Abstract
Fatty infiltration (FI) of rotator cuff (RC) muscles is common in patients with RC tears. Studies have demonstrated that fibro-adipogenic progenitors (FAPs), a population of resident muscle stem cells, are the main contributors of FI, which adversely affects muscle quality and RC repair success. Although FI is common in RC injuries, it is not frequently reported after other musculotendinous injuries. Additionally, studies have shown the development of different pathology patterns across muscle groups suggestive of intrinsic differences in cellular composition and behavior. This study evaluates FAP distribution and differentiation properties across anatomic locations in mice. Muscles from seven different anatomic locations were harvested from PDGFRα-eGFP FAP reporter mice. FAPs were quantified using histology and FACS sorting with BD Aria II with CD31−/CD45−/Integrinα7−/Sca-1+ and PDGFRα reporter signal (n = 3 per muscle). The cells were analyzed for adipogenesis using immunocytochemistry and for proliferation properties with Brdu-Ki67 staining. In a separate group of mice, RC and tibialis anterior muscles received glycerol injection and were harvested after 2 weeks for FI quantification (n = 4). One-way analysis of variance was used for statistical comparisons among groups, with significance at p < 0.05. FAPs from the RC, masseter, and paraspinal muscles were more numerous and demonstrated greater proliferative capacity and adipogenic potency than those from the tibialis anterior and gastrocnemius. The RC demonstrated significantly greater levels of FI than the tibialis anterior after glycerol-injection injury. Clinical Significance: This study suggests differences in FAP distribution and differentiation characteristics may account for the propensity to develop FI in RC tears as compared with other musculotendinous injuries.
Keywords: fatty infiltration, fibro-adipogenic progenitor, paraspinal muscle, rotator cuff tears
Rotator cuff (RC) tears are a common and challenging entity encountered by the health-care system. These injuries are characterized by the development of muscle atrophy, fibrosis, and fatty infiltration (FI), which compromise muscle quality and function.1–3 These factors can also significantly impact the success rates of subsequent repair. Gladstone et al.4 showed that the degree of FI remained constant or progressed after patients underwent repair and found a positive correlation between the degree of FI and RC retear rates. Multiple other studies have described similar findings regarding FI and its detrimental role in RC tears and functional outcomes.5–7
Fibro-adipogenic progenitors (FAPs), a population of resident muscle stems characterized in part by platelet-derived growth factor receptor α (PDGFRα) expression, have recently emerged as a consequential pool of cells with regard to the development of muscle FI, fibrosis, and the regenerative capacity of muscle after injury. These cells rapidly proliferate upon muscle injury, initially providing a transient pro-differentiation environment that can facilitate new myotube formation and muscle regeneration.8,9 However, FAPs continue on to differentiate into adipocytes and fibroblasts, at which point they become the primary source of FI and fibrosis within the muscle.10,11 Our lab has recently demonstrated that FAPs are the major contributor of FI in the RC in our mouse RC tear model.12
Although FI is common in RC tears, this pattern of pathology is not ubiquitous across all types of musculotendinous injuries at all anatomic locations, with the exception of some genetic condition, such as muscular dystrophies, and more global metabolic disturbances such as obesity and type 2 diabetes.13–17 Multiple studies have observed high degrees of FI in paraspinal muscles in patients with congenital and degenerative spine diseases.18–21 Conversely, the development of intramuscular fibrosis rather than FI appears to be the hallmark of volumetric muscle injuries that involve the extremities.22–26 In addition, Davies et al.27 demonstrated different gene expression and histologic profiles in the RC and gastrocnemius muscles in a combined tendon-nerve injury model. RC muscles exhibited increased FI and adipogenic expression as compared with the gastrocnemius at 6 weeks after the same injury, suggesting there are intrinsic differences in FAP activity based on anatomic location.
In this study, we examine FAP distribution and properties among muscles from seven different anatomic locations—masseter (MA), supraspinatus (SS), infraspinatus (IS), paraspinal (PS), gastrocnemius (GA), triceps (TR), and tibialis anterior (TA) in mice. We hypothesize there are intrinsic differences in FAP quantity and adipogenic potency across various muscle tissue in mice.
METHODS
Animal Husbandry
Three-month-old female PDGFRα-eGFP reporter mice were purchased from Jackson laboratory Inc. (Stock No: 007669). After arriving in our VMU, mice were housed in groups of four per cage to allow for ample space to freely exercise when not utilized for specified experiments. The light/dark cycle was kept on a 12/12 h schedule in the housing facility. Health status of the mice was monitored daily by veterinarian staff as well was food and water stores in each cage. All experiments were approved by the Institutional Animal Care and Use Committee of our institution.
Muscle Harvest
Seven different muscles from PDGFRα-eGFP reporter mice (n = 3 per muscle) were harvested separately. The muscles collected included the MA, SS, IS, PS, GA, TR, and TA. All seven muscles underwent digestion separately in 0.2% collagenase for 90 min followed by 0.4% dispase for 30 min. The resulting suspensions were filtered through a 40 μm cell strainer, then pelleted and re-suspended in FACS buffer.
Flow Cytometry
FAPs were isolated and quantified using BD Aria II as defined as CD31−/CD45−/Integrinα7−/Sca-1+/PDGFRα-reporter-signal+ cells per the total number of single non-debris live cells as previously described.10,11 FAPs were cultured in 24-well cell culture plates in standard media (F10, 20% FBS, 10 ng/mL bFGF), fibrogenic media (10 ng/ml TGFβ-1), and adipogenic media (Mesenchymal Stem Cell Adipogenesis Kit; EMD Millipore, Burlington, MA) for 2 weeks before staining and analysis.
Immunocytochemistry
FAP cell proliferation rate was assessed via BrdU and Ki67 staining. On the last day of a 2-week culture period, 10 μM of Brdu was added to the media for 24 h before final fixation. Brdu and Ki67 staining were used to calculate the cell proliferation index as percent of cells staining positive Brdu and Ki67, respectively. To evaluate the percent of cells undergoing fibroblast differentiation, the cells were stained with rabbit anti-mouse Collagen Type I (AB765P; Sigma). To evaluate percent of cells undergoing adipocyte differentiation, the cells were stained with goat anti-mouse perilipin antibody (ab61682; Abeam, Cambridge, MA). The cells were counter-stained with DAPI.
Histology
MA, SS, IS, PS, TR, GA, and TA muscles from another group of PDGFRα-eGFP reporter mice (n = 4) were flash-frozen in liquid nitrogen-cooled isopentane and sectioned (7 μm) with a cryostat. For immunofluorescence, sections were fixed with 4% paraformaldehyde for 10 min, washed in PBST (1XPBS/0.1% TritonX-100), blocked for 1 h in 5% BSA, and then incubated with rabbit anti-laminin antibody (L9393; Sigma, St. Louis, MO) at 4°C overnight. Slides were then washed in PBS and incubated with goat anti-rabbit secondary antibodies (ab150115; Abeam) for 1 h at room temperature. The tissue sections were stained with DAPI and then mounted with Fluoromount G. The percentage of FAPs from the different muscles was calculated using the number PDGFRα-GFP+ cells divided by total cell number seen in the image field.
Glycerol Injection Injury
Intramuscular glycerol injection has been shown to induce muscle FI as an established model of muscle injury.10,28,29 To assess for differences in adipogenic and proliferative potential of FAPs in vivo across muscle groups after same FI-inducing injury, 30 μl of 50% glycerol in 0.9% saline was injected into the mid-bellies of the SS and tibialis anterior muscles, respectively, in a third group of PDGFRα-eGFP reporter mice (n = 4 per subgroup). The muscle samples were harvested 2 weeks after the injection. FI was determined based on the percentage of the image occupied by perilipin staining at the site of injection within the muscle. The percentage of FAPs at 2 weeks post-injection was calculated using of the number PDGFRα-GFP+ cells divided by total cell number seen in the image field.
Statistical Analysis
One-way analysis of variance with Tukey’s post hoc multiple test correction was used for statistical analysis to evaluate the difference among the muscles groups with regard to mean FAP percentage, adipocyte percentage, and Brdu and Ki67 cell proliferation indices. t-Test was used to evaluate the amount of perlipin staining and FAP percentage in the SS and TA after glycerol injection. Significance was defined as p < 0.05. Data are presented as mean ± standard deviation.
RESULTS
Differences in FAP Percentage on FACS Sorting
FAPs were isolated from the seven different muscle groups by FACS (Fig. 1). SS and IS muscles contained the highest percent of FAPs at 17.5 ± 1.0% and 15.7 ± 1.1%, both of which were significantly greater than TR (5.5 ± 1.1%), GA (3.5 ± 0.8%), and the TA (5.3 ± 0.3%) (p < 0.01). The PS contained 10.3 ± 2.1% FAPs, which was significantly greater than TR, GA, and TA (p < 0.01). The MA had significantly more FAPs than the GA at 8.0 ± 0.9% (p < 0.01).
Figure 1.
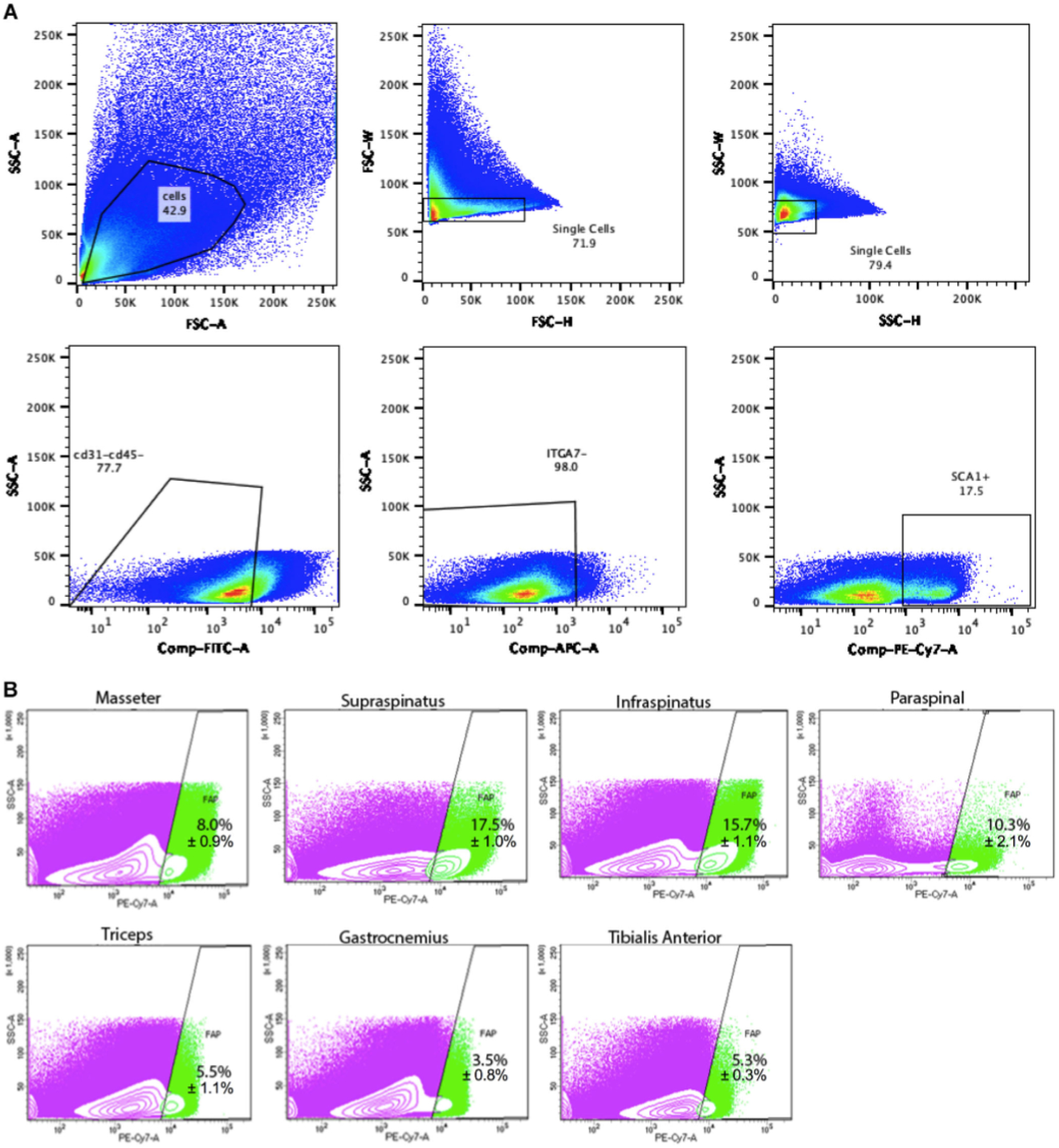
(A) Full fluorescence-activated cell sorting (FACS) gating for the supraspinatus, representative of the FACS sorting that all muscle groups underwent. (B) FACS scatterplots of fibro-adipogenic progenitors (FAPs) isolated from masseter, supraspinatus, infraspinatus, paraspinal, triceps, gastrocnemius, and tibialis anterior muscle (n = 3). Green indicates FAP population isolated. Purple indicates other muscle cells.
Differences in FAP Percentage on Histology
Histologic evaluation of FAP percentage of the seven muscle groups was performed (Fig. 2). The percentage of FAPs with PDGFRα-GFP positivity out of the total nuclei per image field was highest in SS at 20.59 ± 2.8%. Compared with SS, TA had significantly lower FAP percentage at 11.6 ± 2.1% (p < 0.05). The SS also contained significantly more FAPs than the TR (8.8 ± 5.5%; p < 0.01). The FAP percentage for MA, IS, and PS were 18.3 ± 3.8%, 15.2 ± 2.4%, 18.3 ± 3.8%, and in that order. Overall, the more axially located muscles tended to have higher percentages of FAPs, while limb muscles tended have lower percentages.
Figure 2.

(A) Representative images of histology of masseter (MA), supraspinatus (SS), infraspinatus (IS), paraspinal (PS), triceps (TR), gastrocnemius (GA), and tibialis anterior (TA) muscles. Green, PDGFRα-GFP reporter signal. Pink, laminin. Blue, DAPI. (B) Quantification of FAPs from the different muscles calculated using percentage of PDGFRα-GFP reporter signal per number of total nuclei. *p < 0.05, **p < 0.01.
FAPs From Different Muscles Display Variable Differentiation Characteristics In Vitro
FAPs harvested from the different muscle groups were cultured separately in fibrogenic and adipogenic media and analyzed after 2 weeks of incubation (Fig. 3). FAPs collected from SS and PS formed more adipocytes than did FAPs from GA (60 ± 20%, 30 ± 20% vs. 20 ± 4%; p < 0.05) as quantified by perilipin staining, a marker of adipocytes. The SS also displayed significantly more adipocytes than the TA (9 ± 3%; p < 0.05).
Figure 3.

(A) Representative images of fibro-adipogenic progenitors (FAPs) harvested from supraspinatus (SS), paraspinal (PS), gastrocnemius (GA), and tibialis anterior (TA) muscles after 2 weeks of cell culture. Red, collagen type I indicating fibrosis. Green, perilipin A indicating adipogenesis. Blue, DAPI. Scale bars are 50 μm. (B) Quantification of percentage of adipocytes per total nuclei for each muscle. *p < 0.05.
FAPs From Different Muscles Show Different Proliferation Rates In Vitro
The cell proliferation rates of FAPs from different muscles were evaluated using Ki67 and Brdu staining (Fig. 4). FAPs from the SS demonstrated significantly higher cell proliferation rates than GA and TA as visualized using Ki67 (75 ± 14% vs. 9 ± 1.8%, 18 ± 3%; p < 0.05). The PS FAPs also demonstrated significantly higher proliferation rates than GA (50 ± 10%; p < 0.05). A similar pattern was observed using Brdu staining. The Brdu indices for the SS, PS, GA, and TA were 85 ± 12%, 70 ± 15%, 4 ± 4%, and 31 ± 15% in that order. The SS displayed a greater Brdu index than the GA and the TA (p < 0.05). The PS Brdu index was significantly higher than the GA (p < 0.05).
Figure 4.

(A) Representative images of proliferation using Ki67 (red) and Brdu (green) staining of fibro-adipogenic progenitors (FAPs) harvested from supraspinatus (SS), paraspinal (PS), gastrocnemius (GA), and tibialis anterior (TA) muscles after 2 weeks of cell culture. (B) Quantification of Ki67 and Brdu FAP proliferation rates for each muscle. *p < 0.05.
FAPs From the RC Exhibit More Adipogenic and Proliferative Potential In Vivo
The adipogenic and proliferative potential of FAPs in vivo was assessed 2 weeks after intramuscular glycerol injections (Fig. 5). Tissue from SS exhibited significantly more FI at the site of glycerol injection compared with tissue from TA (12.6 ± 3.9% vs. 1.5 ± 1%; p < 0.05). In addition, SS had a greater percentage of FAPs than TA 2 weeks after injection (43.2 ±6.3% vs. 27.1 ± 2.5%; p< 0.01).
Figure 5.

(A) Representative images of adipogenesis of supraspinatus (SS) and tibialis anterior (TA) muscle 2 weeks after intramuscular injection of 30 μl of 50% glycerol in 0.9% saline. Red, perilipin. Green, PDGFRα. Blue, DAPI. (B) Quantification of percent area of positive perilipin staining in image field of each muscle. *p < 0.05. (C) Quantification of fibro-adipogenic progenitors (FAPs) from each muscle calculated using percentage of PDGFRα-GFP reporter signal per number of total nuclei. **p < 0.01.
DISCUSSION
RC tears remain a common and complex injury that result in pain and functional limitations for many patients despite surgical repair. This is due in part to the associated muscle atrophy, fibrosis, and FI that negatively impact muscle integrity and increase the likelihood of retears. FI, in particular, is a limiting factor that may preclude the option to proceed with repair.6,30 In the setting of more global disturbances of muscle structure such the muscular dystrophies or in association with widespread hormonal imbalances such as obesity and diabetes, other muscles can also develop FI.13–17,31 In the case of musculotendinous injuries that orthopedic surgeons more often encounter, the RC has been shown to preferentially develop FI, which suggests there may be intrinsic differences across muscle groups that results in the RC being predisposed to FI.27 The underlying mechanism behind the variable muscle pathology is likely multifactorial. However, given that FAPs are a major contributor of FI in skeletal muscle, it is plausible that the variability in FI across muscle groups is at least partially due to inherent differences in the FAPs residing in different regions.
There is limited literature available that details FAP populations across different anatomic locations, and even fewer studies that examine FAPs specifically from the RC. To our knowledge, this study is the first to examine the distribution and differentiation profile of FAPs within the RC and compare them with FAPs from multiple locations. In this study, we demonstrated that the RC muscles possess greater percentages of FAPs than the hindlimb muscles. Fiore et al.32 reported FAPs account for approximately 2% of mouse TA cells on FACS. Joe et al.8 demonstrated a FAP percentage between 6.2% and 6.7% from FACS sorting of mouse hindlimb muscles. In addition, Uezumi et al.10 found a FAP percentage in mouse hindlimb muscles of 9.6 ± 3.7% on FACS sorting. Considering different tissue preparation and FACS protocols, data from those studies cannot be compared directly. However, our result of 5.3% FAPs in TA muscle falls into the range of those numbers. It is notable that the FAP percentage in SS and IS muscles as tested in this study (17.5% and 15.7%) are markedly higher than that of hindlimb muscles reported in those studies.
Given these results, the tendency of the RC to develop FI after injury may in part be due to the high percentage of FAPs residing in the muscle. Davies et al.33 reported reduced RC fibrosis and FI in mice treated with a TGF-β small molecule inhibitor that promoted FAP apoptosis after SS and IS tendon and suprascapular nerve transection. Lemos et al.34 demonstrated in a transgenic mouse model, in which FAPs lacked apoptotic signaling, that FAPs continued to accumulate after TA muscle injury resulting in increased levels of fibrosis compared with wildtype mice. Furthermore, they reported a reduction in FAP cell number and muscle fibrosis with administration of a pro-FAP-apoptotic tyrosine kinase inhibitor in dystrophic mdx mice. Together, these results suggest that the degree of FAP-related pathology in muscle may depend on the amount of FAPs present in any particular muscle.
Beyond the quantitative difference of FAPs across different muscles, it is also possible that the degree and type of FAP-related pathology that develops after muscle injury may be linked to variable adipogenic differentiation patterns across different muscle groups based on anatomic region. It is not fully understood at this time whether the variety of differentiation properties that emerge could be due to injury-specific signaling, in situ location-specific signaling, or innate differences in FAP populations reflective of yet-to-be described subsets. However, our study details differences in adipogenicity and proliferation rates in vitro among FAPs taken from different locations in uninjured muscle, which suggests innate differences in the behavior of these cells based on anatomic location. In addition, our study found significantly more adipogenesis and increased FAP percentage in SS compared with TA after the same glycerol-induced injury of each muscle. Kang et al.28 found that the adipocyte area increased to approximately 6% in mouse TA muscle 7 days after glycerol injection using triple the dose used in our study. Lukjanenko et al.35 showed similar results as our study regarding TA FI with approximately 1% adipocyte area at 2 weeks using a comparable glycerol dose. Davies et al.27 showed increased FI and adipogenic markers in mouse RC muscles as compared with the GA after an analogous tendon-nerve transection injury was performed at each site. Together, these results further suggest the propensity for RC to form FI may be in part due to a combination of greater FAP baseline percentages, proliferation, and adipogenesis, indicating the presence of intrinsic behavioral differences among FAPs from different anatomic regions. Differences in differentiation profiles of satellite cells (SCs), the other major resident muscle stem cell population, taken from different tissues have also been reported in the literature.36,37
Further supporting the possibility of distinct FAP populations across anatomic sites, Lemos et al.38 described two populations of FAPs: one group derived from neural crest cells (NCFAPs) characterized by yellow fluorescent protein (YFP) expression, a neural crest marker, and another derived from mesenchymal tissues (MFAPs) that do not express YFP. Both cell lines expressed PDGFRα and Sca-1. These two populations were found to have similar abilities to contribute to FI; however, there were notable differences in distribution in muscles from different anatomic locations. NCFAPs comprised 30–100% of the total FAP population in mouse MA muscle depending on the age of the mouse, while MFAPs comprised the remaining percentage. No NCFAPs were found in TA muscles, while MFAPs comprised the entirety of the FAP population at that site. After myotoxin-induced injury to MA, NCFAPs exhibited asymmetric expansion compared with MFAPs, occupying an even greater percentage of total FAPs than observed at baseline. Moreover, NCFAP Brdu positivity increased from 0.81 ± 0.44% at baseline to 70.7 ± 12.2% 3 days after injury, while Brdu positivity in MFAPs only increased from 0.93 ± 0.92% to 48.3% ± 8.3 over the same time period, representing a significant difference in change of proliferation between the cell populations. Similar findings were observed in a study from Paylor et al.39 Of note, in our study MA FAPs were more numerous than hindlimb FAPs, and SS FAPs exhibited significantly higher proliferation indices as demonstrated by Ki67 and Brdu staining. Due to the anatomic proximity of MA and RC muscles, it is possible they possess similar NCFAP:MFAP ratios, which may account for some of the cell behavior differences observed in our study. Future work is needed to characterize potential subsets of FAPs within RC muscles.
Interestingly, our results also showed PS muscles had high percentages of FAPs of which were more adipogenic and had greater cell proliferation rates compared to the hindlimb muscles. Clinically, PS muscles are known to develop FI in a number of spine conditions such as degenerative disc disease and scoliosis.19–21,40,41 Most studies of PS FI center on imaging quantification of FI and correlation to clinical outcomes. Those that include histology of tissue have focused primarily on general muscle fiber architecture and SCs.42 Therefore, the literature on FAP populations within PS muscle and their contributions to FI are scant. Our study shows that relative to the SS, PS muscle contain similar FAP percentages of which have comparable adipogenic and proliferative profiles. It is possible that the tendency to develop FI in PS muscles is driven by similar factors as RC FI as posited in this study. In addition, given PS muscle proximity to the neural tube and origin of neural crest migration during development as well as previous studies identifying NCFAP in Masseter muscle, future work should focus on identifying potential subpopulations of FAPs in PS muscle that may play a role in the tendency to form FI.
In addition to the FAP differences outlined in our study and how they might relate to differences in FI across muscles, it is also important to consider other factors that could play a role. Differences in muscle characteristics such as fiber-type composition, vascularity, and biomechanical function may also impact the type and degree of muscle pathology that develops after different kinds of injury.43–47 For example, FAPs have been shown to cluster in perivascular regions.48 Therefore, the baseline levels and changes in RC vascularity as compared with other muscles may affect associated FAP populations available for downstream FI and fibrosis. Investigation of these potential factors is outside the scope of this study, but additional studies that examine these factors may help to further illuminate the complex and intertwined landscape of actors involved in muscle FI and fibrosis.
There are several limitations to our study. Given the limited studies evaluating the FAP populations across different muscles and the variety of methods used to quantify FAPs in the available literature, direct comparisons with previous studies are challenging in many cases. This study used all female mice; however, it is possible that sex differences exist in regard to FAP quantity and differentiation. Future studies will examine if such differences are present. Additionally, in this study we compare baseline FAP percentages of different muscles; however, it is known that FAPs undergo rapid expansion and contractions in cell number after injury. It is therefore unclear if the differences appreciated in our studies would remain if FAPs were collected after injury of each muscle. Furthermore, it is possible that differences in FAP quantity and differentiation profiles are due in part to variance in FAP subpopulations across muscles. Age-related differences in FAP activation and microenvironments may also play a role in the types and degrees of muscle pathology that develop.49,50 Future work will focus on identifying any age differences, further characterizing FAPs, and investigating if there are any differences among subpopulations in their response to muscle injury that may play a role in pathology differences observed. Studies using human muscle tissue to evaluate if similar FAP patterns exist are also warranted.
In summary, this study demonstrates significant differences in the percentage of FAPs within different muscle groups, as well as differences in FAP differentiation and proliferation characteristics. RC muscle FAPs were of greatest quantity and adipogenic and proliferative capacity which may explain the tendency of the RC to develop FI after injury. These findings may also shed light on new potential therapies that target FAPs in the RC and other FAP-rich muscles to combat FI and improve muscle quality and function.
ACKNOWLEDGMENTS
This study was supported by NIH/NIAMS Research Grant (1R01AR072669-01A1, PI: Feeley), U.S. Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research, and Development Merit Review Grant (1I01BX002680, PI: Kim).
Footnotes
Conflicts of interest: None.
REFERENCES
- 1.Collin P, Matsumura N, Lädermann A, et al. 2014. Relationship between massive chronic rotator cuff tear pattern and loss of active shoulder range of motion. J Shoulder Elbow Surg 23:1195–1202. [DOI] [PubMed] [Google Scholar]
- 2.Melis B, DeFranco MJ, Chuinard C, et al. 2010. Natural history of fatty infiltration and atrophy of the supraspinatus muscle in rotator cuff tears. Clin Orthop Relat Res 468: 1498–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Namdari S, Donegan RP, Dahiya N, et al. 2014. Characteristics of small to medium-sized rotator cuff tears with and without disruption of the anterior supraspinatus tendon. J Shoulder Elbow Surg 23:20–27. [DOI] [PubMed] [Google Scholar]
- 4.Gladstone JN, Bishop JY, Lo IKY, et al. 2007. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med 35:719–728. [DOI] [PubMed] [Google Scholar]
- 5.Lansdown DA, Lee S, Sam C, et al. 2017. A prospective, quantitative evaluation of fatty infiltration before and after rotator cuff repair. Orthop J Sports Med 5:2325967117718537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goutallier D, Postel JM, Bernageau J, et al. 1994. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res 78–83. [PubMed] [Google Scholar]
- 7.Valencia AP, Lai JK, Iyer SR, et al. 2018. Fatty infiltration is a prognostic marker of muscle function after rotator cuff tear. Am J Sports Med 46:2161–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joe AWB, Yi L, Natarajan A, et al. 2010. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol 12:153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quarta M, Cromie M, Chacon R, et al. 2017. Bioengineered constructs combined with exercise enhance stem cell-mediated treatment of volumetric muscle loss. Nat Commun 8:15613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uezumi A, Fukada S, Yamamoto N, et al. 2010. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol 12:143–152. [DOI] [PubMed] [Google Scholar]
- 11.Uezumi A, Ito T, Morikawa D, et al. 2011. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J Cell Sci 124:3654–3664. [DOI] [PubMed] [Google Scholar]
- 12.Liu X, Ning AY, Chen Chang N, et al. 2016. Investigating the cellular origin of rotator cuff muscle fatty infiltration and fibrosis after injury. Muscles Ligaments Tendons J 6:6–15. 10.11138/mltj/2016.6.1.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li W, Zheng Y, Zhang W, et al. 2015. Progression and variation of fatty infiltration of the thigh muscles in Duchenne muscular dystrophy, a muscle magnetic resonance imaging study. Neuromuscul Disord 25:375–380. [DOI] [PubMed] [Google Scholar]
- 14.Jin S, Du J, Wang Z, et al. 2016. Heterogeneous characteristics of MRI changes of thigh muscles in patients with dys-ferlinopathy. Muscle Nerve 54:1072–1079. [DOI] [PubMed] [Google Scholar]
- 15.Matson AP, Kim C, Bajpai S, et al. 2019. The effect of obesity on fatty infiltration of the rotator cuff musculature in patients without rotator cuff tears. Shoulder Elbow 11:30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu H, Ballantyne CM. 2017. Skeletal muscle inflammation and insulin resistance in obesity. J Clin Invest 127:43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dow DF, Mehta K Xu Y, et al. 2018. The relationship between body mass index and fatty infiltration in the shoulder musculature. J Comput Assist Tomogr 42:323–329. [DOI] [PubMed] [Google Scholar]
- 18.Shahidi B, Parra CL, Berry DB, et al. 2017. Contribution of lumbar spine pathology and age to paraspinal muscle size and fatty infiltration. Spine (Phila Pa 1976) 42:616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalichman L, Carmeli E, Been E. 2017. The association between imaging parameters of the paraspinal muscles, spinal degeneration, and low back pain. Biomed Res Int 2017:2562957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hyun SJ, Bae CW, Lee SH, et al. 2016. Fatty degeneration of the paraspinal muscle in patients with degenerative lumbar kyphosis: a new evaluation method of quantitative digital analysis using MRI and CT scan. Clin Spine Surg 29:441–447. [DOI] [PubMed] [Google Scholar]
- 21.Ozcan-Eksi EE, Eksi MS, Akcal MA. 2019. Severe lumbar intervertebral disc degeneration is associated with Modic changes and fatty infiltration in the paraspinal muscles at all lumbar levels, except for L1-L2: a cross-sectional analysis of 50 symptomatic women and 50 age-matched symptomatic men. World Neurosurg 122:e1069–e1077. [DOI] [PubMed] [Google Scholar]
- 22.Corona BT, Wenke JC, Ward CL. 2016. Pathophysiology of volumetric muscle loss injury. Cells Tissues Organs 202: 180–188. [DOI] [PubMed] [Google Scholar]
- 23.Matthias N, Hunt SD, Wu J, et al. 2018. Volumetric muscle loss injury repair using in situ fibrin gel cast seeded with muscle-derived stem cells (MDSCs). Stem Cell Res 27:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Owens BD, Kragh JF Jr., Wenke JC, et al. 2008. Combat wounds in operation Iraqi Freedom and Operation Enduring Freedom. J Trauma 64:295–299. 10.1097/TA.0b013e318163b875 [DOI] [PubMed] [Google Scholar]
- 25.Belmont PJ Jr., McCriskin BJ, Hsiao MS, et al. 2013. The nature and incidence of musculoskeletal combat wounds in Iraq and Afghanistan (2005–2009). J Orthop Trauma 27:e107–e113. [DOI] [PubMed] [Google Scholar]
- 26.Greising SM, Rivera JC, Goldman SM, et al. 2017. Unwavering pathobiology of volumetric muscle loss injury. Sci Rep 7:13179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davies MR, Ravishankar B, Laron D, et al. 2015. Rat rotator cuff muscle responds differently from hindlimb muscle to a combined tendon-nerve injury. J Orthop Res 33:1046–1053. [DOI] [PubMed] [Google Scholar]
- 28.Kang X, Yang M, Shi Y, et al. 2018. Interleukin-15 facilitates muscle regeneration through modulation of fibro/adipogenic progenitors. Cell Commun Signal 16:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahdy MAA. 2018. Glycerol-induced injury as a new model of muscle regeneration. Cell Tissue Res 374:233–241. [DOI] [PubMed] [Google Scholar]
- 30.Burkhart SS, Barth JRH, Richards DP, et al. 2007. Arthroscopic repair of massive rotator cuff tears with stage 3 and 4 fatty degeneration. Arthroscopy 23:347–354. [DOI] [PubMed] [Google Scholar]
- 31.Arrighi N, Moratal C, Clément N, et al. 2015. Characterization of adipocytes derived from fibro/adipogenic progenitors resident in human skeletal muscle. Cell Death Dis 6:e1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fiore D, Judson RN, Low M, et al. 2016. Pharmacological blockage of fibro/adipogenic progenitor expansion and suppression of regenerative fibrogenesis is associated with impaired skeletal muscle regeneration. Stem Cell Res 17:161–169. [DOI] [PubMed] [Google Scholar]
- 33.Davies MR, Liu X, Lee L, et al. 2016. TGF-beta small molecule inhibitor SB431542 reduces rotator cuff muscle fibrosis and fatty infiltration by promoting fibro/adipogenic progenitor apoptosis. PLoS One 11:e0155486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lemos DR, Babaeijandaghi F, Low M, et al. 2015. Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat Med 21:786–794. [DOI] [PubMed] [Google Scholar]
- 35.Lukjanenko L, Brachat S, Pierrel E, et al. 2013. Genomic profiling reveals that transient adipogenic activation is a hallmark of mouse models of skeletal muscle regeneration. PLoS One 8:e71084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Redshaw Z, McOrist S, Loughna P. 2010. Muscle origin of porcine satellite cells affects in vitro differentiation potential. Cell Biochem Funct 28:403–411. [DOI] [PubMed] [Google Scholar]
- 37.Pawlikowski B, Pulliam C, Betta ND, et al. 2015. Pervasive satellite cell contribution to uninjured adult muscle fibers. Skelet Muscle 5:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lemos DR, Paylor B, Chang C, et al. 2012. Functionally convergent white adipogenic progenitors of different lineages participate in a diffused system supporting tissue regeneration. Stem Cells 30:1152–1162. [DOI] [PubMed] [Google Scholar]
- 39.Paylor B, Joe AW, Rossi FMV, et al. 2014. In vivo characterization of neural crest-derived fibro/adipogenic progenitor cells as a likely cellular substrate for craniofacial fibrofatty infiltrating disorders. Biochem Biophys Res Commun 451:148–151. [DOI] [PubMed] [Google Scholar]
- 40.Wan Q, Lin C, Li X, et al. 2015. MRI assessment of paraspinal muscles in patients with acute and chronic unilateral low back pain. Br J Radiol 88:20140546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wajchenberg M, Astur N, Fernandes EA, et al. 2019. Assessment of fatty infiltration of the multifidus muscle in patients with adolescent idiopathic scoliosis through evaluation by magnetic resonance imaging compared with histological analysis: a diagnostic accuracy study. J Pediatr Orthop B 28:362–367. [DOI] [PubMed] [Google Scholar]
- 42.Shahidi B, Hubbard JC, Gibbons MC, et al. 2017. Lumbar multifidus muscle degenerates in individuals with chronic degenerative lumbar spine pathology. J Orthop Res 35:2700–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lovering RM, Russ DW. 2008. Fiber type composition of cadaveric human rotator cuff muscles. J Orthop Sports Phys Ther 38:674–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barton ER, Gimbel JA, Williams GR, et al. 2005. Rat supra-spinatus muscle atrophy after tendon detachment. J Orthop Res 23:259–265. [DOI] [PubMed] [Google Scholar]
- 45.Armstrong RB, Phelps RO. 1984. Muscle fiber type composition of the rat hindlimb. Am J Anat 171:259–272. [DOI] [PubMed] [Google Scholar]
- 46.Gibbons MC, Singh A, Anakwenze O, et al. 2017. Histological evidence of muscle degeneration in advanced human rotator cuff disease. J Bone Joint Surg Am 99:190–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gigliotti D, Xu MC, Davidson MJ, et al. 2017. Fibrosis, low vascularity, and fewer slow fibers after rotator-cuff injury. Muscle Nerve 55:715–726. [DOI] [PubMed] [Google Scholar]
- 48.Iwayama T, Steele C, Yao L, et al. 2015. PDGFRalpha signaling drives adipose tissue fibrosis by targeting progenitor cell plasticity. Genes Dev 29:1106–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sharma AK, Levian B, Shah P, et al. 2019. Aged mice demonstrate greater muscle degeneration of chronically injured rotator cuff. J Orthop Res. 10.1002/jor.24468 [DOI] [PubMed] [Google Scholar]
- 50.Lukjanenko L, Karaz S, Stuelsatz P, et al. 2019. Aging disrupts muscle stem cell function by impairing matricellular WISP1 secretion from fibro-adipogenic progenitors. Cell Stem Cell 24:433–446.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]


