Abstract
A total of 46 brewery and 15 ATCC Pediococcus isolates were ribotyped using a Qualicon RiboPrinter. Of these, 41 isolates were identified as Pediococcus damnosus using EcoRI digestion. Three ATCC reference strains had patterns similar to each other and matched 17 of the brewery isolates. Six other brewing isolates were similar to ATCC 25249. The other 18 P. damnosus brewery isolates had unique patterns. Of the remaining brewing isolates, one was identified as P. parvulus, two were identified as P. acidilactici, and two were identified as unique Pediococcus species. The use of alternate restriction endonucleases indicated that PstI and PvuII could further differentiate some strains having identical EcoRI profiles. An acid-resistant P. damnosus isolate could be distinguished from non-acid-resistant varieties of the same species using PstI instead of EcoRI. 16S rRNA gene sequence analysis was compared to riboprinting for identifying pediococci. The complete 16S rRNA gene was PCR amplified and sequenced from seven brewery isolates and three ATCC references with distinctive riboprint patterns. The 16S rRNA gene sequences from six different brewery P. damnosus isolates were homologous with a high degree of similarity to the GenBank reference strain but were identical to each other and one ATCC strain with the exception of 1 bp in one strain. A slime-producing, beer spoilage isolate had 16S rRNA gene sequence homology to the P. acidilactici reference strain, in agreement with the riboprint data. Although 16S rRNA gene sequencing correctly identified the genus and species of the test Pediococcus isolates, riboprinting proved to be a better method for subspecies differentiation.
Identification of brewery bacterial isolates has traditionally been accomplished biochemically by determining the assimilation and fermentation patterns of a number of carbohydrates and nitrogen sources. Advances have been made in automating and improving detection times using biochemical methods. Both the Biolog system and API Rapid CH kits have proven to be useful for identifying beer spoilage lactic acid bacteria. However, biochemical identification is not accurate for determining the genotypic differences of microorganisms. A more accurate method for genotype determination is that of the molecular biological approach of ribotyping by comparing similarities in the rRNA gene sequences. A more recent ribotyping technique is the patented method called riboprinting. This method is based on restriction endonuclease digestion of bacterial chromosomal DNA, followed by Southern hybridization to probes for sequences in the regions of bacterial DNA coding for the 5S-16S-23S (the Escherichia coli rrnB rRNA operon) rRNA operon (1). The probes have been developed that are directed to highly conserved regions of the rRNA operon present in all eubacteria and can therefore be used for ribotyping most bacteria. Restriction fragments analyzed by probe hybridization range in size from approximately 1 to 50 kb, meaning that the fragments could potentially represent genetic information as far as 50 kb upstream or downstream of the rRNA operons, as well as information within the operons. The fragment sizes and subsequent gel electrophoretic separation patterns can vary with the use of different restriction endonucleases. The number of copies of the rRNA operon has been shown to vary from 1 to 15 copies for different bacteria (4). For example, E. coli contains seven copies of the rRNA operon, which map over a widespread distance in the bacterial chromosome and are not clustered in one region (2). However, the distribution of the operons as well as the copy number varies with different species of bacteria, which in turn will vary the amount of DNA that hybridize with the riboprinting probes.
The Qualicon (DuPont) RiboPrinter is an automated system that takes a purified bacterial suspension, lyses the cells, extracts the DNA, restriction endonuclease digests the DNA, separates the digest on a gel, transfers the DNA bands to a membrane, probes the bands with non-radioisotope-labeled, 5S-16S-23S rRNA-specific probes (Southern hybridization), photographs the membrane, and finally compares the bar code-like pattern to databases in order to identify the genus and species. The entire process takes approximately 8 h and requires only a small amount of growth sample from a petri dish. Although this method and instrument were originally developed to identify the food-borne pathogens, Listeria, Salmonella, and Staphylococcus spp. and E. coli, it has since been used for many applications, including the identification of spoilage bacteria in the brewing industry (3, 5–8, 10; K. J. Bjorkroth, M.-L. Suihko, J. L. Bruce, D. Palmer, and H. J. Korkeala, Abstr. 98th Gen. Meet. Am. Soc. Microbiol., 1998; Y. Motoyama, T. Ogata, and K. Sakai, Abstr. Annu. Meet. Amer. Soc. Brew. Chem., 1997; R. Satokari, A. von Wright, and M.-L. Suihko, Abstr. Annu. Gen. Meet. Eur. Culture Collections Org., p. 16, 1998). The manufacturer has demonstrated with food-borne pathogens that the method is close to 100% accurate at identifying genus and species and often has the ability to differentiate at the subspecies level. This, in turn, has utility in epidemiological studies for tracking isolates. Based on a historical database of isolates encountered in manufacturing facilities, riboprinting may also be useful for predicting the pathogenicity, spoilage capability, or other phenotypic expressions of the organisms.
In a study by Motoyama et al. (6), ribotyping was shown to be useful in identifying brewery Lactobacillus isolates to the subspecies level. However, Storgards and Suihko (10) showed that a number of Lactobacillus lindneri isolates were ribotyped and shown to have almost no variation in their riboprint patterns. This could mean that the technology is not adequate to subspeciate this particular organism or that the organism shows very little genetic variation. It should be noted that only EcoRI DNA digestion was studied and that other restriction endonucleases may have been useful for better differentiation of the isolates. Isolates of other Lactobacillus species studied showed considerable variation in riboprint patterns. In another study by Motoyama et al. (7), the method was shown to be very useful for identifying brewery isolates of Pectinatus spp. In that study, it was shown that greater differentiation between Pectinatus isolates could be obtained when BamHI and HindIII restriction endonucleases were used in addition to EcoRI.
Satokari et al. (Abstr. Annu. Meet. Eur. Culture Collections Org.) have reported the riboprinting of brewery isolates of Pediococcus isolates. Their conclusion, obtained using EcoRI digestion, was that a number of strains could be accurately identified to the species level with some identification at the strain level. The present study presents similar analyses but shows improved subspecies identification using an additional restriction endonuclease and also demonstrates the utility for tracking a troublesome acid-resistant species.
Another method for ribotyping bacteria is to use the molecular biological approach of identifying bacteria based on the DNA sequence of the gene that codes for 16S rRNA. The sequence data for the 16S rRNA gene is highly conserved for different organisms and has also been shown to be very accurate for genus and species identification of eubacteria. We also present here a comparison of 16S rRNA gene sequencing to riboprinting results for identifying pediococci. The riboprinting method is shown to be superior to 16S rRNA sequencing for identifying pediococci to the subspecies level. This, in turn, is very useful to the brewing industry for tracking beer spoilage isolates and for rapidly determining their threat to product stability.
MATERIALS AND METHODS
Pediococcus isolates.
A number of brewing Pediococcus species isolates were subcultured on BMB (Barney-Miller Brewing, Difco product no. T634-17) and Lactobacillus-MRS (Difco product no. 0882-17) agars. Reference strains for different Pediococcus species were obtained from the American Type Culture Collection (ATCC). For riboprinting, isolates were subcultured on MRS agar as recommended by the manufacturer, except that isolates that would not grow on MRS agar were subcultured on BMB agar.
Table 1 lists the Pediococcus spp. that were used in these studies.
TABLE 1.
Pediococcus strains studied
| ATCC strains
|
Brewing isolatesa
|
|||||
|---|---|---|---|---|---|---|
| Species | ATCC no. | Species | ID | Species | ID | |
| P. acidilactici | 25742 | P. damnosus | FWP821 | P. damnosus | IB C2 | |
| P. acidilactici | 33314 | P. damnosus | FW819 | P. damnosus | EBP | |
| P. damnosus | 11308 | P. damnosus | FWM | P. damnosus | IB 1GA | |
| P. damnosus | 11309 | P. damnosus | FWS | P. damnosus | FWP YB5 | |
| P. damnosus | 25248 | Pediococcus sp. | CB-C | P. damnosus | ABP | |
| P. damnosus | 25249 | P. damnosus | TB 10/8 | P. damnosus | FWL-C | |
| P. damnosus | 29358 | P. damnosus | TB9 11/16 | P. damnosus | TB B9 | |
| P. damnosus | 43013 | P. damnosus | EB 103G | P. damnosus | SB L3 | |
| P. dextrinicus | 33087 | Pediococcus sp. | IB Prem2 | P. damnosus | TB-C | |
| P. inopinatus | 49902 | Pediococcus sp. | IB Prem3 | P. damnosus | AB Keg | |
| P. parvulus | 19371 | Pediococcus sp. | Belgium | P. damnosus | AB CO2W | |
| P. pentosaceus | 10791 | Pediococcus sp. | QPN | P. damnosus | AB C2W | |
| P. pentosaceus | 43200 | P. damnosus | ABC K3 | P. damnosus | TB KC1 | |
| P. pentosaceus | 43201 | Pediococcus sp. | Cerw-C | P. damnosus | TB 62 | |
| P. pentosaceus | 33316 | Pediococcus sp. | EB 404A | Pediococcus sp. | SB 15 | |
| Pediococcus sp. | IB B3 | P. damnosus | RB2 | |||
| Pediococcus sp. | RB2 | P. damnosus | IB 2G4 | |||
| P. damnosus | IB 3G4 | P. damnosus | IB 2G7 | |||
| P. damnosus | SB L2 | P. damnosus | IB 2E19 | |||
| P. damnosus | SB L6 | P. damnosus | TB KC21 | |||
| P. damnosus | SB L9 | P. damnosus | IB B3 821 | |||
| P. damnosus | SB L15 | P. damnosus | IB 3G3 | |||
| P. damnosus | SB L16 | P. damnosus | SB L1 | |||
ID, identification designation.
Biochemical identification of Pediococcus isolates.
All of the brewery isolates assigned a species name were identified using API 50 Rapid CHL strips from bioMérieux using carbohydrate assimilation-fermentation of 49 different compounds (and one control). These were incubated at 28°C for 3 to 6 days. The organisms were identified using API's computer database for comparison of assimilation and/or fermentation patterns.
Riboprinting.
Riboprinting was performed using a RiboPrinter Microbial Characterization System (Qualicon, Wilmington, Del.) and following the manufacturer's recommended procedures. Initially, riboprinting used a standard RiboPrinter kit incorporating EcoRI restriction endonuclease supplemented with a lactic agent (supplied for use with identifying lactic acid bacteria). In the first experiments duplicate picks of late-log- to stationary-phase colonies grown on MRS agar were treated by the manufacturer's protocol in duplicate on different days. However, during the course of this study several isolates were shown to deteriorate very quickly upon reaching the stationary growth phase, providing poor DNA isolation, nuclease degradation of DNA, and restriction endonuclease digestion. Therefore, to obtain consistent, reproducible RiboPrinter patterns, it was necessary to use cells in the mid- to late-log phase of growth.
Bacterial DNA isolation and PCR amplification of the 16S rDNA gene.
Test strains were cultured to exponential phase in 10 ml of Lactobacillus-MRS broth. DNA was extracted from the cells and purified by a modification of the method described by Sami et al. (9). Cells were harvested by centrifugation at 10,000 × g for 5 min, followed by resuspension in 2 ml of lysis buffer (10 mM Tris-HCl, 1 mM EDTA, and 0.35 M sucrose [pH 8.0] containing 1 mg of lysozyme and 50 μg of N-acetylmuramidase per ml), and incubated at 37°C for 1 h. Then, 4 ml of CTAB buffer (100 mM Tris-HCl, 1.5 M NaCl, 10 mM EDTA, 2% cetyltrimethylammonium bromide [CTAB] [pH 8.0], and 80 μg of proteinase K per ml) was then added to the digested cells and heated to 50°C for 2 h. After cooling, the mixture was emulsified with 4 ml of chloroform-isoamyl alcohol (97:3) followed by phase separation by centrifugation at 1,000 × g for 5 min. The aqueous phase was removed and extracted two more times with chloroform-isoamyl alcohol. The aqueous phase was then precipitated with 2 volumes of isopropanol and incubation at 4°C for 1 h, and the precipitate was harvested by centrifugation at 10,000 × g for 10 min. The pellet was then rinsed with 70% isopropanol. After it was dried, the pellet was redissolved in 1.0 ml of TE buffer (10 mM Tris-HCl, 1 mM EDTA [pH 8.0]) containing 500 μg of RNase A per ml and incubated at 37°C for 1 h. Then, 0.1 volume of 3 M sodium acetate (pH 5.2) was added, followed by 2 volumes of ethanol. The DNA was then allowed to precipitate overnight at −80°C. The precipitate was removed by centrifugation at 15,000 × g for 10 min at 4°C. After being rinsed with 70% ethanol, the pellet was dried and then resuspended in TE buffer. The 16S rDNA gene was PCR amplified from the isolated DNA using the following primers (based on the E. coli 16S rDNA rrnB sequence; GenBank accession no. U00096): forward primer (beginning at base 5), TGAGAGAGTTTGATCCTGGCTCAG; and reverse primer (beginning with base 1541), AAGGAGGTGATCCAGCCGCA. PCR was accomplished using reagents from a Qiagen Taq PCR Core Kit (Qiagen, Inc., Valencia, Calif.). Thermocycling conditions were as follows: 3 min at 94°C; followed by 30 cycles of 30 s at 94°C, 30 s at 45°C, and 1.5 min at 72°C; followed by 10 s at 72°C and an infinity hold at 4°C. PCR products were analyzed by 1% agarose electrophoresis separation and ethidium bromide staining comparing the bands to a 100-bp ladder molecular weight standard.
Primer design for 16S rRNA gene sequencing and gene assembly.
The following four forward and five reverse primers were designed for PCR amplification of the 16S rRNA gene sequencing the 16S rRNA gene of E. coli rrnB (GenBank accession no. U00096): 5F, TGAAGAGTTTGATCATGGCTCAG; 324F, GACACGGTCCAGACTCCTA; 773F, GGGGAGCAAACAGGATTAGA; 1063F, CGTCAGCTCGTGTTGTGATTTGTT; 1541R, AAGGAGGTGATCCAACCGCA; 1361R, CTGATCCACGATTACTAGCGAT; 996R, TGTCAAGACCAGGTAAGGTTCT; 809R, CGTGGACTACCAGGGTATCTAA; and 332R, CCGTGTCTCAGTTCCAGTGT. The PCR-amplified 16S rRNA genes from each isolate were then sequenced using the nine primers described above with rhodamine dye terminator sequencing and analysis using an ABI Prism 377 Genetic Analyzer (PE Applied Biosystems, Foster City, Calif.). The sequence information was then imported into the DNASTAR, Inc. (Madison, Wis.), SeqMan II and MegAlign software programs for assembly and alignment. Sequences were compared to GenBank reference strains of P. damnosus (GenBank accession no. D87678) and P. acidilactici (GenBank accession no. M58833).
RESULTS
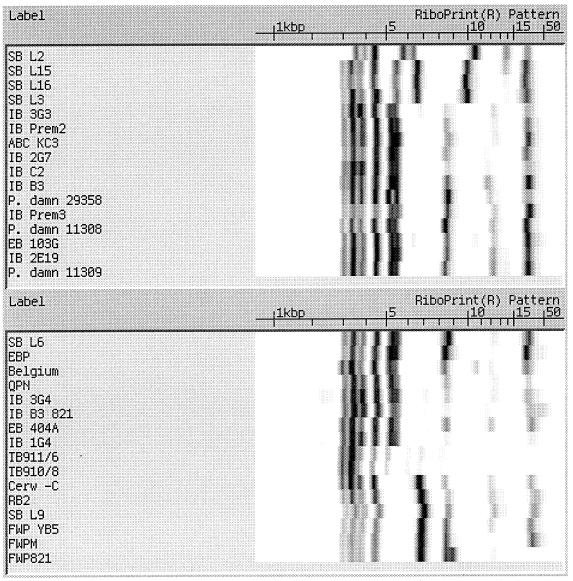
Figure 1 displays the EcoRI RiboPrint results for the 61 Pediococcus isolates tested in this study. The isolates are ordered on the similarity of their RiboPrinter patterns based on the instrument's computer algorithm. The instrument can also be used to display a similarity index to any specified organism, but for this comparison only the similarity grouping is used to show the differences in patterns. Since the Qualicon instrument had a very limited database for industrial isolates of lactic acid bacteria, the riboprint patterns of ATCC reference strains were used to establish typical riboprint patterns for different Pediococcus species. It is noted that FWP 821, FWPS, and FWL-C were all acid-resistant isolates of the same species of P. damnosus and have RiboPrinter patterns with a high degree of similarity. On the other hand, FWPM, FWP YB5, and FW819, which were all identified as non-acid resistant and as the same genus and species (P. damnosus), had riboprints results significantly different from those of the acid-resistant isolates. Of particular interest is the extra band around 9 kbp in the riboprints for the acid-resistant strains. The non-acid-resistant strains were isolated during a 3-year period from the same source in the same brewery as the acid-resistant species listed above. The acid-resistant isolates display a different riboprint pattern with a unique double band in the 8- to 9-kb size range.
FIG. 1.
EcoRI riboprints of Pediococcus isolates and type culture collection reference strains.
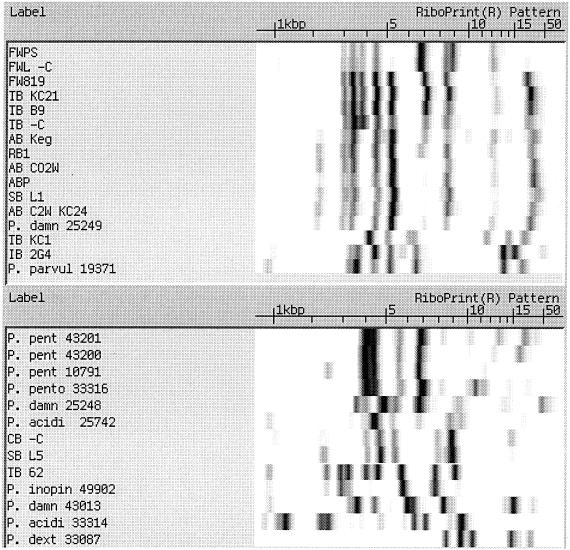
Figure 2 groups EcoRI riboprint patterns for only the ATCC Pediococcus reference strains studied. There were six different P. damnosus strains tested. Three of these, strains 11308, 11309, and 29358, had similar patterns, while the other three, strains 25248, 25249, and 43013, had distinctively different patterns. It is also noted that the P. pentosaceus strains (43201, 43200, 10791, and 33316) all had similar riboprint patterns. P. acidilactici 25742 was identified correctly (according to the instrument's database). In Fig. 1 the RiboPrint pattern for P. acidilactici 33314 showed very strong identity with Staphylococcus warneri (by comparison to the instrument's database). Upon further testing, this strain was shown to be catalase positive and displayed a microscopic morphology similar to Staphylococcus. Therefore, it was concluded that the strain was not a Pediococcus, and it is not known whether the original culture was misidentified or if it had become contaminated. For this reason it was not included as a Pediococcus species in Fig. 2.
FIG. 2.
Comparison of ATCC Pediococcus strains riboprinted with EcoRI.
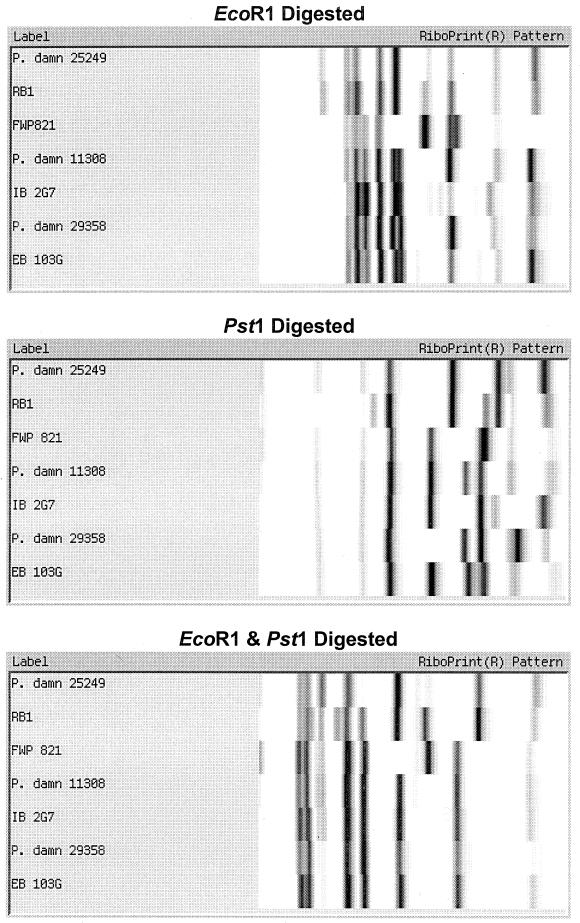
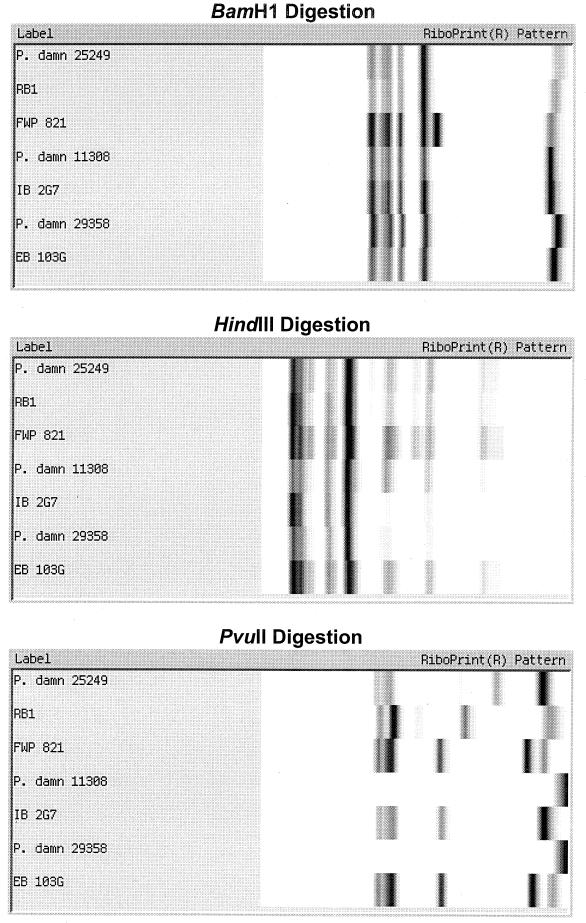
Figure 3 shows the results of comparing seven different randomly chosen Pediococcus isolates using six different restriction endonuclease digestions: EcoRI (the standard method), PstI, EcoRI-PstI, BamHI, HindIII, and PvuII. The results show that EcoRI displayed similar patterns for the first two isolates, a distinctive pattern for the third isolate, and moderate similarity in patterns for the last four isolates. However, the two additional faint bands in the middle of the gel for strain IB 2G7 were seen on replicate samples, allowing it to be distinguished from the other strains. In comparison, PstI digestion produced unique patterns for all seven isolates. Combining EcoRI and PstI did not allow the last four isolates to be differentiated. BamHI digestion showed one unique pattern for strain FWP821, while the other six isolates had similar patterns. HindIII produced a number of minor bands but did not clearly separate the seven isolates. PvuII did not digest strains 11308 and 29358, gave the same pattern for two other isolates, and provided unique patterns for the remaining three isolates.
FIG. 3.
Comparison of seven Pediococcus isolates riboprinted with different restriction endonucleases.
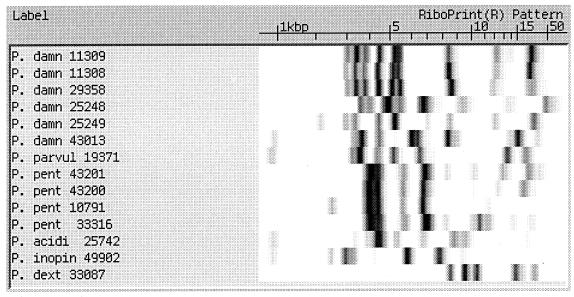
Comparison to 16S RNA-DNA sequencing using the PE Applied Biosystems instrument.
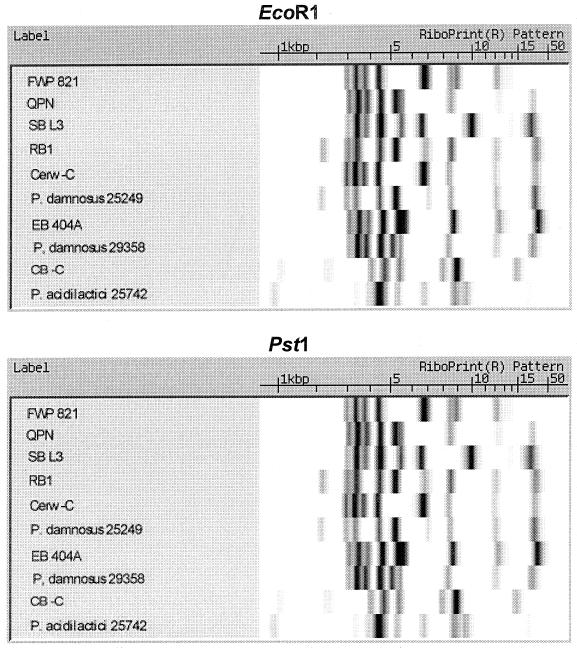
Ten different Pediococcus isolates were selected for 16S rRNA gene sequencing. EcoRI and PstI riboprint patterns of these isolates are presented in Fig. 4. The EcoRI riboprints showed nine different patterns, while the PstI riboprints showed unique patterns for all 10 isolates. Eight of these were shown to be P. damnosus subspecies. One other was an ATCC reference strain for P. acidilactici, strain 25742, and the final strain was a slime-producing, beer spoiler strain whose RiboPrint results showed it to be most closely related to P. acidilactici. When the sequences for the 16S rRNA gene of the six P. damnosus brewery isolates and the ATCC reference strain were compared, there was only a 1-bp difference in one of the brewery isolates. These were compared to a GenBank sequence for P. damnosus (accession no. D87678) and were also shown to have a very high degree of homology with this reference strain. The test P. acidilactici ATCC 25742 strain was also shown to have complete homology with the GenBank reference strain (accession no. M58833). The slime-forming, beer spoilage isolate's 16S rRNA gene sequence had very high homology to the GenBank reference sequence and the ATCC test strain, P. acidilactici ATCC 25742 (Fig. 5).
FIG. 4.
RiboPrint pattern comparisons of 10 different Pediococcus isolates made using EcoRI and PstI restriction endonucleases.
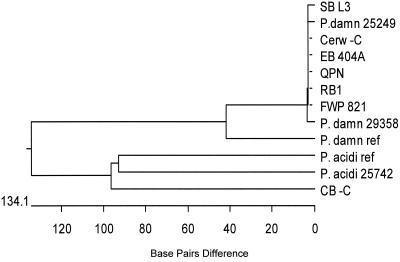
FIG. 5.
Relatedness of Pediococcus isolates based on 16S rRNA gene sequence data.
Figure 5 gives a phylogenetic tree of the strains based on their 16S rRNA gene sequences. As can be seen, all of the P. damnosus brewery isolates and ATCC strains are almost completely identical. Also, the GenBank reference strain shows close homology to the strains tested. The slime-producing beer spoilage Pediococcus isolate, CB-C, which was identified as P. acidilactici by riboprinting, showed very high homology with the GenBank reference strain and the ATCC test strain, P. acidilactici 25742.
Figure 6 presents partial sequence data (from base 70 to 100; all other bases were identical for all of the strains tested) for the 16S rRNA genes for the P. damnosus brewery isolates and two ATCC test strains. The sequences were identical with the exception of base number 87, which is an adenine in strain SB L3 and a guanidine for all of the other isolates.
FIG. 6.
Sequence data for the 16S rRNA gene for P. damnosus isolates.
DISCUSSION
The present study demonstrated that riboprinting is a very useful tool for identifying brewery Pediococcus isolates. The standard EcoRI digestion procedure used with the RiboPrinter did not provide as much differentiation of subspecies as did the use of the alternate enzyme PstI. PvuII also provided some additional differentiation compared to EcoRI but was not as useful as PstI. BamHI and HindIII digestions did not differentiate subspecies as well as EcoRI.
16S ribosomal DNA (rDNA) sequencing of a number of isolates showed that P. damnosus isolates with distinctively different RiboPrinter patterns had identical sequences, except for 1 bp in one strain. The sequence analysis method was very good at identifying the organisms by genus and species but did not differentiate at the subspecies level. The riboprint method, on the other hand, gave the correct genus and species and also allowed the subspeciation of many strains. It was particularly useful in identifying an acid-resistant isolate. An unusual slime-producing, beer spoilage isolate was riboprinted, and 16S rDNA sequence analysis identified it as P. acidilactici. The ability of riboprinting to identify brewing Pediococci at the subspecies level makes the RiboPrint method a powerful tool for tracking isolates in the brewery and for rapidly identifying species that are particularly detrimental to product microbiological stability.
REFERENCES
- 1.Bruce J L, Hubner R J, Cole E M, McDowell C I, Webster J A. Sets of EcoRI fragments containing ribosomal RNA sequences are conserved among different strains of Listeria monocytogenes. Proc Natl Acad Sci USA. 1995;92:5229–5233. doi: 10.1073/pnas.92.11.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellwood M, Nomura M. Chromosomal locations of genes for rRNA in Escherichia coli K-12. J Bacteriol. 1882;149:458–468. doi: 10.1128/jb.149.2.458-468.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Funuahashi W, Suzuki K, Ohtake Y, Yamashita H. Two novel beer-spoilage Lactobacillus species isolated from breweries. J Am Soc Brew Chem. 1998;56:64–69. [Google Scholar]
- 4.Klappenbach J A, Dunbar J M, Schmidt T M. rRNA operon copy number reflects ecological strategies of bacteria. Appl Environ Microbiol. 2000;66:1328–1333. doi: 10.1128/aem.66.4.1328-1333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leisner J J, Pot B, Christensen H, Rusul G, Olsen J, Wee B W, Muhamad K, Ghazali H. Identification of lactic acid bacteria from chili bo, a Malaysian food ingredient. Appl Environ Microbiol. 1999;65:599–605. doi: 10.1128/aem.65.2.599-605.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Motoyama Y, Funahashi W, Ogata T. Characterization of Lactobacillus spp. by ribotyping. J Am Soc Brew Chem. 2000;58:1–3. [Google Scholar]
- 7.Motoyama Y, Ogata T, Sakai K. Characterization of Pectinatus cerevisiiphilus and P. frisingensis by ribotyping. J Am Soc Brew Chem. 1998;56:19–23. [Google Scholar]
- 8.Nakakita Y N, Takahashi T, Sugiyama H, Shigyo T, Shiotsuka K. Isolation of novel beer-spoilage bacteria from the brewery environment. J Am Soc Brew Chem. 1998;56:114–117. [Google Scholar]
- 9.Sami M, Yamashita H, Kadokura H, Ktamoto D, Yoda K, Yamasaki M. A new and rapid method for the determination of beer-spoilage ability of lactobacilli. J Am Soc Brew Chem. 1997;55:137–140. [Google Scholar]
- 10.Storgards E, Suihko M-L. Detection and identification of Lactobacillus lindneri from brewery environments. J Inst Brew. 1998;104:47–54. [Google Scholar]