Abstract
Background:
In March 2020, the Government of Canada introduced measures to reduce intensifying shortages of prescription drugs during the beginning of the COVID-19 pandemic. We sought to assess the extent to which a decline in drug shortages was observed in the months after this policy change.
Methods:
Our data source was the Drug Shortages Canada Database, which reports shortages by drug product, including shortage start and duration. Using a cross-sectional design, we tracked shortage rates of drug products using a 30-day moving average from Apr. 15, 2017, to Apr. 1, 2022. We used autoregressive integrated moving average modelling with a ramp function to determine the significance of trend changes after policy implementation.
Results:
We found that of the 13 329 drug products at risk for shortage, 44.7% (n = 5953) had at least 1 shortage event in the past 5 years. Average daily shortage prevalence rates rose from 901 in April 2017 to a peak of 2345 by April 2020. Significant declines (p = 0.02) ensued shortly thereafter, dropping to a rate of 1611 shortages by the end of the first year after policy implementation. However, we did not observe a significant reduction in shortage rates in the second year (p = 0.2), with rates plateauing below 1500 and then rising back above 1600 by the end of March 2022.
Interpretation:
Drug shortages are common in Canada, including during the initial months of the COVID-19 pandemic. We observed substantial improvements after the implementation of the new measures, but gains appear to have plateaued. Continued vigilance is needed to sustain improvements.
Drug shortages, which are a persistent problem in Canada and around the world, interfere with patients’ ability to consistently take medication to manage chronic diseases; disrupt care providers’ work, as they must spend time struggling to find suitable substitutes for difficult-to-obtain drugs that are free from contraindications given patients’ other medications; and can drive up costs to insurers and patients if alternative products are more costly or are not covered by insurance.1 In addition, some medications used for acute illnesses may not have suitable alternatives, which may increase patient harms. As defined by the American Society of Health-System Pharmacists, drug shortages are “a supply issue that affects how the pharmacy prepares or dispenses a drug product or influences patient care when prescribers must use an alternative agent.”2 The COVID-19 pandemic exposed many vulnerabilities in our social infrastructure, including in health care systems.3 Early in the pandemic, disruptions in supply chains in China and India — which may produce up to 80% of raw ingredients and pharmaceutical products,4,5 although the exact amounts are disputed6 — and an increased demand for certain drugs (e.g., medications used during ventilation) led to concerns that the pandemic would exacerbate existing issues with drug shortages in Canada.
Canadian policy-makers responded to the persistent problems with drug shortages in 2012, with the creation of a database for drug manufacturers to report shortages.1 In 2017, it became mandatory for drug companies to report both anticipated and actual shortages.7 Several important measures were taken in March 2020 to address shortages during the COVID-19 pandemic in an attempt to ensure a steady supply of medication. A key change potentially important to the supply chain occurred Mar. 25, 2020, when the federal government amended the Patent Act so that manufacturers could make, use or sell versions of patented drugs “to the extent necessary to respond to a public health emergency that is a matter of national concern” without having to negotiate with patent holders.8,9 Another key measure for the supply chain was when the federal government issued an Interim Order relevant to certain drugs at high risk for shortage and with high potential to affect the health system, that would permit their importation from other countries provided those products had been approved in a country with a sufficiently rigorous regulatory review process.9–11 These interim measures were made permanent on Mar. 2, 2022.12
Previous studies have investigated factors associated with drug shortages in Canada before the COVID-19 pandemic,1,13 have descriptively tracked shortage frequencies in Canada during COVID-1914,15 or have focused on specific treatments for COVID-19 in Canada.16 However, the impact of these specific measures enacted by the Canadian government during the pandemic is currently unknown. Our objective, therefore, was to assess whether a statistically significant decline in shortages followed the introduction of the federal measures that were introduced in March 2020.
Methods
Study design and setting
Using a cross-sectional design, we tracked changes in drug shortages over time by calculating a standardized daily shortage prevalence rate and used time-series analysis to identify any significant change in trend beginning in April 2020 and April 2021 after the enactment of the interim order.
We tracked drug shortages that were reported in Canada from Apr. 15, 2017, to Apr. 1, 2022.
Data sources
We relied on 3 databases that are publicly available online.17–20 We used extracts from the Drug Shortages Canada Database (DSCD) to establish which drugs were in shortage at what time, and what shortage status was associated with a shortage report at extraction.17 As this reporting is now a legal requirement in Canada, the database provides a uniquely comprehensive perspective of shortages by each products’ Drug Identification Numbers (DINs) as many other countries report only voluntarily. 18 We used extracts from the Drug Product Database (DPD) to establish the overall number of marketed drugs that were subject to shortage reporting.19 We used extracts from Health Canada’s Patent Register to establish separate prevalence rates for patented and nonpatented drugs.20 The extracts were current to Apr. 1, 2022. We linked the 3 databases using their DINs, which are unique 8-digit codes assigned by Health Canada when a drug product is approved.
We excluded any shortage reports that were resolved before our study period, had incomplete shortage duration data or had missing DINs necessary for linkage to the DPD. Furthermore, we excluded shortage reports for veterinary and over-the-counter products because these are not subject to the mandatory requirements for shortage reporting.21 We included all other prescription drugs.
Outcome variable
To track changes in shortage prevalence over time, we calculated a daily shortage prevalence rate (per 10 000 DINs at risk) for every day from Apr. 15, 2017, to Apr. 1, 2022, according to the following equation: daily shortage prevalence rate = number of DINs in shortage on a given day/number of DINs at risk of shortage on the same day × 10 000.
We derived the numerator from the DSCD extracts by totalling the number of DINs that were in shortage on a given day (i.e., the number of DINs having a shortage start date on or before and shortage end date on or after the given day). The denominator was the total number of DINs on the given day that were “at risk” for shortage (i.e., the number of DINs that were both being actively marketed according to Health Canada at that time and that were subject to mandatory requirements for shortage reporting). It was necessary to recalculate the denominator daily because the number of DINs being actively marketed for human use by prescription fluctuates on any given day as Health Canada approves new drug products (brand or generic) and as other drug products are discontinued or withdrawn from the market by the manufacturer for a variety of reasons, including safety. The resulting daily point shortage prevalence rates were expressed as a rate per 10 000 DINs at risk.
Statistical analysis
We performed autoregressive integrated moving average (ARIMA) modelling for each outcome. We incorporated 2 ramp functions into each model, the first starting at Apr. 1, 2020, and the second starting at Apr. 1, 2021, to detect any statistical changes in the time series at these 2 time points, and we considered a p value of less than 0.05 to be significant. For each time series, we assessed stationarity using the Augmented Dickey–Fuller test, and for nonstationarity, we applied differencing until stationarity was reached. Minimizing the standard deviation (SD) of the time series also ensured that the proper order of differencing was applied. Other model parameters, specifically the number of autoregressive or moving average terms, were chosen using the autocorrelation functions and partial autocorrelation functions while taking into consideration the interval of the data (e.g., monthly data may exhibit autocorrelation up to 12 lags, quarterly data up to 4 lags, etc.). We determined model fit using a residual check with the Ljung Box test for white noise and comparison of various model fit statistics such as the Akaike information criterion. The final chosen model for each outcome was the ARIMA(31,1,0) model. As a sensitivity test, we used the raw shortage frequency as an alternative outcome measure rather than the rate per 10 000 DINs to determine the robustness of observed trends and for comparison with other studies using this outcome measure.14
Ethics approval
The study did not require institutional review board approval because it did not include people.
Results
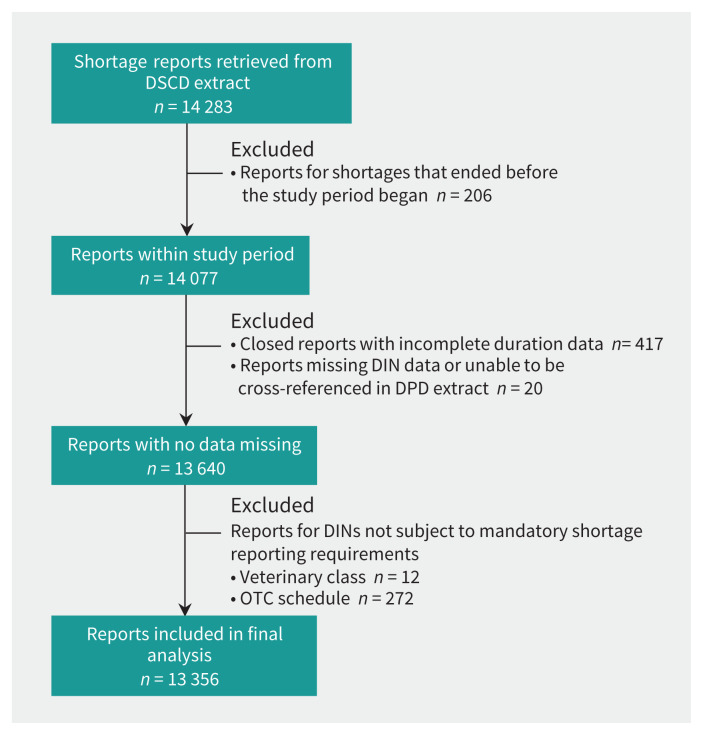
After exclusions (n = 927), we included 93% (n = 13 356) of the shortage reports for analysis (Figure 1). On average, there were 8354 DINs at risk for shortage per day during the 5-year observation window (i.e., they were actively marketed and were subject to mandatory shortage reporting at some point within this time frame). These drug products spanned all therapeutic classes, including both large and small molecule drugs.
Figure 1:
Flow chart of the inclusion and exclusion of drug shortage reports. A total of 14 077 shortage reports from the Drug Shortages Canada Database (DSCD) contributed to daily shortage prevalence on 1 or more days for the observation period of Apr. 15, 2017, through Apr. 1, 2022. Note: DIN = Drug Identification Number, DPD = Drug Product Database, OTC = over the counter.
Of the 13 329 DINs at risk for shortage, we found that 44.7% (n = 5953 DINs) had at least 1 shortage event in the past 5 years. For the entire time frame observed, the average daily prevalence of DINs in shortage was 1803 (21.6, 1803/8354).
Of the 5953 products that had a shortage, many had multiple events during the 5-year period, with an average of 2.1 reports per affected DIN. We found that most shortages (88.45%, n = 11 813) were resolved during the observation period after a mean duration of 140 (SD 247) days or 4.7 (SD 8.2) months (Table 1). Few shortages (11.25%, n = 1502) remained unresolved and had been ongoing for a mean of 922 (SD 850) days or 2.5 (SD 2.3) years. All therapeutic classes were affected by shortages, with the highest mean daily shortage rates in the sensory organ (3771 [SD 648]), cardiovascular (2876 [SD 695]) and dermatological (2613 [SD 580]) classes (Table 2).
Table 1:
Summary of drug shortage reports in Canada over a 5-year observation period (April 2017–April 2022)
| Indicator | Shortage frequency (% of total) | Shortage duration, d | ||
|---|---|---|---|---|
| Mean ± SD | Minimum | Maximum | ||
| Shortage status* | ||||
| Actual | 1502 (11.25) | 922 ± 850 | 2 | 5127 |
| Resolved | 11 813 (88.45) | 140 ± 247 | 1 | 5686 |
| Patent status | ||||
| Patented | 533 (3.99) | 84 ± 244 | 1 | 4019 |
| Nonpatented | 12 823 (96.01) | 233 ± 448 | 1 | 5686 |
| Number of active ingredients | ||||
| Single | 11 704 (87.63) | 230 ± 447 | 1 | 5686 |
| Multiple | 1652 (12.37) | 209 ± 411 | 1 | 3319 |
| Total | 13 356 (100.00) | 227 ± 443 | 1 | 5686 |
Note: SD = standard deviation. We calculated shortage frequency percentages against the total number of reports included in the study (n = 13 356).
Shortages with a current status of “avoided” (n = 11) and “anticipated” (n = 30) are not shown.
Table 2:
Average daily drug shortage prevalence per 10 000 DINs by therapeutic class over a 5-year observation period (April 2017–April 2022)
| Therapeutic group (letter code) | Daily point shortage prevalence rate per 10 000 DINs at risk | ||
|---|---|---|---|
| Mean ± SD | Minimum | Maximum | |
| Alimentary tract and metabolism (A) | 1705 ± 364 | 969 | 2709 |
| Blood and blood forming organs (B) | 600 ± 213 | 163 | 1276 |
| Cardiovascular system (C) | 2876 ± 695 | 1393 | 4268 |
| Dermatologicals (D) | 2613 ± 580 | 1719 | 4350 |
| Genitourinary system and sex hormones (G) | 1657 ± 341 | 647 | 2437 |
| Systemic hormonal preparations (H) | 1101 ± 224 | 612 | 1832 |
| Anti-infectives for systemic use (J) | 1661 ± 265 | 1142 | 2228 |
| Antineoplastic and immunomodulating agents (L) | 897 ± 248 | 472 | 1564 |
| Musculoskeletal system (M) | 1655 ± 299 | 918 | 2222 |
| Nervous system (N) | 1761 ± 178 | 1053 | 2117 |
| Antiparasitic products, insecticides and repellents (P) | 926 ± 640 | 0 | 2333 |
| Respiratory system (R) | 1706 ± 234 | 1198 | 2308 |
| Sensory organs (S) | 3771 ± 648 | 2296 | 5510 |
| Various (V) | 1163 ± 211 | 779 | 1681 |
Note: DIN = Drug Identification Number, SD = standard deviation.
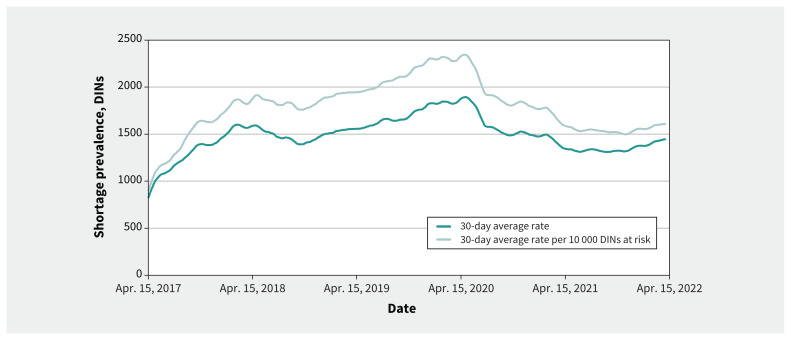
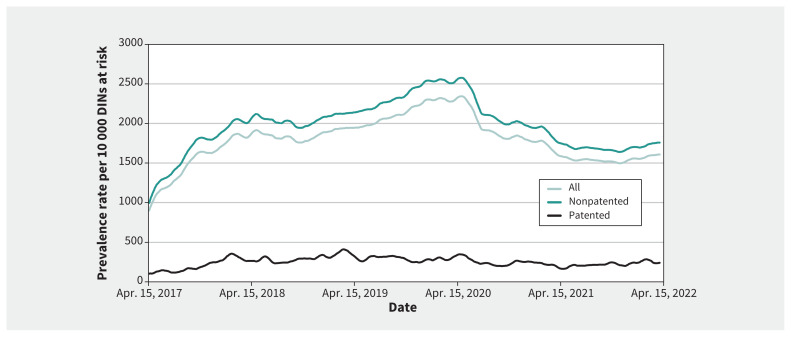
Over the 5-year observation period, the daily shortage prevalence rates rose from a 30-day moving average of 901 on Apr. 15, 2017, to a peak at 2345 per 10 000 DINs by the end of April 2020; a substantial decline ensued thereafter, reaching 1611 shortages by end of year 1. This decline plateaued by year 2, which began and ended at similar rates of around 1600 shortages with a midyear dip below 1500 (Figure 2). The year 1 decline was significant, regardless if it was measured by the rate per 10 000 DINs (p = 0.03) or by raw frequency (p = 0.02), but the year 2 decline was not (rate per 10 000 DINs, p = 0.2; raw frequency, p = 0.160). We observed similar trends for nonpatented DINs (year 1, p = 0.03; year 2, p = 0.3) but not for patented DINs, which showed no significant changes (year 1, p = 0.8; year 2, p = 0.45) (Figure 3).
Figure 2:
Shortage frequency and rate per 10 000 DINs from April 2017 to April 2022. We smoothed all trend lines using a 30-day (lagging) moving average. As mandatory shortage reporting went into effect in March 2017, some lag before reaching full compliance and a plateauing in the shortage rate is expected (such as when the rate flattened around 1600 in October or November 2017, or when the average shortage rate per 10 000 DINs began fluctuating above and below 1800 in 2018); therefore, the data in the initial year after the introduction of this policy in March 2017 should be interpreted with caution. Note: DIN = Drug Identification Number.
Figure 3:
Shortage rates over time before and after the new shortage measures were introduced. We smoothed all trend lines using a simple 30-day (lagging) moving average. The measures to reduce drug shortages caused by the COVID-19 pandemic were implemented on Mar. 25, 2020. Note: DIN = Drug Identification Number.
Interpretation
Our study adds to the literature on drug shortage tracking during the COVID-19 pandemic14–16 by observing that Canada’s high rates of drug shortages declined significantly (especially among nonpatented drugs) in the first year after the institution of interim shortage mitigation policies. These findings potentially justify the March 2022 decision to make the new measures permanent.12 However, 2 unknowns remain: whether the gains in shortage reductions can be further improved or sustained and whether such reductions are causally linked to the policy changes in question.
Regarding whether shortage reductions can be further improved or sustained, it is possible the benefits of the measures may be finite. For example, not all shortages can be resolved through exceptional importation when shortfalls are global. Furthermore, the reforms introduced depend on human action to reduce shortages. Current data22 show that most exceptional importation activity occurred during the summer of 2020 but slowed thereafter (Appendix 1, Supplementary Figure, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.212070/tab-related-content). Although fewer opportunities may exist in subsequent years to resolve shortages via importation, authorities must remain vigilant to maintain the progress made in 2020.
Regarding the question of causality, we cannot rule out the possibility that other unobserved factors may have triggered or contributed to the shortage reductions. For example, declines in overall use of health care services during the pandemic23–25 may have reduced pharmaceutical demand, thereby diminishing shortages until use began rebounding as pandemic restrictions lifted. Demand may have been further smoothed temporarily when pharmacists in some provinces began dispensing only 30-day supplies to reduce supply chain stress in the few months after March 2020.26 These explanations are plausible, but contrary evidence shows use of pharmaceuticals was relatively consistent throughout the pandemic. 27–30 Other possible explanations are that medicine stockpiling during the initial months of the COVID-19 pandemic temporarily suppressed shortage rates; that fewer onsite evaluations occurred during the pandemic, which reduced factory shutdowns in the short term;28,30 or that the fluctuations corresponded with global trends in the ease or difficulty of obtaining raw ingredients from countries like China.31–33 Future research may include studies designed to more directly elucidate the causal story behind the observed declines in shortages, such as qualitative interviews with key informants directly involved with resolving those shortages.
Our finding that patented drugs were not affected by the new policy aligns with other studies that showed that these products are less prone to shortage than off-patent or generic products.1,13 Although our study does not provide an explanation for this phenomenon (e.g., manufacturers may have more resources or incentives to treat more lucrative products with special care34), the implication is that patent protection preventing multiple suppliers from market entry is unlikely to be a major driver of Canada’s drug shortages. Circumstances may arise in which invoking the changes made to the Patent Act in March 2020 are necessary, making it difficult for a study like ours to detect impact. Nevertheless, an exclusive product shortage may be more serious as patients have fewer fallback options, such as was experienced with tocilizumab.16 Therefore, the new flexibilities should be maintained for less common scenarios when authorities can address an urgent shortage by turning to another manufacturer or source, even if the patentee objects.
Our findings also offer updated evidence1,13 on the extensiveness of shortages in Canada with varying degrees of risk by drug class. For example, a retrospective cohort study of all prescription drugs available on the market in Canada between Mar. 14, 2017, and Sept. 12, 2018, also found some clinical areas were affected more often, with drugs for sensory organs still having the highest shortage rates.13 However, it is not always the same products experiencing shortages. This same retrospective cohort study reported that 23% of products had at least 1 shortage over 18 months in 2017–2018.13 With the additional time observed (5 yr, 2017–2022), our finding that 44% had been in shortage underscores how widespread the shortage issue has become (as nearly twice as many drug products had been affected). Under such circumstances, priority setting is a valuable tool for focusing available resources to the most urgent situations first, such as a shortage of valsartan in 2018 that led to about 180 000 Canadians being affected in a single month.35 This highlights the value of prioritizing shortages that pose the greatest risk to patients — an exercise operationalized in March 2020.36
Limitations
Canada’s system of reporting mandatory shortages by manufacturers is imperfect (e.g., alternative medications are not listed, its validity has not been formally studied to our knowledge, it may reflect some fluctuations in reporting compliance [particularly in the first year after mandatory reporting was introduced in 2017]) and does not distinguish “felt shortages” by patients and practitioners. Our estimate of shortages may overrepresent what is experienced anecdotally by patients and practitioners because it is not entirely possible to know the extent to which the shortages are actually causing a clinical impact for patients. Although we provide an overview of the impact on all prescription drug products, there is no perspective on specific severe shortages mitigated via the new measure introduced in March 2020; this is a key area for future research. However, it is important to acknowledge that all shortages have the potential to be clinically significant, including more commonplace instances that may often be disregarded as minor inconveniences. Many studies have found that more than half of patients stop treatment within months after the index prescription, which signals existing barriers to patient adherence.37–39 Even seemingly small differences — such as taking 2 tablets rather than 1 (which may be done to address a shortage) — can result in meaningful changes in treatment persistence at the population level and subsequent clinical outcomes.40 Future research on this topic is ongoing.
Conclusion
Drug shortages are common in Canada, with almost half of all drug products having a shortage at some point in the last 5 years. The new measures to resolve and reduce shortages during the COVID-19 pandemic appeared to have had a subtantial positive impact. These measures should be maintained, sustained and built upon in the long term to minimize the harms caused to patient care and to professional practice.
Supplementary Material
Footnotes
Competing interests: Mina Tadrous has received consultant fees from the Canadian Agency for Drugs and Technologies in Health and Green Shield Canada. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Brian Lau and Cherry Chu performed the research. All of the authors wrote the manuscript, designed the research, analyzed the data, revised the manuscript critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: The authors received no direct financial support for the development of this manuscript. Reed Beall’s work is funded by an O’Brien Institute for Public Health catalyst grant for equitable innovation and access. The funders had no role in the design and execution of this research. Mina Tadrous has received a grant from the Canadian Institutes of Health Research.
Data sharing: The data included in these analyses are available to the public and researchers through Health Canada’s websites and the Drug Shortages Canada website.
References
- 1.Donelle J, Duffin J, Pipitone J, et al. Assessing Canada’s drug shortage problem. Commentary no 515. Toronto: C.D. Howe Institute; 2018. [Google Scholar]
- 2.Ventola CL. The drug shortage crisis in the United States: causes, impact, and management strategies. P&T 2011;36:740–57. [PMC free article] [PubMed] [Google Scholar]
- 3.Hardcastle L, Beall RF. How coronavirus is contributing to drug shortages in Canada. The Conversation 2020. May 13. Available: https://theconversation.com/how-coronavirus-is-contributing-to-drug-shortages-in-canada-137436 (accessed 2022 May 10).
- 4.Drug shortages in Canada. PMPRB Researcher Series. Ottawa: Patented Medicines Prices Review Board (PMPRB); 2020. Available: https://www.canada.ca/content/dam/pmprb-cepmb/documents/consultations/draft-guidelines/2020/PMPRB-webinar-Drug-Shortages-July16-2020-EN.pdf (accessed 2022 May 10). [Google Scholar]
- 5.Cassels A. Most of our prescription drugs are manufactured overseas: But are they safe? CMAJ 2012;184:1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woodcock J. Safeguarding pharmaceutical supply chains in a global economy [testimony]. Silver Spring (MD): US Food and Drug Administration; 2019. Oct. 29. Available: https://www.fda.gov/news-events/congressional-testimony/safeguarding-pharmaceutical-supply-chains-global-economy-10302019 (accessed 2022 May 10). [Google Scholar]
- 7.Guide to reporting drug shortages and discontinuations. Ottawa: Health Canada; modified 2017 Apr. 21. Available: https://www.canada.ca/en/public-health/services/publications/drugs-health-products/reporting-drug-shortages-discontinuations.html (accessed 2022 May 10). [Google Scholar]
- 8.Eggertson L. Canada can override patents to combat drug, equipment shortages during the pandemic. CMAJ 2020;192:E438–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bill C-13. 43rd Parliament, 1st sess. Ottawa: Parliament of Canada; 2020. Available: https://www.parl.ca/LegisInfo/en/bill/43-1/C-13 (accessed 2022 May 10). [Google Scholar]
- 10.List of drugs for exceptional importation and sale. Ottawa: Health Canada; modified 2022 Apr. 26. Available: https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/drug-shortages/list.html (accessed 2022 May 10). [Google Scholar]
- 11.Interim order respecting drugs, medical devices and foods for a special dietary purpose in relation to COVID-19. Ottawa: Health Canada; modified 2022 Mar. 2. Available: https://www.canada.ca/en/health-canada/services/drugs-health-products/compliance-enforcement/covid19-interim-order-drugs-medical-devices-special-foods.html (accessed 2022 May 10). [Google Scholar]
- 12.Drug shortages in Canada: regulations and guidance. Ottawa: Health Canada; modified 2022 Mar. 2. Available: https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/drug-shortages/regulations-guidance.html (accessed 2022 May 10). [Google Scholar]
- 13.Zhang W, Guh DP, Sun H, et al. Factors associated with drug shortages in Canada: a retrospective cohort study. CMAJ Open 2020;8:E535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pipitone J. Assessing Canada’s drug shortage problem [dashboard]. Available: https://drugshortages.pipitone.ca/ (accessed 2022 May 10).
- 15.Gaudette É. COVID-19’s limited impact on drug shortages in Canada. Can Public Policy 2020;46(Suppl 3):S307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verma AA, Pai M, Saha S, et al. Managing drug shortages during a pandemic: tocilizumab and COVID-19. CMAJ 2021;193:E771–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drug Shortages Canada [homepage]. Available: https://www.drugshortagescanada.ca/ (accessed 2022 May 10).
- 18.Drug shortages. Bethesda (MD): American Society of Health-System Pharmacists. Available: https://www.ashp.org/drug-shortages?loginreturnUrl=SSOCheckOnly (accessed 2022 May 10). [Google Scholar]
- 19.Drug Product Database online query. Ottawa: Health Canada; modified 2022 Mar. 10. Available: https://health-products.canada.ca/dpd-bdpp/index-eng.jsp (accessed 2022 May 10). [Google Scholar]
- 20.Patent Register: database download. Ottawa: Health Canada; modified 2012 Dec. 10. Available: https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/patent-register/database-download.html (accessed 2022 May 10). [Google Scholar]
- 21.Archived lists under the Interim Order respecting drugs, medical devices and foods for a special dietary purpose in relation to COVID-19. Ottawa: Health Canada; modified 2022 Mar. 2. Available: https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/drug-medical-device-food-shortages/archived-lists.html#a1 (accessed 2022 May 10). [Google Scholar]
- 22.Knight BD, Shurgold J, Smith G, et al. The impact of COVID-19 on community antibiotic use in Canada: an ecological study. Clin Microbiol Infect 2022;28: 426–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rennert-May E, Leal J, Thanh NX, et al. The impact of COVID-19 on hospital admissions and emergency department visits: a population-based study. PLoS One 2021;16:e0252441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marshall EG, Stock D, Buote R, et al. Emergency department utilization and hospitalizations for ambulatory care sensitive conditions among unattached people actively seeking a primary care provider during the COVID-19 pandemic: a retrospective cohort study [preprint]. medRxiv 2022. Feb. 1. doi: 10.1101/2022.01.31.22270015. [DOI] [Google Scholar]
- 25.Statement on 30-day supply of prescription medications [news release]. Ottawa: Canadian Pharmacists Association; 2020. Mar. 20. Available: https://www.pharmacists.ca/news-events/news/statement-on-30-day-supply-of-prescription-medications/ (accessed 2022 May 10). [Google Scholar]
- 26.COVID-19 Ontario Prescription Drug Utilization Tool. Toronto: Ontario Drug Policy Research Network; 2020. Available: https://odprn.ca/covid19-ontario-prescription-drug-utilization-tool/ (accessed 2022 May 10). [Google Scholar]
- 27.Callaway Kim K, Tadrous M, Kane-Gill SL, et al. Changes in purchases for intensive care medicines during the COVID-19 pandemic: a global time series study. Chest 2021;160:2123–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shakeri A, Gomes T, Suda K, et al. Choppy waters: the importance of accounting for shifting drug utilization during the COVID-19 pandemic in future observational drug-related studies. Basic Clin Pharmacol Toxicol 2021;128:649–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suda KJ, Callaway Kim K, Hernandez I, et al. The global impact of COVID-19 on drug purchases: a cross-sectional time series analysis. J Am Pharm Assoc (2003) 2022;62:766–74.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thacker T. Shanghai lockdown may choke pharma supplies. The Economic Times 2022. Mar. 30. Available: https://economictimes.indiatimes.com/industry/healthcare/biotech/pharmaceuticals/shanghai-lockdown-may-choke-pharma-supplies/articleshow/90527357.cms (accessed 2022 May 10).
- 31.Gupta M, Abdelmaksoud A, Jafferany M, et al. COVID-19 and economy. Dermatol Ther 2020;33:e13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cassels A. Most of our prescription drugs are manufactured overseas: But are they safe? CMAJ 2012;184:1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dave CV, Pawar A, Fox ER, et al. Predictors of drug shortages and association with generic drug prices: a retrospective cohort study. Value Health 2018;21: 1286–90. [DOI] [PubMed] [Google Scholar]
- 34.Fenna J, Chu C, Hassan R, et al. Extent of a valsartan drug shortage and its effect on antihypertensive drug use in the Canadian population: a national cross-sectional study. CMAJ Open 2021;9:E1128–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tier 3 drug shortages. Ottawa: Health Canada; modified 2022 May 9. Available: https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/drug-shortages/tier-3-shortages.html (accessed 2022 May 10). [Google Scholar]
- 36.Campbell DJT, Campbell DB, Ogundeji Y, et al. First-line pharmacotherapy for incident type 2 diabetes: prescription patterns, adherence and associated costs. Diabet Med 2021;38:e14622. [DOI] [PubMed] [Google Scholar]
- 37.Abegaz TM, Shehab A, Gebreyohannes EA, et al. Nonadherence to antihypertensive drugs: a systematic review and meta-analysis. Medicine (Baltimore) 2017;96:e5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lemstra M, Blackburn D, Crawley A, et al. Proportion and risk indicators of nonadherence to statin therapy: a meta-analysis. Can J Cardiol 2012; 28:574–80. [DOI] [PubMed] [Google Scholar]
- 39.Parati G, Kjeldsen S, Coca A, et al. Adherence to single-pill versus free-equivalent combination therapy in hypertension: a systematic review and meta-analysis. Hypertension 2021;77:692–705. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.