ABSTRACT
Objective:
To compare 90-day morbidity in patients undergoing lung lobectomy performed by either robotic-assisted thoracic surgery (RATS) or video-assisted thoracic surgery (VATS). Intraoperative complications, drainage time, length of hospital stay, postoperative pain, postoperative quality of life, and readmissions within 90 days were also compared.
Methods:
This was a two-arm randomized clinical trial including patients with lung lesions (primary lung cancer or lung metastasis) who were candidates for lung lobectomy. Patients with comorbidities that precluded surgical treatment were excluded. All patients followed the same postoperative protocol.
Results:
The overall sample comprised 76 patients (39 in the VATS group and 37 in the RATS group). The two groups were similar regarding gender, age, BMI, FEV1 in % of predicted, and comorbidities. Postoperative complications within 90 days tended to be more common in the VATS group than in the RATS group, but the difference was not significant (p = 0.12). However, when only major complications were analyzed, this tendency disappeared (p = 0.58). Regarding postoperative outcomes, the VATS group had a significantly higher number of readmissions within 90 days than did the RATS group (p = 0.029). No significant differences were found regarding intraoperative complications, drainage time, length of hospital stay, postoperative pain, and postoperative quality of life.
Conclusions:
RATS and VATS lobectomy had similar 90-day outcomes. However, RATS lobectomy was associated with a significant reduction in the 90-day hospital readmission rate. Larger studies are necessary to confirm such a finding.
(ClinicalTrials.gov identifier: NCT02292914 [http://www.clinicaltrials.gov/])
Keywords: Robotic surgical procedures; Thoracic surgery, video-assisted; Lung neoplasms.
RESUMO
Objetivo:
Comparar a morbidade em 90 dias de pacientes submetidos à lobectomia pulmonar por robotic-assisted thoracic surgery (RATS, cirurgia torácica robótica) ou por video-assisted thoracic surgery (VATS, cirurgia torácica videoassistida). Complicações intraoperatórias, tempo de drenagem, tempo de internação hospitalar, dor pós-operatória, qualidade de vida pós-operatória e reinternações em 90 dias também foram comparados.
Métodos:
Ensaio clínico randomizado, com dois braços, incluindo pacientes com lesões pulmonares (câncer de pulmão primário ou metástase pulmonar) candidatos à lobectomia pulmonar. Foram excluídos pacientes com comorbidades que impossibilitassem o tratamento cirúrgico. Todos os pacientes seguiram o mesmo protocolo pós-operatório.
Resultados:
A amostra total foi composta por 76 pacientes (39 no grupo VATS e 37 no grupo RATS). Os dois grupos foram semelhantes quanto a sexo, idade, IMC, VEF1 em % do previsto e comorbidades. Complicações pós-operatórias em 90 dias tenderam a ser mais frequentes no grupo VATS do que no grupo RATS, mas a diferença não foi significativa (p = 0,12). No entanto, quando analisadas apenas as complicações maiores, essa tendência desapareceu (p = 0,58). Quanto aos desfechos pós-operatórios, o grupo VATS apresentou um número significativamente maior de reinternações em 90 dias do que o grupo RATS (p = 0,029). Não foram encontradas diferenças significativas quanto a complicações intraoperatórias, tempo de drenagem, tempo de internação hospitalar, dor pós-operatória e qualidade de vida pós-operatória.
Conclusões:
A lobectomia por RATS e a lobectomia por VATS apresentaram desfechos em 90 dias semelhantes. No entanto, a lobectomia por RATS foi associada a uma redução significativa na taxa de reinternação hospitalar em 90 dias. Estudos maiores são necessários para confirmar esse achado.
(Identificador ClinicalTrials.gov: NCT02292914 [http://www.clinicaltrials.gov/])
Descritores: Procedimentos cirúrgicos robóticos, Cirurgia torácica videoassistida, Neoplasias pulmonares.
INTRODUCTION
Pulmonary lobectomy is the standard treatment for initial lung cancer, and it is also used in selected patients with pulmonary metastases whose characteristics make sublobar resections inadequate. 1 Several studies have compared video-assisted thoracic surgery (VATS) lobectomy with open thoracotomy and demonstrated reduced morbidity and length of hospital stay in favor of the minimally invasive technique without compromising oncologic outcomes. 2 - 5 In spite of the clear advantages of VATS and the fact that it is strongly recommended by guidelines, 1 - 6 thoracotomy has remained the most common approach for lobectomies. 3 - 7 Two-dimensional view and the use of long and inflexible instruments may promote an imprecise dissection and a long and arduous learning curve. These shortcomings might be responsible for the slow implementation of VATS lobectomy worldwide.
Robotic-assisted thoracic surgery (RATS) solves some of the disadvantages of VATS. It offers three-dimensional high-definition view and allows the surgeon to control the camera. Moreover, the robotic platform has endowrist instruments and tremor filtration, allowing a very accurate and safe dissection. Nevertheless, RATS has higher costs that are mainly associated to a large capital investment to acquire the platform. Several studies have confirmed that oncologic outcomes of RATS and VATS lobectomy are equivalent. 8 , 9 With regard to intra- and post-operative outcomes, one study based on a large database showed that RATS was associated to a lower surgical conversion rate, a lower overall postoperative complication rate, and a shorter length of hospital stay 7 ; however, other studies did not find any difference between RATS and VATS. 10 - 12 In spite of the lack of randomized evidence supporting the benefits of RATS, technical improvements that make minimally invasive surgery easier for the surgeon to perform and potential postoperative benefits have resulted in an increase in RATS procedures from 1% to 11% of all lobectomies performed at non-academic hospitals in the United States from 2009 to 2013. 13
In this scenario of uncertainties and high costs, a randomized clinical trial would greatly contribute to this subject by providing important and more accurate information on the outcomes of RATS and VATS lobectomy. Therefore, the primary objective of this trial was to compare the 90-day morbidity rate in patients undergoing either RATS or VATS lobectomy. Secondary outcomes included intraoperative complications, drainage time, length of hospital stay, postoperative pain, postoperative quality of life, and readmissions within 90 days.
METHODS
This was a two-arm randomized clinical trial carried out from April of 2015 to June of 2017 at a university teaching hospital in the city of São Paulo, Brazil. All potential candidates with lung cancer underwent clinical staging by chest CT and PET-CT. Patients with neurological symptoms or pulmonary tumors larger than 3 cm underwent brain MRI. Invasive mediastinal staging was performed by mediastinoscopy or EBUS if suspicious hilar/mediastinal lymph nodes, tumors with central location, or tumors larger than 3 cm were found. After surgical indication was confirmed, all patients were evaluated and approved for surgery by the pulmonology team; only then patients were referred to the research team for trial enrollment evaluation.
The trial inclusion criteria were as follows: eligibility for the treatment of lung cancer or lung metastasis by pulmonary lobectomy; presence of tumor of less than 5 cm in diameter; absence of tumor invasion into the chest wall, diaphragm, mediastinum, or another lung lobe; and clinical and anesthetic evaluation results showing that the patient was able to undergo the proposed procedure. Exclusion criteria were as follows: having previously undergone a thoracic surgical procedure in the hemithorax to be operated on; and being unable to remain on single-lung ventilation during the procedure. All patients signed the written informed consent approved by our institutional research ethics committee (CAAE 21934413.2.0000.0065). This study was also registered at ClinicalTrials.gov (NCT02292914).
The Brazilian Ministry of Health funded the acquisition of the DaVinci Si (Intuitive Surgical Inc., Sunnyvale, CA, USA) robotic system and a limited amount of surgical instruments and disposable materials specific to robotic surgery. Therefore, the sample size was defined by convenience. Surgeons recruited outpatients according to inclusion and exclusion criteria. The clinical research nursing team was responsible for providing and collecting the written informed consent forms, and the research center defined the allocation of the patients using a website software (www.randomization.org, Arlington, VA, USA). We used block randomization to allow an adequate distribution of patients between the two groups. Patients were randomized only after having their surgery scheduled, ensuring allocation concealment. However, the randomization status was not blinded, so both patient and medical staff were aware of the randomization assignment.
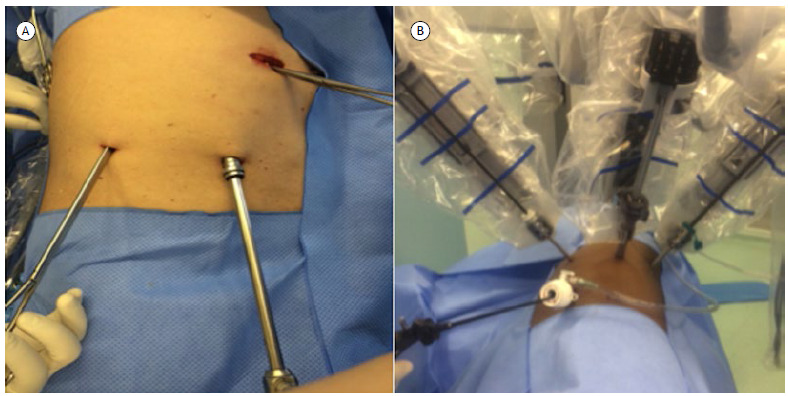
For RATS lobectomies, we used the DaVinci Si robotic system (Innovative Surgical) and the three-arm technique initially described by Ninan & Dylewski. 14 All VATS lobectomies were performed in accordance with the triportal technique. Figure 1 shows photographs of the two surgical approaches. One 28-Fr chest tube was placed in the patients of both groups. All surgical procedures were performed under selective intubation. Radical hilar and mediastinal lymphadenectomy was performed only in patients with primary pulmonary cancer. The same group of surgeons performed both VATS and RATS procedures. The patients were usually transferred to the hospital ward in the postoperative period; only elderly patients with multiple comorbidities or patients with intraoperative complications were sent to the ICU at the end of the procedure. Postoperative analgesia included patient-controlled epidural anesthesia (local anesthetics and opioids), which was discontinued immediately after chest tube removal.
Figure 1. Photographs of the two surgical approaches: in A, video-assisted thoracic surgery; in B, robotic-assisted thoracic surgery.
In the present study the primary outcome was complication rate within 90 days. Postoperative complications were recorded and classified as proposed by Seely et al. 15 As secondary outcomes, we analyzed intraoperative complications, drainage time, length of hospital stay, postoperative pain, postoperative quality of life, and readmissions within 90 days. Drainage time was defined as the interval between surgery and the removal of the chest tube and was measured in days. Length of hospital stay was measured in days after surgery. Postoperative pain was evaluated by a visual analog pain scale 16 on the first, second, and third postoperative days and at the 30-day outpatient visit. We also assessed the need for opioid use at the 30-day outpatient visit. Any hospitalization within the 90-day postoperative period was considered as readmission.
In addition to the variables described above, we collected other pieces of information: demographic characteristics (age, gender, and BMI), FEV1 (in L and % of predicted), smoking status, presence of systemic arterial hypertension, presence of diabetes mellitus, presence of cardiac, hepatic, or renal diseases, and tumor size. We also collected procedure-related information: operative time, conversion to open surgery, need for extended resection, and resected lobe. During the postoperative period, we collected information on length of ICU stay and need for reoperation. When primary cancer was pulmonary, we collected data on the histological type and the pathological stage as per the 8th edition of the TNM staging classification for lung cancer. 17
We used descriptive statistical analyses to summarize the characteristics of the studied patients. We compared categorical variables using the Fisher’s exact test. We tested numerical variables for their distribution using the Shapiro-Wilk and kurtosis tests. We used the t-test and ANOVA to compare continuous variables with normal distribution and the Mann-Whitney and Wilcoxon tests to compare continuous variables with asymmetrical distribution. All analyses were carried out with a level of significance of p < 0.05. The analyzes were performed using the Predictive Analytics Software package, version 18.0 for Windows (SPSS Inc., Chicago, IL, USA).
RESULTS
From June of 2015 to May of 2017, 107 patients were evaluated for trial enrollment. Of these, 80 met the inclusion criteria and were randomized. Four patients were excluded after randomization (3 in the RATS group and 1 in the VATS group): 2 patients had undiagnosed advanced disease at randomization-malignant pleural effusion, in 1; and brain metastasis after reevaluation of brain MRI results, in 1-1 patient had a severe episode of arrhythmia just before the surgery, and we opted for canceling the procedure and referring the patient to stereotaxic radiotherapy, and 1 patient withdrew consent after randomization. Therefore, the overall sample comprised 76 patients (37 and 39 patients in the RATS and VATS groups, respectively). We used intention-to-treat analysis; however, there was no crossover, and all exclusions occurred after randomization, but before the intervention. The flow chart of the patient selection process is depicted in Figure 2, and the characteristics of the patients included in the study are shown in Table 1.
Figure 2. Flow chart of the patient selection process. RATS: robotic-assisted thoracic surgery; VATS: video-assisted thoracic surgery; and PO: postoperative.
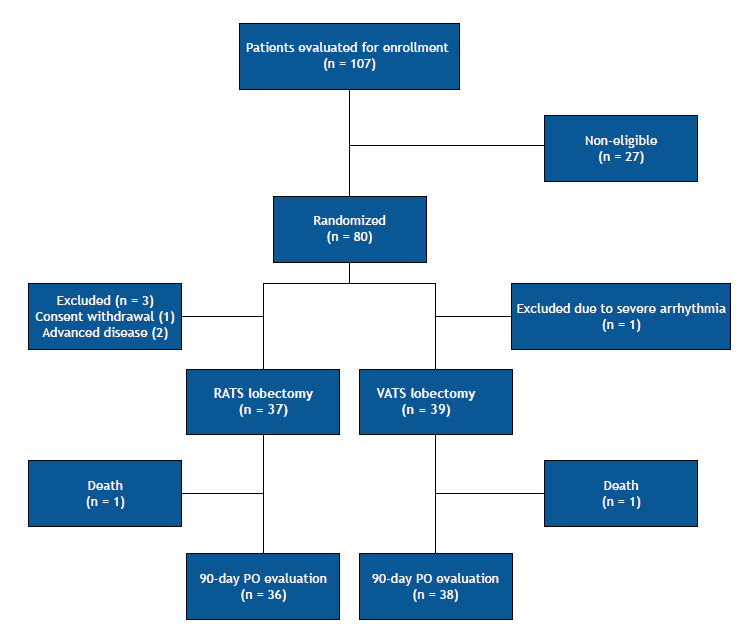
Table 1. Demographic and baseline characteristics of the patients per group (N = 76).a .
| Characteristic | Group | p | |
|---|---|---|---|
| RATS | VATS | ||
| (n = 37) | (n = 39) | ||
| Age, years | 68.4 (65.2-71.5) | 65.7 (61.8-69.5) | 0.31 |
| Female | 20 (54.0%) | 22 (56.4%) | 1.00 |
| BMI, kg/m2 | 27.5 (26.2-28.8) | 26.5 (24.9-28.1) | 0.24 |
| FEV1, L | 2.2 (2.0-2.4) | 2.1 (1.9-2.3) | 0.33 |
| FEV1, % of predicted | 87.3 (81.8-92.8) | 81.5 (77.5-85.5) | 0.19 |
| Never smoker | 12 | 11 | 0.78 |
| COPD | 12 | 18 | 0.28 |
| Morbid obesity (BMI >34.9 kg/m2) | 3 | 3 | 1.00 |
| Hypertension | 24 | 21 | 0.35 |
| Diabetes mellitus | 7 | 11 | 0.42 |
| Cardiac disease | 5 | 3 | 0.47 |
| Liver disease | 2 | 5 | 0.43 |
| Kidney disease | 2 | 1 | 0.61 |
| NSCLC | 34b | 35c | 1.00 |
| Tumor size, cm | 2.32 (1.90-2.74) | 2.45 (2.11-2.79) | 0.65 |
RATS: robotic-assisted thoracic surgery; VATS: video-assisted thoracic surgery; and NSCLC: non-small cell lung cancer. aValues expressed as n or median (95% CI), except where otherwise indicated. bMetastatic breast cancer, in 1; inflammatory myofibroblastic tumor, in 1; and atypical adenomatous hyperplasia, in 1. cMetastatic melanoma, in 1; metastatic renal cell carcinoma, in 2; and small cell lung cancer, in 1.
The overall operative time tended to be longer in the RATS group than in the VATS group, although the difference had no statistical significance-241.7 (218.3-265.1) min vs. 214.4 (200.3-228.5) min (p = 0.06). Neither were any intraoperative complications observed, nor was it necessary any surgical conversion in the RATS group. In the VATS group, there were three cases of intraoperative vascular lacerations (arterial and venous lacerations in 2 and 1, respectively), and two of the procedures were converted to open surgery. No patient required blood transfusion. Table 2 shows the results related to the surgeries performed.
Table 2. Surgical characteristics and intraoperative complications of the patients per group (N = 76).a .
| Variable | Group | p | |
|---|---|---|---|
| RATS | VATS | ||
| (n = 37) | (n = 39) | ||
| Operative time, min | 241.7 (218.3-265.1) | 214.4 (200.3-228.5) | 0.06 |
| Intraoperative complications | 0 | 3b | 0.24 |
| Conversion to open surgery | 0 | 2 | 0.49 |
| Extended resection | 1c | 2d | 0.59 |
| Resected lobe | 0.68 | ||
| RUL | 14 | 13 | |
| RML | 3 | 0 | |
| RLL | 8 | 8 | |
| LUL | 7 | 9 | |
| LLL | 5 | 9 | |
RATS: robotic-assisted thoracic surgery; VATS: video-assisted thoracic surgery; RUL: right upper lobe; RML: right middle lobe; RLL: right lower lobe; LUL: left upper lobe; and LLL: left lower lobe. aValues expressed as n or median (95% CI). bArterial lacerations, in 2; and venous injury, in 1. cWedge resection. dWedge resection and pericardial resection.
With regard to postoperative outcomes (Table 3), the only significant difference observed was in the number of readmissions within 90 days, which were less common in the RATS group (1 patient vs. 8 patients; p = 0.029). In the RATS group, hospital was due to bronchospasm/decompensated COPD, whereas, in VATS group, the causes were empyema (in 2 patients, 1 of whom presenting with prolonged air leak), pneumonia (in 2), prolonged air leak that persisted for two months after lobectomy (n 1)-the patient was readmitted for another video-assisted thoracoscopy (in 1)-pleural effusion (in 1), surgical wound infection (in 1), and severe pain (in 1). Three patients required reoperation due to prolonged air leak (in 2; 1 in the VATS group, and 1 in the RATS group during the same hospitalization period) and empyema (1 in the VATS group). Postoperative complications within 90 days tended to be less common in the RATS group than in the VATS group-7 (18.9%) cases vs. 14 (35.9%) cases-with no statistically significant difference (p = 0.12). Nevertheless, when we considered only major complications (grade ≥ 3, as per the Common Terminology Criteria for Adverse Events, version 4), this tendency disappeared-7 (18.9%) cases vs. 10 (25.6%) cases (p = 0.58). Two patients died (1 in each group), both due to pneumonia and sepsis. All of the postoperative complications are described in Table 4.
Table 3. Postoperative course, postoperative complications, readmissions, and mortality of the patients per group (N = 76).a .
| Variable | Group | p | |
|---|---|---|---|
| RATS | VATS | ||
| (n = 37) | (n = 39) | ||
| ICU time, days | 0 [0-1] | 0 [0-2] | 0.99 |
| Length of hospital stay, days | 3 [2-4] | 4 [2-5] | 0.55 |
| Chest tube time, days | 2 [1-2] | 2 [1-4] | 0.27 |
| Reoperation | 1 (2.7%)b | 2 (5.1%)c | 0.59 |
| Complications within 90 days | 7 (18.9%) | 14 (35.9%) | 0.12 |
| ≥ 3 complications within 90 days | 7 (18.9%) | 10 (25.6%) | 0.58 |
| Readmissions within 90 days | 1 (2.7%) | 8 (20.5%) | 0.029 |
| 90-day mortality | 1 (2.7%) | 1 (2.5%) | 1.0 |
RATS: robotic-assisted thoracic surgery; and VATS: video-assisted thoracic surgery. aValues expressed as median [IQR] or n (%). bProlonged air leak. cProlonged air leak, in 1; and empyema, in 1.
Table 4. Comparison of 90-day postoperative complications in accordance with the Common Terminology Criteria for Adverse Events (version 4) between the groups.a .
| 90-day complications | Any grade | Grade ≥ 3 | ||||
|---|---|---|---|---|---|---|
| RATS | VATS | p | RATS | VATS | p | |
| (n = 37) | (n = 39) | (n = 37) | (n = 39) | |||
| Any | 7 (18.9) | 14 (35.9) | 0.12 | 7 (18.9) | 10 (25.6) | 0.58 |
| Death | 1 (2.7) | 1 (2.5) | 1.00 | |||
| Prolonged air leak | 4 (10.8) | 5 (12.8) | 1.00 | 4 (10.8) | 5 (12.8) | 1.00 |
| Empyema | 0 | 2 (5.1) | 0.49 | 0 | 2 (5.1) | 0.49 |
| Pleural effusion | 0 | 1 (2.5) | 1.00 | 0 | 1 (2.5) | 1.00 |
| Surgical site infection | 0 | 1 (2.5) | 1.00 | 0 | 1 (2.5) | 1.00 |
| Subcutaneous emphysema | 0 | 1 (2.5) | 1.00 | 0 | 0 | 1.00 |
| Acute kidney failure | 1 (2.7) | 2 (5.1) | 1.00 | 1 (2.7) | 2 (5.1) | 1.00 |
| Pyrexia | 0 | 1 (2.5) | 1.00 | 0 | 0 | 1.00 |
| Pneumonia | 1 (2.7) | 1 (2.5) | 1.00 | 1 (2.7) | 1 (2.5) | 1.00 |
| Sepsis | 2 (5.4) | 1 (2.5) | 0.61 | 2 (5.4) | 1 (2.5) | 0.61 |
| Severe pain | 0 | 1 (2.5) | 1.00 | 1 (2.5) | 1.00 | |
| Pulmonary embolism | 1 (2.7) | 0 | 0.48 | 1 (2.7) | 0 | 0.48 |
| Arrhythmia | 1 (2.7) | 0 | 1.00 | 1 (2.7) | 0 | 1.00 |
| Bronchospasm | 1 (2.7) | 2 (5.1) | 1.00 | 1 (2.7) | 0 | 1.00 |
| Atelectasis | 0 | 1 (2.5) | 1.00 | 0 | 0 | 1.00 |
RATS: robotic-assisted thoracic surgery; and VATS: video-assisted thoracic surgery. aValues expressed as n (%).
No significant differences were found between the groups regarding histological types (p = 0.60) or pathological staging (p = 0.36). Among the 34 patients with primary lung cancer in the RATS group, there was N descriptor upstaging in 3: from cN0 to pN1, in 2; and from cN0 to pN2, in 1. Likewise, among the 35 patients with primary lung cancer in the VATS group, N upstaging occurred in 5: from cN0 to pN1, in 2; and from cN0 to pN2, in 3. However, the difference was not statistically significant (p = 0.71). Table S1 (121.3KB, pdf) details such findings.
There were no significant differences regarding the perception of postoperative pain (defined as a score > 2 in the visual analog scale) 16 between the groups either during the first 3 postoperative days or 30 days after surgery. We also evaluated the need for any type of opioid medication 30 days after lobectomy. Once again, no significant difference was found between the groups (p = 0.61). Table S2 (121.3KB, pdf) shows the results regarding postoperative pain.
DISCUSSION
The present study showed a significantly lower 90-day hospital readmission rate in the RATS group when compared with the VATS group (2.7% vs. 20.5%; p = 0.029). Moreover, postoperative complications within 90 days tended to be less common in patients undergoing RATS than in those undergoing VATS, although with no statistical significance (18.9% vs. 35.9%; p = 0.12). This tendency did not occur for major complications (18.9% vs. 25.6%; p = 0.58). The RATS group also showed fewer intraoperative complications and surgical conversions to open thoracotomy than did the VATS group, but again with no statistical significance (respectively, 0 vs. 3; p = 0.24; and 0 vs. 2; p = 0.49). On the other hand, the RATS group tended to have a longer overall operative time: 241.7 (218.3-265.1) min vs. 214.4 (200.3-228.5) min (p = 0.06).
Initially, a few small retrospective studies were published on robotic lobectomy. 8 , 9 Some presented the technique and the experience of a single institution; others compared RATS with either open surgery or VATS. 8 , 9 After a greater dissemination of robotic surgery and a consequent increase in the number of patients operated on by this technique, retrospective studies using large multi-institutional databases have been published and showed similar postoperative outcomes between VATS and RATS. 10 , 11 , 13
More recently, Oh et al. 7 published a study showing some benefits of RATS lobectomy. Compared with the VATS group, the RATS group had a lower surgical conversion rate (6.3% vs. 13.1%; p < 0.0001), a lower overall postoperative complication rate (34.1% vs. 37.6%; p = 0.0061), and a shorter median of length of hospital stay (5 days vs. 6 days; p = 0.006). However, postoperative and 30-day mortality rates were similar: 0.9% vs. 1.2% (p = 0.44) and 1.2% vs. 1.4%; (p = 0.642), respectively. Thus, for the first time, a multi-institutional study with large numbers of patients demonstrated that RATS lobectomy could be associated with improvements in perioperative outcomes when compared with VATS lobectomy. 7 These findings resemble those found in our study, which suggests that RATS lobectomy may be associated with fewer complications.
Our results for postoperative complications within 90 days in the VATS and RATS groups (35.9% vs. 18.9%) were similar to those in the literature. 7 , 10 , 11 Interestingly, patients undergoing VATS had a larger number of isolated minor complications. These cases did not require further interventions or extended hospitalization. We could not identify a specific reason for these findings. The RATS technique might have a more meticulous approach and cause fewer fissures in and less damage to the lungs.
Our results for readmission within 90 days were also similar to those in the literature (18.0%, 16.9%, and 19.8%). 18 - 20 However, patients undergoing RATS had a significantly lower readmission rate than did patients undergoing VATS (2.7% vs. 20.5%; p = 0.029), suggesting a potential benefit that has not been previously described. Both groups had similar preoperative characteristics and were exposed to the same postoperative conditions. The tendency that we observed (fewer complications in the RATS group) seems to have been reflected in the 90-day readmission rate.
In general, our intraoperative outcomes are similar to those of most retrospective studies. 7 , 13 , 21 - 23 We observed operative times similar to those in the literature; moreover, we found a tendency toward a longer operative time for RATS when compared with VATS (241.7 ± 72.6 min vs. 214.4 ± 45.1 min; p = 0.06). 7 , 13 , 21 We believe that we would achieve a significant difference with a larger number of patients. We had neither intraoperative complications nor need for surgical conversion in the RATS group. Similarly to what has previously been published, we identified the need for more surgical conversions with VATS, although this difference was not statistically significant. 7 , 22 , 23
The medians [IQR] in drainage time were not different between the RATS and VATS groups (2 [1-2] days vs. 2 [1-4] days; p = 0.27). The same occurred regarding the length of hospital stay (3 [2-4] days vs. 4 [2-5]; p = 0.55). These findings are compatible with those of most of the previous studies. 7 , 10 , 11 , 21 However, our trial has no statistical power to evaluate such outcomes. Differences could possibly appear with a larger number of patients, as shown in other studies. 7 , 23
This study is a randomized clinical trial. By design, we are eliminating the selection bias inherent to previous retrospective studies, and this was confirmed by the well balanced groups analyzed in our study. However, this study has some limitations. First, the randomization was not blinded; therefore, we could not guarantee the absence of performance and detection biases. We tried to minimize this problem by sticking to rigid guidelines for postoperative management. Sample size was also an important issue. The study budget had a limited amount of resources for robotic surgeries, which allowed the inclusion of up to 40 patients in the RATS group, which might have impacted on statistical power.
In the present study we found that RATS and VATS lobectomy had similar 90-day outcomes. However, RATS lobectomy was associated with a significant reduction in 90-day hospital readmission rate. This gain in safety may help us understand the growth of RATS lobectomy in recent years. 7 Nonetheless, larger studies are necessary to confirm our findings and better explore differences in postoperative complications.
ACKNOWLEDGMENTS
We would like to thank Dr. Ricardo Abdalla for his expertise, commitment, and dedication to tutoring us in the initial cases. In addition, we would like to thank Professors Ivan Ceconello and Ulysses Ribeiro Junior for their unconditional support and for making it possible to implement a robotic surgery program at the Instituto do Câncer do Estado de São Paulo (ICESP). Finally, we would like to thank Evelise Zaidan and the ICESP.
Footnotes
Financial support: The Brazilian Ministry of Health funded the acquisition of the DaVinci Si robotic system, surgical instruments, and disposable materials specific to robotic surgery (2012NE800206).
Study carried out in the Divisão de Cirurgia Torácica, Departamento de Cardiopneumologia, Hospital das Clinicas, Faculdade de Medicina, Universidade de São Paulo, São Paulo (SP) Brasil.
REFERENCES
- 1.National Comprehensive Cancer Network (NCCN) [homepage on the Internet] NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer. Version 2.2020. PA: NCCN; c2021. https://www2.tri-kobe.org/nccn/guideline/lung/english/non_small.pdf [Google Scholar]
- 2.Paul S, Altorki NK, Sheng S, Lee PC, Harpole DH, Onaitis MW. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg. 2010;139(2):366–378. doi: 10.1016/j.jtcvs.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 3.Tsukazan MTR, Terra RM, Vigo Á, Fortunato GA, Camargo SM, de Oliveira HA. Video-assisted thoracoscopic surgery yields better outcomes than thoracotomy for anatomical lung resection in Brazil a propensity score-matching analysis using the Brazilian Society of Thoracic Surgery database. Eur J Cardiothorac Surg. 2018;53(5):993–998. doi: 10.1093/ejcts/ezx442. [DOI] [PubMed] [Google Scholar]
- 4.Falcoz PE, Puyraveau M, Thomas PA, Decaluwe H, Hürtgen M, Petersen RH. Video-assisted thoracoscopic surgery versus open lobectomy for primary non-small-cell lung cancer a propensity-matched analysis of outcome from the European Society of Thoracic Surgeon database. Eur J Cardiothorac Surg. 2016;49(2):602–609. doi: 10.1093/ejcts/ezv154. [DOI] [PubMed] [Google Scholar]
- 5.Bendixen M, Jørgensen OD, Kronborg C, Andersen C, Licht PB. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer a randomised controlled trial. Lancet Oncol. 2016;17(6):836–844. doi: 10.1016/S1470-2045(16)00173-X. [DOI] [PubMed] [Google Scholar]
- 6.Howington JA, Blum MG, Chang AC, Balekian AA, Murthy SC. Treatment of stage I and II non-small cell lung cancer Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e278S–e313S. doi: 10.1378/chest.12-2359. [DOI] [PubMed] [Google Scholar]
- 7.Oh DS, Reddy RM, Gorrepati ML, Mehendale S, Reed MF. Robotic-Assisted, Video-Assisted Thoracoscopic and Open Lobectomy Propensity-Matched Analysis of Recent Premier Data. Ann Thorac Surg. 2017;104(5):1733–1740. doi: 10.1016/j.athoracsur.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 8.Veronesi G, Galetta D, Maisonneuve P, Melfi F, Schmid RA, Borri A. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg. 2010;140(1):19–25. doi: 10.1016/j.jtcvs.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 9.Park BJ, Melfi F, Mussi A, Maisonneuve P, Spaggiari L, Da Silva RK. Robotic lobectomy for non-small cell lung cancer (NSCLC) long-term oncologic results. J Thorac Cardiovasc Surg. 2012;143(2):383–389. doi: 10.1016/j.jtcvs.2011.10.055. [DOI] [PubMed] [Google Scholar]
- 10.Kent M, Wang T, Whyte R, Curran T, Flores R, Gangadharan S. Open, video-assisted thoracic surgery, and robotic lobectomy review of a national database. Ann Thorac Surg. 2014;97(1):236–244. doi: 10.1016/j.athoracsur.2013.07.117. [DOI] [PubMed] [Google Scholar]
- 11.Swanson SJ, Miller DL, McKenna RJ, Jr, Howington J, Marshall MB, Yoo AC. Comparing robot-assisted thoracic surgical lobectomy with conventional video-assisted thoracic surgical lobectomy and wedge resection results from a multihospital database (Premier) J Thorac Cardiovasc Surg. 2014;147(3):929–937. doi: 10.1016/j.jtcvs.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 12.Emmert A, Straube C, Buentzel J, Roever C. Robotic versus thoracoscopic lung resection A systematic review and meta-analysis. Medicine (Baltimore) 2017;96(35):e7633. doi: 10.1097/MD.0000000000007633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Louie BE, Wilson JL, Kim S, Cerfolio RJ, Park BJ, Farivar AS. Comparison of Video-Assisted Thoracoscopic Surgery and Robotic Approaches for Clinical Stage I and Stage II Non-Small Cell Lung Cancer Using The Society of Thoracic Surgeons Database. Ann Thorac Surg. 2016;102(3):917–924. doi: 10.1016/j.athoracsur.2016.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ninan M, Dylewski MR. Total port-access robot-assisted pulmonary lobectomy without utility thoracotomy. Eur J Cardiothorac Surg. 2010;38(2):231–232. doi: 10.1016/j.ejcts.2010.01.047. [DOI] [PubMed] [Google Scholar]
- 15.Seely AJ, Ivanovic J, Threader J, Al-Hussaini A, Al-Shehab D, Ramsay T. Systematic classification of morbidity and mortality after thoracic surgery. Ann Thorac Surg. 2010;90(3):936–942. doi: 10.1016/j.athoracsur.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 16.Schestatsky P, Félix-Torres V, Chaves ML, Câmara-Ehlers B, Mucenic T, Caumo W. Brazilian Portuguese validation of the Leeds Assessment of Neuropathic Symptoms and Signs for patients with chronic pain. Pain Med. 2011;12(10):1544–1550. doi: 10.1111/j.1526-4637.2011.01221.x. [DOI] [PubMed] [Google Scholar]
- 17.Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK. AJCC Cancer Staging Manual. 8. New York: Springer; 2017. [Google Scholar]
- 18.Jean RA, Chiu AS, Boffa DJ, Detterbeck FC, Blasberg JD, Kim AW. When good operations go bad The additive effect of comorbidity and postoperative complications on readmission after pulmonary lobectomy. Surgery. 2018;164(2):294–299. doi: 10.1016/j.surg.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 19.Jean RA, Chiu AS, Hoag JR, Blasberg JD, Boffa DJ, Detterbeck FC. Identifying Drivers of Multiple Readmissions After Pulmonary Lobectomy. Ann Thorac Surg. 2019;107(3):947–953. doi: 10.1016/j.athoracsur.2018.08.070. [DOI] [PubMed] [Google Scholar]
- 20.Stiles BM, Poon A, Giambrone GP, Gaber-Baylis LK, Wu X, Lee PC. Incidence and Factors Associated With Hospital Readmission After Pulmonary Lobectomy. Ann Thorac Surg. 2016;101(2):434–443. doi: 10.1016/j.athoracsur.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Adams RD, Bolton WD, Stephenson JE, Henry G, Robbins ET, Sommers E. Initial multicenter community robotic lobectomy experience comparisons to a national database. Ann Thorac Surg. 2014;97(6):1893–1900. doi: 10.1016/j.athoracsur.2014.02.043. [DOI] [PubMed] [Google Scholar]
- 22.Oh DS, Cho I, Karamian B, DeMeester SR, Hagen JA. Early adoption of robotic pulmonary lobectomy feasibility and initial outcomes. Am Surg. 2013;79(10):1075–1080. doi: 10.1177/000313481307901024. [DOI] [PubMed] [Google Scholar]
- 23.Reddy RM, Gorrepati ML, Oh DS, Mehendale S, Reed MF. Robotic-Assisted Versus Thoracoscopic Lobectomy Outcomes From High-Volume Thoracic Surgeons. Ann Thorac Surg. 2018;106(3):902–908. doi: 10.1016/j.athoracsur.2018.03.048. [DOI] [PubMed] [Google Scholar]


