Abstract
Purpose:
Rituximab and lenalidomide is effective for previously untreated and relapsed/refractory (R/R) indolent non-Hodgkin lymphoma (iNHL). However, long-term survival and predictive biomarkers are not well-described.
Methods:
We conducted two phase 2 open-label trials involving 60 patients with previously untreated and R/R advanced stage iNHL. Patients received lenalidomide and rituximab induction followed by continuous lenalidomide until disease progression or unacceptable toxicity. The primary endpoint was overall response rate (ORR). Correlative studies included plasma cytokine monitoring, flow cytometry of PBMCs (D0, 15, 30, 60), and RNA sequencing of pretreatment tumor biopsies.
Results:
At a median follow-up of 63 months for previously untreated and 100 months for R/R, ORR was 82% for both. The 11 R/R patients who achieved complete remission remained in continuous remission for 16 to 141 months, thereafter. Median overall survival was not reached in the previously untreated and was 140 months (95% CI: 53.4–140) in the R/R group. A mixed effects linear regression model identified significant associations between GranB+ CD8+ T cells and long-term complete response (LTCR) (p=5.3e-4). Furthermore, prior to start of therapy, treatment response could be predicted by B cell and GranB+ CD8+ T cell levels (% total lymphocytes).
Conclusion:
Rituximab plus lenalidomide followed by continuous lenalidomide is effective with manageable toxicity in patients with previously untreated and R/R iNHL. This regimen produces durable remissions, even in heavily pretreated patients, with some lasting greater than 10 years. GranB+ CD8+ T cells, B cells, and plasma IFN-γ allowed prediction of LTCR but need validation in larger trials.
Introduction
Despite the availability of multiple therapeutic modalities and a high response rate to initial therapy, most patients with indolent non-Hodgkin lymphoma (iNHL) will develop recurrent disease and lymphoma-related complications. Immunochemotherapy remains the backbone of initial therapy for patients with iNHL (1). However, toxicity clearly limits efficacy and quality of life. The last decade has heralded a plethora of novel targeted agents, biologics and immune modulators that has significantly changed the management of iNHL.
Rituximab monotherapy produces an overall response rate (ORR) of approximately 20–50% in iNHL (2). While the specific mechanism of action of rituximab is likely multifactorial and incompletely understood, most agree that utilization of host immune effector mechanisms which mediate ADCC is integral (3,4). Lenalidomide is a potent immune modulator which has demonstrated efficacy in a broad range of lymphoma subtypes including iNHL (5). When given in combination with rituximab, lenalidomide augments T- and NK-cell synapse formation to enhance ADCC (6–8).
The combination of rituximab and lenalidomide has produced considerable activity in patients with both untreated and relapsed/refractory (R/R) mantle cell and follicular lymphoma, with ORR ranging from 57% in rituximab refractory patients to over 90% in previously untreated follicular lymphoma (9,10). However, long-term follow-up and survival data is not well-described. Moreover, it is unknown if continuous exposure to lenalidomide enhances durability.
Here, we report long term follow-up of two phase II open-label trials assessing the efficacy and safety of rituximab and lenalidomide induction followed by indefinite continuous lenalidomide in patients with advanced stage untreated and R/R iNHL. In addition, we further used flow cytometry and plasma proteomic analysis to characterize this regimens immune modulating effects and identify biomarkers of response. We also characterized the pretreatment intra-tumoral transcriptome and B cell repertoire. These results will aid the rational design of future clinical trials with these and/or other biologics.
Methods
Trial Oversight
We conducted two multi-center open-label phase 2 trials with untreated and R/R iNHL patients at the University of California, Davis, the Department of Veterans Affairs, Northern California Health Care System and Sutter Pacific Medical Foundation (Santa Rosa, CA). The trials were conducted in accordance with the principles of the Declaration of Helsinki and International Conference on Harmonization Good Clinical Practice guidelines. All the patients provided written informed consent before any trial-related procedures were performed. An independent data and safety monitoring committee and an expert advisory group provided oversight throughout the conduct of the trial. The initial analysis of the R/R trial has been previously reported (20), the untreated trial is being reported here for the first time.
Patient Selection
Patients were eligible for participation in the trials if they were at least 18 years of age, had good functional status defined as an Eastern Cooperative Oncology Group performance status of 0 to 2, had histologically confirmed and measurable iNHL and were assessed as being in need of treatment according to the Groupe d-Etude des Lymphomes Folliculaires (GELF) criteria (11). Indolent NHL subtypes included follicular lymphoma, nodal marginal zone B-cell lymphoma (MZL), extranodal MZL of mucosa-associated lymphoid tissue type, small lymphocytic lymphoma (SLL), lymphoplasmacytic lymphoma or indolent B-cell lymphoma, not otherwise specified (NOS). Patients in the R/R cohort had to have received at least 1 prior treatment with evidence of progression of disease during or after last treatment and had indications for retreatment.
Key exclusion criteria for both trials included pregnant or lactating females, prior lenalidomide use, known intolerance to thalidomide or rituximab or major organ dysfunction defined as any of the following laboratory abnormalities: absolute neutrophil count <1.5 x109 /l; platelet count <100 x x109 /; serum creatinine >221 μmol/l; serum aspartate aminotransferase or alanine aminotransferase values >5 times the upper limit of normal; or serum total bilirubin >34 μmol/l. If patients received previous cancer-directed therapy, all therapies must have been discontinued at least 4 weeks prior to treatment initiation. Additional eligibility criteria and information regarding GELF criteria are provided in the supplementary appendix.
Study Design and Treatment
Treatment consisted of intravenous rituximab 375 mg/m2 which was given weekly for 4 doses starting day 15 of cycle 1 and oral lenalidomide 20 or 25 mg daily which was administered on days 1–21 of a 28-day cycle until disease progression or unacceptable toxicity.
Rituximab was repeated on days 1, 8, 15 and 22 of cycle 5 if patients did not achieve a complete response after cycle 4. Of note, lenalidomide 25 mg daily was initially administered to the first 4 patients in the R/R cohort; 2 patients developed grade 3 tumor lysis syndrome. Therefore, the protocol was amended to reduce the starting dose of lenalidomide to 20 mg daily for the remaining patients in the R/R cohort and for all patients in the previously untreated cohort.Prophylaxis for tumor lysis syndrome with allopurinol 300 mg daily and venous thromboembolism (VTE) with aspirin 81 mg daily was required. Dose reductions and interruptions of lenalidomide were permitted for management of toxic effects.
Assessments
Imaging using conventional or spiral computerized tomography (CT) and/or positron emission tomography (PET)/CT scans to assess disease status was performed at baseline and every 2 months while receiving treatment until a response was noted. Responding patients were reassessed every 4 months thereafter until they were in complete remission (CR) for 5 years and then annually or sooner with lymphoma-related symptoms or at the discretion of the treating investigator. Disease response or progression was assessed according to the International Workshop Lymphoma Response Criteria (12). Patients were assessed for adverse events before each cycle and during scheduled follow-up visits. Toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE Version 4.0, http://ctep.cancer.gov).
Statistical analysis: Clinical data
The primary endpoint was best overall response rate (ORR), which was defined as the proportion of patients whose best response was complete response (CR; confirmed or unconfirmed) or partial response (PR). Patients who were removed from the study without having a response assessment at the end of induction were deemed to be non-evaluable but included in a intent-to-treat (ITT) analysis.. A patient was considered to have stable disease (SD) if they failed to attain the criteria needed for a CR or PR but did not fulfill the criteria for progressive disease (PD). LTCR were defined as a CR lasting greater than 5 years. Secondary endpoints included progression-free survival (PFS), duration of response (DR), time to next therapy (TTNT), overall survival (OS) and safety.
It was hypothesized that untreated and R/R patients with iNHL would have an ORR of 80% or more and 60% or more, respectively, when treated with the combination of lenalidomide and rituximab. Simon’s optimal 2-stage design (13) was used to test the null hypothesis that the ORR would be 60% or less for the untreated cohort and 40% or less for the R/R cohort. A sample size of 30 patients in each cohort would provide 80% power to assess 20% difference with a one-sided alpha level of 0.05. An interim analysis for futility was conducted based on the stopping rules for the Simon’s two-stage design. Based on this interim analysis, enrollment was completed for both cohorts. The Kaplan-Meier method was used to estimate PFS, TTNT, DR and OS. PFS and OS were censored for patients who had not progressed or had not died at the time of last follow-up. Median follow-up in both groups was calculated using a standard univariate analysis as months from date of first treatment to date of death or last known contact, which every occurred first (14,15). The safety analysis included all the patients who received at least one dose of a trial drug. Events were summarized according to cohort.
Correlative studies
Blood samples for correlative analysis was drawn at baseline, days 15, 30, and 60 of treatment. Three analyses were undertaken: 1) flow cytometry of PBMCs to identify T-cell subsets and the magnitude of ADCC (assessed based on BCD) that could predict LTCR, 2) Luminex multi-analyte detection of plasma cytokines, and RNA sequencing (RNA-Seq) of pretreatment tumor biopsy specimens to examine the differences in global gene expression profiles and to conduct B-cell receptor (BCR) repertoire analysis. Detailed methods are available within the supplementary appendix.
Results
Between April of 2008 and November of 2016, 30 previously untreated and 30 R/R patients with iNHL were enrolled. Patient baseline demographics and clinical characteristics are shown in Supplementary Table 1. The data cutoff for this article was May 17, 2020. Median follow-up for the untreated and R/R cohorts were 62.7 months (range 9.0–110.2) and 100 months (range 4.4–140.0), respectively.
Untreated cohort
Safety
Of the 30 patients enrolled 28 (93%) experienced a treatment-related adverse event. Most common grade 3 and 4 toxicities included rash (27%), fatigue (20%), neutropenia (6.7%) and diarrhea (6.7%). Twenty-two patients (73%) had at least one 5 mg lenalidomide dose reduction or treatment interruption. The median time to first dose reduction was 2 months (range 1–12). Second and third 5 mg dose reductions were required in 7(23%) and 5 (17%) of patients, respectively. The median time to second and third dose reductions were 7 months (range 3–26) and 13.5 months (range 4–27), respectively. Four (13%) patients discontinued therapy due to adverse events Other reasons for therapy discontinuation included patient relocation (3.3%), patient preference (10%) and development of a second primary malignancy (3.3%).
One patient (3.3%) had a second primary malignancy and 2 (6.7%) had transformation to DLBCL. One patient developed base of the tongue SCC 16 months after lenalidomide/rituximab initiation.
Efficacy
Among the 27 evaluable patients with untreated iNHL, the ORR was 82% (IIT 77%). Eighteen (64.7%) patients had CR, 4 (14.8%) had PR, 4 (14.8%) had SD and 1 (3.7%) had PD. The median time to best response was 2 months (range 2 to 14 months). Median PFS was 94 months (95% CI: 20.4 to not reached [NR]) and median DR was 92 months (95% CI: 36.2-NR). In patients with disease progression, median TTNT was 97.8 months (95% CI: 63 to NR). Median overall survival was not reached at a median follow-up time of 62.7 months (Figure 1A). At the time of data cutoff, 15 (56%) patients remain in complete remission without further lines of systemic therapy, 10 (37%) of these patients remaining on continuous lenalidomide therapy.
Figure 1.
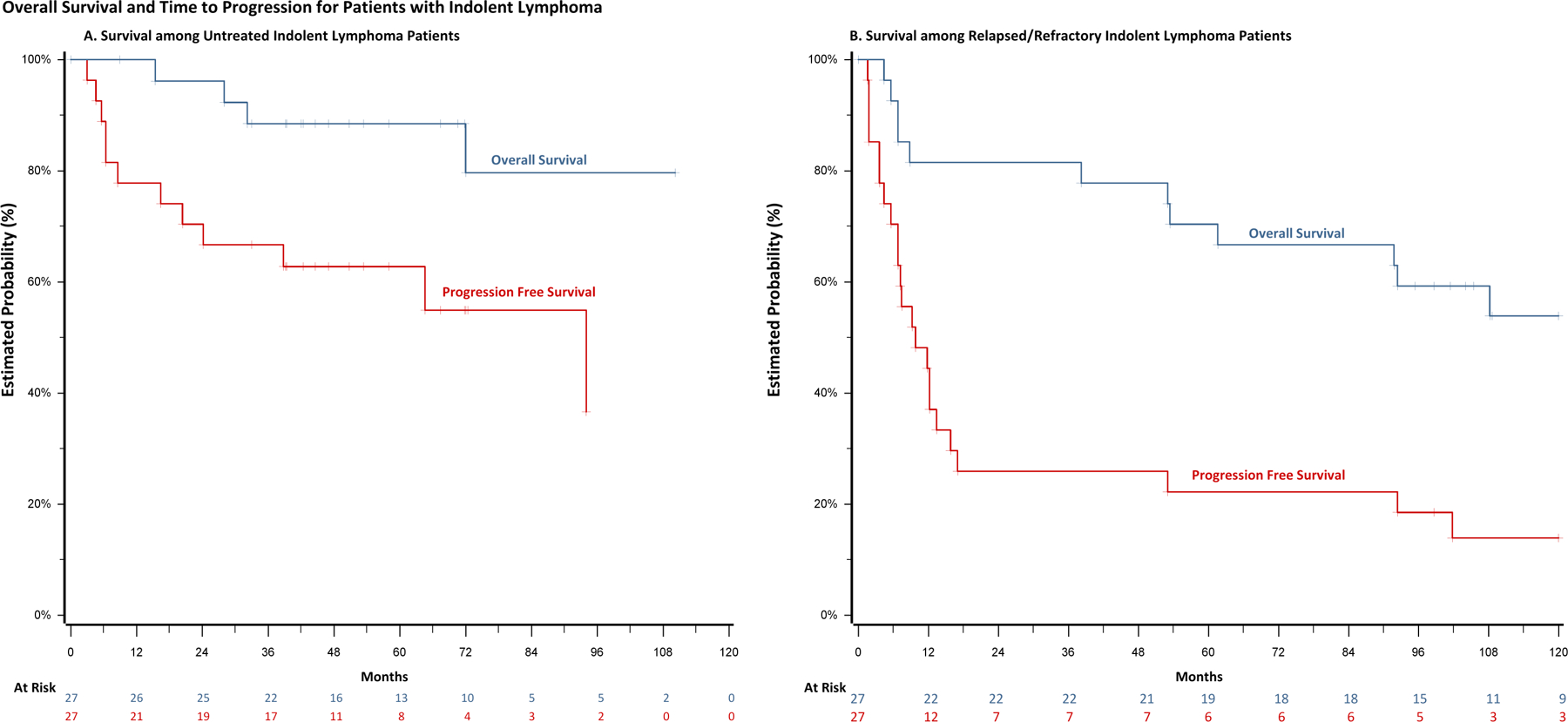
Kaplan-Meier estimates in patients treated with rituximab and lenalidomide. (A) Progression-free survival and overall survival in previously untreated cohort (B) Progression-free survival and overall survival in relapsed/refractory cohort
Relapsed/Refractory cohort
Safety
When lenalidomide 25 mg daily was initially administered on days 1–21 of a 28-day cycle, 2 of the first 4 patients (both with heavily pretreated and bulky disease) developed grade 3 tumor lysis syndrome within the first 2 weeks of therapy with lenalidomide alone. The protocol was subsequently amended to reduce the lenalidomide starting dose to 20 mg daily, and prophylaxis with allopurinol was initiated.
With 30 R/R patients, the most common grade 3 and 4 adverse events were lymphopenia (45%), neutropenia (55%), fatigue (23%) and hyponatremia (9%). Twenty patients (69%) had at least one 5 mg lenalidomide dose reduction or treatment interruption, mostly due to neutropenia or fatigue. The median time to first dose reduction was 1 month (range 1–49). Second and third 5 mg dose reductions were required in 10 (34%) and three (10%) patients, respectively. The median time to second and third dose reductions were 3 months (range 2–70) and 5 months (range 4–31), respectively. Six patients (20%) discontinued therapy due to adverse events that included neutropenia, hyperviscosity, rash, deep vein thrombosis, pulmonary and skin infections. 2 patients (6.7%) discontinued therapy due to relocation and 1 (3.3%) proceeded to transplant.
Two patients (6.7%) had secondary cancers (esophageal adenocarcinoma and myelodysplastic syndrome) and 2 (6.7%) had transformation to DLBCL. Mean duration of lenalidomide exposure was 7.5 months. Both patients with transformed disease had progression and subsequently received other systemic therapies prior to transformation.
Efficacy
Twenty-seven patients completed induction therapy with an ORR of 82%. (77% ITT). Eleven patients (40.7%) had CR, 11 (40.7%) had PR, 1 (3.7%) had SD and 4 (14.8%) had PD. The median time to best response was 2 months (range 2 to 12 months). The median PFS was 9.8 months (95% CI: 5.6 to 15.8) and median DR was 10.1 months (95% CI: 5.6–51.4). The 11 R/R patients who achieved CR remained in continuous remission on lenalidomide with a median duration of 87.3 months (range 16 to 141 months). In patients with disease progression, median TTNT was 28.2 months (95% CI: 8.8 to 56.2). At a median follow-up time of 100 months, the median overall survival was 140 months (95% CI: 53.4–140) (Figure 1B). At the time of data cutoff, 4 (15%) patients remain in complete remission without further lines of systemic therapy, 2 (7.4%) of these patients remaining on continuous lenalidomide therapy at 142 and 154 months.
Correlative Analyses
Samples were available from 34 patients (19 complete responders and 15 partial responders) for plasma cytokine analyses and 13 patients (8 complete responders and 5 partial responders) for lymphocyte flow cytometric analyses.
Plasma IFN-γ as a putative biomarker of response to therapy
Plasma cytokines were measured, and median values were graphed against time according to clinical response (Figure 2 and Supplementary Figure 1). Plasma IFN-γ stood out as being consistently higher at all time points in LTCR (Figure 2A). This difference was most pronounced at baseline (p <0.001) (Figure 2B). To quantitatively evaluate plasma IFN-γ concentrations over time, a mixed effects linear regression model was constructed using log cytokine concentration (pg/ml) as the dependent variable. In this model, time (as a categorical variable) and response to therapy were set as fixed effects and patient was set as random effect. The model revealed that there was a significant increase in IFN-γ concentration in LTCR compared to all other patients (p = 0.0025), which can also be appreciated when each time point was looked at individually (Figure 2A). Given this difference, IFN-γ was evaluated as a single analyte predictive classifier to assess its ability to identify LTCR. A receiver operator characteristic (ROC) curve was constructed and area under the curve (AUC) for D0 was calculated which revealed that elevated IFN-γ γ could distinguish LTCR from all other patients (AUC: 0.81) (Figure 2C). With regards to relative risk (RR), patients with IFN-γ plasma concentrations in the upper quartile had a 3.0 fold increase probability of being a LTCR (95% CI 1.35–6.68) and the RR was 2.5 (95% CI 1.17 – 5.34) for D30 IFN-γ plasma concentrations in the upper quartile.
Figure 2.
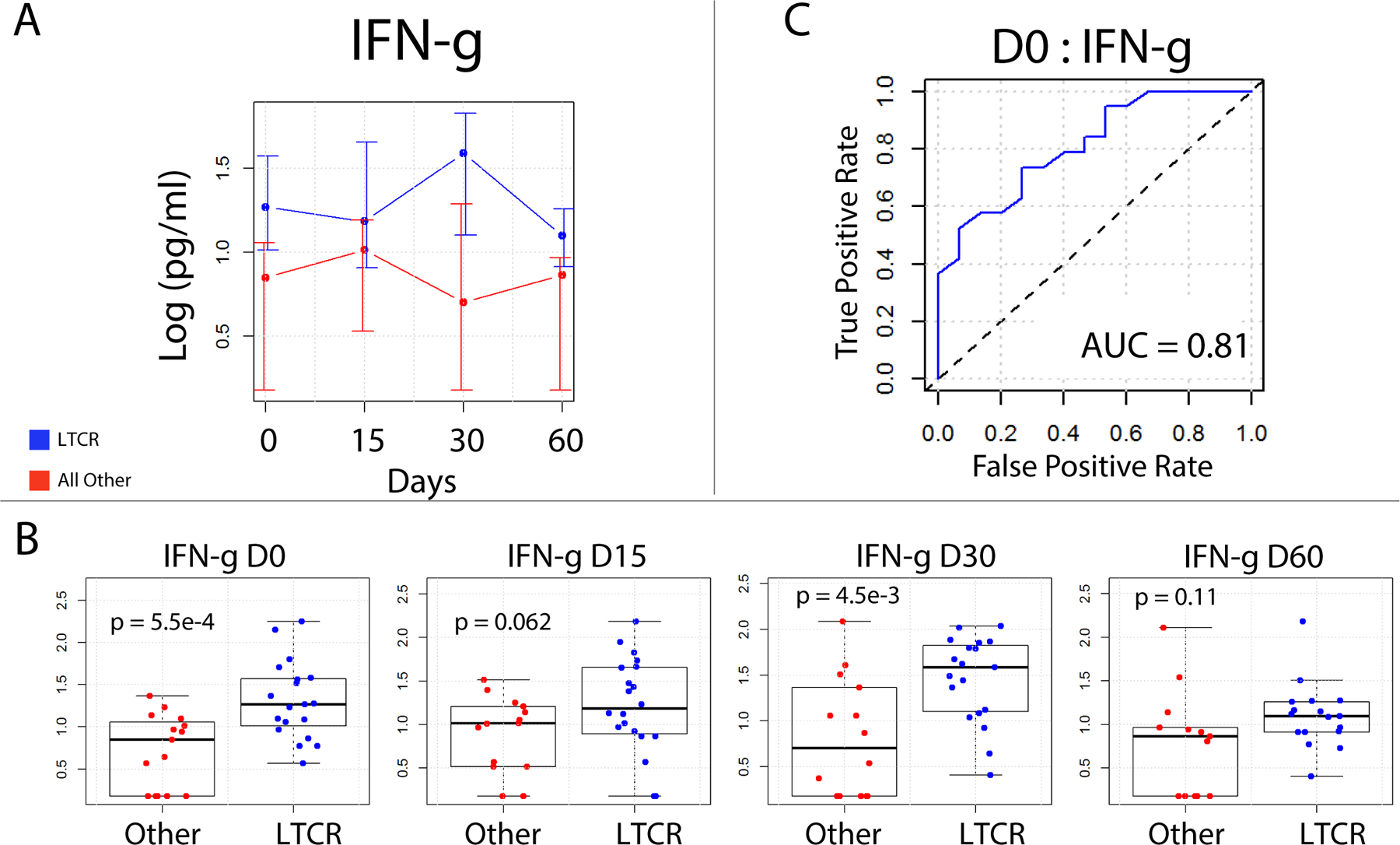
IFN-γ Long term complete responder (LTCR) (blue) versus all others (red) - Plasma was assayed for IFN-γ using the luminex platform. A) Line graph of plasma IFN-γ over time reveals that LTCR tend to have higher plasma IFN-ˠ. Displayed are log median values with error bars indicating 75th and 25th percentiles. B) Individual patient values presented as Box whisker plots over time. The IFN-γ difference between LTCRand all others was most significant on Days 0 and 30, Unpaired Student’s t test. C) Receiver operator characteristic curve for baseline IFN-ˠ values, complete responders versus partial responders. An AUC of 0.81 demonstrates the utility of baseline IFN-ˠ plasma levels as a classifier to identify complete responders from partial responders.
Although no other cytokine was significantly associated with LTCR, a linear mixed effects model demonstrated that CXCL-10 (p = 6.2e-8), GM-CSF (p = 1.7e-5), TNF (p < 2e-16), IL-2 (p = 2.4e-13), IL-8 (p = 1.5e-5) and IL-10 (p < 2e-16) plasma concentrations were significantly altered by therapy. Specifically, following the initiation of therapy, the plasma concentrations of these cytokines increased on D15 and then declined thereafter (Supplementary Figure 1 and Table 2).
Peripheral B cells predict response to therapy
Rituximab-mediated B cell depletion (BCD) has been used as a surrogate for assessing in vivo ADCC. A previous study indicated that the percentage of B cells (compared to the total number of lymphocytes) at baseline could successfully predict responders among R/R B-cell lymphoma patients treated with rituximab and ipilimumab (16). We thus sought to evaluate baseline B-cell percentages and subsequent rituximab-mediated BCD for their ability to predict LTCR. B cells were calculated as the percentage of live CD3- lymphocytes that were CD19 positive. Gating strategies used to identify all cell populations is shown in Supplementary Figure 2. Log B-cell (+/- SD) values were graphed over time and a mixed effects linear regression model was constructed. As expected, this model revealed that peripheral B cells declined over time (p = 1.7e-4). There was also a consistent difference in B-cell percentages between LTCR and all other patients, with the difference being most significant on Day 30, (FC= 2.4, p = 0.01) (Figure 3A and B). In general, responders had fewer B cells and less of a reduction in B cell numbers following administration of rituximab. To evaluate B-cell percentages as a method to predict LTCR, ROC curves were constructed, and AUC values were calculated for each time point. This analysis revealed that B-cell percentages at baseline and on Day 30 could distinguish LTCR from all other responses (AUC 0.72 and 0.92, respectively), with LTCR being associated with lower B cell percentages (Figure 3C).
Figure 3.
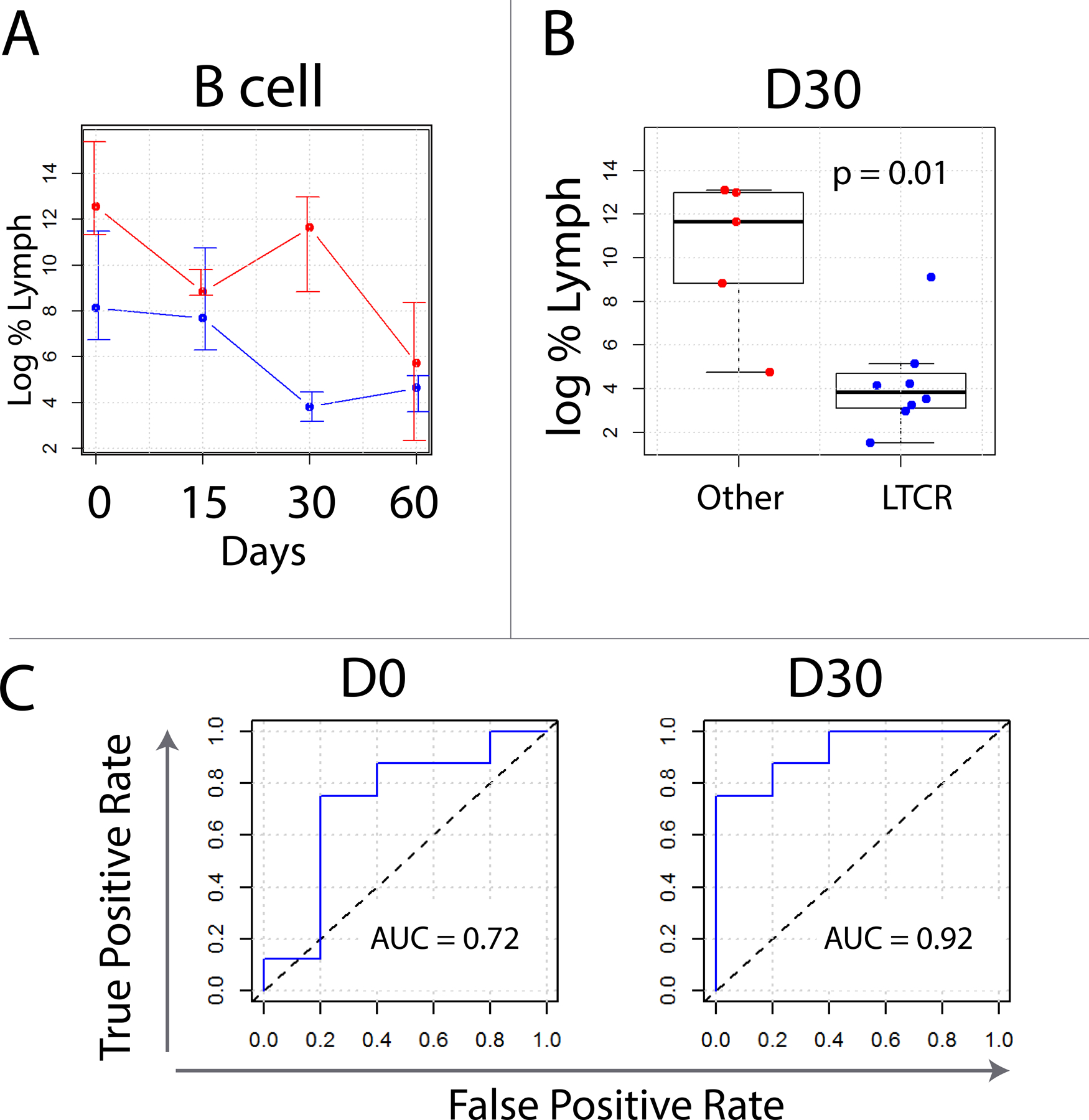
Flow cytometric analysis of B cells in LTCR (blue) versus all other patients (red). (A) Log of median values with error bars indicating 75th and 25th percentiles. Non-LTCR tended to have both more B cells than LTCR as well as less of a Day 30 reduction in B cells following rituximab. Flow gating strategy for these experiments can be found in Supplementary Figure 2. (C) Box whisker plots of quantified B cells on Day 30. When compared to partial responders, complete responders tended to have fewer B cells. (D) Receiver operator characteristic curves constructed for B cells number as a classifier to distinguish LTCR from all others. Area under the curve values are calculated for Days 0 and 30.
The effects of rituximab lenalidomide combination therapy on other immune cell populations
Various immune populations have been implicated in immune recognition of cancer and response to immunotherapy (17,18). Thus, we next sought to more comprehensively evaluate immune cell populations by flow cytometry. For each immune subset log transformed percentages (+/- SD) were graphed against time (Supplemental Figure 3). To evaluate the repeat measure data, a mixed effects linear regression model was constructed (see methods). This revealed that rituximab/lenalidomide therapy also altered the percentages of IFN-γ+ CD4+ T cells, PD1+ CD8+ T cells, and GranB+ CD8+ T cells (p = 8.1e-5, 6.4e-4, and 1.0e-4, respectively; Supplemental Figure 3). Specifically, IFN-γ+ CD4+ T cells and PD1+ CD8+ T cells initially increased, peaking on day D15 and then slowly declined thereafter. Similarly, GranB+ CD8+ T cells steadily increased until Day 30 and then declined.
Therapy-associated alterations in CD107a+ CD8 T cells (p = 5.7e-3), CD107a+ iNKT cells (p = 6.3e-3), CD107a+ NKT cells (p = 1.4e-2), Tfh cells (p = 2.0e-2), CD107a+ NK cells (p = 2.4e-2), NK cells (p = 2.7e-2), iNKT cells (p = 0.3e-2), Tregs (p = 3.1e-2), and GranB+ NK cells (p = 4.6e-2) were also noted (Supplemental Figure 3). Specifically, in response to therapy CD107a+ iNKT cells, CD107a+ NKT cells, CD107a+ NK cells, NK cells, iNKT cells, Tregs, GranB+ NK cells, all increased on D15 and then decreased thereafter (Supplemental Figure 3). Similarly, CD107a+ CD8 T cells continued to increased until D30 and then declined (Supplemental Figure 3). Other cell types were not significantly altered by therapy (p > 0.05, Supplementary Table 3). Finally, the ratio of CD4+ to CD8+ T cells was not significantly altered by therapy (p > 0.05, Supplementary Table 2). With respect to intracellular cytokine concentrations, the amount of IFN-γ per CD4+ T cell continued to rise to Day 60 after therapy (p = 5.6e-8, Supplemental Table 3).
T cells and GranB+ cytotoxic T cells can predict response to therapy
Immune cells such as cytotoxic T cells play a central role in cancer immunosurveillance. We thus sought to characterize how LTCR differed from all other patients with respect to these immune subpopulations. For this analysis immune cell percentages (of total lymphocytes) (+/- SD) were graphed over time and a mixed effects linear regression model was constructed as described in the methods. The mixed effects linear regression model revealed that LTCR differed significantly from all other patients with respect to T cell and GranB+ CD8+ T cell percentages, which were both increased in LTCR at all time points (p = 5.3e-4 and p = 2.5e-3, respectively) (Figure 4A and B). GranB+ NK cells were also notably increased (p = 0.017). (Supplemental Table 3). To determine if T cell and GranB+ CD8+ T cell percentages have utility as LTCR predictive classifiers, receiver operator characteristic curves were constructed and area under the curve (AUC) values were calculated for each time point. This analysis revealed that the percentage of live T cells and GranB+ CD8+ T cells within the lymphocyte gate could predict LTCR at days 15, 30, and 60 (AUC = 0.92 and 0.83, D15; AUC = 1.0 and 1.0, D30; and AUC = 1.0 and 1.0, D60, respectively) (Figure 4C).Further analysis of patients with FL versus all other histologies demonstrated that the predictive potential of the biomarkers was not altered.
Figure 4.
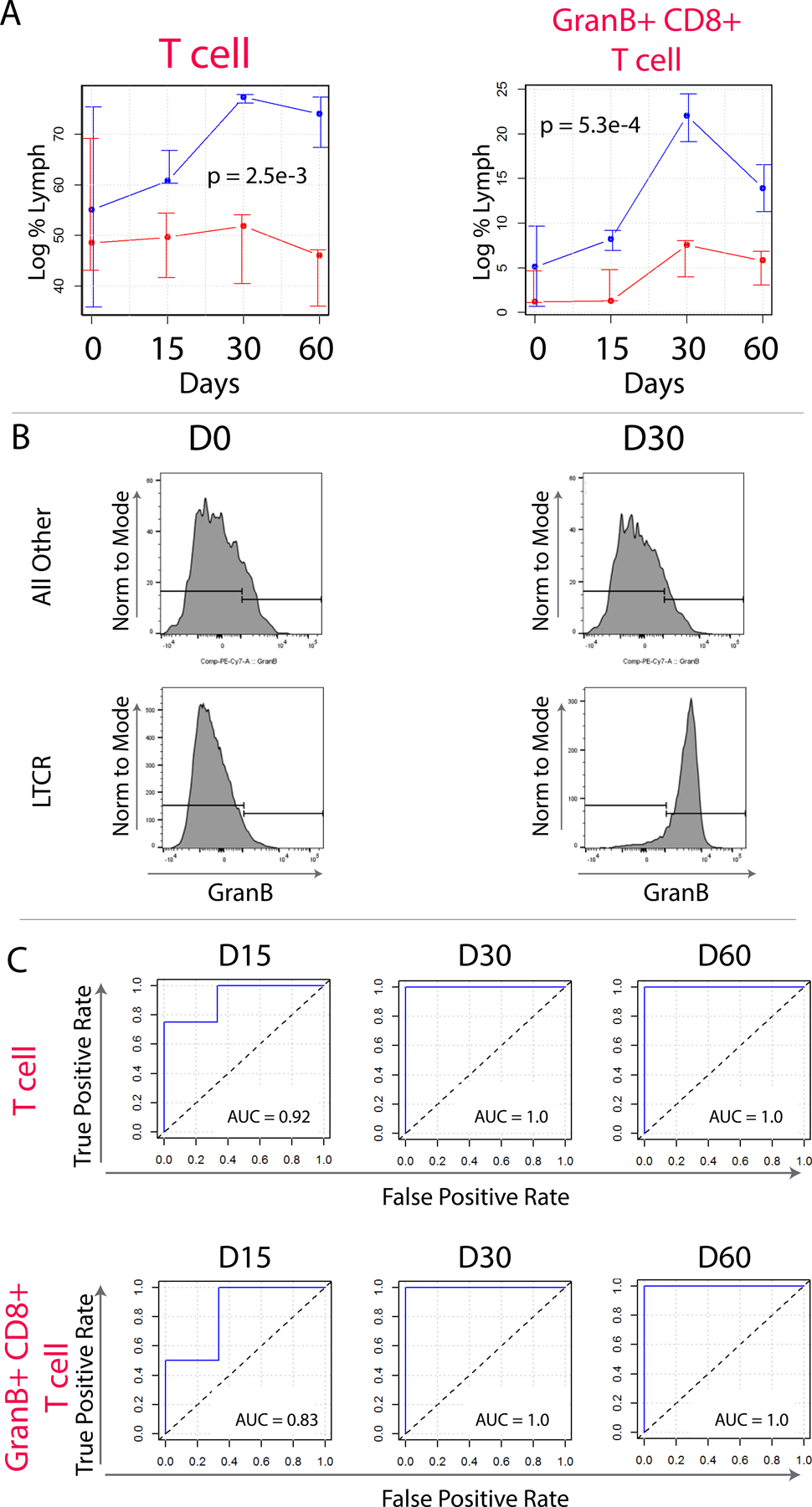
Flow cytometric analysis of T cell subpopulations in LTCR (blue) versus all others (red). (A) median of log median values with error bars indicating 75th and 25th percentiles. Significant differences between LTCR and all others were detected in T cells, and GranB+ CD8+ T cells. Flow cytometry gating strategy used to quantify T cell subpopulations. Is shown in Supplemental Figure 2. (B) Representative dot plot showing the increase in intracellular staining for IFN-α in CD8+ T cells. Responders tended to have more IFN-ˠ-secreting CD8+ T cells at baseline and on day 8 of therapy. (D) Box whisker plots of IFN-ˠ-secreting T cells. Complete responders tended to have more CD8+ IFN-ˠ-secreting T cells on days 0 and 8 when compared to partial responders. Most significant days are also shown for differential expression of CD4+ effector T cells and CD8+ effector memory T cells.
Transcriptomic analysis of LTCR identifies differentially expressed genes
This trial of rituximab lenalidomide combination therapy yielded a cohort of LTCR patients. Thus, it was of interest to characterize the molecular patterns in LTCRs and how they differed from all other patients. To identify differentially expressed genes between these two groups, RNA-Seq of pretreatment biopsies was performed. This analysis revealed a variety of differentially expressed genes (Figure 5A, Supplementary Table 4). Although gene enrichment analysis failed to significantly associate any biological pathways (e.g. KEGG, Reactome, and GO Biological Process) to LTCR patients, several differentially expressed genes were of particular interest. These included two metabolic genes (HMGCS2 and DDC) and G6PC that were strongly downregulated in lymphoma of LTCR (FC<0.001, FDR = 1.2e-16, 2.2e-16, and 2.7e-13, respectively). Of relevance to innate and adaptive immunity, a variety of immunomodulatory genes were also strongly downregulated in lymphoma of LTCR, including the chemokines CXCL5 and CXCL8 (FC<0.001, FDR = 1.3e-5 and 3.0e-5, respectively); IDO2 (FC<0.001, FDR = 7.8e-3), which encodes an immune regulatory enzyme; and DEFA6 (FC<0.001, FDR = 2.2e-9), which encodes the antimicrobial-encoding peptide Defensin Alpha 6. Principal component analysis and hierarchical clustering based on differentially expressed genes failed to separate LTCR patients from all others (Figure 5B and C). Recent studies have identified 26 gene expression classifier, which has been reported to separate diffuse large B-cell lymphoma (DLBCL) patients into subgroup A (low immune) and subgroup B (immune-high)(19), While this has only been validated in DLBCL we sought to assess this panel for a signal in indolent lymphoma however this also failed to cluster LTCR from patients that obtained a PR (Figure 5D).
Figure 5.
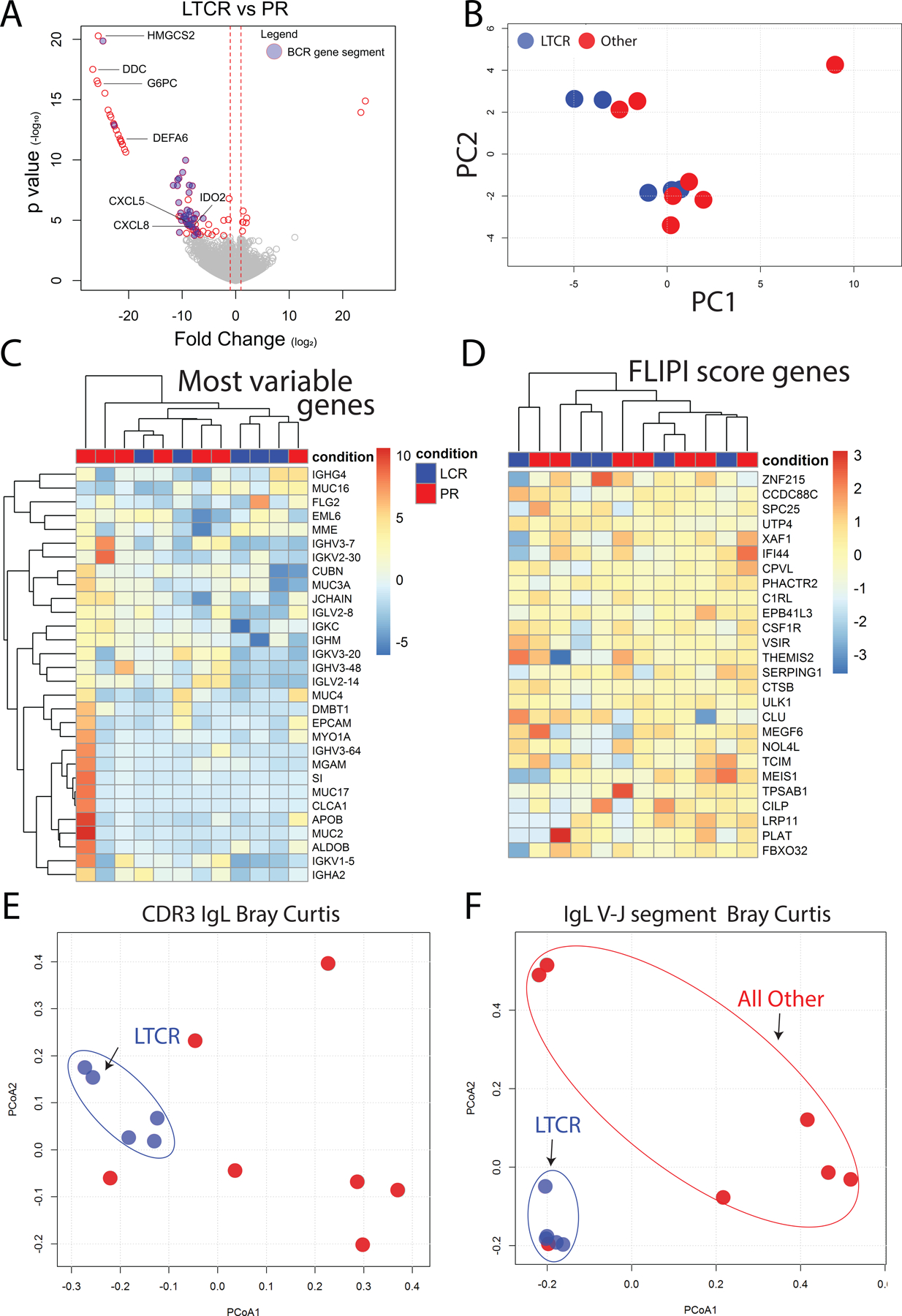
RNA-Seq analysis of pretreatment lymphoma. (A) Volcano plot of differentially expressed genes between long-term complete responders and partial responders (for complete list see Supplemental Table 3). Blue circles indicated differentially expressed BCR gene segments. (B) Principal component analysis based on RNA-Seq gene expression fails to separate long-term complete responders and partial responders. (C) Hierarchical clustering of patients based on the 30 most differentially expressed genes. Long-term complete responders are shown in blue and partial responders in red. For each gene, the intensity of color represents gene expression with red representing higher expression and blue representing lower expression. (D) Hierarchical clustering of patients based on the previously identified 26 genes that separate diffuse large B cell lymphoma patients into “low immune” and “immune-high” subgroups. Again, there is no separation of long-term complete responders and partial responders (E) To visualize BCR repertoire similarity between LTCR and partial responders a PCoA plot of CDR3 Bray Curtis distances of Ig light chains was constructed. Blue circles represent LTCR. (F) PCoA plot of Bray Curtis distances of V-J Ig light chain gene segment usage, again revealing that LTCR cluster together.
Intratumoral B cell repertoire analysis
In addition to a clonal population of malignant B cells, B cell lymphomas are comprised of a heterogeneous population of cells, including non-malignant immune cells. Since flow cytometric analysis of peripheral blood revealed that the percentage of live B cells within the lymphocyte gate could successfully identify LTCR patients (Figure 3C), it was of interest to determine how dissimilar the tumor-infiltrating B cell repertoires of LTCR patients were from all other patient response groups. Also, 32 BCR gene segments were differentially expressed in pretreatment tumor biopsies of LTCR versus all other diagnostic groups (Figure 5A), Thus, BCR sequences were extracted from lymphoma RNA-Seq data and the similarity in CDR3 sequences and V/J gene segment usage between each patient was quantified by calculating the Bray-Curtis dissimilarity (an ecological measure of beta diversity). Principal coordinates analysis (PCoA) plots were then used to visualize this data (Figure 5E and F). The close clustering of LTCR indicates that LTCR intra-tumoral B cell repertoires are more similar with respect to shared CDR3 sequences or BCR gene segment usage, when compared to other response groups.
Discussion
Rituximab plus continuous lenalidomide is effective and associated with acceptable toxicity in patients with both previously untreated and R/R advanced stage iNHL. This regimen produces durable remissions, even in previously treated patients, with some R/R patients having responses lasting greater than 10 years.
While the majority of patients with NHL initially respond to immuno-chemotherapy, most eventually relapse. Resistance and intolerance to chemotherapy increases over time, making the development of well-tolerated non-chemotherapeutic strategies for iNHL of high importance. Many attempts to improve the effectiveness of antibody-based CD20-targeted therapeutics have focused on strategies to enhance host immune effector mechanisms particularly in patients that are considered refractory to anti-CD20-based therapy.
The efficacy of rituximab and lenalidomide combination in both untreated and R/R iNHL has been previously reported in multiple studies (9,10,20–24). The RELEVANCE trial showed rituximab/lenalidomide to have similar responses compared to rituximab/chemotherapy in patients with untreated follicular lymphoma but associated with less toxicity (9). AUGMENT was another trial which showed rituximab/lenalidomide to be superior to rituximab/placebo in patients with R/R follicular lymphoma or MZL (10). Our study, with the longest reported median follow-up of 62.7 months (range 9.0–110.2) for the untreated cohort and 100.0 months (range 4.4–140.0) for R/R, observed ORR of 82% in the untreated cohort and 82% in the R/R cohort with CR rates of 67% and 41%, respectively. Although our study observed comparable response rates, differences in trial design and length of follow-up limit the usefulness of comparisons among these studies. For example, our trial was designed with continuous lenalidomide until disease progression or unacceptable toxicity compared to RELEVANCE and AUGMENT trials which continued lenalidomide for only 18 or 12 months respectively. Moreover, the long-term follow-up provided a patient population that might allow for the identification of biomarkers predictive of very durable remissions, as well as the identification of late effects, including second primary malignancies.
The safety profile of rituximab/lenalidomide in our study was consistent with that of previous studies (9,10,23,24). Over 90% of patients in both cohorts had at least one adverse event and approximately 70% of patients in both cohorts required at least one 5 mg lenalidomide dose reduction or dose interruption, mainly due to neutropenia, lymphopenia, rash and fatigue. Four (13%) and 6 (20%) untreated and R/R iNHL patients discontinued therapy due to adverse events, respectively. The regimen continues to show tolerability with most adverse events being predictable and manageable (25). The incidence of second malignancies in previously treated and R/R cohorts were 3% and 7% respectively which seems consistent with or lower than what has been previously reported (26).
We performed a variety of correlative studies with the goal of identifying biomarkers that could predict LTCR patients. Of the plasma cytokines that were monitored, only IFN-was significantly elevated in LTCR patients. Lenalidomide-mediated IFN-γ production has been observed in previous studies (7,8,27), however in our study the difference in IFN-γ production was most prominent at baseline which has potential clinical significance because it could predict response to therapy.
ADCC is a well-described lymphomacidal mechanism of rituximab and peripheral blood BCD is a known consequence. We hypothesized and previously described the utility of peripheral blood BCD as a surrogate marker for ADCC-mediated anti-lymphoma response (16). In addition, lenalidomide has been shown to enhance ADCC in murine lymphoma models (8,30–33). Indeed, responders tended to have fewer peripheral B cells at baseline and a greater treatment-induced BCD, both of which could predict response to therapy. Specifically, the percentage of live B cells on Day 0 and 30 within the lymphocyte gate could predict LTCR. While lenalidomide has independent B cell depleting effects that may confound interpretation, a previous randomized analysis supports the predictive value of rituximab-mediated BCD (16).
Previous studies have characterized T and NK cells responses to cancer but there are few prospective studies investigating their utility as predictive biomarkers of LTCR (8,27,34–37). GranB, the T-cell and NK-cell effector molecule, has been highly implicated in anti-tumor responses and mechanisms that block GranB are often utilized by cancers to circumvent immune recognition (38). Our current study demonstrates that LTCR tended to have more T cells and GranB+ CD8+ T cells when compared to all other response groups. ROC curves demonstrated the clinical utility of this finding by showing that on days 15, 30, and 60 these differences could classify LTCR from all others. Interestingly, a connection between lenalidomide and granzyme B has been previously reported in an in vitro multiple myeloma model (39).
Similar to the treatment-induced expansion of IFN-γ+ CD4+ T cells reported here, when the PVX-410 vaccine was tested with and without lenalidomide in patients with smoldering multiple myeloma, a similar increase in tetramer-positive IFN-γ + CD8+ T cells was noted in the lenalidomide group (40). Similarly, upregulation of IFN-γ and Th1 immunity was noted in chronic lymphocytic leukemia patients responding to lenalidomide (41).
RNA-Seq analysis of pretreatment tumors revealed a variety of differences between LTCR patients and all others. Of particular interest, the expression of IDO2 was significantly lower in LTCR patients. IDO is involved in tryptophan catabolism. Its upregulation supports immune tolerance and high expression of IDO can limit T cell responses. The differential expression of IDO in LTCR patients is of high relevance as previous studies have demonstrated that 1) IDO expression is one mechanism of tumoral immune escape and 2) that tumoral expression of IDO can predict responses to CHOP-R (42–44). Apart from IDO2, a variety of other immune genes were also downregulated. Of these CXCL8 has been associated DLBCL progression (45).
Using a randomized study design we have previously reported that for patients with R/R NHL, B cell percentage (of total lymphocytes) in peripheral blood at baseline as well as subsequent BCD could successfully classify complete responders (16), a finding that is consistent with our current study (Figure 3). Also, RNA-Seq analysis revealed that 32 BCR gene segments were differentially expressed in LTCR patients when compared to all other patients. Furthermore, PCoA plots of Bray Curtis dissimilarity revealed clustering of LTCR, which indicates similarities in their BCR CDR3 sequences and their BCR V and J gene segment usage. Thus, like the flow cytometric analysis of peripheral blood B cells, there are differences in tumor-infiltrating B cells between LTCR and all other patients. In the future we hope to further develop and validate classifiers capable of identifying advanced B-cell lymphoma patients likely to respond to rituximab-based regimens. Thus far, predictive classifiers based upon non-malignant B-cell percentages, IFN-γ and other immune cell populations have shown great promise.
A potential limitation of this study is the small sample size of each cohort and the lack of a comparator group that did not receive lenalidomide. However, our trial was not designed to specifically address efficacy in comparison with other treatment regimens but rather highlight long-term outcomes of patients treated with this combination as well as an exploratory analysis to identify predictive biomarkers. While the predictive potential of these biomarkers were consistent when FL patients were assessed independently, given the relatively small sample size, further studies are needed to determine the reproducibility of correlative analyses but it is important to note the clinical utility of BCD as a surrogate for response to therapy as described herein has now been demonstrated in two unrelated trials even with heterogenous NHL subtypes (16). Identifying predictive biomarkers such as those described herein remains an important endeavor to advance the field of precision medicine.
In conclusion, the combination of rituximab and lenalidomide followed by continuous lenalidomide is effective and has manageable toxicity in patients with both previously untreated and R/R advanced stage iNHL that remain on long-term lenalidomide therapy. With long term-follow up, this regimen continues to demonstrate durable remissions, even in heavily pretreated patients, with some lasting greater than 10 years. Increased IFN-γ and BCD were identified as potential predictors of LTCR and should be further validated in future studies.
Supplementary Material
Translational Relevance.
While validation in randomized trials will be required, this study supports our hypothesis that continuous lenalidomide may increase the durability of response without a significant increase in toxicity. Based on the demonstrated ability of lenalidomide to enhance antibody-dependent cell mediated cytotoxicity (ADCC) we used BCD as a surrogate biomarker of lenalidomide enhanced, rituximab-mediated ADCC which predicted LTCR in a significant number of patients. Similar to previous studies GranB+, CD8+ T cells and plasma IFN-γ also predicted CR which translated into LTCR. Intra-tumoral B cell repertoire analysis generated a unique finding that identified repertoire clustering in LTCR. While the long-term follow-up of this study allowed for assessment of the durability of responses, it also facilitated the identification of biomarkers that may predict LTCR all of which require validation in larger clinical trials
Funding:
This study was supported by research funding from Celgene and by NCI-P30CA09337. EM was supported by (K24 AR077313–02).
Footnotes
Declarations:
Ethics approval and consent to participate
This study was carried out in compliance with the protocol and Good Clinical Practice, as described in the International Council for Harmonisation Harmonized Tripartite Guidelines for Good Clinical Practice 1996 and the Declaration of Helsinki, concerning medial research in humans. This study was reviewed and approved by a properly constituted Institutional Review Board of the University of California, Davis. IRB Number is 876213. Written informed consent was obtained from every study participant
Consent for publication: Not applicable
Availability of data and materials:
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflict-of-interest disclosure: J.M.T. recieved research funding from Celgene, Novartis, Spectrum, and Takada. J.M.T., received honoraria Seattle Genetics. BAJ: Consulting/advising – AbbVie, Amgen, Celgene, Genentech, GlycoMimetics, Jazz, Takeda, Tolero, Treadwell; Travel reimbursement – AbbVie, Amgen; Grant/research support to institution – 47, AbbVie, Accelerated Medical Diagnostics, Amgen, AROG, Celgene, Daiichi Sankyo, Esanex, F. Hoffmann-La Roche, Forma, Genentech/Roche, GlycoMimetics, Hanmi, Incyte, Jazz, LP Therapeutics, Pfizer, Pharmacyclics, Sigma Tau. CP: Acrotech: HonorariaAB, AR and PK declare no competing interests.
References:
- 1.Gribben JG. How I treat indolent lymphoma. Blood 2007;109(11):4617–26 doi 10.1182/blood-2006-10-041863. [DOI] [PubMed] [Google Scholar]
- 2.Sousou T, Friedberg J. Rituximab in indolent lymphomas. Seminars in hematology 2010;47(2):133–42 doi 10.1053/j.seminhematol.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiner GJ. Rituximab: mechanism of action. Semin Hematol 2010;47(2):115–23 doi 10.1053/j.seminhematol.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zahavi D, AlDeghaither D, O’Connell A, Weiner LM. Enhancing antibody-dependent cell-mediated cytotoxicity: a strategy for improving antibody-based immunotherapy. Antibody Therapeutics 2018;1(1):7–12 doi 10.1093/abt/tby002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arora M, Gowda S, Tuscano J. A comprehensive review of lenalidomide in B-cell non-Hodgkin lymphoma. Ther Adv Hematol 2016;7(4):209–21 doi 10.1177/2040620716652861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramsay AG, Clear AJ, Kelly G, Fatah R, Matthews J, Macdougall F, et al. Follicular lymphoma cells induce T-cell immunologic synapse dysfunction that can be repaired with lenalidomide: implications for the tumor microenvironment and immunotherapy. Blood 2009;114(21):4713–20 doi 10.1182/blood-2009-04-217687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramsay AG, Johnson AJ, Lee AM, Gorgün G, Le Dieu R, Blum W, et al. Chronic lymphocytic leukemia T cells show impaired immunological synapse formation that can be reversed with an immunomodulating drug. J Clin Invest 2008;118(7):2427–37 doi 10.1172/jci35017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu L, Adams M, Carter T, Chen R, Muller G, Stirling D, et al. lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ tumor cells. Clin Cancer Res 2008;14(14):4650–7 doi 10.1158/1078-0432.Ccr-07-4405. [DOI] [PubMed] [Google Scholar]
- 9.Morschhauser F, Fowler NH, Feugier P, Bouabdallah R, Tilly H, Palomba ML, et al. Rituximab plus Lenalidomide in Advanced Untreated Follicular Lymphoma. N Engl J Med 2018;379(10):934–47 doi 10.1056/NEJMoa1805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leonard JP, Trneny M, Izutsu K, Fowler NH, Hong X, Zhu J, et al. AUGMENT: A Phase III Study of Lenalidomide Plus Rituximab Versus Placebo Plus Rituximab in Relapsed or Refractory Indolent Lymphoma. J Clin Oncol 2019;37(14):1188–99 doi 10.1200/jco.19.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brice P, Bastion Y, Lepage E, Brousse N, Haïoun C, Moreau P, et al. Comparison in low-tumor-burden follicular lymphomas between an initial no-treatment policy, prednimustine, or interferon alfa: a randomized study from the Groupe d’Etude des Lymphomes Folliculaires. Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol 1997;15(3):1110–7 doi 10.1200/jco.1997.15.3.1110. [DOI] [PubMed] [Google Scholar]
- 12.Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol 1999;17(4):1244 doi 10.1200/jco.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 13.Simon R Optimal two-stage designs for phase II clinical trials. Control Clin Trials 1989;10(1):1–10 doi 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 14.Clark TG, Bradburn MJ, Love SB, Altman DG. Survival analysis part I: basic concepts and first analyses. Br J Cancer 2003;89(2):232–8 doi 10.1038/sj.bjc.6601118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials 1996;17(4):343–6 doi 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 16.Tuscano JM, Maverakis E, Groshen S, Tsao-Wei D, Luxardi G, Merleev AA, et al. A Phase I Study of the Combination of Rituximab and Ipilimumab in Patients with Relapsed/Refractory B-Cell Lymphoma. Clin Cancer Res 2019;25(23):7004–13 doi 10.1158/1078-0432.Ccr-19-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005;105(8):3051–7 doi 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 18.Degli-Esposti MA, Smyth MJ. Close encounters of different kinds: dendritic cells and NK cells take centre stage. Nat Rev Immunol 2005;5(2):112–24 doi 10.1038/nri1549. [DOI] [PubMed] [Google Scholar]
- 19.Al-Khenaizan S Lichen planus occurring after hepatitis B vaccination: a new case. J Am Acad Dermatol 2001;45(4):614–5 doi 10.1067/mjd.2001.114590. [DOI] [PubMed] [Google Scholar]
- 20.Tuscano JM, Dutia M, Chee K, Brunson A, Reed-Pease C, Abedi M, et al. Lenalidomide plus rituximab can produce durable clinical responses in patients with relapsed or refractory, indolent non-Hodgkin lymphoma. Br J Haematol 2014;165(3):375–81 doi 10.1111/bjh.12755. [DOI] [PubMed] [Google Scholar]
- 21.Kiesewetter B, Willenbacher E, Willenbacher W, Egle A, Neumeister P, Voskova D, et al. A phase 2 study of rituximab plus lenalidomide for mucosa-associated lymphoid tissue lymphoma. Blood 2017;129(3):383–5 doi 10.1182/blood-2016-06-720599. [DOI] [PubMed] [Google Scholar]
- 22.Zucca E, Rondeau S, Vanazzi A, Østenstad B, Mey UJM, Rauch D, et al. Short regimen of rituximab plus lenalidomide in follicular lymphoma patients in need of first-line therapy. Blood 2019;134(4):353–62 doi 10.1182/blood-2018-10-879643. [DOI] [PubMed] [Google Scholar]
- 23.Martin P, Jung SH, Pitcher B, Bartlett NL, Blum KA, Shea T, et al. A phase II trial of lenalidomide plus rituximab in previously untreated follicular non-Hodgkin’s lymphoma (NHL): CALGB 50803 (Alliance). Ann Oncol 2017;28(11):2806–12 doi 10.1093/annonc/mdx496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Becnel MR, Nastoupil LJ, Samaniego F, Davis RE, You MJ, Green M, et al. Lenalidomide plus rituximab (R(2) ) in previously untreated marginal zone lymphoma: subgroup analysis and long-term follow-up of an open-label phase 2 trial. Br J Haematol 2019;185(5):874–82 doi 10.1111/bjh.15843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheson BD, Morschhauser F, Martin P. Management of Adverse Events From the Combination of Rituximab and Lenalidomide in the Treatment of Patients With Follicular and Low-Grade Non-Hodgkin Lymphoma. Clinical Lymphoma Myeloma and Leukemia 2020. doi 10.1016/j.clml.2020.03.009. [DOI] [PubMed]
- 26.Mozas P, Nadeu F, Rivas-Delgado A, Rivero A, Garrote M et al. Patterns of change in treatment, response, and outcome in patients with follicular lymphoma over the last four decades: a single-center experience. Blood Cancer Journal 2020;10(31):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gandhi AK, Kang J, Havens CG, Conklin T, Ning Y, Wu L, et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN.). Br J Haematol 2014;164(6):811–21 doi 10.1111/bjh.12708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol 2004;75(2):163–89 doi 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 29.Street SE, Trapani JA, MacGregor D, Smyth MJ. Suppression of lymphoma and epithelial malignancies effected by interferon gamma. J Exp Med 2002;196(1):129–34 doi 10.1084/jem.20020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reddy N, Hernandez-Ilizaliturri FJ, Deeb G, Roth M, Vaughn M, Knight J, et al. Immunomodulatory drugs stimulate natural killer-cell function, alter cytokine production by dendritic cells, and inhibit angiogenesis enhancing the anti-tumour activity of rituximab in vivo. Br J Haematol 2008;140(1):36–45 doi 10.1111/j.1365-2141.2007.06841.x. [DOI] [PubMed] [Google Scholar]
- 31.Hernandez-Ilizaliturri FJ, Reddy N, Holkova B, Ottman E, Czuczman MS. Immunomodulatory drug CC-5013 or CC-4047 and rituximab enhance antitumor activity in a severe combined immunodeficient mouse lymphoma model. Clin Cancer Res 2005;11(16):5984–92 doi 10.1158/1078-0432.Ccr-05-0577. [DOI] [PubMed] [Google Scholar]
- 32.Zhang L, Qian Z, Cai Z, Sun L, Wang H, Bartlett JB, et al. Synergistic antitumor effects of lenalidomide and rituximab on mantle cell lymphoma in vitro and in vivo. Am J Hematol 2009;84(9):553–9 doi 10.1002/ajh.21468. [DOI] [PubMed] [Google Scholar]
- 33.Gribben JG, Fowler N, Morschhauser F. Mechanisms of Action of Lenalidomide in B-Cell Non-Hodgkin Lymphoma. J Clin Oncol 2015;33(25):2803–11 doi 10.1200/jco.2014.59.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schafer PH, Gandhi AK, Loveland MA, Chen RS, Man HW, Schnetkamp PP, et al. Enhancement of cytokine production and AP-1 transcriptional activity in T cells by thalidomide-related immunomodulatory drugs. J Pharmacol Exp Ther 2003;305(3):1222–32 doi 10.1124/jpet.102.048496. [DOI] [PubMed] [Google Scholar]
- 35.Yang Y, Shaffer AL 3rd, Emre NC, Ceribelli M, Zhang M, Wright G, et al. Exploiting synthetic lethality for the therapy of ABC diffuse large B cell lymphoma. Cancer Cell 2012;21(6):723–37 doi 10.1016/j.ccr.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang LH, Kosek J, Wang M, Heise C, Schafer PH, Chopra R. Lenalidomide efficacy in activated B-cell-like subtype diffuse large B-cell lymphoma is dependent upon IRF4 and cereblon expression. Br J Haematol 2013;160(4):487–502 doi 10.1111/bjh.12172. [DOI] [PubMed] [Google Scholar]
- 37.Hagner PR, Chiu H, Ortiz M, Apollonio B, Wang M, Couto S, et al. Activity of lenalidomide in mantle cell lymphoma can be explained by NK cell-mediated cytotoxicity. Br J Haematol 2017;179(3):399–409 doi 10.1111/bjh.14866. [DOI] [PubMed] [Google Scholar]
- 38.Medema JP, de Jong J, Peltenburg LT, Verdegaal EM, Gorter A, Bres SA, et al. Blockade of the granzyme B/perforin pathway through overexpression of the serine protease inhibitor PI-9/SPI-6 constitutes a mechanism for immune escape by tumors. Proc Natl Acad Sci U S A 2001;98(20):11515–20 doi 10.1073/pnas.201398198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neuber B, Dai J, Waraich WA, Awwad MHS, Engelhardt M, Schmitt M, et al. Lenalidomide overcomes the immunosuppression of regulatory CD8(+)CD28(-) T-cells. Oncotarget 2017;8(58):98200–14 doi 10.18632/oncotarget.21516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nooka AK, Wang ML, Yee AJ, Kaufman JL, Bae J, Peterkin D, et al. Assessment of Safety and Immunogenicity of PVX-410 Vaccine With or Without Lenalidomide in Patients With Smoldering Multiple Myeloma: A Nonrandomized Clinical Trial. JAMA Oncol 2018;4(12):e183267 doi 10.1001/jamaoncol.2018.3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aue G, Sun C, Liu D, Park JH, Pittaluga S, Tian X, et al. Activation of Th1 Immunity within the Tumor Microenvironment Is Associated with Clinical Response to Lenalidomide in Chronic Lymphocytic Leukemia. J Immunol 2018;201(7):1967–74 doi 10.4049/jimmunol.1800570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katz JB, Muller AJ, Prendergast GC. Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunol Rev 2008;222:206–21 doi 10.1111/j.1600-065X.2008.00610.x. [DOI] [PubMed] [Google Scholar]
- 43.Ninomiya S, Hara T, Tsurumi H, Hoshi M, Kanemura N, Goto N, et al. Indoleamine 2,3-dioxygenase in tumor tissue indicates prognosis in patients with diffuse large B-cell lymphoma treated with R-CHOP. Ann Hematol 2011;90(4):409–16 doi 10.1007/s00277-010-1093-z. [DOI] [PubMed] [Google Scholar]
- 44.Yoshikawa T, Hara T, Tsurumi H, Goto N, Hoshi M, Kitagawa J, et al. Serum concentration of L-kynurenine predicts the clinical outcome of patients with diffuse large B-cell lymphoma treated with R-CHOP. European Journal of Haematology 2010;84(4):304–9 doi 10.1111/j.1600-0609.2009.01393.x. [DOI] [PubMed] [Google Scholar]
- 45.Nie M, Yang L, Bi X, Wang Y, Sun P, Yang H, et al. Neutrophil Extracellular Traps Induced by IL8 Promote Diffuse Large B-cell Lymphoma Progression via the TLR9 Signaling. Clin Cancer Res 2019;25(6):1867–79 doi 10.1158/1078-0432.Ccr-18-1226. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


