alexander GC. Mind the gap: understanding the effects of pharmaceutical direct-to-consumer advertising. Med Care 2014; 52(4): 291–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crowe K Following the money between patient groups and Big Pharma. CBC News, March 21, 2018. https://www.cbc.ca/news/health/second-opinion-patient-advocacy-pharmaceutical-industry-funding-drug-prices-1.4539271 (accessed December 12, 2020). [Google Scholar]
- 43.Fontanarosa PB, Rennie D, DeAngelis CD. Postmarketing Surveillance—Lack of Vigilance, Lack of Trust. JAMA 2004; 292(21): 2647–50. [DOI] [PubMed] [Google Scholar]
- 44.Rennie D When Evidence Isn’t: Trials, Drug Companies and the FDA. Journal of law and policy 2008; 15: 2. [Google Scholar]
- 45.Humphreys K, Caulkins JP, Felbab-Brown V. What the US and Canada can learn from other countries to combat the opioid crisis. Brookings Institute; 2020. [Google Scholar]
- 46.Interagency Pain Research Coordinating Committee. National Pain Strategy Overview. June 11, 2021. 2021. https://www.iprcc.nih.gov/national-pain-strategy-overview (accessed June 12 2021).
- 47.Institute for Safe Medication Practices Canada. Opioid Stewardship. 2021. https://www.ismp-canada.org/opioid_stewardship/ (accessed July 21 2021).
- 48.Furlan AD, MacDougall P, Pellerin D, et al. Overview of four prescription monitoring/review programs in Canada. Pain Res Manag 2014; 19(2): 102–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pardo B Do more robust prescription drug monitoring programs reduce prescription opioid overdose? Addiction 2017; 112(10): 1773–83. [DOI] [PubMed] [Google Scholar]
- 50.Buchmueller TC, Carey C. The Effect of Prescription Drug Monitoring Programs on Opioid Utilization in Medicare. American Economic Journal: Economic Policy 2018; 10(1): 77–112. [Google Scholar]
- 51.Chiu AS, Jean RA, Hoag JR, Freedman-Weiss M, Healy JM, Pei KY. Association of Lowering Default Pill Counts in Electronic Medical Record Systems With Postoperative Opioid Prescribing. JAMA Surg 2018; 153(11): 1012–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meara E, Frank RG. Spending on substance abuse treatment: how much is enough? Addiction (Abingdon, England) 2005; 100(9): 1240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Humphreys K, McGovern M, McLellan AT. Integrated care for substance use disorder. In: Miller SC, Fiellin DA, Rosenthal RN, eds. The ASAM Principles of Addiction Medicine. Washington, D.C.: American Society of Addiction Medicine; 2018. [Google Scholar]
- 54.Polydorou S, Gunderson EW, Levin FR. Training physicians to treat substance use disorders. Curr Psychiatry Rep 2008; 10(5): 399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koh HK, Kerlikowske RG, Botticelli MP. A Smarter War on Drugs. JAMA 2018; 320(22): 2301–2. [DOI] [PubMed] [Google Scholar]
- 56.Green TC, Clarke J, Brinkley-Rubinstein L, et al. Postincarceration Fatal Overdoses After Implementing Medications for Addiction Treatment in a Statewide Correctional System. JAMA Psychiatry 2018; 75(4): 405–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.American Bar Association Criminal Justice Section. Collateral Consequences of Criminal Convictions Judicial Bench Book, 2018.
- 58.Haffajee RL, Faherty LJ, Zivin K. Pregnant Women with Substance Use Disorders — The Harm Associated with Punitive Approaches. New England Journal of Medicine 2021; 384(25): 2364–7. [DOI] [PubMed] [Google Scholar]
- 59.Planet Ark Recycling Near You. Unwanted and out-of-date Medicines. 2020. https://recyclingnearyou.com.au/medicines/ (accessed December 13, 2020).
- 60.Stevens MP. Horizontal versus vertical infection prevention strategies. International Journal of Infectious Diseases 2014; 21: 37. [DOI] [PubMed] [Google Scholar]
- 61.Hemel DJ, Ouellette LL. Innovation institutions and the opioid crisis†. Journal of Law and the Biosciences 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stanczyk MA, Kandasamy R. Biased agonism: the quest for the analgesic holy grail. PAIN Reports 2018; 3(3): e650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Humphreys K Avoiding globalisation of the prescription opioid epidemic. The Lancet 2017; 390(10093): 437–9. [DOI] [PubMed] [Google Scholar]
- 64.Galofaro C, D’Emilio F. AP: Purdue foreign arm caught up in opioid probe in Europe. Associated Press, May 29, 2019. https://apnews.com/article/d384c975e039474e8b93e11b9ace18e0 (accessed December 12, 2020). [Google Scholar]
- 65.Action on Smoking and Health. ASH Fact sheet: Tobacco and the Developing World, 2019.
- 66.Brandt AM. FDA Regulation of Tobacco — Pitfalls and Possibilities. New England Journal of Medicine 2008; 359(5): 445–8. [DOI] [PubMed] [Google Scholar]
- 67.Humphreys K, Caulkins JP, Felbab-Brown V. Opioids of the Masses. Foreign Affairs 2018; 97(3): 118–29. [Google Scholar]
- 68.American Medical Association. Issue brief: Reports of increases in opioid- and other drug-related overdose and other concerns during COVID pandemic. 2020. https://www.ama-assn.org/system/files/2020-12/issue-brief-increases-in-opioid-related-overdose.pdf (accessed December 12, 2020).
- 69.U.S. Food and Drug Administration. Timeline of Selected FDA Activities & Significant Events Addressing Opioid Misuse & Abuse. Not dated. https://www.fda.gov/media/85029/download (accessed December 12, 2020). [Google Scholar]
- 70.Cuéllar M-F, Humphreys KN. The Political Economy of the Opioid Epidemic. Yale Law & Policy Review; 2020; 38. [Google Scholar]
- 71.Gossett TD, Finney FT, Hu HM, et al. New Persistent Opioid Use and Associated Risk Factors Following Treatment of Ankle Fractures. Foot Ankle Int 2019; 40(9): 1043–51. [DOI] [PubMed] [Google Scholar]
- 72.Kaafarani HMA, Han K, El Moheb M, et al. Opioids After Surgery in the United States Versus the Rest of the World: The International Patterns of Opioid Prescribing (iPOP) Multicenter Study. Ann Surg 2020; 272(6): 870–86. [DOI] [PubMed] [Google Scholar]
- 73.Humphreys K Avoiding globalisation of the prescription opioid epidemic. Lancet 2017; 390(10093): 437–9. [DOI] [PubMed] [Google Scholar]
- 74.International Narcotics Control Board. Narcotic Drugs 2013. 2013. https://www.incb.org/documents/Narcotic-Drugs/Technical-Publications/2013/Narcotic_Drugs_Report_2013.pdf (accessed December 14, 2020).
- 75.Watson ST. Name of convicted donor coming off UB Pharmacy School building. Buffalo News, June 3, 2019. https://buffalonews.com/news/local/name-of-convicted-donor-coming-off-ub-pharmacy-school-building/article_09388600-0b5a-51fe-9819-04a0b295ac87.html (accessed November 10, 2020). [Google Scholar]
- 76.Balko R The War Over Prescription Painkillers. HuffPost, 2012. https://www.huffpost.com/entry/prescription-painkillers_b_1240722 (accessed. [Google Scholar]
- 77.Humphreys K We can’t fight opioids by controlling demand alone. The Washington Post, July 5, 2019, 2019. https://www.washingtonpost.com/outlook/we-cant-fight-opioids-by-controlling-demand-alone/2019/07/05/d025358e-7e2d-11e9-8ede-f4abf521ef17_story.html (accessed December 13, 2020). [Google Scholar]
- 78.Quinones S Dreamland: The True Tale of America’s Opiate Epidemic. New York, NY: Bloomsbury; 2015. [Google Scholar]
- 79.Muhuri PK, Gfroerer JC, Davies MC. Associations of Nonmedical Pain Reliever Use and Initiation of Heroin Use in the United States. CBHSQ Data Review, 2013. https://www.samhsa.gov/data/sites/default/files/DR006/DR006/nonmedical-pain-reliever-use-2013.htm (accessed December 13, 2020). [Google Scholar]
- 80.Compton WM, Jones CM, Baldwin GT. Relationship between Nonmedical Prescription-Opioid Use and Heroin Use. New England Journal of Medicine 2016; 374(2): 154–63. [DOI] [PubMed] [Google Scholar]
- 81.Pitt AL, Humphreys K, Brandeau ML. Modeling Health Benefits and Harms of Public Policy Responses to the US Opioid Epidemic. American Journal of Public Health 2018; 108(10): 1394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Frank RG, Pollack HA. Addressing the Fentanyl Threat to Public Health. New England Journal of Medicine 2017; 376(7): 605–7. [DOI] [PubMed] [Google Scholar]
- 83.National Institute on Drug Abuse. Prescription opioid use is a risk factor for heroin use. 2015. https://www.drugabuse.gov/publications/research-reports/prescription-opioids-heroin/prescriptionopioid-use-risk-factor-heroin-use (accessed December 4, 2020).
- 84.Nechuta SJ, Tyndall BD, Mukhopadhyay S, McPheeters ML. Sociodemographic factors, prescription history and opioid overdose deaths: a statewide analysis using linked PDMP and mortality data. Drug and Alcohol Dependence 2018; 190: 62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hoffman KM, Trawalter S, Axt JR, Oliver MN. Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proceedings of the National Academy of Sciences of the United States of America 2016; 113(16): 4296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morden NE, Chyn D, Wood A, Meara E. Racial Inequality in Prescription Opioid Receipt — Role of Individual Health Systems. New England Journal of Medicine 2021; 385(4): 342–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Horwitz S, Cenziper D, Rich S. As opioids flooded tribal lands across the U.S., overdose deaths skyrocketed. The Washington Post. 2020. [Google Scholar]
- 88.Webster PC. Indigenous Canadians confront prescription opioid misuse. The Lancet 2013; 381(9876): 1447–8. [DOI] [PubMed] [Google Scholar]
- 89.Hedegaard H, Bastian B, Trinidad J, Spencer M, Warner M. Regional Differences in the Drugs Most Frequently Involved in Drug Overdose Deaths: United States, 2017. National Vital Statistics Reports 2019; 68(12). [PubMed] [Google Scholar]
- 90.Spencer MR, Warner M, Bastian BA, Trinidad JP, Hedegaard H. Drug Overdose Deaths Involving Fentanyl, 2011–2016. Natl Vital Stat Rep 2019; 68(3): 1–19. [PubMed] [Google Scholar]
- 91.Levin D The Class of 2000 ‘Could Have Been Anything.’ Until Opioids Hit. The New York Times, December 2, 2019. https://www.nytimes.com/interactive/2019/12/02/us/opioid-crisis-high-school-teenagers.html (accessed November 1, 2020). [Google Scholar]
- 92.Lynch B What a difference a year makes: Woman focused on detox after addiction. Charleston Gazette-Mail, January 8, 2017. https://www.wvgazettemail.com/news/health/what-a-difference-a-year-makes-woman-focused-on-detox-after-addiction/article_8366e4cb-4209-5bf1-be53-94e1e52c13f2.html (accessed November 1, 2020). [Google Scholar]
- 93.Bramham D ‘I don’t want Nick to be only a statistic’. Vancouver Sun, June 20, 2020. https://vancouversun.com/news/daphne-bramham-i-dont-want-nick-to-be-only-a-statistic (accessed October 10, 2020). [Google Scholar]
- 94.Krebs EE, Bergman AA, Coffing JM, Campbell SR, Frankel RM, Matthias MS. Barriers to Guideline-Concordant Opioid Management in Primary Care—A Qualitative Study. The Journal of Pain 2014; 15(11): 1148–55. [DOI] [PubMed] [Google Scholar]
- 95.Friedman J, Mann NC, Hansen H, et al. Racial/Ethnic, Social, and Geographic Trends in Overdose-Associated Cardiac Arrests Observed by US Emergency Medical Services During the COVID-19 Pandemic. JAMA Psychiatry 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pardo B Supplying synthetic opioids during a pandemic: An early look at North America. Int J Drug Policy 2021; 93: 102833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Westhoff B. Fentanyl, Inc.: How Rogue Chemists Created the Deadliest Wave of the Opioid Epidemic. New York, NY: Grove Atlantic; 2019. [Google Scholar]
- 98.Palamar JJ, Le A, Carr TH, Cottler LB. Shifts in drug seizures in the United States during the COVID-19 pandemic. Drug Alcohol Depend 2021; 221: 108580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Department of Health and Human Services. Practice Guidelines for the Administration of Buprenorphine for Treating Opioid Use Disorder. 2021.
- 100.Alexander GC, Stoller KB, Haffajee RL, Saloner B. An Epidemic in the Midst of a Pandemic: Opioid Use Disorder and COVID-19. Annals of internal medicine 2020; 173(1): 57–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Clarke H, Weinrib A, Kotteeswaran Y, Katz J, Yu A, Tanguay R. Remote buprenorphine-naloxone initiation as an essential service for people with chronic pain and opioid dependence during the COVID-19 pandemic: Case reports, clinical pathways, and implications for the future. Can J Pain 2020; 4(1): 224–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cance JD, Doyle E. Changes in Outpatient Buprenorphine Dispensing During the COVID-19 Pandemic. JAMA 2020; 324(23): 2442–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Clark SA, Davis C, Wightman RS, et al. Using telehealth to improve buprenorphine access during and after COVID-19: A rapid response initiative in Rhode Island. Journal of substance abuse treatment 2021; 124: 108283-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huskamp HA, Busch AB, Uscher-Pines L, Barnett ML, Riedel L, Mehrotra A. Treatment of Opioid Use Disorder Among Commercially Insured Patients in the Context of the COVID-19 Pandemic. JAMA 2020; 324(23): 2440–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Duncan A, Sanders N, Schiff M, Winkelman TNA. Adaptations to jail-based buprenorphine treatment during the COVID-19 pandemic. J Subst Abuse Treat 2021; 121: 108161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bandara S, Kennedy-Hendricks A, Merritt S, Barry CL, Saloner B. Early Effects of COVID-19 on Programs Providing Medications for Opioid Use Disorder in Jails and Prisons. J Addict Med 2020; 14(5): e257–e60. [DOI] [PubMed] [Google Scholar]
- 107.Donelan CJ, Hayes E, Potee RA, Schwartz L, Evans EA. COVID-19 and treating incarcerated populations for opioid use disorder. J Subst Abuse Treat 2021; 124: 108216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Volkow ND. Collision of the COVID-19 and Addiction Epidemics. Annals of internal medicine 2020; 173(1): 61–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shover CL, Falasinnu TO, Freedman RB, Humphreys K. Emerging Characteristics of Isotonitazene-Involved Overdose Deaths: A Case-Control Study. J Addict Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.British Columbia Coroners Service. Illicit Drug Toxicity Deaths in BC January 1, 2011 – April 30, 2021 2021.
- 111.King County. Overdose deaths. 2020. https://www.kingcounty.gov/depts/health/examiner/services/reports-data/overdose.aspx (accessed May 1, 2020).
- 112.Rao IJ, Humphreys K, Brandeau ML. Effectiveness of Policies for Addressing the US Opioid Epidemic: A Model-Based Analysis from the Stanford-Lancet Commission on the North American Opioid Crisis. The Lancet Regional Health - Americas 2021: 100031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Goodman-Meza D, Medina-Mora ME, Magis-Rodríguez C, Landovitz RJ, Shoptaw S, Werb D. Where Is the Opioid Use Epidemic in Mexico? A Cautionary Tale for Policymakers South of the US-Mexico Border. American journal of public health 2019; 109(1): 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Goodman-Meza D, Friedman J, Kalmin MM, et al. Geographical and socioeconomic disparities in opioid access in Mexico, 2015–19: a retrospective analysis of surveillance data. The Lancet Public Health 2021; 6(2): e88–e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Keefe PR. The Family That Built an Empire of Pain. The New Yorker, October 23, 2017. https://www.newyorker.com/magazine/2017/10/30/the-family-that-built-an-empire-of-pain (accessed December 12, 2020). [Google Scholar]
- 116.Herper M An Opioid Spray Showered Billionaire John Kapoor In Riches. Now He’s Feeling The Pain. Forbes, Oct 24, 2016, 2016. https://www.forbes.com/sites/matthewherper/2016/10/04/death-kickbacks-and-a-billionaire-the-story-of-a-dangerous-opioid/?sh=b23d5186e3f9 (accessed. [Google Scholar]
- 117.Higham S, Horwitz S, Rich S. 76 billion opioid pills: Newly released federal data unmasks the epidemic. The Washington Post, July 26, 2019. https://www.washingtonpost.com/investigations/76-billion-opioid-pills-newly-released-federal-data-unmasks-the-epidemic/2019/07/16/5f29fd62-a73e-11e9-86dd-d7f0e60391e9_story.html (accessed December 13, 2020). [Google Scholar]
- 118.Eyre E Death in Mud Lick: A Coal Country Fight Against the Drug Companies That Delivered the Opioid Epidemic. New York, NY: Simon and Schuster; 2020. [Google Scholar]
- 119.Johns MW. Self-poisoning with barbiturates in England and Wales during 1959–74. Br Med J 1977; 1(6069): 1128–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lembke A, Papac J, Humphreys K. Our Other Prescription Drug Problem. The New England journal of medicine 2018; 378(8): 693–5. [DOI] [PubMed] [Google Scholar]
- 121.Agarwal SD, Landon BE. Patterns in Outpatient Benzodiazepine Prescribing in the United States. JAMA Network Open 2019; 2(1): e187399–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Watson GL, Arcona AP, Antonuccio DO. The ADHD Drug Abuse Crisis on American College Campuses. Ethical Hum Psychol Psychiatry 2015; 17(1): 5–21. [Google Scholar]
- 123.Hadland SE, Cerdá M, Li Y, Krieger MS, Marshall BDL. Association of Pharmaceutical Industry Marketing of Opioid Products to Physicians With Subsequent Opioid Prescribing. JAMA Intern Med 2018; 178(6): 861–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nguyen TD, Bradford WD, Simon KI. Pharmaceutical payments to physicians may increase prescribing for opioids. Addiction 2019; 114(6): 1051–9. [DOI] [PubMed] [Google Scholar]
- 125.Van Zee A The promotion and marketing of oxycontin: commercial triumph, public health tragedy. American journal of public health 2009; 99(2): 221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hadland SE, Rivera-Aguirre A, Marshall BDL, Cerdá M. Association of Pharmaceutical Industry Marketing of Opioid Products With Mortality From Opioid-Related Overdoses. JAMA Network Open 2019; 2(1): e186007–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Taitsman JK, VanLandingham A, Grimm CA. Commercial Influences on Electronic Health Records and Adverse Effects on Clinical Decision-making. JAMA Intern Med 2020; 180(7): 925–6. [DOI] [PubMed] [Google Scholar]
- 128.United States Department of Justice Office of Public Affairs. Electronic Health Records Vendor to Pay $145 Million to Resolve Criminal and Civil Investigations. 2020.
- 129.Ventola CL. Direct-to-Consumer Pharmaceutical Advertising: Therapeutic or Toxic? P T 2011; 36(10): 669–84. [PMC free article] [PubMed] [Google Scholar]
- 130.Schwartz LM, Woloshin S. Medical Marketing in the United States, 1997–2016. JAMA 2019; 321(1): 80–96. [DOI] [PubMed] [Google Scholar]
- 131.Office of Budget. HHS FY2016 Budget in Brief. 2015. https://www.hhs.gov/about/budget/budget-in-brief/fda/index.html#:~:text=food%20safety%20activities.-,FDA%20Programs,9%20percent%2C%20above%20FY%202015. (accessed December 13, 2020).
- 132.Garcia A Super Bowl drug ad spurs big backlash. CNN, February 12, 2016. https://money.cnn.com/2016/02/11/news/super-bowl-painkiller-constipation-ad/#:~:text=A%20Super%20Bowl%20ad%20for,epidemic%20that’s%20plaguing%20the%20country. (accessed December 12, 2020). [Google Scholar]
- 133.Horwitz S, Higham S, Bennett D, Kornfield M. Inside the opioid industry’s marketing machine. The Washington Post, December 6, 2019. https://www.washingtonpost.com/graphics/2019/investigations/opioid-marketing/ (accessed December 12, 2020). [Google Scholar]
- 134.Shapiro BT. Positive Spillovers and Free Riding in Advertising of Prescription Pharmaceuticals: The Case of Antidepressants. Journal of Political Economy 2017; 126(1): 381–437. [Google Scholar]
- 135.Tufts University Office of the President. Important Announcement. 2019.
- 136.Persaud N Questionable content of an industry-supported medical school lecture series: a case study. Journal of Medical Ethics 2014; 40(6): 414–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Larkin I, Ang D, Steinhart J, et al. Association Between Academic Medical Center Pharmaceutical Detailing Policies and Physician Prescribing. JAMA 2017; 317(17): 1785–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Eisenberg MD, Stone EM, Pittell H, McGinty EE. The Impact Of Academic Medical Center Policies Restricting Direct-To-Physician Marketing On Opioid Prescribing. Health Affairs 2020; 39(6): 1002–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gregory A Scientists quit back pain campaign over links to opioid giant Grünenthal. The Sunday Times, August 30, 2020, 2020. https://www.thetimes.co.uk/article/scientists-quit-back-pain-campaign-over-links-to-opioid-giant-gruenenthal-txtclkdvd (accessed December 14, 2020). [Google Scholar]
- 140.Bachhuber MA, Saloner B, Cunningham CO, Barry CL. Medical cannabis laws and opioid analgesic overdose mortality in the United States, 1999–2010. JAMA Intern Med 2014; 174(10): 1668–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shover CL, Davis CS, Gordon SC, Humphreys K. Association between medical cannabis laws and opioid overdose mortality has reversed over time. Proceedings of the National Academy of Sciences 2019; 116(26): 12624–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Humphreys K, Saitz R. Should Physicians Recommend Replacing Opioids With Cannabis? JAMA 2019. [DOI] [PubMed] [Google Scholar]
- 143.Shover CL, Vest NA, Chen D, et al. Association of State Policies Allowing Medical Cannabis for Opioid Use Disorder With Dispensary Marketing for This Indication. JAMA Network Open 2020; 3(7): e2010001–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Daly R More States Explore Laws on Gifts to Physicians. Psychiatric News, March 19, 2010, 2010. https://psychnews.psychiatryonline.org/doi/full/10.1176/pn.45.6.psychnews_45_6_014 (accessed. [Google Scholar]
- 145.Sullivan T Massachusetts Pharmaceutical and Device Code of Conduct: Companies Won’t Have to Report Payments Covered by Federal Sunshine Act; Gift Ban Still In Place. Policy & Medicine, May 6, 2018, 2018. https://www.policymed.com/2014/06/massachusetts-pharmaceutical-and-device-code-of-conduct-companies-wont-have-to-report-payments-covered-by-federal-sunshin.html (accessed. [Google Scholar]
- 146.Dieners P, Kemmner C. Germany: Pharmaceutical Advertising Laws and Regulations 2020. 2020.
- 147.Government of Canada. Restricting the Marketing and Advertising of Opioids. 2019. https://www.canada.ca/en/health-canada/services/substance-use/problematic-prescription-drug-use/opioids/responding-canada-opioid-crisis/advertising-opioid-medications.html (accessed December 13, 2020).
- 148.Jeanne Shaheen U.S. Senator for New Hampshire. Shaheen Introduces Legislation to Close Big Pharma Advertising Tax Loophole. 2019.
- 149.Harris Biden. Healthcare. 2020. https://joebiden.com/healthcare/ (accessed December 12, 2020).
- 150.Council of Medical Specialty Societies. Code for Interactions with Companies, 2015. [PubMed]
- 151.Education ACfCM. Standards for Commercial Support: Standards to Ensure the Independence of CME Activities, 2021.
- 152.Piller C Is FDA’s revolving door open too wide? Science 2018; 361(6397): 21-. [DOI] [PubMed] [Google Scholar]
- 153.Sarasohn J Tauzin to Head Drug Trade Group. The Washington Post, December 16, 2004. https://www.washingtonpost.com/archive/politics/2004/12/16/tauzin-to-head-drug-trade-group/71c7aadc-e195-4f68-abbe-b09d5464c207/ (accessed December 14, 2020). [Google Scholar]
- 154.Higham S, Bernstein L. The Drug Industry’s Triumph Over the FDA. The Washington Post, October 15, 2017. https://www.washingtonpost.com/graphics/2017/investigations/dea-drug-industry-congress/ (accessed. [Google Scholar]
- 155.Keefe PR. The Sackler Family’s Plan to Keep Its Billions. The New Yorker, October 4, 2020, 2020. https://www.newyorker.com/news/news-desk/the-sackler-familys-plan-to-keep-its-billions (accessed December 12, 2020). [Google Scholar]
- 156.Bien J, Prasad V. Future jobs of FDA’s haematology-oncology reviewers. BMJ 2016; 354: i5055. [DOI] [PubMed] [Google Scholar]
- 157.U.S. Food and Drug Administration. Postmarketing Requirements and Commitments: Reports, 2020.
- 158.Spelsberg A, Prugger C, Doshi P, et al. Contribution of industry funded post-marketing studies to drug safety: survey of notifications submitted to regulatory agencies. BMJ 2017; 356: j337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Heyward J, Olson L, Sharfstein JM, Stuart EA, Lurie P, Alexander GC. Evaluation of the Extended-Release/Long-Acting Opioid Prescribing Risk Evaluation and Mitigation Strategy Program by the US Food and Drug Administration: A Review. JAMA Intern Med 2019. [DOI] [PubMed] [Google Scholar]
- 160.Guadamuz JS, Qato DM, Alexander GC. Use of Risk Evaluation and Mitigation Strategies by the US Food and Drug Administration, 2008–2019. JAMA 2020; 324(3): 299–301. [DOI] [PubMed] [Google Scholar]
- 161.Zisblatt L, Hayes SM, Lazure P, Hardesty I, White JL, Alford DP. Safe and competent opioid prescribing education: Increasing dissemination with a train-the-trainer program. Subst Abus 2017; 38(2): 168–76. [DOI] [PubMed] [Google Scholar]
- 162.Traynor K Advisers say FDA’s opioid REMS program needs improvement. Am J Health Syst Pharm 2016; 73(13): 940–2. [DOI] [PubMed] [Google Scholar]
- 163.Anna Lembke’s Comments for the Open Public Hearing portion of the May 3–4, 2016 meeting regarding the Opioid Analgesics REMS. Drug Safety and Risk Management Advisory Committee and the Anesthetic and Analgesic Drug Products Advisory Committee; 2016. [Google Scholar]
- 164.Rollman JE, Heyward J, Olson L, Lurie P, Sharfstein J, Alexander GC. Assessment of the FDA Risk Evaluation and Mitigation Strategy for Transmucosal Immediate-Release Fentanyl Products. Jama 2019; 321(7): 676–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Angell M FDA: This Agency Can Be Dangerous. The New York Review of Books. https://www.nybooks.com/articles/2010/09/30/agency-can-be-dangerous/ (accessed September 30, 2010). [Google Scholar]
- 166.U.S. Food and Drug Administration. Prescription Drug User Fee Act; Public Meeting; Request for Comments. Federal Register, July 19, 2016, 2016. https://www.federalregister.gov/documents/2016/07/19/2016-16916/prescription-drug-user-fee-act-public-meeting-request-for-comments (accessed. [Google Scholar]
- 167.United States General Accounting Office. OxyContin Abuse and Diversion and Efforts to Address the Problem, 2003.
- 168.Baker DW. The Joint Commission’s Pain Standards: Origins and Evolution, 2017.
- 169.Retraction of WHO guidance on opioid use. Bull World Health Organ 2020; 98(1): 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.deShazo RD, Johnson M, Eriator I, Rodenmeyer K. Backstories on the US Opioid Epidemic. Good Intentions Gone Bad, an Industry Gone Rogue, and Watch Dogs Gone to Sleep. Am J Med 2018; 131(6): 595–601. [DOI] [PubMed] [Google Scholar]
- 171.Meier B In Guilty Plea, OxyContin Maker to Pay $600 Million. The New York Times, May 10, 2007. https://www.nytimes.com/2007/05/10/business/11drug-web.html (accessed December 14, 2020). [Google Scholar]
- 172.Zaring DT. Against Being Against the Revolving Door. University of Illinois Law Review 2013; 2013(2): 507–50. [Google Scholar]
- 173.Baldwin TS 2057 (115th): Pharmaceutical Regulation Conflict of Interest Act. 2017.
- 174.National Conference of State Legislatures. Revolving Door Prohibitions. September 8, 2020. 2020. https://www.ncsl.org/research/ethics/50-state-table-revolving-door-prohibitions.aspx (accessed July 21, 2021.
- 175.Maskell J Post-Employment, “Revolving Door,” Laws for Federal Personnel 2014.
- 176.Supreme Court of the United States. Citizens United v. Federal Election Commission. 2009. [Google Scholar]
- 177.Mulvihill G, Whyte LE, Weider B. Drugmakers fought state opioid limits amid crisis. Associated Press, September 18, 2016. https://www.heraldnews.com/news/20160918/drugmakers-fought-state-opioid-limits-amid-crisis (accessed December 14, 2020). [Google Scholar]
- 178.Ornstein C, Weber T. American Pain Foundation Shuts Down as Senators Launch Investigation of Prescription Narcotics. ProPublica; May 8, 2012. https://www.propublica.org/article/senate-panel-investigates-drug-company-ties-to-pain-groups (accessed December 14, 2020). [Google Scholar]
- 179.Ornstein C, Weber T. The Champion of Painkillers. ProPublica, December 23, 2011. https://www.propublica.org/article/the-champion-of-painkillers (accessed December 14, 2020). [Google Scholar]
- 180.American Pain Foundation. 2010. Annual Report.
- 181.Charity Commission for England and Wales. Pain UK: regulatory compliance case conclusions, 2019.
- 182.McCoy MS, Carniol M, Chockley K, Urwin JW, Emanuel EJ, Schmidt H. Conflicts of Interest for Patient-Advocacy Organizations. New England Journal of Medicine 2017; 376(9): 880–5. [DOI] [PubMed] [Google Scholar]
- 183.Rose SL, Highland J, Karafa MT, Joffe S. Patient Advocacy Organizations, Industry Funding, and Conflicts of Interest. JAMA Intern Med 2017; 177(3): 344–50. [DOI] [PubMed] [Google Scholar]
- 184.Ozieranski P, Csanádi M, Rickard E, Mulinari S. Under-reported relationship: a comparative study of pharmaceutical industry and patient organisation payment disclosures in the UK (2012–2016). BMJ Open 2020; 10(9): e037351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Federal Trade Commission. FTC Brings First Case Challenging Fake Paid Reviews on an Independent Retail Website. 2019. https://www.ftc.gov/news-events/press-releases/2019/02/ftc-brings-first-case-challenging-fake-paid-reviews-independent (accessed November 10, 2020).
- 186.Scott MJ. Ripping Up the Astroturf: Regulating Deceptive Corporate Advertising Methods. Iowa Law Review 2019; 105: 431–61. [Google Scholar]
- 187.Kahneman D. Thinking, Fast and Slow. New York, NY: Farrar, Strous and Giroux; 2011. [Google Scholar]
- 188.Weick KE. Small wins: Redefining the scale of social problems. American Psychologist 1984; 39(1): 40–9. [Google Scholar]
- 189.U. S. Food Drug Administration. Statement from FDA Commissioner Scott Gottlieb, M.D., on agency’s approval of Dsuvia and the FDA’s future consideration of new opioids. 2018.
- 190.Furlan AD, Sandoval JA, Mailis-Gagnon A, Tunks E. Opioids for chronic noncancer pain: a meta-analysis of effectiveness and side effects. CMAJ 2006; 174(11): 1589–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Krebs EE, Gravely A, Nugent S, et al. Effect of Opioid vs Nonopioid Medications on Pain-Related Function in Patients With Chronic Back Pain or Hip or Knee Osteoarthritis Pain: The SPACE Randomized Clinical Trial. JAMA 2018; 319(9): 872–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Humphreys K, Weisner C. Use of Exclusion Criteria in Selecting Research Subjects and Its Effect on the Generalizability of Alcohol Treatment Outcome Studies. American Journal of Psychiatry 2000; 157(4): 588–94. [DOI] [PubMed] [Google Scholar]
- 193.Yusuf S, Collins R, Peto R. Why do we need some large, simple randomized trials? Statistics in Medicine 1984; 3(4): 409–20. [DOI] [PubMed] [Google Scholar]
- 194.Institute of Medicine Committee on Advancing Pain Research C, Education. The National Academies Collection: Reports funded by National Institutes of Health. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 195.Hoy D, March L, Brooks P, et al. The global burden of low back pain: estimates from the Global Burden of Disease 2010 study. Annals of the Rheumatic Diseases 2014; 73(6): 968–74. [DOI] [PubMed] [Google Scholar]
- 196.GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020; 396(10258): 1204–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults - United States, 2016. MMWR Morb Mortal Wkly Rep 2018; 67(36): 1001–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Buchbinder R, Underwood M, Hartvigsen J, Maher CG. The Lancet Series call to action to reduce low value care for low back pain: an update. PAIN 2020; 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Dieleman JL, Cao J, Chapin A, et al. US Health Care Spending by Payer and Health Condition, 1996–2016. JAMA 2020; 323(9): 863–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.National Institutes of Health. Estimates of Funding for Various Research, Condition, and Disease Categories (RCDC). 2020. https://report.nih.gov/funding/categorical-spending#/ (accessed December 13, 2020).
- 201.Humphreys K Reconciling the Present and the Future in Opioid Prescription Policy: An Ethical Dilemma. Pain Medicine 2018; 19(8): 1514–5. [DOI] [PubMed] [Google Scholar]
- 202.Webster F, Rice K, Katz J, Bhattacharyya O, Dale C, Upshur R. An ethnography of chronic pain management in primary care: The social organization of physicians’ work in the midst of the opioid crisis. PLOS ONE 2019; 14(5): e0215148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Green CA, McCarty D, Mertens J, et al. A qualitative study of the adoption of buprenorphine for opioid addiction treatment. Journal of substance abuse treatment 2014; 46(3): 390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sinha CB, Bakshi N, Ross D, Krishnamurti L. Management of Chronic Pain in Adults Living With Sickle Cell Disease in the Era of the Opioid Epidemic: A Qualitative Study. JAMA Network Open 2019; 2(5): e194410–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wertli MM, Reich O, Signorell A, Burgstaller JM, Steurer J, Held U. Changes over time in prescription practices of pain medications in Switzerland between 2006 and 2013: an analysis of insurance claims. BMC Health Serv Res 2017; 17(1): 167-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Tsang A, Von Korff M, Lee S, et al. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain 2008; 9(10): 883–91. [DOI] [PubMed] [Google Scholar]
- 207.Mackey S, Kao MC. Managing twin crises in chronic pain and prescription opioids. Bmj 2019; 364: l917. [DOI] [PubMed] [Google Scholar]
- 208.Medicines and Healthcare products Regulatory Agency. Drug Safety Update, 2020. [Google Scholar]
- 209.Levy N, Lord LJ, Lobo DN. UK recommendations on opioid stewardship. BMJ 2021; 372: m4901. [DOI] [PubMed] [Google Scholar]
- 210.Canadian Deprescribing Network. The Canadian Deprescribing Network is dedicated to raising awareness of medication safety, deprescribing and safer alternatives to risky medications. 2020. https://www.deprescribingnetwork.ca (accessed October 11, 2020).
- 211.U.S. Department of Health and Human Services (HHS). HHS Guide for Clinicians on the Appropriate Dosage Reduction or Discontinuation of Long-Term Opioid Analgesics, 2019.
- 212.U. S. Drug Enforcement Administration. “Pill Mill” doctor pleads guilty to opioid distribution, admits signing prescriptions for dead and jailed patients. 2020.
- 213.The U. S. District Attorney’s Office District of Connecticut. APRN Who Received Kickbacks from Insys Therapeutics for Prescribing Fentanyl Spray is Sentenced. 2019.
- 214.Funk C, Kennedy B. Public confidence in scientists has remained stable for decades. Fact Tank, 2020. https://www.pewresearch.org/fact-tank/2020/08/27/public-confidence-in-scientists-has-remained-stable-for-decades/ (accessed December 14, 2020). [Google Scholar]
- 215.Davis CS, Lieberman AJ, Hernandez-Delgado H, Suba C. Laws limiting the prescribing or dispensing of opioids for acute pain in the United States: A national systematic legal review. Drug and Alcohol Dependence 2019; 194: 166–72. [DOI] [PubMed] [Google Scholar]
- 216.Davis CS, Piper BJ, Gertner AK, Rotter JS. Opioid Prescribing Laws Are Not Associated with Short-term Declines in Prescription Opioid Distribution. Pain Med 2020; 21(3): 532–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Funk C, Kennedy B, Johnson C. Trust in Medical Scientists Has Grown in U.S., but Mainly Among Democrats. Pew Research Center. 2020. May 21, 2020. [Google Scholar]
- 218.National Academy of Medicine. First, Do No Harm: Marshaling Clinician Leadership to Counter the Opioid Epidemic. Washington, DC: National Academy of Medicine, 2017. [PubMed] [Google Scholar]
- 219.Gellad WF, Good CB, Shulkin DJ. Addressing the Opioid Epidemic in the United States: Lessons From the Department of Veterans Affairs. JAMA Intern Med 2017; 177(5): 611–2. [DOI] [PubMed] [Google Scholar]
- 220.Frank JW, Carey E, Nolan C, et al. Increased Nonopioid Chronic Pain Treatment in the Veterans Health Administration, 2010–2016. Pain Med 2019; 20(5): 869–77. [DOI] [PubMed] [Google Scholar]
- 221.Hadlandsmyth K, Mosher H, Vander Weg MW, Lund BC. Decline in Prescription Opioids Attributable to Decreases in Long-Term Use: A Retrospective Study in the Veterans Health Administration 2010–2016. J Gen Intern Med 2018; 33(6): 818–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Centers for Disease Control and Prevention. Prescription Drug Monitoring Programs (PDMPs). 2020. https://www.cdc.gov/drugoverdose/pdmp/states.html#:~:text=A%20prescription%20drug%20monitoring%20program,a%20nimble%20and%20targeted%20response. (accessed December 13, 2020).
- 223.McDonald DC, Carlson KE. Estimating the Prevalence of Opioid Diversion by “Doctor Shoppers” in the United States. PLOS ONE 2013; 8(7): e69241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Delgado MK, Shofer FS, Patel MS, et al. Association between Electronic Medical Record Implementation of Default Opioid Prescription Quantities and Prescribing Behavior in Two Emergency Departments. J Gen Intern Med 2018; 33(4): 409–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Babor TF, Caulkins J, Fischer B, et al. Drug Policy and the Public Good: a summary of the second edition. Addiction 2019; 114(11): 1941–50. [Google Scholar]
- 226.Strang J, Babor T, Caulkins J, Fischer B, Foxcroft D, Humphreys K. Drug policy and the public good: evidence for effective interventions. Lancet 2012; 379(9810): 71–83. [DOI] [PubMed] [Google Scholar]
- 227.Fairley M, Humphreys K, Joyce VR, et al. Cost-effectiveness of Treatments for Opioid Use Disorder. JAMA Psychiatry 2021; 78(7): 767–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Santo T Jr, Clark B, Hickman M, et al. Association of Opioid Agonist Treatment With All-Cause Mortality and Specific Causes of Death Among People With Opioid Dependence: A Systematic Review and Meta-analysis. JAMA Psychiatry 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Day E, Mitcheson L. Psychosocial interventions in opiate substitution treatment services: does the evidence provide a case for optimism or nihilism? Addiction 2017; 112: 132Y 1336. [DOI] [PubMed] [Google Scholar]
- 230.Best JHMGMFEFANJSD. Use of non-prescribed methadone and other illicit drugs during methadone maintenance treatment. Drug and Alcohol Review 2000; 19(1): 9–16. [Google Scholar]
- 231.Hall WD, Strang J. Alcohol problems need more attention in patients receiving long-term opioid substitution therapy. The Lancet Psychiatry 2017; 4(4): 265–6. [DOI] [PubMed] [Google Scholar]
- 232.Tjagvad C, Skurtveit S, Linnet K, Andersen LV, Christoffersen DJ, Clausen T. Methadone-Related Overdose Deaths in a Liberal Opioid Maintenance Treatment Programme. European Addiction Research 2016; 22(5): 249–58. [DOI] [PubMed] [Google Scholar]
- 233.Strang J, Hall W, Hickman M, Bird SM. Impact of supervision of methadone consumption on deaths related to methadone overdose (1993–2008): analyses using OD4 index in England and Scotland. BMJ 2010; 341: c4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Best D, Day E, Morgan B, Oza T, Copello A, Gossop M. What treatment means in practice: An analysis of the delivery of evidence-based interventions in criminal justice drug treatment services in Birmingham, England. Addiction Research & Theory 2009; 17(6): 678–87. [Google Scholar]
- 235.National Center for Injury Prevention and Control. Integrating & Expanding Prescription Drug Monitoring Program Data: Lessons from Nine States, 2017.
- 236.McLellan AT, Arndt IO, Metzger DS, Woody GE, O’Brien CP. The effects of psychosocial services in substance abuse treatment. JAMA 1993; 269(15): 1953–9. [PubMed] [Google Scholar]
- 237.Carroll KM, Weiss RD. The Role of Behavioral Interventions in Buprenorphine Maintenance Treatment: A Review. Am J Psychiatry 2017; 174(8): 738–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Hunter SB, Dopp AR, Ober AJ, Uscher-Pines L. Clinician perspectives on methadone service delivery and the use of telemedicine during the COVID-19 pandemic: A qualitative study. Journal of Substance Abuse Treatment 2021; 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Joseph G, Torres-Lockhart K, Stein MR, Mund PA, Nahvi S. Reimagining patient-centered care in opioid treatment programs: Lessons from the Bronx during COVID-19. J Subst Abuse Treat 2021; 122: 108219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Lembke A Unsafe Supply: Why Making Controlled Prescription Drugs Available for Unsupervised Use Will Not Target the Syndemic of HIV, Hepatitis C, Overdose, and COVID-19-- A Commentary on Bonn et al. (2020). J Stud Alcohol Drugs 2020; 81(5): 564–5. [PubMed] [Google Scholar]
- 241.Brooks L Scottish government urged to declare drug addiction emergency. The Guardian, November 1, 2019. http://www.theguardian.com/society/2019/nov/01/scottish-government-urged-to-declare-drug-addiction-emergency (accessed October 10, 2020). [Google Scholar]
- 242.Sun EC, Dixit A, Humphreys K, Darnall BD, Baker LC, Mackey S. Association between concurrent use of prescription opioids and benzodiazepines and overdose: retrospective analysis. BMJ 2017; 356: j760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Shover CL, Falasinnu TO, Dwyer CL, et al. Steep increases in fentanyl-related mortality west of the Mississippi River: Recent evidence from county and state surveillance. Drug and Alcohol Dependence 2020: 108314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Humphreys K, Malenka RC, Knutson B, MacCoun RJ. Brains, environments, and policy responses to addiction. Science 2017; 356(6344): 1237–8. [DOI] [PubMed] [Google Scholar]
- 245.Jalal H, Buchanich JM, Roberts MS, Balmert LC, Zhang K, Burke DS. Changing dynamics of the drug overdose epidemic in the United States from 1979 through 2016. Science 2018; 361(6408): eaau1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Yang LH, Wong LY, Grivel MM, Hasin DS. Stigma and substance use disorders: an international phenomenon. Curr Opin Psychiatry 2017; 30(5): 378–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Botticelli MP, Koh HK. Changing the Language of Addiction. JAMA 2016; 316(13): 1361–2. [DOI] [PubMed] [Google Scholar]
- 248.Kelly JF, Greene MC, Abry A. A US national randomized study to guide how best to reduce stigma when describing drug-related impairment in practice and policy. Addiction 2021; 116(7): 1757–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Henderson S Opioid Crisis vs. the War on Drugs: A Double Standard?, 2017.
- 250.D’Aunno T Analysis by T. D’Aunno of 346 clinics in the National Drug Abuse Treatment System Survey study,. In: Humphreys K, editor.; 2020. [Google Scholar]
- 251.McLellan AT, Carise D, Kleber HD. Can the national addiction treatment infrastructure support the public’s demand for quality care? J Subst Abuse Treat 2003; 25(2): 117–21. [PubMed] [Google Scholar]
- 252.Hoffman KA, Ford JH, Tillotson CJ, Choi D, McCarty D. Days to treatment and early retention among patients in treatment for alcohol and drug disorders. Addict Behav 2011; 36(6): 643–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Andrews CM, Shin HC, Marsh JC, Cao D. Client and program characteristics associated with wait time to substance abuse treatment entry. Am J Drug Alcohol Abuse 2013; 39(1): 61–8. [DOI] [PubMed] [Google Scholar]
- 254.World Health Organization. The top 10 causes of death. 2018. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed December 12, 2020).
- 255.Medicaid Innovation Accelerator Program. Collaborative Models for Medication-Assisted Treatment: Key Elements of Vermont’s Hub-and-Spoke System, 2019.
- 256.Rawson RA. Vermont Hub-and-Spoke Model of Care for Opioid Use Disorders: An Evaluation: Vermont Center on Behavior and Health, 2017.
- 257.Brooklyn JR, Sigmon SC. Vermont Hub-and-Spoke Model of Care for Opioid Use Disorder: Development, Implementation, and Impact. J Addict Med 2017; 11(4): 286–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Darfler K, Sandoval J, Pearce Antonini V, Urada D. Preliminary results of the evaluation of the California Hub and Spoke Program. Journal of Substance Abuse Treatment 2020; 108: 26–32. [DOI] [PubMed] [Google Scholar]
- 259.Substance Abuse and Mental Health Services Administration. TI-18–009 Individual Grant Awards. 2019. https://www.samhsa.gov/grants/awards/2019/ti-18-009 (accessed November 2, 2020).
- 260.Stoffelmayr BE, Benishek LA, Humphreys K, Lee JA, Mavis BE. Substance abuse prognosis with an additional psychiatric diagnosis: understanding the relationship. J Psychoactive Drugs 1989; 21(2): 145–52. [DOI] [PubMed] [Google Scholar]
- 261.Douglas KR, Chan G, Gelernter J, et al. Adverse childhood events as risk factors for substance dependence: partial mediation by mood and anxiety disorders. Addict Behav 2010; 35(1): 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Williams AR, Nunes EV, Bisaga A, Levin FR, Olfson M. Development of a Cascade of Care for responding to the opioid epidemic. The American Journal of Drug and Alcohol Abuse 2019; 45(1): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Yedinak JL, Goedel WC, Paull K, et al. Defining a recovery-oriented cascade of care for opioid use disorder: A community-driven, statewide cross-sectional assessment. PLOS Medicine 2019; 16(11): e1002963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.ASAM Continuum. What are the ASAM Levels of Care? 2015. https://www.asamcontinuum.org/knowledgebase/what-are-the-asam-levels-of-care/ (accessed December 13, 2020).
- 265.U.S. Department of Health and Human Services (HHS) Office of the Surgeon General. Facing Addiction in America: The Surgeon General’s Spotlight on Opioids. Washington, DC, 2018. [PubMed] [Google Scholar]
- 266.Humphreys K, Wing S, McCarty D, et al. Self-help organizations for alcohol and drug problems: Toward evidence-based practice and policy. Journal of Substance Abuse Treatment 2004; 26(3): 151–8. [DOI] [PubMed] [Google Scholar]
- 267.Kelly JF, White WL. Addiction Recovery Management: Theory, Research and Practice: Humana Press; 2011. [Google Scholar]
- 268.Mitchell TH. The Big Fix: Hope After Heroin. New York, NY: Seal Press; 2016. [Google Scholar]
- 269.Sixx N Take it from a recovering addict, a lot more could be done to end the opioid crisis. The Los Angeles Times, September 29, 2017. https://www.latimes.com/opinion/op-ed/la-oe-sixx-opioid-crisis-20170929-story.html (accessed November 6, 2020). [Google Scholar]
- 270.Hampton R American Fix: Inside the Opioid Addiction Crisis - and How to End It|Paperback. New York, NY: All Points Books; 2018. [Google Scholar]
- 271.Dorsey T The Rooms Project: Tarah. 2017. http://www.theroomsproject.org/tarah/ (accessed November 2, 2020).
- 272.Woods JS, Joseph H. Reducing stigma through education to enhance Medication-Assisted Recovery. J Addict Dis 2012; 31(3): 226–35. [DOI] [PubMed] [Google Scholar]
- 273.Humphreys K, Piot P. Scientific evidence alone is not sufficient basis for health policy. BMJ 2012; 344: e1316. [DOI] [PubMed] [Google Scholar]
- 274.Babor T, Caulkins JP, Fischer B, et al. Drug Policy and the Public Good. 2nd ed. Oxford, U.K.: Oxford University Press; 2018. [Google Scholar]
- 275.Witkiewitz K, Kirouac M, Roos CR, et al. Abstinence and low risk drinking during treatment: Association with psychosocial functioning, alcohol use, and alcohol problems 3 years following treatment. Psychol Addict Behav 2018; 32(6): 639–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Harvey LM, Fan W, Cano MÁ, et al. Psychosocial intervention utilization and substance abuse treatment outcomes in a multisite sample of individuals who use opioids. Journal of Substance Abuse Treatment 2020; 112: 68–75. [DOI] [PubMed] [Google Scholar]
- 277.Weiss RD, Griffin ML, Marcovitz DE, et al. Correlates of Opioid Abstinence in a 42-Month Posttreatment Naturalistic Follow-Up Study of Prescription Opioid Dependence. J Clin Psychiatry 2019; 80(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Monico LB, Gryczynski J, Mitchell SG, Schwartz RP, O’Grady KE, Jaffe JH. Buprenorphine Treatment and 12-step Meeting Attendance: Conflicts, Compatibilities, and Patient Outcomes. J Subst Abuse Treat 2015; 57: 89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Rosenstein RJ. Fight Drug Abuse, Don’t Subsidize It. The New York Times, August 27, 2018. https://www.nytimes.com/2018/08/27/opinion/opioids-heroin-injection-sites.html (accessed December 14, 2020). [Google Scholar]
- 280.Pardo B, Caulkins JP, Kilmer B. Assessing the Evidence on Supervised Drug Consumption Sites. Santa Monica, CA: RAND Corporation, 2018. [Google Scholar]
- 281.Abraham AJ, Andrews CM, Grogan CM, et al. The Affordable Care Act Transformation of Substance Use Disorder Treatment. American journal of public health 2017; 107(1): 31–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Andrews CM, Grogan CM, Smith BT, et al. Medicaid Benefits For Addiction Treatment Expanded After Implementation Of The Affordable Care Act. Health Aff (Millwood) 2018; 37(8): 1216–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Humphreys K, Frank RG. The Affordable Care Act will revolutionize care for substance use disorders in the United States. Addiction 2014; 109(12): 1957–8. [DOI] [PubMed] [Google Scholar]
- 284.Andrews CM, Humphreys K. Investing in Medicaid to End the Opioid Epidemic. Psychiatr Serv 2019; 70(7): 537. [DOI] [PubMed] [Google Scholar]
- 285.Humphreys K How Medicaid Can Strengthen the National Response to the Opioid Epidemic. Am J Public Health 2018; 108(5): 589–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Sharp A, Jones A, Sherwood J, Kutsa O, Honermann B, Millett G. Impact of Medicaid Expansion on Access to Opioid Analgesic Medications and Medication-Assisted Treatment. Am J Public Health 2018; 108(5): 642–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Saloner B, Levin J, Chang H-Y, Jones C, Alexander GC. Changes in Buprenorphine-Naloxone and Opioid Pain Reliever Prescriptions After the Affordable Care Act Medicaid Expansion. JAMA Network Open 2018; 1(4): e181588–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Kravitz-Wirtz N, Davis CS, Ponicki WR, et al. Association of Medicaid Expansion With Opioid Overdose Mortality in the United States. JAMA network open 2020; 3(1): e1919066–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Grogan CM, Andrews C, Abraham A, et al. Survey Highlights Differences In Medicaid Coverage For Substance Use Treatment And Opioid Use Disorder Medications. Health Aff (Millwood) 2016; 35(12): 2289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Centers for Medicare and Medicaid Services. Medicare Coverage of Substance Abuse Services, 2016.
- 291.Department of Health and Human Services. Patient Protection and Affordable Care Act; HHS Notice of Benefit and Payment Parameters for 2019, 2018. [PubMed]
- 292.Tran Smith B, Seaton K, Andrews C, et al. Benefit requirements for substance use disorder treatment in state health insurance exchanges. Am J Drug Alcohol Abuse 2018; 44(4): 426–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.New York State Office of the Attorney General. A.G. Schneiderman Announces Settlement With Emblem Health For Wrongly Denying Mental Health And Substance Abuse Treatment For Thousands Of New York Members. 2014.
- 294.Beetham T, Saloner B, Gaye M, Wakeman SE, Frank RG, Barnett ML. Therapies Offered at Residential Addiction Treatment Programs in the United States. JAMA 2020; 324(8): 804–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Harris AHS, Weisner CM, Chalk M, Capoccia V, Chen C, Thomas CP. Specifying and Pilot Testing Quality Measures for the American Society of Addiction Medicine’s Standards of Care. Journal of addiction medicine 2016; 10(3): 148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Hoffman KA, Green CA, Ford JH 2nd, Wisdom JP, Gustafson DH, McCarty D. Improving quality of care in substance abuse treatment using five key process improvement principles. J Behav Health Serv Res 2012; 39(3): 234–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Ayu AP, Schellekens AFA, Iskandar S, Pinxten L, De Jong CAJ. Effectiveness and Organization of Addiction Medicine Training Across the Globe. European Addiction Research 2015; 21(5): 223–39. [DOI] [PubMed] [Google Scholar]
- 298.Babor T Alcohol: No Ordinary Commodity: Research and Public Policy. 2nd Edition ed. Oxford, U.K.: Oxford Medical Publications; 2003. [Google Scholar]
- 299.McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA 2000; 284(13): 1689–95. [DOI] [PubMed] [Google Scholar]
- 300.Compton WM, Dawson D, Duffy SQ, Grant BF. The effect of inmate populations on estimates of DSM-IV alcohol and drug use disorders in the United States. The American journal of psychiatry 2010; 167(4): 473–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Messina N, Grella CE, Cartier J, Torres S. A randomized experimental study of gender-responsive substance abuse treatment for women in prison. Journal of Substance Abuse Treatment 2010; 38(2): 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.McKenzie M, Nunn A, Zaller ND, Bazazi AR, Rich JD. Overcoming obstacles to implementing methadone maintenance therapy for prisoners: implications for policy and practice. J Opioid Manag 2009; 5(4): 219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Feucht TE, Keyser A. Reducing Drug Use in Prisons: Pennsylvania’s Approach, 1999.
- 304.Logan MW, Link NW. Taking Stock of Drug Courts: Do They Work? Victims & Offenders 2019; 14(3): 283–98. [Google Scholar]
- 305.Kilmer B, Caulkins J, DuPont RL, Humphreys K. Reducing substance use in criminal justice populations. In: Miller SC DAF, Rosenthal RN, R., & Saitz R, ed. Principles of Addiction Medicine. 6 ed. Washington, D.C.: American Society of Addiction Medicine; 2019: 1768–74. [Google Scholar]
- 306.Bird SM, McAuley A, Perry S, Hunter C. Effectiveness of Scotland’s National Naloxone Programme for reducing opioid-related deaths: a before (2006–10) versus after (2011–13) comparison. Addiction (Abingdon, England) 2016; 111(5): 883–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Supreme Court of the United States. Brown, Governor of California, et al. v. Plata et al. 2010.
- 308.Charuvastra A, Stein J, Schwartzapfel B, et al. Hepatitis B vaccination practices in state and federal prisons. Public Health Rep 2001; 116(3): 203–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Parmar MKB, Strang J, Choo L, Meade AM, Bird SM. Randomized controlled pilot trial of naloxone-on-release to prevent post-prison opioid overdose deaths. Addiction (Abingdon, England) 2017; 112(3): 502–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Congressman Tonko Paul D.. Tonko, Turner Reintroduce Bipartisan Addiction Treatment Bill. 2019.
- 311.Martin RA, Gresko SA, Brinkley-Rubinstein L, Stein LAR, Clarke JG. Post-release treatment uptake among participants of the Rhode Island Department of Corrections comprehensive medication assisted treatment program. Prev Med 2019; 128: 105766. [DOI] [PubMed] [Google Scholar]
- 312.Brinkley-Rubinstein L, Peterson M, Clarke J, et al. The benefits and implementation challenges of the first state-wide comprehensive medication for addictions program in a unified jail and prison setting. Drug and Alcohol Dependence 2019; 205: 107514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Bird M, Lofstrom M, Martin B, Raphael S, Nguyen V. The Impact of Proposition 47 on Crime and Recidivism: Public Policy Institute of California, 2018.
- 314.Kleiman MAR. When Brute Force Fails: How to Have Less Crime and Less Punishment. Princeton, NJ: Princeton University Press; 2010. [Google Scholar]
- 315.Anglin MD, Nosyk B, Jaffe A, Urada D, Evans E. Offender diversion into substance use disorder treatment: the economic impact of California’s proposition 36. Am J Public Health 2013; 103(6): 1096–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Bernard CL, Rao IJ, Robison KK, Brandeau ML. Health outcomes and cost-effectiveness of diversion programs for low-level drug offenders: A model-based analysis. PLoS medicine 2020; 17(10): e1003239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Substance Abuse and Mental Health Services Administration. Clinical Guidance for Treating Pregnant and Parenting Women With Opioid Use Disorder and Their Infants Rockville, MD: Substance Abuse and Mental Health Services Administration; 2018.
- 318.Nader MA, Czoty PW, Nader SH, Morgan D. Nonhuman primate models of social behavior and cocaine abuse. Psychopharmacology (Berl) 2012; 224(1): 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Phillips KA, Bales KL, Capitanio JP, et al. Why primate models matter. American Journal of Primatology 2014; 76(9): 801–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Case A, Deaton A. Deaths of Despair and the Future of Capitalism. Princeton, NJ: Princeton University Press; 2020. [Google Scholar]
- 321.Pickett K, Wilkinson R. The Spirit Level: Why More Equal Societies Almost Always Do Better. London, U.K.: Bloomsbury Press; 2009. [Google Scholar]
- 322.Ruhm CJ. Deaths of Despair or Drug Problems? NBER Working Paper 2018. [Google Scholar]
- 323.Alpert A, Evans WN, Lieber EMJ, Powell D. Origins of the Opioid Crisis and Its Enduring Impacts. NBER Working Paper 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Humphreys K, Gifford E. Religion, Spirituality, and the Troublesome Use of Substances. In: Miller WR, Carroll KM, eds. Rethinking Substance Abuse: What the Science Shows, and What We Should Do about It. New York, NY: Guilford Press; 2006: 257–74. [Google Scholar]
- 325.Courtwright D The Age of Addiction: How Bad Habits Became Big Business. Cambridge, MA: The Belknap Press of Harvard University Press; 2019. [Google Scholar]
- 326.Proctor RN. Golden Holocaust: Origins of the Cigarette Catastrophe and the Case for Abolition. Berkeley, CA: University of California Press; 2012. [Google Scholar]
- 327.Kilpatrick DG, Acierno R, Saunders B, Resnick HS, Best CL, Schnurr PP. Risk factors for adolescent substance abuse and dependence: Data from a national sample. Journal of Consulting and Clinical Psychology 2000; 68(1): 19–30. [DOI] [PubMed] [Google Scholar]
- 328.Jones DJ, Lewis T, Litrownik A, et al. Linking Childhood Sexual Abuse and Early Adolescent Risk Behavior: The Intervening Role of Internalizing and Externalizing Problems. Journal of Abnormal Child Psychology 2013; 41(1): 139–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Esposito CL, Clum GA. Social support and problem-solving as moderators of the relationship between childhood abuse and suicidality: Applications to a delinquent population. Journal of Traumatic Stress 2002; 15(2): 137–46. [DOI] [PubMed] [Google Scholar]
- 330.Teesson M, Newton NC, Slade T, et al. Combined prevention for substance use, depression, and anxiety in adolescence: a cluster-randomised controlled trial of a digital online intervention. The Lancet Digital Health 2020; 2(2): e74–e84. [DOI] [PubMed] [Google Scholar]
- 331.University of Washington Center for Communities That Care. Communities that Care Plus. 2020. https://www.communitiesthatcare.net (accessed November 2, 2020).
- 332.Alcohol Public Policy Group. Alcohol: No Ordinary Commodity – a summary of the second edition. Addiction 2010; 105(5): 769–79. [DOI] [PubMed] [Google Scholar]
- 333.Barr A, Gibbs CR. Breaking the Cycle? Intergenerational Effects of an Anti-Poverty Program in Early Childhood. 2017. [Google Scholar]
- 334.Social Programs that Work. Nurse-Family Partnership. 2020. https://evidencebasedprograms.org/about-us/ (accessed November 2, 2020).
- 335.Schweinhart LJ. Benefits, Costs, and Explanation of the High/Scope Perry Preschool Program. Society for Research in Child Development 2003. [Google Scholar]
- 336.Barnett S, Belfield C, Montie J, Nores M, Scheweihart L, Xiang Z. Lifetime Effects: The High/Scope Perry Preschool Study Through Age 40. Web: High/Scope Press.; 2005. [Google Scholar]
- 337.Campbell FA, Ramey CT, Pungello E, Sparling J, Miller-Johnson S. Early Childhood Education: Young Adult Outcomes From the Abecedarian Project. Applied Developmental Science 2002; 6(1): 42–57. [Google Scholar]
- 338.Canadian Institute for Health Information. Opioid Prescribing in Canada: How Are Practices Changing? Ottawa, ON, 2019. [Google Scholar]
- 339.Howard R, Fry B, Gunaseelan V, et al. Association of Opioid Prescribing With Opioid Consumption After Surgery in Michigan. JAMA Surg 2019; 154(1): e184234–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Choose PT. In 2016 Prescribed Opioids Resulted in 3.3 Billion Unused Pills. https://www.choosept.com/didyouknow/detail/in-2016-prescribed-opioids-resulted-in-3-3-billion (accessed December 12, 2020).
- 341.Arnarsson A, Kristofersson GK, Bjarnason T. Adolescent alcohol and cannabis use in Iceland 1995–2015. Drug Alcohol Rev 2018; 37 Suppl 1: S49–s57. [DOI] [PubMed] [Google Scholar]
- 342.Jonsson G, Milkman H. Perspective: Iceland Succeeds at Preventing Teenage Substance Use. In: Stephens M, El-Sholkamy MM, Moonesar IA, Awamleh R, eds. Future Governments (Actions and Insights - Middle East North Africa, Vol 7) Bingley, UK: Emerald Publishing Limited; 2019: 315–24. [Google Scholar]
- 343.Kristjansson AL, Mann MJ, Sigfusson J, Thorisdottir IE, Allegrante JP, Sigfusdottir ID. Development and Guiding Principles of the Icelandic Model for Preventing Adolescent Substance Use. Health Promot Pract 2020; 21(1): 62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Young E How Iceland Got Teens to Say No to Drugs. The Atlantic, January 19, 2017. https://www.theatlantic.com/health/archive/2017/01/teens-drugs-iceland/513668/ (accessed January 19, 2017). [Google Scholar]
- 345.Raitasalo K, Kraus L, Bye EK, et al. Similar countries, similar factors? Studying the decline of heavy episodic drinking in adolescents in Finland, Norway and Sweden. Addiction 2021; 116(1): 62–71. [DOI] [PubMed] [Google Scholar]
- 346.Planet Youth. Communities: Countries introduced to the Icelandic model. 2020. https://planetyouth.org/get-involved/communities/ (accessed November 3, 2020).
- 347.California Product Stewardship Council. Products | CPSC. 2020. https://www.calpsc.org/products (accessed December 13, 2020).
- 348.Albert S, Brason FW 2nd, Sanford CK, Dasgupta N, Graham J, Lovette B. Project Lazarus: community-based overdose prevention in rural North Carolina. Pain Med 2011; 12 Suppl 2: S77–85. [DOI] [PubMed] [Google Scholar]
- 349.Stewart H, Malinowski A, Ochs L, Jaramillo J, McCall K 3rd, Sullivan M. Inside Maine’s Medicine Cabinet: Findings From the Drug Enforcement Administration’s Medication Take-Back Events. American journal of public health 2015; 105(1): e65–e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.United States Government Accountability O. Preventing Drug Abuse. 2017. https://www.gao.gov/assets/690/687719.pdf (accessed November 8, 2020).
- 351.Johnson K, Rigg KK, Hopkins Eyles C. Receiving addiction treatment in the US: Do patient demographics, drug of choice, or substance use disorder severity matter? Int J Drug Policy 2020; 75: 102583. [DOI] [PubMed] [Google Scholar]
- 352.Shover CL, Humphreys K. Predictors of availability of long-acting medication for opioid use disorder. Drug Alcohol Depend 2019; 204: 107586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Lehman WEK, Simpson DD, Knight DK, Flynn PM. Integration of treatment innovation planning and implementation: strategic process models and organizational challenges. Psychology of addictive behaviors : journal of the Society of Psychologists in Addictive Behaviors 2011; 25(2): 252–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Humphreys K, Pollack HA. How Should the United States Respond to the Opioid Addiction and Overdose Epidemic? In: Goldman HH, Frank RG, Morrissey JP, eds. The Palgrave Handbook of American Mental Health Policy. New York, NY: Springer International Publishing; 2020: 259–95. [Google Scholar]
- 355.Larance B, Dobbins T, Peacock A, et al. The effect of a potentially tamper-resistant oxycodone formulation on opioid use and harm: main findings of the National Opioid Medications Abuse Deterrence (NOMAD) study. The Lancet Psychiatry 2018; 5(2): 155–66. [DOI] [PubMed] [Google Scholar]
- 356.Becker WC, Fiellin DA. Abuse-Deterrent Opioid Formulations — Putting the Potential Benefits into Perspective. New England Journal of Medicine 2017; 376(22): 2103–5. [DOI] [PubMed] [Google Scholar]
- 357.Reuter P, Caulkins JP, Midgette G. Heroin use cannot be measured adequately with a general population survey. Addiction 2021; n/a(n/a). [DOI] [PubMed] [Google Scholar]
- 358.Kilmer B, Caulkins JP. Hard drugs demand solid understanding: Column. USA Today, March 8, 2014. https://www.usatoday.com/story/opinion/2014/03/08/heroin-abuse-hoffman-research-column/6134337/ (accessed November 10, 2020). [Google Scholar]
- 359.Oquendo MA, Volkow ND. Suicide: A Silent Contributor to Opioid-Overdose Deaths. New England Journal of Medicine 2018; 378(17): 1567–9. [DOI] [PubMed] [Google Scholar]
- 360.Rockett IRH, Caine ED, Banerjee A, et al. Fatal self-injury in the United States, 1999–2018: Unmasking a national mental health crisis. EClinicalMedicine 2021; 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.National Drug Early Warning System. Introducing the new NDEWS Coordinating Center at UF. 2020. https://ndews.org (accessed November 10, 2020).
- 362.European Monitoring Centre on Drugs and Drug Addiction. Wastewater analysis and drugs — a European multi-city study. 2020. https://www.emcdda.europa.eu/topics/pods/waste-water-analysis_en (accessed November 10, 2020).
- 363.Cornelius K Sewage Is Helping Cities Flush Out the Opioid Crisis. Scientific American, May 25, 2018. https://www.scientificamerican.com/article/sewage-is-helping-cities-flush-out-the-opioid-crisis/ (accessed. [Google Scholar]
- 364.Kennedy DM. Don’t Shoot: One Man, A Street Fellowship, and the End of Violence in Inner-City America. New York, NY: Bloomsbury; 2011. [Google Scholar]
- 365.Zhu Y, Wienecke CFR, Nachtrab G, Chen X. A thalamic input to the nucleus accumbens mediates opiate dependence. Nature 2016; 530(7589): 219–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Schmid CL, Kennedy NM, Ross NC, et al. Bias Factor and Therapeutic Window Correlate to Predict Safer Opioid Analgesics. Cell 2017; 171(5): 1165–75.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Gillis A, Kliewer A, Kelly E, et al. Critical Assessment of G Protein-Biased Agonism at the μ-Opioid Receptor. Trends in Pharmacological Sciences 2020; 41(12): 947–59. [DOI] [PubMed] [Google Scholar]
- 368.Whitehouse.gov. Advisory to Financial Institutions on Illicit Financial Schemes and Methods Related to the Trafficking of Fentanyl and Other Synthetic Opioids, 2019.
- 369.NASA Tournament Lab. The Opioid Detection Challenge. 2019. https://www.opioiddetectionchallenge.com (accessed September 6, 2020).
- 370.Collins FS, Schwetz TA, Tabak LA, Lander ES. ARPA-H: Accelerating biomedical breakthroughs. Science 2021; 373(6551): 165–7. [DOI] [PubMed] [Google Scholar]
- 371.Kalkman GA, Kramers C, van Dongen RT, van den Brink W, Schellekens A. Trends in use and misuse of opioids in the Netherlands: a retrospective, multi-source database study. The Lancet Public Health 2019; 4(10): e498–e505. [DOI] [PubMed] [Google Scholar]
- 372.International Narcotics Control Board. Narcotic Drugs, 2019.
- 373.Curtis HJ, Croker R, Walker AJ, Richards GC, Quinlan J, Goldacre B. Opioid prescribing trends and geographical variation in England, 1998–2018: a retrospective database study. Lancet Psychiatry 2019; 6(2): 140–50. [DOI] [PubMed] [Google Scholar]
- 374.Krawczyk N, Greene MC, Zorzanelli R, Bastos FI. Rising Trends of Prescription Opioid Sales in Contemporary Brazil, 2009–2015. American Journal of Public Health 2018; 108(5): 666–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Blanch B, Pearson SA, Haber PS. An overview of the patterns of prescription opioid use, costs and related harms in Australia. Br J Clin Pharmacol 2014; 78(5): 1159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Knaul FM, Bhadelia A, Rodriguez NM, Arreola-Ornelas H, Zimmermann C. The Lancet Commission on Palliative Care and Pain Relief: findings, recommendations, and future directions. The Lancet Global Health 2018; 6: S5–S6. [Google Scholar]
- 377.Centers for Disease Control and Prevention. U.S. Opioid Dispensing Rate Maps. 2021. https://www.cdc.gov/drugoverdose/maps/rxrate-maps.html.
- 378.Böckerman P, Haapanen M, Hakulinen C, Karhunen H, Maczulskij T. Determinants of prescription opioid use: population-based evidence from Finland. Addiction 2021; 116(1): 170–5. [DOI] [PubMed] [Google Scholar]
- 379.Ryan H, Girion L, Glover S. OxyContin goes global — “We’re only just getting started”. Los Angeles Times, December 18, 2016. https://www.latimes.com/projects/la-me-oxycontin-part3/ (accessed November 10, 2020). [Google Scholar]
- 380.Varney S How big pharma is targeting India’s booming opioid market. The Guardian, August 27, 2019, 2019. https://www.theguardian.com/world/2019/aug/27/india-opioids-crisis-us-pain-narcotics (accessed November 10, 2020). [Google Scholar]
- 381.Varney S Opioid addiction rising in India as US drugmakers push painkillers. The Guardian, August 28, 2019, 2019. https://www.theguardian.com/world/2019/aug/28/india-opioids-addiction-us-drugmakers-push-painkillers (accessed November 10, 2020). [Google Scholar]
- 382.Kinetz E Fake doctors, misleading claims drive OxyContin China sales. Associated Press. 2019. November 19, 2019. [Google Scholar]
- 383.Furlan AD, Harvey AM, Chadha R. Warning from Canada: Latin America, South Africa and India may face an opioid epidemic in the coming years. J Glob Health 2020; 10(1): 010324-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Mann B Federal Judge Approves Landmark $8.3 Billion Purdue Pharma Opioid Settlement. NPR, November 17, 2020, 2020. https://www.npr.org/2020/11/17/936022386/federal-judge-approves-landmark-8-3-billion-purdue-pharma-opioid-settlement (accessed December 9, 2020). [Google Scholar]
- 385.Victor RG, Lynch K, Li N, et al. A Cluster-Randomized Trial of Blood-Pressure Reduction in Black Barbershops. New England Journal of Medicine 2018; 378(14): 1291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.National Heart Lung and Blood Institute. One year later, barbershop intervention continues to lower blood pressure in black men. 2018.
- 387.Pellowski JA, Kalichman SC, White D, Amaral CM, Hoyt G, Kalichman MO. Real-time medication adherence monitoring intervention: test of concept in people living with HIV infection. J Assoc Nurses AIDS Care 2014; 25(6): 646–51. [DOI] [PMC free article] [PubMed] [Google Scholar]


