Abstract
Mutations in the protein kinase A (PKA) regulatory subunit type 1A (PRKAR1A) and armadillo repeat-containing 5 (ARMC5) genes cause Cushing syndrome (CS) due to primary pigmented nodular adrenocortical disease (PPNAD) and primary bilateral macronodular adrenocortical hyperplasia (PBMAH), respectively. Between the two genes, ARMC5 is highly polymorphic with several variants in the population, whereas PRKAR1A has very little, if any, non-pathogenic variation in its coding sequence. We tested the hypothesis that ARMC5 variants may affect the clinical presentation of PPNAD and CS among patients with PRKAR1A mutations. In this study, 91 patients with PPNAD due to PRKAR1A mutations were tested for abnormal cortisol secretion or CS and for ARMC5 sequence variants. Abnormal cortisol secretion was present in 71 of 74 patients with ARMC5 variants, whereas 11 of 17 patients negative for ARMC5 variants did not present hypercortisolemia. Presence of ARMC5 variants was a statistically strong predictor of CS among patients with PRKAR1A mutations (p<0.001). Among patients with CS due to PPNAD, ARMC5 variant was associated with lower cortisol levels at baseline (p=0.04) and after high dose dexamethasone administration (p=0.02). The ARMC5 p.I170V variant increased ARMC5 protein accumulation in vitro and decreased viability of NCI-H295 cells (but not HEK 293T cells). PPNAD tissues with ARMC5 variants showed stronger ARMC5 protein expression versus those that carried a normal ARMC5 sequence. Taken together, our results suggest that ARMC5 variants among patients with PPNAD due to PRKAR1A defects may play the role of a genetic modifier for the presence and severity of hypercortisolemia.
Keywords: Cortisol, adrenocortical hyperplasia, PRKAR1A gene, ARMC5 gene
INTRODUCTION
Cyclic adenosine monophosphate (cAMP)-dependent protein kinase or PKA mediates most cAMP signaling in the adrenal cortex through its regulatory (R) and catalytic (C) subunits (Almeida & Stratakis 2011, de Joussineau et al. 2012). There are four different R-subunits (RIα, RIβ, RIIα and RIIβ) and four C-subunits (Cα, Cβ, and Cγ, and PRKX); each are coded by their own genes (PRKAR1A, PRKAR1B, PRKAR2A, PRKAR2B, PRKACA, PRKACB, PRKACG, and PRKAX, respectively) (Almeida & Stratakis 2011). RIα, Cα and Cβ defects have been implicated in corticotrophin (ACTH)-independent, adrenocortical Cushing syndrome (CS) (Hannah-Shmouni & Stratakis 2020, Stratakis 2014, Espiard et al. 2018). PRKAR1A mutations cause Carney complex (CNC), a rare multiple neoplasia syndrome inherited in an autosomal dominant manner (Kirschner et al. 2000, Horvath et al. 2010), and manifesting with primary pigmented nodular adrenocortical disease (PPNAD), as well as (rarely) cortisol-producing adenomas (CPA) causing CS (Bertherat et al. 2009, Bertherat et al 2003). The importance of the role of cAMP-signaling for adrenocortical function and regulation of cortisol secretion was further enhanced by the discovery of cAMP-phosphodiesterase 11 A (PDE11A) defects in association with PPNAD and PPNAD-like lesions and CS (Horvath et al. 2006).
cAMP-signaling defects and abnormal PKA activity have also been implicated in another form of adrenocortical CS, primary bilateral macronodular adrenocortical hyperplasia (PBMAH) (Bordeau et al. 2006, Almeida et al., 2011, Almeida et al., 2012). In both PPNAD and PBMAH, regardless of the underlying primary defect, other genes or pathways appear to play a modifier role in the clinical phenotype of the affected patients and/or the onset and severity of hypercortisolemia and CS. For example, somatic CTNNB1 mutations have been described in patients with PRKAR1A defects that present with a CPA in addition to PPNAD and severe CS (Tadjine et al. 2008). Furthermore, PDE11A variants may increase the severity of CS in PPNAD (Libé et al., 2015), and participate in the pathogenesis of other adrenocortical lesions, from PBMAH to CPAs, and possibly adrenocortical cancer (Vezzosi et al. 2012, Libé et al., 2008).
Mutations of the armadillo repeat-containing 5 (ARMC5) gene are the main causative genetic defect of PBMAH (Assié et al. 2013, Faucz et al. 2104), although others exist, too, from GNAS1 to MEN1 and GIPR (Hsiao et al. 2009, Lecoq et al. 2017). In addition to PDE11A (Vezzosi et al. 2012, Libé et al., 2008).), it has long been suspected that additional genes or pathways (Bourdeau et al. 2004, Bimpaki et al. 2010) may modify the phenotype of CS among patients with PBMAH that can be very variable, from asymptomatic to cyclical and, less frequently, severe CS (Hsiao et al. 2009). Yet, little is known about the newly identified ARMC5 gene and its possible interactions or regulators.
The highly polymorphic ARMC5 gene in the general population is widely expressed in human tissues, particularly in the adrenal cortex (Berthon et al. 2017a). Conversely, PRKAR1A is also ubiquitously expressed but, unlike ARMC5, does not have frequent coding variants in the general population (Horvath et al. 2010, Bertherat et al. 2009). Hypercortisolemia due to PRKAR1A defects may also lead to CS of varying degrees of severity: PPNAD can be insidious or lead to early onset, pediatric CS (Sarlis et al. 1997, Pereira et al. 2010, Lowe et al. 2017). Like CTNNB1 (Tadjine 2008) and PDE11A (Libé et al. 2011) defects in the past, in this study, we tested the hypothesis that ARMC5 variants may play a role in the adrenocortical expression of a PRKAR1A-inactivating mutation, causative of PPNAD and CS. The data suggest that ARMC5 may have a role in regulating cortisol secretion in PPNAD.
MATERIALS & METHODS
Clinical and DNA studies
Patients were evaluated at the NIH Clinical Center between 1995 and 2020 under protocol 95-CH-0059. Ethics approval was granted from the Institutional Review Boards of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) (until 2010) and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (2010 to present), NIH (Clinical Trial Registration no. NCT00001452). For all patients, informed consents were obtained; assents were signed by minors and consents obtained from their parents, as appropriate.
A total of 91 patients with Carney complex and PPNAD who were evaluated at the National Institutes of Health (NIH) for hypercortisolemia and CS, and were older than 20 years of age at the time of the last follow-up or had already developed CS were included in the study. Patients were initially examined for clinical features of Carney complex per established guidelines (Stratakis & Raygada 1993). The biochemical diagnosis of hypercortisolemia relied upon elevations in 24-h urinary free cortisol (UFC) above the upper cutoffs of reference intervals; loss of diurnal circadian rhythm in midnight salivary cortisol; and lack of suppression or a paradoxical response of serum cortisol following the overnight high dose dexamethasone suppression test, as detailed elsewhere (Stratakis & Raygada 1993, Stratakis et al. 1999, Tirosh et al. 2016). ACTH-independent hypercortisolemia was ascertained through ACTH measurement, second-tier biochemical testing and radiographic imaging. Tumor samples were obtained from patients, as previously described (Bertherat et al. 2003, Horvath et al. 2006).
DNA was extracted from peripheral blood and fresh-frozen tissues, as previously described (Kirschner et al. 2000, Horvath et al. 2010, Bertherat et al. 2009, Libé et al. 2011). Sequencing for PRKAR1A and ARMC5 genes was obtained, as we have described elsewhere (Kirschner et al. 2000, Horvath et al. 2010, Bertherat et al. 2009, Assié 2013, Faucz et al. 2014). The coding and the flanking intronic sequences were sequenced for both genes.
In silico modeling
Germline variants in ARMC5 were evaluated by MutationTaster (http://www.mutationtaster.org), Polymorphism Phenotyping v2 algorithm tool (PolyPhen-2) (http://genetics.bwh-.harvard.edu/pph2), and SIFT (Sorting Tolerant From Intolerant) algorithm (http://sift.jcvi.org) to predict the possible impact of the amino acid substitution on the structure and function of a human protein, as we have described elsewhere (Assié et al. 2013).
DNA constructs, cell culture, and ARMC5 expression studies
The human ARMC5 WT (NM_001105247.1) coding sequence was cloned into the pCMV6-Entry vector with C-terminal Myc-DDK tag (RC226267, Origene, Rockville, MD, USA). The p.Ile170Val variant was introduced into the human ARMC5 WT template using the QuikChange Lightning Site-directed Mutagenesis Kit (210518–5, Agilent Technologies, Santa Clara, CA, USA), following the manufacturer’s protocol. The following primers were used for mutagenesis:
ARMC5-Ile170Val MUT _F: GCCGTTCGGTTCTGGACGCTGTCTGTCTTCATG
ARMC5-Ile170Val MUT_R: CATGAAGACAGACAGCGTCCAGAACCGAACGGC
Cell culture
Human Embryonic Kidney 293T cells (HEK 293T) were grown in Dulbecco’s modified Eagle’s medium (DMEM, 10313, Gibco) supplemented with 10% fetal bovine serum (100–106, Gemini Bio Products, USA) and 1% antibiotic (Penicillin-Streptomycin – 15140–148, Gibco, USA). NCI-H295 cells were grown in Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F-12, A4192002, Gibco), supplemented with 2% fetal bovine serum (100–106, Gemini Bio Products), 1% antibiotic (Penicillin-Streptomycin – 15140–148, Gibco) and 5μg/ml insulin, 5μg /ml transferrin and 5ng/ml sodium selenite (1% ITS – 41400045, Thermo Fisher, USA). Cell were incubated in a humidified atmosphere at 37°C with 5% CO2.
Analysis of protein expression in vitro
HEK 293T and NCI-H295 cells were seeded into 6-well plates at a density of 3×105 and 5×105 cells per well respectively. After 24h of incubation, HEK 293T cells were transfected with Lipofectamine 2000 (11668030, Invitrogen, USA) using Opti-MEM I Reduced Serum Medium (31985–070, Gibco) and NCI-H295 cells were transfected with Effectene Transfection Reagent (301425, Qiagen, Germany) following manufacturer’s protocol. 500ng of each vector was used for the transfection and the empty pCMV6-Entry vector was used as a negative control. After 24 hours of transfection, cells were washed with PBS and resuspended in 50μl of ice-cold lysis buffer (Tris-HCl 10 mM, pH 7,5, NaCl 150 mM, EDTA 1 mM, EGTA 1 mM, SDS 0.1%, Nonidet P-40 1%) containing a cocktail of protease and phosphatase inhibitors (PPC1010, Sigma-Aldrich, USA). The collected cells were incubated for 30 minutes on ice and centrifuged for 15 minutes at 4 °C, 13000 rpm. Total protein concentration of supernatant was determined by Pierce™ BCA Protein Assay (23227, Thermo Scientific), following the manufacturer’s protocol. After quantification of protein extracts, 50μg of total proteins of each sample were separated by electrophoresis in 10% polyacrylamide gel under denaturing conditions (SDS-PAGE). Proteins were transferred to a nitrocellulose membrane (1620115, BioRad, USA) and Western Blot was performed using antibodies against DDK diluted 1:1000 (NB600–345, Novus Biologicals, USA), and GAPDH diluted 1:2000 (SC-32233, Santa Cruz Biotechnology, USA). Fluorescent secondary antibodies (827–08364 IRDye 800CW Goat anti-Mouse and 926–68073 IRDye 680RD Donkey anti Rabbit, LiCor, USA) diluted 1:20000 and Odyssey CLx Imaging System (Licor, USA) were used to acquire the signal of the bands. Densitometric quantification was performed using ImageJ software and a ratio between the intensity of ARMC5 and GAPDH bands was calculated.
Cell viability studies
HEK 293T and NCI-H295 cells were seeded into 96-well plates at a density of 5×103 and 1×104 cells per well respectively. Cells were transfected as described above for 2, 24 and 48 hours. In the end of incubation time, MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium salt) was added to a final concentration of 0.5 mg/mL into the culture media and incubated for 3 h at 37°C. Media was removed and 200 μL of isopropanol containing acetic acid 0.04M was added into the wells to solubilize the product reduced by MTT and absorbance was measured at 570 nm.
Immunohistochemistry (IHC)
IHC was performed on serial sections from paraffin-embedded adrenal tissue from selected patients. Slides were deparaffinized in Histoclear (HS-202, National diagnostics, USA) and rehydrated through ethanol gradient. Epitope retrieval was done in Vector Antigen Retrieval Solution (H3300, Vector Labs, USA) at 95°C. ARMC5 antibody (NBP1–94024, Novus, USA) was diluted 1:50 and sections were incubated overnight at 4°C. Detection of the primary antibody was performed using an anti-rabbit antibody coupled to biotin (111–065-144, Jackson Immuno Research Laboratory, USA) followed by streptavidin-HRP amplification (016–030-084, Jackson Immuno Research Laboratory). HRP activity was detected with 3,3’-diaminobenzidinetetrahydrochloride (DAB) (SK-4105, Vector Labs). Negative controls were not used since the tissue itself showed positive and negative ARMC5 staining. Representative pictures of the staining were taken in an inverted light microscope at 5x and 10x objective lenses. Quantification of DAB positive staining was performed using ImageJ software and optical density (OD) was calculated as follows: OD = log(maximum intensity/mean intensity).
Statistics
Categorical data are presented as percentage and proportions and were analyzed using χ2 test or Fisher exact test as appropriate. Continuous data were checked for normality based on histogram distribution and the Shapiro-Wilk statistical test. Based on the above results, continuous data normally distributed are presented as mean (standard deviation, SD) and were compared between groups using student’s t-test. Continuous data not normally distributed are presented as median (inter-quartile range: 25th – 75th percentile) and were compared between groups using Wilcoxon rank-signed test. Hazard risk analysis for diagnosis of CS based on ARMC5 genotype status was performed using cox proportional hazard and is presented as hazard ratio (HR, 95% confidence intervals). Statistical analysis of clinical data was performed in R. Data related to functional assays are presented as the mean (standard error of the mean) and P-value was obtained by two-way ANOVA followed by Tukey’s multiple comparisons test carried out using the software GraphPadPrism 6 (GraphPad®).
RESULTS
ARMC5 germline variants in patients with PPNAD and PRKAR1A defects
The germline variants in ARMC5 that were identified among patients with PPNAD carriers of PRKAR1A mutations are listed in Table 1. They have all been described previously, and most have been predicted to be benign by all in silico analyses.
Table 1: ARMC5 variants and in silico prediction.
ARMC5 variants found in germline DNA of PPNAD individuals with Cushing syndrome. Minor allele frequency (MAF) and in silico prediction for pathogenicity are listed for the variants found.
| Intronic | c.DNA | Protein | MAF (%) gnomAD |
CNC* | CNC**+ Cushing's |
Pathogenicity (Varsome) |
Pathogenicity (Mutation Taster) |
Pathogenicity (SIFT) |
Conservation (GERP RS) |
rs number |
|---|---|---|---|---|---|---|---|---|---|---|
| c.41T>A | p.F14Y | 4.84 | 1.65 | 2.02 | Benign | Polymorphism | Tolerated | 4.95 | rs151069962 | |
| c.328G>A | p.A110T | 0.31 | 0.55 | 0.68 | Benign | Polymorphism | Tolerated | 2.5 | rs201768837 | |
| c.438G>A | p.R146 | 0.83 | 1.1 | 1.35 | Benign | Disease causing | N/A | 2.53 | rs201280100 | |
| c.508A>G | p.I170V | 3.64 | 2.19 | 2.7 | Benign | Polymorphism | Tolerated | 4.15 | rs35923277 | |
| c.909G>C | p.L303 | 0.03 | 0.55 | 0.68 | Benign | Disease causing | N/A | 3.5199 | rs377221865 | |
| c.968G>C | p.G323A | 0.0287 | 0.55 | 0.68 | Benign | Polymorphism | Tolerated | 3.5499 | rs35461188 | |
| c.1223A>G | p.Q408R | 0.317 | 0.55 | 0.68 | Benign | Polymorphism | Tolerated | 4.67 | rs141923065 | |
| c.1520C>T | p.P507L | 2.12 | 2.19 | 2.7 | Benign | Polymorphism | Tolerated | 0.4519 | rs142376949 | |
| c.1641G>A | p.A547 | 0.487 | 0.55 | 0.68 | Benign | Disease causing | N/A | −7.38 | rs61732352 | |
| c.1740C>T | p.L580 | 0.01569 | 0.55 | 0.68 | Uncertain | Disease causing | N/A | −11.3999 | rs201720272 | |
| c.1842C>G | p.L614 | 6.55 | 4.94 | 6.08 | Benign | Polymorphism | N/A | −3.43 | rs55800131 | |
| c.2114C>T | p.A705V | 40.6 | 45.05 | 53.38 | Benign | Polymorphism | Damaging*** | −7.2699 | rs11150624 | |
| g.1978A>G | c.475+58A>G | N/A | 29.5 | 18.13 | 14.19 | Benign | Polymorphism | N/A | −0.666 | rs9926717 |
91 patients with a total of 182 alleles examined
74 patients with 148 alleles examined
predicted as damaging in only one out of 11 prediction tools
N/A: Not applicable
GERP: Genomic Evolutionary Rate Profiling (GERP) is a conservation score calculated by quantifying substitution deficits across multiple alignments of orthologues using the genomes of 35 mammals. It ranges from −12.3 to 6.17, with 6.17 being the most conserved. GERP RS: typical score. It quantifies position-specific constraint in terms of rejected substitutions (RS) by estimating the actual number of substitutions at that site and subtracting it from the number expected assuming neutrality.
Among the 91 patients that met the eligibility criteria for the study (PRKAR1A mutation status confirmed and adequate screening for hypercortisolemia or CS), there were 74 patients that had an ARMC5 variant; among them, 71 (96%) had CS and only 3 (4%) did not have CS. Among those that did not have an ARMC5 variant (N=17) 6 (35%) had CS and 11 (65%) did not. This clear excess of PPNAD patients with an ARMC5 variant that had CS was highly statistically significant (p<0.001). There were no specific ARMC5 variants that were reliably statistically overrepresented among patients with CS, given the small numbers and the rarity of some of the sequences. Data is shown in the Supplementary Table 1. As expected from its population frequency (gnomAD database), the p.A705V variant was the most frequent; it was even more frequent among patients with CS, but the difference was not significant (p=0.11).
Clinical data analysis for patients with CS and ARMC5 variant carriers vs. non-carriers
Collective clinical data for patients diagnosed with CS are presented in Table 2. There were no differences in the median age of diagnosis of CS between patients with no ARMC5 variants and those with a variant (18.9 vs. 19.4, respectively) or in mean BMI z-score (1.5 vs. 1.4, respectively). However, patients with ARMC5 variants had consistently lower cortisol levels that those without variants: median midnight cortisol levels (7.7 vs. 17.6 μg/dl, respectively, p= 0.06), median urinary free cortisol (UFC) levels adjusted for upper limit of normal (ULN) (0.4-fold increase vs. 2.34-fold increase, respectively, p= 0.01), and median cortisol levels in response to high dose dexamethasone adjusting for baseline cortisol (12.2 vs. 25.8 μg/dl, respectively, p= 0.02) were lower. These differences did not depend on the presence of particular variants, when we analyzed the data per the most frequent ARMC5 sequence alterations. No difference in the prevalence of disorders of glucose metabolism or elevated blood pressure were observed between patients with and without ARMC5 variants.
Table 2: Clinical profile of patients with Cushing syndrome.
Clinical and biochemical data of patients with Cushing syndrome included in the study overall and according to ARMC5 genotype. Values represent the median (Min-Max).
| No ARMC5 variant | ARMC5 variant present | Overall | p-value* | |
|---|---|---|---|---|
| Age at diagnosis, years 1 | 18.9 (17.5-21.5), n=6 | 19.4 (13.6-34.1), n= 59 | 19.4 (13.6-33.5) | 0.73 |
| Serum midnight cortisol, μg/dL 1 | 17.6 (14.4-20.9), n= 5 | 7.7 (4.3-14.9), n=47 | 8.2 (4.7-16.1) | 0.06 |
| Serum morning cortisol, μg/dL 1 | 20.9 (15.5-21.4), n=5 | 12.1 (9.2-16.6), n=48 | 12.5 (10.1-18.6) | 0.04 |
| 24-hour UFC corrected for ULN, xfold change 1 | 2.34 (1.8-3.3), n=4 | 0.38 (0.1-0.5), n=33 | 0.44 (0.15-0.91) | 0.01 |
| Serum cortisol after low dose DST, μg/dL 1 | 26 (17.5-36.7), n= 3 | 10.4 (6.8-16.5), n= 24 | 12.1 (7.1-17.4) | 0.26 |
| Serum cortisol after high dose DST, μg/dL 1 | 25.8 (21.4-32.2), n= 4 | 12.2 (7.4-16.7), n= 30 | 13.4 (7.5-19.3) | 0.02 |
| Weight z-score at diagnosis 1 | 0.8 (−1.1- 1.5), n=5 | 1.5 (0.6- 2.0), n=49 | 1.3 (0.5-2.0) | 0.33 |
| BMI z-score at diagnosis | 1.5 (1.1), n=5 | 1.4 (1.0), n= 49 | 1.4 (1.0) | 0.86 |
| Fasting Glucose, mg/dL 1 | 89 (81.3-93.3), n=4 | 85 (80-92), n= 33 | 87 (80-92) | 0.87 |
| Fasting Insulin, mIU/L 1 | 8.7, n=1 | 9.1 (6.9-14.2), n=29 | 9 (7-13.8) | 0.86 |
| Diagnosis of prediabetes or diabetes, n(%) | Yes: 1 (50) No: 1 (50) |
Yes: 5 (18.5) No: 22 (81.5) |
Yes: 6 (20.7) No: 23 (79.3) |
0.38 |
| Diagnosis of prehypertension or hypertension, n(%) | Yes: 4 (80) No: 1 (20) |
Yes: 32 (66.7) No:16 (33.3) |
Yes: 36 (68) No: 17 (32) |
0.99 |
: indicates the number of available data for each variable
Indicates not normally distributed data
Corresponds to comparison between patients without ARMC5 variant and with ARMC5 variant
BSA: Body surface area, DST: Dexamethasone suppression test, UFC: Urinary free cortisol
On hazard risk assessment analysis of diagnosis of CS based on ARMC5 genotype status, patients with ARMC5 variants tended to be diagnosed with CS earlier (Figure 1A), however, the numbers of patients without ARMC5 variants was small compared to those with a variant [HR: 1.93 (0.83–4.52), p= 0.13], and no statistical significant differences were observed. When we examined individual variants with sufficient number of events, it appeared that the higher risk was identified in patients with a single ARMC5 variant (Figure 1B), the p.I170V [HR: 3.67 (0.84–15.96), p= 0.08]. Although patients with the p.I170V variant were also carriers for the common p.A705V variant, when we looked only at the p.A705V variant compared to patients with no ARMC5 variant the risk was lower [HR: 1.77 (0.74–4.28), p= 0.20]. Indeed, the four patients that had CS due to PPNAD and carried the p.I170V ARMC5 variant, not only developed CS at an earlier age, but also all had severe CS and required bilateral adrenalectomy.
Figure 1: Event plot of the time of Cushing syndrome (CS) diagnosis.
A. Age of PPNAD patients at Cushing syndrome (CS) diagnosis based on the presence of any variant in the ARMC5 gene. B. Age of PPNAD patients at CS diagnosis based on the presence of the p.I170V ARMC5 variant compared to patients with no ARMC5 variants.
In vitro expression and proliferation studies
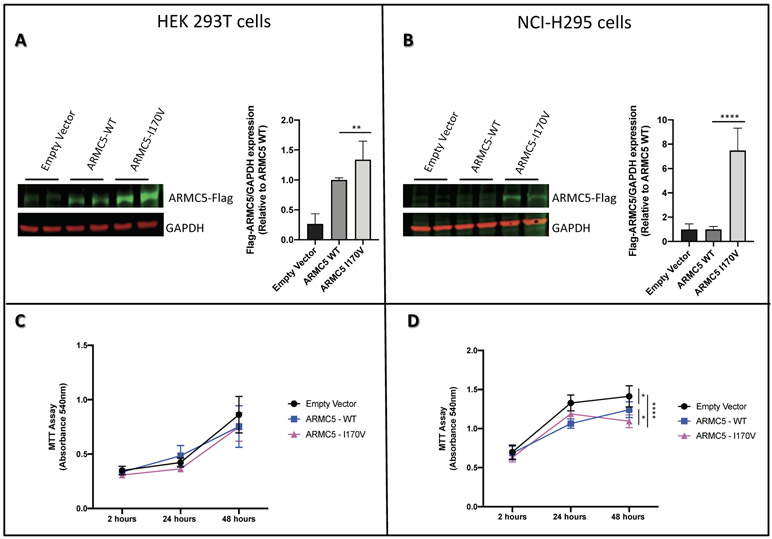
When a flag-tagged construct bearing the p.I170V ARMC5 variant was expressed in HEK 293T and NCI-H295 cells, it led to higher expression of the protein in both cell types, although more so in the adrenocortical NCI-H295 cells (Figure 2A and 2B). As a complementary study of the role of ARMC5 p.I170V variant in the protein expression, MTT assay was performed to check the if this variant would affect the viability of the two types of cells. Our results show that, after 48 hours of transfection, the viability of the HEK 293T cells (Figure 2C) was not affected, whereas the NCI-H295 cells transfected with the ARMC5 WT protein or the one carrying the p.I170V variant had decreased viability compared to the empty vector; the p.I170V ARMC5 variant decreased the viability of the NCI-H295 cells significantly more than the WT suggesting a possible role of this variant in protein translation (Figure 2D).
Figure 2: Functional study of ARMC5 p.I170V variant.
Western blot assay of ARMC5 in A. HEK 293T cells and B. NCI-H295 cells transfected with plasmids expressing wild type or mutated p.I170V ARMC5. GAPDH was used as an internal loading control. Data are expressed as fold of change from relative expression of ARMC5/GAPDH ± SEM; **p = 0.0098, ****p < 0.0001. Blots shown are representative of two independent experiments. MTT assay of C. HEK 293T cells and D. NCI-H295 cells after transfection with plasmids expressing wild type or mutated p.I170V ARMC5. Measurements was performed 2, 24 and 48 hours after transfection. Data represents values of two independent experiments ± SEM; *p<0.05, ****p<0.0001.
Immunohistochemistry (IHC)
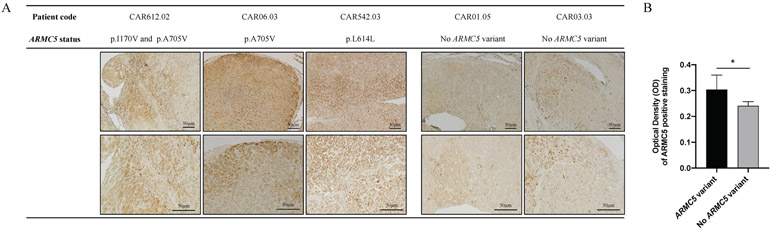
IHC showed that immunostaining for ARMC5 was stronger among adrenal tumor samples that carried an ARMC5 variant. Moreover, ARMC5 expression showed to be widely distributed in samples of patients that had ARMC5 variants compared to samples of patients that did not have ARMC5 variants (Figure 3).
Figure 3: ARMC5 expression in adrenal of patients with PRKAR1A mutation.
A. Immunohistochemistry staining of ARMC5 in adrenal glands of patients who carry PRKAR1A mutation, have Cushing’s syndrome (CS) and went to adrenalectomy. Representative pictures were taken in an inverted light microscope at 5x and 10x objective lenses. B. Quantification of DAB ARMC5 positive staining using ImageJ software. *p=0.0193.
DISCUSSION
The present study shows that germline variants in ARMC5 among patients with PRKAR1A defects and PPNAD may play the role of a modifier of the phenotype of hypercortisolemia and/or CS: patients with ARMC5 variants had hypercortisolemia and/or CS more frequently than those without any ARMC5 sequence variants. This is consistent with our hypothesis when we started this investigation. On the other hand, patients with ARMC5 variants had overall lower cortisol levels than those without (Table 2).
At first glance, these two sets of data are contradictory. Yet, we know that ARMC5 in vitro decreased steroidogenesis in NCI-H295 adrenocortical cells (Assié et al. 2014, Espiard et al. 2015) and that the Armc5 haploinsufficient mouse showed decreased corticosterone secretion, before it reverted to increased glucocorticoids and a PBMAH-like phenotype (33). The function of ARMC5 is unknown but the normal protein, when overexpressed in adrenocortical cell cultures (NCI-H295), appears to increase apoptosis (Assié et al. 2013, Berthon et al. 2017a, Espiard et al. 2015). In our experiments, normal ARMC5 decreased the viability of NCI-H295 cells (Figure 2). Interestingly, this effect of ARMC5 appeared to be cell-specific: it was not observed in the non-adrenal HEK 293T cells. In the same experiments, the p.I170V ARMC5 variant, which was seen in 4 of our patients with PPNAD and severe CS, decreased the viability of the H295R cells significantly more than the WT (Figure 2D). It is noteworthy, that the p.I170V variant increased the presence of the ARMC5 protein in both HEK 293T and NCI-H295 cells (Figure 2A and 2B) but affected viability only in the latter. Corroborating this data, ARMC5 expression was also increased in adrenal tissues of PPNAD patients with CS that carried the p.I170V variant compared to patients that did not carry any ARMC5 variants (Figure 3).
How does one reconcile these data? We would have to speculate, given the absence of information on the exact function of ARMC5 in the adrenal cortex; we may rely on what others have speculated on what may be the way ARMC5 mediates tumorigenicity. One assumption is that under physiological conditions, ARMC5 is a negative regulator of adrenocortical proliferation and glucocorticoid secretion Berthon et al. 2017b, Hu et al. 2017); its deficiency and/or dysfunction may lead to compensatory proliferation and eventually overgrowth and tumor formation along with excess steroid hormone secretion Assié et al 2013, Espiard et al. 2015, Berthon et al. 2017b). Recent data indicate that cullin 3 targets ARMC5 for ubiquitination and degradation, a finding that may suggest that increased presence of ARMC5 may be undesirable under physiologic adrenocortical function (Cavalcante et al. 2020).
The possible interaction between ARMC5 and PKA remains a matter of speculation, too, although it is suggested by the data in this paper. ARMC5 may be implicated in the regulation of adrenocortical b-catenin (CTNNB1), a protein with which it shares a structure and early developmental adrenal expression (Berthon et al. 2017a, Berthon et al. 2017b), and one that is involved in PRKAR1A’s tumorigenicity Almeida & Stratakis, 2011, Tadjine et al. 2008). CTNNB1 mutations were found in the somatic state in CPAs that developed in patients with PPNAD and severe forms of CS (Tadjine et al. 2008). This was the first demonstration that other genetic events can modify PRKAR1A’s phenotypic effects. PDE11A variants were also shown to lead more frequently to the development of CS among patients with PPNAD (Libé et al. 2011).
A limitation for this study includes its retrospective nature which led unavoidably to missing data. In addition, we identified a small number of patients without ARMC5 variants which results in small number of patients in the group comparisons. Although we present statistical analyses of the results, the reader should critically review the data and we acknowledge that final conclusions are difficult to be drawn. However, this remains to our knowledge the largest study on the investigation of ARMC5 variants in patients with CNC. Additionally, it should be mentioned that patients with CNC undergo regular testing for CS given their predisposition for PPNAD, which however may differ depending on their family and personal preferences, or whether they are the first probands diagnosed with the disease in their family or not. Furthermore, although the screening protocol used at our institute has remained the same over the last years, we acknowledge that our screening testing may differ from other hospitals. Thus, the timing of diagnosis and markers of CS may not be as reliable factors as the presence or not of CS.
In conclusion, this study shows that germline variants in ARMC5 were found more frequent among patients with PRKAR1A mutations and hypercortisolemia and/or CS than those that never developed high cortisol levels. The data suggest that ARMC5 can be involved in regulating PKA signaling and cortisol secretion in a state of PRKAR1A deficiency, although the way the two molecular pathways interact remains unelucidated.
Supplementary Material
Supplementary Table 1: ARMC5 variants in Cushing syndrome patients. ARMC5 variants at protein level found in germline DNA of PPNAD individuals with Cushing syndrome. Number of mutated and total alleles found in gnomAD data base and in the studied cohort are shown.
Acknowledgments
Funding: This work was funded by the NIH Intramural Grant Z01-HD008920–01 of the Eunice Kennedy Shriver National Institute for Child Health & Human Development Division of Intramural Research (DIR) to Dr. Constantine A. Stratakis.
Footnotes
Disclosure statement: C.A.S. holds patents on the function of the PRKAR1A, PDE11A, and GPR101 genes and related issues; his laboratory has also received research funding on the GPR101 gene, abnormal growth hormone secretion and its treatment by Pfizer, Inc; F.R.F. holds patent on the GPR101 gene and/or its function. The other authors have nothing to disclose.
REFERENCES
- Almeida MQ, Stratakis CA 2011a. How does cAMP/protein kinase A signaling lead to tumors in the adrenal cortex and other tissues? Mol Cell Endocrinol. Apr 10;336(1–2):162–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida MQ, Harran M, Bimpaki EI, Hsiao HP, Horvath A, Cheadle C, Watkins T, Nesterova M, Stratakis CA 2011. Integrated genomic analysis of nodular tissue in macronodular adrenocortical hyperplasia: progression of tumorigenesis in a disorder associated with multiple benign lesions. J Clin Endocrinol Metab. Apr;96(4):E728–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida MQ, Azevedo MF, Xekouki P, Bimpaki EI, Horvath A, Collins MT, Karaviti LP, Jeha GS, Bhattacharyya N, Cheadle C, et al. 2012. Activation of cyclic AMP signaling leads to different pathway alterations in lesions of the adrenal cortex caused by germline PRKAR1A defects versus those due to somatic GNAS mutations. J Clin Endocrinol Metab. Apr;97(4):E687–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assié G, Libé R, Espiard S, Rizk-Rabin M, Guimier A, Luscap W, Barreau O, Lefèvre L, Sibony M, Guignat L, et al. 2013. ARMC5 mutations in macronodular adrenal hyperplasia with Cushing’s syndrome. N. Engl. J. Med 369:2105–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertherat J, Groussin L, Sandrini F, Matyakhina L, Bei T, Stergiopoulos S, Papageorgiou T, Bourdeau I, Kirschner LS, Vincent-Dejean C, et al. 2003. Molecular and functional analysis of PRKAR1A and its locus (17q22–24) in sporadic adrenocortical tumors: 17q losses, somatic mutations, and protein kinase A expression and activity. Cancer Res. Sep 1;63(17):5308–19. [PubMed] [Google Scholar]
- Bertherat J, Horvath A, Groussin L, Grabar S, Boikos S, Cazabat L, Libe R, René-Corail F, Stergiopoulos S, Bourdeau I, et al. 2009. Mutations in regulatory subunit type 1A of cyclic adenosine 5’-monophosphate-dependent protein kinase (PRKAR1A): phenotype analysis in 353 patients and 80 different genotypes. J Clin Endocrinol Metab. Jun;94(6):2085–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdeau I, Antonini SR, Lacroix A, Kirschner LS, Matyakhina L, Lorang D, Libutti SK, Stratakis CA 2004. Gene array analysis of macronodular adrenal hyperplasia confirms clinical heterogeneity and identifies several candidate genes as molecular mediators. Oncogene. Feb 26;23(8):1575–85. [DOI] [PubMed] [Google Scholar]
- Bourdeau I, Matyakhina L, Stergiopoulos SG, Sandrini F, Boikos S, Stratakis CA 2006. 17q22–24 chromosomal losses and alterations of protein kinase a subunit expression and activity in adrenocorticotropin-independent macronodular adrenal hyperplasia. J Clin Endocrinol Metab. Sep;91(9):3626–32. [DOI] [PubMed] [Google Scholar]
- Berthon A, Faucz F, Bertherat J, Stratakis CA 2017a. Analysis of ARMC5 expression in human tissues. Mol. Cell. Endocrinol 441:140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthon A, Faucz FR, Espiard S, Drougat L, Bertherat J, Stratakis CA 2017b. Age-dependent effects of Armc5 haploinsufficiency on adrenocortical function. Hum. Mol. Gen 18:3495–3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimpaki EI, Iliopoulos D, Moraitis A, Stratakis CA 2010. MicroRNA signature in massive macronodular adrenocortical disease and implications for adrenocortical tumourigenesis. Clin. Endocrinol. (Oxf) Jun;72(6):744–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalcante IP, Vaczlavik A, Drougat L, Lotfi CFP, Hecale-Perlemoine K, Ribes C, Rizk-Rabin M, Clauser E, Fragoso MCBV, Bertherat J, et al. 2020 Cullin 3 targets the tumor suppressor gene ARMC5 for ubiquitination and degradation. Endocr Relat Cancer. Feb 1. pii: ERC-19–0502.R1. [DOI] [PubMed] [Google Scholar]
- de Joussineau C, Sahut-Barnola I, Levy I, Saloustros E, Val P, Stratakis CA, Martinez A 2012. The cAMP pathway and the control of adrenocortical development and growth. Mol Cell Endocrinol. Mar 31;351(1):28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espiard S, Drougat L, Libe R, Assie G, Perlemoine K, Guignat L, Barrande G, Brucker-Davis F, Doullay F, Lopez S, et al. 2015. ARMC5 Mutations in a Large Cohort of Primary Macronodular Adrenal Hyperplasia: Clinical and Functional Consequences. J. Clin. Endocrinol. Metab 100:E926–E935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espiard S, Knape MJ, Bathon K, Assié G, Rizk-Rabin M, Faillot S, Luscap-Rondof W, Abid D, Guignat L, Calebiro D, et al. 2018. Activating PRKACB somatic mutation in cortisol-producing adenomas. JCI Insight. Apr 19;3(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faucz FR, Zilbermint M, Lodish MB, Szarek E, Trivellin G, Sinaii N, Berthon A, Libé R, Assié G, Espiard S, et al. 2014. Macronodular Adrenal Hyperplasia due to Mutations in an Armadillo Repeat Containing 5 (ARMC5) Gene: A Clinical and Genetic Investigation. J. Clin. Endocrinol. Metab 99: E1113–E1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannah-Shmouni F, Stratakis CA 2020. A gene-based classification of primary adrenocortical hyperplasias. Horm Metab Res Mar;52(3):133–141. [DOI] [PubMed] [Google Scholar]
- Horvath A, Boikos S, Giatzakis C, Robinson-White A, Groussin L, Griffin KJ, Stein E, Levine E, Delimpasi G, Hsiao HP, et al. 2006. A genome-wide scan identifies mutations in the gene encoding phosphodiesterase 11A4 (PDE11A) in individuals with adrenocortical hyperplasia. Nat Genet Jul;38(7):794–800. [DOI] [PubMed] [Google Scholar]
- Horvath A, Bertherat J, Groussin L, Guillaud-Bataille M, Tsang K, Cazabat L, Libe R, Remmers E, Rene-Corail F, Faucz FR, et al. 2010. Mutations and polymorphisms in the gene encoding regulatory subunit type 1-alpha of protein kinase A (PRKAR1A): an update. Hum Mutat. 31:369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao HP, Kirschner LS, Bourdeau I, Keil MF, Boikos SA, Verma S, Robinson-White AJ, Nesterova M, Lacroix A, Stratakis CA 2009. Clinical and genetic heterogeneity, overlap with other tumor syndromes, and atypical glucocorticoid hormone secretion in adrenocorticotropin-independent macronodular adrenal hyperplasia compared with other adrenocortical tumors. J Clin Endocrinol Metab. Aug;94(8):2930–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Lao L, Mao J, Jin W, Luo H, Charpentier T, Qi S, Peng J, Hu B, Marcinkiewicz MM, et al. 2017. Armc5 deletion causes developmental defects and compromises T-cell immune responses. Nat Commun. Feb 7;8:13834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner LS, Carney JA, Pack SD, Taymans SE, Giatzakis C, Cho YS, Cho-Chung YS, Stratakis CA 2000. Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit in patients with the Carney complex. Nat Genet. 26:89–92. [DOI] [PubMed] [Google Scholar]
- Lecoq AL, Stratakis CA, Viengchareun S, Chaligné R, Tosca L, Deméocq V, Hage M, Berthon A, Faucz FR, Hanna P, et al. 2017. Adrenal GIPR expression and chromosome 19q13 microduplications in GIP-dependent Cushing’s syndrome. JCI Insight. Sep 21;2(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe KM, Young WF Jr, Lyssikatos C, Stratakis CA, Carney JA 2017. Cushing Syndrome in Carney Complex: Clinical, Pathologic, and Molecular Genetic Findings in the 17 Affected Mayo Clinic Patients. Am J Surg Pathol. Feb;41(2):171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libé R, Fratticci A, Coste J, Tissier F, Horvath A, Ragazzon B, Rene-Corail F, Groussin L, Bertagna X, Raffin-Sanson ML, et al. 2008. Phosphodiesterase 11A (PDE11A) and genetic predisposition to adrenocortical tumors. Clin Cancer Res. Jun 15;14(12):4016–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libé R, Horvath A, Vezzosi D, Fratticci A, Coste J, Perlemoine K, Ragazzon B, Guillaud-Bataille M, Groussin L, Clauser E, et al. 2011. Frequent phosphodiesterase 11A gene (PDE11A) defects in patients with Carney complex (CNC) caused by PRKAR1A mutations: PDE11A may contribute to adrenal and testicular tumors in CNC as a modifier of the phenotype. J Clin Endocrinol Metab. Jan;96(1): E208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira AM, Hes FJ, Horvath A, Woortman S, Greene E, Bimpaki E, Alatsatianos A, Boikos S, Smit JW, Romijn JÁ et al. 2010. Association of the M1V PRKAR1A mutation with primary pigmented nodular adrenocortical disease in two large families. J Clin Endocrinol Metab. Jan;95(1):338–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarlis NJ, Chrousos GP, Doppman JL, Carney JA, Stratakis CA 1997. Primary pigmented nodular adrenocortical disease: reevaluation of a patient with carney complex 27 years after unilateral adrenalectomy. J Clin Endocrinol Metab. Apr;82(4):1274–8. [DOI] [PubMed] [Google Scholar]
- Stratakis CA 2014. E pluribus unum? The main protein kinase A catalytic subunit (PRKACA), a likely oncogene, and cortisol-producing tumors. J Clin Endocrinol Metab. 99:3629–3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratakis CA, Raygada M 1993. Carney Complex. In: Adam MP, Ardinger HH, Pagon RA, et al. , eds. GeneReviews. Seattle (WA): University of Washington, Seattle. [PubMed] [Google Scholar]
- Stratakis CA, Sarlis N, Kirschner LS, Carney JA, Doppman JL, Nieman LK, Chrousos GP, Papanicolaou DA 1999. Paradoxical response to dexamethasone in the diagnosis of primary pigmented nodular adrenocortical disease. Ann Intern Med. 131(8):585–591. [DOI] [PubMed] [Google Scholar]
- Tadjine M, Lampron A, Ouadi L, Horvath A, Stratakis CA, Bourdeau I 2008. Detection of somatic beta-catenin mutations in primary pigmented nodular adrenocortical disease (PPNAD). Clin Endocrinol (Oxf). Sep;69(3):367–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirosh A, Lodish MB, Papadakis GZ, Lyssikatos C, Belyavskaya E, Stratakis CA 2016. Diurnal Plasma Cortisol Measurements Utility in Differentiating Various Etiologies of Endogenous Cushing Syndrome. Horm Metab Res. 48(10):677–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzosi D, Libé R, Baudry C, Rizk-Rabin M, Horvath A, Levy I, René-Corail F, Ragazzon B, Stratakis CA, Vandecasteele G et al. 2012. Phosphodiesterase 11A (PDE11A) gene defects in patients with acth-independent macronodular adrenal hyperplasia (AIMAH): functional variants may contribute to genetic susceptibility of bilateral adrenal tumors. J Clin Endocrinol Metab. Nov;97(11):E2063–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: ARMC5 variants in Cushing syndrome patients. ARMC5 variants at protein level found in germline DNA of PPNAD individuals with Cushing syndrome. Number of mutated and total alleles found in gnomAD data base and in the studied cohort are shown.