Abstract
Background:
The muscle quality of the rotator cuff (RC), measured by atrophy and fatty infiltration (FI), is a key determinant of outcomes in RC injury and repair. The ability to regenerate muscle after repair has been shown to be limited.
Purpose:
To determine if there is a source of resident endogenous stem cells, fibroadipogenic progenitor cells (FAPs), within RC injury patients, and if these cells are capable of adipogenic, fibrogenic, and pro-myogenic differentiation.
Study Design:
Controlled laboratory study.
Methods:
A total of 20 patients between the ages of 40 and 75 years with partial- or full-thickness RC tears of the supraspinatus and evidence of atrophy and FI Goutallier grade 1, 2, or 3 were selected from 2 surgeons at an orthopaedic center. During the surgical repair procedure, supraspinatus muscle biopsy specimens were obtained for analysis as were deltoid muscle biopsy specimens to serve as the control. FAPs and satellite cells were quantified using fluorescence-activated cell sorting. Muscle FI and fibrosis was quantified using Oil Red O and Masson trichrome staining. FAP differentiation and gene expression profiles were compared across tear sizes after culture in adipogenic, fibrogenic, and beta-3 agonist (amibegron) conditions. Analysis of variance was used for statistical comparisons between groups, with P < .05 as statistically significant.
Results:
Histologic analysis confirmed the presence of fat in biopsy specimens from patients with full-thickness tears. There were more FAPs in the full-thickness tear group compared with the partial-thickness tear group (9.43% ± 4.25% vs 3.84% ± 2.54%; P < .01). Full-thickness tears were divided by tear size, with patients with larger tears having significantly more FAPs than those with smaller tears. FAPs from muscles with full-thickness tendon tears had more adipogenic and fibrogenic potential than those with partial tears. Induction of a beige adipose tissue (BAT) phenotype in FAPs was possible, as demonstrated by increased expression of BAT markers and pro-myogenic genes including insulin-like growth factor 1 and follistatin.
Conclusion:
Endogenous FAPs are present within the RC and likely are the source of FI. These FAPs were increased in muscles with in larger tears but are capable of adopting a pro-myogenic BAT phenotype that could be utilized to improve muscle quality and patient function after RC repair.
Keywords: shoulder, rotator cuff, stem cell, fibroadipogenic progenitor
Rotator cuff (RC) injuries are the most common upper extremity cause for physician visits in the United States, ranking only behind back pain and neck pain for musculoskeletal physician visits.51 As the RC ages, it becomes susceptible to degenerative tears, which can lead to shoulder pain and dysfunction. Secondary muscle degradation after RC tears, including atrophy and fatty infiltration (FI), has been shown to directly determine the clinical outcome of patients with this injury.13,23,24 Up to 60% to 70% of patients with large and massive RC tears fail surgical repair, and 60% of anterior supraspinatus tears develop muscle degeneration, suggesting that muscle degeneration is more common than initially suspected.35
Despite the known importance of muscle degeneration after RC tears, relatively little is known about the cellular pathophysiology that occurs in patients with RC tears. In small-animal models, it has been shown that fibroadipogenic progenitor (FAP) cells are the key cell that mediates the transition to FI within RC muscle after injury.32 It has recently been determined that this cell line expands after RC injury, that it co-localizes to fat deposits within the muscle, and that outcomes of RC injury and repair are dependent on FAP presence in a small-animal model.6,50
Despite the correlation of FAPs with the development of FI, it does not suggest that the presence of FAPs is entirely deleterious. Recently, FAPs have been demonstrated to possess the capacity to transition to a beige fat phenotype both within RC tissue and in other muscle injury models. Brown and beige adipose tissue (BAT) serve a unique role in thermogenesis and energy production.17,40 The discovery of BAT as a potential metabolic source has gained considerable interest for the treatment of a variety of conditions, including diabetes.4,37 Unlike white adipose tissue that secretes primarily adipokines, BAT has been found to secrete factors that have a prominent role in promoting muscle growth and satellite cell (SC) population expansion.40,43 Putting these 2 bodies of research together, it stands to reason that if there are FAPs present within human RC tears, there may be an inducible source of stem cells that can be driven to a beige fat phenotype, improving muscle quality after RC injury and repair.
The purpose of this study was to evaluate the FAP cell population, gene expression, and differentiation capability in patients with RC tears. We hypothesized that patients with partial and small tears would have higher numbers of FAPs, and patients with large tears would not have significant numbers of FAPs.
METHODS
Patient Recruitment and Biopsy
The institutional review board (IRB) approved the study before commencement (IRB No. 18–26760). Patients were selected from 2 surgeons (B.T.F., C.B.M.) in the University of California, San Francisco Sports Medicine Clinic between June 2019 and October 2019. Inclusion criteria were patients aged 45–76 years with a partial- or full-thickness tear of the supraspinatus (SS) tendon, and the presence of atrophy and FI Goutallier grade 1, 2, or 3. Grade 4 was excluded, as a majority of these patients undergo reverse shoulder arthroplasty. Tear size was measured on the coronal T2-weighted magnetic resonance imaging (MRI) slice at the greater tuberosity footprint. Patients were divided into the following groups: partial-thickness, small (0–1 cm), medium (1–3 cm), and large (3–5 cm) tears. A total of 20 patients were enrolled. Patient data are shown in Table 1.
TABLE 1.
Descriptive Data of Included Patients and Tear Size
| Variable | Value |
|---|---|
|
| |
| Mean age, y (range) | 59.5 (45–76) |
| Sex, n | |
| Male | 14 |
| Female | 6 |
| Tear size | |
| Partial | 4 |
| Small | 4 |
| Medium | 5 |
| Large | 7 |
Patients gave consent in the clinic or on the day of surgery to the research coordinator or attending surgeon. At the time of surgery, the muscle belly of the SS was identified after the procedure (RC repair) was completed. The biopsy specimen was obtained from the superior aspect of the SS muscle, approximately 2 to 3 cm from the muscle-tendon junction, using a pituitary rongeur (Figure 1). The samples were approximately 5 to 7 mg each. As a control, a similar-sized sample of deltoid muscle was obtained from each patient. The sample was placed on ice and transferred for histologic or cell processing.
Figure 1.
Arthroscopic supraspinatus biopsy with a pituitary rongeur.
Histologic Processing
Muscle specimens were flash frozen by immersion in isopentane cooled with liquid nitrogen and sectioned (10 μm) with a cryostat. For immunofluorescence staining, sections were fixed with 2% paraformaldehyde for 10 minutes, washed in phosphate-buffered saline (PBS) and Triton (1× PBS/0.1% Triton X-100), blocked in 5% bovine serum albumin for 1 hour, and then incubated with primary antibodies at 4°C overnight.49 Slides were then washed in PBS and incubated with secondary antibodies (1:200) for 1 hour at room temperature. Tissue sections were mounted with VectaShield with DAPI. For evaluation of fibrosis, staining was carried out using a Masson trichrome kit (American Mastertech) per the manufacturer’s instructions. FI was assessed with Oil Red O staining.31
Immunofluorescence Staining
Samples were fixed in 4% paraformaldehyde for 30 minutes, rinsed 3 times in PBS for 10 minutes, placed in 0.1 M glycine (Fisher Scientific; diluted in PBS) for 30 minutes, and washed again 3 times in PBS for 10 minutes. They were then immersed in 100% methanol for 5 minutes at –20°C, rinsed 3 times in PBS for 10 minutes, and covered with blocking solution (0.2% Triton X-100, 2% bovine serum albumin in PBS) for 1 hour at room temperature. Primary antibodies (Developmental Studies Hybridoma Bank) against uncoupling protein 1 (UCP-1) were diluted in a block mix and added to the sections for overnight incubation at 4°C.2 The next day, samples were rinsed 3 times in PBS for 10 minutes and incubated in a mixture containing rhodamine-conjugated α-bungarotoxin (T0195; Sigma-Aldrich; diluted 1:200) and fluorescein isothiocyanate–conjugated donkey anti-mouse immunoglobulin (ab150 109; Abcam; diluted 1:250) at room temperature for 120 minutes. Sections were then rinsed for 20 minutes in PBS and mounted with coverslips using VectaShield with DAPI (H-1200; Vector Laboratories).
Image Capture and Quantification
Histology images were observed on an optical microscope (Axio Imager; Zeiss), and fluorescent images were acquired using an Axio Observer D1 fluorescence microscope. Pictures were analyzed using image analysis software (ImageJ; National Institutes of Health). All pictures were assessed by 2 blinded reviewers (M.L, O.A.). The area fraction of fibrosis was calculated by dividing the Aniline Blue staining collagenous fibrotic area by the entire sample area. Similarly, the area fraction of FI was assessed by dividing the Oil Red O staining fat area by the entire sample area.
Fluorescence-Activated Cell Sorting Analysis
Muscle was digested with 0.2% collagenase for 90 minutes followed by 0.4% dispase for 30 minutes. FAPs were isolated from human specimens using Beckton Dickinson Aria II with propidium iodide (PI) live and dead staining, and further isolated using CD31–, CD45–, CD184–, CD29–, CD56–, CD34+, and PDGFRα+ markers.46 SCs were sorted with PI live and dead staining, and further isolated using CD31–, CD45–, CD184+, CD29–, CD56+, CD34–, and PDGFRα– markers.6 Cell numbers for FAPs and SCs were reported as a percentage of live cells. The cells were then used for either polymerase chain reaction (PCR) analysis or differentiation evaluation.
Cell Differentiation
To determine if there are differences in the ability of FAPs to differentiate, cells from partial-thickness and full-thickness tears were placed in appropriate media conditions. FAPs were cultured in 24-well cell culture plates in standard media (F10 + 20% fetal bovine serum + 1% anti-biotics), fibrogenic media (10 ng/mL transforming growth factor [TGF]-β1), or adipogenic media (StemPro kit; Thermo Fisher) for 2 weeks. To test the ability of these cells to undergo BAT differentiation, they were also treated with the beta-3 adrenergic agonist amibegron. Cells in this group were treated with adipogenic media with 10 μM amibegron (Sigma) for 2 weeks. At 2 weeks, cells were assessed for fibrogenesis and adipogenesis by fixation with 4% paraformaldehyde and stained for collagen 1 for quantification of a fibrosis index and perilipin A for an adipogenic index.
Gene Expression
Total RNA for the samples was extracted using Trizol reagent (Fisher Scientific) according to the instructions. The Transcriptor First Strand cDNA Synthesis Kit (Roche Applied Bioscience Inc) was applied to synthesize cDNA. We performed reverse transcription PCR to quantify the expression of genes using SYBR Green Detection and an Applied Biosystems Prism 7900HT detection system (Applied Biosystems Inc). Sequences of the primers for target genes were summarized (Table 2). The expression level of each gene was normalized to that of the housekeeping gene of S26. Fold changes relative to sham controls were calculated using ΔΔCT.
TABLE 2.
Gene Function and RT-PCR Expression Primersa
| Gene | Function | Primer Sequence |
|---|---|---|
|
| ||
| Adiponectin | Protein hormone secreted by adipose tissue, increases fatty acid oxidation and insulin sensitivity | Forward: GCAGTCTGTGGTTCTGATTCCATAC Reverse: GCCCTTGAGTCGTGGTTTCC |
| PPARγ | Nuclear receptor that acts as transcription factor, activates adipogenesis, increases insulin sensitivity | Forward: GCAGTCTGTGGTTCTGATTCCATAC Reverse: GCCCTTGAGTCGTGGTTTCC |
| FABP4 | Cytoplasmic protein found in adipocytes that binds lipids, involved in lipid transport and metabolism | Forward: ATGGGATGGAAAATCAACCA Reverse: TGCTTGCTAAATCAGGGAAAA |
| CEBP | Transcription factor, promotes adipocyte proliferation and differentiation | Forward: ATGGGATGGAAAATCAACCA Reverse: TGCTTGCTAAATCAGGGAAAA |
| Leptin | Protein hormone secreted by adipocytes acts on brain to inhibit hunger/food intake | Forward: AGGGAGACCGAGCGCTTTC Reverse: TGCATCTCCACACACCAAACC |
| CD36 | Scavenger receptor found on adipocytes involved in import of lipids and adipocyte differentiation | Forward: GGGAAAGTCACTGCGACATGAT Reverse: ACGTCGGATTCAAATACAGCATAGA |
| UCP-1 | Mitochondrial protein that disrupts electron transport chain and produces heat, marker of brown fat | Forward: GTGTGCCCAACTGTGCAATG Reverse: CCAGGATCCAAGTCGCAAGA |
| PRDM-16 | Transcription regulator that displays histone methyltransferase activity and activates brown fat phenotype | Forward: CCACCAGCGAGGACTTCA Reverse: GGAGGACTCTCGTAGCTCGAA |
| MTUS-1 | Tumor suppressor protein, promotes brown fat differentiation | Forward: GGCATAGCTGGCCAAACAA Reverse: CACTTGGGAGAAGGCTTAGAATAAA |
| EVA-1 | Cell adhesion protein, expressed in white fat | Forward: GATGCCTCCATCCTTCTCTG Reverse: TTCCGGTAATGCTGGAAGAG |
| ZIC1 | Zinc-finger protein, brown fat marker | Forward: GGGAGAAGCCCTTCAAGTGCGAGTTTGAGGG Reverse: CGCAGGGTTCTTTCAGTAATGTTGTGTATAC |
| KCNK3 | Potassium channel protein, brown fat marker | Forward: GCAGACGCAGCCGCAGTATG Reverse: GCCTGGCCGTTGTGCGTGAGCAGGG |
| COL1A | Major component of type 1 collagen | Forward: CCATGCTCGCCTTTCTGCTCCTTT Reverse: CACTTGGGTGTTTGAGCATTGCCT |
| COL3A | Major component of type 3 collagen | Forward: TTGGCAGCAACGACACAGAAACTG Reverse: TTGAGTGCAGGGTCAGCACTACTT |
| αSMA | Protein that forms microfilaments, expressed in fibroblasts | Forward: TGCCTGCATGGGCAAGTGA Reverse: CTGGGCAGCGGAAACGGCA |
| F288 | Follistatin isoform, inhibits TGF-(b and myostatin, promotes myogenesis | Forward: CTCTGCCAGTTCATGGAGGA Reverse: TCCTTGCTCAGTTCGGTCTT |
| F315 | Follistatin isoform, inhibits TGF-(b and myostatin, promotes myogenesis | Forward: TGGACCGAGGAGGACGTGAA Reverse: AGACGCAGCGGGGTTTGTTC |
| IGF-1 | Growth hormone secreted by liver, increases glucose uptake, protein synthesis | Forward: GGCATAGCTGGCCAAACAA Reverse: CACTTGGGAGAAGGCTTAGAATAAA |
RT-PCR, reverse transcription polymerase chain reaction; TGF, transforming growth factor.
Statistical Analysis
The study was powered to determine differences in FAPs between deltoid muscle and full-thickness tears. On the basis of animal studies and preliminary data, 10 patients were required to detect a significant difference between groups.25,26,39 To determine differences in tear types, additional patients were added to the study. Analysis of variance was used for statistical comparisons between groups, with P < .05 as statistically significant. Data are presented as mean ± SD.
RESULTS
Histologic Evaluation of RC Tears
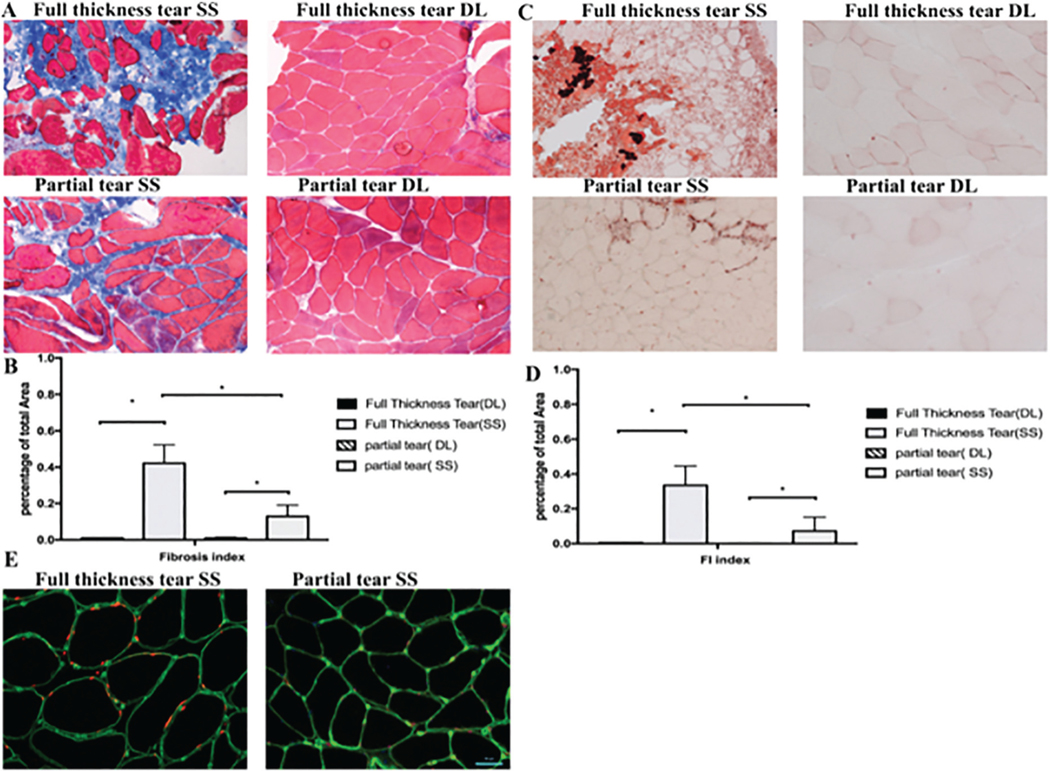
Histologic staining with Masson trichrome and Oil Red O demonstrated that there was significantly more fibrotic tissue and fat present in patients with medium and large tears than in patients with partial and small tears (Figure 2). Fat quantification showed that full-thickness tears had approximately 25% fat on histology (range, 14%−44%), compared with less than 10% in partial tears (range, 3%−13%), and there was no fat present within the deltoid muscle. Importantly, the biopsy technique was able to preserve muscle architecture. Immunofluorescent staining demonstrated that FAP cells were more prevalent in patients with full-thickness tears, with the cells localized outside the myotubes (Figure 2E).
Figure 2.
(A) Representative trichrome straining of supraspinatus (SS) and deltoid (DL) biopsy specimens. (B) There was a higher percentage of collagen staining in the full-thickness tears compared with the partial tear and DL. (C) Oil Red O evaluation of fatty infiltration (FI) of full-thickness and partial-thickness tears in the SS compared with the control DL. (D) Quantification of FI with significantly more fat in the full-thickness than the partial-thickness tears. (E) Typical immunostaining of full-thickness tears compared with partial tears. Green stains are laminin and red is stained for fibroadipogenic progenitors. *P < .05.
FAPs, but Not SC Number, Increase With Tear Size
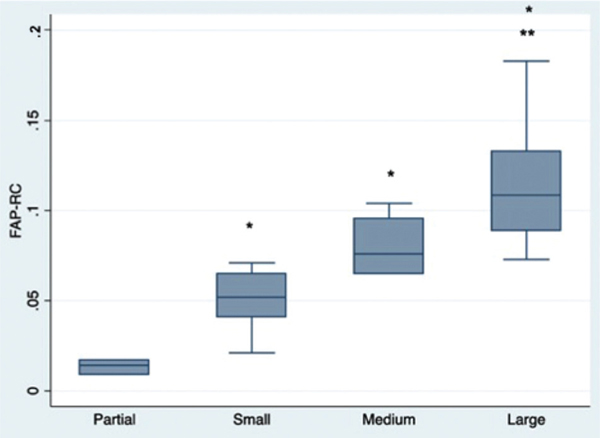
While there was no significant difference in RC SC number in different tear sizes (0.19% ± 0.02% in partial tear vs 0.35% ± 0.21% in full-thickness tear; P = .066), there were significantly more RC FAPs in patients with full-thickness tears when compared with patients with partial-thickness tears (9.43% ± 4.25% vs 3.84% ± 2.54%; P < .01). When patients with full-thickness tears were divided by tear size, there was also a significant difference in FAPs between small and large tears, with larger tears consistently having more FAPs present (Figure 3). There was also a significant correlation with FAP number and Goutallier grade (R2 = 0.74; P < .01). In comparison, there was no difference in deltoid FAP percentage among all RC tear thicknesses (0.41% ± 0.11% partial vs 1.00% ± 1.01% full; P = .336) or sizes (0.73% ± 0.26% small vs 0.58% ± 0.08% medium vs 1.34% ± 1.38% large; P = .484). The same was true for deltoid SC percentages across tear thicknesses (0.11% ± 0.02% partial vs 0.19% ± 0.16% full; P = .413) and sizes (0.25% ± 0.19% small vs 0.33% ± 0.21% medium vs 0.10% ± 0.00% large; P = .403).
Figure 3.
Fibroadipogenic progenitor (FAP) cell counts from supraspinatus biopsy specimens from different size rotator cuff (RC) tears using fluorescence-activated cell sorting. *P < .05 compared with partial tears; **P < .05 compared with small tear size.
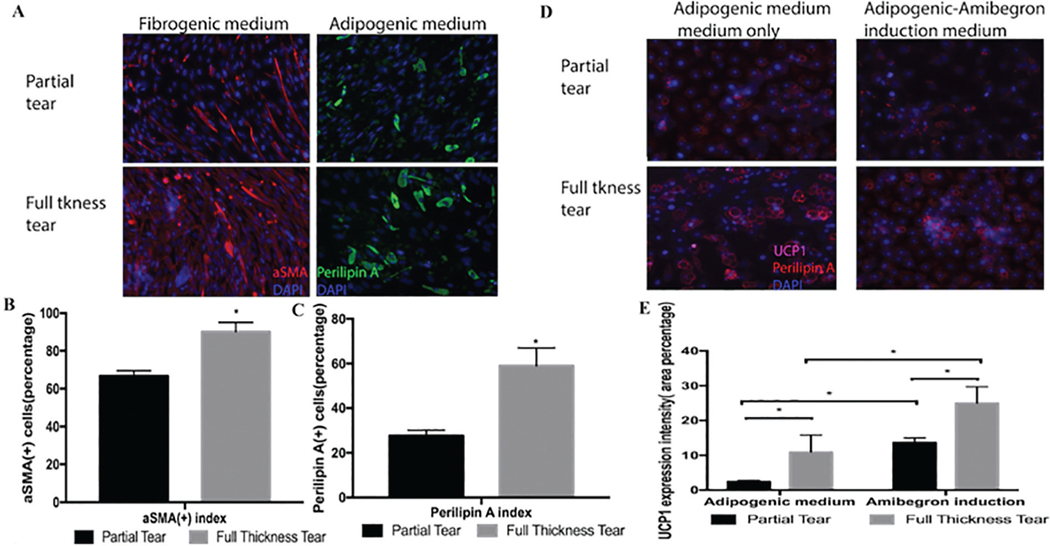
FAPs From Muscles With Full-Thickness Tears Are More Inducible to Fibrotic Tissue and Adipogenic Tissue
To determine the cellular plasticity of FAPs and to confirm their ability to differentiate, FAPs from partial tears and full-thickness tears were placed in either fibrogenic or adipogenic media for 2 weeks. In fibrogenic media, 68.2% ± 5.5% of the FAPs from partial-thickness tears expressed the fibrotic marker a-smooth muscle actin, compared with more than 90.2% ± 7.6% of the FAPs from full-thickness tears (P < .05). Similarly, FAPs from partial-thickness tears had less adipogenic differentiation compared with FAPs from full-thickness tears (32.9% ± 3.7% vs 60.7% ± 10.1%; P < .05) (Figure 4). To test the ability of FAPs to differentiate into beige fat, FAPs were obtained from partial- and full-thickness tears and treated with either adipogenic or adipogenic and amibegron-induced media. In both partial- and full-thickness tears, there was increased UCP-1 expression compared with adipogenic media alone (Figure 4, D and E), confirming that these cells can differentiate into beige fat, but more so in patients with full-thickness tears.
Figure 4.
(A) Treatment with fibrogenic and adipogenic media led to more fibrogenic and adipogenic differentiation in fibroadipogenic progenitor from full-thickness tears compared with partial tears. (B) Quantification of fibroblastic cells. (C) Quantification of adipocytes. (D) Treatment with amibegron results in increased beige fat differentiation, more so in FAPs from full-thickness tears compared with partial tears. (E) Quantification of beige fat differentiation. *P < .05.
Gene Expression in FAPs
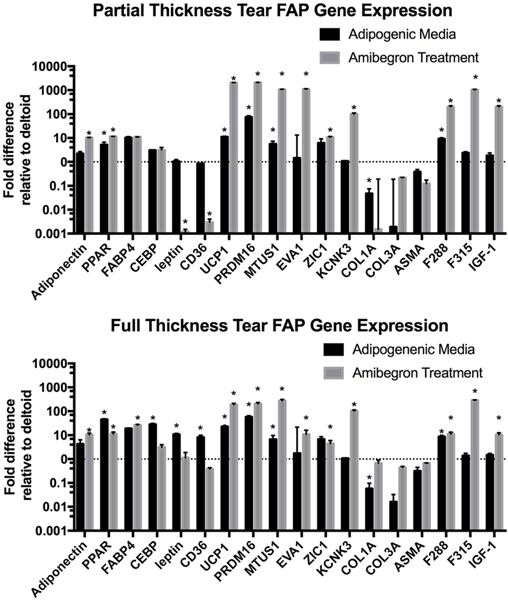
To test the ability of FAPs from partial- and full-thickness tears to differentiate into BAT, FAPs from both the RC and deltoid were treated with amibegron for 2 weeks, and gene expression was tested. The results are summarized in Figure 5. After treatment with amibegron, there was a significant increase in adipogenic genes in the RC FAPs, including PPARγ and FABP4, compared with those from the patient’s deltoid. There was no increase in fibrogenic genes. There was a marked increase in beige fat genes including UCP-1 and PR/Set Domain 16 (PRDM-16), both specific to BAT. Most importantly, there was a significant increase in pro-myogenic genes including both types of follistatin (F288, F315) and insulin-like growth factor 1 (IGF-1). Both F315 and IGF-1 were significantly increased with amibegron treatment in FAPs from both partial- and full-thickness tears compared with adipogenic media treatment alone.
Figure 5.
Reverse transcription polymerase chain reaction analysis of gene expression of fibroadipogenic progenitors (FAPs) obtained from partial rotator cuff (RC) tears (top graph) and full RC tears (bottom graph) compared with FAPs obtained from the patient’s deltoid muscle after 2 weeks of treatment with either adipogenic media alone (black bars) or 10 μM amibegron (gray bars). Y axis represents logarithmic scale. *P < .05.
DISCUSSION
In this study, we confirmed that FAPs are a stem cell population that is present in patients with RC tears and, contrary to our initial hypothesis, that there are more FAPs present in patients with larger, full-thickness tears. Importantly, these cells have the capability to differentiate into white fat, which suggests that these are the primary cell source for FI. They also represent an endogenous stem cell source that is capable of induction to a beige fat phenotype, as demonstrated by increased expression of UCP-1 and PRDM-16. Furthermore, these cells displayed drastically increased follistatin and IGF-1 with amibegron treatment, suggestive of their potential to drive RC regeneration with either pharmacologic or transplantation techniques.
Several studies have evaluated muscle architecture and cell type in patients with RC tears. Gibbons et al12 evaluated 23 patients with RC tears. They found that a significant amount of the muscle was replaced with connective tissue, with increased vascularity and inflammatory cells, but did not specifically evaluate stem cell populations. In this study, we specifically evaluated for the presence of SCs and FAPs, and the amount of FI was quantified as well. The degree of FI was consistent with that found in other studies and correlated with the number of FAPs present as well as FI present on MRI as measured by Goutallier grade. While the overall number of SCs did not change significantly with tear size, it is important to know that there are SCs present, as this suggests that there is still a cell population capable of myogenic differentiation even in patients with large full-thickness tears. Another study found that SCs from muscles with full-thickness tears had differentiation capability but limited regeneration ability.33 This study did not evaluate regenerative potential specifically but supports the notion that SCs are present in patients with cuff tears. Meyer et al33 found that SCs obtained from human cuff tears are able to grow in culture and engraft when injected into injured mouse muscle, and they were able to contribute to muscle hypertrophy and regeneration regardless of tear severity. Thus, the presence of SCs suggests that muscle in the RC retains its regenerative capacity given the proper activation.
FAPs were originally described by Uezumi et al.47 They defined a collection of muscle resident progenitor cells that were able to differentiate into both adipocytes and fibroblasts. Subsequent studies have shown that these cells have a role in pathologic muscle processes, including muscular dystrophy.46,48 In animal models of RC injury, these cells have been found to be present as well, as identified by the marker PDGFRα/β, and were shown to expand after RC injury and co-localize to fat deposits within the muscle.6,9,50 Inhibition of TGF-β resulted in a decrease in FI because of FAP cell apoptosis, confirming the importance of these FAP cells in FI.7 Conditional cellular depletion of FAPs has been shown to decrease FI in animal models, further supporting the role of these cells in the development of FI.27
The presence of FAPs within RC muscle has not previously been described in humans, although there is convincing data from the animal studies described above that this stem cell type would be present.6,7 Given what is known of the pattern of FAP rapid expansion after muscle injury followed by cell number contraction, we hypothesized that chronic tear states, which often result in larger tears, would further deplete the FAP pool.20,47 SCs have also been shown to deplete over time.5,8 Contrary to our hypothesis, however, FAPs increased with tear size, which suggests that while they have an active role in the development of FI, they do not deplete in the setting of a chronic tear. It could be possible in patients with massive tears with more chronicity that FAPs do become depleted, but these patients were not included in the study, as advanced FI is more likely to not be repairable and thus these patients are more likely to undergo a reverse shoulder arthroplasty.
The fact that FAPs are present and increase with larger tear sizes suggests that these cells are the primary source for FI, as FI increases with tear size. To test the cellular plasticity of FAPs, cells were treated with specific media conditions in vitro. Stimulation of FAPs from muscles with partial tears with adipogenic media resulted in a significant increase in adipogenic differentiation, but not as much as with FAPs from muscles with full-thickness tears. A study that examined FAP populations across multiple uninjured muscle groups in mice found that FAPs in the RC were of highest concentration and displayed the greatest proliferative and adipogenic capacity.25 However, our study demonstrates that there is also a spectrum of FAP quantity and differentiation profiles within the RC across different tear sizes. This suggests a difference in gene expression and epigenetic changes in FAPs in partial- and full-thickness tears that result in a difference in their ability to differentiate into white fat, consistent with other studies of beige fat differentiation.17 These differences in FAP behavior may be a contributing factor for the profound fatty degeneration often seen in massive RC tears. However, the mechanism that governs these changes is not known at this time and will be the subject of additional studies.
Additionally, the role of fibrosis in the pathogenesis of RC disease is also not well-studied. While fibrosis is a clear pathologic mechanism in other muscle disease states,10,15,36 its role in RC disease is unclear, possibly due to the fact that it is not as easy to measure on clinical MRI scans as fat quantity is. Thus, while we see increased fibrosis and more fibrogenic potential in FAPs from muscles with full-thickness tears, the clinical significance of this finding at this time is not clear. Previous studies have demonstrated the ability to reduce FAP-derived fibrosis in in vitro and in vivo animal studies using small molecule inhibitors, which may be of some benefit to RC muscle quality and function.7,19,30 Utilization of animal gait analysis and isolated muscle force measurements may help delineate the effects of RC fibrosis from those of FI.
The functional purpose of FAPs also is not well-defined, but their presence and differentiation capabilities may indicate that there is a resident stem cell population that could function to assist in muscle regeneration given the proper stimulus. While adipose deposits within and surrounding muscle are thought to be an entirely deleterious process, recent studies highlight the metabolic importance of brown and beige adipose tissue. Beige fat was recently found to resemble classic brown fat, although it shares developmental origins with white fat and has been found to be present in the supraclavicular region in humans.43 The best-known function of brown and beige fat is its thermogenic capacity, enabled by the selective expression of UCP-1, which stimulates thermogenesis by uncoupling cellular respiration and mitochondrial adenosine tri-phosphate (ATP) synthesis.1,22,45 Recent studies have suggested other important roles of beige fat beyond thermogenesis, including a role in regulating muscle quality.17,21,37
Brown and beige fat have been shown to secrete anabolic and myogenic factors, such as follistatin.2,29,38,41,42,44,52 Mice FAPs have demonstrated the ability to adopt a beige fat phenotype characterized by elevated expression of UCP-1.14,49 Meyer et al34 showed that RC epimuscular fat displayed a beige fat signature and increased myotube formation in co-culture experiments. Transplantation of BAT into RC muscles after cardiotoxin injury increased muscle mass, contractile force, and fiber cross-sectional area.3 Beigelike FAPs transplanted into the RC in a delayed mouse RC repair model improved shoulder gait function, increased RC vascularity, and diminished muscle atrophy, FI, and fibrosis.26 Furthermore, Wang et al49 showed that reversal of RC FI in mice after suprascapular nerve compression and release likely involved the “browning” of white adipocytes.
In this study, we demonstrated that human FAPs obtained from patients with partial- and full-thickness RC tears were able to be stimulated into beige fat with the administration of amibegron, a potent beta-3 adrenergic agonist. FAPs treated with amibegron expressed factors consistent with beige fat, including UCP-1 and PRDM-16. Interestingly, pro-myogenic genes such as IGF-1 and follistatin (expressed through its 2 subtypes) were markedly upregulated. These findings confirm previous studies’ demonstration of beige fat follistatin expression.2,44 These data suggest a possible functional role of FAPs in the ability to improve muscle quality after RC repair if directed toward a more beneficial phenotype as opposed to their usual white adipocyte and fibroblast fates. In muscles with a full-thickness tear state, FAPs may proliferate and differentiate eventually into white adipose and result in FI if no beige fat stimulus is received. However, given the correct stimulus, through either pharmacologic modification or beige fat transplantation, FAPs, from either white fat or the dedifferentiated FAP pool, may be able to differentiate into beige fat to provide the local gene and protein expression necessary for muscle regeneration after RC repair.
There are several weaknesses of this study. First, the overall sample size is relatively small. While a larger patient study has considerable benefits, the current sample size is consistent with other published literature, and the study was powered to detect a difference in FAP number between deltoid muscle and SS muscle in full-thickness tears. Second, the location of the sample may not be representative of the entire stem cell population. Currently, we are not aware of studies that have observed regional differences in FAP numbers or phenotype within entire muscle samples or how these cells are distributed throughout the muscle. Other studies have suggested that FAPs are localized in the perivascular area, but this would lead to a generalized distribution.18 Future studies will investigate how location within the muscle can affect FAP number and phenotype. Third, there was not a quantitative measure of FI on MRI with an advanced sequence such as iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL).28 While this would improve the correlation of fat seen on histologic analysis with quantitative MRI measures, it was not the primary goal of the study, and IDEAL is not able to differentiate between fat types. To determine BAT quantity, positron emission tomography MRI would need to be performed, which is beyond the scope of this current study.11,16 Finally, the mechanism by which FAPs appear to proliferate and differentiate is not elucidated in this study. This is primarily an observational study, and therefore the underlying change, whether from altered mechanical forces, use, or changes in inflammation, oxygenation, or innervation to RC muscles, is not defined in this study. Currently, animal models are being utilized to determine the underlying cause of FAP changes after RC tears.
CONCLUSION
In conclusion, we found that human RC tears result in an increase in the number of FAPs that correlates with larger tears, contrary to our hypothesis. FAPs increased with tear size, but importantly, even FAPs from patients with large tears were inducible to either adipogenic or fibrogenic cells, suggesting that these cells retain their ability to differentiate. Treatment with amibegron, a beta-3 agonist, allowed FAPs to differentiate into beige fat, with increased expression of UCP-1. These cells were able to secrete several factors important for muscle regeneration, including IGF-1 and follistatin. Thus, RC muscle has an endogenous, inducible stem cell source that can be utilized to improve muscle quality and patient function after RC repair.
Acknowledgments
One or more of the authors has declared the following potential conflict of interest or source of funding: B.T.F. reports funding in support of submitted work by an NIH/NIAMS research grant (1R01AR072669-01A1, PI: B.T.F.) and a U.S. Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development Merit Review Grant (1I01BX002680); B.T.F. is a consultant for Kaliber Labs; and has received hospitality payments from Zimmer Biomet Holdings. C.B.M.’s institution receives research grant money from the NIH, Histogenics, Zimmer, and Samumed, unrelated to the submitted work. This author has received consulting fees from Stryker, Conmed, Tornier, Wright Medical Technology, and Zimmer Biomet Holdings; hospitality payments from Arthrex; and education payments from Pacira Pharmaceuticals Inc. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
REFERENCES
- 1.Bartesaghi S, Hallen S, Huang L, et al. Thermogenic activity of UCP1 in human white fat-derived beige adipocytes. Mol Endocrinol. 2015;29(1):130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braga M, Reddy ST, Vergnes L, et al. Follistatin promotes adipocytedifferentiation, browning, and energy metabolism. J Lipid Res. 2014;55(3):375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bryniarski AR, Meyer GA. Brown fat promotes muscle growth duringregeneration. J Orthop Res. 2019;37(8):1817–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen P, Levy JD, Zhang Y, et al. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell. 2014;156(1–2):304–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins CA, Zammit PS, Ruiz AP, et al. A population of myogenic stemcells that survives skeletal muscle aging. Stem Cells. 2007;25(4):885–894. [DOI] [PubMed] [Google Scholar]
- 6.Davies MR, Garcia S, Tamaki S, et al. Muscle stem cell activation ina mouse model of rotator cuff injury. J Orthop Res. 2018;36(5):13701376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies MR, Liu X, Lee L, et al. TGF-beta small molecule inhibitorSB431542 reduces rotator cuff muscle fibrosis and fatty infiltration by promoting fibro/adipogenic progenitor apoptosis. PLoS One. 2016;11(5):e0155486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Day K, Shefer G, Shearer A, et al. The depletion of skeletal muscle satellite cells with age is concomitant with reduced capacity of single progenitors to produce reserve progeny. Dev Biol. 2010;340(2):330–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eliasberg CD, Dar A, Jensen AR, et al. Perivascular stem cells diminish muscle atrophy following massive rotator cuff tears in a small animal model. J Bone Joint Surg Am. 2017;99(4):331–341. [DOI] [PubMed] [Google Scholar]
- 10.Garg K, Ward CL, Hurtgen BJ, et al. Volumetric muscle loss: persistent functional deficits beyond frank loss of tissue. J Orthop Res. 2015;33(1):40–46. [DOI] [PubMed] [Google Scholar]
- 11.Gariani K, Gariani J, Amzalag G, et al. Hybrid PET/MRI as a tool todetect brown adipose tissue: proof of principle. Obes Res Clin Pract. 2015;9(6):613–617. [DOI] [PubMed] [Google Scholar]
- 12.Gibbons MC, Sato EJ, Bachasson D, et al. Muscle architecturalchanges after massive human rotator cuff tear. J Orthop Res. 2016;34(12):2089–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gladstone JN, Bishop JY, Lo IK, et al. Fatty infiltration and atrophy ofthe rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med. 2007;35(5):719–728. [DOI] [PubMed] [Google Scholar]
- 14.Gorski T, Mathes S, Krutzfeldt J. Uncoupling protein 1 expression inadipocytes derived from skeletal muscle fibro/adipogenic progenitors is under genetic and hormonal control. J Cachexia Sarcopenia Muscle. 2018;9(2):384–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gumucio JP, Korn MA, Saripalli AL, et al. Aging-associated exacerbation in fatty degeneration and infiltration after rotator cuff tear. J Shoulder Elbow Surg. 2014;23(1):99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holstila M, Pesola M, Saari T, et al. MR signal-fat-fraction analysisand T2* weighted imaging measure BAT reliably on humans without cold exposure. Metabolism. 2017;70:23–30. [DOI] [PubMed] [Google Scholar]
- 17.Inagaki T, Sakai J, Kajimura S. Transcriptional and epigenetic controlof brown and beige adipose cell fate and function. Nat Rev Mol Cell Biol. 2016;17(8):480–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwayama T, Steele C, Yao L, et al. PDGFRalpha signaling drives adipose tissue fibrosis by targeting progenitor cell plasticity. Genes Dev. 2015;29(11):1106–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jensen AR, Kelley BV, Mosich GM, et al. Neer Award 2018: plateletderived growth factor receptor alpha co-expression typifies a subset of platelet-derived growth factor receptor beta-positive progenitor cells that contribute to fatty degeneration and fibrosis of the murine rotator cuff. J Shoulder Elbow Surg. 2018;27(7):1149–1161. [DOI] [PubMed] [Google Scholar]
- 20.Joe AW, Yi L, Natarajan A, et al. Muscle injury activates residentfibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12(2):153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kajimura S, Spiegelman BM, Seale P. Brown and beige fat: physiological roles beyond heat generation. Cell Metab. 2015;22(4):546559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keipert S, Jastroch M. Brite/beige fat and UCP1—is it thermogenesis? Biochim Biophys Acta. 2014;1837(7):1075–1082. [DOI] [PubMed] [Google Scholar]
- 23.Kim JR, Cho YS, Ryu KJ, et al. Clinical and radiographic outcomesafter arthroscopic repair of massive rotator cuff tears using a suture bridge technique: assessment of repair integrity on magnetic resonance imaging. Am J Sports Med. 2012;40(4):786–793. [DOI] [PubMed] [Google Scholar]
- 24.Kim SJ, Jung M, Lee JH, et al. Arthroscopic repair of anterosuperior rotator cuff tears: in-continuity technique vs. disruption of subscapularissupraspinatus tear margin: comparison of clinical outcomes and structural integrity between the two techniques. J Bone Joint Surg Am. 2014;96(24):2056–2061. [DOI] [PubMed] [Google Scholar]
- 25.Lee C, Agha O, Liu M, et al. Rotator cuff fibro-adipogenic progenitors demonstrate highest concentration, proliferative capacity, and adipogenic potential across muscle groups. J Orthop Res. 2020;38(5): 1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee C, Liu M, Agha O, et al. Beige fibro-adipogenic progenitor transplantation reduces muscle degeneration and improves function in a mouse model of delayed repair of rotator cuff tears. J Shoulder Elbow Surg. 2020;29(4):719–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee L, Liu X, Nguyen C, et al. The role of beige adipocytes in fattyinfiltration of the rotator cuff muscle. Trans Orthop Res Soc. 2017;63:1126. [Google Scholar]
- 28.Lee S, Lucas RM, Lansdown DA, et al. Magnetic resonance rotatorcuff fat fraction and its relationship with tendon tear severity and subject characteristics. J Shoulder Elbow Surg. 2015;24(9):1442–1451. [DOI] [PubMed] [Google Scholar]
- 29.Lee SJ, Lee YS, Zimmers TA, et al. Regulation of muscle mass by follistatin and activins. Mol Endocrinol. 2010;24(10):1998–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lemos DR, Babaeijandaghi F, Low M, et al. Nilotinib reduces musclefibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat Med. 2015;21(7):786–794. [DOI] [PubMed] [Google Scholar]
- 31.Liu X, Laron D, Natsuhara K, et al. A mouse model of massive rotatorcuff tears. J Bone Joint Surg Am. 2012;94(7):e41. [DOI] [PubMed] [Google Scholar]
- 32.Liu X, Ning AY, Chang NC, et al. Investigating the cellular origin ofrotator cuff muscle fatty infiltration and fibrosis after injury. Muscles Ligaments Tendons J. 2016;6(1):6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meyer GA, Farris AL, Sato E, et al. Muscle progenitor cell regenerative capacity in the torn rotator cuff. J Orthop Res. 2015;33(3):421429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyer GA, Gibbons MC, Sato E, et al. Epimuscular fat in the humanrotator cuff is a novel beige depot. Stem Cells Transl Med. 2015;4(7):764–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Namdari S, Donegan RP, Dahiya N, et al. Characteristics of small tomedium-sized rotator cuff tears with and without disruption of the anterior supraspinatus tendon. J Shoulder Elbow Surg. 2014;23(1):20–27. [DOI] [PubMed] [Google Scholar]
- 36.Ogura Y, Tajrishi MM, Sato S, et al. Therapeutic potential of matrixmetalloproteinases in Duchenne muscular dystrophy. Front Cell Dev Biol. 2014;2:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohyama K, Nogusa Y, Shinoda K, et al. A synergistic antiobesityeffect by a combination of capsinoids and cold temperature through promoting beige adipocyte biogenesis. Diabetes. 2016;65(5):14101423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rahman S, Lu Y, Czernik PJ, et al. Inducible brown adipose tissue, orbeige fat, is anabolic for the skeleton. Endocrinology. 2013;154(8): 2687–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma AK, Levian B, Shah P, et al. Aged mice demonstrate greatermuscle degeneration of chronically injured rotator cuff. J Orthop Res. 2020;38(2):320–328. [DOI] [PubMed] [Google Scholar]
- 40.Sharp LZ, Shinoda K, Ohno H, et al. Human BAT possesses molecularsignatures that resemble beige/brite cells. PLoS One. 2012;7(11):e49452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharples AP, Al-Shanti N, Hughes DC, et al. The role of insulin-likegrowth factor binding protein 2 (IGFBP2) and phosphatase and tensin homologue (PTEN) in the regulation of myoblast differentiation and hypertrophy. Growth Horm IGF Res. 2013;23(3):53–61. [DOI] [PubMed] [Google Scholar]
- 42.Sidis Y, Mukherjee A, Keutmann H, et al. Biological activity of follistatin isoforms and follistatin-like-3 is dependent on differential cell surface binding and specificity for activin, myostatin, and bone morphogenetic proteins. Endocrinology. 2006;147(7):3586–3597. [DOI] [PubMed] [Google Scholar]
- 43.Sidossis L, Kajimura S. Brown and beige fat in humans: thermogenicadipocytes that control energy and glucose homeostasis. J Clin Invest. 2015;125(2):478–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh R, Braga M, Pervin S. Regulation of brown adipocyte metabolism by myostatin/follistatin signaling. Front Cell Dev Biol. 2014;2:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tseng YH, Kokkotou E, Schulz TJ, et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454(7207):1000–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uezumi A, Fukada S, Yamamoto N, et al. Identification and characterization of PDGFRalpha 1 mesenchymal progenitors in human skeletal muscle. Cell Death Dis. 2014;5:e1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uezumi A, Fukada S, Yamamoto N, et al. Mesenchymal progenitorsdistinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol. 2010;12(2):143–152. [DOI] [PubMed] [Google Scholar]
- 48.Uezumi A, Ito T, Morikawa D, et al. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J Cell Sci. 2011;124(pt 21):3654–3664. [DOI] [PubMed] [Google Scholar]
- 49.Wang Z, Feeley BT, Kim HT, et al. Reversal of fatty infiltration aftersuprascapular nerve compression release is dependent on UCP1 expression in mice. Clin Orthop Relat Res. 2018;476(8):1665–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Z, Liu X, Davies MR, et al. A mouse model of delayed rotatorcuff repair results in persistent muscle atrophy and fatty infiltration. Am J Sports Med. 2018;46(12):2981–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamamoto A, Takagishi K, Osawa T, et al. Prevalence and risk factors of a rotator cuff tear in the general population. J Shoulder Elbow Surg. 2010;19(1):116–120. [DOI] [PubMed] [Google Scholar]
- 52.Yamashita H, Sato N, Kizaki T, et al. Norepinephrine stimulates theexpression of fibroblast growth factor 2 in rat brown adipocyte primary culture. Cell Growth Differ. 1995;6(11):1457–1462. [PubMed] [Google Scholar]