Dear Editor,
Myelin oligodendrocyte glycoprotein (MOG) antibody-associated disease (MOGAD) is an idiopathic inflammatory disease of the central nervous system. Its various presentations include optic neuritis (ON), myelitis, acute disseminated encephalomyelitis (ADEM), and brainstem syndrome.1 Pediatric MOGAD is often monophasic, but up to 53% of patients are at risk of relapses and potential neurological disabilities.1 The EU pediatric MOG consortium consensus currently suggests four first-line maintenance treatments: azathioprine, mycophenolate mofetil (MMF), rituximab, and intravenous immunoglobulin (IVIG).2 However, these monotherapies are only partially effective, with relapses reportedly seen in 20%–75% of patients.2 We report a case of pediatric MOGAD that was refractory to several immunotherapies, wherein relapse was successfully prevented with a combination of IVIG and MMF.
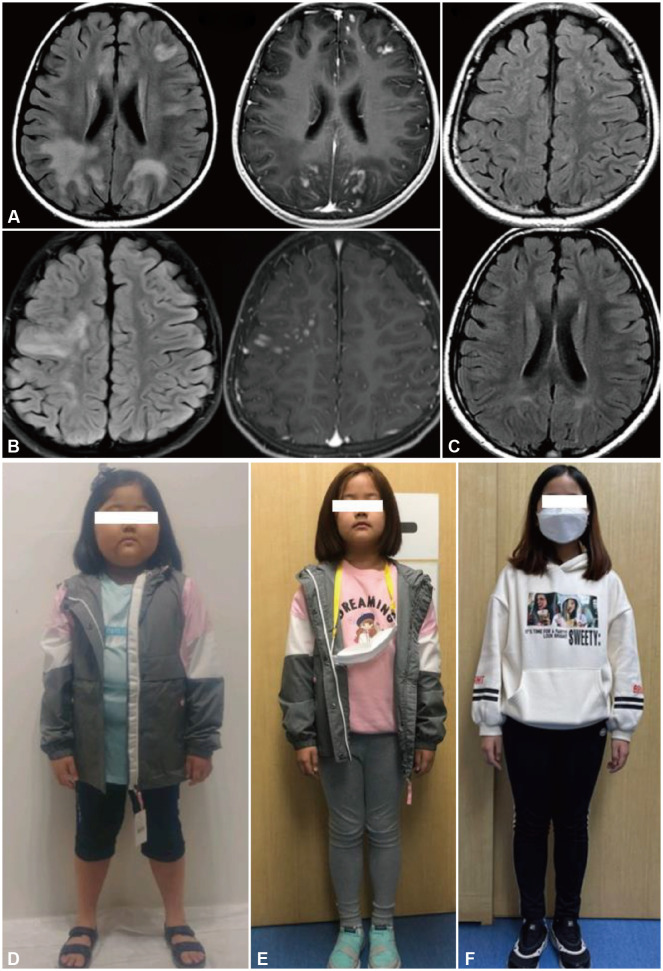
The patient was an 8-year-old female with MOGAD who had experienced six relapses over 2 years despite receiving treatment with several immunotherapies. She presented with acute left-sided ON in September 2017 at the age of 6 years, when she was 120 cm tall and weighed 21 kg. A cerebrospinal fluid examination revealed pleocytosis (white blood cells at 24 cells/mm3) and negative oligoclonal bands. At nadir, there was no light perception in the left eye; however, her visual acuity recovered following intravenous methylprednisolone therapy (IVMP) (1 g/day for 5 days). In December 2017 she experienced a relapse of ADEM, presenting with fever and lethargy. Multifocal, hyperintense, subcortical, and periventricular white-matter lesions with contrast enhancement were observed in brain magnetic resonance imaging (MRI) (Fig. 1A). Spine MRI showed a short-segment T2-weighted hyperintense lesion without enhancement at the C3 level. Multiple sclerosis was diagnosed, and interferon beta-1a was initiated after IVMP. In March 2018, right-sided ON occurred after oral prednisolone had been tapered out, and so interferon beta-1a was replaced with MMF (750 mg/day). However, right ON recurred 3 months later, and so MMF was replaced with interferon beta-1b. In February 2019 (4 months after tapering out steroids) she experienced an ADEM relapse, presenting with fever, seizures, and extensive brain lesions in the right hemisphere (Fig. 1B). The anti-MOG antibody test was positive. She fully recovered after IVMP administration for 5 days. Monthly IVIG (0.4 g/kg) and low-dose oral prednisolone were administered as long-term maintenance therapy. The steroid dose was reduced from 15 mg to 7.5 mg after 5 months of IVIG therapy. However, ADEM recurred 3 weeks later, and she recovered after IVMP was reinstated. At the time of her first visit to our center in October 2019, she was on monthly IVIG and oral steroid therapy (15 mg/day). There was no neurological disability, but she had cushingoid features with growth delay due to long-term steroid use (126 cm tall and weighing 42 kg when aged 8 years) (Fig. 1D). Her blood test was positive for anti-MOG antibody (3+) and negative for anti-aquaporin-4 antibody.3 A more-effective steroid-sparing agent was required to prevent relapse, and so MMF (1,000 mg/day) was added to the monthly IVIG therapy (0.4 mg/kg). The oral steroid was tapered out over 2 months. She experienced no relapses and lost the cushingoid features during 2 years of combined IVIG and MMF treatment, after which she was 150 cm tall and weighed 48 kg (Fig. 1F). In follow-up MRI performed 2 years later, most T2-weighted hyperintense lesions had disappeared and there were no new lesions (Fig. 1C). During this time, the semiquantitative grade of anti-MOG antibody staining decreased from 3+ to 1+, but it remained positive.
Fig. 1. Brain magnetic resonance imaging (MRI) findings and photographs of this patient. A: Fluid-attenuated inversion recovery (FLAIR) MRI at the first acute disseminated encephalomyelitis attack revealed multifocal hyperintense subcortical and periventricular white-matter lesions, and a gadolinium-enhanced T1-weighted image showed corresponding enhancement of the lesion. B: Similar FLAIR-hyperintense lesions were observed in the right hemisphere during the fifth episode. C: In follow-up MRI performed 2 years after initiating combination therapy with intravenous immunoglobulin (IVIG) and mycophenolate mofetil (MMF), most of the FLAIR-hyperintense lesions disappeared, and no new lesions were found. D-F: Photographs of the patient at the initial visit in October 2019 (D) and after 1 year (E) and 2 years (F) of IVIG and MMF combination therapy.
There is no consensus regarding escalation strategies for pediatric patients with relapsing MOGAD that is refractory to the conventional immunotherapies. Escalation with rituximab or IVIG has been recommended in cases of further relapses despite receiving azathioprine or MMF.2 However, rituximab appears less effective for MOGAD, with relapse occurring in 67% of patients.2 Although maintenance IVIG appears promising for refractory relapsing patients,4,5 this has only been reported in a few patients, and our patient experienced relapse despite receiving IVIG when the steroid dose was tapered. Chronic steroid therapy in children should be avoided since they are more vulnerable to the adverse effects of long-term steroids, which can affect growth and immunity and cause adrenal suppression.6 Since combination therapies targeting different pathways exert a synergistic effect, a combination of immunotherapies might be necessary for patients who do not respond to monotherapy. An in vitro study documented an additive effect of IVIG and mycophenolic acid on the inhibition of cell proliferation in mixed lymphocyte reactions.7 The effectiveness of combined treatment with IVIG and MMF was also reported in myositis refractory to other immunotherapies.8 In the present case, a combination of IVIG and MMF was effective in preventing relapse for 2 years, and steroid therapy was successfully discontinued.
The combination of IVIG and MMF could be a well-tolerated and effective steroid-sparing treatment option for pediatric patients with relapsing MOGAD that is refractory to the conventional therapeutic regimens. Further studies are necessary to determine the optimal maintenance treatment in relapsing pediatric patients with MOGAD.
Footnotes
Ethics Statement: The study was approved by the local ethics committee (NCC2022-0007). The patient and her parents provided written informed consent.
- Conceptualization: Su-Hyun Kim.
- Data curation: Ki Hoon Kim, Su-Hyun Kim.
- Formal analysis: Ki Hoon Kim.
- Investigation: all authors.
- Methodology: Su-Hyun Kim.
- Resources: Su-Hyun Kim, Ho Jin Kim.
- Supervision: Ho Jin Kim, Su-Hyun Kim.
- Validation: Su-Hyun Kim.
- Visualization: Ki Hoon Kim.
- Writing—original draft: Ki Hoon Kim, Su-Hyun Kim.
- Writing—review & editing: all authors.
Conflicts of Interest: Ho Jin Kim received a grant from the National Research Foundation of Korea and research support from Aprilbio and Eisai; received consultancy/speaker fees from Alexion, Aprilbio, Biogen, Celltrion, Daewoong, Eisai, GC Pharma, HanAll BioPharma, MDimune, Merck Serono, Novartis, Roche, Sanofi Genzyme, Teva-Handok, UCB, and Viela Bio; is a co-editor for the Multiple Sclerosis Journal and an associated editor for the Journal of Clinical Neurology (he was not involved in the editorial evaluation or decision to publish this article). All remaining authors have declared no conflicts of interest.
Funding Statement: The measurement of MOG and anquaporin-4 antibody in this study was supported by National Research Foundation of Korea (2018R1A5A2023127) (Dr. Kim HJ).
Availability of Data and Material
Data sharing not applicable to this article as no datasets were generated or analyzed during the study.
References
- 1.Cobo-Calvo A, Ruiz A, Rollot F, Arrambide G, Deschamps R, Maillart E, et al. Clinical features and risk of relapse in children and adults with myelin oligodendrocyte glycoprotein antibody-associated disease. Ann Neurol. 2021;89:30–41. doi: 10.1002/ana.25909. [DOI] [PubMed] [Google Scholar]
- 2.Bruijstens AL, Wendel EM, Lechner C, Bartels F, Finke C, Breu M, et al. E.U. paediatric MOG consortium consensus: part 5 - Treatment of paediatric myelin oligodendrocyte glycoprotein antibody-associated disorders. Eur J Paediatr Neurol. 2020;29:41–53. doi: 10.1016/j.ejpn.2020.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Kim Y, Hyun JW, Woodhall MR, Oh YM, Lee JE, Jung JY, et al. Refining cell-based assay to detect MOG-IgG in patients with central nervous system inflammatory diseases. Mult Scler Relat Disord. 2020;40:101939. doi: 10.1016/j.msard.2020.101939. [DOI] [PubMed] [Google Scholar]
- 4.Hacohen Y, Wong YY, Lechner C, Jurynczyk M, Wright S, Konuskan B, et al. Disease course and treatment responses in children with relapsing myelin oligodendrocyte glycoprotein antibody-associated disease. JAMA Neurol. 2018;75:478–487. doi: 10.1001/jamaneurol.2017.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen JJ, Flanagan EP, Bhatti MT, Jitprapaikulsan J, Dubey D, Lopez Chiriboga ASS, et al. Steroid-sparing maintenance immunotherapy for MOG-IgG associated disorder. Neurology. 2020;95:e111–e120. doi: 10.1212/WNL.0000000000009758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deshmukh CT. Minimizing side effects of systemic corticosteroids in children. Indian J Dermatol Venereol Leprol. 2007;73:218–221. doi: 10.4103/0378-6323.33633. [DOI] [PubMed] [Google Scholar]
- 7.Sharma KG, Radha R, Pao A, Amet N, Baden L, Jordan SC, et al. Mycophenolic acid and intravenous immunoglobulin exert an additive effect on cell proliferation and apoptosis in the mixed lymphocyte reaction. Transpl Immunol. 2010;23:117–120. doi: 10.1016/j.trim.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Danieli MG, Calcabrini L, Calabrese V, Marchetti A, Logullo F, Gabrielli A. Intravenous immunoglobulin as add on treatment with mycophenolate mofetil in severe myositis. Autoimmun Rev. 2009;9:124–127. doi: 10.1016/j.autrev.2009.04.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the study.