Abstract
Hydrocotyle pseudoconferta was an important medicinal plant. The complete plastid genome of this species was reported for the first time. The full length of the complete chloroplast genome is 153,302 bp, with a typical quadripartite organization: a large single-copy (LSC) region of 84,417 bp, a small single-copy (SSC) region of 18,767 bp, and a pair inverted repeat regions (IRa and IRb) with 25,059 bp for each. The complete chloroplast genome of H. pseudoconferta encoded 133 genes, comprising 86 protein-coding genes, 37 tRNA genes, 8 rRNA genes, and 2 pseudogenes. The phylogenetic analysis suggested the closest relationship between H. pseudoconferta and Hydrocotyle nepalensis.
Keywords: Araliaceae, Hydrocotyle, chloroplast genome, phylogenetic analysis
Hydrocotyle pseudoconferta Masamune 1932 is a medicinal herb species of the genus Hydrocotyle Tourn. ex L. The genus was formerly classified in the family Apiaceae and later transferred into Araliaceae inferred from a limited number of DNA fragments (Chandler and Plunkett 2004; Plunkett et al. 2004). In recent years, comparative analysis of the complete chloroplast genome sequences has been used as an effective tool for plant phylogeny analysis. The relationships among Hydrocotyle, Apiaceae, and Araliaceae may also be elucidated by the same means. Many chloroplast genomes of Apiaceae and Araliaceae have been reported, with only three species of Hydrocotyle included (Downie and Jansen 2015; Ge et al. 2017; Wen et al. 2021). We herein assembled and annotated the complete chloroplast genome sequence of H. pseudoconferta as supplementary material for further study.
Species of H. pseudoconferta is naturally distributed from southern China to Myanmar, and narrowly grew in wet valleys at altitudes of 800–1500 m (Sheh et al. 2005). The solitary axillary sessile umbel is the main characteristic that distinguishes this species from other Hydrocotyle species. Fresh leaves of H. pseudoconferta (Collection number: wj_2021072302) were collected from Cangnan county, Zhejiang province, China (27°27′56.18″N, 120°19′0.64″E). The voucher specimen (no. NAS00637160) was deposited in the herbarium of Nanjing Botanical Garden Mem. Sun Yat-Sen (http://www.cnbg.net, Zeng-lai Xu, 1355655293@qq.com). The total genomic DNA was extracted with a modified CTAB method (Doyle 1987) and sequenced paired-end (PE) using Illumina Novaseq platform (Illumina novaseq6000, Illumina, San Diego, CA). The raw reads were assembled using NOVOPlasty 4.3.1 (Dierckxsens et al. 2017) and then annotated using Geneious 11.1.5 (Kearse et al. 2012).
The complete chloroplast genome of H. pseudoconferta (GenBank accession: OK585058) is 153,302 bp in length, with 37.6% GC contents and was consisted of four regions: including two inverted repeat regions (IRa and IRb, 25,059 bp for each) separated by a large single-copy gene region (LSC, 84,417 bp) and a small single-copy gene region (SSC, 18,767 bp). The chloroplast genome has 133 genes in total, including 86 protein-coding genes, 37 tRNA genes, 8 rRNA genes, and 2 pseudogenes.
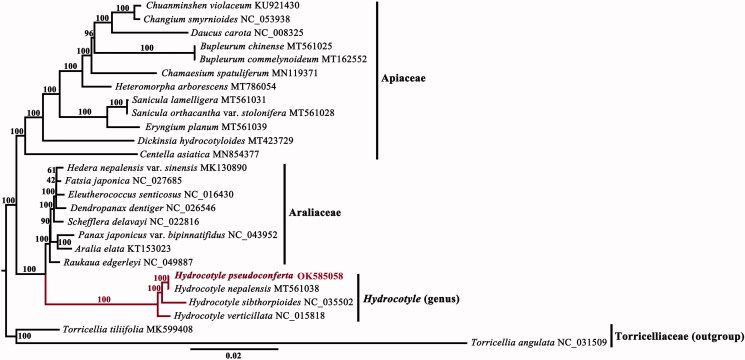
The complete chloroplast genomes of 26 species (involving 12 Apiaceae species, 12 Araliaceae species, and 2 outgroups belonging to Torricelliaceae) were selected to reconstruct the phylogenetic position of this species. Data matrices were aligned using MAFFT v7 (Katoh and Standley 2013). A maximum-likelihood (ML) phylogenetic tree was generated based on a data matrix of a concatenation of 77 protein-coding sequences, implemented with RAxML v8 (Stamatakis 2014) under the GTR + G model for 1000 bootstrap replicates (Figure 1). The phylogenetic analysis suggested that Hydrocotyle was recovered as a sister group of Araliaceae, and H. pseudoconferta is the closest sister group of Hydrocotyle nepalensis Hook. 1822 within the genus. This study extends our comprehension of chloroplast genome evolution in Hydrocotyle.
Figure 1.
The maximum-likelihood (ML) phylogenetic tree reconstructed from protein-coding sequences of 26 complete chloroplast genomes. Numbers beside each node indicate bootstrap support values.
Funding Statement
This work was supported by the Foundation of Jiangsu Key Laboratory for the Research and Utilization of Plant Resources under Grant nos. JSPKLB202016, JIBTF202101, and JSPKLB201834; and The Natural Science Foundation of Jiangsu Province under Grant number BK20200294.
Author contributions
Chun-Feng Song and Jun Wen were involved in the conception and design; Bao-Cheng Wu and Hui-Min Li collected the leaf and specimen material of H. pseudoconferta; Jun Wen analyzed the data and drafted the paper; Wei Zhou revised the paper critically for intellectual content. All authors agree to be accountable for all aspects of the work and the final approval of the version to be published.
Ethical approval
No ethical issues were involved in this study. The collection of plant sample was legal and reasonable. Voucher specimen has been deposited in the herbarium of Nanjing Botanical Garden Mem. Sun Yat-Sen (http://www.cnbg.net, Zeng-lai Xu, 1355655293@qq.com). Information on the voucher specimen and who identified it were included in the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data that support the findings of this study are openly available in GenBank of NCBI at https://www. ncbi.nlm.nih.gov/under the Accession no. OK585058. The associated BioProject, SRA, and Bio-Sample numbers are PRJNA772920, SRR16548130, and SAMN22420039, respectively.
References
- Chandler GT, Plunkett GM.. 2004. Evolution in Apiales: nuclear and chloroplast markers together in (almost) perfect harmony. Bot J Linn Soc. 144(2):123–147. [Google Scholar]
- Dierckxsens N, Mardulyn P, Smits G.. 2017. NOVOPlasty: de novo assembly of organelle genomes from whole genome data. Nucleic Acids Res. 45(4):e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downie SR, Jansen RK.. 2015. A comparative analysis of whole plastid genomes from the Apiales: expansion and contraction of the inverted repeat, mitochondrial to plastid transfer of DNA, and identification of highly divergent noncoding regions. Syst Bot. 40(1):336–351. [Google Scholar]
- Doyle JJ. 1987. A rapid DNA isolation procedure for small amounts of fresh leaf tissue. Phytochem Bull. 19(1):11–15. [Google Scholar]
- Ge L, Shen L, Chen Q, Li X, Zhang L.. 2017. The complete chloroplast genome sequence of Hydrocotyle sibthorpioides (Apiales: Araliaceae). Mitochondrial DNA B Resour. 2(1):29–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Standley DM.. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30(4):772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, et al. 2012. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 28(12):1647–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plunkett GM, Chandler GT, Lowry PP, Pinney SM, Sprenkle TS, van Wyk B-E, Tilney PM.. 2004. Recent advances in understanding Apiales and a revised classification. S Afr J Bot. 70(3):371–381. [Google Scholar]
- Sheh ML, Watson MF, Cannon JFM.. 2005. Hydrocotyle Linnaeus. In: Wu ZY, Raven PH, editors. Flora of China. Beijing and St. Louis: Science Press and Missouri Botanical Garden Press. pp. 14–17. [Google Scholar]
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 30(9):1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J, Xie D-F, Price M, Ren T, Deng Y-Q, Gui L-J, Guo X-L, He X-J.. 2021. Backbone phylogeny and evolution of Apioideae (Apiaceae): new insights from phylogenomic analyses of plastome data. Mol Phylogenet Evol. 161:107183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available in GenBank of NCBI at https://www. ncbi.nlm.nih.gov/under the Accession no. OK585058. The associated BioProject, SRA, and Bio-Sample numbers are PRJNA772920, SRR16548130, and SAMN22420039, respectively.