Abstract
BACKGROUND AND PURPOSE:
Pathogenic somatic variants affecting the genes Histone 3 Family 3A and 3B (H3F3) are extensively linked to the process of oncogenesis, in particular related to central nervous system tumors in children. Recently, H3F3 germline missense variants were described as the cause of a novel pediatric neurodevelopmental disorder. We aimed to investigate patterns of brain MR imaging of individuals carrying H3F3 germline variants.
MATERIALS AND METHODS:
In this retrospective study, we included individuals with proved H3F3 causative genetic variants and available brain MR imaging scans. Clinical and demographic data were retrieved from available medical records. Molecular genetic testing results were classified using the American College of Medical Genetics criteria for variant curation. Brain MR imaging abnormalities were analyzed according to their location, signal intensity, and associated clinical symptoms. Numeric variables were described according to their distribution, with median and interquartile range.
RESULTS:
Eighteen individuals (10 males, 56%) with H3F3 germline variants were included. Thirteen of 18 individuals (72%) presented with a small posterior fossa. Six individuals (33%) presented with reduced size and an internal rotational appearance of the heads of the caudate nuclei along with an enlarged and squared appearance of the frontal horns of the lateral ventricles. Five individuals (28%) presented with dysgenesis of the splenium of the corpus callosum. Cortical developmental abnormalities were noted in 8 individuals (44%), with dysgyria and hypoplastic temporal poles being the most frequent presentation.
CONCLUSIONS:
Imaging phenotypes in germline H3F3-affected individuals are related to brain features, including a small posterior fossa as well as dysgenesis of the corpus callosum, cortical developmental abnormalities, and deformity of lateral ventricles.
Histones are nuclear proteins that bind to DNA in the nucleus and help condense it into chromatin.1 Histones are dynamically decorated with posttranslational modifications, which regulate the processes of DNA repair, gene expression, mitosis, and meiosis. Abnormal dysregulation of these posttranslational modifications has been linked to cancer, neurodevelopmental syndromes, psychiatric disorders, and cardiovascular disease.2-5 Therefore, histone biology is critical to understanding the pathophysiology of many diseases and developing treatments.
Pathogenic somatic variants affecting H3F3 have been extensively linked to the epigenetic process of oncogenesis. In particular, when these variants involve 2 critical amino acids, p.Lys27 and p.Gly34, they are linked to central nervous system in children (p.Lys27 is linked to diffuse midline gliomas, and p.Gly34 is linked to supratentorial hemispheric gliomas).6-8 Currently, these variants represent a major molecular feature for accurate classification of these neoplasms according to the World Health Organization.9
Expanding the correlation of this gene with human disease, Bryant et al10 have recently demonstrated that H3F3 plays a major role during embryogenesis, and causative pathogenic variants in these genes are associated with neurocognitive delay along with other symptoms such as seizures and hypotonia. In the present study, we sought to investigate the value of brain MR imaging in individuals carrying H3F3A or H3F3B germline variants, looking for an imaging pattern that would be recognizable for diagnostic purposes.
MATERIALS AND METHODS
Individual Population
This study included individuals with proved H3F3 pathogenic and likely pathogenic variants that are causative of disease and with available clinical MR imaging scans of the brain. Individuals and their families were collected prospectively from the Myelin Disorders Bioregistry Project with approval from the institutional review board at Children’s Hospital of Philadelphia (institutional review board approval No. IRB 14–011236). Written informed consent for the collection of clinical information, neuroimaging, and genetic information was obtained for each study participant.
Abstraction of Clinical Data
Clinical and demographic data were retrieved from available medical records of affected individuals. Genetic testing reports were reviewed, or variants were provided by the referring provider and classified using the American College of Medical Genetics criteria for variant curation. All clinical and molecular data were reviewed by a board-certified clinical and/or clinical molecular geneticist.
MR Imaging Technique
We retrospectively reviewed all available brain MR imaging studies of the included subjects. Images were acquired at either 1.5T or 3T MR using different imaging protocols including at least sagittal and axial T1WI and T2WI. MR images not allowing adequate visual assessment were excluded.
Imaging Analysis
MR images of all individuals were reviewed independently by 2 pediatric neuroradiologists (C.A.P.F.A. and F.D.) with final consensus agreement in searching for structural features involving the posterior fossa, major commissural structures, and cortex, along with abnormalities in the ventricular system, basal ganglia, and thalamus. Additional evaluation, measurements, and ratios of the posterior fossa and corpus callosum, both evaluated in the sagittal midline, were performed to confirm the size abnormalities.11-15 Detailed analysis of characteristics of white matter myelination was also performed.
Statistical Analysis
Statistical analyses were performed using R statistical and computing software (http://www.r-project.org) and R studio (http://rstudio.org/download/desktop). A 2-tailed P < .05 was considered statistically significant. The Shapiro-Wilk test was used to assess the normality of continuous variables, which were presented as median and first and third quartiles (1Q–3Q). Categoric variables were presented as counts and percentages. Mann-Whitney U or Student t tests were used to compare continuous variables, and the Fisher exact test was used to compare categoric variables between clinical data and MR imaging findings. For statistical analysis, individuals were divided into 2 groups, nonachievement milestones versus normal achievement plus development delay. Delayed and normal developmental milestones were grouped together due to the small number of subjects with normal development. Delayed milestones were defined as sitting after 6 months, walking after 20 months, and first word after 12 months of age.
RESULTS
On the basis of the inclusion criteria, a cohort of 18 individuals (mean age, 4.5 years; range, 1.9–12.1 years; 10 males/8 females) with proved H3F3 variants causative of disease were included in this study. All individuals underwent brain MR imaging. These individuals had 1 of 2 genotypes: H3F3A variants (n = 11) or H3F3B variants (n = 7). The details of each individual’s demographic, clinical, and genetic information are given in Table 1 and the Online Supplemental Data. The overall imaging findings are described in Table 2.
Table 1:
Demographic, genetic, and clinical information of individuals with disease-causing missense variantsa
| Characteristics | Individuals (n = 18) |
|---|---|
| Age at last evaluation (yr) | 4.46 (1.9–12.1) |
| Sex: female/male | (8:10) |
| H3F3 variant | |
| H3F3A | 11 (61) |
| H3F3B | 7 (39) |
| Microcephaly | 8 (44) |
| Seizures | 10 (56) |
| Febrile | 5 (50) |
| Nonfebrile | 5 (50) |
| Sitting (n = 17) | |
| Normal | 1 (6) |
| Delayed | 12 (71) |
| Not achieved | 4 (23) |
| Walking | |
| Normal | 2 (11) |
| Delayed | 10 (56) |
| Not achieved | 6 (33) |
| Speaking | |
| Normal | 1 (6) |
| Delayed | 7 (38) |
| Not achieved | 10 (56) |
Categoric variables are described as number (percentage). Continuous variables are described as median (1Q–3Q).
Table 2:
Abnormal imaging information of individuals with disease-causing H3F3 missense variantsa
| Imaging Features | Individuals(n = 18) |
|---|---|
| Small posterior fossa | 13 (72) |
| Cerebellum | 2 (11) |
| Brainstem | 2 (11) |
| Thalamus | 0 |
| Caudate | 8 (44) |
| Putamen | 0 |
| Globus pallidum | 0 |
| Corpus callosum | 5 (28) |
| Fourth ventricle | 6 (33) |
| Lateral ventricle | 7 (38) |
| White matter | 2 |
| Cortex | 8 (44) |
| Optic nerves and chiasm | 1 (6) |
| Temporal lobes/hippocampus | 8 (44) |
| Clivus/sella | 4 (22) |
Categoric variables are described as number (percentage).
Posterior Fossa
Thirteen of 18 individuals (72%) presented with a verticalized tentorium and low insertion of the torcula, along with features suggestive of a small posterior fossa and hypoplasia of the occipital bone, later confirmed with additional posterior fossa measurements and ratios (Online Supplemental Data).13-15 The occipital bone, measured by the supraoccipital line, of those patients with a small posterior fossa was disproportionally small, and it was significantly smaller (P = .001) compared with the subjects with a normal posterior fossa. Of those, 7 individuals (7/13 54%) had posterior fossa structures (brainstem and cerebellum) that appeared crowded (Fig 1A–D). A low disposition of the cerebellar tonsils fitting in the Chiari I deformity criteria15 was observed in 4 individuals. Further mild malformative features of the brainstem were observed in 2 children: both with abnormal anterior-posterior pattern of malformations, one with disproportional predominance of the midbrain (Online Supplemental Data) and the other with disproportional size reduction of the midbrain compared with the medulla oblongata (Online Supplemental Data). Hypoplasia of the clivus and signs of platybasia were noted in 3 individuals.
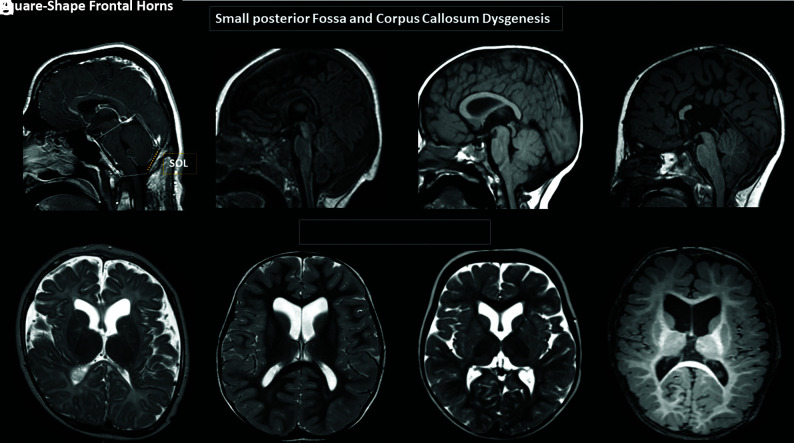
FIG 1.
Brain MR images. A and D, Variable degrees of corpus callosum deformities, particularly involving the body and splenium, noting partial agenesis in D. Deformed morphology of the posterior fossa, with variable degrees of low insertion of the torcula and size reduction of the supraoccipital line (SOL). Note the crowded appearance of the structures in the posterior fossa along with low disposition of the cerebellar tonsils, fitting in the Chiari I deformity criteria in C and D. E–H, Variable degrees of reduced size and/or internal rotational appearance of the head of the caudate nuclei, resulting in an enlarged and squared appearance of the frontal horns.
Major Commissures
Malformative features of the corpus callosum, accompanied or not accompanied by anterior commissure hypoplasia, were present in 5 individuals (28%). The involvement of the splenium of the corpus callosum was the most remarkable feature, being absent or elongated and hypoplastic according to the patient’s age in all 5 cases. Agenesis of the body and splenium of the corpus callosum was observed in 1 case (5.5%) (Fig 1A–D and Online Supplemental Data).
Cortex
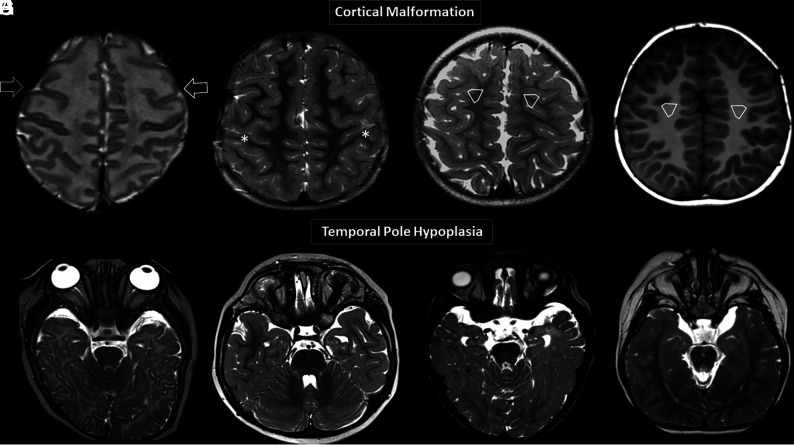
The cerebral cortex of 8 individuals (44%) presented with malformative features. Six of them showed variable degrees of diffuse dysgyria, (Fig 2A–D) 1 anterior pachygyria, and 1 diffuse simplified cortical appearance. Along with the cortical abnormalities, all 8 individuals also had bilateral temporal lobe hypoplasia (Fig 2E–H).
FIG 2.
Brain MR images. Axial T2WI (A–C) and axial T1WI (D) showing 4 different patients with variable degrees of diffuse abnormal orientation and morphology of the gyri and sulci. Note particular abnormal morphology involving both frontal lobes (open arrows, A), a deformed perirolandic region (asterisks, B), and abnormal gyration of the medial frontal lobes in C and D (open arrowheads, C and D). Axial T2WI (E–H) shows 4 different patients with temporal pole hypoplasia.
Basal Ganglia and Lateral Ventricles
Six individuals (33%) presented with a relatively reduced size and/or internal rotational appearance of the heads of the caudate nuclei. These features resulted in a characteristic deformity of the lateral ventricles, with an enlarged and squared appearance of the frontal horns (Fig 1E–H).
Clinical Correlation
Our entire cohort (n = 18) presented with at least 1 clinical symptom, including microcephaly, the presence of seizures, and delayed or not delayed achieved development milestones (Table 1).
No statistically significant (P < .05) correlation between clinical symptoms (absence of achievement of gross motor and speaking milestones, presence of seizures) and main imaging findings (small posterior fossa, basal ganglia abnormalities, and corpus callosum and cortical malformations) was found. However, all individuals included in our cohort except for 1 had severe clinical symptoms and marked abnormalities involving the brain. Moreover, 50% of individuals presenting with seizures also had malformative features involving the cortex (Online Supplemental Data).
DISCUSSION
Somatic variants in H3F3 are well-known promoters of oncogenesis;16-19 however, the role of germline variants remain underrecognized. The recent discovery of disease-associated missense variants in H3F3 that cause a neurodevelopmental disorder, but not cancer, profoundly impacts histone biology research.10 In the present study, we sought to investigate the value of brain MR imaging of individuals carrying H3F3 germline variants, assessing imaging patterns and the neurodegenerative clinical symptoms. We found a constellation of malformative features of the brain, including a small posterior fossa, along with changes in the basal ganglia, cortex, and corpus callosum.
Neuroimaging plays an important role in the diagnosis of pediatric glial tumors related to pathogenic somatic variants affecting Histone 3 Family 3A and 3B.9,16,20-22 Because some recent studies have demonstrated the critical importance of histone turnover in neuronal transcription and plasticity in the mammalian brain23-25 and Bryant et al10 have demonstrated the role of germline variants of H3F3 during embryogenesis as a causative factor of neurocognitive delay in young individuals, we have investigated the potential presentation of malformative features in the brain MR imaging of these individuals and how these would impact the patient’s prognosis.
Individuals with disease-causing germline variants in histone 3.3 in our cohort had a characteristic clinical background, usually presenting with neurocognitive delay, seizures, and microcephaly. On neuroimaging, our cohort also shared some similar features; the most prevalent was a small posterior fossa (13/18 individuals), with some of them presenting with Chiari I deformity and malformative features affecting the brainstem or cerebellum. There is a wide spectrum of congenital abnormalities associated with a small posterior fossa, including developmental malformations caused by a genetic defect26-28 as well as disruptive causes due to injury of a structure with normal developmental potential.29-32 Understanding the spectrum of congenital posterior fossa anomalies and their diagnostic criteria is of paramount importance for optimal therapy, accurate prognosis, and correct genetic counseling.29
We have encountered further neuroimaging findings in our cohort of individuals with disease-causing germline missense variants in H3F3. The findings included enlarged frontal horns of the lateral ventricles, reflecting reduced size and/or an internal rotational appearance of the nuclei of the caudate heads, dysgenesis of the corpus callosum, and malformations of cortical development, such as dysgyria. Cortical malformation implies abnormalities in both the migration of neurons to the cortex and abnormal cortical organization33,34 and may underlie the relatively high frequency of epilepsy in those individuals.35,36
No statistical significance (P < .05) was found correlating milestones delay (in sitting, walking, and speaking the first word) or the presence of seizures with the main imaging findings, including a small posterior fossa, basal ganglia abnormalities, and corpus callosum and cortical malformations. Fifty percent of individuals presenting with seizures also had malformative features involving the cortex, suggesting a potential correlation between both.
Despite presenting promising findings, our study also had limitations, including the retrospective nature of our study design. Our cohort was biased because all individuals included in our study except for 1 had severe clinical symptoms and significant abnormalities involving the brain, making correlative analysis more difficult. The other limitation was that we had a relatively small sample size of individuals who had brain MR imaging assessment, though it represents almost half of the 43 reported individuals in the literature. On the other hand, we found consistent neuroimaging findings among our cohort, which is helpful for guiding appropriate genetic investigations of these individuals. Our imaging findings may reflect the spectrum of abnormalities seen in individuals with germline variants in histone 3.3; however, these results need to be validated in a larger cohort with broader disease severity.
CONCLUSIONS
The current series, including a subset of individuals previously reported in the original work of Bryant et al,10 represents the largest cohort of neuroradiologically characterized subjects carrying disease-causing H3F3 germline variants. The identified constellation of neuroimaging findings, namely a small posterior fossa, reduced size and/or a rotational appearance of the nuclei of the caudate heads, dysgenesis of the corpus callosum, and malformations of cortical development, offer novel diagnostic pattern able to guide the diagnosis of H3F3-related disorder.
Acknowledgments
We are grateful to the patients and their families for their involvement in this study. We also thank Lydia Sheldon, MSEd, for helpful editing suggestions.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- 1.Mariño-Ramírez L, Kann MG, Shoemaker BA, et al. Histone structure and nucleosome stability. Expert Rev Proteomics 2005;2:719–29 10.1586/14789450.2.5.719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao Z, Shilatifard A. Epigenetic modifications of histones in cancer. Genome Biol 2019;20:245 10.1186/s13059-019-1870-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim JH, Lee JH, Lee IS, et al. Histone lysine methylation and neurodevelopmental disorders. Int J Mol Sci 2017;18:1404 10.3390/ijms18071404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peter CJ, Akbarian S. Balancing histone methylation activities in psychiatric disorders. Trends Mol Med 2011;17:372–79 10.1016/j.molmed.2011.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bagchi RA, Weeks KL. Histone deacetylases in cardiovascular and metabolic diseases. J Mol Cell Cardiol 2019;130:151–59 10.1016/j.yjmcc.2019.04.003 [DOI] [PubMed] [Google Scholar]
- 6.Sturm D, Witt H, Hovestadt V, et al. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell 2012;22:425–37 10.1016/j.ccr.2012.08.024 [DOI] [PubMed] [Google Scholar]
- 7.Aboian MS, Solomon DA, Felton E, et al. Imaging characteristics of pediatric diffuse midline gliomas with histone H3 K27M mutation. AJNR Am J Neuroradiol 2017;38:795–800 10.3174/ajnr.A5076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurokawa R, Baba A, Kurokawa M, et al. Neuroimaging features of diffuse hemispheric glioma, H3 G34-mutant: a case series and systematic review. J Neuroimaging 2022;32:17–27 10.1111/jon.12939 [DOI] [PubMed] [Google Scholar]
- 9.Louis DN, Perry A, Wesseling P, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol 2021;23:1231–51 10.1093/neuonc/noab106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bryant L, Li D, Cox SG, et al. Histone H3.3 beyond cancer: germline mutations in Histone 3 Family 3A and 3B cause a previously unidentified neurodegenerative disorder in 46 patients. Sci Adv 2020;6:eabc9207 10.1126/sciadv.abc9207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garel C, Cont I, Alberti C, et al. Biometry of the corpus callosum in children: MR imaging reference data. AJNR Am J Neuroradiol 2011;32:1436–43 10.3174/ajnr.A2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jandeaux C, Kuchcinski G, Ternynck C, et al. Biometry of the cerebellar vermis and brain stem in children: MR imaging reference data from measurements in 718 children. AJNR Am J Neuroradiol 2019;40:1835–41 10.3174/ajnr.A6257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marin-Padilla M, Marin-Padilla TM. Morphogenesis of experimentally induced Arnold–Chiari malformation. J Neurol Sci 1981;50:29–55 10.1016/0022-510x(81)90040-x [DOI] [PubMed] [Google Scholar]
- 14.Nishikawa M, Sakamoto H, Hakuba A, et al. Pathogenesis of Chiari malformation: a morphometric study of the posterior cranial fossa. J Neurosurg 1997;86:40–47 10.3171/jns.1997.86.1.0040 [DOI] [PubMed] [Google Scholar]
- 15.Poretti A, Ashmawy R, Garzon-Muvdi T, et al. Chiari type 1 deformity in children: pathogenetic, clinical, neuroimaging, and management aspects. Neuropediatrics 2016;47:293–307 10.1055/s-0036-1584563 [DOI] [PubMed] [Google Scholar]
- 16.Schwartzentruber J, Korshunov A, Liu XY, et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 2012;482:226–31 10.1038/nature10833 [DOI] [PubMed] [Google Scholar]
- 17.Weinberg DN, Allis CD, Lu C. Oncogenic mechanisms of histone H3 mutations. Cold Spring Harb Perspect Med 2017;7:a026443 10.1101/cshperspect.a026443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khuong-Quang DA, Buczkowicz P, Rakopoulos P, et al. K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol 2012;124:439–47 10.1007/s00401-012-0998-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng J, Hao S, Pan C, et al. The H3.3 K27M mutation results in a poorer prognosis in brainstem gliomas than thalamic gliomas in adults. Hum Pathol 2015;46:1626–32 10.1016/j.humpath.2015.07.002 [DOI] [PubMed] [Google Scholar]
- 20.Castel D, Philippe C, Calmon R, et al. Histone H3F3A and HIST1H3B K27M mutations define two subgroups of diffuse intrinsic pontine gliomas with different prognosis and phenotypes. Acta Neuropathol 2015;130:815–27 10.1007/s00401-015-1478-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aboian MS, Tong E, Solomon DA, et al. Diffusion characteristics of pediatric diffuse midline gliomas with histone H3-K27M mutation using apparent diffusion coefficient histogram analysis. AJNR Am J Neuroradiol 2019;40:1804–10 10.3174/ajnr.A6302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piccardo A, Tortora D, Mascelli S, et al. Advanced MR imaging and 18F-DOPA PET characteristics of H3K27M-mutant and wild-type pediatric diffuse midline gliomas. Eur J Nucl Med Mol Imaging 2019;46:1685–94 10.1007/s00259-019-04333-4 [DOI] [PubMed] [Google Scholar]
- 23.Maze I, Wenderski W, Noh K-M, et al. Critical role of histone turnover in neuronal transcription and plasticity. Neuron 2015;87:77–94 10.1016/j.neuron.2015.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deal RB, Henikoff JG, Henikoff S. Genome-wide kinetics of nucleosome turnover determined by metabolic labeling of histones. Science 2010;328:1161–64 10.1126/science.1186777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dion MF, Kaplan T, Kim M, et al. Dynamics of replication-independent histone turnover in budding yeast. Science 2007;315:1405–08 10.1126/science.1134053 [DOI] [PubMed] [Google Scholar]
- 26.Hennekam RC, Biesecker LG, Allanson JE, et al. ; Elements of Morphology Consortium. Elements of morphology: general terms for congenital anomalies. Am J Med Genet A 2013;161A:2726–33 10.1002/ajmg.a.36249 [DOI] [PubMed] [Google Scholar]
- 27.Doherty D, Millen KJ, Barkovich AJ. Midbrain and hindbrain malformations: advances in clinical diagnosis, imaging, and genetics. Lancet Neurol 2013;12:381–93 10.1016/S1474-4422(13)70024-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boycott KM, Flavelle S, Bureau A, et al. Homozygous deletion of the very low density lipoprotein receptor gene causes autosomal recessive cerebellar hypoplasia with cerebral gyral simplification. Am J Hum Genet 2005;77:477–83 10.1086/444400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bosemani T, Orman G, Boltshauser E, et al. Congenital abnormalities of the posterior fossa. Radiographics 2015;35:200–20 10.1148/rg.351140038 [DOI] [PubMed] [Google Scholar]
- 30.Limperopoulos C, Soul JS, Gauvreau K, et al. Late gestation cerebellar growth is rapid and impeded by premature birth. Pediatrics 2005;115:688–95 10.1542/peds.2004-1169 [DOI] [PubMed] [Google Scholar]
- 31.Messerschmidt A, Prayer D, Brugger PC, et al. Preterm birth and disruptive cerebellar development: assessment of perinatal risk factors. Eur J Paediatr Neurol 2008;12:455–60 10.1016/j.ejpn.2007.11.003 [DOI] [PubMed] [Google Scholar]
- 32.Steggerda SJ, Leijser LM, Wiggers-de Bruïne FT, et al. Cerebellar injury in preterm infants: incidence and findings on US and MR images. Radiology 2009;252:190–99 10.1148/radiol.2521081525 [DOI] [PubMed] [Google Scholar]
- 33.Barkovich AJ, Kuzniecky RI, Jackson GD, et al. Classification system for malformations of cortical development: update 2001. Neurology 2001;57:2168–78 10.1212/wnl.57.12.2168 [DOI] [PubMed] [Google Scholar]
- 34.Barkovich AJ, Kuzniecky RI, Jackson GD, et al. A developmental and genetic classification for malformations of cortical development. Neurology 2005;65:1873–87 10.1212/01.wnl.0000183747.05269.2d [DOI] [PubMed] [Google Scholar]
- 35.Shorvon S, Guerrini R, Trinka E, et al. The Causes of Epilepsy: Diagnosis and Investigation. Cambridge University Press; 2019 [Google Scholar]
- 36.Barkovich AJ, Guerrini R, Kuzniecky RI, et al. A developmental and genetic classification for malformations of cortical development: update 2012. Brain 2012;135:1348–69 10.1093/brain/aws019 [DOI] [PMC free article] [PubMed] [Google Scholar]