Abstract
BACKGROUND:
Although the flow diverter has advantages in the treatment of intracranial aneurysms, pooled studies that directly compare it with conventional endovascular treatments are rare.
PURPOSE:
Our aim was to compare the safety and efficacy of flow-diverter and conventional endovascular treatments in intracranial aneurysms.
DATA SOURCES:
We performed a comprehensive search of the literature using PubMed, EMBASE, and the Cochrane Database.
STUDY SELECTION:
We included only studies that directly compared the angiographic and clinical outcomes of flow-diverter and conventional endovascular treatments.
DATA ANALYSIS:
Random effects or fixed effects meta-analysis was used to pool the cumulative rate of short- and long-term angiographic and clinical outcomes.
DATA SYNTHESIS:
Eighteen studies with 1001 patients with flow diverters and 1133 patients with conventional endovascular treatments were included; 1015 and 1201 aneurysm procedures were performed, respectively. The flow-diverter group had aneurysms of a larger size (standard mean difference, 0.22; 95% CI, 0.03–0.41; P = .026). There was a higher risk of complications in the flow-diverter group compared with the conventional endovascular group (OR, 1.4; 95% CI, 1.01–1.96; P = .045) during procedures. The follow-up angiographic results of flow-diverter treatment indicated a higher rate of complete occlusion (OR, 2.55; 95% CI, 1.70–3.83; P < .001) and lower rates of recurrence (OR, 0.24; 95% CI, 0.12–0.46; P < .001) and retreatment (OR, 0.31; 95% CI, 0.21–0.47; P < .001).
LIMITATIONS:
Limitations include a retrospective, observational design in some studies, high heterogeneity, and selection bias.
CONCLUSIONS:
Compared with the conventional endovascular treatments, the placement of a flow diverter may lead to more procedure-related complications, but there is no difference in safety, and it is more effective in the long term.
Rapid technologic advances in endovascular treatments have been transforming the treatment modalities of intracranial aneurysms (IAs) in recent years. The Guglielmi detachable coil (Stryker), introduced in the early 1990s, provided an alternative to traditional surgical clipping in the treatment of IAs.1 After that, reconstructive techniques such as balloon-assisted coiling (BAC) and stent-assisted coiling (SAC), were initially used.2,3 Most recently, low-profile visualized intraluminal support (LVIS; MicroVention), a self-expandable, recyclable, braided stent, has also been widely adopted in clinical practice.4
Compared with these standard and conventional stent methods, flow diverters (FDs), like the Pipeline Embolization Device (PED; Medtronic) approved by the US Food and Drug Administration in 2011,5,6 have greater metal coverage and have broader indications for the treatment of complex aneurysms, such as large and giant ICA aneurysms and fusiform, dissecting, and blood blister–like aneurysms.7,8 However, the high rate of aneurysm rupture, procedural mortality, and morbidity after placement of FDs has also raised many concerns.9 It is crucial to assess the risk-benefit ratio for treatment with FDs by comparing it with conventional endovascular (CEV) treatments. However, early pooled analyses focused on only single-arm studies without directly comparing them. In our present work, we conducted a meta-analysis directly comparing the short- and long-term angiographic and clinical outcomes of the 2 methods in the past decade since the introduction of FDs.
MATERIALS AND METHODS
Search Strategy and Selection Criteria
Our searches of PubMed, EMBASE, and the Cochrane Database followed the principles of the common evidence medicine framework Patient Population, Intervention, Control, and Outcome: Did adult patients with intracranial aneurysms (patient population) who underwent an FD procedure (intervention) have better clinical outcomes, higher rates of aneurysm occlusion, and lower rates of mortality and procedure-related complications (outcomes) compared with patients who underwent the CEV (control) treatments from January 2010 to December 2020? Titles, abstracts, and keywords were searched using combinations of the terms including the following: “intracranial aneurysm,” “cerebral aneurysm,” “endovascular,” “flow diverter,” “flow diverting,” “Pipeline,” “PED,” “Surpass,” and “Tubridge.” For detailed strategies, see the Online Supplemental Data. This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses.10 The systematic review protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO, ID: CRD42021282218). References generated from these searches were imported into the reference manager EndNote X9 (Thompson), and 2 authors (C.Z. and W.T.) systematically screened the references independently according to the inclusion criteria. Any discrepancies were resolved after discussion with the third author (S.L.). The inclusion criteria were the following: 1) direct comparison of FD and CEV treatment, including coiling alone, stent alone, SAC, BAC, and LVIS; 2) patients 18 years of age or older with intracranial aneurysms; and 3) detailed follow-up of angiographic and clinical outcomes. The exclusion criteria were the following: 1) fewer than 10 participants in either group; 2) no report of outcome variables; and 3) studies primarily focused on animals. Additionally, studies were included only if they were original articles published in English. Review articles, abstracts, case reports, systematic reviews and meta-analyses, letters to the editor, reviews, editorials, commentaries, studies on animal models, and basic science studies were not considered.
Data Extraction and Quality Assessment
A review and the data extraction of all included studies were performed by 3 authors (C.Z., W.T., and S.L.) independently. Any disagreements were resolved by consensus in meetings with all authors. Extracted study and patient characteristics included the author, year of publication, sex, age, hypertension, aneurysm size, number of participants in each group, follow-up duration, and the study design type, ie, whether the patient was matched by age, sex, aneurysm size, or aneurysm morphology. The periprocedural mortality, procedure-related complications such as ischemia and hemorrhage, the immediate occlusion rates, and good outcomes (mRS 0–2) were extracted for each study. The follow-up angiographic and clinical outcomes were also included.
The quality of included studies was assessed using the Newcastle-Ottawa Scale for cohort studies.11 This scale rates studies on the basis of 3 major aspects: selection, comparability, and ascertainment of the outcome of interest. We indicated high-quality choices by adding stars to the questions in each aspect if available. More stars indicated higher-quality studies. We included all eligible studies regardless of their assessed quality.
Statistical Analysis
All statistical analyses were performed using R version 4.1.0 (http://www.r-project.org). Dichotomous data from included studies were used to generate ORs, and continuous data were used for standard mean difference (SMD) with 95% confidence intervals by the DerSimonian and Laird models using the inverse-variance weighting method. A random effects model was used if the outcome had high heterogeneity and was noted as I2 > 50%; otherwise, the fixed-effects model was applied. The sources of heterogeneity were explored by subgroup analyses, meta-regression, and sensitivity analyses by the sequential exclusion of 1 study at a time. Publication bias was evaluated using a funnel plot based on the Egger regression test. Statistical significance was identified with P < .05.
RESULTS
Selected Studies
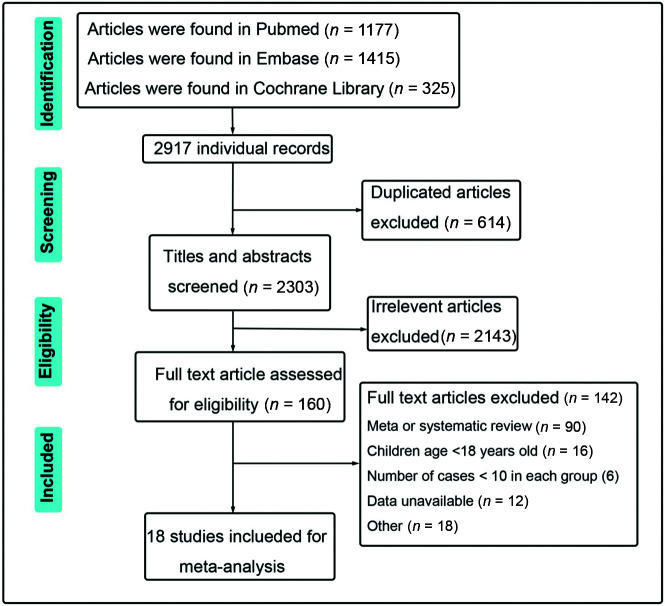
A total of 18 articles met the eligibility criteria for the meta-analysis after the full-text screening of 1001 patients with FDs and 1133 with CEV treatments, including 1015 and 1201 aneurysm procedures in the FD and CEV groups, respectively.12-29 The flow chart and selection process are shown in Fig 1. Among the selected studies, 15 used the PED, 1 used Pipeline or Surpass stent (Stryker Neurovascular), and 2 studies used the Tubridge (MicroPort Medical Company) as the only endovascular tool in the FD group. The Surpass and Pipeline stents without embolization tools were used in 2 studies in combination with other FD devices. Many different methods were applied in the conventional group. Detailed descriptions of the included studies are listed in the Online Supplemental Data. Eight matched studies were identified using propensity score matching analysis or other methods by matching patient age, sex, aneurysm size, or aneurysm morphology. All selected studies scored at least 6 stars in the Newcastle-Ottawa Scale grading system, indicating the high quality of these cohort studies (Table 1).
FIG 1.
The flow chart of selecting eligible studies in the present work.
Table 1:
The quality assessments based on Newcastle-Ottawa Scale for included cohort studies
| Author | Selection | Comparability | Outcome | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Year | A | B | C | D | E | F | G | H | Quality Scores | |
| Chalouhi et al15 | 2013 | * | * | * | * | * | * | * | * | 8 |
| Zhang et al29 | 2016 | * | * | * | * | * | * | * | 7 | |
| Lanzino et al20 | 2012 | * | * | * | * | * | * | * | 7 | |
| Chalouhi et al14 | 2014 | * | * | * | * | * | * | * | * | 8 |
| Chalouhi et al13 | 2017 | * | * | * | * | * | * | * | * | 8 |
| Salem et al24 | 2020 | * | * | * | * | * | * | * | * | 8 |
| Durst et al17 | 2016 | * | * | * | * | * | * | * | 7 | |
| Yupeng Zhang et al28 | 2019 | * | * | * | * | * | * | * | 7 | |
| Lu et al22 | 2019 | * | * | * | * | * | * | 6 | ||
| Silva et al25 | 2019 | * | * | * | * | * | * | * | 7 | |
| Adeeb et al12 | 2017 | * | * | * | * | * | * | 6 | ||
| Petr et al23 | 2016 | * | * | * | * | * | * | * | 7 | |
| Zanaty et al27 | 2014 | * | * | * | * | * | * | * | 7 | |
| Di Maria et al16 | 2015 | * | * | * | * | * | * | * | 7 | |
| Kim et al19 | 2014 | * | * | * | * | * | * | * | 7 | |
| Enriquez-Marulanda et al18 | 2019 | * | * | * | * | * | * | * | 7 | |
| Liu et al21 | 2018 | * | * | * | * | * | * | * | 7 | |
| Wang et al26 | 2019 | * | * | * | * | * | * | 6 |
Note:—Asterisk indicates that the included study meet the quality assessment criteria.
A, Representativeness of the exposed cohort.
B, Selection of the nonexposed cohort.
C, Ascertainment of exposure.
D, Demonstration that the outcome of interest was not present at the start of the study.
E, Comparability of cohorts on the basis of the design or analysis.
F, Assessment of outcome.
G, Was follow-up long enough for outcomes to occur?
H, Adequacy of follow-up of cohorts.
Patient Characteristics
Four usual variables were selected, including age, sex, hypertension, and the diameter of the aneurysm. There were no significant differences between the FD and CEV groups in terms of age (SMD, −0.23; 95% CI, −0.55–0.09; P = .166), proportion of women (OR, 1.02; 95% CI, 0.79–1.32; P = .864), and hypertension rates (OR, 1.19; 95% CI, 0.82–1.72; P = .357). Compared with the CEV group, the FD group had larger aneurysms (SMD, 0.22; 95% CI, 0.03–0.41; P = .026) (Online Supplemental Data).
Procedural Outcomes
Results were inconclusive about the risk of periprocedural mortality in the FD group compared with the conventional group (OR, 1.81; 95% CI, 0.73–4.48; P = .197), and there was no significant difference in the risk of periprocedural ischemia (OR, 0.85; 95% CI, 0.53–1.36; P = .505) and hemorrhage (OR, 1.51; 95% CI, 0.80–2.86; P = .204). Intriguingly, the combination of procedure-related complications (including ischemia, hemorrhage, mortality, and visual impairment) was statistically significant, with the FD group having a higher risk of procedural complications than the CEV group (OR, 1.4; 95% CI, 1.01–1.96; P = .045) (Online Supplemental Data). No significant differences were observed about immediate occlusions (OR, 0.27; 95% CI, 0.04–1.69; P = .16) (Online Supplemental Data). Subsequently, similar rates of good outcomes (mRS 0–2) at discharge were observed between the 2 groups (OR, 0.43; 95% CI, 0.15–1.23; P = .117) (Online Supplemental Data).
Long-term Angiographic and Clinical Outcomes
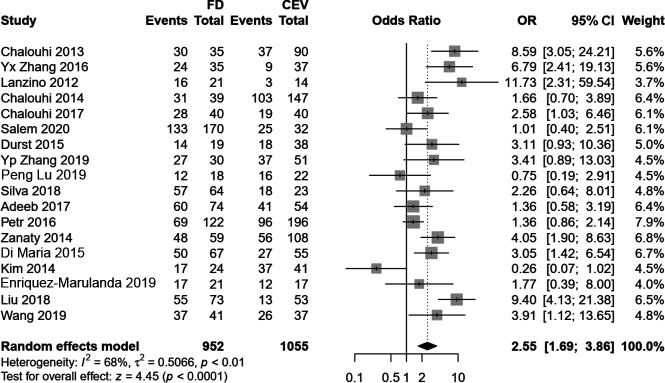
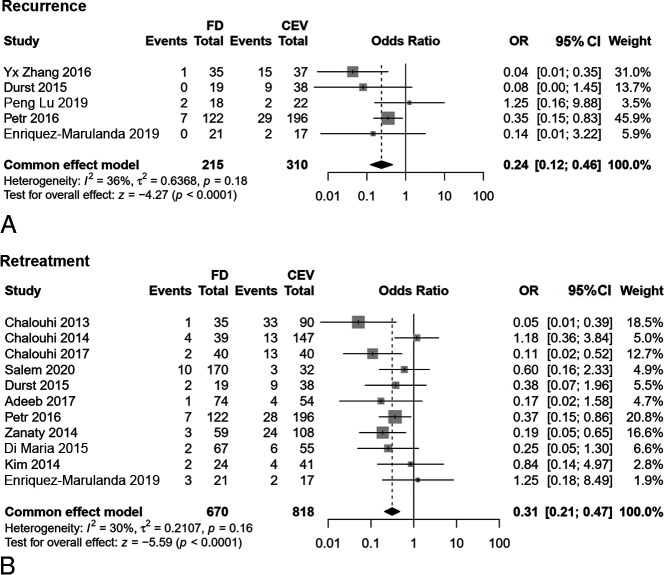
In contrast to the results of immediate occlusions, the follow-up angiographic results after flow diversion indicated higher rates of complete occlusion (OR, 2.55; 95% CI, 1.70–3.83; P < .001) but with a high heterogeneity of I2 = 68% (Fig 2). Moreover, during follow-up, the FD group had lower recurrence rates after removal of the aneurysms under angiography (OR, 0.24; 95% CI, 0.12–0.46; P < .001) and retreatment (OR, 0.31; 95% CI, 0.21–0.47; P < .001) (Fig 3). There were no statistical differences of delayed complications (OR, 1.14; 95% CI, 0.46–2.84; P = .775) and follow-up clinical outcomes (OR, 1.24; 95% CI, 0.82–1.88; P = .304) between the FD and CEV groups (Online Supplemental Data). Table 2 summarizes all the results of this meta-analysis.
FIG 2.
The complete occlusion rate of FD and CEV treatments at the last follow-up.
FIG 3.
The comparison of recurrence (A) and retreatment (B) of FD and CEV treatment.
Table 2:
Summaries of all results of present work
| Variables | Studies, No. | FD, No. | CEV, No. | OR/SMD | 95% CI | I2 | P Value |
|---|---|---|---|---|---|---|---|
| Age | 9 | 361 | 522 | –0.23a | –0.55–0.09 | 78% | .166 |
| Female | 17 | 910 | 1076 | 1.02 | 0.79–1.32 | 7% | .864 |
| Hypertension | 6 | 272 | 210 | 1.19 | 0.82–1.72 | 0% | .357 |
| Diameter of aneurysm | 10 | 464 | 712 | 0.22a | 0.03–0.41 | 52% | .026 |
| Periprocedural death | 17 | 910 | 1076 | 1.81 | 0.73–4.48 | 0% | .197 |
| Periprocedural ischemia | 16 | 848 | 1053 | 0.85 | 0.53–1.36 | 0% | .505 |
| Periprocedural hemorrhage | 16 | 848 | 1053 | 1.51 | 0.80–2.8 | 0% | .204 |
| Procedure-related complications | 17 | 910 | 1076 | 1.4 | 1.01–1.96 | 0% | .045 |
| Immediate occlusion | 7 | 302 | 405 | 0.27 | 0.04–1.69 | 92% | .16 |
| mRS at discharge | 6 | 249 | 263 | 0.43 | 0.15–1.23 | 0% | .117 |
| Follow-up occlusion | 18 | 952 | 1055 | 2.55 | 1.70–3.83 | 68% | <.001 |
| Recurrence | 5 | 215 | 310 | 0.24 | 0.12–0.46 | 38% | <.001 |
| Retreatment | 11 | 670 | 818 | 0.31 | 0.21–0.47 | 33% | <.001 |
| Delayed complications | 11 | 582 | 708 | 1.14 | 0.46–2.84 | 59% | .775 |
| mRS at follow-up | 14 | 763 | 883 | 1.24 | 0.82–1.88 | 0% | .304 |
Represents the SMD result.
Subgroup, Meta-regression, and Sensitivity Analysis
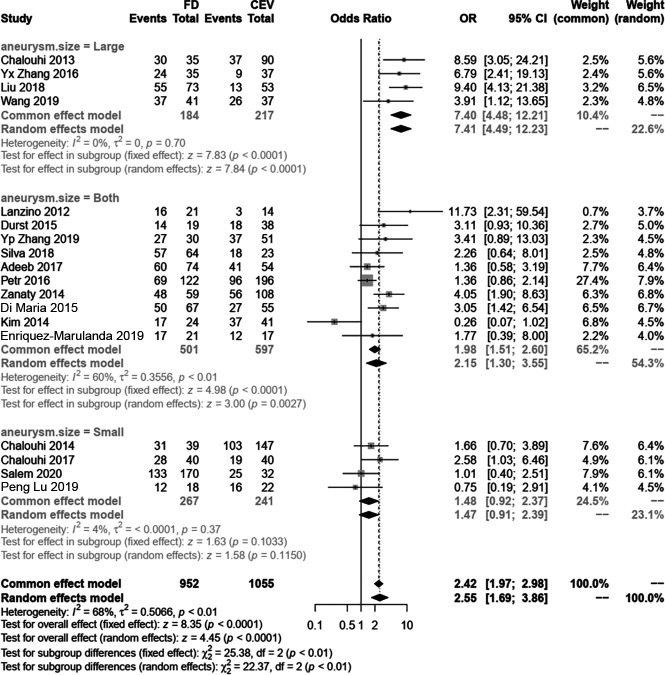
To discover the source of heterogeneity in follow-up occlusions, we conducted subgroup analyses, meta-regression, and sensitivity analyses. First, we divided the included studies into 2 groups, matched and nonmatched. In the subgroup analysis, the matched group indicates that the FD group had a higher rate of follow-up occlusion (OR, 3.33; 95% CI, 1.86–5.98; P < .001) and the I2 decreased to 58%, but in the nonmatched group, the I2 increased to 73% (Online Supplemental Data). Because no evident changes were observed after dividing the study designs into subgroups, we further divided these studies into 3 groups according to reported aneurysm sizes: large aneurysm group (diameter, >10 mm), small aneurysm (<4 mm), and both. In our analysis, the I2 decreased to 0% and 6% in the large and small groups, respectively, but remained at 60% in studies that did not distinguish among the sizes of aneurysms (Fig 4). Therefore, we postulated that the source of heterogeneity of the follow-up occlusion rate was due to aneurysm size. We also conducted a meta-regression that showed that neither the published years (β, −0.1043; 95% CI, −0.287–0.078; P = .262) nor age (β, 0.0544; 95% CI, −0.065–0.174; P = .373) affected the outcome (Online Supplemental Data). Furthermore, the sensitivity analysis showed that the results of follow-up occlusions were not influenced by the leaving-one-out method (Online Supplemental Data). Finally, the funnel plot revealed that there was no publication bias, with all studies exhibiting symmetric distributions (Online Supplemental Data).
FIG 4.
The subgroup analysis based on the aneurysm size to find the source of heterogeneity for the follow-up complete occlusion rate.
DISCUSSION
CEV treatments, including coiling alone,30 SAC,31 and BAC,32 have been widely used in the treatment of intracranial aneurysms. Aneurysms unfavorable for simple coiling require deployment of a stent across the aneurysm neck to prevent coil migration, while the high bleeding risk due to dual-antiplatelet therapy during the perioperative period can lead to a poor prognosis.33 In contrast, dual-antiplatelet medication was not obligatory for the BAC embolization technique, which was accompanied by low thrombosis formation, first reported by Moret et al34 in 1997. However, the risk of recurrence and retreatment of aneurysms treated by coil embolization can reach 20% and 10%, respectively, based on a meta-analysis across all aneurysm sizes.35 The role of conventional and standard endovascular tools in the treatment of IAs was challenged when FD devices were introduced. The PED,36 as the first commercially available FD on the US market, presented its safety and effectiveness in the clinic.37 Failures or complications associated with the FD were also reported, such as remaining filling, postprocedural rupture, postprocedural thrombosis, and ischemic stroke.38,39 Thus, the feasibility, safety, and efficacy of the FD versus conventional standard treatments are still elusive and controversial. To our knowledge, this is the first meta-analysis that directly compares both techniques, without considering aneurysm size and location, in terms of immediate and long-term angiographic and clinical outcomes.
In the present study, a total of 18 studies from the past decade, including 2000 patients (2200 aneurysms), were selected. The covariates age, sex, and risk factors, such as hypertension, did not show statistical difference, but we observed that the size of aneurysms in the FD group were larger than that of CEV group. Originally, the FD was intended for treatment of complex and large or giant aneurysms,15 and across time, the FD was indicated for small aneurysms.14 Furthermore, due to major injuries caused by clipping or bypass microsurgeries, patients and surgeons preferred the FD to remove large/giant aneurysms out of the circulation while protecting the perforating artery.However, the conventional treatments for large/giant aneurysms may cause stent malposition and endoleaks, leading to recurrence and retreatment. Thus, in the real world, the CEV group had smaller aneurysms than the FD group.
Both short- and long-term angiographic and clinical outcomes are commonly reported, including procedure-related complications, immediate occlusion rates, mRS scores at discharge, occlusion rates at the last follow-up, delayed complications, and mRS scores at the last follow-up. For these observed variables, only the occlusion rate at the last follow-up was reported in all included cohorts; therefore, a funnel plot of this variable was depicted to detect the publication bias of all the studies. We extracted only the complete occlusion data according to the Raymond classification, except for Wang et al,26 who selected the O’Kelly-Marotta grading scale as the standard criterion. Aggregation of the data about ischemia, hemorrhage, and cranial nerve deficits and other complications indicated that the risk of procedure-related complications due to the FD was higher than that of the CEV group, which was consistent with an early meta-analysis.40 Rupture with poor prognoses was reported in about 81.3% of patients experiencing death or poor neurologic outcomes after FD treatment.41,42 Using a numeric method, Cebral et al43 found that the increased pressure in aneurysms following FD treatment may contribute to rupture, which was proved by another simulation study.44 An early single-arm meta-analysis found that procedure-related permanent morbidity and mortality rates reached 5% and 4%, respectively, in FD treatments. High rates of intraparenchymal hemorrhage, postprocedural SAH, and ischemic stroke were also reported.45 Our meta-analysis provides more representative data by directly comparing the safety of FD and CEV treatments.
The long-term follow-up angiographic results indicated the superiority of the FD with a higher complete occlusion rate and lower recurrence and retreatment rates. In series studies, complete occlusion was noted in 63% of aneurysms in early postmarket results,8 82.6% of aneurysms in the study were not restricted to the circle of Willis,46 and 93.9% of aneurysms had a stent placed within an FD.47 The occlusion rate at the last follow-up treatment with an FD can even reach 100%.48 In this pooled study, the complete occlusion rate of the FD group was 2.5-fold that of the CEV group. Nevertheless, high heterogeneity was also observed. After the subgroup meta-regression and sensitivity analyses, we found that the heterogeneity was due to aneurysm size, which implied that a study adjusting for aneurysm diameter may be better when exploring the effectiveness and safety of flow-diverting in the future. Accompanied by a high complete occlusion rate, the rates of recurrence and retreatment in the FD group were lower than those in the CEV group. However, in terms of long-term clinical outcomes, there were no significant differences between the 2 groups.
The significant findings of this work were the following: 1) aneurysms treated with an FD were larger than those in the CEV group; 2) the procedure-related complications occurred more often during FD placement; 3) compared with the FD group, the CEV group had a lower rate of complete obliteration during angiography; and 4) the recurrence and retreatment rates of the FD group were lower than those of the CEV group.
There are some limitations to our study. First, as we determined, aneurysm size influenced the analysis and contributed to the heterogeneity of the results. It is better to divide this variable into small, medium, and large groups. In addition, the status (ruptured or unruptured),49,50 anatomic location,51 and aneurysm type52 may also affect the final results, but we neglected to include these factors in our study. This omission is because research studies that directly compare FD and CEV treatments are rare, and these factors were not taken into consideration in the original studies. However, these confounding effects were resolved as much as possible by subgrouping analyses and meta-regression. Second, the findings of recurrence and retreatment differences were based on data from a small subset of the included studies. A large data set is needed to verify these results. Third, multiple FDs and CEV treatments included may introduce heterogeneity. Last, the periprocedural risk that occurs with retreatment was not pooled because such results were not recorded in the original articles.
CONCLUSIONS
Our meta-analysis directly compared the effectiveness and safety of FD and CEV treatment in the immediate and long term. Compared with the CEV treatment, the placement of an FD may lead to more procedure-related complications, but there is not a difference of safety and it is more effective in the long term.
ABBREVIATIONS:
- BAC
balloon-assisted coiling
- CEV
conventional endovascular
- FD
flow diverter
- IA
intracranial aneurysm
- SAC
stent-assisted coiling
- SMD
standard mean difference
Footnotes
Registration ID: CRD42021282218
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- 1.Guglielmi G, Viñuela F, Dion J, et al. Electrothrombosis of saccular aneurysms via endovascular approach, Part 2: preliminary clinical experience. J Neurosurg 1991;75:8–14 10.3171/jns.1991.75.1.0008 [DOI] [PubMed] [Google Scholar]
- 2.Higashida RT, Smith W, Gress D, et al. Intravascular stent and endovascular coil placement for a ruptured fusiform aneurysm of the basilar artery: case report and review of the literature. J Neurosurg 1997;87:944–49 10.3171/jns.1997.87.6.0944 [DOI] [PubMed] [Google Scholar]
- 3.Moret J, Cognard C, Weill A, et al. Reconstruction technic in the treatment of wide-neck intracranial aneurysms: long-term angiographic and clinical results—apropos of 56 cases. J Neuroradiol 1997;24:30–44 [PubMed] [Google Scholar]
- 4.Zhang X, Zhong J, Gao H, et al. Endovascular treatment of intracranial aneurysms with the LVIS device: a systematic review. J Neurointerv Surg 2017;9:553–57 10.1136/neurintsurg-2016-012403 [DOI] [PubMed] [Google Scholar]
- 5.Becske T, Kallmes DF, Saatci I, et al. Pipeline for uncoilable or failed aneurysms: results from a multicenter clinical trial. Radiology 2013;267:858–68 10.1148/radiol.13120099 [DOI] [PubMed] [Google Scholar]
- 6.Nelson PK, Lylyk P, Szikora I, et al. The Pipeline Embolization Device for the intracranial treatment of aneurysms trial. AJNR Am J Neuroradiol 2011;32:34–40 10.3174/ajnr.A2421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chalouhi N, Jabbour P, Singhal S, et al. Stent-assisted coiling of intracranial aneurysms: predictors of complications, recanalization, and outcome in 508 cases. Stroke 2013;44:1348–53 10.1161/STROKEAHA.111.000641 [DOI] [PubMed] [Google Scholar]
- 8.Kan P, Siddiqui AH, Veznedaroglu E, et al. Early postmarket results after treatment of intracranial aneurysms with the Pipeline Embolization Device: a U.S. multicenter experience. Neurosurgery 2012;71:1080–87; discussion 7–8 10.1227/NEU.0b013e31827060d9 [DOI] [PubMed] [Google Scholar]
- 9.Li W, Tian Z, Zhu W, et al. Hemodynamic analysis of postoperative rupture of unruptured intracranial aneurysms after placement of flow-diverting stents: a matched case-control study. AJNR Am J Neuroradiol 2019;40:1916–23 10.3174/ajnr.A6256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, et al. ; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–05 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 12.Adeeb N, Griessenauer CJ, Foreman PM, et al. Comparison of stent-assisted coil embolization and the Pipeline Embolization Device for endovascular treatment of ophthalmic segment aneurysms: a multicenter cohort study. World Neurosurg 2017;105:206–12 10.1016/j.wneu.2017.05.104 [DOI] [PubMed] [Google Scholar]
- 13.Chalouhi N, Daou B, Barros G, et al. Matched comparison of flow diversion and coiling in small, noncomplex intracranial aneurysms. Neurosurgery 2017;81:92–97 10.1093/neuros/nyw070 [DOI] [PubMed] [Google Scholar]
- 14.Chalouhi N, Starke RM, Yang S, et al. Extending the indications of flow diversion to small, unruptured, saccular aneurysms of the anterior circulation. Stroke 2014;45:54–58 10.1161/STROKEAHA.113.003038 [DOI] [PubMed] [Google Scholar]
- 15.Chalouhi N, Tjoumakaris S, Starke RM, et al. Comparison of flow diversion and coiling in large unruptured intracranial saccular aneurysms. Stroke 2013;44:2150–54 10.1161/STROKEAHA.113.001785 [DOI] [PubMed] [Google Scholar]
- 16.Di Maria F, Pistocchi S, Clarençon F, et al. Flow diversion versus standard endovascular techniques for the treatment of unruptured carotid-ophthalmic aneurysms. AJNR Am J Neuroradiol 2015;36:2325–30 10.3174/ajnr.A4437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durst CR, Starke RM, Clopton D, et al. Endovascular treatment of ophthalmic artery aneurysms: ophthalmic artery patency following flow diversion versus coil embolization. J Neurointerv Surg 2016;8:919–22 10.1136/neurintsurg-2015-011887 [DOI] [PubMed] [Google Scholar]
- 18.Enriquez-Marulanda A, Salem MM, Ascanio LC, et al. No differences in effectiveness and safety between Pipeline Embolization Device and stent-assisted coiling for the treatment of communicating segment internal carotid artery aneurysms. Neuroradiol J 2019;32:344–52 10.1177/1971400919845368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim LJ, Tariq F, Levitt M, et al. Multimodality treatment of complex unruptured cavernous and paraclinoid aneurysms. Neurosurgery 2014;74:51–61; discussion 61; quiz 61 10.1227/NEU.0000000000000192 [DOI] [PubMed] [Google Scholar]
- 20.Lanzino G, Crobeddu E, Cloft HJ, et al. Efficacy and safety of flow diversion for paraclinoid aneurysms: a matched-pair analysis compared with standard endovascular approaches. AJNR Am J Neuroradiol 2012;33:2158–61 10.3174/ajnr.A3207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu JM, Zhou Y, Li Y, et al. Parent artery reconstruction for large or giant cerebral aneurysms using the Tubridge flow diverter: a multicenter, randomized, controlled clinical trial (PARAT). AJNR Am J Neuroradiol 2018;39:807–16 10.3174/ajnr.A5619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu P, Zhang Y, Niu H, et al. Comparison of endovascular treatment for middle cerebral artery aneurysm with a low-profile visualized intraluminal support stent or Pipeline Embolization Device. Exp Ther Med 2019;18:2072–78 10.3892/etm.2019.7775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petr O, Brinjikji W, Cloft H, et al. Current trends and results of endovascular treatment of unruptured intracranial aneurysms at a single institution in the flow-diverter era. AJNR Am J Neuroradiol 2016;37:1106–13 10.3174/ajnr.A4699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salem MM, Ravindran K, Enriquez-Marulanda A, et al. Pipeline Embolization Device versus stent-assisted coiling for intracranial aneurysm treatment: a retrospective propensity score-matched study. Neurosurgery 2020;87:516–22 10.1093/neuros/nyaa041 [DOI] [PubMed] [Google Scholar]
- 25.Silva MA, See AP, Khandelwal P, et al. Comparison of flow diversion with clipping and coiling for the treatment of paraclinoid aneurysms in 115 patients. J Neurosurg 2019;130:1505–08 10.3171/2018.1.JNS171774 [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Jia L, Duan Z, et al. Endovascular treatment of large or giant non-saccular vertebrobasilar aneurysms: Pipeline Embolization Devices versus conventional stents. Front Neurosci 2019;13:1253 10.3389/fnins.2019.01253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zanaty M, Chalouhi N, Starke RM, et al. Flow diversion versus conventional treatment for carotid cavernous aneurysms. Stroke 2014;45:2656–61 10.1161/STROKEAHA.114.006247 [DOI] [PubMed] [Google Scholar]
- 28.Yupeng Zhang Y, Liang F, Zhang Y, et al. Exploring the feasibility of Pipeline Embolization Device compared with stent-assisted coiling to treat non-saccular, unruptured, intradural vertebral artery aneurysms. Front Neurol 2019;10:275 10.3389/fneur.2019.00275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, Zhou Y, Yang P, et al. Comparison of the flow diverter and stent-assisted coiling in large and giant aneurysms: safety and efficacy based on a propensity score-matched analysis. Eur Radiology 2016;26:2369–77 10.1007/s00330-015-4052-1 [DOI] [PubMed] [Google Scholar]
- 30.Park HK, Horowitz M, Jungreis C, et al. Endovascular treatment of paraclinoid aneurysms: experience with 73 patients. Neurosurgery 2003;53:14–23; discussion 24 10.1227/01.neu.0000068789.08955.1c [DOI] [PubMed] [Google Scholar]
- 31.Hetts SW, Turk A, English JD, et al. ; Matrix and Platinum Science Trial Investigators. Stent-assisted coiling versus coiling alone in unruptured intracranial aneurysms in the Matrix and Platinum Science Trial: safety, efficacy, and mid-term outcomes. AJNR Am J Neuroradiol 2014;35:698–705 10.3174/ajnr.A3755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malek AM, Halbach VV, Phatouros CC, et al. Balloon-assist technique for endovascular coil embolization of geometrically difficult intracranial aneurysms. Neurosurgery 2000;46:1397–406; discussion 406–07 10.1097/00006123-200006000-00022 [DOI] [PubMed] [Google Scholar]
- 33.Amenta PS, Dalyai RT, Kung D, et al. Stent-assisted coiling of wide-necked aneurysms in the setting of acute subarachnoid hemorrhage: experience in 65 patients. Neurosurgery 2012;70:1415–29; discussion 29 10.1227/NEU.0b013e318246a4b1 [DOI] [PubMed] [Google Scholar]
- 34.Moret J, Cognard C, Weill A, et al. The “Remodelling Technique” in the treatment of wide neck intracranial aneurysms. angiographic results and clinical follow-up in 56 cases. Interv Neuroradiol 1997;3:21–35 10.1177/159101999700300103 [DOI] [PubMed] [Google Scholar]
- 35.Ferns SP, Sprengers ME, van Rooij WJ, et al. Late reopening of adequately coiled intracranial aneurysms: frequency and risk factors in 400 patients with 440 aneurysms. Stroke 2011;42:1331–37 10.1161/STROKEAHA.110.605790 [DOI] [PubMed] [Google Scholar]
- 36.Nossek E, Chalif DJ, Chakraborty S, et al. Concurrent use of the Pipeline Embolization Device and coils for intracranial aneurysms: technique, safety, and efficacy. J Neurosurg 2015;122:904–11 10.3171/2014.12.JNS141259 [DOI] [PubMed] [Google Scholar]
- 37.Xiang J, Ma D, Snyder KV, et al. Increasing flow diversion for cerebral aneurysm treatment using a single flow diverter. Neurosurgery 2014;75:286–94; discussion 94 10.1227/NEU.0000000000000409 [DOI] [PubMed] [Google Scholar]
- 38.Bonney PA, Connor M, Fujii T, et al. Failure of flow diverter therapy: predictors and management strategies. Neurosurgery 2020;86:S64–73 10.1093/neuros/nyz305 [DOI] [PubMed] [Google Scholar]
- 39.Townsend RK, Wolfe SQ, Anadani M, et al. Endovascular management of acute postprocedural flow diverting stent thrombosis. J Neurointerv Surg 2020;12:67–71 10.1136/neurintsurg-2019-014944 [DOI] [PubMed] [Google Scholar]
- 40.Domingo RA, Tripathi S, Perez-Vega C, et al. Treatment of posterior circulation non-saccular aneurysms with flow diversion versus stent-assisted coiling: a systematic review and meta-analysis. J Neurointerv Surg 2021;13:159–63 10.1136/neurintsurg-2020-016294 [DOI] [PubMed] [Google Scholar]
- 41.Rouchaud A, Brinjikji W, Lanzino G, et al. Delayed hemorrhagic complications after flow diversion for intracranial aneurysms: a literature overview. Neuroradiology 2016;58:171–77 10.1007/s00234-015-1615-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turowski B, Macht S, Kulcsár Z, et al. Early fatal hemorrhage after endovascular cerebral aneurysm treatment with a flow diverter (SILK-Stent): do we need to rethink our concepts? Neuroradiology 2011;53:37–41 10.1007/s00234-010-0676-7 [DOI] [PubMed] [Google Scholar]
- 43.Cebral JR, Mut F, Raschi M, et al. Aneurysm rupture following treatment with flow-diverting stents: computational hemodynamics analysis of treatment. AJNR Am J Neuroradiol 2011;32:27–33 10.3174/ajnr.A2398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hassan T, Ahmed YM, Hassan AA. The adverse effects of flow-diverter stent-like devices on the flow pattern of saccular intracranial aneurysm models: computational fluid dynamics study. Acta Neurochir (Wien) 2011;153:1633–40 10.1007/s00701-011-1055-9 [DOI] [PubMed] [Google Scholar]
- 45.Brinjikji W, Murad MH, Lanzino G, et al. Endovascular treatment of intracranial aneurysms with flow diverters: a meta-analysis. Stroke 2013;44:442–47 10.1161/STROKEAHA.112.678151 [DOI] [PubMed] [Google Scholar]
- 46.Pistocchi S, Blanc R, Bartolini B, et al. Flow diverters at and beyond the level of the circle of Willis for the treatment of intracranial aneurysms. Stroke 2012;43:1032–38 10.1161/STROKEAHA.111.636019 [DOI] [PubMed] [Google Scholar]
- 47.Ocal O, Peker A, Balci S, et al. Placement of a stent within a flow diverter improves aneurysm occlusion rates. AJNR Am J Neuroradiol 2019;40:1932–38 10.3174/ajnr.A6237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mazur MD, Kilburg C, Wang V, et al. Pipeline Embolization Device for the treatment of vertebral artery aneurysms: the fate of covered branch vessels. J Neurointerv Surg 2016;8:1041–47 10.1136/neurintsurg-2015-012040 [DOI] [PubMed] [Google Scholar]
- 49.Bhatia KD, Kortman H, Orru E, et al. Periprocedural complications of second-generation flow diverter treatment using Pipeline Flex for unruptured intracranial aneurysms: a systematic review and meta-analysis. J Neurointerv Surg 2019;11:817–24 10.1136/neurintsurg-2019-014937 [DOI] [PubMed] [Google Scholar]
- 50.Cagnazzo F, di Carlo DT, Cappucci M, et al. Acutely ruptured intracranial aneurysms treated with flow-diverter stents: a systematic review and meta-analysis. AJNR Am J Neuroradiol 2018;39:1669–75 10.3174/ajnr.A5730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cagnazzo F, Perrini P, Dargazanli C, et al. Treatment of unruptured distal anterior circulation aneurysms with flow-diverter stents: a meta-analysis. AJNR Am J Neuroradiol 2019;40:687–93 10.3174/ajnr.A6002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rouchaud A, Brinjikji W, Cloft HJ, et al. Endovascular treatment of ruptured blister-like aneurysms: a systematic review and meta-analysis with focus on deconstructive versus reconstructive and flow-diverter treatments. AJNR Am J Neuroradiol 2015;36:2331–39 10.3174/ajnr.A4438 [DOI] [PMC free article] [PubMed] [Google Scholar]