Abstract
Kanack and colleagues analyze anti-platelet factor 4 antibodies from 5 patients with vaccine-induced thrombotic thrombocytopenia (VITT) secondary to COVID-19 adenoviral vaccination and antibodies from patients with spontaneous heparin-induced thrombocytopenia (HIT) and classical HIT. VITT antibodies are monoclonal or oligoclonal, similar to spontaneous HIT, whereas classical HIT antibodies are polyclonal. Heparin inhibits antibody-induced platelet activation in VITT, suggesting that heparin should be considered for the treatment of VITT.
TO THE EDITOR:
Recently developed vaccines have produced salutary effects on hospitalizations and deaths related to SARS-CoV-2 (COVID-19).1 Two vaccines, ChAdOx1 nCoV-19 (AstraZeneca) and Ad26.COV2.S (Janssen/Johnson & Johnson), have been primarily associated with a rare adverse reaction, vaccine-induced immune thrombotic thrombocytopenia (VITT).2-12 VITT is characterized by strong anti-platelet factor 4 (PF4) antibodies, thrombocytopenia, and thrombosis, and has caused significant morbidity and mortality.13 VITT shares a key feature with another well-studied entity, heparin-induced thrombocytopenia (HIT)14 in that the generated antibodies recognize PF4. We embarked on a study to further characterize anti-PF4 antibodies in patients with VITT. Methods used in the study are provided in the supplemental data file, available on the Blood Web site. Briefly, all 5 patients with VITT in our study experienced thrombocytopenia and thrombosis and all but 1 were treated with intravenous immunoglobulin (IVIg) (supplemental Figure 1 and “VITT patient clinical histories” in the supplemental data file). All patients had strong positive results in solid-phase enzyme-linked immunosorbent assays (ELISAs), but results from serotonin release assay (SRA; performed in the presence of low concentrations of heparin) were variably positive between patients and within the same patient over time (supplemental Figure 1). All 5 patients tested positive in an assay that used PF4-treated platelets, the PF4-dependent P-Selectin Expression Assay (PEA: 48%, 68%, 61%, 68%, and 73% in VITT patients 1 through 5, respectively. Negative control PEA values ranged from 1% to 8%; data not shown).
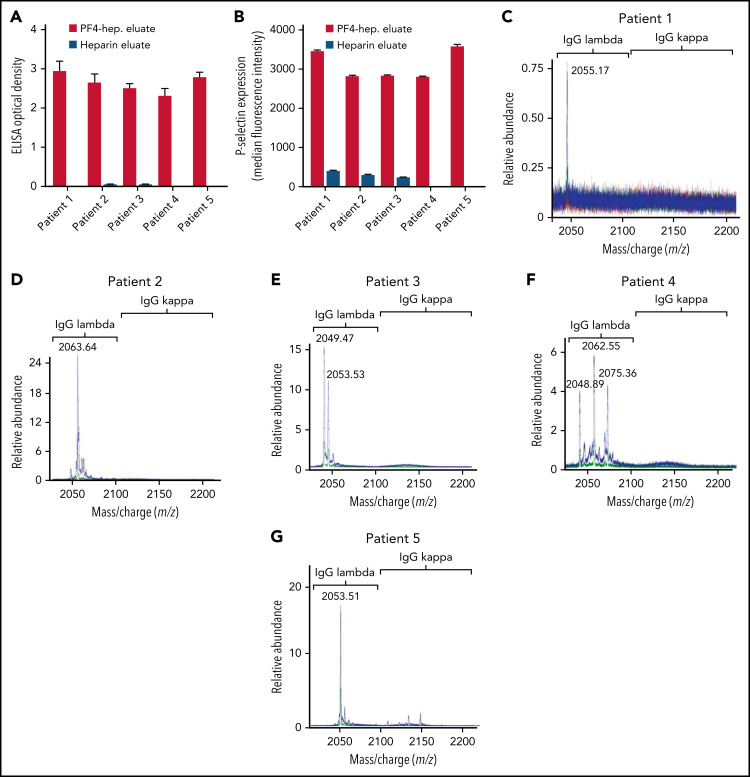
Techniques used for anti-PF4 antibody isolation and characterization by mass spectrometry are schematically presented in supplemental Figure 2 and described in detail in the supplemental data file. To ensure that the techniques used effectively depleted anti-PF4 antibodies from the VITT native samples, both the native sample and the native sample treated with PF4-heparin beads were subjected to testing in the PF4-polyanion ELISA. Results demonstrated little to no unbound anti-PF4 antibody in the bead-treated native samples (supplemental Figure 3A). In all 5 patients with VITT, the majority of anti-PF4 antibodies were of the immunoglobulin G1 (IgG1) subclass (supplemental Figure 3B), and platelet FcγRIIa blockade with antibody IV.3 abrogated VITT anti-PF4 antibody-mediated platelet activation (supplemental Figure 3C). VITT anti-PF4 antibodies eluted from PF4-treated heparin sepharose beads or heparin (control) beads were tested in the PF4-polyanion ELISA (Figure 1A) and PEA (Figure 1B). Results demonstrated that antibodies eluted from PF4-treated heparin beads, but not control beads, bound PF4-polyanion complexes strongly and activated platelets in the PEA, which confirmed the specific isolation of anti-PF4 antibodies. Liquid chromatography electrospray ionization quadrupole time-of-flight mass spectrometry (LC-ESI-QTOF MS) was performed on these antibody eluates. Monoclonal anti-PF4 antibodies were seen in patients 1, 2, and 5 (Figure 1C-D,G), whereas biclonal and triclonal anti-PF4 antibodies were noted in patients 3 and 4, respectively (Figure 1E-F). Of note, anti-PF4 antibodies from all patients with VITT contained λ light chains. These monoclonal and oligoclonal antibodies, although prominent upon evaluation of the isolated anti-PF4 antibody, were not evident above the patients’ IgG polyclonal background. Neither immunofixation electrophoresis (supplemental Figure 4) nor LC-ESI-QTOF MS (supplemental Figure 5A) performed on native serum from patients with VITT identified monoclonal or oligoclonal bands or antibodies, respectively.
Figure 1.
VITT antibodies are monoclonal or oligoclonal. (A-B) Eluates from PF4-heparin beads and control heparin beads were evaluated in PF4-polyanion ELISA for binding to PF4-polyvinylsulfonate complexes and for platelet activation in the PEA. Means and SD (n = 3) are shown. Control (heparin) bead studies were not performed with patients 4 and 5 because of limited sample volume. (C-G) Displayed are LC-ESI-QTOF MS light chain +11 (mass to charge [m/z]) distributions from anti-PF4 antibodies isolated from 5 patients with VITT. In the spectra, green represents the distribution of all λ-containing Ig’s, red represents the +11 m/z distribution of all κ-containing Ig’s, and blue represents the +11 m/z light chain distribution of κ and λ light chains associated with an IgG heavy chain. The number listed above the peaks indicates the +11 m/z ratio of the identified light chain. The x-axis shows m/z ratios, and the y-axis shows the relative abundance of the monoclonal or oligoclonal antibody identified.
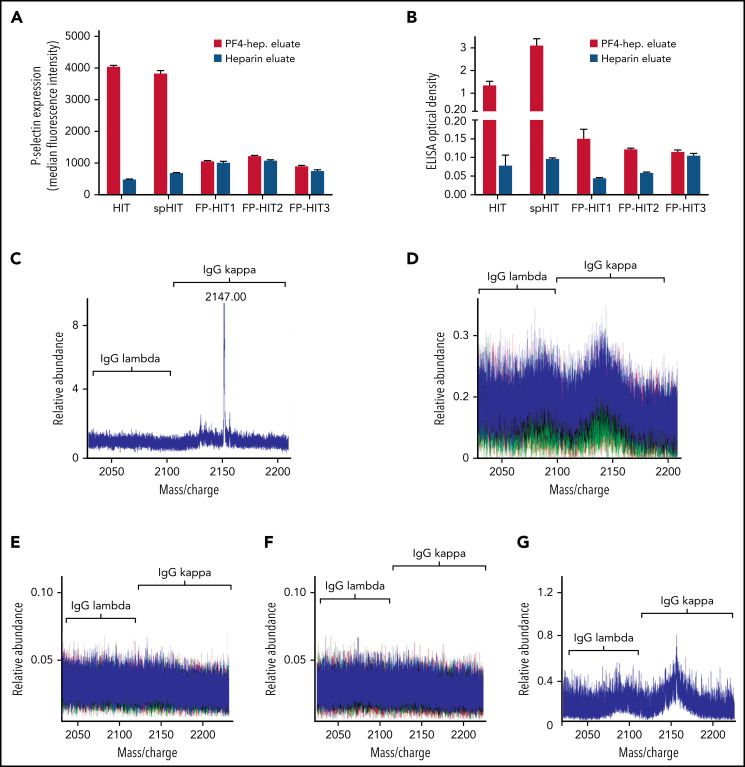
Comparative studies were performed to assess anti-PF4 antibody clonality in a patient with spontaneous HIT (spHIT; ELISA optical density [OD], 2.37; SRA-positive),15 which, like VITT, develops in the absence of proximate heparin exposure, in a patient with classical heparin-induced HIT (HIT; ELISA OD, 2.500; SRA-positive), and 3 patients with positive PF4-polyanion ELISA but negative SRA results after heparin exposure during cardiac surgery (false-positive [FP] ELISA antibodies [FP-HIT]; ELISA-positive with OD of 0.429 [FP-HIT1], 0.426 [FP-HIT2], and 0.802 [FP-HIT3], all SRA-negative). As expected, isolated anti-PF4 HIT and spHIT antibodies strongly activated platelets and bound to PF4-polyanion targets (Figure 2A-B) whereas eluates from the 3 patients with FP-ELISA results did not activate platelets and produced only minimal binding to PF4-polyanion complexes (Figure 2A-B). The patient with spHIT demonstrated a relatively abundant IgG κ monoclonal anti-PF4 antibody (Figure 2C), while the patient with classical HIT had polyclonal anti-PF4 antibodies (Figure 2D). Effective depletion of anti-PF4 antibodies from the spHIT native serum sample was confirmed before these studies were performed. Mean PF4-polyanion ELISA OD was 2.37 (0.023 standard deviation [SD]) in the native sample and 0.10 (0.008 SD) in the anti-PF4–depleted sample, respectively (data not shown). Anti-PF4 antibodies from 2 patients with non-activating anti-PF4 antibodies (FP-HIT1 and FP-HIT2) were below the level of detection by MS (Figure 2E-F), but low-level polyclonal antibodies were noted in FP-HIT3 (Figure 2G). Of note, evaluation of the entire serum IgG repertoire of one of the patients with FP-ELISA antibodies (FP-HIT1) demonstrated a monoclonal antibody (supplemental Figure 5B) that was not isolated non-specifically by our techniques. In addition, eluates from control (heparin sepharose) beads showed no eluted IgGs, demonstrating that PF4 bound to the beads was critical for isolation of anti-PF4 antibodies (supplemental Figure 6A-D). Testing of patient 4 at 6 weeks after acute presentation revealed persistent antibodies that recognized PF4-polyanion complexes although at lower levels compared with the acute samples (Figure 1A vs supplemental Figure 7A). Similarly, although the PEA was still positive, it was lower in the follow-up sample relative to acute serum (41% [supplemental Figure 7B] vs 68%), and triclonal VITT antibodies were still detectable by MS but in lower relative abundance (Figure 1F vs supplemental Figure 7C).
Figure 2.
Anti-PF4 antibody characterization in spHIT, HIT, and patients with ELISA-positive but non-activating anti-PF4 antibodies (FP-HIT). (A-B) Eluates from PF4-heparin beads and control heparin beads were evaluated for platelet activation in the PEA and PF4-polyanion ELISA. Means and SD (n = 3) are shown. (C-F) Shown are LC-ESI-QTOF MS +11 light chain distributions from anti-PF4 antibodies isolated from patients with (C) spHIT, (D) HIT, and (E-G) FP-HIT. In the spectra, green represents the distribution of all λ-containing Ig’s, red represents the distribution of all κ-containing Ig’s, and blue represents the light chain distribution of κ and λ light chains associated with an IgG heavy chain. The numbers listed above peaks depict the identified light chain’s m/z ratio. The x-axis shows m/z ratios, and the y-axis depicts the relative abundance of the monoclonal or oligoclonal antibody identified.
Murray et al16 have recently used MS for the sensitive and specific detection of monoclonal proteins in multiple myeloma and related disorders.16,17 Using this technique, we demonstrated that anti-PF4 antibodies in VITT are monoclonal or oligoclonal, whereas HIT antibodies were confirmed to be polyclonal, which is consistent with current dogma.18-20 Due to the limited number of samples tested, it is too early to know whether λ light chain restriction seen with all 5 patients tested is characteristic of VITT antibodies. ELISA-reactive but non-activating anti-PF4 antibodies were either below the limit of detection by MS or polyclonal in nature. Interestingly, anti-PF4 antibodies in spontaneous HIT, like in VITT, were monoclonal (albeit with the κ light chain in the patient tested). Importantly, to exclude the possibility that the techniques used to isolate anti-PF4 antibodies in this study resulted in the selective enrichment of only those antibodies that had the highest affinity for PF4, thereby producing an artificial oligoclonal antibody pattern by MS, we ensured that there was a significant depletion of anti-PF4 antibodies from the native sample after the affinity purification process. The majority of VITT antibodies were of the IgG1 subclass, similar to anti-PF4 antibodies seen in HIT.21 Results also revealed persistent platelet-activating and strongly ELISA-binding antibodies in both native sera and isolated anti-PF4 antibody fractions obtained from 2 patients at ∼1.5 months (patient 4) and ∼ 2.5 months (patient 1) after initial presentation, consistent with recent data on significantly longer persistence of anti-PF4 antibodies in VITT relative to HIT.22-24 These findings are consistent with the possibility that single or very few clones producing anti-PF4 antibodies in VITT are more active or persistent than multiple clones that produce polyclonal antibodies in classical HIT. Clonal restriction of anti-PF4 antibodies in VITT seen here is also consistent with very limited epitope specificity of VITT antibodies within the heparin-binding domain of PF4 as shown recently by Huynh and colleagues.25
In summary, we demonstrate that VITT is caused by monoclonal or oligoclonal anti-PF4 antibodies in contrast to HIT, where polyclonal antibodies are generated. In addition, monoclonal anti-PF4 antibodies were also seen in spontaneous HIT, a condition that like VITT, develops in the absence of proximate heparin exposure. Studies characterizing anti-PF4 antibody–producing cells in these syndromes should significantly enhance our understanding of their pathophysiology.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
The authors thank Stephanie Hafner for exceptional research coordination.
This work was supported, in part, by grants from the National Institutes of Health, National Heart, Lung, and Blood Institute (HL133479, HL158932 [A.P.], HL148120 [R.W.] and HL130724 [D.W.]).
Footnotes
The online version of this article contains a data supplement.
Authorship
Contribution: A.J.K., A.B., G.G., M.Y.A.-I., B.S., N.P.S., and A.G. provided critical input on laboratory and clinical elements of the study; A.J.K., B.S., and N.P.S. performed anti-PF4 antibody isolation and ELISA studies and assessed platelet activation; M.C.K. performed MS studies; M.C., M.N., K.A.M., K.J.S., R.W., and D.W. provided helpful advice on clinical and laboratory aspects of the manuscript; D.L.M. and A.P. conceived the experimental plan and directed the laboratory studies; A.J.K., B.S., and A.P. wrote the first draft; and all authors provided input and approved the final version.
Conflict-of-interest disclosure: A.P. has pending/issued patents (Mayo Clinic, Retham Technologies, and Versiti), equity ownership in Retham Technologies, and serves on the advisory board of Veralox Therapeutics. D.M. has pending/issued patents (Mayo Clinic). The remaining authors declare no competing financial interests.
Correspondence: Anand Padmanabhan, Mayo Clinic, 200 1st St SW, Rochester, MN 55905; e-mail: padmanabhan.anand@mayo.edu; and David L. Murray, Mayo Clinic, 200 1st St SW, Rochester, MN 55905; e-mail: murray.david@mayo.edu.
REFERENCES
- 1.Creech CB, Walker SC, Samuels RJ. SARS-CoV-2 vaccines. JAMA. 2021;325(13):1318-1320. [DOI] [PubMed] [Google Scholar]
- 2.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384(22):2092-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(22):2124-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scully M, Singh D, Lown R, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021; 384(23):2202-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muir KL, Kallam A, Koepsell SA, Gundabolu K. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination. N Engl J Med. 2021;384(20):1964-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.See I, Lale A, Marquez P, et al. Case series of thrombosis with thrombocytopenia syndrome after COVID-19 vaccination-United States, December 2020 to August 2021. Ann Intern Med. 2022; 175(4):513-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abou-Ismail MY, Moser KA, Smock KJ, Lim MY. Vaccine-induced thrombotic thrombocytopenia following Ad26.COV2.S vaccine in a man presenting as acute venous thromboembolism. Am J Hematol. 2021;96(9):E346-E349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.George G, Friedman KD, Curtis BR, Lind SE. Successful treatment of thrombotic thrombocytopenia with cerebral sinus venous thrombosis following Ad26.COV2.S vaccination. Am J Hematol. 2021;96(8): E301-E303. [DOI] [PubMed] [Google Scholar]
- 9.Bayas A, Menacher M, Christ M, Behrens L, Rank A, Naumann M. Bilateral superior ophthalmic vein thrombosis, ischaemic stroke, and immune thrombocytopenia after ChAdOx1 nCoV-19 vaccination. Lancet. 2021;397(10285):e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bourguignon A, Arnold DM, Warkentin TE, et al. Adjunct immune globulin for vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med. 2021;385(8):720-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tiede A, Sachs UJ, Czwalinna A, et al. Prothrombotic immune thrombocytopenia after COVID-19 vaccination. Blood. 2021;138(4): 350-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makris M, Pavord S, Lester W, Scully M, Hunt B. Vaccine-induced immune thrombocytopenia and thrombosis (VITT). Res Pract Thromb Haemost. 2021;5(5):e12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pavord S, Scully M, Hunt BJ, et al. Clinical features of vaccine-induced immune thrombocytopenia and thrombosis. N Engl J Med. 2021; 385(18):1680-1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warkentin TE. Laboratory diagnosis of heparin-induced thrombocytopenia. Int J Lab Hematol. 2019;41(suppl 1):15-25. [DOI] [PubMed] [Google Scholar]
- 15.Irani M, Siegal E, Jella A, Aster R, Padmanabhan A. Use of intravenous immunoglobulin G to treat spontaneous heparin-induced thrombocytopenia. Transfusion. 2019;59(3):931-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murray DL, Puig N, Kristinsson S, et al. Mass spectrometry for the evaluation of monoclonal proteins in multiple myeloma and related disorders: an International Myeloma Working Group Mass Spectrometry Committee Report. Blood Cancer J. 2021;11(2):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murray DL, Dasari S. Clinical mass spectrometry approaches to myeloma and amyloidosis. Clin Lab Med. 2021;41(2): 203-219. [DOI] [PubMed] [Google Scholar]
- 18.Li ZQ, Liu W, Park KS, et al. Defining a second epitope for heparin-induced thrombocytopenia/thrombosis antibodies using KKO, a murine HIT-like monoclonal antibody. Blood. 2002;99(4): 1230-1236. [DOI] [PubMed] [Google Scholar]
- 19.Huynh A, Arnold DM, Kelton JG, et al. Characterization of platelet factor 4 amino acids that bind pathogenic antibodies in heparin-induced thrombocytopenia. J Thromb Haemost. 2019;17(2):389-399. [DOI] [PubMed] [Google Scholar]
- 20.Staibano P, Arnold DM, Bowdish DM, Nazy I. The unique immunological features of heparin-induced thrombocytopenia. Br J Haematol. 2017;177(2):198-207. [DOI] [PubMed] [Google Scholar]
- 21.Arepally G, McKenzie SE, Jiang XM, Poncz M, Cines DB. Fc gamma RIIA H/R 131 polymorphism, subclass-specific IgG anti-heparin/platelet factor 4 antibodies and clinical course in patients with heparin-induced thrombocytopenia and thrombosis. Blood. 1997;89(2):370-375. [PubMed] [Google Scholar]
- 22.Warkentin TE, Kelton JG. Temporal aspects of heparin-induced thrombocytopenia. N Engl J Med. 2001;344(17):1286-1292. [DOI] [PubMed] [Google Scholar]
- 23.Kanack AJ, Singh B, George G, et al. Persistence of Ad26.COV2. S-associated vaccine-induced immune thrombotic thrombocytopenia (VITT) and specific detection of VITT antibodies. Am J Hematol. 2022;97(5):519-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schönborn L, Thiele T, Kaderali L, et al. Most anti-PF4 antibodies in vaccine-induced immune thrombotic thrombocytopenia are transient. Blood. 2022;139(12):1903-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huynh A, Kelton JG, Arnold DM, Daka M, Nazy I. Antibody epitopes in vaccine-induced immune thrombotic thrombocytopaenia. Nature. 2021;596(7873):565-569. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.