Abstract
Experiences with one’s own infant attune the parent nervous system to infant stimuli. To explore the effects of motherhood on brain activity patterns, electroencephalogram (EEG) was recorded while primipara mothers of 3- and 6-month-olds viewed images of faces of their own child and an unfamiliar but appearance-matched child. Mothers of 3- and 6-month-olds showed equivalent early-wave (N/P1 “visual” and N170 “face-sensitive”) responses to own and unfamiliar baby faces but differentiating late-wave (N/P600 “familiar/novel”) activity to own versus unfamiliar infant faces. Based on 3 months experience with their own infant’s face, mothers’ brain patterns give evidence of distinctive late-wave (recognition) sensitivity.
Little is more compelling to a new parent than the sights, sounds, smells, and somatosensory stimulation of their infant (Barratt & Fleming, 2011; Bornstein, 2002, 2013). A smiling face, a hunger cry, a unique odor, and a special touch are powerful motivators for a mother to respond to her infant through caregiving, holding, speech, or play. Newborn babies and young infants communicate their needs and physiological states mainly through vocalizations and facial expressions. Faces are particularly significant biological and social stimuli. They are privileged in perception (Palermo & Rhodes, 2007; Vuilleumier & Pourtois, 2007; Zebrowitz, 2006), and the faces of human infants appear to be especially captivating (Brosch, Sander, & Scherer, 2007). The ethologist Konrad Lorenz (1943, 1971) famously identified a constellation of morphological characteristics that distinguish infant from mature faces. That special physiognomy in infants includes a head large in proportion to the body, a protruding forehead that is sizable relative to the rest of the face, substantial relatively low-set eyes, and round protruding cheeks (see also Eibl-Eibesfeldt, 1970; Glocker et al., 2009). Notably, an enhanced ability to encode faces develops in mothers during late pregnancy, perhaps as an evolutionary adaptation that prepares women for the protective and nurturing demands of motherhood by increasing their general emotional sensitivity and vigilance (Fullgrabe, 2002; Pearson, Lightman, & Evans, 2009; Purhonen, Valkonen-Korhonen, & Lehtonen, 2008). In addition, a growing body of research points to neurobiological supports for the experience of parenthood on processing information that pertains to infants generally and to parents’ own infants specifically (Bornstein, 2013; Caria et al., 2012; Kim et al., 2010). To investigate the neurobiological underpinnings of this specific phenomenon, in this study we recorded and compared mothers’ brain responses to the face of their own young infant versus the face of an unfamiliar infant.
In the last decade, hemodynamic and electrophysiological brain imaging has revealed associations between central nervous system (CNS; and other, e.g., limbic) structures and putative caregiving propensities and functions in humans. Based on a developing literature using functional magnetic resonance imaging (fMRI), for example, a number of brain regions have been implicated in adults’ processing of facial stimuli of children, and parents of their own children compared to unfamiliar children (see Swain, Lorberbaum, Kose, & Strathearn, 2007, for a review). In a typical study, mothers (and sometimes fathers) are presented with pictures or videos of infants while in the scanner. Parents have shown enhanced activity to own infant stimuli in a variety of brain regions—striate and extrastriate, intraparietal sulcus and the precuneus, nucleus accumbens, orbitofrontal cortex, and anterior cingulate, and amygdala and insula—associated with cognition, motivation, emotion, and motor outputs (e.g., Bartels & Zeki, 2004; Leibenluft, Gobbini, Harrison, & Haxby, 2004; Lenzi et al., 2008; Nitschke et al., 2004; Noriuchi, Kikuchi, & Senoo, 2008; Ranote et al., 2004; Strathearn, Li, Fonagy, & Montague, 2008). In parenting terms, these areas may subserve infant-directed attentiveness, approach, caring, and empathy as well as social bonding (Bornstein et al., 1996).
fMRI provides excellent spatial resolution (in the millimeter range) and is useful in identifying structures thought to support perception, cognition, emotion, and behavior associated with parenting. By contrast, magnetoencephalography (MEG) and electroencephalography (EEG)/event-related potentials (ERPs) have excellent temporal resolution (in the millisecond range) and thus allow for monitoring and measuring noninvasively the time course and processing stages of neuronal activity related to attention, detection, and stimulus processing (Luck, 2005; Wild-Wall, Dimigen, & Sommer, 2008). There are adaptive reasons to focus on temporal parameters of maternal brain responses (Tamis-LeMonda, Bornstein, Baumwell, & Damast, 1996). Rapid identification of one’s own child is vital to child survival, it figures fundamentally in social interaction, and it presumably calls on neurobiological mechanisms deeply embedded in the parent brain. Moreover, baby physiognomy changes especially quickly, so reading, recognizing, and responding appropriately to facial features and expressions require constant and relatively rapid updating (Gauthier, Tarr, Anderson, Skudlarski, & Gore, 1999; Kuchuk, Vibbert, & Bornstein, 1986). As Rutherford and Mayes (2011) have noted, ERP techniques are exceptionally apposite to study intuitive parenting (Papoušek & Papoušek, 2002), aspects of which occur within time frames too brief to be captured by functional neuroimaging, self-report measures, or behavioral observations.
Surprisingly few studies have addressed the comparative advantage in adults for infant stimuli by assessing brain responses in the temporal domain, but two general findings have emerged. First, infant stimuli appear to be privileged, and second mothers may have a processing advantage over non-mothers. Using MEG, Kringelbach et al. (2008) found that activity in the medial orbitofrontal cortex, an area implicated in reward, occurred within a seventh of a second in response to generic unfamiliar infant faces. This finding pointed to a very early appearing specific neural signature for infant versus adult faces. Proverbio, Brignone, Matarazzo, Del Zotto, and Zani (2006) examined time-locked EEG responses in adults to images of unfamiliar infants. Female and male parents and nonparents saw four different infant facial expressions (pleasure, comfort, discomfort, and distress). Females showed larger amplitude responses to infant stimuli, regardless of parent status or infant facial expression. Notably, Proverbio et al. also reported that late components of the ERP were sensitive to infant facial expression. Noll, Mayes, and Rutherford (2012) also used the ERP to investigate the impact of parental status (mother, non-mother) on early visual processing of generic infant faces. P1 and N170 components were elicited by infant face stimuli independent of parental status. Finally, studies that used similar ERP techniques in the auditory domain have revealed that women respond significantly more to an infant cry than to an emotionally neutral vocalization, that mothers respond more than non-mothers to infant cries, and that evoked responses to infant crying, compared with responses to other auditory stimuli, habituate more slowly in mothers (Purhonen, Kilpeläinen-Lees, et al., 2001; Purhonen, Pääkkönen, Yppärilä, Lehtonen, & Karhu, 2001; Purhonen et al., 2008).
FACES AND ERPS
In the present study, we explored electrophysiological correlates of seeing one’s own versus an unfamiliar infant face in new mothers with different amounts of experience. Face processing is carried out by a network of occipitotemporal regions within the ventral visual stream (e.g., Haxby, Hoffman, & Gobbini, 2000; Kanwisher, McDermott, & Chun, 1997; Puce, Allison, Asgari, Gore, & McCarthy, 1996; Vuilleumier & Pourtois, 2007). It is believed that different kinds of facial information are processed by distinct and specialized brain sub-systems. Moreover, it is generally accepted that face processing occurs in two broad stages. The prevailing distributed model of face processing distinguishes between an early perceptual stage of structural encoding, where individual face features and their spatial configuration are analyzed, and a later recognition stage, where structural information is compared with stored face representations (Bruce & Young, 1986; Haxby et al., 2000). Responding to a face as familiar implies that the face activates an encoded visual representation of the familiar person. In short, identification of faces likely consists of multiple specialized processes. Moreover, the temporal and spatial features of these processes are thought to be identifiable in face-specific modulations of the ERP. That is, different components of the ERP waveform reflect different stages of facial information processing and index distinct perceptuocognitive mechanisms. Guided by the extant literature, therefore, we focused on early- and late-wave potentials of evoked responses to familiar and unfamiliar infant faces.
The “familiar” stimuli used in most face-processing research have consisted of participants’ own faces, faces of well-known individuals (parents, professors, and politicians), or faces that the participant studied before the experiment (e.g., Begleiter, Porjesz, & Wang, 1995; Caharel, Courtay, Bernard, Lalonde, & Rebaï, 2005; Hautecoeur et al., 1993; Renault, Signoret, Debruille, Breton, & Bolgert, 1989; Smith & Halgren, 1987). Here, we contrasted mothers’ own infant faces with appearance-matched unfamiliar infant faces. New parents spend great amounts of time attending to their infant (Bornstein, 2002). This natural situation provides an opportunity to evaluate the effects of parenthood on early- and late-wave components of the ERP thought to be involved in perceptual and cognitive aspects of face processing. We think that personally familiar faces, like one’s own infant, in contrast with familiar faces from the public domain, should have engendered representations in memory that are especially early appearing, consistent, and robust (see Caharel et al., 2005; Tong & Nakayama, 1999).
EARLY-WAVE POTENTIALS (N/P1 AND N170)
N/P1
The N/P1 is so called because it is the first negative- or positive-going component and its peak is normally observed in around 80 to 130 msec after stimulus onset (Mangun, 1995; Spehlmann, 1965). Current source density maps and structural MRI localize its neurological source some-where over the ventrolateral prestriate cortex (Brodmann’s Area 18; Di Russo, Martinez, & Hillyard, 2003; Martinez et al., 1999). N/P1 responses, which are widely observed in visual ERP tasks, are taken to reflect processes of early sensation and attention (Luck, Heinze, Mangun, & Hillyard, 1990): hence, the P1 “effect” in selective attention. Van Voorhis and Hillyard (1977) found that the P1 had a greater positive amplitude when the target was presented in the attended field than when it was presented outside the attended field. Mangun and colleagues (1991, 1997; but see Luck et al., 1994) associated the P1 with activation in the posterior fusiform gyrus. P1 is therefore an index of early visual processing. Notably, Noll et al. (2012) found P1 responses to be equivalent in mothers and nonmothers.
N170
Allison et al. (1994) recorded ERPs intracranially to faces and non-face stimuli and identified a face-specific negative-going potential with a latency of about 200 msec. Shortly afterward, Bentin, Allison, Puce, Perez, and McCarthy (1996) reported that faces elicit a negative-going potential with a latency peak at 170 msec (N170). Bentin et al. (1996; Bentin & Deouell, 2000) obtained the face-specific N170 to intact upright faces, and also to inverted faces or isolated eyes (but see Eimer, 2000), and they argued that the N170 reflects face-specific structural encoding prior to later higher-order processing stages involved in face identification or recognition (see also Eimer, 2000; Eimer & Holmes, 2007). No N170 was triggered by cars, hands, furniture, or even by scrambled faces. Neural responses associated with the N170 occur automatically, perhaps reflecting obligatory processing of facial information, and specificity of the N170 to human faces, along with its insensitivity to non-facial stimuli, suggests that the N170 reflects the activity of cells tuned to detect human faces and/or face components.
Past studies examining the influence of face familiarity have shown that N170 responses to well-known faces are equivalent to N170 responses to the faces of strangers (Bentin & Deouell, 2000; Bentin et al., 1996; Eimer, 2000; Herzmann, Schweinberger, Sommer, & Jentzsch, 2004; Jemel, Pisani, Calabria, Crommelinck, & Bruyer, 2003). Neither repetition nor priming affect N170 amplitudes for face stimuli (Eimer, 2000; Schweinberger, Pickering, Jentzsch, Burton, & Kaufmann, 2002). Thus, the N170 is largely insensitive to variation in facial familiarity. These results therefore support consensus that the N170 indexes structural representations of the general face category rather than previously stored representations of particular faces (Bentin & Deouell, 2000; Eimer, 2000). In brief, we evaluated the N/P1, which assesses processing demands at the level of basic sensory and attentional characteristics, and the N170, which is specific to faces relative to non-facial stimuli, encodes facial structure and configuration, and is insensitive to (at least short-lived) facial familiarity. We regarded the P1 as a general marker of early visual processing, and the N170 was utilized as a neural marker of early face processing. For these reasons, we scrutinized both the N/P1 and the N170 in mothers’ responses to own versus unfamiliar infant faces, and we expected to see N/P1 and N170 responses to both classes of faces, but we expected no stimulus-related differences at either temporal location on the waveform.
LATE-WAVE POTENTIALS (N/P600)
In contrast to early waves, responding to specific characteristics of a face and recognizing a face as familiar or unfamiliar are thought to occur at later stages in neurological processing: For example, Eimer (2000) reported late-wave ERP differences between famous and non-famous faces, Proverbio et al. (2006) heightened sensitivity of late ERP components to infant facial expression, Grasso, Moser, Dozier, and Simons (2009) late-wave sensitivity to own versus other child, and Doi and Shinohara (2012) larger amplitude late waves when mothers heard their own infant’s crying.
Overt face recognition in normal observers takes on the order of 650 msec (Barrett, Rugg, & Perrett, 1988), but covert face recognition is evidenced using ERPs at somewhat shorter intervals, ~N400–500 msec following stimulus onset (Bobes, Quiñonez, Perez, Leon, & Valdés-Sosa, 2007; Eimer, 2000; Nelson, Thomas, de Haan, & Wewerka, 1998; Tanaka, Curran, Porterfield, & Collins, 2006). Covert recognition is also robust as face familiarity is preserved even in prospagnosic patients like P.C. who “recognized” faces as evidenced in a late-wave component that peaked between 700 and 800 msec after the stimulus onset (Renault et al., 1989; see also Bobes et al., 2004). Accumulating evidence indicates that a family of distinct but overlapping late-wave components at divergent distributions over the scalp, each sensitive to different experimental factors, reflects distinct mental operations (Soltani & Knight, 2000; Squires, Squires, & Hillyard, 1975).
This N/P600 complex has been elicited in both visual and auditory experiments (Hagoort, 2007; Kaan & Swaab, 2003) and implicated in an extensive literature on attentional orienting to recollected information (Buckner, Kahn, Shannon, & Wagner, 2005; Langeslag, Franken, & Van Strien, 2008; Langeslag, Jansma, Franken, & Van Strien, 2007; Rugg, Otten, & Henson, 2002) as well as explicit recognition memory (Dolcos & Cabeza, 2002; Friedman & Johnson, 2000; Mecklinger, 2000; Münte, Urbach, Düzel, & Kutas, 2000; Olofsson, Nordin, Sequiera, & Polich, 2008; Polich, 2007; Righi et al., 2012; Rosenkrants & Polich, 2008; Wilding, 2002; Yovel & Paller, 2004). Germane here, it has been associated with cognitive evaluation stages of face processing that take place after initial (N170) perceptual stages of face processing (Soltani & Knight, 2000). Late-stage processing has been implicated in sustained and elaborated stimulus analysis after stimulus identification (Ritter & Ruchkin, 2006).
Different experimental conditions appear to selectively enhance late-wave ERPs by location and polarity (Loveless, Simpson, & Näätänen, 1987). For example, recognition (e.g., of deeply studied words) is based on both familiarity (indexed by a mid-frontal old/new effect) and recollection (indexed by a parietal old/new effect). Recollection and familiarity are therefore dissociated in retrieval-related ERPs (Rugg & Curran, 2007). The frontal “old/new” effect is a late-wave negative ERP (N600) correlate of familiarity-driven recognition (Rugg, 1995; Yonelinas, Otten, Shaw, & Rugg, 2005). By contrast, recollection is remembering an item together with retrieving physical, contextual, or other source-specifying information about prior occurrences of the item (Mecklinger, 2000). The parietal “old/new” effect is a late-wave positive ERP (P600) correlate of recollection (Dennis, Finnigan, Geffen, & Humphreys, 2002; Rugg & Curran, 2007). The parietal old/new effect is elicited by items subjected to deep study, is linked to the recollection of specific information, and reflects attentional orienting and representation of recollected information (Rugg & Henson, 2002; Wagner et al., 2005; Wilding & Rugg, 1996). Numerous fMRI studies confirm recollection-sensitive activity in this region (Buckner et al., 2005; Ecker, Zimmer, Groh-Bordin, & Mecklinger, 2007; Herron, Henson, & Rugg, 2004; Tsivilis & Otten, 2001; Vilberg, Moosavi, & Rugg, 2006; Woodruff, Hayama, & Rugg, 2006; Yonelinas et al., 2005).
In brief, the N/P600 complex encompasses temporally overlapping but spatially and functionally differentiated ERP components originating in frontal and parietal regions, respectively, and is associated with recognition and “depth of processing” recollection. For these reasons, we also focused on the N/P600 complex in mothers’ responses to own versus unfamiliar infant faces, and here we expected to find stimulus-related differences in late-wave temporal and spatial components of the ERP.
PRESENT STUDY
In the present study, we explored electrophysiological correlates of own infant versus unfamiliar infant face processing by new mothers. The natural circumstance of the great investment of new mothers in their young infants, accompanied by close and consistent mother–infant interaction in the first months, provide an opportunity to evaluate the effects of stimulus familiarity and recollection of a unique and evolutionarily freighted circumstance on components of the EEG thought to be involved in face processing, recognition, and recollection. To assess these face effects on fast- and slow-wave brain potentials, mothers of young infants viewed photographs of their own infant and an unfamiliar infant matched for age, skin tone, head shape, and eye and hair color. Amplitude and latency were quantified for ERP components of interest (N/P1, N170, and N/P600) at different relevant scalp locations (frontal, occipital, parietal, right temporal, left temporal). These potentials assess perceptual, attentive, and sustained/evaluative processes, and so in analyzing them we were able to explore different stages in mothers’ processing of own versus unfamiliar infant faces. If infant faces stimulate sensory and perceptual processes, we expected stimulus-equivalent very early responses in mothers. If the experience of motherhood facilitates face recognition and recollection for one’s own infant specifically, we expected to see familiar versus unfamiliar face differences in late-wave frontal and parietal potentials. We also tested mothers with 3 versus 6 months experience with their infants to ascertain if duration of experience after parturition affects face processing.
METHOD
Participants
Twenty-two primiparas of 3-month-old (n = 10) and 6-month-old infants (n = 12), M age = 32.06 years (SD = 4.66) participated. Participants were middle- to upper-middle socioeconomic status (Hollingshead, 1975). An additional 11 mothers were tested, but their data were not included due to experimenter error or equipment failure (5) or failure to meet the trial criterion for inclusion (6). Preliminary analyses revealed no differences in brain activity as a function of infant gender (n = 11 girls), so all subsequent analyses are collapsed by infant gender.
Stimuli
At the start of the laboratory visit, digital photographs of infants’ faces were taken following informed consent. Infants were placed in an upright infant seat that was draped with a gray cloth. A second cloth was wrapped around the infant’s neck and torso to eliminate the view of clothing. Multiple photographs were taken to select one in which each infant’s facial expression was neutral. We held infant facial expression constant as the N170 is modulated by emotional expression (e.g., Blau, Maurer, Tottenham, & McCandliss, 2007). For example, smiles, as compared to neutral expressions, increase the subjective familiarity of faces (Baudouin, Gilibert, Sansone, & Tiberghien, 2000), and the amplitude of the N170 is increased for crying versus smiling faces (Doi & Shinohara, 2012). Also, recognition of personally familiar faces is facilitated by displays of neutral, as compared to happy and angry, expressions (Endo, Endo, Kirita, & Maruyama, 1992). Each mother’s infant’s face (own) was paired with another infant face (unfamiliar) from our laboratory archive of images captured under identical conditions with respect to lighting, background, framing, and camera angle. Based on experimenter consensus, each unfamiliar infant was selected to closely match each mother’s own infant’s skin tone, head shape, age, and eye and hair color. Face images (12.55° by 15.94°) were presented to mothers on a computer screen against a black background.
Procedure
Participants sat approximately 65 cm in front of the display and were instructed to minimize head and eye movements while fixating the screen. Mothers were presented 36 trials of the image of their own infant and 36 trials of the image of the unfamiliar infant, for a total of 72 trials presented in a uniquely randomized order for each mother. On each trial, a 100-msec baseline period with a fixation point preceded stimulus presentation. The stimulus appeared for 500 msec and was followed by a variable 1,800- to 2,200-msec inter-trial interval during which the computer screen was blue.
Recording and Segmenting of EEG
EEG was recorded with the EGI (Electrical Geodesics Incorporated, Eugene, OR) 128-channel EEG recording system (Net Station 4.1.1). The signal was referenced to the vertex, recorded with 20K amplification, at a sampling rate 250 Hz, with band pass filters set at 0.1–100 Hz, and with no more than 80 Ω impedance. Recordings were digitally filtered with a 40-Hz low-pass filter and segmented into own and unfamiliar face trials using Net Station 4.3 Waveform Tools. A segmented trial consisted of 100 msec before the stimulus was presented and 1,000 msec after the stimulus was presented. Recordings were inspected for artifacts (signal amplitude exceeding 200 μ Volts or a differential amplitude exceeding 100 μ Volts), and a trial was excluded if more than 20% of the channels exceeded these thresholds. Participants needed a minimum of 10 artifact-free trials per face category to be included. The numbers of trials completed that were free of gross artifacts were 24.96 (SD = 6.65) for their own infant and 24.95 (SD = 8.21) for the unfamiliar infant and did not differ between stimulus conditions, t(21) 0.05, ns.
The EEG for each channel was averaged across trials separately for each face category. The data were average referenced, and a baseline correction was applied to the 100 msec prestimulus recording interval. Analyses were conducted for clusters of electrodes at midline frontal (Fz), central (Cz), occipital (Oz), and temporal (Tl, Tr) sites, following the 128 to 10–20 conversion suggested by Reynolds and Richards (2005), and parietal left (Pl) and right (Pr), following Yang, Perfetti, and Schmalhofer (2007; see Table 1).
TABLE 1.
Sensor Clusters Used for Event-Related Potential Measurement
| Site | EGI GSN Sensors |
|---|---|
|
| |
| Fz | 4, 10, 11, 16, 19, 20 |
| Cz | 7, 32, 55, 81, 107 |
| Pl | 38, 43, 52, 53, 54, 61, 60 |
| Pz | 61, 62, 68, 69 |
| Pr | 79, 80, 86, 87, 88, 93, 94 |
| Tl | 58, 59, 64, 65, 66 |
| Oz | 72, 73, 76, 77 |
| Tr | 85, 91, 92, 96, 97 |
Note. EGI = Electrical Geodesics Incorporated.
GSN = Geodesic Sensor Net.
RESULTS
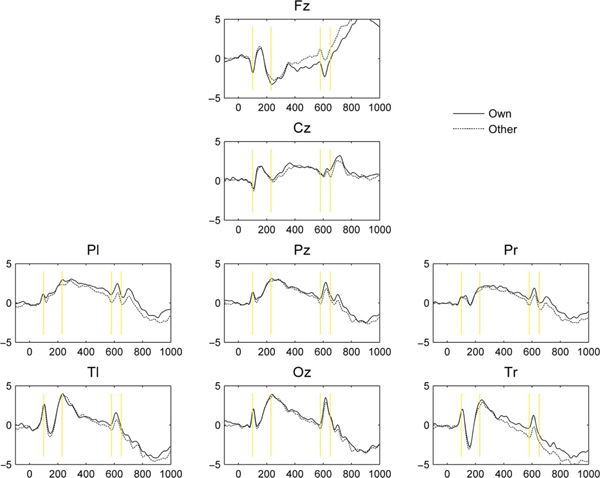
The segmented ERP waveforms were preprocessed using EEGLAB (Delorme & Makeig, 2004) running in Matlab v7.1. An independent components analysis (ICA) was conducted to decompose the signal into separate information sources. An algorithm developed by Mognon, Jovicich, Bruzzone, and Buiatti (2011), automatic EEG artifact detection based on joint use of spatial and temporal features (ADJUST), was used to identify and remove components corresponding to four classes of source artifact: eye blink, vertical eye movement, horizontal eye movement, and generic discontinuity. For each participant, cleaned data were averaged over channels within site clusters and then averaged over trials by stimulus condition. Grand averages were inspected for the presence of discrete components (Figure 1). Three deflections were observed.
FIGURE 1.
Grand average amplitude by channel clusters. Solid lines represent participants’ responses to their own infants’ faces and dashed lines represent responses to unfamiliar infants’ faces.
Very early deflections peaked between 75 and 125 msec going negative at anterior sites and positive at posterior sites. The presence of these very early components was verified by zeroing amplitude at the window onset for each case and testing mean amplitude through the window against zero via one-sample t tests for each site. Amplitude magnitudes differed from zero at all recording sites, ts ranging from 2.16, p =.043, at Cz to 4.62, p <.001, at Tr. A second early deflection peaked between 100 and 230 msec going positive at anterior sites and negative at posterior sites. Positive amplitude magnitudes differed at Fz, t(21) = 2.61, p =.016, and Cz, t(21) = 4.87, p <.001, negative magnitudes at Tl, t(21) = −2.56, p = .018, and Tr, t(21) = −2.89, p =.009. Late deflections going negative at anterior sites and positive at posterior sites were observed between 580 and 650 msec. These deflections differed from zero at all sites except Cz, ts ranging from 2.33, p = .03, at Tr to 4.82, p < .001, at Pz. To compare peaks and their latencies of the early N/P1 and late N/P600 responses across conditions, sites, and age groups, values were analyzed with ANOVAs that included familiarity condition (own vs. unfamiliar) and site contrasts (left vs. right sites and midline vs. lateral sites) as within-subjects factors and infant age (3 vs. 6 months) as a between-subjects factor. To compare the magnitudes of peaks across sites, the signs of the peaks at Fz and Cz were reversed to match those at posterior sites. Following the conventions of previous research, N170 responses were analyzed at left and right temporal sites.
N/P1
The analysis of peaks revealed a significant site contrast with larger peaks at midline sites (M = − 0.99, SD = 0.80) than lateral sites (M = −0.77, SD = 0.67), F(1, 20) = 7.41, p = .013, ηp2 = .27. No significant effects emerged for familiarity condition, F(1, 20) = 0.21, p = .65, or infant age, F(1, 20) = 0.72, p = .41. The analysis of latencies revealed no effects of familiarity condition, F(1, 20) = 0.12, p = .73, site, F(2, 19) = 0.91, p = .42, or infant age, F(1, 20) = 1.96, p = .18.
N170
The analysis of peaks revealed no significant effects of familiarity condition, F(1, 20) = 0.15, p = .70, side (left vs. right), F(1, 20) = 2.70, p = .12, or infant age, F(1, 20) = 1.74, p = .20. The analysis of latencies revealed no significant effects of familiarity condition, F(1, 20) = 1.36, p = .26, side, F(1, 20) = 3.11, p = .09, or infant age, F(1, 20) = 0.56, p = .46.
N/P600
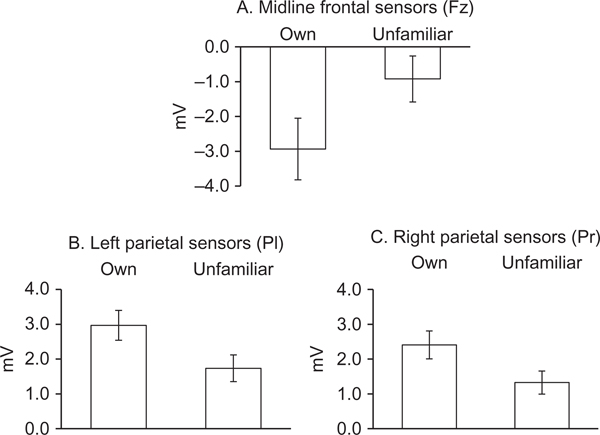
The analysis revealed larger peaks in response to own infant faces (M = 2.40 mv, SD = 2.46) than to unfamiliar infant faces (M = 1.41, SD = 1.65), F(1,20) = 4.87, p = .039, ηp2 = .20. Also, peaks in the left hemisphere (M = 2.06, SD = 1.91) were larger than those on the right (M = 1.42, SD = 1.47), F(1,20) = 5.66, p = .027, ηp2 = .22. A marginally significant interaction emerged between face condition and the midline versus lateral sites contrast, F(1,20) = 3.74, p = .067, ηp2 = .16. To examine the interaction and localize the familiarity condition differences observed in the main analysis, t tests were conducted separately at each site, collapsing across age (Figure 2). Analysis of the N600 at Fz and Cz sites revealed a larger peak at Fz for own infant faces (M = −2.93, SD = 4.15) than for unfamiliar infant faces (M = –0.92, SD = 3.10), t(21) = 2.33, p = .029 (Figure 3); conditions did not differ at Cz. Analyses of the P600 at parietal, temporal, and occipital sites revealed a larger peak at Pl for own infant faces (M = 2.97, SD = 2.01) than for unfamiliar infant faces (M = 1.74, SD = 1.79), t(21) = 2.68, p = .014, and a larger peak at Pr for own infant faces (M = 2.41, SD = 1.88) than for unfamiliar infant faces (M = 1.33, SD = 1.55), t(21) = 2.71, p = .013 (Figure 3). The analysis of latencies revealed no significant effects of familiarity condition, F(1, 20) = 0.99, p = .33, site, F(2, 19) = 2.18, p = .14, or infant age, F(1, 20) = 3.19, p = .09.
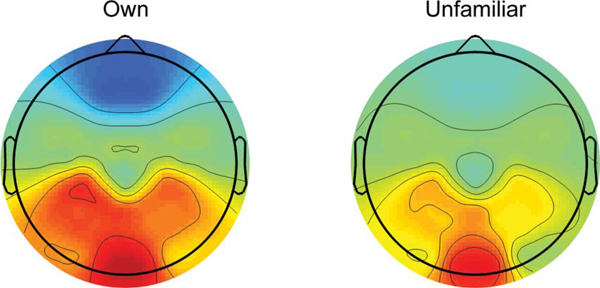
FIGURE 2.
Topographic plot of N/P600 amplitude peaks, across sites analyzed by face familiarity condition. Warm colors represent positive responses, and cool colors represent negative responses.
FIGURE 3.
Mean N/P600 response by familiarity condition at frontal and parietal sites.
DISCUSSION
To explore the effects of motherhood on ERP components associated with own infant face processing, mothers viewed faces of two infants—one of the infants was their own, and the other was an unfamiliar infant matched for multiple perceptual features—while their EEG was recorded. Infant faces evoked N/P1 and N170 potentials in mothers, as expected, and their amplitudes and latencies showed no differences in mothers between own and unfamiliar infant faces. In contrast, mothers showed greater N/P600 amplitudes to their own infant’s face than to an unfamiliar infant’s face and did so specifically and as expected at frontal and parietal sites, respectively. The present study reveals the temporal course with which familiarity exerts influences on neural processing of one’s own infant’s face in mothers.
N/P1 responses are commonly observed in visual ERP tasks and are understood to reflect early sensation and attention (e.g., Luck, 2005). N/P1 responses were equivalent between face conditions in the present study. This finding indicates that later differences observed are a result of higher-order stimulus processing.
The ERP literature has established that an enhanced negative deflection appears approximately 170 msec after stimulus onset in response to face stimuli relative to nonface objects (Bentin et al., 1996). The N170 is normally unaffected by face familiarly or repetition, which indicates that early visual processing is elicited automatically by structural characteristics of a face (Bentin & Deouell, 2000; Eimer & Holmes, 2007). Our findings are consistent with this literature: Mothers responded to infant faces with discernible N170s, but they responded equally to faces of their own infant and an unfamiliar infant at this early structural stage of face processing. These results also accord with those of Doi and Shinohara (2012) who found N170 amplitudes did not differ according to the familiarity of an infant face. They suggested that their finding might indicate that, at initial perceptual stages of face processing (which are likely subserved by fusiform gyrus, superior temporal sulcus, and the occipital face area; Minnebusch & Daum, 2009), infant faces are processed in a similar manner regardless of familiarity. Thus, the N170 appears to reflect instinctive responsiveness (in these mothers) to infant facial stimuli and that responsiveness is triggered regardless of kinship to the infant. Discriminating brain responses toward own infant faces unfolded shortly afterward, however.
Mothers showed higher N/P600 amplitudes to their own infant’s face than to an unfamiliar infant’s face, and did so specifically and as expected at frontal and parietal sites. Such late-wave potentials are believed to reflect higher-order cognitive control processes beyond more basic automatic ones (Schupp, Junghofer, Weike, & Hamm, 2004). Perhaps because of their significance, own infant faces are selected by the brain for sustained processing, which likely results in more elaborated or evaluative stimulus analysis (Cuthbert, Schnupp, Bradley, Birmbauer, & Lang, 2000; Lang, Bradley, & Cuthbert, 1997). Furthermore, the significantly nonzero N170 indicates the stimuli were processed as faces rather than generic objects or patterns; the N/P600 is likely showing recognition of faces rather than generic items. Recognition and recollection are associated, respectively, with late-wave negative and positive components of the ERP. Consistent with prior studies using verbal or visual stimulus materials that interpreted effects between ~500 and ~650 msec as reflections of recollection (Mecklinger, 2000), the present results indicate that late-wave ERP correlates of recognition are also reliably observed using own child faces as stimulus materials (see also Bobes et al., 2007; Doi & Shinohara, 2012; Grasso et al., 2009; Münte, Matzke, & Johannes, 1997; Proverbio et al., 2006). These results suggest that mothers’ brains are modified by specific experiences with their own infant’s face, and the increased amplitudes of late waves suggest a heightened sensitivity (recognition and recollection) to one’s own infant in mothers within the first months of becoming a parent.
The observation of a left hemisphere advantage for face response latency is somewhat surprising given usual evidence for right-hemisphere localization for face processing (e.g., Bentin et al., 1996; Damasio, Damasio, & van Hoesen, 1982). Most studies in this tradition have utilized adult faces for stimuli and have included both men and women as participants. Stimulus face, age, and gender, therefore, may be part of the distinctive pattern observed here. As noted earlier, Proverbio et al. (2006) examined adult brain responses to infant faces, and they also compared responses between genders of participants. They observed the conventional right-hemisphere advantage in men, but not in women. Noll et al. (2012) found no lateralization of N170 amplitude in mothers. Everhart, Shucard, Quatrin, and Shucard (2001) compared facial recognition ERPs between hemispheres and genders in children 8 to 11 years of age and found a in boys, but a left-hemisphere advantage in girls. Thus, laterality in face processing may depend more on stimulus face, respondent age and gender, and dependent measure than has previously been appreciated. This finding merits further experimental attention.
On what bases might the late-wave recognition effects observed here occur? Childbearing and childrearing are accompanied by rapid structural nervous system, hormonal, and behavioral adjustments (Bornstein, 2013). Prospective longitudinal study of gray matter changes using voxel-based morphometry on high-resolution magnetic resonance images of mothers’ brains has revealed increases in gray matter volume of the prefrontal cortex, parietal lobes, and mid-brain areas between 2–4 weeks and 3–4 months postpartum (Kim et al., 2010). Moreover, these effects are associated with positive maternal perceptions of her baby. Also, single neurons in the superior temporal sulcus of the monkey detect familiar faces (Perrett et al., 1984). Our ERP findings articulate with these results and are complemented by fMRI findings with parents reviewed earlier.
Hormones may also play a role. Hormones activate key brain regions that augment mothers’ attraction to infant cues, enhance their affective state, and render them attentive and sensitive to infants so that mothers learn from their experiences with, and behave appropriately toward, their infants (see Bornstein, 2013, for a review). For example, the first months of parenting are associated with a rise in oxytocin (OT) suggesting that OT increases in parents as their relationship with their infant consolidates (Feldman, 2012; Fleming, Ruble, Krieger, & Wong, 1997; Lambert & Kinsley, 2012). Indeed, generally higher levels of OT are associated with more sensitive and synchronous parental behaviors in mothers (Feldman, Weller, Zagoory-Sharon, & Levine, 2007). Germane to the present study, OT also enhances memory for familiar faces (Guastella, Mitchell, & Dadds, 2008).
Do the effects we observed depend on a biological connection between mother and infant? Perhaps not. Both birth and adoptive mothers exhibit larger late-wave amplitudes toward own children as compared with unfamiliar children and adult stimuli (Grasso et al., 2009). It may be, therefore, that faces with great personal significance lead to more robust representations by conferring them rapid processing and ease of retrieval (Tong & Nakayama, 1999). Social parenting appears to constitute the effective circumstance contra biological connection (Leon, 2002).
Limitations and Future Directions
This initial study of the brain’s sensitivity to own infant stimuli has some design limitations and inspires a host of related questions. For example, we did not include faces of infants who were familiar, but not kin, to the mothers or non-infant faces as controls, preventing us from determining whether effects of infant identity are attributable to kinship or perceptual familiarity (Caharel et al., 2005; Furl, van Rijsbergen, Treves, Friston, & Dolan, 2007). Future research should address this issue.
Relative to men, women appear to pay more attention to social or reproductive-related stimuli (Proverbio, Zani, & Adorni, 2008); they recognize emotional facial expressions (Cellerino, Borghetti, & Sartucci, 2004; Hall & Matsumoto, 2004; Thayer & Johnsen, 2000); and their ERPs to adult facial expressions are larger (Orozco & Ehlers, 1998). To address additional questions of underlying mechanism and neuroplasticity, an additional next step might be to explore whether the effects reported here for own infants’ faces is limited to mothers. Fathers, like mothers, recognize the face of their newborn (Kaitz, Good, Rokem, & Eidelman, 1988). A similar electrophysiological study with fathers might help to ascertain whether the patterns of activation observed when mothers view their own infant reflects a general parental sensitivity, rather than one that is exclusively maternal. Few neurobiological studies have included fathers, but in one exception Seifritz et al. (2003) assessed female and male parents’ and nonparents’ responses to crying and laughter of unfamiliar infants. Females showed greater prefrontal activation than males to infant vocalizations regardless of parent status. Proverbio et al. (2006) found N170 amplitudes in mothers were larger than in fathers, suggesting that parental status may modulate the structural encoding of infant faces more strongly in women than in men. However, neural plasticity in parenthood is not restricted to biological changes that accompany pregnancy, as fathers in Seifritz et al. (2003) showed similar effects as mothers in greater activation at multiple anatomical sites to infant crying than laughing.
Another future question pertains to the developmental and experiential time course of these kinds of effects. We found no differences in mothers with 3 or 6 months of experience. This result implies some (at least, short-term) stability in brain responsivity. However, it also raises other questions. Could the differentiated effects of own infant face on late-wave ERPs occur earlier than 3 months? Perceiving and recognizing older versus younger infants might involve different sets of neural circuits, and different amounts of experience may be operative. Variations in infant affective facial expressions (happy vs. neutral vs. sad) too may influence parental brain responses. We presented infants displaying neutral facial expressions. Doi and Shinohara (2012) measured ERPs in mothers while they observed crying or smiling by their own or unfamiliar infants embedded within a series of neutral expressions. The amplitude of the face-specific N170 component was greater for crying regardless of familiarity.
Finally, different sample populations may show individual differences in neural responses to infant cues. This study involved a normative community parent sample, but non-parents and parents with a clinical disease (depression, abuse and neglect, or substance abuse) likely process infant cues in different ways. ERPs could constitute biomarkers of these sensitivities, as they allow access to the time course of information processing and provide information about the processing stages of infant cues. Research already suggests that the amplitudes of the N170 elicited by neutral, crying, or laughing faces of unfamiliar infants are attenuated and undifferentiated in neglectful mothers relative to larger and differentiated ones in healthy controls (Rodrigo et al., 2011). Thus, a diminished N170 may index reduced maternal sensitivity to infant facial expressions of distress. Likewise, depressed individuals show reduced accuracy in recognizing facial expressions and increased memory for negative faces (Leppänen, 2006), and depressed mothers show a positive correlation between symptom severity and N170 amplitude (Noll et al., 2012). Mothers’ alertness or attunement to their infants’ needs could depend, at least in part, on the adequacy of these neural mechanisms (Bornstein, Tamis-LeMonda, Hahn, & Haynes, 2008).
Conclusions
Survival of our species rests on protecting and nurturing vulnerable offspring. Human mothers recognize the faces, cries, odors, and tactile characteristics of their newborns (Corter & Fleming, 2002; Green & Gustafson, 1983; Kaitz, Rokem, & Eidelman, 1988; Kaitz, Good, Rokem, & Eidelman, 1987, 1988; Kaitz, Lapidot, Bronner, & Eidelman, 1992; Porter, Cernoch, & McLaughlin, 1983). ERP analysis allowed us to gain important information about the time course and neural processing stages for own infant faces in first-time mothers, moving from sensory attentiveness to the structural analysis of faces to their recognition and recollection. Here, we found ERP differences in responding to images of own versus unfamiliar infants in mothers with as little as 3 months’ experience with their infants. These findings point to a heightened biological sensitivity to own infant within the first months of becoming a mother.
ACKNOWLEDGMENTS
We thank A. Bradley, A. Dovidio, P. Horn, and C. Padilla.
This research was supported by the Intramural Research Program of the NIH, NICHD.
Contributor Information
Marc H. Bornstein, Child and Family Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Maryland
Martha E. Arterberry, Department of Psychology, Colby College, Waterville, Maine
Clay Mash, Child and Family Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Maryland.
REFERENCES
- Allison T, Ginter H, McCarthy G, Nobre AC, Puce A, Luby M, & Spencer DD (1994). Face recognition in human extrastriate cortex. Journal of Neurophysiology, 71, 821–825. [DOI] [PubMed] [Google Scholar]
- Barratt J, & Fleming AS (2011). All mothers are not created equal: Neural and psychobiological perspectives on mothering and the importance of individual differences. Journal of Child Psychology and Psychiatry, 52, 368–397. doi: 10.1111/j.1469-7610.2010.02306.x [DOI] [PubMed] [Google Scholar]
- Barrett SE, Rugg MD, & Perrett DI (1988). Event-related potentials and the matching of familiar and unfamiliar faces. Neuropsychologia, 1, 105–117. doi: 10.1016/0028-39328890034-6 [DOI] [PubMed] [Google Scholar]
- Bartels A, & Zeki S (2004). The neural correlates of maternal and romantic love. Neuroimage, 21, 1155–1166. doi: 10.1016/j.neuroimage.2003.11.003 [DOI] [PubMed] [Google Scholar]
- Baudouin JY, Gilibert D, Sansone D, & Tiberghien G (2000).When the smile is a cue to familiarity. Memory, 8, 285–292. doi: 10.1080/09658210050117717 [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, & Wang W (1995). Event-related brain potentials differentiate priming and recognition to familiar and unfamiliar faces. Electroencephalography and Clinical Neurophysiology, 94, 41–49. doi: 10.1016/0013-4694(94)00240-L [DOI] [PubMed] [Google Scholar]
- Bentin S, Allison T, Puce A, Perez E, & McCarthy G (1996). Electrophysiological studies of face perception in humans. Journal of Cognitive Neuroscience, 8, 551–565. doi: 10.1162/jocn.1996.8.6.551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentin S, & Deouell LY (2000). Structural encoding and identification in face processing: ERP evidence for separate processes. Cognitive Neuropsychology, 17, 35–54. doi: 10.1080/026432900380472 [DOI] [PubMed] [Google Scholar]
- Blau VC, Maurer U, Tottenham N, & McCandliss BD (2007). The face-specific N170 component is modulated by emotional facial expression. Behavioral and Brain Functions, 3. doi: 10.1186/1744-9081-3-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobes MA, Lopera F, Garcia M, Díaz Comas L, Galan L, & Valdes-Sosa M (2004). Brain potentials reflect covert recognition in a case of prosopagnosia. Cognitive Neuropsychology, 21, 691–718. [DOI] [PubMed] [Google Scholar]
- Bobes MA, Quiñonez I, Perez J, Leon I, & Valdes-Sosa M (2007). Brain potentials reflect access to visual and emotional memories for faces. Biological Psychology, 75, 146–153. doi: 10.1016/j.biopsycho.2007.01.006 [DOI] [PubMed] [Google Scholar]
- Bornstein MH (2002). Parenting infants. In Bornstein MH (Ed.), Handbook of parenting vol. 1 children and parenting (2nd ed., pp. 3–43). Mahwah, NJ: Erlbaum. [Google Scholar]
- Bornstein MH (2013). Mother-infant attunement: A multilevel approach via body, brain, and behavior. In Legerstee M, Haley DW, & Bornstein MH (Eds.), The infant mind: Origins of the social brain (pp. 266–298). New York, NY: Guilford. [Google Scholar]
- Bornstein MH, Tamis-LeMonda CS, Hahn C-S, & Haynes OM (2008). Maternal responsiveness to very young children at three ages: Longitudinal analysis of a multidimensional modular and specific parenting construct. Developmental Psychology, 44, 867–874. [DOI] [PubMed] [Google Scholar]
- Bornstein MH, Tamis-LeMonda CS, Pascual L, Haynes OM, Painter K, Galperín C, & Pêcheux M-G (1996). Ideas about parenting in Argentina, France, and the United States. International Journal of Behavioral Development, 19, 347–367. [Google Scholar]
- Brosch T, Sander D, & Scherer KR (2007). That baby caught my eye . . . Attention capture by infant faces. Emotion, 7, 685–689. doi: 10.1037/1528-3542.7.3.685 [DOI] [PubMed] [Google Scholar]
- Bruce V, & Young A (1986). Understanding face recognition. British Journal of Psychology, 77, 305–327. doi: 10.1111/j.2044-8295.1986.tb02199.x [DOI] [PubMed] [Google Scholar]
- Buckner RL, Kahn I, Shannon BJ, & Wagner AD (2005). Parietal lobe contributions to episodic memory retrieval. Trends Cognitive Science, 9, 445–453. doi: 10.1016/j.tics.2005.07.001 [DOI] [PubMed] [Google Scholar]
- Caharel S, Courtay N, Bernard C, Lalonde R, & Rebaï M (2005). Familiarity and emotional expression influence an early stage of face processing: An electrophysiological study. Brain and Cognition, 59, 96–100. doi: 10.1016/j.blandc.2005.05.005 [DOI] [PubMed] [Google Scholar]
- Caria A, de Falco S, Venuti P, Lee S, Esposito G, Rigo P, . . . Bornstein MH (2012). Species-specific response to human infant faces in the premotor cortex. NeuroImage, 60, 884–893. doi: 10.1016/j.neuroimage.2011.12.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellerino A, Borghetti D, & Sartucci C (2004). Sex differences in face gender recognition in humans. Brain Research Bulletin, 63, 443–449. doi: 10.1016/j.brainresbull.2004.03.010 [DOI] [PubMed] [Google Scholar]
- Corter CM, & Fleming AS (2002). Psychobiology of maternal behavior in human beings. In Bornstein MH (Ed.), Handbook of parenting vol. 2 biology and ecology of parenting (2nd ed., pp. 141–181). Mahwah, NJ: Erlbaum. [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley NM, Birbaumer N, & Lang PJ (2000). Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biological Psychology, 52, 95–111. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Damasio H, & van Hoesen GW (1982). Prosopagnosia: Anatomical basis and neurobehavioral mechanisms. Neurology, 32, 331–341. [DOI] [PubMed] [Google Scholar]
- Delorme A, & Makeig S (2004). EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134, 9–21. doi: 10.1016/j.jneumeth.2003.10.009 [DOI] [PubMed] [Google Scholar]
- Dennis S, Finnigan S, Geffen G, & Humphreys MS (2002). ERP “old/new” effects: Memory strength and decisional factor(s). Neuropsychologia, 40, 2288–2304. doi: 10.1016/S0028-3932(02)00113-6 [DOI] [PubMed] [Google Scholar]
- Di Russo F, Martinez A, & Hillyard SA (2003, May). Source analysis of event-related cortical activity during visuo-spatial attention. Cerebral Cortex, 13(5), 486–499. doi: 10.1093/cercor/13.5.486 [DOI] [PubMed] [Google Scholar]
- Doi H, & Shinohara K (2012). Event-related potentials elicited in mothers by their own and unfamiliar infants’ faces with crying and smiling expression. Neuropsychologia, 50, 1297–1307. doi: 10.1016/j.neuropsychologia.2012.02.013 [DOI] [PubMed] [Google Scholar]
- Dolcos F, & Cabeza R (2002). Event-related potentials of emotional memory: Encoding pleasant, unpleasant, and neutral pictures. Cognitive, Affective, & Behavioral Neuroscience, 2, 252–263. doi: 10.3758/CABN.2.3.252 [DOI] [PubMed] [Google Scholar]
- Ecker UKH, Zimmer HD, Groh-Bordin C, & Mecklinger A (2007). Context effects of familiarity are familiarity effects of context—An electrophysiological study. International Journal of Psychophysiology, 64, 146–156. doi: 10.16/j.ijpsycho.2007.01.005 [DOI] [PubMed] [Google Scholar]
- Eibl-Eibesfeldt I (1970). Ethology: The biology of behavior. Oxford, England: Holt, Rinehart, & Winston. [Google Scholar]
- Eimer M (2000). Event-related brain potentials distinguish processing stages involved in face perception and recognition. Clinical Neurophysiology, 111, 694–705. doi: 10.1016/S1388-2457(99)00285-0 [DOI] [PubMed] [Google Scholar]
- Eimer M, & Holmes A (2007). Event-related brain potential correlates of emotional face processing. Neuropsychologia, 45, 15–31. doi: 10.1016/j.neuropsychologia.2006.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo N, Endo M, Kirita T, & Maruyama K (1992). The effect of expression on face recognition. Tohoku Psychologia Folia, 52, 37–44. [Google Scholar]
- Everhart DE, Shucard JL, Quatrin T, & Shucard DW (2001). Sex-related ERP differences during face processing and facial affect. Neuropsychology, 15, 329–341. doi: 10.1037/0894-4105.15.3.329 [DOI] [PubMed] [Google Scholar]
- Feldman R (2012). Bio-behavioral synchrony: A model for integrating biological and microsocial behavioral processes in the study of parenting. Parenting: Science and Practice, 12, 154–164. doi: 10.1080/15295192.2012.683342 [DOI] [Google Scholar]
- Feldman R, Weller A, Zagoory-Sharon O, & Levine A (2007). Evidence for a neuroendocrinological foundation of human affiliation: Plasma oxytocin levels across pregnancy and the postpartum period predict mother-infant bonding. Psychological Science, 18, 965–970. doi: 10.1111/j.1467-9280.2007.02010.x [DOI] [PubMed] [Google Scholar]
- Fleming AS, Ruble D, Krieger H, & Wong PY (1997). Hormonal and experiential correlates of maternal responsiveness during pregnancy and the puerperium in human mothers. Hormones and Behavior, 31, 145–158. doi: 10.1006/hbeh.1997.1376 [DOI] [PubMed] [Google Scholar]
- Friedman D, & Johnson RE (2000). Event-related potential (ERP) studies of memory encoding and retrieval: A selective review. Microscopy Research and Technique, 51, 6–28. doi: 10.1002/1097-0029(20001001)51:1 [DOI] [PubMed] [Google Scholar]
- Fullgrabe U (2002). Psychologie der eigensicherung: Uberleben ist kein zufall [The psychology of self-protection: Survival is not an accident]. Stuttgart, Germany: Boorberg Verlag. [Google Scholar]
- Furl N, van Rijsbergen N, Treves A, Friston KJ, & Dolan RJ (2007). Experience-dependent coding of facial expression in superior temporal sulcus. Proceedings of the National Academy of Sciences of the United States of America, 104, 13485–13489. doi: 10.1073/pnas.0702548104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ, Anderson AW, Skudlarski P, & Gore JC (1999). Activation of the middle fusiform area increases with expertise in recognizing novel objects. Nature Neuroscience, 6, 568–573. doi: 10.1038/9224 [DOI] [PubMed] [Google Scholar]
- Glocker ML, Langleben DD, Ruparel K, Loughead JW, Valdez JN, Griffin MD, . . . Gur RC (2009). Baby schema modulates the brain reward system in nulliparous women. Proceedings of the National Academy of Sciences, 106, 9115–9119. doi: 10.1073/pnas.0811620106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso DJ, Moser JS, Dozier M, & Simons R (2009). ERP correlates of attention allocation in mothers processing faces of their children. Biological Psychology, 18, 95–102. doi: 10.1016/j.biopsycho.2009.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JA, & Gustafson GE (1983). Individual recognition of human infants on the basis of cries alone. Developmental Psychobiology, 16, 485–493. doi: 10.1002/dev.420160604 [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, & Dadds MR (2008). Oxytocin increases gaze to the eye-region of human faces. Biological Psychiatry, 63, 3–5. doi: 10.1016/j.biopsych.2007.06.026 [DOI] [PubMed] [Google Scholar]
- Hagoort P (2007). The memory, unification and control (MUC) model of language. In Meyer AS, Wheeldon LR, & Krott A (Eds.), Automaticity and control in language processing (pp. 243–270). New York, NY: Psychology Press. [Google Scholar]
- Hall JA, & Matsumoto D (2004). Gender differences in judgments of multiple emotions from facial expressions. Emotion, 4, 201–206. doi: 10.1037/1528-3542.4.2.201 [DOI] [PubMed] [Google Scholar]
- Hautecœur P, Debruyne P, Forzy G, Gallois P, Hache JC, & Dereux J-F (1993). Potentiels évoqués visuels et reconnaissance des visages: Influence de la célébrité et de I’expression émotionnelle [Visual evoked potentials and facial recognition: Influence of renown and emotional expression]. Revue Neurologique (Paris), 149, 207–212. [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, & Gobbini MI (2000). The distributed human neural system for face perception. Trends in Cognitive Sciences, 4, 223–233. doi: 10.1016/S1364-6613(00)01482-0 [DOI] [PubMed] [Google Scholar]
- Herron JE, Henson RNA, & Rugg MD (2004). Probability effects on the neural correlates of retrieval success: An fMRI study. Neuroimage, 21, 302–310. doi: 10.1016/j.neuroimage.2003.09.039 [DOI] [PubMed] [Google Scholar]
- Herzmann G, Schweinberger SR, Sommer W, & Jentzsch I (2004). What’s special about personally familiar faces? A multimodal approach. Psychophysiology, 41, 688–701. doi: 10.1111/j.1469-8986.2004.00196.x [DOI] [PubMed] [Google Scholar]
- Hollingshead AB (1975). Four-factor index of social status (Unpublished Manuscript). Yale University, New Haven, CT. [Google Scholar]
- Jemel B, Pisani M, Calabria M, Crommelinck M, & Bruyer R (2003). Is the N170 for faces cognitively penetrable? Evidence from repetition priming of Mooney faces of familiar and unfamiliar persons. Cognitive Brain Research, 17, 431–446. doi: 10.1016/S0926-6410(03)00145-9 [DOI] [PubMed] [Google Scholar]
- Kaan E, & Swaab T (2003). Repair, revision, and complexity in syntactic analysis: An electrophysiological differentiation. Journal of Cognitive Neuroscience, 15(1), 98–110. doi: 10.1162/089892903321107855 [DOI] [PubMed] [Google Scholar]
- Kaitz M, Good A, Rokem AM, & Eidelman AI (1987). Mothers’ recognition of their newborns by olfactory cues. Developmental Psychobiology, 20, 587–591. doi: 10.1002/dev.420200604 [DOI] [PubMed] [Google Scholar]
- Kaitz M, Good A, Rokem AM, & Eidelman AI (1988). Mothers’ and fathers’ recognition of their new-borns’ photographs during the postpartum period. Journal of Developmental & Behavioral Pediatrics, 9, 223–226. doi: 10.1097/00004703-198808000-00008 [DOI] [PubMed] [Google Scholar]
- Kaitz M, Lapidot P, Bronner R, & Eidelman AI (1992). Parturient women can recognize their infants by touch. Developmental Psychology, 28, 35–39. doi: 10.1037/0012-1649.28.1.35 [DOI] [Google Scholar]
- Kaitz M, Rokem AM, & Eidelman AI (1988). Infants’ face-recognition by primiparous and multiparous women. Perceptual & Motor Skills, 67, 495–502. doi: 10.2466/pms.1988.67.2.495 [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, & Chun MM (1997). The fusiform face area: A module in human extrastriate cortex specialized for face perception. Journal of Neuroscience, 17, 4302–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Leckman JF, Mayes LC, Feldman R, Wang X, & Swain JE (2010). The plasticity of human maternal brain: Longitudinal changes in brain anatomy during the early postpartum period. Behavioral Neuroscience, 124, 695–700. doi: 10.1037/a0020884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, Lehtonen A, Squire S, Harvey AG, Craske MG, Holliday IE, . . . Stein A (2008). A specific and rapid neural signature for parental instinct. PloS ONE, 3, e1664. doi: 10.1371/journal.pone.0001664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchuk A, Vibbert M, & Bornstein MH (1986).The perception of smiling and its experiential correlates in 3-month-old infants. Child Development, 57, 1054–1061. [PubMed] [Google Scholar]
- Lambert KG, & Kinsley CH (2012). Brain and behavioral modifications that accompany the onset of motherhood. Parenting: Science and Practice, 12, 74–88. doi: 10.1080/15295192.2012.638868 [DOI] [Google Scholar]
- Lang P, Bradley MM, & Cuthbert BN (1997). Motivated attention: Affect, activation, and action. In Lang P, Simons RF, & Balaban M (Eds.), Attention and orienting: Sensory and motivational processes (pp. 97–136). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Langeslag SJE, Franken IHA, & Van Strien JWV (2008). Dissociating love-related attention from task-related attention: An event-related potential oddball study. Neuroscience Letters, 431, 236–240. doi: 10.1016/j.neulet.2007.11.044 [DOI] [PubMed] [Google Scholar]
- Langeslag SJE, Jansma BM, Frankem IHA, & Van Strien JW (2007). Event-related potential responses to love-related facial stimuli. Biological Psychology, 76, 109–115. doi: 10.1016/j.biopsycho.2007.06.007 [DOI] [PubMed] [Google Scholar]
- Leibenluft E, Gobbini MI, Harrison T, & Haxby JV (2004). Mothers’ neural activation in response to pictures of their children and other children. Biological Psychiatry, 56, 225–232. doi: 10.1016/j.biopsych.2004.05.017 [DOI] [PubMed] [Google Scholar]
- Lenzi D, Trentini C, Pantano P, Macaluso E, Iacoboni M, Lenzi GL, & Ammaniti M (2008). Neural basis of maternal communication and emotional expression processing during infant preverbal stage. Cerebral Cortex. 19, 1124–1133. doi: 10.1093/cercor/bhn153 [DOI] [PubMed] [Google Scholar]
- Leon IG (2002). Adoption losses: Naturally occurring or socially constructed? Child Development, 73, 652–663. doi: 10.1111/1467-8624.00429 [DOI] [PubMed] [Google Scholar]
- Leppänen JM (2006). Emotional information processing in mood disorders: A review of behavioral and neuroimaging findings. Current Opinion in Psychiatry, 19, 34–39. [DOI] [PubMed] [Google Scholar]
- Lorenz K (1943). Die angeborenen Formen möglicher Erfahrung [Innate form of potential experience]. Zeitschrift für Tierpsychologie, 5, 235–309. doi: 10.1111/j.1439-0310.1943.tb00655.x [DOI] [Google Scholar]
- Lorenz K (1971). Studies in animal and human behavior (Vol. II). London, England: Methuen. [Google Scholar]
- Loveless NE, Simpson M, & Näätänen R (1987). Frontal negative and parietal positive components of the slow wave dissociated. Psychophysiology, 24, 340–345. doi: 10.1111/j.1469-8986.1987.tb00305.x [DOI] [PubMed] [Google Scholar]
- Luck SJ (2005). An introduction to the event-related potential technique. Boston, MA: MIT Press. [Google Scholar]
- Luck SJ, Heinze HJ, Mangun GR, & Hillyard SA (1990). Visual event-related potentials index focused attention within bilateral stimulus arrays. II. Functional dissociation of P1 and N1 components. Electroencephalography and Clinical Neurophysiology, 75, 528–542. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA, Mouloua M, Woldorff MG, Clark VP, & Hawkins HL (1994). Effect of spatial cueing on luminance detectability: Psychophysical and electrophysiological evidence for early selection. Journal of Experimental Psychology: Human Perception and Performance, 20, 887–904. [DOI] [PubMed] [Google Scholar]
- Mangun GR (1995). Neural mechanisms of visual selective attention. Psychophysiology, 32, 4–18. [DOI] [PubMed] [Google Scholar]
- Mangun GR, & Hillyard SA (1991). Modulations of sensory-evoked brain potentials indicate changes in perceptual processing during visual-spatial priming, Journal of Experimental Psychology: Human Perception and Performance, 17, 1057–1074. [DOI] [PubMed] [Google Scholar]
- Mangun GR, Hopfinger JB, Kussmaul CL, Fletcher EM, & Heinze HJ (1997). Covariations in ERP and PET measures of spatial selective attention in human extrastriate visual cortex. Human Brain Mapping, 5, 273–279. doi: 10.1002/(SICI)1097-0193(1997)5:4 [DOI] [PubMed] [Google Scholar]
- Martinez A, Anllo-Vento L, Sereno MI, Frank LR, Buxton RB, Dubowitz DJ, . . . Hillyard SA (1999). Involvement of striate and extrastriate visual cortical areas in spatial attention. Nature Neuroscience, 2(4), 364–369. [DOI] [PubMed] [Google Scholar]
- Mecklinger A (2000). Interfacing mind and brain: A neurocognitive model of recognition memory. Psychophysiology, 37, 565–582. doi: 10.1111/1469-8986.3750565 [DOI] [PubMed] [Google Scholar]
- Minnebusch DA, & Daum I (2009). Neuropsychological mechanisms of visual face and body perception. Neuroscience Biobehavioral Review, 33, 1133–1144. doi: 10.1016/j.neubiorev.2009.05.008 [DOI] [PubMed] [Google Scholar]
- Mognon A, Jovicich J, Bruzzone L, & Buiatti M (2011). ADJUST: An automatic EEG artifact detector based on the joint use of spatial and temporal features. Psychophysiology, 48, 229–240. doi: 10.1111/j.1469-8986.2010.01061.x [DOI] [PubMed] [Google Scholar]
- Münte TF, Matzke M, & Johannes S (1997). Brain activity associated with syntactic incongruencies in words and pseudo-words. Journal of Cognitive Neuroscience, 9, 318–329. doi: 10.1162/jocn.1997.9.3.318 [DOI] [PubMed] [Google Scholar]
- Münte TF, Urbach TP, Düzel E, & Kutas M (2000). Event-related brain potentials in the study of human cognition and neuropsychology. In Boller F, Grafman J, & Rizzolatti G (Eds.), Handbook of neuropsychology (Vol. 1, 2nd ed., (pp. 139–235). Amsterdam, The Netherlands: Elsevier Science Publishers. [Google Scholar]
- Nelson CA, Thomas KM, de Haan M, & Wewerka SS (1998). Delayed recognition memory in infants and adults as revealed by event-related potentials. International Journal of Psychophysiology, 29, 145–165. doi: 10.1016/S0167-8760(98)00014-2 [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Nelson EE, Rusch BD, Fox AS, Oakes TR, & Davidson RJ (2004). Orbitofrontal cortex tracks positive mood in mothers viewing pictures of their newborn infants. Neuroimage, 21, 583–592. doi: 10.1016/j.neuroimage.2003.10.005 [DOI] [PubMed] [Google Scholar]
- Noll LK, Mayes LC, & Rutherford HJV (2012). Investigating the impact of parental status and depression symptoms on the early perceptual coding of infant faces: An event-related potential study. Social Neuroscience, 7, 525–536. doi: 10.1080/17470919.2012.672457 [DOI] [PubMed] [Google Scholar]
- Noriuchi M, Kikuchi Y, & Senoo A (2008). The functional neuroanatomy of maternal love: Mother’s response to infant’s attachment behaviors. Biological Psychiatry, 63, 415–423. doi: 10.1016/j.biopsych.2007.05.018 [DOI] [PubMed] [Google Scholar]
- Olofsson JK, Nordin S, Sequeira H, & Polich J (2008). Affective picture processing: An integrative review of ERP findings. Biological Psychology, 77, 247–265. doi: 10.1016/j.biopsycho.2007.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco S, & Ehlers C (1998). Gender differences in electrophysiological responses to facial stimuli. Biological Psychiatry, 44, 281–289. doi: 10.1016/S0006-3223(97)00487-3 [DOI] [PubMed] [Google Scholar]
- Palermo R, & Rhodes G (2007). Are you always on my mind? A review of how face perception and attention interact. Neuropsychologia, 45, 75–92. doi: 10.1016/j.neuropsychologia.2006.04.025 [DOI] [PubMed] [Google Scholar]
- Papoušek H, & Papoušek M (2002). Intuitive parenting. In Bornstein MH (Ed.), Handbook of parenting vol. 2 biology and ecology of parenting (2e, pp. 183–203. Mahwah, NJ: Erlbaum. [Google Scholar]
- Pearson RM, Lightman SL, & Evans J (2009). Emotional sensitivity for motherhood: Late pregnancy is associated with enhanced accuracy to encode emotional faces. Hormones and Behavior, 56, 557–563. doi: 10.1016/j.yhbeh.2009.09.013 [DOI] [PubMed] [Google Scholar]
- Perrett DI, Smith PAJ, Potter DD, Mistlin AJ, Head AS, Milner AD, & Jeeves MA (1984). Neurons responsive to faces in the temporal cortex: Studies of functional organization, sensitivity to identity and relation to perception. Human Neurobiology, 3, 197–208. [PubMed] [Google Scholar]
- Polich J (2007). Updating P300: An integrative theory of P3a and P3b. Clinical Neurophysiology, 118, 2128–2148. doi: 10.1016/j.clinph.2007.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter RH, Cernoch JM, & McLaughlin FJ (1983). Maternal recognition of neonates through olfactory cues. Physiology & Behavior, 30, 151–154. doi: 10.1016/0031-9384(83)90051-3 [DOI] [PubMed] [Google Scholar]
- Proverbio AM, Brignone V, Matarazzo S, Del Zotto M, & Zani A (2006). Gender and parental status affect the visual cortical response to infant facial expression. Neuropsychologia, 44, 2987–2999. doi: 10.1016/j.neuropsychologia.2006.06.015 [DOI] [PubMed] [Google Scholar]
- Proverbio AM, Zani A, & Adorni R (2008). The left fusiform area is affected by written frequency of words. Neuropsychologia, 48, 2292–2299. doi: 10.1016/j.neuropsychologia.2008.03.024 [DOI] [PubMed] [Google Scholar]
- Puce A, Allison T, Asgari M, Gore JC, & McCarthy G (1996). Differential sensitivity of human visual cortex to faces, letter strings, and textures: A functional magnetic resonance imaging study. Journal of Neuroscience, 15, 5205–5215. doi: 10.1006/nimg.2000.0612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purhonen M, Kilpeläinen-Lees R, Pääkkönen A, Yppärilä H, Lehtonen J, & Karhu J (2001). Effects of maternity on auditory event-related potentials to human sound. Neuroreport, 12, 2975–2979. [DOI] [PubMed] [Google Scholar]
- Purhonen M, Pääkkönen A, Yppärilä H, Lehtonen J, & Karhu J (2001). Dynamic behavior of the auditory N100 elicited by a baby’s cry. International Journal of Psychophysiology, 41, 271–278. doi: 10.1016/S0167-8760(01)00139-8 [DOI] [PubMed] [Google Scholar]
- Purhonen M, Valkonen-Korhonen M, & Lehtonen J (2008). The impact of stimulus type and early motherhood on attentional processing. Developmental Psychobiology, 50, 600–607. doi: 10.1002/dev.20321 [DOI] [PubMed] [Google Scholar]
- Ranote S, Elliott R, Abel KM, Mitchell R, Deakin JF, & Appleby L (2004). The neural basis of maternal responsiveness to infants: An fMRI study. Neuroreport, 15, 1825–1829. doi: 10.1097/01.wnr.0000137078.64128.6a [DOI] [PubMed] [Google Scholar]
- Renault B, Signoret JL, Debruille B, Breton F, & Bolgert F (1989). Brain potentials reveal covert facial recognition in prosopagnosia. Neuropsychologia, 27, 905–912. doi: 10.1016/0028-3932(89)90066-3 [DOI] [PubMed] [Google Scholar]
- Reynolds GD, & Richards JE (2005). Familiarization, attention, and recognition memory in infancy: An event-related potential and cortical source localization study. Developmental Psychology, 41, 598–615. doi: 10.1037/0012-1649.41.4.598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righi S, Marzi T, Toscani M, Baldassi S, Ottonello S, & Viggiano MP (2012). Fearful expressions enhance recognition memory: Electrophysiological evidence. Acta Psychologica (Amst), 139, 7–18. doi: 10.1016/j.actpsy.2011.09.015 [DOI] [PubMed] [Google Scholar]
- Ritter W, & Ruchkin DS (2006). A review of event-related potential components discovered in the context of studying P3. Annals of the New York Academy of Sciences, 658, 1–32. doi: 10.1111/j.1749-6632.1992.tb22837.x [DOI] [PubMed] [Google Scholar]
- Rodrigo MJ, León I, Quiñones I, Lage A, Byrne S, & Bobes MA (2011). Brain and personality bases of insensitivity to infant cues in neglectful mothers: An event-related potential study. Development and Psychopathology, 23, 163–176. doi: 10.1017/S0954579410000714 [DOI] [PubMed] [Google Scholar]
- Rosenkrants B, & Polich J (2008). Affective ERP processing in a visual oddball task: Arousal, valence, and gender. Clinical Neurophysiology, 119, 2260–2265. doi: 10.1016/j.clinph.2008.07.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg MD (1995). ERP studies of memory. In Rugg MD & Coles MGH (Eds.), Electrophysiology of mind (pp. 132–170). New York, NY: Oxford University Press. [Google Scholar]
- Rugg MD, & Curran T (2007). Event-related potentials and recognition memory. Trends in Cognitive Sciences, 11, 251–257. doi: 10.1016/j.tics.2007.04.004 [DOI] [PubMed] [Google Scholar]
- Rugg MD, & Henson RNA (2002). Episodic memory retrieval: An (event-related) functional neuroimaging perspective. In Parker AE, Wilding EL, & Bussey T (Eds.), The cognitive neuroscience of memory encoding and retrieval (pp. 3–37). New York, NY: Psychology Press. [Google Scholar]
- Rugg MD, Otten LJ, & Henson RNA (2002). The neural basis of episodic memory: Evidence from functional neuroimaging. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 357, 1097–1110. doi: 10.1098/rstb.2002.1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford HJV, & Mayes LC (2011). Primary maternal preoccupation: Using neuroimaging techniques to explore the parental brain. Psyche, 65, 973–988. [Google Scholar]
- Schupp HT, Junghofer M, Weike AI, & Hamm AO (2004). The selective processing of briefly presented affective pictures: An ERP analysis. Psychophysiology, 41, 441–449. doi: 10.1111/j.1469-8986.2004.00174.x [DOI] [PubMed] [Google Scholar]
- Schweinberger SR, Pickering EC, Jentzsch I, Burton AM, & Kaufmann JM (2002). Event-related brain potential evidence for a response of inferior temporal cortex to familiar face repetitions. Cognitive Brain Research, 14, 398–409. doi: 10.1016/S0926-6410(02)00142-8 [DOI] [PubMed] [Google Scholar]
- Seifritz E, Esposito F, Neuhoff JG, Lüthi A, Mustovic H, Dammann G,...Di Salle F (2003). Differential sex-independent amygdale response to infant crying and laughing in parents versus nonparents. Biological Psychiatry, 54, 1367–1375. doi: 10.1016/S0006-3223(03)00697-8 [DOI] [PubMed] [Google Scholar]
- Smith ME, & Halgren E (1987). Event-related potentials elicited by familiar and unfamiliar faces. Current Trends in Event-Related Potential Research (EEG Suppl.), 40, 422–426. doi: 10.1016/0028-3932(88)90034-6 [DOI] [PubMed] [Google Scholar]
- Soltani M, & Knight RT (2000). Neural origins of the P300. Critical Reviews in Neurobiology, 14, 199–224. [PubMed] [Google Scholar]
- Spehlmann R (1965). The average electrical responses to diffuse and to patterned light in the human. Electorencephology and Clinical Neuropsychology, 19, 560–569. doi: 10.1016/0013-4694(65)90241-5 [DOI] [PubMed] [Google Scholar]
- Squires NK, Squires KC, & Hillyard SA (1975). Two varieties of long-latency positive waves evoked by unpredictable auditory stimuli in man. Electroencephalography and Clinical Neurophysiology, 4, 387–401. doi: 10.1016/0013-4694(75)90263-1 [DOI] [PubMed] [Google Scholar]
- Strathearn L, Li J, Fonagy P, & Montague PR (2008). What’s in a smile? Maternal brain responses to infant facial cues. Pediatrics, 122, 40–51. doi: 10.1542/peds.2007-1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Lorberbaum JP, Kose S, & Strathearn L (2007). Brain basis of early parent-infant interactions: Psychology, physiology, and in vivo functional neuroimaging studies. Journal of Child Psychology and Psychiatry, 48, 262–287. doi: 10.1111/j.1469-7610.2007.01731.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamis-LeMonda CS, Bornstein MH, Baumwell L, & Damast AM (1996). Responsive parenting in the second year: Specific influences on children’s language and play. Early Development and Parenting, 5, 173–183. [Google Scholar]
- Tanaka JW, Curran T, Porterfield AL, & Collins D (2006). Activation of preexisting and acquired face representations: The N250 event-related potential as an index of face familiarity. Journal of Cognitive Neuroscience, 18, 1488–1497. doi: 10.1162/jocn.2006.18.9.1488 [DOI] [PubMed] [Google Scholar]
- Thayer JF, & Johnsen BH (2000). Sex differences in judgment of facial affect: A multivariate analysis of recognition errors. Scandinavian Journal of Psychology, 41, 243–246. doi: 10.1111/1467-9450.00193 [DOI] [PubMed] [Google Scholar]
- Tong F, & Nakayama K (1999). Robust representations for faces: Evidence from visual search. Journal of Experiment Psychology: Human Perception and Performance, 25, 1016–1035. doi: 10.1037/0096-1523.25.4.1016 [DOI] [PubMed] [Google Scholar]
- Tsivilis D, & Otten LJ (2001). Context effects on the neural correlates of recognition memory: An electrophysiological study. Neuron, 31, 497–505. doi: 10.1016/S0896-6273(01)00376-2 [DOI] [PubMed] [Google Scholar]
- Van Voorhis S, & Hillyard SA (1977). Visual evoked potentials and selective attention to points in space. Perception & Psychophysics, 22(1), 54–62. doi: 10.3758/BF03206080 [DOI] [Google Scholar]
- Vilberg KL, Moosavi RF, & Rugg MD (2006). The relationship between electrophysiological correlates of recollection and amount of information retrieved. Brain Research, 1122, 161–170. doi: 10.1016/j.brainres.2006.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, & Pourtois G (2007). Distributed and interactive brain mechanisms during emotion face perception: Evidence from functional neuroimaging. Neuropsychologia, 45, 174–194. doi: 10.1016/j.neuropsychologia.2006.06.003 [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, & Buckner RL(2005). Parietal lobe contributions to episodic memory retrieval. Trends in Cognitive Sciences, 9, 445–453. doi: 10.1016/j.tics.2005.07.001 [DOI] [PubMed] [Google Scholar]
- Wilding EL (2002). In what way does the parietal ERP old/new effect index recollection? International Journal of Psychophysiology, 77, 81–87. doi: 10.1016/S0167-8760(99)00095-1 [DOI] [PubMed] [Google Scholar]
- Wilding EL, & Rugg MD (1996). An event-related potential study of recognition memory with and without retrieval of source. Brain, 119, 889–905. doi: 10.1093/brain/119.3.889 [DOI] [PubMed] [Google Scholar]
- Wild-Wall N, Dimigen O, & Sommer W (2008). Interaction of facial expressions and familiarity: ERP evidence. Biological Psychology, 77, 138–149. doi: 10.1016/j.biopsycho.2007.10.001 [DOI] [PubMed] [Google Scholar]
- Woodruff CC, Hayama HR, & Rugg MD (2006). Electrophysiological dissociation of the neural correlates of recollection and familiarity. Brain Research, 1100, 125–135. doi: 10.1016/j.brainres.2006.05.019 [DOI] [PubMed] [Google Scholar]
- Yang CL, Perfetti CA, & Schmalhofer F (2007). Event-related potential indicators of text integration across sentence boundaries. Journal of Experimental Psychology: Learning, Memory, and Cognition, 33, 55–89. doi: 10.1037/0278-7393.33.1.55 [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Otten LJ, Shaw KN, & Rugg MD (2005). Separating the brain regions involved in recollection and familiarity in recognition memory. Journal of Neuroscience, 25, 3002–3008. doi: 10.1523/JNEUROSCI.5295-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yovel G, & Paller KA (2004). The neural basis of the butcher-on-the-bus phenomenon: When a face seems familiar but is not remembered. Neuroimage, 21, 789–800. doi: 10.1016/j.neuroimage.2003.09.034 [DOI] [PubMed] [Google Scholar]
- Zebrowitz LA (2006). Finally, faces find favor. Social Cognition, 24, 657–701. doi: 10.1521/soco.2006.24.5.657. [DOI] [Google Scholar]