Abstract
Background:
Early in the COVID-19 pandemic, the South Asian community in the Greater Toronto Area (GTA) was identified as having risk factors for exposure and specific barriers to accessing testing and reliable health information, rendering them particularly vulnerable to SARS-CoV-2 infection. We sought to investigate the burden of SARS-CoV-2 infection among South Asian people in the GTA, and to characterize the demographic characteristics, risk perceptions and trusted sources of health information in this group.
Methods:
We conducted a cross-sectional analysis from the baseline assessment of participants in a prospective cohort study. Participants from the GTA were enrolled from Apr. 14 to July 28, 2021. Seropositivity for antispike and antinucleocapsid antibodies was determined from dried blood spots, and estimates of seropositivity were age and sex standardized to the South Asian population in Ontario. Demographic characteristics, risk perceptions and sources of COVID-19 information were collected via questionnaire and reported descriptively.
Results:
Among the 916 South Asian participants enrolled (mean age 41 yr), the age- and sex-standardized seropositivity was 23.6% (95% confidence interval 20.8%–26.4%). Of the 693 respondents to the questionnaire, 228 (32.9%) identified as essential workers, and 125 (19.1%) reported living in a multigenerational household. A total of 288 (49.4%) perceived that they were at high COVID-19 risk owing to their geographic location, and 149 (34.3%) owing to their type of employment. The top 3 most trusted sources of information related to COVID-19 included health care providers and public health, traditional media sources and social media.
Interpretation:
By the third wave of the COVID-19 pandemic, about one-quarter of a sample of South Asian individuals in Ontario had serologic evidence of prior SARS-CoV-2 infection. Insight into factors that put certain populations at risk can help future pandemic planning and disease control efforts.
The novel SARS-CoV-2 caused an outbreak that was declared a global pandemic in March 2020. Within Canada, COVID-19 hot spots emerged, and attention was drawn to these regions by the high infection and hospitalization rates and the need to transfer patients outside of these regions to receive intensive care. South Asian people (i.e., who originate from the Indian subcontinent) are the largest nonwhite ethnic group in Canada1 and have been disproportionally affected by COVID-19.2 More than half of the residents of the Regional Municipality of Peel, in the Greater Toronto Area (GTA), identify as South Asian.3
The Peel Region emerged as a hot spot in Ontario, accounting for 22% of provincial cases during the second wave, beginning in September 2020,2 while making up only 10% of the province’s population, with the City of Brampton as the epicentre.4 Public Health Ontario provided indirect measures of the impact on South Asian populations, showing the rates of infection, hospitalization, intensive care unit admission and death by quintiles of increasing ethnic diversity in Ontario.5 People in the most diverse neighbourhoods were more likely to be new immigrants, younger and living in larger households than those living in less diverse communities. They also had 3 times the rate of infection, 4 times the rate of hospitalization, 4 times the rate of intensive care unit admission and 2 times the rate of death from COVID-19 compared with their counterparts in less diverse communities.5
Early in the pandemic, there was limited availability of laboratory testing for SARS-CoV-2 infection throughout Ontario. Furthermore, some South Asian individuals faced barriers in accessing testing, as well as reliable information, at the outset of the pandemic.6 In hot-spot communities like Peel, this was compounded by other barriers to accessing health care that predated the pandemic, which resulted in a high burden of infection. 2 However, action by local and provincial public health officials, combined with advocacy from community groups and the media,7 resulted in increased resources for testing and prioritization of the vaccine rollout, which coincided with the third wave and commenced in April 2021.8
We report the seroprevalence of SARS-CoV-2 infection in South Asian people from Ontario, Canada, who were recruited during Wave 3 of the pandemic, and report the demographic characteristics, risk perceptions and trusted sources of health information in this high-risk ethnic group.
Methods
The COVID CommUNITY study is a prospective cohort study focused on South Asian adults living in Canada, in the provinces of Ontario and British Columbia. In this cross-sectional analysis, we present data from the baseline assessment of the Ontario subcohort recruited during Wave 3 of the pandemic in Ontario (from Apr. 14 to July 28, 2021). This study was funded by the COVID-19 Immunity Task Force (CITF), and funds were distributed from the Public Health Agency of Canada.
Setting
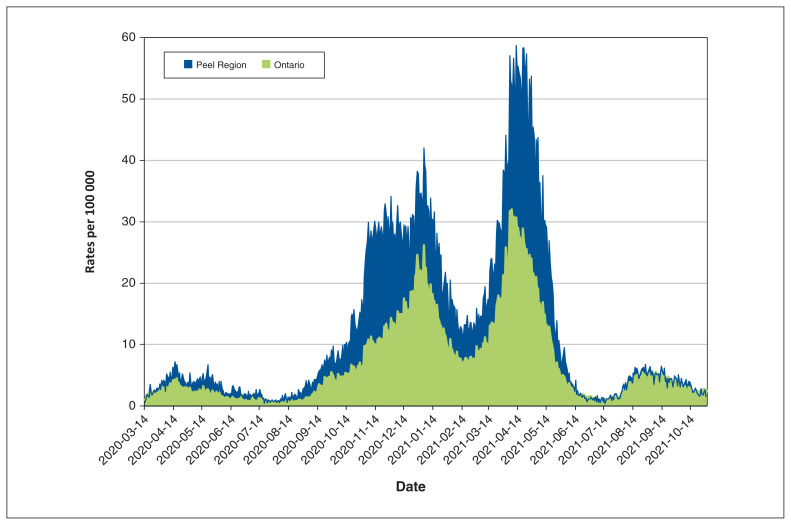
The Peel Region is made up of a town and 2 cities — Caledon, Brampton and Mississauga. Brampton has a population of 600 000, and more than one-third of residents are South Asian, increasing in some areas to nearly two-thirds.9 Data from Public Health Ontario showed that the burden of infection was higher in the Peel Region than in the rest of Ontario during our study period (Figure 1).
Figure 1:
Daily rates of SARS-CoV-2 infection in Ontario and the Peel Region, Mar. 14, 2020, to Oct. 31, 2021. Data from Public Health Ontario: COVID-19 pandemic comparing Peel Region with Ontario.10
Between April and July 2021, during our recruitment period, vaccine eligibility evolved swiftly in the Peel Region, such that on Apr. 1, vaccines were available only to those with the highest risk health conditions and seniors (age ≥ 65 yr), but by May 6, all Peel residents aged 18 years and older were eligible to receive the SARS-CoV-2 vaccine.
Study population
Adults (age ≥ 18 yr) of South Asian ethnicity were eligible for the COVID CommUNITY study (the screening form is available in Appendix 1, available at www.cmajopen.ca/content/10/3/E599/suppl/DC1). South Asian ethnicity was self-reported and defined by parental South Asian ancestry from the Indian subcontinent, Africa, Caribbean and Guyana.1
Recruitment
Recruitment was predominantly from vaccination centres in Brampton immediately after participants received dose 1 (prevaccination group) or more than 24 hours after they received dose 1 or 2 (postvaccination group). A smaller proportion of participants were recruited from places of worship, from SARS-CoV-2 testing centres in Brampton, through social media (i.e., Facebook and Instagram) and by inviting participants of South Asian origin from an existing cohort study.11
Community engagement was performed by building on the research team’s long-standing work in the South Asian community, including our research collaboration with Peel Public Health and our strong relationships with South Asian advocacy groups. In addition, when we advertised the study through social media, we used multiple South Asian languages. Our recruiters were of South Asian origin and spoke multiple South Asian languages.
Data collection
All participants provided informed consent. At the time of consent, questions were asked of participants by research personnel to collect key sociodemographic information and vaccination status. All participants were encouraged to complete a questionnaire online via an emailed link (the questionnaire is available in Appendix 2, available at www.cmajopen.ca/content/10/3/E599/suppl/DC1). The questionnaire collected additional information regarding employment type, health history, prior SARS-CoV-2 infection, perception of risk for SARS-CoV-2 infection, and primary and trusted sources of COVID-19 information. If participants did not complete the questionnaire online, study staff called participants to encourage them to complete the questionnaire, and administered a subset of questions (first visit short questionnaire, Appendix 2) by telephone.
Dried blood spots were collected by trained research personnel at recruitment sites, or through the mail for participants who were recruited through social media or existing cohorts (i.e., did not have an in-person visit with research personnel). For home collection, an instructional video and self-addressed stamped envelope was provided for mail returns. Blood was collected on Whatman 903 protein saver cards (GE Healthcare, Biosciences Corporation).
Essential work was defined as follows: health care workers; employees in a food manufacturing or distribution centre who could not work from home; retail employees working in a store, either in an essential retail setting or in a non-essential retail setting; and grocery, foodbank, nonclinical pharmacy, restaurant, LCBO, wholesale and general goods workers (https://www.retailcouncil.org/province/ontario/ontario-to-open-vaccinations-to-include-frontline-retail-workers-on-monday-may-10-2021/). Respondents’ perception of risk was rated on a 5-point scale (1 = strongly disagree and 5 = strongly agree), with statements such as “I am at high risk of COVID-19 because of my location.”
We present income in 2 ways — first, at the neighbourhood level, which corresponds to the median household income of the forward sortation area (FSA) where the participant lives, using 2015 Census tract data; and second, using the same FSA income divided by the self-reported number of household members and categorized into 5 groups (≤ $14 999, $15 000–$19 999, $20 000–$24 999, $25 000–$30 000 and ≥ $30 001 per household member). We present the former to characterize the neighbourhoods represented in our study, and we use the latter to characterize the affluence of households.
As a sensitivity analysis, we looked at the seropositivity estimates over the self-reported income categories, and the same divided by household members and categorized. These analyses support the findings of seropositivity across the FSA income/household members.
Laboratory measurements
Batched specimens of dried blood spots were sent for analysis to the CITF-funded laboratory of an author (M.-A.L.) for analysis and were processed and analyzed using a high-throughput enzyme-linked immunosorbent assay (ELISA).12,13 The main assays focused on the parallel detection of immunoglobulin G (IgG) against the spike trimer (S) and the nucleocapsid (N) protein, with standardized antigens to S and N produced by the National Research Council Canada laboratory. This method has been used in prior studies,14 and detailed methods are described elsewhere.14,15 The IgG-based ELISAs measure antibody levels in single-point measurements in reference to a standard antibody curve to accurately distinguish individuals with previous infection or vaccination from those who have not been infected or vaccinated (area under the receiver operating curve > 0.96 for each assay).
Evidence of previous infection was defined in prevaccinated participants (i.e., those unvaccinated or those submitting samples immediately after receiving dose 1 of a vaccine) as individuals with test results having signal-to-cutoff ratios of 1 or greater for both anti-S and anti-N IgG, or anti-S of 1 or greater and a history of SARS-CoV-2 infection. Evidence of previous infection among vaccinated individuals (i.e., those who submitted a dried blood spot sample > 1 day after vaccination) was defined as an anti-N IgG result of 1 or greater. A range of strict definition and lenient definition in the prevaccination group was also examined and defined as anti-S IgG and anti-N IgG of 1 or greater, and anti-S IgG of 1 or greater, respectively.15 The false discovery rate was set at 2% for the anti-S IgG and at 3% for anti-N IgG; these cut-offs have more than 98% accuracy when validated by the National Microbiology Laboratory reference panel.13 Plots were also examined to ensure these cut-offs were reasonable based on visual inspection to rule out cohort-specific effects. The mean titres of background seasonal coronavirus antibodies in the Ottawa region were used as reference.12,15 The performance of the assay was equal or superior to clinical serologic diagnostic platforms in regard to sensitivity, specificity, positive predictive value and negative predictive value of 100% (95% confidence interval [CI] 72.2–100), even when using a wide range of prevalence estimates.
Statistical analysis
The raw proportion of participants who had evidence of prior SARS-CoV-2 infection was evaluated graphically in comparison with the infection rate in similar FSAs reported by ICES, with a line of best fit drawn, and Pearson r value calculated. Overall seropositivity estimates were age and sex standardized to population numbers for South Asian people in Ontario from the Statistics Canada 2016 Census.1 Seropositivity with 95% CIs for various demographic descriptors was estimated from generalized mixed models to account for the multiple responders per household (intraclass correlation coefficient of 0.17).
Sources of health information and perception of SARS-CoV-2 risk are reported as percentages or as means and standard deviations (SDs) for the overall cohort, and for comparing seropositive participants with noninfected participants. Nonresponders to the follow-up questionnaire were compared with responders for age, sex, income, location and seroprevalence. As this sample was not a random sample of the population, the risk of bias for seroprevalence was assessed with a modified Joanna Briggs Institute Checklist for Prevalence Studies.16 This tool evaluates the external and internal validity of the study using the CoCoPop mnemonic (condition, context and population).17 The overall risk of bias for seroprevalence was determined based on the criteria met and the impact any criteria that were not met would have on the validity and reliability of the prevalence estimate.16 Items include questions on the sampling frame, sampling method, sample size, coverage, validity and reliability of the condition measured, statistical analysis and response rate (Appendix 3, Supplementary Table 1, available at www.cmajopen.ca/content/10/3/E599/suppl/DC1).
Ethics approval
The study was approved by the Hamilton Integrated Research Ethics Board (13323 — Mar. 24, 2021).
Results
Recruitment into the COVID CommUNITY study in Ontario began on Apr. 14, 2021. A total of 939 participants were recruited and had a dried blood spot collected up to July 28, 2021. Of 939 participants, 916 participants had their dried blood spots analyzed, and 693 (75.7%) of these participants completed the questionnaire online or a subset of questions by telephone (Appendix 3, Supplementary Figure 1). Participants who did not complete the survey were older and more likely to be seropositive, although no significant differences in location of recruitment, FSA household income or sex were observed (Appendix 3, Supplementary Table 2). The 916 participants represent 770 unique households, with 640 of the households having a single respondent.
Demographic characteristics
Characteristics of the cohort are shown in Table 1. The mean age of participants was 41.5 (SD 14.4) years, and 49.2% (n = 451) were women. Briefly, 91.7% (n = 830) of participants lived in the Peel Region, and most (82.0%, n = 742) were from Brampton. About two-thirds of participants (65.4%, n = 469) were born in Canada or had lived in Canada for more than 10 years.
Table 1:
Demographic characteristics of participants in the COVID CommUNITY study in Ontario
| Characteristic | No. (%) of participants n = 916 |
|---|---|
| Sex, n = 916 | |
| Female | 451 (49.2) |
| Male | 462 (50.4) |
| Self-described | 3 (0.3) |
| Age group, yr, n = 906 | |
| 18–24 | 95 (10.5) |
| 25–34 | 233 (25.7) |
| 35–44 | 247 (27.3) |
| 45–54 | 157 (17.3) |
| 55–64 | 94 (10.4) |
| ≥ 65 | 80 (8.8) |
| Vaccinated, n = 916 | |
| Yes, 2 doses | 65 (7.1) |
| Yes, 1 dose | 393 (42.9) |
| No | 458 (50.0) |
| Self-reported history of previous SARS-CoV-2 infection, n = 699 | |
| Yes | 88 (12.6) |
| No | 600 (85.8) |
| Unknown | 11 (1.6) |
| Median household income (2015) based on FSA, $, n = 904 | |
| 40 000–59 999 | 9 (1.0) |
| 60 000–79 999 | 97 (10.7) |
| 80 000–99 999 | 570 (63.1) |
| ≥ 100 000 | 228 (25.2) |
| Essential worker,* n = 693 | |
| Yes | 228 (32.9) |
| No | 352 (50.8) |
| Prefer not to answer | 113 (16.3) |
| Currently employed, n = 693 | |
| Yes | 435 (62.8) |
| No | 169 (24.4) |
| Prefer not to answer | 89 (12.8) |
| Completed education, n = 693 | |
| High school or less | 121 (17.5) |
| College, trade, certificate | 89 (12.8) |
| University degree | 453 (65.4) |
| Prefer not to answer | 30 (4.3) |
| Multigenerational household, n = 654 | |
| Yes | 125 (19.1) |
| No | 451 (69.0) |
| Prefer not to answer | 78 (11.9) |
| Time in Canada, yr, n = 717 | |
| ≤ 10 | 223 (31.1) |
| > 10 | 384 (53.6) |
| Born in Canada | 85 (11.9) |
| Prefer not to answer | 25 (3.5) |
| Mother tongue,† n = 732 | |
| Punjabi or Urdu | 363 (49.6) |
| Hindi | 83 (11.3) |
| Gujarati | 106 (14.5) |
| Other South Asian languages | 152 (20.8) |
| English | 54 (7.4) |
| Prefer not to answer | 4 (0.5) |
| Medical history, n = 679 | |
| Cardiovascular disease (MI, angioplasty or stroke) | 21 (3.1) |
| Chronic medical condition requiring medication, n = 666 | |
| Hypertension | 48 (7.2) |
| Diabetes | 53 (8.0) |
| Arthritis | 10 (1.5) |
| Chronic lung disease | 1 (0.2) |
| Cancer | 1 (0.2) |
| Smoking status, n = 641 | |
| Never | 555 (86.6) |
| Former | 53 (8.3) |
| Current | 33 (5.1) |
| Location, n = 905 | |
| Region of Peel | 830 (91.7) |
| City of Brampton | 742 (82.0) |
| Town of Caledon | 39 (4.3) |
| City of Mississauga | 49 (5.4) |
Note: FSA = forward sortation area, MI = myocardial infarction.
Examples of essential work: food processing, manufacturing or distribution; transportation; health care; education; utilities.
Multiple answers can be selected.
From the group of participants who most fully completed the questionnaires, we observed that most participants (78.2%, n = 542) had completed postsecondary education, and 62.8% (n = 435) were employed. A total of 32.9% (n = 228) of participants’ jobs were classified as essential work using the criteria of the Government of Ontario (e.g., food manufacturing and transportation workers), and an additional 16.3% (n = 113) of participants preferred not to answer this question. Although multigenerational household data were incomplete, of the 654 participants who completed this section, 19.1% (n = 125) reported living in a multigenerational household, with an additional 11.9% (n = 78) who preferred not to answer.
The most common mother tongue languages reported included Punjabi or Urdu (49.6%, n = 363), Gujarati (14.5%, n = 106) and Hindi (11.3%, n = 83). Demographic characteristics by pre- or postvaccination status are provided in Appendix 3, Supplementary Table 3.
Seropositivity
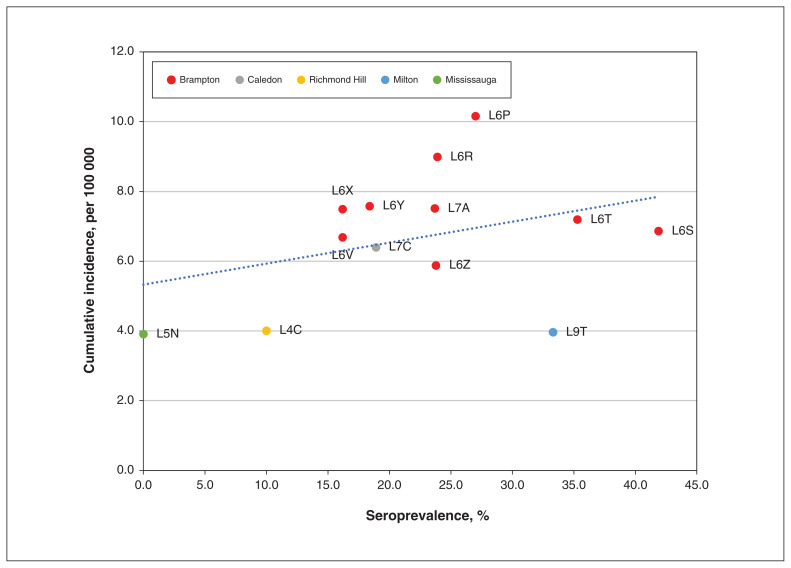
We found a moderate association (r = 0.35, p = 0.2) between the raw proportion of seropositive cases by geographic region (FSA) and the cumulative incidence of SARS-CoV-2 infection rate (Figure 2). The age- and sex-standardized seropositivity for previous infection was 23.6% (95% CI 20.8%–26.4%).
Figure 2:
Cumulative incidence of cases of SARS-CoV-2 infection (per 100 000) as of Oct. 3, 2021, by seroprevalence by geographic region (forward sortation area). Cumulative incidence data from ICES.18 Seroprevalence data from the COVID CommUNITY study.
Among the 458 participants without a prior vaccination, the age- and sex-standardized seroprevalence was 26.9% (95% CI 22.8%–31.0%) compared with 21.3% (95% CI 17.5%–25.1%) in the 458 participants who had received at least 1 dose of the SARS-CoV-2 vaccine (393 had received 1 dose, 65 had received 2 doses). The overall seropositivity ranged from 22.7%, applying the strict definition of seropositivity, to 27.4%, using the lenient definition. The risk of bias was classified as moderate, attributed to the complex, multi-pronged, nonprobability sampling method of recruitment (Appendix 3, Supplementary Table 1).
With adjustment for multiple respondents per household, the overall seropositivity was 22.9% (95% CI 20.1%–26.1%). The adjusted seropositivity was higher in participants who were male, were older, had less education, were living in multigenerational households, had lower FSA income per household size and were from the City of Brampton. Of participants who were seropositive, 53.3% (n = 81) did not report previous SARS-CoV-2 infection (Table 2).
Table 2:
Seroprevalence by demographic characteristics
| Characteristic | No. of samples tested | No. of positives | Seropositive, % (95% CI)* |
|---|---|---|---|
| Overall | 916 | 212 | 22.9 (20.1–26.1) |
| Sex | |||
| Female | 451 | 96 | 21.0 (17.3–25.4) |
| Male | 462 | 115 | 24.9 (20.9–28.8) |
| Self-described | 3 | 1 | – |
| Age group, yr | |||
| 18–24 | 95 | 22 | 22.6 (14.8–32.8) |
| 25–34 | 233 | 49 | 20.9 (15.8–27.1) |
| 35–44 | 247 | 54 | 21.5 (16.5–27.6) |
| 45–54 | 157 | 36 | 23.2 (16.8–31.0) |
| 55–64 | 94 | 28 | 29.6 (20.6–40.5) |
| ≥ 65 | 80 | 22 | 28.1 (18.5–40.1) |
| Vaccinated† | |||
| No | 458 | 107 | 23.0 (19.1–27.4) |
| Yes, 1 dose | 393 | 93 | 23.7 (19.5–28.6) |
| Yes, 2 doses | 65 | 12 | 18.8 (10.7–30.9) |
| History of previous SARS-CoV-2 infection | |||
| Yes | 88 | 69 | 78.1 (67.5–85.9) |
| No | 600 | 81 | 13.5 (10.9–16.7) |
| Unknown | 11 | 2 | 18.3 (4.3–52.8) |
| Median household income based on FSA, $ | |||
| 40 000–59 999 | 9 | 1 | 10.6 (1.3–52.4) |
| 60 000–79 999 | 97 | 22 | 22.1 (14.4–32.4) |
| 80 000–99 999 | 570 | 130 | 22.7 (19.1–26.6) |
| ≥ 100 000 | 228 | 56 | 24.6 (19.0–31.3) |
| Essential worker | |||
| Yes | 228 | 50 | 22.1 (16.8–28.4) |
| No | 352 | 73 | 20.6 (16.3–25.5) |
| Prefer not to answer | 113 | 21 | 18.3 (11.8–27.2) |
| Completed education | |||
| High school or less | 121 | 31 | 25.4 (17.9–34.7) |
| College, trade, certificate | 89 | 18 | 20.1 (12.6–30.5) |
| University degree | 453 | 89 | 19.6 (16.0–23.9) |
| Prefer not to answer | 30 | 6 | 19.4 (8.4–38.8) |
| Multigenerational household | |||
| Yes | 125 | 30 | 23.1 (16.0–32.1) |
| No | 451 | 88 | 19.7 (16.0–24.0) |
| Prefer not to answer | 78 | 15 | 18.7 (11.1–29.8) |
| Location | |||
| Region of Peel | 830 | 194 | 23.2 (20.2–26.5) |
| City of Brampton | 742 | 178 | 23.8 (20.6–27.3) |
| City of Caledon | 39 | 7 | 18.4 (8.6–34.9) |
| City of Mississauga | 49 | 9 | 18.4 (9.4–32.9) |
| FSA income by household size categories | |||
| Lowest income | 80 | 23 | 28.0 (18.5–39.9) |
| Category 2 | 121 | 22 | 18.0 (11.8–26.6) |
| Category 3 | 152 | 34 | 22.4 (16.0–30.5) |
| Category 4 | 117 | 21 | 18.1 (11.7–26.9) |
| Highest income | 102 | 17 | 16.8 (10.4–26.1) |
Note: CI = confidence interval, FSA = forward sortation area.
Seropositivity estimates and 95% CI estimates from mixed models accounting for multiple responders per household. For overall model, the intraclass correlation coefficient = 0.17.
Test interpretation for vaccinated individuals differed from that for nonvaccinated individuals; see the Methods section.
Risk perception
Of those who completed the risk perception section of the questionnaire (Table 3), 89.0% (n = 519) reported that COVID-19 posed a major threat to the South Asian community, 49.4% (n = 288) agreed or strongly agreed that they were at high risk of COVID-19 because of their location, 34.3% (n = 149) reported being at high risk because of their work or profession, 13.9% (n = 81) because of socializing or lifestyle and 13.6% (n = 79) because of their housing situation.
Table 3:
Risk perception*
| Risk statement | N | Strongly disagree | Disagree | Neutral | Agree | Strongly agree | Prefer not to answer |
|---|---|---|---|---|---|---|---|
| COVID-19 poses a major threat to our community | 583 | 10 (1.7) | 4 (0.7) | 34 (5.8) | 149 (25.6) | 370 (63.5) | 16 (2.7) |
| The situation around COVID-19 is overexaggerated or overblown | 582 | 154 (26.5) | 190 (32.6) | 106 (18.2) | 61 (10.5) | 48 (8.2) | 23 (4.0) |
| I am at high risk of COVID-19 because of my location | 583 | 41 (7.0) | 89 (15.3) | 147 (25.2) | 185 (31.7) | 103 (17.7) | 18 (3.1) |
| I am at high risk of COVID-19 because of my housing situation | 583 | 165 (28.3) | 217 (37.2) | 107 (18.4) | 50 (8.6) | 29 (5.0) | 15 (2.6) |
| I am at high risk of COVID-19 because of my profession or my work | 435 | 103 (23.7) | 84 (19.3) | 85 (19.5) | 82 (18.9) | 67 (15.4) | 14 (3.2) |
| I am at high risk of COVID-19 because of my lifestyle (socializing or working in a crowded place) | 583 | 189 (32.4) | 199 (34.1) | 100 (17.2) | 47 (8.1) | 34 (5.8) | 14 (2.4) |
| If I get exposed to [SARS-CoV-2] or contract COVID-19, I am likely to have serious symptoms because of age and/or pre-existing conditions | 583 | 136 (23.3) | 162 (27.8) | 149 (25.6) | 73 (12.5) | 40 (6.9) | 23 (3.9) |
| If I get exposed to [SARS-CoV-2] or contract COVID-19, I am likely to need hospitalization because of my age and/or pre-existing conditions | 582 | 138 (23.7) | 173 (29.7) | 163 (28.0) | 49 (8.4) | 36 (6.2) | 23 (4.0) |
Response captured on 5-point scale of strongly disagree = 1, disagree, neutral, agree, or strongly agree = 5.
Sources of health information
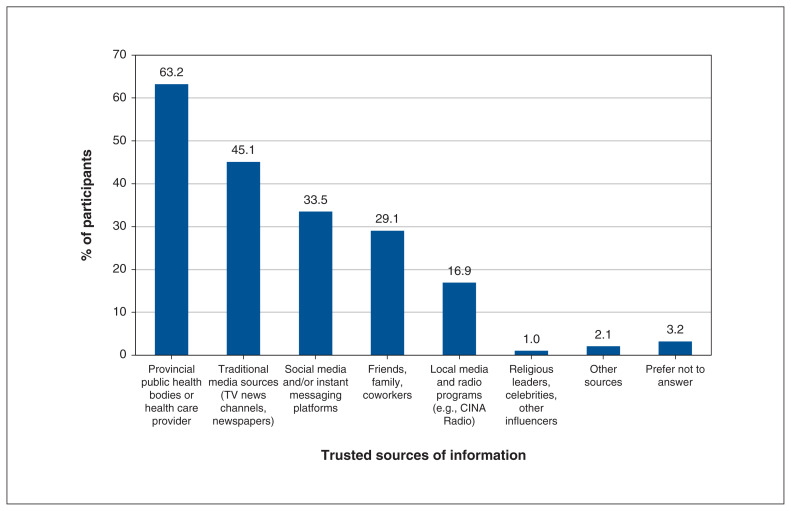
Among those who completed the health information sources section of the questionnaire, the top-ranked sources of health information included health care providers or provincial public health bodies (63.2%, n = 370), traditional media sources (TV news channels, newspapers; 45.1%, n = 264), social media (33.5%, n = 196), and friends, family or coworkers (29.1%, n = 170) (Figure 3).
Figure 3:
Top-ranked sources of COVID-19 health information according to respondents (n = 585 respondents).
Interpretation
Among a sample of South Asian individuals recruited primarily from a designated hot spot in the GTA, we report a seroprevalence of SARS-CoV-2 of 23.6% (95% CI 20.8%–26.4%), standardized by age and sex to the Ontario South Asian population. The range of seropositivity using the strict definition to lenient definition was 22.7% to 27.4%. This report reaffirms other sources of evidence that racialized groups in general, as well as the South Asian community, specifically in the GTA, represent a high-risk group for SARS-CoV-2 infection.2
Nearly 90% of our participants reported that COVID-19 posed a major threat to the South Asian community, about half reported that their risk of acquiring COVID-19 was high because of their location, and more than 30% reported that their risk was high because of their type of employment. In contrast, a lower proportion of participants perceived their risk of COVID-19 to be from their lifestyle, such as socializing, or their housing situation.
Almost one-third of participants in our study reported doing essential work, and 1 in 5 respondents reported living in a multigenerational household, although some respondents preferred not to answer these questions. Participants who indicated they preferred not to answer may reflect the stigmatization they felt during the initial waves of the pandemic. The proportion of essential workers and those living in multigenerational households may be underestimates, especially considering our observation that participants who did not complete the follow-up survey, where this information was collected, were more likely to be seropositive for SARS-CoV-2 (Appendix 3, Supplementary Table 2). Immigrants are overrepresented in jobs designated as essential work, including meat, food and beverage processing plants, trucking, and in long-term care as nurse aides and orderlies. The reasons for overrepresentation include the low pay and lack of benefits and protections, features not desired by others who can pursue jobs in other sectors.19,20
Previous studies have also reported higher SARS-CoV-2 seropositivity among individuals performing essential work, those living in larger households and those of lower socioeconomic status, and among immigrants and visible minority groups.20–23 A study from Alberta conducted from June 2020 to January 2021 reported a high seropositivity of 19.8%, which reflected an outbreak in a large meat-packing plant.21 Higher seropositivity of two-to threefold has also been reported in ethnic minority groups, including Black and South Asian people, compared with white people living in the United Kingdom (1 reference from preprint),22,23 and comparing essential with nonessential workers in Toronto, Canada.20 Compared with population-based seroprevalence studies conducted during the third wave of the pandemic in Canada, such as a report by Canadian Blood Services showing a seropositivity for previous infection of 3.95%,24 we observed a relatively high rate of seropositivity among South Asian people in the GTA (23.6%). This is similar to a seropositivity rate (22%) reported among 1100 incarcerated men in Canada, which was attributed to enhanced daily interactions in a congregate setting.25
Previous studies had serious limitations with respect to assessing seroprevalence of SARS-CoV-2 by ethnicity. Before the COVID-19 pandemic, ethnicity data were not routinely collected or made publicly available in Canada.26 As a surrogate, in areas where a single ethnic group was concentrated, infection and hospitalization rates were examined by postal code, and then inferred to be representative of high-density ethnic populations.5 While our seropositivity data by FSA show a moderate correlation with the cumulative incidence of SARS-CoV-2 infection in Peel (Figure 2), overall, our data provide direct evidence of the high infection rate experienced by South Asian people in this region. Our data suggest, therefore, that the true exposure to SARS-CoV-2 was appreciably higher than the reported cumulative incidence of disease. Further, we observed that some communities with low-to-moderate reported cumulative incidence may have strikingly high seropositivity. Our findings show that the seropositivity rate was higher among men than women, and was higher among participants with prior SARS-CoV-2 infection, less educational attainment and lower income per household size, and among those living in Brampton and in multigenerational households.
Our work has important lessons for the management of pandemics in high-risk communities and provides guidance for future public health studies. During the initial waves of the pandemic, it was unclear whether standard public health messaging by public health officials at the federal, provincial and local levels was reaching communities at high risk, such as the South Asian community.27 Our data, collected during the third pandemic wave, show that the top sources of information used by our cohort included information from health care providers or public health, traditional media sources and social media (Figure 3). It is possible that our findings reflect the impact of the activities of South Asian advocacy groups to raise awareness about health risks and testing for SARS-CoV-2 infection, including provision of health information in multiple South Asian languages. For example, at the outset of the second wave of the pandemic, a physician-led group, the South Asian COVID Task Force, opened a culturally sensitive testing centre in the Embassy Grand Convention Centre (funded and supported by Peel Public Health28), with Punjabi signage and information translated into multiple South Asian languages, as outlined in media reports.29 Another advocacy group, the South Asian Health Network, advocated for isolation centres, sick pay and time off work for testing and vaccination for essential workers, and regular COVID-19 information sessions.30 Finally, Peel Public Health worked closely with community leaders to provide outreach on site for essential workers, to initiate testing at workplaces and to develop mass vaccine centres with cultural sensitivity.26 By April 2021, 10.6% of Peel residents had received at least 1 dose of a SARS-CoV-2 vaccine, and 1.5% had received 2 doses. By July 2021, these figures were 70.6% and 59.3%, respectively.4
Limitations
Limitations of our analysis include recruitment from vaccination centres, which may overrepresent health-conscious individuals and underrepresent the vaccine hesitant, which may have resulted in an underestimate of true seroprevalence (Appendix 3, Supplementary Table 1). Further, the history of SARS-CoV-2 infection was self-reported, which is less reliable than a laboratory-confirmed diagnosis. Although members of the same household were allowed to be enrolled in this study, the seropositivity estimates were adjusted for this clustering. The risk of bias was classified as moderate, attributed to the nonprobability sampling method.
Conclusion
The COVID-19 pandemic was particularly devastating for the South Asian community in the GTA. In this analysis, we show that the seropositivity data confirm a high infection rate of 23.6% from samples collected during the peak of Wave 3. Planning for future COVID-19 waves should incorporate an understanding of sociocultural determinants of health of such high-risk communities.
Supplementary Material
Acknowledgements
The authors acknowledge the funder of this work, COVID-19 Immunity Task Force, Public Health Agency of Canada. They acknowledge the advocacy support of the South Asian COVID-19 Task Force, the South Asian Health Network and Peel Public Health. Sonia Anand holds a Canada Research Chair (Tier 1) Ethnic Diversity and Cardiovascular Disease, and Michael G. DeGroote Heart and Stroke Foundation Chair in Population Health. Marc-André Langlois holds a Canada Research Chair in Molecular Virology and Intrinsic Immunity. Dawn Bowdish holds a Canada Research Chair in Aging and Immunity.
Footnotes
Competing interests: Shelly Bolotin is co-investigator on several COVID-19 grants funded by Canadian Institutes of Health Research, the COVID-19 Immunity Task Force, the Canadian Immunization Research Network and the Public Health Agency of Canada. She is director of the Centre for Vaccine Preventable Diseases at the University of Toronto; the centre is supported by the Dalla Lana School of Public Health (DLSPH), which receives funding from government, philanthropic, not-for-profit and private sector organizations. Private sector funding sources include vaccine manufacturers. A set of governance processes are in place at the DLSPH to ensure independent operation of the centre. All funding is received under agreements that are aligned with policies of the University of Toronto and DLSPH that safeguard academic freedom of faculty and students. Decisions on private sector support are made in consultation with the dean, relevant faculty and the Office of Advancement. In addition, the Centre for Vaccine Preventable Diseases receives oversight from the dean of the DLSPH and a Senior Advisory Committee of the University of Toronto. Mark Loeb has received vaccine advisory board consulting fees from Seqirus, Pfizer, Merck, Sanofi and Medicago; grant funding for a vaccine trial from Seqirus; and in-kind vaccine from Sanofi for a trial. He is on the Vaccine Data Safety Monitoring Board for Medicago, National Institutes of Health NIH and CanSino Biologics.
This article has been peer reviewed.
Contributors: Sonia Anand, Shrikant Bangdiwala, Shelly Bolotin, Dawn Bowdish, Rahul Chanchlani, Russell de Souza, Dipika Desai, Marc-André Langlois, Scott Lear, Mark Loeb, Lawrence Loh, Zubin Punthakee, Gita Wahi and Natalie Williams contributed to the conception and design of the work. Sujane Kandasamy, Farah Khan, Zainab Khan, Jayneel Limbachia and Baanu Manoharan contributed to the acquisition of the data. Corey Arnold, Marc-André Langlois, Kiran Nakka, Martin Pelchat and Karleen Schulze contributed to the analysis of the data. Sonia Anand, Shrikant Bangdiwala, Shelley Bolotin, Dawn Bowdish, Marc-André Langlois, Scott Lear, Mark Loeb and Karleen Schulze contributed to the interpretation of the data. Sonia Anand, Baanu Manoharan and Karleen Schulze contributed to drafting the work, and all other authors revised it critically for important intellectual content. All authors gave final approval of the version to be published. All authors agree to be accountable for all aspects of the work.
Funding: This study was funded by the COVID-19 Immunity Task Force, and funds were distributed from the Public Health Agency of Canada.
Data sharing: The data from the COVID CommUNITY study contains identifiable information that cannot be given to an outside group and is not publicly available as per the study consent guidelines. Nevertheless, requests for collaboration will be considered.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/10/3/E599/suppl/DC1.
References
- 1.Canada [Country] (table). Census profile. 2016 Census. Ottawa: Statistics Canada; 2017. [accessed 2021 Nov. 29]. Cat no 98-316-X2016001. Available: https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/prof/index.cfm?Lang=E. [Google Scholar]
- 2.McKenzie K, Petersen S. Tracking COVID-19 through race-based data. Toronto: Wellesley Institute and Ontario Health; 2021. [accessed 2022 Apr. 22]. Available: https://www.ontariohealth.ca/sites/ontariohealth/files/2021-08/Tracking%20COVID%2019%20Through%20Race%20Based%20Data-EN.pdf. [Google Scholar]
- 3.2016 Census Bulletin: Immigration and ethnic diversity. Toronto: Region of Peel; 2017. [accessed 2021 Nov. 29]. Available: https://www.peelregion.ca/planning-maps/CensusBulletins/2016-immigration-ethnic-diversity.pdf. [Google Scholar]
- 4.COVID-19 in Peel: Dashboard and information about the status of COVID-19. Toronto: Region of Peel; [accessed 2021 Nov. 29]. updated 2022. Available: https://www.peelregion.ca/coronavirus/case-status/ [Google Scholar]
- 5.Weekly epidemiologic summary: COVID-19 in Ontario: focus on September 13, 2020 to September 19, 2020. Toronto: Ontario Agency for Health Protection and Promotion (Public Health Ontario); 2020. [accessed 2021 Nov. 29]. Available: https://files.ontario.ca/moh-covid-19-weekly-epi-report-en-2020-09-19.pdf. [Google Scholar]
- 6.Draaisma M, Ricci T. Ontario faces pressure to shift COVID-19 vaccine 2nd doses to hot-spot communities. CBC News. 2021. Jun 7, [accessed 2022 June 2]. Available: https://www.cbc.ca/news/canada/toronto/second-doses-hot-spots-covid-19-vaccines-ontario-1.6057141.
- 7.McKenzie K. Socio-demographic data collection and equity in Covid-19 in Toronto. EClinicalMedicine. 2021;34:100812. doi: 10.1016/j.eclinm.2021.100812.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Overview Peel’s community mass vaccination plan (MVP) Toronto: Region of Peel; 2021. [accessed 2021 Nov. 19]. Available: https://www.peelregion.ca/coronavirus/vaccine/_media/community-mass-vaccination-plan-framework.pdf. [Google Scholar]
- 9.Brampton CY. Census. Ottawa: Statistics Canada; 2017. [accessed 2021 Nov. 29]. [Census subdivision], Ontario and Peel, RM [Census division], Ontario(table) Census profile 2016. Cat no 98-316-X2016001. Available: https://www12.statcan.gc.ca/censusrecensement/2016/dp-pd/prof/index.cfm?Lang=E. [Google Scholar]
- 10.Ontario COVID-19 Data Tool. Toronto: Public Health Ontario; 2021. [accessed 2021 Nov. 29]. Available: https://www.publichealthontario.ca/en/data-and-analysis/infectious-disease/covid-19-data-surveillance/covid-19-data-tool?tab=trends. [Google Scholar]
- 11.Anand SS, Samaan Z, Middleton C, et al. A digital health intervention to lower cardiovascular risk: a randomized clinical trial. JAMA Cardiol. 2016;1:601–6. doi: 10.1001/jamacardio.2016.1035. [DOI] [PubMed] [Google Scholar]
- 12.Colwill K, Galipeau Y, Stuible M, et al. A scalable serology solution for profiling humoral immune responses to SARS-CoV-2 infection and vaccination. Clin Transl Immunology. 2022;11:e1380. doi: 10.1002/cti2.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cholette F, Mesa C, Harris A, et al. Dried blood spot specimens for SARS-CoV-2 antibody testing: a multi-site, multi-assay comparison. PLoS One. 2021;16:e0261003. doi: 10.1371/journal.pone.0261003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang X, Sharma A, Pasic M, et al. Assessment of SARS-CoV-2 seropositivity during the first and second viral waves in 2020 and 2021 among Canadian adults. JAMA Netw Open. 2022;5:e2146798. doi: 10.1001/jamanetworkopen.2021.46798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galipeau Y, Siragam V, Laroche G, et al. Relative ratios of human seasonal coronavirus antibodies predict the efficiency of cross-neutralization of SARS-CoV-2 spike binding to ACE2. EBioMedicine. 2021;74:103700. doi: 10.1016/j.ebiom.2021.103700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bobrovitz N, Arora RK, Cao C, et al. Global seroprevalence of SARS-CoV-2 antibodies: a systematic review and meta-analysis. PLoS One. 2021;16:e0252617. doi: 10.1371/journal.pone.0252617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munn Z, Moola S, Lisy K, et al. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid-Based Healthc. 2015;13:147–53. doi: 10.1097/XEB.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 18.ICES COVID-19 Dashboard. Applied health research questions (AHRQ) Toronto: ICES; [accessed 2021 Nov. 11]. updated 2021 Dec. 21. Available: https://www.ices.on.ca/~/media/Files/COVID-19/ICES-COVID19-Vaccination-Data-by-FSA.ashx?la=en-CA. [Google Scholar]
- 19.Guidance on essential services and functions in Canada during the COVID-19 pandemic. Ottawa: Public Safety Canada; [accessed 2021 Apr. 10]. updated 2021 Oct. 14. Available: https://www.publicsafety.gc.ca/cnt/ntnl-scrt/crtcl-nfrstrctr/esf-sfe-en.aspx. [Google Scholar]
- 20.Rao A, Ma H, Moloney G, et al. A disproportionate epidemic: COVID-19 cases and deaths among essential workers in Toronto, Canada. Ann Epidemiol. 2021;63:63–7. doi: 10.1016/j.annepidem.2021.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charlton CL, Nguyen LT, Bailey A, et al. Pre-vaccine positivity of SARS-CoV-2 antibodies in Alberta, Canada during the first two waves of the COVID-19 pandemic. Microbiol Spectr. 2021;9:e0029121. doi: 10.1128/Spectrum.00291-21.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ward H, Atchison C, Whitaker M, et al. Antibody prevalence for SARS-CoV-2 following the peak of the pandemic in England: REACT2 study in 100 000 adults [preprint] medRxiv. 2020. Aug 21, doi: [DOI]
- 23.Chen X, Chen Z, Azman AS, et al. Serological evidence of human infection with SARS-CoV-2: a systematic review and meta-analysis. Lancet Glob Health. 2021;9:E598–E609. doi: 10.1016/S2214-109X(21)00026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.COVID-19 Seroprevalence Report March 11th, 2022. Ottawa: Canadian Blood Services; 2022. [accessed 2021 Apr. 21]. Available: https://www.covid19immunitytaskforce.ca/wp-content/uploads/2022/03/covid-19-full-report-jan-2022.pdf. [Google Scholar]
- 25.Kronfi N, Dussault C, Maheu-Giroux M, et al. Seroprevalence and risk factors for SARS-CoV-2 among incarcerated adult men in Quebec, Canada (2021) Clin Infect Dis. 2022 Jan 17;:ciac031. doi: 10.1093/cid/ciac031.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson E, Edjoc R, Atchessi N, et al. COVID-19: a case for the collection of race data in Canada and abroad. Can Commun Dis Rep. 2021;47:300–4. doi: 10.14745/ccdr.v47i78a02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bangdiwala SI, Gomez A, Monsalves MJ, et al. Statistical considerations when communicating health risks: experiences from Canada, Chile, Ecuador, and England facing COVID-19. Soc Health Sci. 2021;19:52–79. [Google Scholar]
- 28.South Asian COVID Task Force [homepage] [accessed 2021 Nov. 29]. Available: https://www.southasiancovidtf.ca/about-us/
- 29.Gamrot S. New COVID-19 testing centre to open in Brampton. Brampton Guardian. 2021. Jan 21, [accessed 2021 Nov. 29]. Available: https://www.bramptonguardian.com/news-story/10314339-new-covid-19-testing-centre-to-open-in-brampton/
- 30.South Asian Health Network. COVID-19 original resources. [accessed 2022 Apr. 14]. Available: https://southasianhealthnetwork.ca/covid-resources.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.