Abstract
Introduction:
The relationship between cognitive function and frailty in older, long-term breast cancer survivors was examined.
Materials and Methods:
Breast cancer survivors who were diagnosed and treated at 60 years of age or above and were 5–15 year disease-free survivors and non-cancer controls matched on age and education were evaluated with neuropsychological tests and the Comprehensive Geriatric Assessment which was used to assess frailty based on a deficit accumulation frailty index (DAFI).
Results:
Unadjusted regression analyses revealed that cancer survivors scored significantly lower on the Language (P=0.015), Attention, Processing Speed, Executive Function (APE) (P=0.015), and Learning and Memory (LM) (P=0.023) domains compared to controls. However, only the LM domain remained significantly different (P=0.002) in the adjusted analysis. Survivors had significantly higher DAFI scores compared to controls (p=0.006) and significantly more survivors were categorized as pre-frail or frail (35%) compared to controls (23%, p=0.009). Increasing frailty scores were associated with worse cognitive performance across all domains (all Ps ≤ 0.004). For the LM domain, there was a significant interaction (P = 0.019) between DAFI score and survivorship vs control status. Survivors demonstrated a significant linear decline in LM scores as DAFI scores increased, whereas controls demonstrated comparable scores between the robust and pre-frail DAFI groups, demonstrating decline in the frailty group only.
Conclusion:
Older, long-term breast cancer survivors had lower cognitive performance and higher levels of frailty compared to controls. For the Learning and Memory domain, the decline in performance began in the pre-frail range for survivors, but not controls.
Keywords: Older breast cancer survivor, cognition frailty
Introduction
There are approximately 17 million cancer survivors in the United States; 3.5 million are breast cancer survivors (1). Further, approximately 60% of survivors are 65 years of age or older (1). Given the predicted expediential growth of the cancer survivor population and the increasing percentage of older cancer survivors, there is a critical need to understand the issues facing older cancer survivors. Cognitive decline is one of the most feared consequences of aging (2), yet little is known about the impact of cancer and cancer treatments on cognitive aging in older, long-term survivors of breast cancer. Most of the research examining the cognitive effects of breast cancer treatments have been conducted with younger women; however, an emerging literature with older women with breast cancer suggest that age is a risk factor for post-treatment cognitive decline (3–9).
The importance of studying older breast cancer survivors is amplified by evidence suggesting that cancer treatments may accelerate aging on a biological level (9–10). Therefore, the cognitive effects of cancer treatments may reflect specific effects on brain structure and function and an acceleration of normal cognitive aging (9). Although the biology of aging is not completely understood, one perspective is that aging is associated with increased damage accumulation across multiple biological systems resulting in decreased resiliency (11). Frailty is a clinical manifestation of this process as measured by increasing burden of comorbidity and loss of functional capacity (12). Studies have found a relationship between frailty and cognitive decline in older women with breast cancer assessed pre- to post-treatment (13–15), but little is known about how a history of treatment for breast cancer affects cognitive aging in long-term breast cancer survivors who were diagnosed and treated as older adults.
Our research group is examining the trajectory of cognitive aging in older, disease-free, long-term breast cancer survivors who were assessed 5–15 years after diagnosis and followed for two years. Age and education matched controls without a cancer history were assessed at the same time intervals. This paper addresses the first primary hypothesis, which predicted that, at the first assessment, cancer survivors would have lower performance on neuropsychological testing compared to controls. Further, we examined the relationship between frailty and cognitive function in cancer survivors and controls in order to test whether a history of diagnosis and treatment for breast cancer interacted with frailty to negatively impact cognitive outcomes.
Patients and Methods
Breast cancer survivors were identified through the survivorship clinics at Memorial Sloan Kettering Cancer Center (MSK) and City of Hope Comprehensive Cancer Center (COH), supplemented at each site by recruitment through the Army of Women. Survivors were eligible if they were diagnosed with stage 0-III breast cancers and treated at age 60 or above and were 5–15-year disease-free survivors at the time of enrollment. All primary treatments (surgery, chemotherapy, and radiation therapy) were completed at the time of enrollment; however, 25% of survivors were receiving endocrine therapy at enrollment. Survivors were excluded based on the following criteria: metastatic disease, score of 11 or greater (indicating risk of dementia) on the Blessed Orientation-Memory-Concentration (BOMC) Test (16), previous history of cancer (except non-melanoma skin cancer), treatment with chemotherapy for non-cancer conditions, neurobehavioral risk factors, including history of neurologic disorder (e.g., Parkinson’s disease, seizure, dementia), alcohol/substance abuse, head trauma requiring hospitalization or evidence of structural brain changes on imaging; and severe psychiatric disorder (e.g., schizophrenia, bipolar disorder). Additionally, survivors that had a recurrence of breast cancer during follow up were removed from the study. Recruitment was targeted so that approximately 50% had a history of treatment with chemotherapy (MSK=81, COH=79) or no chemotherapy (MSK=87, COH=81).
Female noncancer controls (MSK=77, COH=85) who met the same inclusion criteria (except for diagnosis of cancer) and exclusion criteria were recruited through community advertisement and the Army of Women. Noncancer controls were frequency matched on age and education. The institutional review boards of MSK and COH approved all methods and procedures.
Toward the end of the study, the age at diagnosis was lowered to 55 to increase the number of survivors who had been treated with chemotherapy. As a result, 23 participants between 55–60 were recruited (11 treated with chemotherapy, 5 not treated with chemotherapy, and 7 controls). Assessments occurred at enrollment and at 8, 16, and 24-month follow-ups. The assessment battery included standardized neuropsychological tests, self-report of cognitive function, and the Comprehensive Geriatric Assessment (17–18). The neuropsychological measures were categorized into domains based on previous studies (7) and clinical judgment of the neuropsychologists involved with the study (JR, ER, SP) informed by a factor analysis.
Each test score was standardized (z-score) according to the healthy control group, and then a mean of standardized scores within the domain calculated for each participant. Individual test scores were checked for deviation from a normal distribution, and for those that differed the Box-Cox algorithm (19) was used to determine a suitable power transformation prior to group comparisons and domain score calculations. Below are the tests administered categorized by domain.
Cognitive Reserve:
Wide Range Achievement Test 4 (WRAT4) (20)
Language:
Category Fluency (21); Boston Naming Test (22), Controlled Oral Word Association Test (23)
Attention, Processing Speed, Executive Function:
Digit Symbol (22); Trail Making A and B (24); DKEFS Color-Word Naming (25); NAB Digits Forward and Backward (26–27); NAB Driving Scenes (26–27).
Learning and Memory:
NAB List Learning (26–27): Trial 1, Semantic Clustering, List A Immediate, List A Delayed, Long Delay, List B Immediate, New Recognition Index; Logical Memory Part 1 and 2 (WMS-R, 28).
The Patients Assessment of Own Functioning Inventory (PAOFI, 29) was administered as the self-report measure of cognitive function. Additional self-report measures assessed: 1) Functional Status: Instrumental Activities of Daily Living (IADL) Subscale of the Multidimensional Functional Assessment Questionnaire (30); Medical Outcomes Study (MOS) Physical Health, Social Limitations, and Social Support Scales (30); Karnofsky Self-Reported Performance Status Scale; and Self-report of the number of falls in the last 6 months; 2) Comorbidity: Physical Health Section Older American Resources & Services Questionnaire (OARS) (31) and a single sum of the 14 items; 3) Depression: Center for Epidemiological Study – Depression (32); 4) Anxiety: Spielberger State Anxiety Inventory (33); and 5) Fatigue: Fatigue Symptom Inventory (34). Finally, the timed get up a go task was administered (35).
Deficit Accumulation Frailty Index (DAFI score): Frailty was quantified as a score ranging between zero and one based on up to 44 possible frailty indicators, as described by Cohen et.al. (36). For each indicator (e.g., recent weight loss, limited ability to climb one flight of stairs, a diagnosis of arthritis) the participant scored a zero, one, or two based on whether the indicator showed absent, intermediate, or most adverse risk, respectively. The deficit accumulation frailty index (DAFI) score was then calculated as the sum of these individual indicator scores divided by the maximum possible score. In cases where an indicator variable was missing, the item was excluded from both numerator and denominator; the score was calculated for all participants for whom at least 35 indicators were assessed (2 participants were excluded). Continuous DAFI scores were than used to classify participants as robust (DAFI < 0.2), pre-frail (0.2 ≤ DAFI < 0.35), or frail (DAFI ≥ 0.35). Since we were interested in the relationship between frailty and cognition, self-report of cognitive function was not included as a frailty indicator. Additionally, to utilize the same criteria for survivors and controls, breast cancer history was not included as an indicator (3% of the sample had a history of another type of cancer, which was included as a frailty indicator).
Statistical Analysis. Participant demographic and clinical characteristics are described overall and by cancer survivorship status and, for survivors, by treatment status (i.e., those who received chemotherapy compared to those who did not). Next, neurocognitive test and domain scores were compared across the three treatment/survivorship groups. For this comparison, means were computed for description, but differences were tested using regression models with adjustment for age group (i.e., over 75 versus younger), education, and study site. Depression and fatigue were also added as covariates because of significantly higher scores in survivors compared to controls. Finally, both the main effect of frailty and its moderating effect on the group effects were assessed using another series of regression models. For main effects of frailty on neurocognitive function, we regressed the standardized domain scores on continuous frailty scores, both with and without adjustment for age, education, and study site, depression and fatigue. For moderating effects, we expanded these adjusted models to also include a main effect for cancer survivorship group and the interaction between continuous frailty score and the survivorship indicator. All analyses were conducted in SAS version 9.4 (Cary, NC).
Site Differences.
Participants at the two clinical sites were compared on demographic characteristics using a series of Chi-square and independent samples t-tests, illuminating important differences. Although the two groups were comparable on distributions of survivorship and cancer treatment group, frailty distribution, age, ethnicity, marital status, and BMI, significant differences were found on race (10% Black at MSK compared to 5% at COH; 2% Asian or Pacific Islander at MSK compared to 5% at COH), education (47% at MSK with a graduate degree compared to 20% at COH), employment status (23% at MSK employed compared to 14% at COH), and smoking history (55% at MSK compared to 37% at COH). Further, the two samples differed significantly on the Language domain, likely due to the higher education level at MSK.
Results
Table 1 provides a description of demographic and medical variables among the participants. The groups were well matched on most variables. However, there was a significant age difference, not surprisingly, more survivors not treated with chemotherapy fell into the 75–79 and 80+ age groups (15% and 7% among chemotherapy group, 26% and 21% among non-chemotherapy group). Additionally, significantly more survivors exposed to chemotherapy reported a history of smoking (53%) compared to either survivors not treated with chemotherapy (44%) or controls (40%). Approximately equal numbers of survivors who were treated with chemotherapy (72%) or not exposed to chemotherapy (77%) were treated with endocrine therapies. Survivors not exposed to chemotherapy were more likely to have ER positive (88% vs. 73%) or PR positive (76% vs. 55%) tumors and less likely to have HER2 positive (6% vs. 17%) tumors. Finally, survivors had significantly higher depression and fatigue scores compared to controls.
Table 1.
Participant characteristics by treatment group
| No. of Patients (%) | |||||
|---|---|---|---|---|---|
| Characteristic | Overall | Chemo | No Chemo | Control | p-value |
| (n = 490) | (n = 160) | (n = 168) | (n = 162) | ||
| Age, M (SD) [n = 489] | 72.6 (6.0) | 70.9 (5.1) | 74.6 (5.9) | 72.1 (6.4) | <.001 |
| Race | |||||
| White | 415 (85%) | 129 (81%) | 141 (84%) | 145 (90%) | .282 |
| Black | 37 (8%) | 16 (10%) | 15 (9%) | 6 (4%) | |
| Asian/PI | 17 (3%) | 8 (5%) | 5 (3%) | 4 (2%) | |
| Other | 14 (3%) | 5 (3%) | 5 (3%) | 4 (2%) | |
| Missing | 7 (1%) | 2 (1%) | 2 (1%) | 3 (2%) | |
| Ethnicity | |||||
| Hispanic | 49 (10%) | 18 (11%) | 12 (7%) | 19 (12%) | .310 |
| Non-Hispanic | 432 (88%) | 138 (86%) | 153 (91%) | 141 (87%) | |
| Missing | 9 (2%) | 4 (3%) | 3 (2%) | 2 (1%) | |
| Education | |||||
| Less than college | 196 (40%) | 67 (42%) | 71 (42%) | 58 (36%) | .382 |
| College or more | 292 (60%) | 92 (58%) | 96 (57%) | 104 (64%) | |
| Missing | 2 (0%) | 1 (1%) | 1 (1%) | 0 (0%) | |
| WRAT, M (SD) [n = 487] | 63.1 (5.5) | 62.4 (6.5) | 63.4 (5.1) | 63.5 (4.6) | .137 |
| Employment | |||||
| Employed FT | 40 (8%) | 20 (13%) | 8 (5%) | 12 (7%) | .065 |
| Employed PT | 48 (10%) | 13 (8%) | 13 (8%) | 22 (14%) | |
| Unemployed | 23 (5%) | 5 (3%) | 9 (5%) | 9 (6%) | |
| Retired | 351 (72%) | 111 (69%) | 126 (75%) | 114 (70%) | |
| Missing | 28 (6%) | 11 (7%) | 12 (7%) | 5 (3%) | |
| Marital Status | |||||
| Married/DP | 341 (70%) | 104 (65%) | 128 (76%) | 109 (67%) | .246 |
| Widowed | 78 (16%) | 29 (18%) | 18 (11%) | 31 (19%) | |
| Divorced | 12 (2%) | 6 (4%) | 3 (2%) | 3 (2%) | |
| Single | 54 (11%) | 18 (11%) | 17 (10%) | 19 (12%) | |
| Missing | 5 (1%) | 3 (2%) | 2 (1%) | 0 (0%) | |
| Smoking Hx | |||||
| Yes | 224 (46%) | 85 (53%) | 74 (44%) | 65 (40%) | .049 |
| No | 264 (54%) | 74 (46%) | 93 (55%) | 97 (60%) | |
| Missing | 2 (0%) | 1 (1%) | 1 (1%) | 0 (0%) | |
| BMI, M (SD) [n = 480] | 27.4 (5.4) | 27.8 (5.8) | 27.4 (4.8) | 27.1 (5.5) | .515 |
| Frailty | |||||
| Robust | 336 (69%) | 103 (64%) | 109 (65%) | 124 (77%) | .125 |
| Pre-frail | 118 (24%) | 42 (26%) | 45 (27%) | 31 (19%) | |
| Frail | 34 (7%) | 14 (9%) | 13 (8%) | 7 (4%) | |
| Missing | 2 (<1%) | 1 (1%) | 1 (1%) | 0 (0%) | |
| Comorbidity Count, M (SD) | 2.1 (1.5) | 2.2 (1.6) | 2.3 (1.5) | 1.7 (1.4) | .001 |
| Endocrine Therapy | |||||
| Ever (n = 314) | 234 (75%) | 110 (72%) | 124 (77%) | NA | .298 |
| At Assessment 1 (n = 319) | 80 (25%) | 52 (34%) | 28 (17%) | NA | <.001 |
| Cancer Characteristics | |||||
| ER Positive (n = 297) | 237 (80%) | 111 (73%) | 126 (88%) | NA | .001 |
| PR Positive (n = 292) | 190 (65%) | 84 (55%) | 106 (76%) | NA | <.001 |
| HER2 (FISH) Positive (n = 266) | 32 (12%) | 25 (17%) | 7 (6%) | NA | .006 |
| Tumor size (cm), M (SD) | 1.7 (1.4) | 2.2 (1.5) | 1.2 (1.2) | NA | <.001 |
| Years since diagnosis, M (SD) | 8.0 (2.7) | 8.1 (2.7) | 8.0 (2.6) | NA | .574 |
| Site | |||||
| COH | 245 (50%) | 79 (49%) | 81 (48%) | 85 (52%) | .728 |
| MSK | 245 (50%) | 81 (51%) | 87 (52%) | 77 (48%) | |
| Baseline Psych [n = 488] | |||||
| FSI Disruption, M (SD) | 8.1 (11.0) | 9.5 (12.4) | 8.9 (10.8) | 5.9 (9.3) | .008 |
| STAI State Sum, M (SD) | 25.8 (7.3) | 26.8 (8.3) | 25.5 (6.9) | 25.0 (6.6) | .076 |
| CESD Sum, M (SD) | 6.8 (7.1) | 7.9 (8.8) | 6.6 (6.1) | 5.9 (5.9) | .043 |
| CESD, Meets Criteria | 45 (9%) | 21 (13%) | 15 (9%) | 9 (6%) | .060 |
| PAOFI Total, M (SD) | 25.2 (18.0) | 29.2 (21.8) | 23.7 (14.9) | 22.9 (16.1) | .003 |
Neuropsychological Performance
Unadjusted regression analyses (Table 2) revealed that cancer survivors scored significantly lower on the Language (P=0.015), APE (P=0.015), and Learning and Memory (P=0.023) domains compared to controls. After adjusting for age, education, site, depression and fatigue, only the Learning and Memory domain remained significantly different (P=0.002). Analysis of survivors who had been exposed to chemotherapy compared to those not exposed to chemotherapy revealed no significant differences on any of the domains either in unadjusted or adjusted analyses (Supplementary Table 1).
Table 2.
Test and domain scores (SD) for survivors and controls
| Domain / Test | Survivors, M (SD) n = 328 |
Controls, M (SD) n = 162 |
Unadj. p val |
Adj. p val |
|---|---|---|---|---|
| Language | −0.18 (0.83) | 0.00 (0.76) | 0.015 | 0.128 |
| APE | −0.16 (0.67) | 0.00 (0.70) | 0.015 | 0.167 |
| Learning and Memory | −0.23 (0.79) | 0.00 (0.74) | 0.002 | 0.023 |
| PAOFI Total1 (n = 488) | 26.38 (18.78) | 22.94 (16.14) | 0.063 | 0.630 |
Raw means are shown, but these scores were transformed according to the Box-Cox algorithm and the transformations used for statistical comparisons. Minor intermittent missingness resulted in samples sizes between 488 and 490 for individual tests. Adjusted models include age (years), education (college or above vs less), site, CES-D, and FSI average.
Relationship between Frailty and Cognitive Function
Analysis of DAFI scores revealed that survivors had significantly higher scores compared to controls (p=0.006) and that significantly more survivors were categorized as pre-frail or frail (35%) compared to controls (23%, p=0.009). Survivors were more likely to report “heart trouble” (p=0.05) and high blood pressure (p=0.03) compared to controls.
Utilizing DAFI scores as a continuous variable revealed that higher frailty scores were significantly associated with poorer performance across Language, APE, and Learning and Memory domains for the combined sample of survivors and controls in the unadjusted and adjusted analyses (all Ps ≤ 0.004). Overall, there was a linear reduction in performance in each domain with increasing frailty scores.
For the Learning and Memory domain, there was a significant interaction (P = 0.019) between DAFI score and survivorship vs. control status. As depicted in Figure 1, survivors demonstrated a significant linear decline in Learning and Memory scores as DAFI scores increased, whereas controls demonstrated comparable Learning and Memory scores between the robust and pre-frail DAFI groups, but lower scores in the frail group. A similar pattern was seen for the APE domain, although the interaction was not significant (P=0.081).
Figure 1:
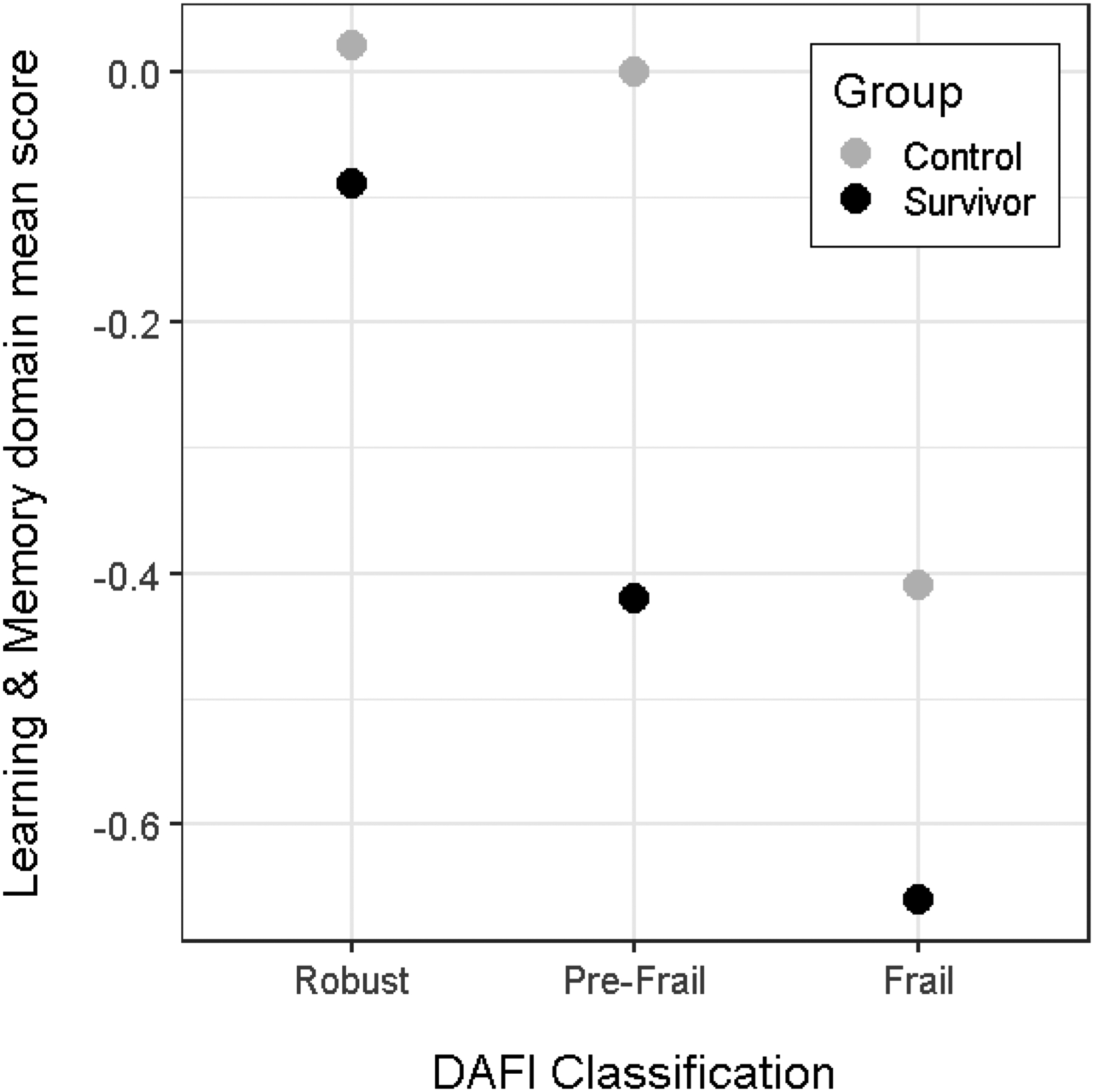
Interaction of Frailty Categorization and Survivor vs. Control Status for the Learning and Memory Domain
Self-Report of Cognitive Function
Similar analyses of PAOFI scores revealed no significant differences between survivors and controls on the total score. Comparing survivors exposed to chemotherapy vs. those who were not, there was a trend for survivors exposed to chemotherapy to have higher total scores (P=0.080) in the unadjusted analysis but not in the adjusted analysis. No significant interaction between DAFI score and survivors vs. controls was found for the total score.
Discussion
The results of this study demonstrated that women who were diagnosed and treated for breast cancer primarily at age 60 or older and were 5–15 year disease-free survivors scored significantly lower than age- and education-matched non-cancer controls on the Learning and Memory domain. Memory problems are the most commonly reported problems and one of the greatest concerns among older individuals in general and cancer survivors in particular (9). Importantly, there were no differences in cognitive function is survivors exposed to chemotherapy vs. those not exposed to chemotherapy suggesting that multiple aspects of breast cancer treatment, including endocrine therapy, effect cognition. These results are consistent with previous research finding that cognitive deficits can persist for many years post-treatment (37–38).
The study revealed that survivors had higher DAFI scores and were more likely to be categorized as pre-frail or frail. Further, higher frailty scores were associated with lower cognitive performance on the Language, APE, and Learning and Memory domains across the combined sample of survivors and controls. The association between frailty and cognitive function and the higher prevalence of survivors categorized as pre-frail or frail may partially explain the difference in cognitive performance in the survivor compared to the control group.
Cancer survivors had higher frailty scores even though neither the diagnosis of breast cancer nor the report of cognitive problems was included in the calculation of the DAFI score. Cancer treatments have well known late effects such as cardiotoxicity, alteration of the hormonal function, weight gain, etc., and functional declines up to 12 months post-treatment all of which contribute to increasing levels of frailty (39).
Finally, there was a significant interaction of group (survivor vs. controls) and frailty score for the Learning and Memory domain. Survivors demonstrated a linear decrease in performance with increasing DAFI score, whereas, for controls, decreased cognitive performance was only seen in those categorized as frail. A similar pattern was seen for the APE domain, although the interaction was not statistically significant. The finding that survivors who meet criteria for frailty have lower cognitive performance is consistent with previous research (13, 15). Importantly, the robust cancer survivors do not differ in cognitive performance compared to controls. These survivors likely represent resilient older adults who are either resistant to toxicities of treatment or are able to recover over time (39). The novel finding is that cancer survivors who meet criteria for pre-frailty have lower cognitive performance compared to controls who are pre-frail.
One hypothesis is that cancer treatments accelerate biological aging through a variety of mechanisms including increased DNA damage, inflammation, and oxidative stress (9, 40). Frailty measures like the DAFI typically assess deficits that can be observed (disease states like hypertension, diabetes etc or behavioral changes like slowed walking speed) or perceived by the individual (pain, fatigue, etc), but often do not include biomarkers of aging. However, human and animal studies have demonstrated that cancer treatments activate biomarkers of aging like p16INK, ARF and AKT (10, 41–42). It is possible that cancer survivors have higher levels of damage to the biological system compared to controls due to their exposure to cancer treatments. Therefore, the accumulation of deficits in the pre-frail range may have a larger impact on the biological system in general and cognitive function in particular. These results suggest that the assessment of cognitive function in older adults needs to be understood within the context of the broader biological / functional system since greater deficit accumulation, even within the pre-frail range, is associated with poorer cognitive function.
The most commonly endorsed frailty indicators (50% or greater) were: arthritis, high blood pressure, elevated BMI, polypharmacy, fatigue, increased time in bed, needing help with meal preparation and chores, and change in social activities. A combination of these indicators would not necessarily trigger concerns about cognitive function. Therefore, this finding has important clinical implications including: 1) survivors who are pre-frail may require closer monitoring of cognitive function because they may be vulnerable to clinically significant cognitive decline and 2) pre-frail survivors may benefit from interventions targeting cognitive function specifically or that slow the progression along the frailty continuum generally.
The self-report data showed a less robust and consistent pattern compared to the performance-based data from neuropsychological testing which is the opposite pattern found in many studies. This may be related to older controls also reporting more cognitive problems related to aging in general.
This is the first study that we are aware of that examined cognitive function in long-term breast cancer survivors, the majority of whom were diagnosed and treated above the age of 60 and were 5–15 year survivors at the time of diagnosis. Strengths of the study include a large sample of survivors and controls recruited at two major comprehensive cancer centers located on opposite coasts, assessment of cognitive function and psychological function along with the Comprehensive Geriatric Assessment. Despite these strengths, limitations should be noted including that the sample consisted of primarily Caucasian, highly educated participants and few participants were categorized as frail; therefore, we could not examine the relationship of cognition and frailty along the full frailty continuum.
In summary, several important findings emerged from this study. First, the majority of older breast cancer survivors were robust and the cognitive performance for robust survivors was similar to robust controls. However, significantly more survivors were pre-frail or frail and differences in cognitive performance in the Learning and Memory were seen in pre-frail survivors compared to controls. These results have important clinical implications for geriatricians and clinicians who care for long-term breast cancer survivors and illustrate the importance of assessing cognitive function pre-frail survivors who are vulnerable to cognitive decline.
Supplementary Material
Table 3.
Mean scores (SD) by frailty group and survivorship status
| Domain/Measure | Group | Robust (n = 336) |
Pre-Frail (n = 118) |
Frail (n = 34) |
Interaction p-value |
|---|---|---|---|---|---|
| Language | Controls | 0.07 (0.75) | −0.19 (0.74) | −0.42 (0.77) | 0.936 |
| Survivors | −0.10 (0.81) | −0.22 (0.83) | −0.73 (0.83) | ||
| APE | Controls | 0.04 (0.68) | −0.02 (0.76) | −0.65 (0.41) | 0.081 |
| Survivors | −0.03 (0.60) | −0.29 (0.69) | −0.73 (0.75) | ||
| Learning & Memory | Controls | 0.02 (0.74) | 0.00 (0.75) | −0.41 (0.60) | 0.019 |
| Survivors | −0.09 (0.74) | −0.42 (0.83) | −0.66 (0.79) | ||
| PAOFI Total | Controls | 177.61 (13.99) | 166.75 (16.70) | 166.71 (31.86) | 0.182 |
| Survivors | 177.03 (13.70) | 164.86 (20.76) | 150.96 (25.01) |
Acknowledgements:
This research was supported by grants from the National Cancer Institute (TA: R01 CA172119, U54 CA137788, P30 CA008748) and the American Cancer Society (SKP: RSG-17-023-01-CPPB)
Footnotes
Conflict of Interest: The authors have no conflicts of interest to disclose.
References
- 1.Cancer Treatment and Survivorship Facts and Figures: 2019–2021 American Cancer Society, 2020. [Google Scholar]
- 2.Fried TR, Bradley EH, Towle VR, Allore H. Understanding the Treatment Preferences of Seriously Ill Patients New England Journal of Medicine 2002; 346: 1061–1066. [DOI] [PubMed] [Google Scholar]
- 3.Yamada TH, Denburg NL, Beglinger LJ, Schultz SK: Neuropsychological outcomes of older breast cancer survivors: Cogntive features ten or more years after chemotherapy. Journal of Neuropsychiatry and Clinical Neurosciences 2010; 22: 48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hurria A, Rosen C, Hudis C, et al. : Cognitive function of older patients receiving adjuvant chemotherapy for breast cancer: A pilot study in breast cancer. Journal of the American Geriatric Society 2006; 98: 1742–1745, [DOI] [PubMed] [Google Scholar]
- 5.Hurria A, Goldfarb S, Rosen C, et al. Effect of adjuvant breast cancer chemotherapy on cognitive function from the older patient’s perspective. Breast Cancer Research and Treatment 2006; 98: 343–348. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen CM, Yamada TH, Beglinger L, Cavanaugh JE, Denburg N, Schultz SK. Cognitive features ten or more years after successful breast cancer survival: Comparisons across types of cancer interventions. Psychooncology 2013; 22: 862–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mandelblatt JS, Small BJ, Luta G, Hurria A, Jim H, McDonald BC, et al. Cancer-related cognitive outcomes among older breast cancer survivors in the thing and living with cancer study. J Clin Oncol 2018; 36: 3211–3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mandelblatt JS, Jacobsen PB, Ahles TA. Cognitive effects of cancer systemic therapy: Implications for the care of older patients and survivors J Clin Oncol 2014; 32: 2617–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahles TA, Root JC. Cognitive effects of cancer and cancer treatments. Annu Rev Clin Psychol 2018; 14: 435–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hk Sanoff, Deal AM, Krishnamurthy J, Torrice C, Dillon P, Sorrentino J et al. Effect of cytotoxic chemotherapy on molecular age in patients with breast cancer J Natl Cancer Inst 2014; 106, dju057 doi: 10.1093/jnci/dju057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gavrilov LA, Gavrilova NS. The reliability-engineering approach to the problem of biological aging. Ann NY Acad Sci 2004; 1019: 509–512. [DOI] [PubMed] [Google Scholar]
- 12.Mitnitski A, Rockwood K. Aging as a process of deficit accumulation: Its utility and origin. In Yashin AI, Jazwinski SM (eds): Aging and Health – A Systems Biology Perspective. Interdiscipl Top Gerontol Basel, Karger, 2015, vol 40, pp 85–98. [DOI] [PubMed] [Google Scholar]
- 13.Magnuson A, Lei L, Gilmore N, Kleckner AS, Lin FV, Ferguson R, et al. Longitudinal relationship between frailty and congition in patients age > 50 years with breast cancer. J Am Geriatr Soc 2019; 67: 928–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mandelblatt JS, Clapp JD, Luta G, Faul LA, Tallarico MD, McClendon TD et al. Long-term trajectories of self-reported cognitive function in a cohort of older survivors of breast cancer: CALGB 369901 (Alliance). Cancer 2016; 122: 3555–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mandelblatt JS, Zhou X, Small BJ, Ahn J, Zhai W, Ahles TA et al. Deficits accumulation frailty trajectories of older breast cancer survivors and matched non-cancer controls: The Thinking and Living with Cancer (TLC) study. JNCI, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katzman R, Brown T, Fuld P et al. Validation of a short Orientation-Memory-Concentration Test of cognitive impairment. Am J Psychiatry. 1983;140: 734–739 [DOI] [PubMed] [Google Scholar]
- 17.Hurria A, Togawa K, Mohile SG, Owusu C, Klepin HD, Gross CP, et al. Predicting chemotherapy in older adults with cancer: A prospective multicenter study. J Clin Oncol 2011; 29: 3457–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohile SG, Dale W, Soerfield MR, Schonberg MA, Boyd CM, Burhenn PS et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol 2018; 36: 2326–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Box GEP, Cox DR. “An analysis of transformations”. Journal of the Royal Statistical Society, Series B 1964; 26: 211–252. [Google Scholar]
- 20.Wilkinson G, Robertson G. Wide Range Achievement Test 4 (WRAT4). Lutz, FL: Psychological Assessment Resources, 2006. [Google Scholar]
- 21.Benton AL. Neuropsychological assessment. Annu Rev Psychol 45:1–23: 1994. [DOI] [PubMed] [Google Scholar]
- 22.Weintraub S, Salmon D, Mercaldo N, Ferris S, et al. : The alzheimer’s disease centers’ Uniform Data Set (UDS): The neuropsychological test battery. Alzheimer Disease and Associated Disorders 23: 91–101: 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benton A Differntial behavioral effects in frontal lobe disease. Neuropsychologia 6: 53–60: 2006 [Google Scholar]
- 24.Reitan R, Wolfson D. The Halstead-Reitan neuropsychological test battery. Tucson, AZ, Neuropsychological Press, 1985. [Google Scholar]
- 25.Delis DC, Kaplan E, Kramer JH: Delis-Kaplan Executive Function System. San Antonio, TX: The Psychological Corporation, 2001 [Google Scholar]
- 26.Stern RA, White T. Neuropsychological Assessment Battery (NAB). Psychological Assessment Resources (PAR), Lutz, Fl. 2003. [Google Scholar]
- 27.Stern RA, White T. NAB Psychometric and Technical Manual. Lutz, FL: Psychological Assessment Resources, 2003. [Google Scholar]
- 28.WMS-R - Wechsler D WMS-R: Wechsler Memory Scale–Revised. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- 29.Van Dyk K, Ganz PA, Ercoli L, Petersen et al. : Measuring cognitive complaints in breast cancer survivors: Psychometric properties of the patient’s assessment of own functioning inventory Supportive Care in Cancer 2016: 24: 4939–4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stewart AL, Ware JE: Measuring functioning and well-being: The Medical Outcomes Study approach 1992: Durham NC, Duke University Press. [Google Scholar]
- 31.Fillenbaum GG, Smyer MA. The development, validity, and reliability of the OARS multidimensional functional assessment questionnaire. Journal of gerontology. 36: 428–434:1981. [DOI] [PubMed] [Google Scholar]
- 32.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Pscyhol Measurement 1:385–401:1977. [Google Scholar]
- 33.Spielberger CD. Manual for the State-Trait Anxiety Inventory (STAI: Form Y). Consulting Psychologists Press, Palo Alto. 1983. [Google Scholar]
- 34.Hann DM, Jacobsen PB, Martin SC, et al. : Fatigue in women treated with bone marrow transplantation forbreast cancer: A comparison with women with no history of cancer. Support Care Cancer 5: 44–52: 1997 [DOI] [PubMed] [Google Scholar]
- 35.Owsley C, Sloane M, McGwin G, Jr., Ball K. Timed instrumental activities of daily living tasks: relationship to cognitive function and everyday performance assessments in older adults. Gerontology 48: 254–265: 2002. [DOI] [PubMed] [Google Scholar]
- 36.Cohen HJ, Smith D, Sun CL, Filo J, Katheria V, Hurria A, et al. Frailty as determined by a comprehensive geriatric assessment derived deficit accumulation index in older patients with cancer treated with chemotherapy. Cancer 2016; 15: 3865–3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahles TA, Saykin AJ, Furstenberg CT, Cole B, et al. : Neuropsychological impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. Journal of Clinical Oncology 20: 485–493: 2002. [DOI] [PubMed] [Google Scholar]
- 38.Koppelmans V, Breteler MMB, Boogerd W, Seynaeve C. et al. : Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. Journal of Clinical Oncology 30: 1080–1086: 2012. [DOI] [PubMed] [Google Scholar]
- 39.Hurria A, Soto-Perez-de-Celis E, Allred JB, Cohen HJ, et al. :. Functional Decline and Resilience in Older Women Receiving Adjuvant Chemotherapy for Breast Cancer. J Am Geriatr Soc. 67: 920–927, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahles TA, Saykin AJ: Candidate mechanisms for chemotherapy-induced cognitive changes. Nature Reviews Cancer 7:192–201: 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salas-Ramiriz KY, Bagnall C, Fria C Abdali SA, Ahles TA et al. : Doxorubicin and cyclophosphamide induce cognitive dysfunction and activate ERK and AKT signaling pathways. Behavioural Brain Research 292: 133–141: 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bagnall-Moreau C, Chaudhry S, Salas-Ramirez K, Ahles TA, et al. : Chemotherapy-induced cognitive impairment is associated with increased inflammation and oxidative damage in the hippocampus. Molecular Neurobiology 56: 7159–7172: 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


