Abstract
Transcranial direct current stimulation (tDCS) is a safe and well-tolerated noninvasive method for stimulating the brain that is rapidly developing into a treatment method for various neurological and psychiatric conditions. In particular, there is growing evidence of a therapeutic role for tDCS in ameliorating or delaying the cognitive decline in Alzheimer’s disease (AD). We provide a brief overview of the current development and application status of tDCS as a nonpharmacological therapeutic method for AD and mild cognitive impairment (MCI), summarize the levels of evidence, and identify the improvements needed for clinical applications. We also suggest future directions for large-scale controlled clinical trials of tDCS in AD and MCI, and emphasize the necessity of identifying the mechanistic targets to facilitate clinical applications.
Keywords: transcranial direct current stimulation, Alzheimer disease, clinical trial
INTRODUCTION
Alzheimer’s disease (AD) represents a major global health challenge whose prevalence is increasing along with the aging population. There is an urgent need for effective treatment options for the 24 million people currently living with AD.1 Unfortunately, despite intensive drug development and research, AD remains without a cure, and current treatment options offer inadequate benefit in delaying its progression. While we await a cure for AD, our near-term approach is to improve its care by preserving functioning and delaying decline.
Noninvasive brain stimulation is rapidly developing as a nondrug treatment for use in a wide range of neurological and psychiatric conditions.2 One extensively investigated technique is transcranial direct current stimulation (tDCS), which involves delivering a low-intensity sustained electrical current to cortical tissue via scalp electrodes with the goal of modulating brain excitability and plasticity.3 Based on the cumulative effects of tDCS, a period of repeated sessions (e.g., daily) can be used to induce alterations in brain function that will have clinical effects.4,5,6
tDCS is a safe and well-tolerated treatment approach7,8 that can be easily combined with simultaneous rehabilitative activities (e.g., cognitive, motor, or psychotherapeutic). Directing tDCS at a brain region activated during training can enhance the potency of that training, presumably based on the mechanisms via which tDCS can boost ongoing plasticity.9,10,11,12 Since tDCS devices can be wearable and portable, they offer the further advantage of providing treatment to patients at home and in other locations away from the clinic through telehealth.13,14 In this manner, patients can access tDCS treatment away from the clinic and thereby receive the necessary number of daily repeated applications over time so as to maximize its clinical benefit.
There is growing evidence of a therapeutic role for tDCS in ameliorating or delaying the cognitive decline in AD. As reviewed in detail elsewhere,6,15 clinical trials have found tDCS to be safe and tolerable for use in the AD population, with therapeutic benefits of preserving or even improving cognitive functioning in the short term. However, clinical applications of tDCS remains restricted by the need for definitive clinical trials and the identification of the mechanisms underlying its beneficial effects. Here we focus on optimizing the design of large-scale controlled clinical trials and identifying the mechanistic targets to facilitate clinical applications of tDCS.
OVERVIEW OF tDCS
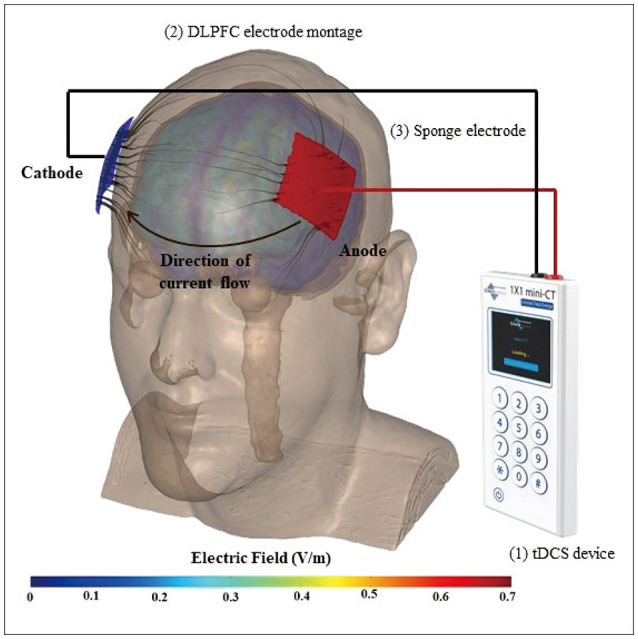
tDCS is a noninvasive brain stimulation technique that involves passing a low-amplitude direct current (typically 1–2 mA) through the brain via electrodes on the scalp (Fig. 1). The placement locations of the electrodes are determined based on the targeted brain region. A portion of the applied current that crosses the brain leads to polarization of neurons,16 which in turn modulates excitability, synaptic efficacy,17 oscillations,18 plasticity,19,20 and other cellular processes that are key to cognitive function and learning.21 The electrode polarity (anode or cathode) determines the direction of current flow, which influences the outcomes.8,22
Fig. 1. Transcranial direct current stimulation (tDCS) equipment, working principle, and modeling of the electric field distribution. (1) tDCS device: programmable session type (active or sham), stimulation duration, and current intensity. (2) Dorsolateral prefrontal cortex (DLPFC) electrode montage (left to right, F3-F4 according to the international 10-20 system). (3) Sponge electrodes.
Based on hundreds of clinical trials in patients with a wide range of neurological and psychiatric conditions, including studies of patients with mild cognitive impairment (MCI) and AD, tDCS has an extensive record of safety and tolerability.7,23,24 Its advantages over other stimulation methods such as transcranial magnetic stimulation (TMS) include easier use, lower cost, and better tolerability (e.g., not associated with seizures25,26). As also reported previously, the most-common side effects involve transient sensations at the electrode site and include tingling, itching, and a sensation of warmth, with no increased risk in the elderly.7,27 The safety and tolerability records extracted across our remotely supervised tDCS (RS-tDCS) randomized controlled trials (RCTs)10,28,29,30 showed that the side effects in a subsample of older participants (n=45, age range=65–78 years) with various neurological diseases (e.g., multiple sclerosis, Parkinson’s disease, and cerebellar ataxia) did not differ in either nature or frequency from those in a subsample of young adults (old vs. young adults: tingling, 71% vs. 68%; itching, 35% vs. 41%; warmth sensation, 37% vs. 42%).
CLINICAL POTENTIAL OF tDCS IN AD
Notwithstanding the hundreds of clinical trials to date studying the clinical effects of tDCS, there remains the need for Class I/II evidence to advance its clinical use.2 While the findings have largely been encouraging, most clinical trials have been limited by small samples and/or suboptimal dosing. As summarized in Table 1, several RCTs of tDCS have been conducted in AD or MCI over the last decade.
Table 1. Summary of recent randomized controlled trials of tDCS in AD and MCI.
| Study | Participants | Active tDCS | Sham tDCS | Intensity, dose, and duration | Combination with another intervention | Assessment outcomes | Findings |
|---|---|---|---|---|---|---|---|
| Rasmussen et al.83 | Mild-to-moderate AD (n=19: active=10; sham=9) | Anodal: left DLPFC | For 1 min, same montage | 2-mA HD-tDCS for 20 min/session, 3 sessions/day for 2 days (total 6 sessions) | None | MMSE, clock-drawing test, TMT part A, RBANS, MRI including DTI | Short-term improvement in delayed-recall memory and global cognition. Positive correlation between FA in the anterior thalamic radiation with delayed memory score |
| He et al.82 | MCI (n=43: active=24; sham=19) | Anodal: left DLPFC | For 1 min, same montage | 1-mA HD-tDCS for 20 min/day, 5 sessions/week for 2 weeks (total 10 sessions) | None | MMSE, MoCA, resting-state fMRI | No improvement in cognition. Altered intensity and synchrony of brain activity in multiple regions on fMRI |
| Gangemi et al.43 | Mild AD (n=26: active=13 and 9; sham=13 and 9) | Anodal: left frontotemporal | For 10 sec, same montage | Study 1: 2 mA for 20 min/day for 10 days (total 10 sessions) Study 2: 10 days/month for 8 months (total 80 sessions) |
None | MMSE, MODA, EEG | Both short-term and long-term effects on the prevention of cognitive decline |
| Im et al.38 | Mild AD (n=18: active=11; sham=7) | Anodal: left DLPFC Cathodal: right DLPFC |
30-sec ramp up and 30-sec ramp down, same montage | 2 mA for 30 min/day for 6 months (total 180 sessions) | None | MMSE, CDR and CDR-SOB, digit span, BNT, RCFT, SVLT, executive function tests, [18F]FDG PET | Long-term improvements in global cognition and language function. Marginally improved executive functions. Left middle/inferior temporal glucose metabolism was preserved on PET |
| Lu et al.44 | MCI (n=201: active=69 and 68; sham=64) | Anodal: left lateral temporal cortex | For 30 sec, same montage | 2 mA, 20 min/day, 3 sessions/week for 3 months (total 36 sessions) | WMT | N-back task, ADAS-Cog, MMSE, CDR-SOB, NPI, CVFT, digit span, logical memory, TMT | The combined tDCS-WMT group showed greater short-term improvements in delayed-recall and working memory, and long-term improvement in logical memory relative to single-modality groups |
| Gomes et al.110 | MCI (n=58: active=29; sham=29) | Anodal: left DLPFC | For 30 sec, same montage | 2 mA, 30 min/day, 2 sessions/week for 5 weeks (total 10 sessions) | None | CAMCOG, MMSE, TMT, semantic verbal fluency (animals), BNT, clock-drawing test, word-list memory, direct and indirect digit order, N-back task | Short-term improvement in memory recall, verbal fluency, and executive functioning |
| Khedr et al.80 | Mild-to-moderate AD (n=46: active=23; sham=23) | Anodal: left and right temporoparietal | For 30 sec, same montage | 2 mA, 20 min/day, 5 sessions/week for 2 weeks (total 10 sessions) | None | MMSE, MoCA, clock-drawing test, Cornell depression scale, serum tau, Aβ 1–42, lipid peroxidase | Short-term improvement in global cognition and depression, also correlated with increased plasma Aβ 1–42 levels. |
| Bystad et al.45 | Mild-to-moderate AD (n=25: active=12; sham=13) | Anodal: left temporal Cathodal: right frontal |
For 30 sec, same montage | 2 mA, 30 min/day, 6 sessions in 10 days (total 6 sessions) | None | MMSE, CVLT, clock-drawing test, TMT, WAIS, Cornell depression scale | No significant improvement in cognition |
| Yun et al.81 | MCI (n=16: active=8; sham=8) | Anodal: left DLPFC Cathodal: right DLPFC |
For 20 sec, same montage | 2 mA, 20 min/day, 3 sessions/week for 3 weeks (total 9 sessions) | None | Modified MMQ, [18F]FDG PET | Short-term improvement of the memory strategies, increased regional cerebral metabolism on PET |
| Khedr et al.84 | Mild-to-moderate AD (n=34: active=11 and 12; sham=11) | Anodal: left DLPFC Cathodal: left DLPFC |
For 30 sec, same montage | 2 mA, 25 min/day, daily for 10 days (total 10 sessions) | None | MMSE, WAIS-III motor cortical excitability, P300 event-related potentials | Both anodal and cathodal tDCS improved short-term cognition and reduced the P300 latency |
| Cotelli et al.46 | Mild-to-moderate AD (n=36: active=12 and 12; sham=12) | Anodal: left DLPFC | For 20 sec at the beginning and end, same montage | 2 mA, 25 min/day, 5 sessions/week for 2 weeks (total 10 sessions) | Individualized computerized memory training | FNAT, MMSE, ADL, IADL, Tinetti scale, NPI, Picture naming task, BADA, RBMT, Rey Auditory Verbal Learning Test, complex figure copying, TMT | No significant additive effect on the general improvement by memory training |
| Suemoto et al.47 | Moderate AD (n=40: active=20; sham=20) | Anodal: left DLPFC | For 20 sec, same montage | 2 mA, 20 min/day, 3 sessions/week for 2 weeks (total 6 sessions) | None | Apathy Scale, NPI, ADAS-Cog | No significant difference in apathy or cognition |
AD, Alzheimer’s disease; ADAS-Cog, Alzheimer’s Disease Assessment Scale–Cognitive Subscale; ADL, activities of daily living; BADA, Battery for Analysis of Aphasic Deficits; BNT, Boston Naming Test; CAMCOG, Cambridge Cognition Examination; CDR, Clinical Dementia Rating; CDR-SOB, Clinical Dementia Rating–Sum of Boxes; CVFT, Category Verbal Fluency Test; DLPFC, dorsolateral prefrontal cortex; DTI, diffusion-tensor imaging; EEG, electroencephalography; FA, fractional anisotropy; fMRI, functional magnetic resonance imaging; FNAT, Face–Name Association Memory Task; HD, high definition; IADL, Instrumental Activities of Daily Living; MCI, mild cognitive impairment; MMQ, Multifactorial Memory Questionnaire; MMSE, Mini-Mental Status Examination; MoCA, Montreal Cognitive Assessment; MODA, Milan Overall Dementia Assessment; MRI, magnetic resonance imaging; NPI, Neuropsychiatric Inventory; PET, positron-emission tomography; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status; RBMT, Rivermead Behavioral Memory Test; RCFT, Rey Complex Figure Test; SVLT, Seoul Verbal Learning Test; tDCS, transcranial direct current stimulation; TMT, Trail-Making Test; WAIS, Wechsler Adult Intelligence Scale; WMT, working memory training.
Dosing considerations
Preclinical model studies indicate that tDCS produces lasting changes in brain excitability (e.g., hemodynamic response, functional connectivity, and neuroplastic changes31,32,33,34) that are cumulative over repeated sessions, which is consistent with clinical neurophysiology.23,35,36,37,38 Further, multiple-session protocols induce larger effects in terms of behavioral changes than do single-session protocols.38,39,40,41,42
Several studies have adopting 10 or fewer applications,6 which means that dosing may have been suboptimal for definitive evaluations of its clinical effectiveness. It is notable that recent studies involving relatively large numbers of total sessions over extended periods tended to show more-robust improvements in cognition with more-persistent effects,38,43,44 relative to trials with smaller numbers of sessions over shorter periods.42,45,46,47,48 This effect is mirrored through consistent findings across clinical studies, including in our own trials, showing that 1) a single tDCS session does not induce any meaningful behavioral response,42 and 2) behavioral changes only follow a sustained period of daily treatment.49,50 Multiple RCTs have shown that repeated application (i.e., ≥20 daily sessions) of tDCS targeting the dorsolateral prefrontal cortex (DLPFC) improves cognitive training outcomes in healthy aging adults.11,42,48 Importantly, these changes have also been linked to increases in the functional connectivity in the DLPFC11 and in frontal lobe neurotransmitter concentrations.51
In previous trials there have also been wide variations in the dosing parameters applied to the electrode montage (functional targeting vs. lesion-based targeting), electrical current intensity, stimulation duration, and the number of applied stimuli, as well as in the application of simultaneously paired training activities across study designs. Four of the 12 RCTs listed in Table 1 involved MCI patients, and the remainder involved patients with mild or moderate AD. Almost all of these studies used anodal tDCS (i.e., with the anode electrode over the target region) with a current of 2 mA for 20–30 min per session. The nominal target region varied, although the most-common target region was the DLPFC (left>right), F3-F4 according to the international 10-20 system), while temporal targeting has been applied less, hence warranting further studies. The total number of sessions varied markedly between 6 and 180, with the overall treatment duration varying between 2 days and 8 months.
We now know that neuroplasticity can occur throughout the lifespan, and the neuroplastic effects induced by tDCS have been demonstrated to be preserved in healthy elderly individuals.51,52,53,54 However, critical guidance concerning tDCS dosing parameters is needed in order to maximize its clinical benefit in the presence of neurophysiological age-related changes;55 for example, age may affect the optimal current intensity27,56,57,58 and stimulation duration.59 Further, various factors such as the brain state, brain atrophy, and hormonal levels are likely to influence individual differences in response, and so they can ultimately be used to identify those who are most likely to benefit from treatment.6,27,60,61
Simultaneous pairing of tDCS with cognitive training
tDCS can target the neuromodulation of regions underlying cognitive impairments or be paired with simultaneous cognitive training. When paired together with training tasks, the targeted region (functional targeting) is engaged both through the stimulation and simultaneous activity, which may produce a synergistic benefit. In MCI and AD, investigators have taken approaches to employ neuromodulation with tDCS with the goal of both improving cognitive functioning and ultimately delaying or even preventing its progressive decline. Studies have targeted stimulation of frontal or temporal regions, often pairing with simultaneous cognitive training exercises.44,46 This approach activates the target region by the activity during the stimulation, and may lead to improved cognitive training outcomes.
Only two RCTs have used cognitive training as an intervention combined with tDCS (see Table 1)—such a combined intervention may be especially effective in potentiating and strengthening the learning process.62 The simultaneous delivery of tDCS with cognitive training is broadly thought to increase potential neuronal firing and synaptic activity, which will selectively activate and reinforce the regions engaged in the cognitive activity,63,64,65 with these positive effects being transferred to similar tasks.66,67,68,69 The findings of recent electrophysiological studies analyzing the mechanism of tDCS suggest that the long-term effects of tDCS are due to the enhancement of neural plasticity by modulating long-term potentiation and depression in relevant neural networks.20,65 This combined approach may have the added benefit of prolonging learning effects after the treatment has ceased.70,71,72,73 As demonstrated by our previous work across neurodegenerative disorders, pairing DLPFC tDCS with cognitive training increases learning and performance outcomes, and leads specifically to improvements in measures of vigilance.10,12,74,75,76,77,78
Long-term efficacy
No previous study has addressed the important question of the persistence of benefits. Due to the small number of trials with follow-ups longer than 1 month, further studies are needed to determine whether the established effects of tDCS persist over time once treatment has been discontinued. This question is critical when considering the potential of tDCS in preventing or delaying cognitive decline in MCI and AD patients over time.79
THE NEED FOR MECHANISTIC INSIGHT
For the outcome measurements of the latest RCTs in Table 1, basic and global cognitive assessment tools such as the Mini-Mental Status Examination, Montreal Cognitive Assessment, and Clinical Dementia Rating were used the most, with most studies also including more-comprehensive measures for specific domains. Only one of the studies that evaluated psychiatric symptoms found a significant improvement in depression.80
However, only a handful of studies have used neuroimaging or electrophysiological methods as secondary outcomes. Im et al.38 and Yun et al.81 used [18F]FDG positron emission tomography (PET) to visualize changes in the cerebral glucose metabolism after tDCS, and both studies found significant positive effects. Recent studies using resting-state functional magnetic resonance imaging or diffusion tensor imaging have also demonstrated the utility of advanced neuroimaging tools for assessing the outcome of tDCS.82,83 Khedr et al.84 measured motor cortical excitability using TMS and P300 latency in event-related potentials at baseline and after tDCS sessions. In a subsequent study they measured the following blood markers for AD at baseline and after tDCS: tau, Aβ 1–42, and lipid peroxidase.80 Thus, promising findings from the small number of trials using multimodal outcome measures warrant further studies taking advantage of the rapid advances in technology in clinical neuroscience in order to understand the complex therapeutic mechanisms of tDCS.
Various neurotransmitters have been suggested to be involved in the mechanisms of the changes resulting from tDCS applications, including dopamine, acetylcholine, serotonin, glutamate, and GABA.85 For example, a PET investigation with [11C]raclopride demonstrated that applying a single session of tDCS to the DLPFC induced dopamine release in the ventral striatum, which was associated with attention enhancement in healthy humans.86 Likewise, there is good evidence from both animal and human studies for the strong involvement of the serotonin system in the antidepressant effect of tDCS on the DLPFC.87,88 More importantly for AD, there are accumulating data suggesting the pivotal role of the cholinergic neurons and their synaptic modulation in the changes induced by tDCS.85,89,90 Neurophysiological studies in animals have shown that cholinergic modulation facilitates long-term potentiation.91,92 Previous human studies of tDCS combined with an acetylcholinesterase inhibitor or nicotine patch also demonstrated the significant contribution of the cholinergic pharmacodynamic status on the different levels of neuroplasticity induced by tDCS.93,94 In addition, recent tDCS investigations involving MCI and AD patients demonstrated significant increases in cortical metabolism in the areas relevant to cholinergic synaptic innervation, such as the prefrontal, anterior cingulate, and medial and lateral temporal cortices.38,81 A recent study utilizing magnetic resonance spectroscopy demonstrated increased GABA and a decreased ratio of glutamate to GABA after tDCS in older adults with or without MCI.95 To our knowledge, that is the only previous clinical trial of tDCS in AD or MCI that has employed outcome measures that can detect specific changes in each of those neurotransmitters, which should be seriously considered when planning future studies.
HOME-BASED tDCS USING REMOTE SUPERVISION IN DESIGNING CLINICAL TRIALS
Both tDCS and cognitive training must be dosed in a sustained and cumulative manner to induce an effect. However, as detailed above, previous trials of tDCS and cognitive training in AD or MCI have only allowed restricted conclusions to be drawn,96 due in part to the potential underdosing in terms of the frequency and number of treatment sessions being lower than those required for an optimal effect.97,98,99
We believe that trial designs have been restricted by practical obstacles associated with accessing treatment in the clinic. The logistical constraints of clinic-based treatment—such as participant time and travel as well as costs for daily visits—have been a primary and practical limitation for the field in terms of sample size and suboptimal dosing of the number of sessions. To enhance access to treatment and enable protocols with extended treatment periods, we have validated an RS-tDCS protocol that delivers treatment at home to as to overcome practical barriers to access and thereby facilitate cumulative dosing. There has been a strong demand for such at-home treatment across various neurological conditions, including for people with multiple sclerosis, Parkinson’s disease, traumatic brain injury, and cerebellar ataxia, which have led to clinical applications.10,13,14,28,29,30,100,101,102,103,104 In the RS-tDCS protocol, participants are provided with remotely controlled tDCS devices, and they are extensively trained in their safe and effective operation. We apply real-time supervision in each tDCS session through live videoconferencing with extensively trained personnel who follow rigorous go/no-go criteria during the daily treatments in order to ensure that the protocol complies with the laboratory standards of the tDCS devices.13,100,105
The telehealth delivery of tDCS results in rapid enrollment and high retention and adherence rates in repeated and extended sessions (e.g., >97% completion rates across RCTs to date).10,13,14,28,29,30,100,101,102,103,104 Extensive testing over >7 years (covering >9,000 at-home tDCS sessions involving >400 patients to date) has verified the feasibility of our RS-tDCS procedures for use across all ages (18–80 years), including in those with advanced cognitive or motor disabilities and/or low technical experience, and it also reaches those whose poor socioeconomic healthcare status impedes their inclusion in RCTs. Further, the RS-tDCS platform has facilitated continuing enrollments in ongoing RCTs during the restrictions to clinical research associated with the COVID-19 pandemic, with >100 participants completing all study procedures from home.106,107,108,109 Only one previous study evaluated tDCS in the home setting, by providing unsupervised tDCS to patients with mild AD over 6 months and without concurrent cognitive training activity.38 That study confirmed the safety and feasibility of the home use of tDCS in the AD population, and also demonstrated the mild general cognitive improvements achieved, indicating that remotely supervised home-based trials can facilitate the design of further clinical trials.38
CONCLUSIONS AND FUTURE DIRECTIONS
Further investigations are needed into the efficacy of tDCS and cognitive training with sufficient dosing over longer follow-ups. The inclusion of biomarkers for guidance is critical to advancing the field and developing new therapeutic targets. With the goal of delaying progression, the MCI population represents the earliest and best target group for further evaluation. The effects on neuroplasticity in MCI can be investigated by including a sensitive biomarker of the underlying mechanism. Finally, the additive effects of the combined intervention of tDCS and cognitive training have not been thoroughly evaluated relative to applying tDCS and cognitive training separately in a study design with adequate intensity, duration, and scientific rigor.
Future clinical trials of tDCS in AD and MCI need to be performed under optimized conditions based on the accumulating evidence for the importance of a longer follow-up. Namely, a sufficient number of sessions over an extended period should be administered in conjunction with cognitive training. Outcomes should be assessed using a multimodal approach that includes neuroimaging, electrophysiological, or other AD-specific biomarkers in combination with conventional neuropsychological testing. In order to gain a better understanding of the mechanism so as to guide AD treatments, further studies employing a specific biomarker that can sensitively detect modulation of the targeted neural system are necessary. We propose that home-based RS-tDCS is a practical solution for promoting the clinical use of tDCS by the MCI and AD population. The next investigative steps could lead to tDCS and other noninvasive brain stimulation approaches representing an important clinical tool for the care of AD patients.
Footnotes
- Conceptualization: Leigh E. Charvet, Min-Jeong Kim.
- Supervision: Leigh E. Charvet, Marom Bikson, Nikhil Palekar.
- Visualization: Giuseppina Pilloni.
- Writing—original draft: Giuseppina Pilloni, Min-Jeong Kim.
- Writing—review & editing: all authors.
Conflicts of Interest: Min-Jeong Kim, a contributing editor of the Journal of Clinical Neurology, was not involved in the editorial evaluation or decision to publish this article.
Funding Statement: None
Availability of Data and Material
Data sharing not applicable to this article as no datasets were generated or analyzed during the study.
References
- 1.Mayeux R, Stern Y. Epidemiology of Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006239. doi: 10.1101/cshperspect.a006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fregni F, El-Hagrassy MM, Pacheco-Barrios K, Carvalho S, Leite J, Simis M, et al. Evidence-based guidelines and secondary meta-analysis for the use of transcranial direct current stimulation in neurological and psychiatric disorders. Int J Neuropsychopharmacol. 2021;24:256–313. doi: 10.1093/ijnp/pyaa051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist. 2011;17:37–53. doi: 10.1177/1073858410386614. [DOI] [PubMed] [Google Scholar]
- 4.Pelletier SJ, Cicchetti F. Cellular and molecular mechanisms of action of transcranial direct current stimulation: evidence from in vitro and in vivo models. Int J Neuropsychopharmacol. 2014;8:pyu047. doi: 10.1093/ijnp/pyu047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohammadi A. Induction of neuroplasticity by transcranial direct current stimulation. J Biomed Phys Eng. 2016;6:205–208. [PMC free article] [PubMed] [Google Scholar]
- 6.Indahlastari A, Hardcastle C, Albizu A, Alvarez-Alvarado S, Boutzoukas EM, Evangelista ND, et al. A systematic review and meta-analysis of transcranial direct current stimulation to remediate age-related cognitive decline in healthy older adults. Neuropsychiatr Dis Treat. 2021;17:971–990. doi: 10.2147/NDT.S259499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T, et al. Safety of transcranial direct current stimulation: evidence based update 2016. Brain Stimul. 2016;9:641–661. doi: 10.1016/j.brs.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni AR, Celnik P, et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol. 2016;127:1031–1048. doi: 10.1016/j.clinph.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kronberg G, Rahman A, Sharma M, Bikson M, Parra LC. Direct current stimulation boosts hebbian plasticity in vitro. Brain Stimul. 2020;13:287–301. doi: 10.1016/j.brs.2019.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charvet L, Shaw M, Dobbs B, Frontario A, Sherman K, Bikson M, et al. Remotely supervised transcranial direct current stimulation increases the benefit of at-home cognitive training in multiple sclerosis. Neuromodulation. 2018;21:383–389. doi: 10.1111/ner.12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nissim NR, O’Shea A, Indahlastari A, Kraft JN, von Mering O, Aksu S, et al. Effects of transcranial direct current stimulation paired with cognitive training on functional connectivity of the working memory network in older adults. Front Aging Neurosci. 2019;11:340. doi: 10.3389/fnagi.2019.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elmasry J, Loo C, Martin D. A systematic review of transcranial electrical stimulation combined with cognitive training. Restor Neu rol Neurosci. 2015;33:263–278. doi: 10.3233/RNN-140473. [DOI] [PubMed] [Google Scholar]
- 13.Charvet LE, Shaw MT, Bikson M, Woods AJ, Knotkova H. Supervised transcranial direct current stimulation (tDCS) at home: a guide for clinical research and practice. Brain Stimul. 2020;13:686–693. doi: 10.1016/j.brs.2020.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Shaw M, Pilloni G, Charvet L. Delivering transcranial direct current stimulation away from clinic: remotely supervised tDCS. Mil Med. 2020;185(Suppl 1):319–325. doi: 10.1093/milmed/usz348. [DOI] [PubMed] [Google Scholar]
- 15.Siegert A, Diedrich L, Antal A. New methods, old brains-a systematic review on the effects of tDCS on the cognition of elderly people. Front Hum Neurosci. 2021;15:730134. doi: 10.3389/fnhum.2021.730134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radman T, Ramos RL, Brumberg JC, Bikson M. Role of cortical cell type and morphology in subthreshold and suprathreshold uniform electric field stimulation in vitro. Brain Stimul. 2009;2:215–228. 228.e1–228.e3. doi: 10.1016/j.brs.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rahman A, Reato D, Arlotti M, Gasca F, Datta A, Parra LC, et al. Cellular effects of acute direct current stimulation: somatic and synaptic terminal effects. J Physiol. 2013;591:2563–2578. doi: 10.1113/jphysiol.2012.247171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reato D, Rahman A, Bikson M, Parra LC. Effects of weak transcranial alternating current stimulation on brain activity-a review of known mechanisms from animal studies. Front Hum Neurosci. 2013;7:687. doi: 10.3389/fnhum.2013.00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG, et al. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron. 2010;66:198–204. doi: 10.1016/j.neuron.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kronberg G, Bridi M, Abel T, Bikson M, Parra LC. Direct current stimulation modulates LTP and LTD: activity dependence and dendritic effects. Brain Stimul. 2017;10:51–58. doi: 10.1016/j.brs.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson MP, Rahman A, Lafon B, Kronberg G, Ling D, Parra LC, et al. Animal models of transcranial direct current stimulation: methods and mechanisms. Clin Neurophysiol. 2016;127:3425–3454. doi: 10.1016/j.clinph.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, et al. Transcranial direct current stimulation: state of the art 2008. Brain Stimul. 2008;1:206–223. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Brunoni AR, Nitsche MA, Bolognini N, Bikson M, Wagner T, Merabet L, et al. Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain Stimul. 2012;5:175–195. doi: 10.1016/j.brs.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nikolin S, Huggins C, Martin D, Alonzo A, Loo CK. Safety of repeated sessions of transcranial direct current stimulation: a systematic review. Brain Stimul. 2018;11:278–288. doi: 10.1016/j.brs.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 25.Vanneste S, Plazier M, Ost J, van der Loo E, Van de Heyning P, De Ridder D. Bilateral dorsolateral prefrontal cortex modulation for tinnitus by transcranial direct current stimulation: a preliminary clinical study. Exp Brain Res. 2010;202:779–785. doi: 10.1007/s00221-010-2183-9. [DOI] [PubMed] [Google Scholar]
- 26.Priori A, Hallett M, Rothwell JC. Repetitive transcranial magnetic stimulation or transcranial direct current stimulation? Brain Stimul. 2009;2:241–245. doi: 10.1016/j.brs.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 27.Thomas C, Datta A, Woods A. Effect of aging on cortical current flow due to transcranial direct current stimulation: considerations for safety. Annu Int Conf IEEE Eng Med Biol Soc. 2018;2018:3084–3087. doi: 10.1109/EMBC.2018.8513014. [DOI] [PubMed] [Google Scholar]
- 28.Pilloni G, Shaw M, Feinberg C, Clayton A, Palmeri M, Datta A, et al. Long term at-home treatment with transcranial direct current stimulation (tDCS) improves symptoms of cerebellar ataxia: a case report. J Neuroeng Rehabil. 2019;16:41. doi: 10.1186/s12984-019-0514-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Charvet LE, Dobbs B, Shaw MT, Bikson M, Datta A, Krupp LB. Remotely supervised transcranial direct current stimulation for the treatment of fatigue in multiple sclerosis: results from a randomized, sham-controlled trial. Mult Scler. 2018;24:1760–1769. doi: 10.1177/1352458517732842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dobbs B, Pawlak N, Biagioni M, Agarwal S, Shaw M, Pilloni G, et al. Generalizing remotely supervised transcranial direct current stimulation (tDCS): feasibility and benefit in Parkinson’s disease. J Neuroeng Rehabil. 2018;15:114. doi: 10.1186/s12984-018-0457-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korai SA, Ranieri F, Di Lazzaro V, Papa M, Cirillo G. Neurobiological after-effects of low intensity transcranial electric stimulation of the human nervous system: from basic mechanisms to metaplasticity. Front Neurol. 2021;12:587771. doi: 10.3389/fneur.2021.587771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cavaleiro C, Martins J, Gonçalves J, Castelo-Branco M. Memory and cognition-related neuroplasticity enhancement by transcranial direct current stimulation in rodents: a systematic review. Neural Plast. 2020;2020:4795267. doi: 10.1155/2020/4795267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma M, Farahani F, Bikson M, Parra LC. Weak DCS causes a relatively strong cumulative boost of synaptic plasticity with spaced learning. Brain Stimul. 2022;15:57–62. doi: 10.1016/j.brs.2021.10.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim SJ, Kim BK, Ko YJ, Bang MS, Kim MH, Han TR. Functional and histologic changes after repeated transcranial direct current stimulation in rat stroke model. J Korean Med Sci. 2010;25:1499–1505. doi: 10.3346/jkms.2010.25.10.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monte-Silva K, Kuo MF, Hessenthaler S, Fresnoza S, Liebetanz D, Paulus W, et al. Induction of late LTP-like plasticity in the human motor cortex by repeated non-invasive brain stimulation. Brain Stimul. 2013;6:424–432. doi: 10.1016/j.brs.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 36.Meesen RL, Thijs H, Leenus DJ, Cuypers K. A single session of 1 mA anodal tDCS-supported motor training does not improve motor performance in patients with multiple sclerosis. Restor Neurol Neurosci. 2014;32:293–300. doi: 10.3233/RNN-130348. [DOI] [PubMed] [Google Scholar]
- 37.Zanão TA, Moffa AH, Shiozawa P, Lotufo PA, Benseñor IM, Brunoni AR. Impact of two or less missing treatment sessions on tDCS clinical efficacy: results from a factorial, randomized, controlled trial in major depression. Neuromodulation. 2014;17:737–742. doi: 10.1111/ner.12167. [DOI] [PubMed] [Google Scholar]
- 38.Im JJ, Jeong H, Bikson M, Woods AJ, Unal G, Oh JK, et al. Effects of 6-month at-home transcranial direct current stimulation on cognition and cerebral glucose metabolism in Alzheimer’s disease. Brain Stimul. 2019;12:1222–1228. doi: 10.1016/j.brs.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song S, Zilverstand A, Gui W, Li HJ, Zhou X. Effects of single-session versus multi-session non-invasive brain stimulation on craving and consumption in individuals with drug addiction, eating disorders or obesity: a meta-analysis. Brain Stimul. 2019;12:606–618. doi: 10.1016/j.brs.2018.12.975. [DOI] [PubMed] [Google Scholar]
- 40.Lau CI, Liu MN, Chang KC, Chang A, Bai CH, Tseng CS, et al. Effect of single-session transcranial direct current stimulation on cognition in Parkinson’s disease. CNS Neurosci Ther. 2019;25:1237–1243. doi: 10.1111/cns.13210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saxena V, Pal A. Role of transcranial direct current stimulation in the management of Alzheimer’s disease: a meta-analysis of effects, adherence and adverse effects. Clin Psychopharmacol Neurosci. 2021;19:589–599. doi: 10.9758/cpn.2021.19.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horvath JC, Forte JD, Carter O. Quantitative review finds no evidence of cognitive effects in healthy populations from single-session transcranial direct current stimulation (tDCS) Brain Stimul. 2015;8:535–550. doi: 10.1016/j.brs.2015.01.400. [DOI] [PubMed] [Google Scholar]
- 43.Gangemi A, Colombo B, Fabio RA. Effects of short- and long-term neurostimulation (tDCS) on Alzheimer’s disease patients: two randomized studies. Aging Clin Exp Res. 2021;33:383–390. doi: 10.1007/s40520-020-01546-8. [DOI] [PubMed] [Google Scholar]
- 44.Lu H, Chan SSM, Chan WC, Lin C, Cheng CPW, Linda Chiu Wa L. Randomized controlled trial of TDCS on cognition in 201 seniors with mild neurocognitive disorder. Ann Clin Transl Neurol. 2019;6:1938–1948. doi: 10.1002/acn3.50823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bystad M, Grønli O, Rasmussen ID, Gundersen N, Nordvang L, Wang-Iversen H, et al. Transcranial direct current stimulation as a memory enhancer in patients with Alzheimer’s disease: a randomized, placebo-controlled trial. Alzheimers Res Ther. 2016;8:13. doi: 10.1186/s13195-016-0180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cotelli M, Manenti R, Brambilla M, Petesi M, Rosini S, Ferrari C, et al. Anodal tDCS during face-name associations memory training in Alzheimer’s patients. Front Aging Neurosci. 2014;6:38. doi: 10.3389/fnagi.2014.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suemoto CK, Apolinario D, Nakamura-Palacios EM, Lopes L, Leite RE, Sales MC, et al. Effects of a non-focal plasticity protocol on apathy in moderate Alzheimer’s disease: a randomized, double-blind, sham-controlled trial. Brain Stimul. 2014;7:308–313. doi: 10.1016/j.brs.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 48.Talsma LJ, Kroese HA, Slagter HA. Boosting cognition: effects of multiple-session transcranial direct current stimulation on working memory. J Cogn Neurosci. 2017;29:755–768. doi: 10.1162/jocn_a_01077. [DOI] [PubMed] [Google Scholar]
- 49.Pilloni G, Bikson M, Badran BW, George MS, Kautz SA, Okano AH, et al. Update on the use of transcranial electrical brain stimulation to manage acute and chronic COVID-19 symptoms. Front Hum Neu rosci. 2020;14:595567. doi: 10.3389/fnhum.2020.595567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pilloni G, Choi C, Coghe G, Cocco E, Krupp LB, Pau M, et al. Gait and functional mobility in multiple sclerosis: immediate effects of transcranial direct current stimulation (tDCS) paired with aerobic exercise. Front Neurol. 2020;11:310. doi: 10.3389/fneur.2020.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alvarez-Alvarado S, Boutzoukas EM, Kraft JN, O’Shea A, Indahlastari A, Albizu A, et al. Impact of transcranial direct current stimulation and cognitive training on frontal lobe neurotransmitter concentrations. Front Aging Neurosci. 2021;13:761348. doi: 10.3389/fnagi.2021.761348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Farnad L, Ghasemian-Shirvan E, Mosayebi-Samani M, Kuo MF, Nitsche MA. Exploring and optimizing the neuroplastic effects of anodal transcranial direct current stimulation over the primary motor cortex of older humans. Brain Stimul. 2021;14:622–634. doi: 10.1016/j.brs.2021.03.013. [DOI] [PubMed] [Google Scholar]
- 53.Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- 54.Pascual-Leone A, Taylor MJ. A developmental framework of brain and cognition from infancy to old age. Brain Topogr. 2011;24:183–186. doi: 10.1007/s10548-011-0197-7. [DOI] [PubMed] [Google Scholar]
- 55.Habich A, Fehér KD, Antonenko D, Boraxbekk CJ, Flöel A, Nissen C, et al. Stimulating aged brains with transcranial direct current stimulation: opportunities and challenges. Psychiatry Res Neuroim aging. 2020;306:111179. doi: 10.1016/j.pscychresns.2020.111179. [DOI] [PubMed] [Google Scholar]
- 56.Antonenko D, Nierhaus T, Meinzer M, Prehn K, Thielscher A, Ittermann B, et al. Age-dependent effects of brain stimulation on network centrality. Neuroimage. 2018;176:71–82. doi: 10.1016/j.neuroimage.2018.04.038. [DOI] [PubMed] [Google Scholar]
- 57.Ghasemian-Shirvan E, Mosayebi-Samani M, Farnad L, Kuo MF, Meesen RLJ, Nitsche MA. Age-dependent non-linear neuroplastic effects of cathodal tDCS in the elderly population: a titration study. Brain Stimul. 2022;15:296–305. doi: 10.1016/j.brs.2022.01.011. [DOI] [PubMed] [Google Scholar]
- 58.Indahlastari A, Albizu A, Boutzoukas EM, O’Shea A, Woods AJ. White matter hyperintensities affect transcranial electrical stimulation in the aging brain. Brain Stimul. 2021;14:69–73. doi: 10.1016/j.brs.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hanley CJ, Alderman SL, Clemence E. Optimising cognitive enhancement: systematic assessment of the effects of tDCS duration in older adults. Brain Sci. 2020;10:304. doi: 10.3390/brainsci10050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Laakso I, Tanaka S, Koyama S, De Santis V, Hirata A. Inter-subject variability in electric fields of motor cortical tDCS. Brain Stimul. 2015;8:906–913. doi: 10.1016/j.brs.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 61.Krause B, Cohen Kadosh R. Not all brains are created equal: the relevance of individual differences in responsiveness to transcranial electrical stimulation. Front Syst Neurosci. 2014;8:25. doi: 10.3389/fnsys.2014.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Choe J, Coffman BA, Bergstedt DT, Ziegler MD, Phillips ME. Transcranial direct current stimulation modulates neuronal activity and learning in pilot training. Front Hum Neurosci. 2016;10:34. doi: 10.3389/fnhum.2016.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bikson M, Name A, Rahman A. Origins of specificity during tDCS: anatomical, activity-selective, and input-bias mechanisms. Front Hum Neurosci. 2013;7:688. doi: 10.3389/fnhum.2013.00688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gomez Palacio Schjetnan A, Faraji J, Metz GA, Tatsuno M, Luczak A. Transcranial direct current stimulation in stroke rehabilitation: a review of recent advancements. Stroke Res Treat. 2013;2013:170256. doi: 10.1155/2013/170256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brasil-Neto JP. Learning, memory, and transcranial direct current stimulation. Front Psychiatry. 2012;3:80. doi: 10.3389/fpsyt.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boggio PS, Ferrucci R, Rigonatti SP, Covre P, Nitsche M, Pascual-Leone A, et al. Effects of transcranial direct current stimulation on working memory in patients with Parkinson’s disease. J Neurol Sci. 2006;249:31–38. doi: 10.1016/j.jns.2006.05.062. [DOI] [PubMed] [Google Scholar]
- 67.Richmond LL, Wolk D, Chein J, Olson IR. Transcranial direct current stimulation enhances verbal working memory training performance over time and near transfer outcomes. J Cogn Neurosci. 2014;26:2443–2454. doi: 10.1162/jocn_a_00657. [DOI] [PubMed] [Google Scholar]
- 68.Segrave RA, Arnold S, Hoy K, Fitzgerald PB. Concurrent cognitive control training augments the antidepressant efficacy of tDCS: a pilot study. Brain Stimul. 2014;7:325–331. doi: 10.1016/j.brs.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 69.Andrews SC, Hoy KE, Enticott PG, Daskalakis ZJ, Fitzgerald PB. Improving working memory: the effect of combining cognitive activity and anodal transcranial direct current stimulation to the left dorsolateral prefrontal cortex. Brain Stimul. 2011;4:84–89. doi: 10.1016/j.brs.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 70.Waters-Metenier S, Husain M, Wiestler T, Diedrichsen J. Bihemispheric transcranial direct current stimulation enhances effector-independent representations of motor synergy and sequence learning. J Neurosci. 2014;34:1037–1050. doi: 10.1523/JNEUROSCI.2282-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saucedo Marquez CM, Zhang X, Swinnen SP, Meesen R, Wenderoth N. Task-specific effect of transcranial direct current stimulation on motor learning. Front Hum Neurosci. 2013;7:333. doi: 10.3389/fnhum.2013.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reis J, Fischer JT, Prichard G, Weiller C, Cohen LG, Fritsch B. Time- but not sleep-dependent consolidation of tDCS-enhanced visuomotor skills. Cereb Cortex. 2015;25:109–117. doi: 10.1093/cercor/bht208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, et al. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci U S A. 2009;106:1590–1595. doi: 10.1073/pnas.0805413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brunoni AR, Vanderhasselt MA. Working memory improvement with non-invasive brain stimulation of the dorsolateral prefrontal cortex: a systematic review and meta-analysis. Brain Cogn. 2014;86:1–9. doi: 10.1016/j.bandc.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 75.Sarkar A, Dowker A, Cohen Kadosh R. Cognitive enhancement or cognitive cost: trait-specific outcomes of brain stimulation in the case of mathematics anxiety. J Neurosci. 2014;34:16605–16610. doi: 10.1523/JNEUROSCI.3129-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McIntire LK, McKinley RA, Goodyear C, Nelson J. A comparison of the effects of transcranial direct current stimulation and caffeine on vigilance and cognitive performance during extended wakefulness. Brain Stimul. 2014;7:499–507. doi: 10.1016/j.brs.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 77.Nelson JT, McKinley RA, Golob EJ, Warm JS, Parasuraman R. Enhancing vigilance in operators with prefrontal cortex transcranial direct current stimulation (tDCS) Neuroimage. 2014;85 Pt 3:909–917. doi: 10.1016/j.neuroimage.2012.11.061. [DOI] [PubMed] [Google Scholar]
- 78.Gill J, Shah-Basak PP, Hamilton R. It’s the thought that counts: examining the task-dependent effects of transcranial direct current stimulation on executive function. Brain Stimul. 2015;8:253–259. doi: 10.1016/j.brs.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 79.Chu CS, Li CT, Brunoni AR, Yang FC, Tseng PT, Tu YK, et al. Cognitive effects and acceptability of non-invasive brain stimulation on Alzheimer’s disease and mild cognitive impairment: a component network meta-analysis. J Neurol Neurosurg Psychiatry. 2021;92:195–203. doi: 10.1136/jnnp-2020-323870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Khedr EM, Salama RH, Abdel Hameed M, Abo Elfetoh N, Seif P. Therapeutic role of transcranial direct current stimulation in Alzheimer disease patients: double-blind, placebo-controlled clinical trial. Neurorehabil Neural Repair. 2019;33:384–394. doi: 10.1177/1545968319840285. [DOI] [PubMed] [Google Scholar]
- 81.Yun K, Song IU, Chung YA. Changes in cerebral glucose metabolism after 3 weeks of noninvasive electrical stimulation of mild cognitive impairment patients. Alzheimers Res Ther. 2016;8:49. doi: 10.1186/s13195-016-0218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.He F, Li Y, Li C, Fan L, Liu T, Wang J. Repeated anodal high-definition transcranial direct current stimulation over the left dorsolateral prefrontal cortex in mild cognitive impairment patients increased regional homogeneity in multiple brain regions. PLoS One. 2021;16:e0256100. doi: 10.1371/journal.pone.0256100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rasmussen ID, Boayue NM, Mittner M, Bystad M, Grønli OK, Vangberg TR, et al. High-definition transcranial direct current stimulation improves delayed memory in Alzheimer’s disease patients: a pilot study using computational modeling to optimize electrode position. J Alzheimers Dis. 2021;83:753–769. doi: 10.3233/JAD-210378. [DOI] [PubMed] [Google Scholar]
- 84.Khedr EM, Gamal NF, El-Fetoh NA, Khalifa H, Ahmed EM, Ali AM, et al. A double-blind randomized clinical trial on the efficacy of cortical direct current stimulation for the treatment of Alzheimer’s disease. Front Aging Neurosci. 2014;6:275. doi: 10.3389/fnagi.2014.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Medeiros LF, de Souza IC, Vidor LP, de Souza A, Deitos A, Volz MS, et al. Neurobiological effects of transcranial direct current stimulation: a review. Front Psychiatry. 2012;3:110. doi: 10.3389/fpsyt.2012.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fukai M, Bunai T, Hirosawa T, Kikuchi M, Ito S, Minabe Y, et al. Endogenous dopamine release under transcranial direct-current stimulation governs enhanced attention: a study with positron emission tomography. Transl Psychiatry. 2019;9:115. doi: 10.1038/s41398-019-0443-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brunoni AR, Kemp AH, Shiozawa P, Cordeiro Q, Valiengo LC, Goulart AC, et al. Impact of 5-HTTLPR and BDNF polymorphisms on response to sertraline versus transcranial direct current stimulation: implications for the serotonergic system. Eur Neuropsychopharma col. 2013;23:1530–1540. doi: 10.1016/j.euroneuro.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 88.Cambiaghi M, Buffelli M, Masin L, Valtorta F, Comai S. Transcranial direct current stimulation of the mouse prefrontal cortex modulates serotonergic neural activity of the dorsal raphe nucleus. Brain Stimul. 2020;13:548–550. doi: 10.1016/j.brs.2020.01.012. [DOI] [PubMed] [Google Scholar]
- 89.Newman EL, Gupta K, Climer JR, Monaghan CK, Hasselmo ME. Cholinergic modulation of cognitive processing: insights drawn from computational models. Front Behav Neurosci. 2012;6:24. doi: 10.3389/fnbeh.2012.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hansen N. Action mechanisms of transcranial direct current stimulation in Alzheimer’s disease and memory loss. Front Psychiatry. 2012;3:48. doi: 10.3389/fpsyt.2012.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hasselmo ME, Barkai E. Cholinergic modulation of activity-dependent synaptic plasticity in the piriform cortex and associative memory function in a network biophysical simulation. J Neurosci. 1995;15:6592–6604. doi: 10.1523/JNEUROSCI.15-10-06592.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Patil MM, Linster C, Lubenov E, Hasselmo ME. Cholinergic agonist carbachol enables associative long-term potentiation in piriform cortex slices. J Neurophysiol. 1998;80:2467–2474. doi: 10.1152/jn.1998.80.5.2467. [DOI] [PubMed] [Google Scholar]
- 93.Kuo MF, Grosch J, Fregni F, Paulus W, Nitsche MA. Focusing effect of acetylcholine on neuroplasticity in the human motor cortex. J Neu rosci. 2007;27:14442–14447. doi: 10.1523/JNEUROSCI.4104-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thirugnanasambandam N, Grundey J, Adam K, Drees A, Skwirba AC, Lang N, et al. Nicotinergic impact on focal and non-focal neuroplasticity induced by non-invasive brain stimulation in non-smoking humans. Neuropsychopharmacology. 2011;36:879–886. doi: 10.1038/npp.2010.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lengu K, Ryan S, Peltier SJ, Tyszkowski T, Kairys A, Giordani B, et al. Effects of high definition-transcranial direct current stimulation on local GABA and glutamate levels among older adults with and without mild cognitive impairment: an exploratory study. J Alzheim ers Dis. 2021;84:1091–1102. doi: 10.3233/JAD-201091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cruz Gonzalez P, Fong KNK, Chung RCK, Ting KH, Law LLF, Brown T. Can transcranial direct-current stimulation alone or combined with cognitive training be used as a clinical intervention to improve cognitive functioning in persons with mild cognitive impairment and dementia? A systematic review and meta-analysis. Front Hum Neurosci. 2018;12:416. doi: 10.3389/fnhum.2018.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu CS, Rau A, Gallagher D, Rajji TK, Lanctôt KL, Herrmann N. Using transcranial direct current stimulation to treat symptoms in mild cognitive impairment and Alzheimer’s disease. Neurodegener Dis Manag. 2017;7:317–329. doi: 10.2217/nmt-2017-0021. [DOI] [PubMed] [Google Scholar]
- 98.Rajji TK. Transcranial magnetic and electrical stimulation in Alzheimer’s disease and mild cognitive impairment: a review of randomized controlled trials. Clin Pharmacol Ther. 2019;106:776–780. doi: 10.1002/cpt.1574. [DOI] [PubMed] [Google Scholar]
- 99.Nardone R, Bergmann J, Christova M, Caleri F, Tezzon F, Ladurner G, et al. Effect of transcranial brain stimulation for the treatment of Alzheimer disease: a review. Int J Alzheimers Dis. 2012;2012:687909. doi: 10.1155/2012/687909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kasschau M, Sherman K, Haider L, Frontario A, Shaw M, Datta A, et al. A protocol for the use of remotely-supervised transcranial direct current stimulation (tDCS) in multiple sclerosis (MS) J Vis Exp. 2015;106:e53542. doi: 10.3791/53542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shaw MT, Best P, Frontario A, Charvet LE. Telerehabilitation benefits patients with multiple sclerosis in an urban setting. J Telemed Telecare. 2021;27:39–45. doi: 10.1177/1357633X19861830. [DOI] [PubMed] [Google Scholar]
- 102.Agarwal S, Pawlak N, Cucca A, Sharma K, Dobbs B, Shaw M, et al. Remotely-supervised transcranial direct current stimulation paired with cognitive training in Parkinson’s disease: an open-label study. J Clin Neurosci. 2018;57:51–57. doi: 10.1016/j.jocn.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 103.Clayton AM, Howard J, Dobbs B, Shaw MT, Charvet LE. Remotely supervised transcranial direct current stimulation after ECT improves mood and cognition in a patient with multiple sclerosis: a case study. J ECT. 2018;34:e15. doi: 10.1097/YCT.0000000000000474. [DOI] [PubMed] [Google Scholar]
- 104.Eilam-Stock T, George A, Charvet LE. Cognitive telerehabilitation with transcranial direct current stimulation improves cognitive and emotional functioning following a traumatic brain injury: a case study. Arch Clin Neuropsychol. 2021;36:442–453. doi: 10.1093/arclin/acaa059. [DOI] [PubMed] [Google Scholar]
- 105.Charvet LE, Kasschau M, Datta A, Knotkova H, Stevens MC, Alonzo A, et al. Remotely-supervised transcranial direct current stimulation (tDCS) for clinical trials: guidelines for technology and protocols. Front Syst Neurosci. 2015;9:26. doi: 10.3389/fnsys.2015.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.NYU Langone Health. Assessment of TDCS-induced neuronal responses in multiple sclerosis (MS) with advanced MRI [Internet] [cited 2020 Sep 28]. ClinicalTrials.gov identifier: NCT03564496. Available from: https://clinicaltrials.gov/ct2/show/NCT03564496 .
- 107.NYU Langone Health. A pilot trial of remotely-supervised transcranial direct current stimulation (RS-TDCS) to enhance motor learning in progressive multiple sclerosis (MS) [Internet] [cited 2020 Sep 28]. ClinicalTrials.gov identifier: NCT03499314. Available from: https://clinicaltrials.gov/ct2/show/NCT03499314 .
- 108.NYU Langone Health. tDCS for the management of multiple sclerosis related fatigue [Internet] [cited 2020 Sep 29]. ClinicalTrials.gov identifier: NCT03838770. Available from: https://clinicaltrials.gov/ct2/show/NCT03838770 .
- 109.VA Office of Research and Development. Remotely supervised transcranial direct current stimulation for persistent post-traumatic headache [Internet] [cited 2020 Sep 30]. ClinicalTrials.gov identifier: NCT04012853. Available from: https://clinicaltrials.gov/ct2/show/NCT04012853 .
- 110.Gomes MA, Akiba HT, Gomes JS, Trevizol AP, de Lacerda ALT, Dias ÁM. Transcranial direct current stimulation (tDCS) in elderly with mild cognitive impairment: a pilot study. Dement Neuropsychol. 2019;13:187–195. doi: 10.1590/1980-57642018dn13-020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the study.


