Abstract
Introduction:
The corticobasal syndrome (CBS) is associated with several neuropathologic disorders, including corticobasal degeneration (CBD) and Alzheimer’s disease (AD).
Method:
In this report, we studied 43 AD patients with CBS (AD-CBS) and compared them with 42 AD patients with typical amnestic syndrome (AD-AS), as well as 15 cases of CBD-CBS.
Results:
Unlike AD-AS, AD-CBS had prominent motor problems, including limb apraxia (90%), myoclonus (81%) and gait disorders (70%). Alien limb phenomenon was reported in 26% and cortical sensory loss in 14%. Language problems were also more frequent in CBS-AD and memory impairment was less frequent. AD-CBS had more tau pathology in peri-Rolandic cortices, but less in superior temporal cortex than AD-AS. In addition, AD-CBS had greater neuronal loss in the substantia nigra.
Discussion:
AD-CBS is a clinicopathological subtype of AD with an atypical distribution of Alzheimer type tau pathology. Greater neuronal loss in substantia nigra may contribute to Parkinsonism that is not a feature of typical AD.
Keywords: Alzheimer’s disease, corticobasal syndrome, neuropathology, tau, neurodegeneration
1. Introduction
Alzheimer disease (AD) typically presents with episodic memory impairment followed by cognitive deterioration in other domains, such as executive function, praxis, and visuospatial skills [1]. It is increasingly recognized that pathologically confirmed AD can have atypical clinical presentations, such as posterior cortical syndrome [2], aphasia [3] (especially the logopenic variant [4]), and frontal lobe syndrome associated with dysexecutive and behavioral changes [5, 6]. Uncommonly, AD may present with limb apraxia [7] and other features of the corticobasal syndrome (CBS) [8]. CBS has asymmetric, higher-order cortical dysfunction (e.g., apraxia and cortical sensory deficits) and extrapyramidal motor dysfunction [9]. Current clinical criteria of CBD define several clinical presentations of CBD, only one of which is CBS [8]. The latter is a progressive asymmetric motor disorder (at least two of dystonia, Parkinsonism, or myoclonus) with non-motor features (at least two of limb apraxia, cortical sensory loss, or alien limb phenomenon) [8]. CBS was once thought to be specific for CBD [9], but autopsy series show other pathologic processes, including AD, frontotemporal lobar degeneration with TDP-43 pathology (FTLD-TDP), progressive supranuclear palsy, and Pick’s disease [8, 10–14].
AD-CBS is increasingly recognized as an atypical variant of AD, and antemortem imaging for amyloid or analyses of cerebrospinal fluid Aβ have been proposed as means to assist in the differential diagnosis of CBD-CBS and AD-CBS [8, 15]. To date, most clinicopathologic studies of AD-CBS have been relatively small patient series [16–21] or individual case reports [13, 17, 18, 22]. Predicting the pathologic underpinnings of CBS is of increasing interest as disease-modifying therapies are being developed for both AD [23] and tauopathies [24]. In this report, we studied 43 pathologically-proven AD patients presenting with antemortem clinical features of CBS and compare them to 15 patients with CBS and CBD pathology (CBD-CBS), as well as 42 patients with AD and a typical amnestic clinical presentation (AD-AS).
2. Material and methods
2.1. Case material
All cases were submitted to or autopsied by the brain bank for neurodegenerative disorders at Mayo Clinic in Jacksonville, Florida. The left or right hemibrain was fixed in 10% formalin, and the opposite hemibrain was frozen at −80°C. In this study, histologic studies of 4 AD-CBS (9%), 9 AD-AS (21%) and 3 CBD-CBS (20%) were from the right hemibrain. Formalin-fixed tissue was sampled with standardized dissection methods and embedded in paraffin blocks. Most of the CBD cases were acquired through the CurePSP brain bank [25]. AD cases were acquired from a range of sources and types of studies, including prospective longitudinal research studies as part of the Mayo Clinic Alzheimer’s Disease Research Center (P50 AG16574), but also from neurologists and specialists in academic medical centers (see Acknowledgement Section). Many of the AD patients were enrolled in the State of Florida Alzheimer Disease Initiative [26]. One AD-CBS and two AD-AS were Hispanic, while all others were Caucasian.
Clinical information (age at death, sex, clinical diagnosis, disease duration, and family history) was abstracted from medical records. The specialty of the clinician of record (internist, psychiatrist, general neurologist, movement disorder specialist or behavioral neurologist) differed between AD-AS and AD-CBS and between AD-AS and CBD-CBS, but not between AD-CBS and CBD-CBS. The clinician of record for patients with CBS, regardless of underlying pathology, was more likely to have been a movement disorder specialist.
2.2. Demographics and clinical presentation
We identified 58 cases of AD-CBS among 1,693 pathologically confirmed AD (3.5%), all meeting criteria for intermediate-to-high likelihood AD using NIA-AA criteria [27]. For clinicopathologic studies, we excluded AD cases with concomitant Lewy bodies, ranging from brainstem-predominant to diffuse cortical Lewy bodies, since this pathology could independently contribute to an atypical parkinsonian syndrome [14]. We permitted cases with Lewy-related pathology confined to the amygdala [28], given that this pathology is not associated with atypical parkinsonian syndrome [29]. After applying exclusion criteria, there were 43 autopsy-confirmed cases of AD with CBS (AD-CBS), including one that was reported previously [30]. For comparison of clinicopathological features with AD-CBS, we matched AD-CBS with 42 patients with autopsy-confirmed AD and typical amnestic clinical presentations. We made an effort to match AD-CBS with AD-AS cases for age, sex, age at onset, age at death and disease duration, as well as for Braak neurofibrillary tangle stage [31] and Thal amyloid phase [32].
As an additional comparison group, we included a series of 15 patients with autopsy-proven CBD and an antemortem clinical syndrome consistent with CBS (CBD-CBS), matched as closely as possible for demographic features. There was insufficient antemortem biomarker data, such as beta-amyloid positron emission tomography and cerebrospinal fluid beta-amyloid and tau, to permit evaluation of biomarkers in differential diagnosis of AD-CBS and CBD-CBS. None of the CBD-CBS cases had Lewy body pathology.
All cases were screened for presence of TAR DNA binding protein of 43 kDa (TDP-43) pathology in a section that contained the amygdala. Demographic and pathologic data for the three groups are summarized in Table 1.
Table 1.
Demographics AD-CBS and Controls
| p-values | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| AD-AS n=42 | AD-CBS n=43 | CBD-CBS n=15 | AD-AS vs AD-CBS | AD-AS vs CBD-CBS | AD-CBS vs CBD-CBS | |
|
| ||||||
| Clinical features | ||||||
|
| ||||||
| Sex, male (%) | 18 (43%) | 17 (40%) | 10 (63%) | |||
| Age at onset, years | 63 (57,73) | 61 (55,69) | 64 (55,69) | |||
| Age at death, years | 73 (67, 78) | 70 (64,77) | 71 (62, 77) | |||
| Disease duration | 9 (7, 11) | 9 (7, 1) | 7 (5, 8) | 0.05 | 0.02 | |
|
| ||||||
| Neuropathologic features | ||||||
|
| ||||||
| Brain weight | 960 (920, 1070) | 980 (860, 1060) | 1160 (1040, 1295) | <0.001 | <0.001 | |
| Braak stage | VI | VI | II-III (I, III) | <0.001 | <0.001 | |
| Thal phase | 5 | 5 | 1 (0, 3) | <0.001 | <0.001 | |
| TDP-43 | 12/42 (28%) | 7/43 (16%) | 2/15 (13%) | 0.008 | ||
| Amygdala Lewy bodies | 6 | 5 | none | |||
|
| ||||||
| Regions of cortical atrophy at autopsy | ||||||
|
| ||||||
| Superior frontal gyrus | 0 (0%) | 4 (10%) | 6 (40%) | 0.05 | <0.001 | 0.01 |
| Paracentral lobule | 2 (5%) | 9 (22%) | 2 (13%) | 0.03 | ||
| Temporal, anterior | 8 (21%) | 1 (2%) | 0 (0%) | 0.01 | ||
| Parietal | 9 (23%) | 10 (24%) | 1 (7%) | |||
| Occipital | 13 (33%) | 19 (43%) | 3 (20%) | 0.05 | ||
All variables are analyzed with Kruskal-Wallis ANOVA on Ranks and displayed as median (25th and 75th range), unless otherwise noted. Post hoc pairwise comparisons were performed with Mann-Whitney rank sum test. Only statistically significant p-values are shown.
2.2.1. Histopathologic studies
Gross and microscopic neuropathologic assessments were performed by standardized procedures and were evaluated by a single neuropathologist (DWD). In addition to histologic evaluation with hematoxylin and eosin stains, presence and severity of Alzheimer pathology was assessed with thioflavin-S fluorescent microscopy. Braak neurofibrillary tangle stages and Thal amyloid phases were assigned to each case based upon lesion counts in cortical and subcortical areas with thioflavin-S fluorescent microscopy [33]. The severity of neuronal loss was assessed in the basal nucleus of Meynert on a four point scale: none, mild, moderate, and severe.
2.2.2. Immunohistochemistry
Immunohistochemistry was performed on 5-μm thick sections of formalin-fixed, paraffin embedded tissue. Glass-mounted sections were de-parafinized in xylene and rehydrated in ethanol and distilled water. Immunohistochemistry for TDP-43 used a rabbit polyclonal antibody to a mid-region neoepitope (MC2085; from Leonard Petrucelli, PhD, Mayo Clinic Jacksonville); for tau immunochemistry, we used an antibody to phospho-serine 202 (CP13; mouse monoclonal; from Peter Davies, PhD, Feinstein Institute, North Shore Hospital, NY); and for microglia we used a monoclonal antibody to a member of the lysosomal/endosomal-associated membrane glycoprotein (LAMP) family (CD68; mouse monoclonal; DAKO). The following regions were evaluated: cingulate gyrus, superior frontal gyrus, motor cortex, somatosensory cortex and corpus callosum, as well as the hippocampus. In the latter, hippocampal subfields were analyzed separately (CA4, CA2, CA1 and subiculum).
Immunoperoxidase staining was performed on a DAKO AutostainerPlus (Agilent/DAKO, Santa Clara, CA) with the DAKO EnVisionTM+ system-HRP with diaminobenzidine as the chromogen. Nonspecific antibody binding was blocked with normal goat serum.
2.2.3. Substantia nigra neurodegeneration
Neuronal loss in the ventrolateral region of the substantia nigra was assessed in a single transverse section of midbrain at the level of the third cranial nerve [34] using a four point scale – none (0), mild (1), moderate (2), or severe (3). This scoring scheme was shown previously to correlate with striatal dopaminergic nerve terminal loss assessed with tyrosine hydroxylase immunohistochemistry [35].
2.2.4. Image analysis
Digital microscopy methods have been described previously [36]. Briefly, immunostained glass slides were scanned on an Aperio ScanScope XT slide scanner (Aperio Technologies/Leica Biosystems, Buffalo Grove, IL), producing high resolution digital images. Digital image analysis was performed using Aperio ImageScope software. Regions of interests were outlined on digital images. A color deconvolution algorithm was used to count the number of pixels that were positive with the chromogen. The output variable was percentage of strong positive staining relative to total pixels in the region of interest. The region of interest for cerebral cortex was defined as the entire thickness of the cortical ribbon in a region where the pial surface was parallel to the cortical gray-white junction. Regions of interest were also traced in the corpus callosum and peri-Rolandic white matter beneath the motor cortex.
2.2.5. Statistical analyses
Sigma Plot Version 12 (Systat Software, San Jose, CA) was used for statistical analyses. Due to small sample sizes, non-parametric Kruskal-Wallis analysis of variance on ranks (ANOVA) was performed on quantitative measures to assess differences in the median values. Post hoc pairwise comparisons were performed using Mann-Whitney rank sum test. For categorical data (e.g., sex and clinical specialty), a Chi-square test was used to compare group differences. Fisher’s exact test was used for comparison of pairwise categorical data if the counts were less than 5. A statistically significant difference was considered for two sided P < 0.05.
3. Results
3.1. Clinical characteristics of CBS-AD
All AD-CBS cases met criteria for probable CBS using criteria of Armstrong et al. [8]. Demographics and gross neuropathologic characteristics of cases in this study are shown in Table 1. Although AD usually presented with episodic memory problems, the initial symptoms of AD-CBS were more variable (Table 2). Initial clinical features included language problems and asymmetric limb apraxia more often than episodic memory impairment. Early episodic memory problems were significantly less frequent in AD-CBS (26%) than in AD-AS (93%).
Table 2.
Initial signs and symptoms of AD-CBS and controls
| p-values | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| AD-AS n=42 | AD-CBS n=43 | CBD-CBS n=15 | AD-AS vs AD-CBS | AD-AS vs CBD-CBS | AD-CBS vs CBD-CBS | |
|
| ||||||
| Cortical/cognitive signs | ||||||
|
| ||||||
| Language | 2 (5%) | 11 (26%) | 3 (21%) | 0.02 | ||
| Limb apraxia | 0 (0%) | 14 (31%) | 7 (50%) | <0.001 | <0.001 | |
| Memory impairment | 39 (93%) | 10 (23%) | 0 (0%) | <0.001 | <0.001 | |
| Visual problems | 1 (2%) | 2 (5%) | 0 (0%) | |||
| Personality change | 1 (2%) | 0 (0%) | 0 (0%) | |||
|
| ||||||
| Motor signs | ||||||
|
| ||||||
| Gait abnormalities | 0 (0%) | 3 (7%) | 2 (14%) | |||
| Tremors | 0 (0%) | 1 (2%) | 1 (7%) | |||
| Myoclonus | 0 (0%) | 2 (5%) | 0 (0%) | |||
|
| ||||||
| Clinician of record | ||||||
|
| ||||||
| Internist | 2 | 1 | 0 | |||
| Psychiatrist | 1 | 1 | 0 | |||
| Neurologist | 22 | 7 | 0 | |||
| Movement disorder specialist | 1 | 18 | 13 | |||
| Behavioral neurologist | 16 | 16 | 2 | |||
Data are displayed as frequency of a given clinical feature (percent of total in that group). Post hoc pairwise comparison analysis is performed with Mann-Whitney rank sum test. Only statistically significant p-values are shown.
Motor signs or symptoms were common in AD-CBS. In 12 patients (29%), initial complaints were motor problems, including asymmetric motor incoordination, gait abnormalities, tremor, or myoclonus (Table 2). During the disease course, 39 (90%) patients with AD-CBS had limb apraxia, and 28 (65%) had language problems; however, memory problems were prominent in only 23 (53%) patients. Visuospatial problems were recorded in 26 patients (60%), and visual neglect was noted in 8 patients (19%). Behavioral abnormalities were present in 17 patients (40%) during the disease course. A core feature of CBS, the alien limb phenomenon, was reported in 11 cases (26%), and cortical sensory loss in 6 cases (14%). Other motor signs or symptoms included myoclonus (81%), gait problems (70%) and rigidity (67%). Tremor and dystonia were less frequent (36% and 17%, respectively). Hallucinations or delusions, or both, were noted in 13 cases (30%) of AD-CBS. Falls were noted in 13 (28%) of AD-CBS. Comparing AD-CBS to CBD-CBS, myoclonus and behavioral problems were significantly more frequent in AD-CBS (Figure 1).
Fig. 1. Clinical features during the disease course of AD-CBS and controls.
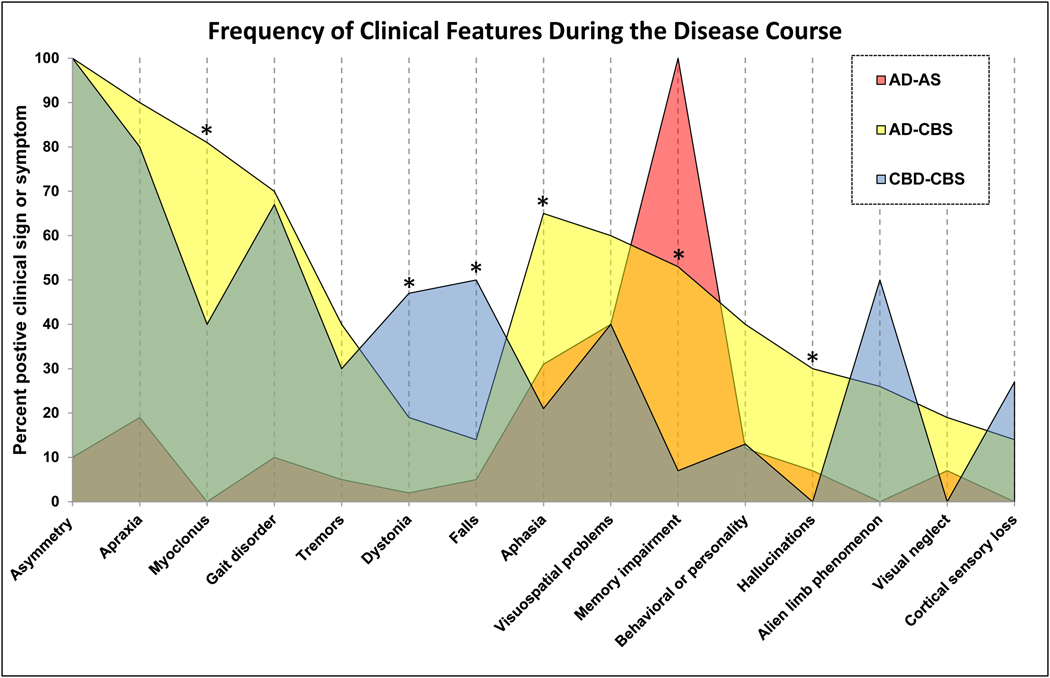
Data are displayed as frequency of given clinical feature (percent of total in that group). Post hoc pairwise comparison analysis is performed with Mann-Whitney rank sum test. The asterisks (*) indicate significant differences between AD-CBS and CBD-CBS (myoclonus - P=0.006, aphasia = P=0.007, memory impairment; P=0.004, hallucinations or delusions; P=0.01, dystonia; P=0.04, and falls; P=0.03)
3.2.1. Pathologic findings
Most cases of pathologically confirmed CBD had atrophy in superior frontal gyrus (Figure 2), often accompanied by topographically-aligned thinning of the corpus callosum [37]. We assessed brain atrophy patterns in photographs of the fixed hemisphere of AD-CBS, CBD-CBS and AD-AS blinded to diagnostic groups (Table 1). There were differences between AD-CBS, CBD-CBS and AD-AS in superior frontal gyrus, with CBD-CBS showing more frequent atrophy than AD-CBS. AD-AS had significant less paracentral lobule atrophy compared with AD-CBS (Table 1). In addition, AD-CBS tended to have more atrophy in occipital cortex compared with CBD-CBS (Table 1). We did not find differences in thickness of the corpus callosum between AD-CBS and AD-AS (Table 3).
Fig. 2. Macroscopic examples of AD-CBS and AD-AS.
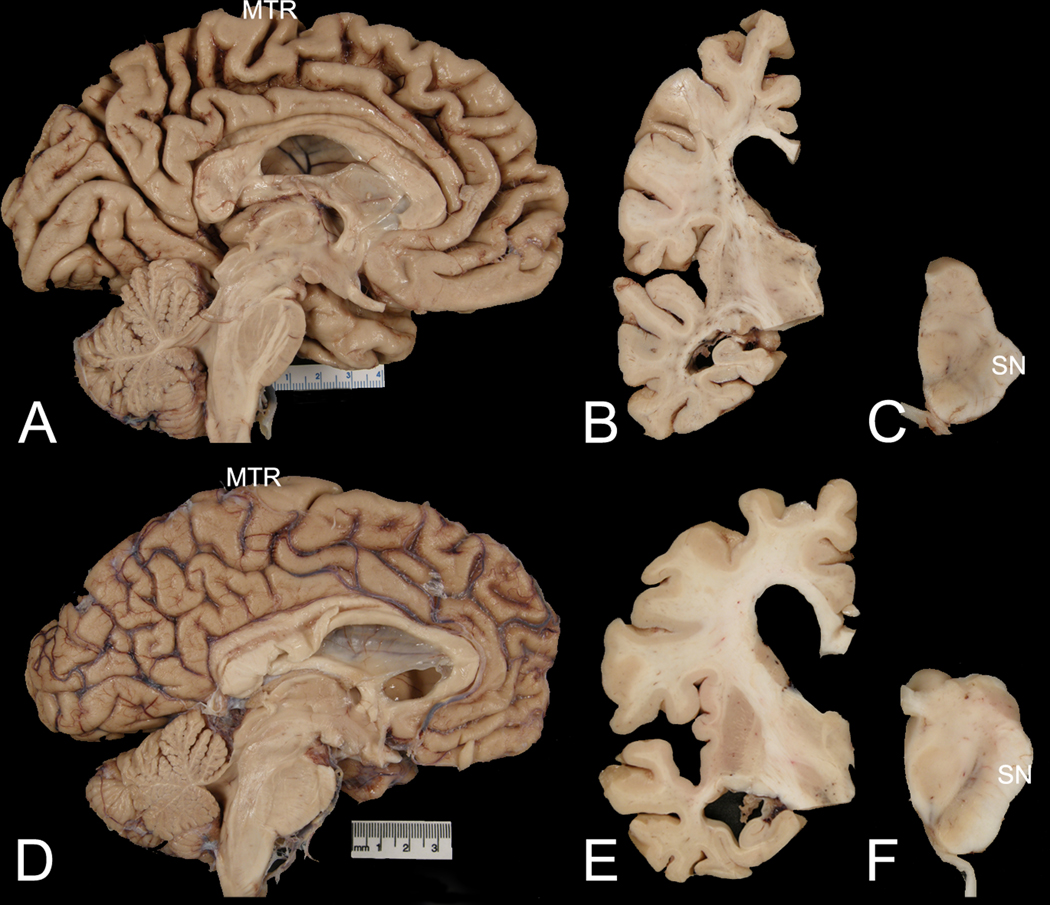
Comparison of representative cases of AD-CBS (A, B, C) and AD-AS (D, E, F). The midsagittal view shows marked medial frontal atrophy, especially in paracentral lobule and peri-Rolandic region (MTR) in AD-CBS (A), but not in AD-AS (D). Coronal sections show ventricular enlargement, with disproportionate enlargement of frontal compared with temporal horns of the lateral ventricle in AD-CBS (B) compared with AD-AS (E). Transverse sections of the midbrain show decreased neuromelanin pigment in the lateral substantia nigra (SN) in AD-CBS (C) compared with AD-AS (F).
Table 3.
Neuropathological characteristics of AD-CBS and controls
| p-values | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| AD-AS n=42 | AD-CBS n=43 | CBD-CBS n=15 | AD-AS vs AD-CBS | AD-AS vs CBD-CBS | AD-CBS vs CBD-CBS | |
|
| ||||||
| Thickness of corpus callosum | ||||||
|
| ||||||
| 3.6 (3.1, 4.4) | 3.3 (2.7, 4.4) | 3.4 (2.6, 4.1) | ||||
|
| ||||||
| NFT counts in neocortex with thioflavin S fluorescent microscopy | ||||||
|
| ||||||
| MF | 11 (6, 15) | 8 (5, 16) | 0 (0, 0) | <0.001 | <0.001 | |
| ST | 15 (12, 22) | 10 (6, 15) | 0 (0, 0) | 0.002 | <0.001 | <0.001 |
| IP | 15 (11, 21) | 12 (8, 20) | 0 (0, 0) | <0.001 | <0.001 | |
| MTR | 2 (1, 3) | 4 (2, 6) | 0 (0, 0) | <0.001 | <0.001 | <0.001 |
| VC | 3 (2, 6) | 3 (1, 7) | 0 (0, 0) | <0.001 | <0.001 | |
|
| ||||||
| SP counts in neocortex with thioflavin S fluorescent microscopy | ||||||
|
| ||||||
| MTR | 26 (16, 38) | 34 (20, 43) | 0 (0, 0) | <0.001 | <0.001 | |
| VC | 50 (30, 50) | 40 (30, 46) | 0 (0, 8) | <0.001 | <0.001 | |
|
| ||||||
| Neuronal loss scores on hematoxylin eosin stained sections | ||||||
|
| ||||||
| nbM | 3 (2, 3) | 3 (2, 3) | 0 (0, 0) | <0.001 | <0.001 | |
| SN | 0.5 (0, 1) | 1 (0.5, 1.5) | 3 (2.9, 3.0) | <0.001 | <0.001 | <0.001 |
|
| ||||||
| Phospho-tau burden with CP-13 immunohistochemistry | ||||||
|
| ||||||
| CING | 12 (5.3, 18) | 7.7 (4.5, 17) | 3.7(2.7, 4.6) | <0.001 | 0.005 | |
| SFG | 15 (8.1, 22) | 11 (6.8, 24) | 4.2 (2.7,7.5) | <0.001 | <0.001 | |
| CC | 0.17 (0.1, 0.3) | 0.11 (0.1, 0.2) | 0.82(0.6,1.8) | 0.01 | <0.001 | <0.001 |
| MTR | 3.8 (1.5, 8.6) | 10 (6.5, 14) | 9.1 (7.7, 14.8) | <0.001 | <0.001 | |
| SS | 7.1 (4.0, 12) | 12.8 (6.7, 22) | 1.6 (0.9, 4.2) | 0.007 | <0.001 | <0.001 |
| WM (MCtx) | 0.2 (0.1,0.3) | 0.2 (0.1, 0.3) | 4.1 (2.3, 6.1) | <0.001 | <0.001 | |
|
| ||||||
| Microglial burden with CD68 immunohistochemistry | ||||||
|
| ||||||
| CING | 0.16 (0.08, 0.26) | 0.09 (0.05, 0.22) | 0.14 (0.09, 0.28) | |||
| SFG | 0.19 (0.15, 0.29) | 0.16 (0.07, 0.31) | 0.19 (0.12, 0.34) | |||
| CC | 0.62 (0.42, 0.87) | 0.52 (0.30, 0.73) | 0.97 (0.61, 1.2) | 0.01 | <0.001 | |
| MTR | 0.17 (0.11, 0.23) | 0.2 (0.11, 0.31) | 0.56 (0.32, 0.87) | <0.001 | <0.001 | |
| SS | 0.17 (0.12, 0.23) | 0.24(0.12, 0.32) | 0.20 (0.12, 0.38) | |||
| WM (MCtx) | 0.35 (0.26, 0.66) | 0.44 (0.24, 0.71) | 1.5 (1.2, 2.6) | <0.001 | <0.001 | |
All variables are analyzed with Kruskal-Wallis ANOVA on Ranks and displayed as median (25th and 75th range), unless otherwise noted. Post hoc pairwise comparisons were performed with Mann-Whitney rank sum test. Only statistically significant p-values are shown. Corpus callosum thickness measures are in centimeters. NFT counts are the maximal number in a representative 400x field. Tau and microglia burden are derived from image analysis of digitized images of stained tissue sections and represent the ratio of positively stained pixels to the total number of pixels in the given region of interest. Abbreviations: NFT = neurofibrillary tangles, SP = senile plaques (both with thioflavin S fluorescent microscopy), MF = middle frontal gyrus, ST = superior temporal gyrus, IP = inferior parietal lobule, MTR = motor cortex, VC = primary visual cortex, nbM = basal nucleus of Meynert, SN = substantia nigra pars compacta, ventrolateral tier, CING = anterior cingulate gyrus, SFG = superior frontal gyrus, CC = corpus callosum at level of anterior cingulate gyrus, MTR = motor cortex, SS = somatosensory cortex, WM (MTR) = white matter beneath motor cortex.
Regarding AD-related pathology, AD-CBS and AD-AS had similar Braak NFT stage and Thal amyloid phase based upon study design (Table 1); but when quantitative indices were evaluated rather than topographic staging, we found significantly fewer NFT in superior temporal cortex (p=0.002), and significantly more NFT in motor cortex (p<0.001) in AD-CBS compared with AD-AS (Table 3). In the hippocampus, there were trends for lower NFT counts in AD-CBS compared with AD-AS, especially in CA1 and subiculum (not shown). We compared scores for neuronal loss in basal nucleus of Meynert and substantia nigra. Neuronal loss in the basal nucleus of Meynert was greater in AD-AS and AD-CBS than in CBD-CBS, but there was no difference between AD-CBS and AD-AS. In contrast, neuronal loss in substantia nigra was significantly different in the 3 groups, with greater neuronal loss in AD-CBS than AD-AS. As expected, CBD-CBS had significantly greater neuronal loss in substantia nigra than both AD-AS and AD-CBS. There were no significant differences between AD-CBS and AD-AS in regions assessed for senile plaque counts or for Thal amyloid phase. Both senile plaque counts and Thal phase were significantly less in CBD-CBS.
3.2.2. Tau pathology
We used immunohistochemistry with a phospho-tau antibody and image analysis of burden of tau pathology in various brain regions (Figure 3). In both AD-CBS and AD-AS, tau burden was greater in cingulate gyrus than in CBD-CBS. In contrast, tau burden was significant greater in peri-Rolandic cortices, namely motor cortex (p<0.001) and somatosensory cortex (p=0.007), in AD-CBS compared with AD-AS (Table 3). While tau burden in motor cortex was similar in AD-CBS and CBD-CBS, there was less tau burden in somatosensory cortex in CBD-CBS than in AD-CBS. White matter tau pathology is a characteristic feature of CBD [38]; therefore, we assessed tau burden in both corpus callosum and peri-Rolandic white matter. As expected, CBD-CBS had high density of tau pathology in corpus callosum and peri-Rolandic white matter, but white matter tau pathology was similar in AD-CBS than AD-AS (Figure 3 and Table 3).
Fig. 3. Phospho-tau immunohistochemistry of AD-CBS and AD-AS.
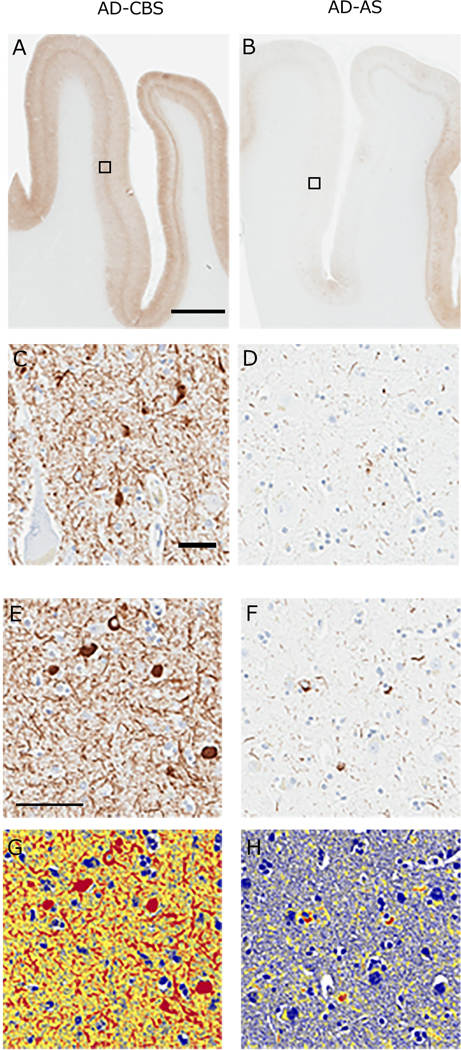
Peri-Rolandic cortices of representative cases of AD-CBS (A and D) and AD-AS (B and D) immunostained for phospho-tau. Boxed areas of motor cortex in A and B are shown at higher magnification in C and D. Representative higher magnification images of phospho-tau immunostaining in motor cortex of AD-CBS (E) and AD-AS (F) after application of the image analysis color deconvolution algorithm on the same images in (G) and (H). Strong positive pixels are shown in red. Tau burden is the ratio of strong positive pixels to all pixels in the region of interest. The analysis does not discriminate between NFT and neuropil threads. Note higher density of tau pathology in motor cortex of AD-CBS compared with AD-AS. Scale bars: A - 150-μm, C - 20-μm, E - 50-μm.
3.2.3. Microglial pathology
We also assessed microglial pathology using CD68 as a marker of activated microglia and macrophages. Of the three groups evaluated, CBD-CBS tended to have the greatest microglial burden in several brain regions, but there were few differences between AD-CBS and AD-AS. CBD-CBS had significantly greater CD68 burden in motor cortex, peri-Rolandic white matter and corpus callosum compared with AD-AS and AD-CBS (Table 3).
4. Discussion
CBS is a distinctive clinical syndrome with heterogeneous underlying neuropathology, including CBD, PSP, FTLD-TDP, Pick’s disease, Lewy body disease, and AD. CBD and PSP are the most common pathological findings in patients with antemortem CBS [39]. The diagnostic accuracy of CBS with CBD pathology is poor (not more than 50%; based upon unpublished observations from the CurePSP Brain Bank). Although the frequency is difficult to assess from the medical literature due to small and selective case series, individual case reports, and lack of systematic surveys of large autopsy cohorts [40], AD is recognized as one of the pathologic substrates of CBS. The frequency ranges from less than 10% [12] to over 20% [11, 20]. In this study, we focused on AD-CBS and report the largest series to date. Cases were drawn from a brain bank for neurodegenerative disorders, with a particular focus on both AD and atypical parkinsonian disorders; 58 cases of AD-CBS were identified in 1,693 autopsy-confirmed cases of AD (3.5%).
The most common clinical presentation of AD-CBS was cognitive dysfunction (74%), but memory deficits were not always the initial feature. Less than 25% of AD-CBS had early memory problems. Of the other core clinical features of CBS, alien limb phenomenon was noted in 26% and cortical sensory loss in 14%. Myoclonus was even more frequent (81%). Motor problems preceded cognitive symptoms in 10 cases (23%) (Table 2). Over the disease course, the most common higher order cortical dysfunction in AD-CBS was limb apraxia (90%). The most common motor disorders were myoclonus (81%), gait disorder (70%), and rigidity (67%).
Relatively few reports of AD-CBS have been based on autopsy-proven cases of AD-CBS and AD-AS. Hu et al. studied five patients with AD-CBS and eleven with CBS not due to AD and found that supportive features such as myoclonus and tremor may help differentiate AD-CBS from CBS not due to AD (myoclonus: 80% vs 18%; tremor: 0% vs 73%) [16]. Lee et al. reported that visual neglect was frequent in AD-CBS [20].
The largest studies of AD-CBS are based on antemortem AD biomarker profiles. Given that Alzheimer type pathology can occur in CBD, this type of study has its drawbacks. Nevertheless, McMillan et al. compared clinical features of 12 patients with AD-CBS and 23 patients with CBS not due to AD and reported higher frequency of asymmetric rigidity compared with AD-CBS [41]. They did not differ in dementia severity or frequency of extrapyramidal signs. Di Stefano, et al. reported 8 patients with AD-CBS and 37 patients with CBS not due to AD, and reported that AD-CBS had more frequent phonemic paraphasias (62%), myoclonus (50%) and rigidity (88%) than CBS not due to AD [42].
Although challenging, there may be clinical features that assist in differentiating AD-CBS from other disorders presenting as CBS. In this study, the most common combination of features in AD-CBS was apraxia, myoclonus, and language problems. In CBD, language impairments are increasingly recognized as a common and frequent presenting feature. Aphasia has been reported to occur in 40% at presentation and in 52% over the disease course [8]. Although the subtype of aphasia was difficult to categorize in our retrospective study, a logopenic-like aphasia with anomia, word retrieval problems and poor sentence repetition may be useful in diagnosis of AD-CBS, because logopenic aphasia is often associated with AD pathology [43]. In this study, visuospatial problems were frequent during the disease course in AD-CBS (60%), and six patients had visuospatial problems as key clinical feature. Boyd et al. suggested that visuoperceptual deficits may assist in differentiating AD-CBS from CBS not due to AD pathology [44]. Lee, et al. also reported spatial disorientation in 22% and visual neglect in 44% of nine autopsy-proven cases of AD-CBS [20]. Further investigation on the value of visuospatial impairments in the differential diagnosis of CBS are needed.
The current study supports the notion that myoclonus may help differentiate AD-CBS from CBD-CBS [16]. Myoclonus was detected in 81% of AD-CBS, but in only 40% of CBD-CBS. It is noteworthy that two cases of AD-CBS had myoclonus as an initial feature, but it was not detected as an initial feature in any patient with CBD-CBS.
Hallucinations or delusions, or both, were noted in 13 cases (30%) of AD-CBS, but not in CBD-CBS. The frequency of hallucinations has not been thoroughly investigated in AD-CBS, and the effects of levodopa treatment complicate this finding [8]. Additional studies are needed to determine the value of hallucinations in the differential diagnosis of CBS.
In this study, six patients with AD-CBS had clinical features of posterior cortical atrophy (PCA) syndrome without necessarily meeting research criteria for PCA [45]. While not significantly different from AD-AS (33%), they tended to be different from CBD-CBS (20%). Antemortem imaging studies have also suggested that posterior cortical atrophy might differentiate CBD-CBS from AD-CBS [46]. It is of interest that posterior cortical dysfunction can be associated with visual neglect [20]. In the present series, eight AD-CBS patients had visual neglect, but it was not recorded in CBD-CBS. In contrast to AD-AS and AD-CBS, cortical atrophy was more frequent in superior frontal gyrus of CBD-CBS. This suggests that in patients with CBS, distribution of cortical atrophy may assist in the differential diagnosis, with occipital atrophy, sometimes associated with visual neglect, favoring AD-CBS and superior frontal gyrus atrophy favoring CBD-CBS.
Although asymmetry has been stressed as a core feature of CBD [20], asymmetric clinical presentations are not predictive of CBD. Moreover, patients with autopsy-confirmed CBD can have symmetric clinical features and symmetric cortical atrophy on antemortem neuroimaging [47]. In the present series, all CBS patients had asymmetry of motor signs or symptoms given that the selection criteria were stipulated to be CBS [8].
While corpus callosum atrophy is frequent in CBD, we found no difference in the thickness of corpus callosum between AD-CBS and CBD-CBS. This is similar to the observations of Lee, et al. [20]. Our study furthered suggests that clinical features in AD-CBS are not driven by white matter pathology, since we found no differences between AD-AS and AD-CBS for burden of tau or activated microglia, the latter assessed by CD68 immunohistochemistry. On the other hand, we found significant differences in regional tau pathology, with increased tau burden in peri-Rolandic cortices (motor cortex and somatosensory cortex) of AD-CBS and significantly decreased tau pathology in limbic and multimodal association cortices (cingulate gyrus and superior frontal gyrus) compared with AD-AS (Table 3).
Regarding Parkinsonism, we found more neuronal loss in substantia nigra in AD-CBS compared with AD-AS, but both had significantly less neuronal loss than in CBD-CBS (Table 4). Our findings differ from previous reports of no difference in substantia nigra degeneration between AD and AD-CBS [12, 17, 21]. This may be related to differences in assessment of neuronal loss in the substantia nigra. In the present study, neuronal loss was specifically assessed in the ventrolateral cell group, which is selectively vulnerable to neuronal loss in Parkinsonian disorders [34] and which has been shown to correlate with striatal dopaminergic degeneration [35]. The substantia nigra cell groups evaluated for neuronal loss is not always clearly defined in other studies.
There are strengths and weaknesses in this study. A weakness of the study is its retrospective nature, and as result we did not have standardization of medical documentation. Nevertheless, most of the AD-CBS patients were evaluated by experts in behavioral neurology or movement disorders, often in clinics in academic medical centers (see acknowledgements). The strengths of this study are that all cases had systematic and standardized neuropathologic evaluations, with semi-quantitative assessment of neuronal and glial lesions, supplemented with measures of tau burden objectively measured with digital pathologic methods.
In summary, we find that AD-CBS is a variant of AD with atypical distribution of tau pathology, particularly in peri-Rolandic cortices. It differs from AD-AS by more severe neuronal loss in ventrolateral substantia nigra, a finding that may be associated with Parkinsonism. Myoclonus, logopenic type aphasia, posterior cortical syndromes and evidence of occipital atrophy may help differentiate AD-CBS from CBD-CBS. Early onset of myoclonus may also support a diagnosis of AD-CBS over CBD-CBS, while early visuospatial problems, visual neglect and early memory deficits would favor AD-CBS. Together with these clinical features, structural and molecular neuroimaging (amyloid and tau PET) and CSF biomarkers needs to be evaluated and would be expected to increase the antemortem diagnostic accuracy of AD-CBS.
Research in context.
1. Systematic review
Review of the medical literature and personal experience from the brain bank at Mayo Clinic suggest that diagnostic accuracy of corticobasal degeneration (CBD) is poor (less than 50%).
2. Interpretation
We studied clinical and pathological features that differentiate AD-CBS from CBD-CBS, based on retrospective review of medical records and quantitative neuropathologic methods in the world’s largest autopsy series of AD-CBS. While there are inherent limitations of retrospective analyses, we found early myoclonus and visual neglect may assist in differentiating AD-CBS from CBD-CBS. AD-CBS had greater tau pathology in peri-Rolandic cortices, but less in the temporal cortex than AD-AS. Substantia nigra neuronal loss was also greater.
3. Future directions
Molecular and neuroimaging biomarkers will be needed to increase diagnostic accuracy of CBS. Differentiating CBD-CBS from AD-CBS is important since tau pathology in AD is different, namely 3R+4R tau, and 4R-tau specific therapies may not be beneficial in AD.
Acknowledgments
We thank the patients and their families who donated brains to help further our knowledge of neurodegeneration. We are grateful to Linda Rousseau and Virginia Phillips for histological support; and to Monica Castanedes-Casey for immunohistochemistry support. Cases were referred to the brain bank for neurodegenerative diseases from a wide range of sources and the contributions of the following clinicians for diagnostic evaluation of CBS-AD patients is gratefully acknowledged: Garrett Alexander (Emory University), Karen Bell (Columbia University), Bradley Boeve (Mayo Clinic), Kevin Boylan (Mayo Clinic), Melanie Brandabur (The Parkinson Institute), David Burks (University of Florida), Joan Camprodon (Harvard University), Howard Crystal (Albert Einstein College of Medicine), Daniel Drubach (Mayo Clinic), Michael Geschwind (University of California San Francisco), Timothy Hain (Northwestern University), Argye Hillis (Johns Hopkins University), Kenneth Heilman (University of Florida), Jorge Juncos (Emory University), Margery Mark (Robert Wood Johnson Medical School), Norman Relkin (Cornell University), David Solomon (Johns Hopkins University), Daniel Tarsy (Harvard University), Edward Urban (University of Florida), Ray Watts (Emory University), and Kyle Womack (University of Texas Southwestern). This work is supported by NIH grants P01 NS84974, U54 NS100693 and P50 AG16574.
Footnotes
Conflict of interest statement: The authors have declared that no conflict of interest exists.
References
- [1].Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014;13:614–29. [DOI] [PubMed] [Google Scholar]
- [2].Panegyres PK, Goh J, McCarthy M, Campbell AI. The Nature and Natural History of Posterior Cortical Atrophy Syndrome: A Variant of Early-onset Alzheimer Disease. Alzheimer Dis Assoc Disord. 2017;31:295–306. [DOI] [PubMed] [Google Scholar]
- [3].Josephs KA, Whitwell JL, Duffy JR, Vanvoorst WA, Strand EA, Hu WT, et al. Progressive aphasia secondary to Alzheimer disease vs FTLD pathology. Neurology. 2008;70:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gorno-Tempini ML, Brambati SM, Ginex V, Ogar J, Dronkers NF, Marcone A, et al. The logopenic/phonological variant of primary progressive aphasia. Neurology. 2008;71:1227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Johnson JK, Head E, Kim R, Starr A, Cotman CW. Clinical and pathological evidence for a frontal variant of Alzheimer disease. Arch Neurol. 1999;56:1233–9. [DOI] [PubMed] [Google Scholar]
- [6].Ossenkoppele R, Pijnenburg YA, Perry DC, Cohn-Sheehy BI, Scheltens NM, Vogel JW, et al. The behavioural/dysexecutive variant of Alzheimer’s disease: clinical, neuroimaging and pathological features. Brain. 2015;138:2732–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Snowden JS, Thompson JC, Stopford CL, Richardson AM, Gerhard A, Neary D, et al. The clinical diagnosis of early-onset dementias: diagnostic accuracy and clinicopathological relationships. Brain. 2011;134:2478–92. [DOI] [PubMed] [Google Scholar]
- [8].Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, Borroni B, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology. 2013;80:496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gibb WR, Luthert PJ, Marsden CD. Corticobasal degeneration. Brain. 1989;112 ( Pt 5):1171–92. [DOI] [PubMed] [Google Scholar]
- [10].Hassan A, Whitwell JL, Josephs KA. The corticobasal syndrome-Alzheimer’s disease conundrum. Expert Rev Neurother. 2011;11:1569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ling H, O’Sullivan SS, Holton JL, Revesz T, Massey LA, Williams DR, et al. Does corticobasal degeneration exist? A clinicopathological re-evaluation. Brain. 2010;133:2045–57. [DOI] [PubMed] [Google Scholar]
- [12].Boeve BF, Lang AE, Litvan I. Corticobasal degeneration and its relationship to progressive supranuclear palsy and frontotemporal dementia. Ann Neurol. 2003;54 Suppl 5:S15–9. [DOI] [PubMed] [Google Scholar]
- [13].Chand P, Grafman J, Dickson D, Ishizawa K, Litvan I. Alzheimer’s disease presenting as corticobasal syndrome. Mov Disord. 2006;21:2018–22. [DOI] [PubMed] [Google Scholar]
- [14].Kasanuki K, Josephs KA, Ferman TJ, Murray ME, Koga S, Konno T, et al. Diffuse Lewy body disease manifesting as corticobasal syndrome: A rare form of Lewy body disease. Neurology. 2018;91:e268–e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sha SJ, Ghosh PM, Lee SE, Corbetta-Rastelli C, Jagust WJ, Kornak J, et al. Predicting amyloid status in corticobasal syndrome using modified clinical criteria, magnetic resonance imaging and fluorodeoxyglucose positron emission tomography. Alzheimers Res Ther. 2015;7:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hu WT, Rippon GW, Boeve BF, Knopman DS, Petersen RC, Parisi JE, et al. Alzheimer’s disease and corticobasal degeneration presenting as corticobasal syndrome. Mov Disord. 2009;24:1375–9. [DOI] [PubMed] [Google Scholar]
- [17].Okazaki K, Fu YJ, Nishihira Y, Endo M, Fukushima T, Ikeuchi T, et al. Alzheimer’s disease: report of two autopsy cases with a clinical diagnosis of corticobasal degeneration. Neuropathology. 2010;30:140–8. [DOI] [PubMed] [Google Scholar]
- [18].Duker AP, Espay AJ, Wszolek ZK, Rademakers R, Dickson DW, Kelley BJ. Atypical motor and behavioral presentations of Alzheimer disease: a case-based approach. Neurologist. 2012;18:266–72. [DOI] [PubMed] [Google Scholar]
- [19].Josephs KA, Whitwell JL, Boeve BF, Knopman DS, Petersen RC, Hu WT, et al. Anatomical differences between CBS-corticobasal degeneration and CBS-Alzheimer’s disease. Mov Disord. 2010;25:1246–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lee SE, Rabinovici GD, Mayo MC, Wilson SM, Seeley WW, DeArmond SJ, et al. Clinicopathological correlations in corticobasal degeneration. Ann Neurol. 2011;70:327–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ouchi H, Toyoshima Y, Tada M, Oyake M, Aida I, Tomita I, et al. Pathology and sensitivity of current clinical criteria in corticobasal syndrome. Mov Disord. 2014;29:238–44. [DOI] [PubMed] [Google Scholar]
- [22].Doran M, du Plessis DG, Enevoldson TP, Fletcher NA, Ghadiali E, Larner AJ. Pathological heterogeneity of clinically diagnosed corticobasal degeneration. J Neurol Sci. 2003;216:127–34. [DOI] [PubMed] [Google Scholar]
- [23].Sperling RA, Rentz DM, Johnson KA, Karlawish J, Donohue M, Salmon DP, et al. The A4 study: stopping AD before symptoms begin? Sci Transl Med. 2014;6:228fs13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Boxer AL, Lang AE, Grossman M, Knopman DS, Miller BL, Schneider LS, et al. Davunetide in patients with progressive supranuclear palsy: a randomised, double-blind, placebo-controlled phase 2/3 trial. Lancet Neurol. 2014;13:676–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Josephs KA, Dickson DW. Diagnostic accuracy of progressive supranuclear palsy in the Society for Progressive Supranuclear Palsy brain bank. Mov Disord. 2003;18:1018–26. [DOI] [PubMed] [Google Scholar]
- [26].Barker WW, Luis CA, Kashuba A, Luis M, Harwood DG, Loewenstein D, et al. Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis Assoc Disord. 2002;16:203–12. [DOI] [PubMed] [Google Scholar]
- [27].Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 2012;123:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Uchikado H, Lin WL, DeLucia MW, Dickson DW. Alzheimer disease with amygdala Lewy bodies: a distinct form of alpha-synucleinopathy. J Neuropathol Exp Neurol. 2006;65:685–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ferman TJ, Arvanitakis Z, Fujishiro H, Duara R, Parfitt F, Purdy M, et al. Pathology and temporal onset of visual hallucinations, misperceptions and family misidentification distinguishes dementia with Lewy bodies from Alzheimer’s disease. Parkinsonism Relat Disord. 2013;19:227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Imamura A, Wszolek ZK, Lucas JA, Dickson DW. Corticobasal syndrome with Alzheimer’s disease pathology. Mov Disord. 2009;24:152–3; author reply 3. [DOI] [PubMed] [Google Scholar]
- [31].Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59. [DOI] [PubMed] [Google Scholar]
- [32].Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–800. [DOI] [PubMed] [Google Scholar]
- [33].Murray ME, Lowe VJ, Graff-Radford NR, Liesinger AM, Cannon A, Przybelski SA, et al. Clinicopathologic and 11C-Pittsburgh compound B implications of Thal amyloid phase across the Alzheimer’s disease spectrum. Brain. 2015;138:1370–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Dickson DW, Braak H, Duda JE, Duyckaerts C, Gasser T, Halliday GM, et al. Neuropathological assessment of Parkinson’s disease: refining the diagnostic criteria. Lancet Neurol. 2009;8:1150–7. [DOI] [PubMed] [Google Scholar]
- [35].Kasanuki K, Heckman MG, Diehl NN, Murray ME, Koga S, Soto A, et al. Regional analysis and genetic association of nigrostriatal degeneration in Lewy body disease. Mov Disord. 2017;32:1584–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Murray ME, Przybelski SA, Lesnick TG, Liesinger AM, Spychalla A, Zhang B, et al. Early Alzheimer’s disease neuropathology detected by proton MR spectroscopy. J Neurosci. 2014;34:16247–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ling H, Kovacs GG, Vonsattel JP, Davey K, Mok KY, Hardy J, et al. Astrogliopathy predominates the earliest stage of corticobasal degeneration pathology. Brain. 2016;139:3237–52. [DOI] [PubMed] [Google Scholar]
- [38].Dickson DW, Kouri N, Murray ME, Josephs KA. Neuropathology of frontotemporal lobar degeneration-tau (FTLD-tau). J Mol Neurosci. 2011;45:384–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Dickson DW, Ahmed Z, Algom AA, Tsuboi Y, Josephs KA. Neuropathology of variants of progressive supranuclear palsy. Curr Opin Neurol. 2010;23:394–400. [DOI] [PubMed] [Google Scholar]
- [40].Kovacs GG, Molnar K, Laszlo L, Strobel T, Botond G, Honigschnabl S, et al. A peculiar constellation of tau pathology defines a subset of dementia in the elderly. Acta Neuropathol. 2011;122:205–22. [DOI] [PubMed] [Google Scholar]
- [41].McMillan CT, Boyd C, Gross RG, Weinstein J, Firn K, Toledo JB, et al. Multimodal imaging evidence of pathology-mediated disease distribution in corticobasal syndrome. Neurology. 2016;87:1227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Di Stefano F, Kas A, Habert MO, Decazes P, Lamari F, Lista S, et al. The phenotypical core of Alzheimer’s disease-related and nonrelated variants of the corticobasal syndrome: A systematic clinical, neuropsychological, imaging, and biomarker study. Alzheimers Dement. 2016;12:786–95. [DOI] [PubMed] [Google Scholar]
- [43].Mesulam MM, Rogalski EJ, Wieneke C, Hurley RS, Geula C, Bigio EH, et al. Primary progressive aphasia and the evolving neurology of the language network. Nat Rev Neurol. 2014;10:554–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Boyd CD, Tierney M, Wassermann EM, Spina S, Oblak AL, Ghetti B, et al. Visuoperception test predicts pathologic diagnosis of Alzheimer disease in corticobasal syndrome. Neurology. 2014;83:510–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Crutch SJ, Schott JM, Rabinovici GD, Murray M, Snowden JS, van der Flier WM, et al. Consensus classification of posterior cortical atrophy. Alzheimers Dement. 2017;13:870–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Whitwell JL, Jack CR Jr., Boeve BF, Parisi JE, Ahlskog JE, Drubach DA, et al. Imaging correlates of pathology in corticobasal syndrome. Neurology. 2010;75:1879–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hassan A, Whitwell JL, Boeve BF, Jack CR Jr., Parisi JE, Dickson DW, et al. Symmetric corticobasal degeneration (S-CBD). Parkinsonism Relat Disord. 2010;16:208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]


