Abstract
Purpose
To comprehensively evaluate the effect on angiogenesis of heme-binding protein 1 (Hebp1) in the treatment of diabetes-induced erectile dysfunction.
Materials and Methods
Mouse corpus cavernosum endothelial cells and pericytes were used for in vitro study. Four groups of mice were used: control nondiabetic mice and streptozotocin-induced diabetic mice receiving two intracavernous injections of phosphate-buffered saline, Hebp1 (1 µg), or Hebp1 (5 µg). The function of Hebp1 in diabetic conditions was evaluated by tube formation assay, aorta ring assay, migration assay, intracavernous pressure, immunofluorescence staining, and Western blot experiments.
Results
We report that Hebp1 is more highly expressed in mouse corpus cavernosum pericytes and can effectively promote endothelial cell angiogenesis under high-glucose conditions. Following exogenous administration of Hebp1 protein, we found that elevated Hebp1 levels can improve the erectile function of diabetic mice, which is achieved by reducing reactive oxygen species levels and activating the PI3K/AKT/eNOS signaling pathway.
Conclusions
Our findings demonstrate that Hebp1 can promote angiogenesis and improve erectile function under diabetic conditions.
Keywords: Diabetes mellitus, Erectile dysfunction, Heme-binding proteins, Pericytes
Graphical Abstract
INTRODUCTION
Erectile dysfunction (ED) is a vascular disease that affects up to 75% of men with diabetes globally [1]. Oral phosphodiesterase 5 inhibitors are most commonly used in the treatment of ED; however, because of the insufficient bioavailability of endogenous nitric oxide as the result of severe vascular disease and neuropathy, the efficacy of these drugs is reduced in men with diabetes [2]. Recently, various gene expression profiling studies, such as RNA-sequencing analysis in mouse corpus cavernosum endothelial cells (MCECs) and microarray analysis of penile tissue and corpus cavernosum pericytes, have provided an unprecedented database for us to use to develop therapeutic targets for ED, but these studies are only in the initial stages [3,4,5]. Therefore, it is necessary to evaluate more novel candidates and determine their role in promoting neurovascular regeneration in diabetic ED.
Pericytes surround endothelial cells and play an important role in vasoconstriction and stabilization [6]. As reservoirs of mesenchymal stem cells, they can differentiate into cells with completely different phenotypes and functions, such as vascular smooth muscle cells, endothelial cells, fibroblasts, osteoblasts, and chondrocytes [7]. Through previous screening of physiological and pathological gene expression profiles [5], we found that heme-binding protein 1 (Hebp1) may be a potential new therapeutic candidate for diabetesinduced ED. Hebp1, an intracellular tetrapyrrole-binding protein, is expressed in various tissues [8]. The processing of Hebp1 by cathepsin D produces a peptide called F2L that inhibits formyl peptide receptor- and formyl peptide receptor-like 1-induced signaling and blocks superoxide generation in human neutrophils [9]. However, the biological function of Hebp1 remains unclear. Superoxide is the key reactive oxygen species (ROS) involved in the pathophysiological mechanisms of ED [10]. Therefore, we hypothesized that Hebp1 may play an important role in pathophysiological conditions such as diabetic ED.
In this study, we have, for the first time, assessed Hebp1 expression in primary cultured MCECs and primary cultured mouse corpus cavernosum pericytes (MCPs) under normal and high-glucose (HG) conditions. We also demonstrated that pericyte-derived Hebp1 induces endothelial cell angiogenesis under HG conditions and that exogenous recombinant mouse Hebp1 protein can reduce ROS levels and activate the PI3K/AKT/eNOS signaling pathway, thereby improving erectile function in a diabetic ED mouse model.
MATERIALS AND METHODS
1. Ethics statement and study design
All experimental animal studies were approved by the Institutional Animal Care and Use Committee of Inha University (approval no. INHA 200309-691). The aim of the present study was to evaluate the effect of Hebp1 in alleviating diabetes-induced ED. We administered Hebp1 protein in the penises of diabetic mice and evaluated detailed mechanisms in mice with streptozotocin (STZ)-induced diabetes and age-matched controls as well as primary cultures of MCECs and MCPs. All parameters of genetically modified mice and diabetic mice were compared with those of littermate controls.
2. Animals and treatment
In total, 65 adult male C57BL/6J mice (8 weeks old) were used in this study (10 for MCEC culture; 10 for MCP culture; 5 for ex vivo mouse aortic ring assay; 20 for in vivo erectile function and western blot study; and 20 for histological examinations). Diabetes was induced in 8-week-old C57BL/6J mice by intraperitoneal injections of STZ (50 mg/kg) for 5 consecutive days, as described previously [11]. Four randomly distributed groups of mice were studied: control nondiabetic mice and mice with STZ-induced diabetes that received two successive intracavernous injections of phosphate-buffered saline (PBS, 20 µL, days -3 and 0) or recombinant mouse Hebp1 (days -3 and 0; 1 µg or 5 µg in 20 µL PBS; Mybiosource Inc., San Diego, CA, USA) into the midportion of the corpus cavernosum (n=5 per group). We compressed the penis with a vascular clamp at the base immediately before injection and then used a 30-gauge insulin syringe for intracavernous injection. After the injection, the clamp was left in place for 30 minutes to restrict blood flow out of the penis. Fasting and postprandial blood glucose levels were determined with an Accu-Check blood glucose meter (Roche Diagnostics, Mannheim, Baden-Württemberg, Germany) before the erectile function test. After erectile function was assessed, only the penis tissues of the mice were harvested for further studies. No mice were found dead during any of the experimental procedures.
3. Cell culture and treatment
The MCECs were prepared and maintained as previously described [12]. After the cells were confluent and spread over the bottom of the dish, only sprouting cells were used for subculture. The primary culture of MCPs was performed as previously described [13]. All cells from passages 2 to 3 were used in the experiments.
For glucose condition studies, the MCECs or MCPs were serum-starved for 24 hours and then exposed to normal glucose (NG, 5 mM glucose, Sigma-Aldrich) or HG (30 mM glucose) conditions for 3 days at 37℃ in a humidified 5% CO2 atmosphere [14].
4. Tube formation assay
Tube formation assays were performed as described previously [12,15]. Tube formation was monitored for 18 hours under a phase-contrast microscope (CKX41, Olympus, Tokyo, Japan), and the number of master junctions from seven separate experiments was determined by using ImageJ software (National Institutes of Health, Montgomery, MD, USA).
5. Aortic ring assay
Aortic ring assays were performed as described previously [15]. Explants from normal adult male C57BL/6J mice (8 weeks old) were cultured in complement M199 for 5 days in NG (5 mM) or HG (30 mM) medium, PBS, or Hebp1 (200 ng/mL). The images of aortic segments and sprouting cells were captured using a phase-contrast microscope (Olympus).
6. Cell migration assay
To create uniform scratches during cell migration, the pretreated MCECs were seeded into the SPLScar™Block system (SPL Life Sciences, Pocheon, Korea) at >95% confluence in 60-mm culture dishes as described previously [15]. Images were captured using a phase-contrast microscope (Olympus).
7. shRNA delivery by lentiviral infection
For MCP lentiviral infection, lentiviral small hairpin RNA (shRNA) scramble control (shCon) particles (Origene Technologies Inc., Rockville, MD, USA) or Hebp1-mouse shRNA lentiviral particles (4 unique 29mer target-specific shRNA, Origene Technologies Inc.) were added to the culture medium at the indicated doses. The original lentiviral stock was 1×107 TU/mL. The sequences of the shRNAs targeting mouse Hebp1 were as follows: TL513407VC, CCGTAACGAGGTCTGGCTTGTGAAGGCAT; TL513407VD, ATGCCAAGGAAGCAGACTATGTTGCTCAT; TL513407VA, TGTCTCCTATGAGGAAAGAGCCTGTGAAG; and TL513407VB, GGTGGCACCAATGACAAAGGAGTCGGCAT. Experiments were performed 3 days after lentivirus infection.
8. Measurement of erectile function
Erectile function was measured as previously described [16]. The maximal intracavernous pressure (ICP) and the area under the curve (total ICP) were recorded during tumescence. Mean systemic blood pressure (MSBP) was measured using a tail-cuff blood pressure system (Visitech Systems, Apex, NC, USA).
9. Histological examinations
For immunofluorescence microscopy, frozen tissue sections (12-µm thick) were incubated with antibodies against the following primary antibodies: NG2 (1:100, Millipore, Temecula, CA, USA), PECAM-1 (1:100, Millipore), nitrotyrosine (1:100, Sigma-Aldrich), or βIII tubulin (1:100, Abcam, Cambridge, MA, USA) at 4℃ overnight. After several washes with PBS, the samples were incubated with tetramethyl rhodamine isothiocyanate- or fluorescein isothiocyanate-conjugated secondary antibodies (1:100, Zymed Laboratories, South San Francisco, CA, USA) for 2 hours at room temperature. Samples were mounted in a solution containing 4,6-diamidino-2-phenylindole (DAPI, Vector Laboratories Inc., Burlingame, CA, USA) for nuclei staining, and fluorescent signals were visualized using a confocal microscope (K1-Fluo, Nanoscope Systems Inc., Daejeon, Korea). Quantitative analysis of histological examinations was performed using ImageJ software.
10. In situ detection of superoxide anions
Hydroethidine (1:5,000, Molecular Probes, Eugene, OR, USA) is an oxidative fluorescent dye used to assess the level of superoxide anions in situ, as previously described [17]. The number of ethidium bromide fluorescence-positive endothelial cells in four different areas was determined with a screen magnification of ×400.
11. Western blotting
For the immunoblot analyses, equal amounts of protein were electrophoresed on sodium dodecyl sulfate-polyacrylamide gels (12%) and transferred to polyvinylidene fluoride membranes. After blocking with 5% skim milk (BD Biosciences, Franklin Lakes, NJ, USA) for 1 hour at room temperature, the membranes were probed with antibodies against p-PI3K (Tyr458, 1:1,000, Cell Signaling, Beverly, MA, USA), PI3K (1:1,000, Cell Signaling), p-AKT (Ser473, 1:1,000, Cell Signaling), AKT (1:1,000, Cell Signaling), p-eNOS (Ser1177, 1:500, Cell Signaling), eNOS (1:500, Cell Signaling), or β-actin (1:5,000, Cell Signaling). The results were quantified with densitometry using ImageJ software.
12. Statistical analysis
The results are expressed as the mean±standard error of the mean of values from four or more independent experiments. Statistical analyses were performed using one-way ANOVA followed by Tukey’s post-hoc tests using GraphPad Prism version 8 (GraphPad Software Inc., San Diego, CA, USA). Values of p<0.05 were considered statistically significant; individual p-values are indicated by asterisks in figure legends.
RESULTS
1. Metabolic variables
Fasting and postprandial blood glucose concentrations in mice with STZ-induced diabetes were significantly higher than in control mice. In addition, body weight was significantly lower in mice with STZ-induced diabetes than in controls. However, the body weight and blood glucose levels of the diabetic mice did not differ significantly regardless of treatment (Table 1).
Table 1. Physiological and metabolic parameters at 2 weeks after treatment with heme-binding protein 1 (Hebp1).
| Variable | Control | Streptozotocin-induced diabetic mice | ||
|---|---|---|---|---|
| Phosphate-buffered saline | Hebp1 | |||
| 1 μg | 5 μg | |||
| Body weight (g) | 31.4±0.4 | 24±0.3* | 24.4±0.4* | 23.9±0.3* |
| Fasting glucose (mg/dL) | 102.4±1.8 | 553±18.1* | 563.4±18.2* | 541.2±17.6* |
| Postprandial glucose (mg/dL) | 168.8±12.1 | 598.2±1.8* | 599.8±0.2* | 597.8±2.2* |
| Mean systolic blood pressure (mm Hg) | 102.0±1.6 | 105.8±2.4 | 101.4±2.8 | 102.2±2.4 |
Values are presented as mean±standard error of the mean for n=5 animals per group.
*p<0.05 vs. control group.
2. Hebp1 expression increases in pericytes and decreases under high-glucose conditions
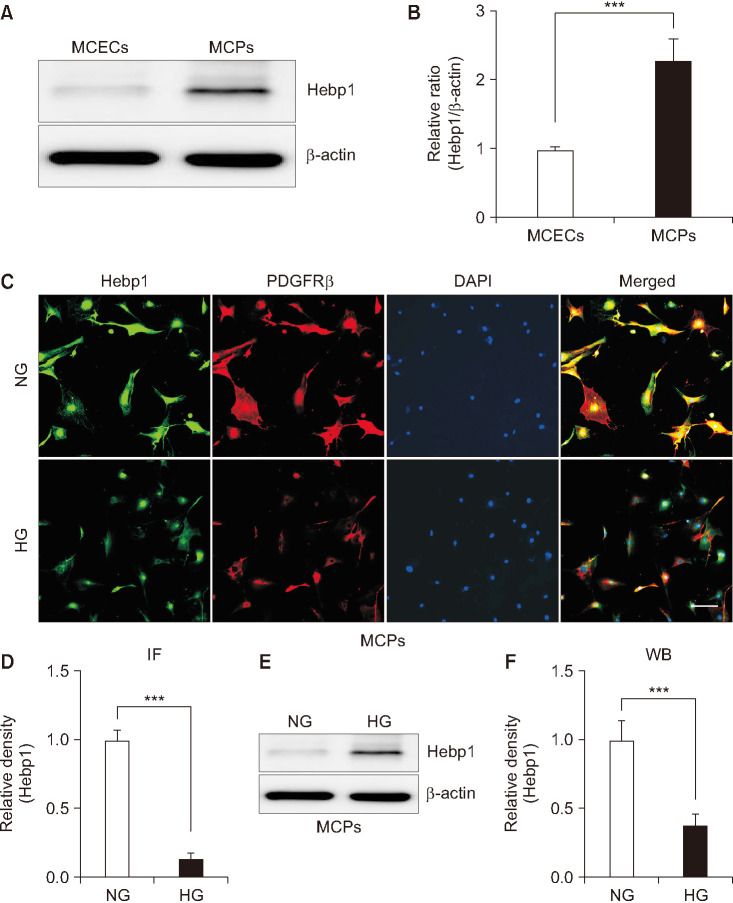
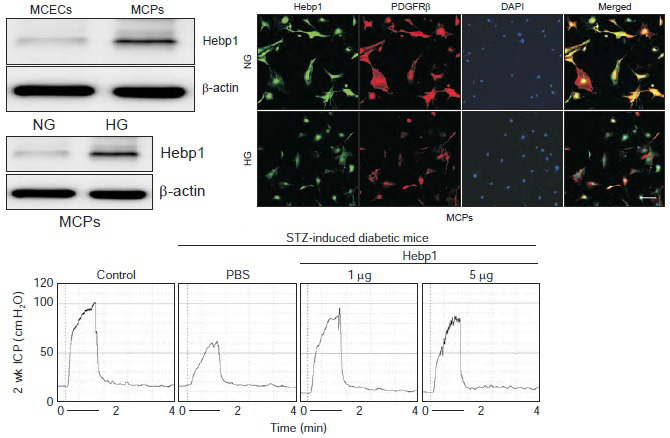
To comprehensively evaluate the effect of Hebp1 in pericytes, we first evaluated the expression of Hebp1 protein in primary cultured MCPs and MCECs and found that the expression of Hebp1 was higher in MCPs than in MCECs (Fig. 1A, B). Next, using immunofluorescent staining (Fig. 1C, D) and western blot analysis (Fig. 1E, F), we found that the expression of Hebp1 in MCPs exposed to HG conditions was significantly lower than that in NG conditions. These results are consistent with our previous study [5], in which we reported that Hebp1 expression is reduced in the corpus cavernosum tissue of diabetic mice and that decreased Hebp1 expression may be related to diabetes-induced pericyte dysfunction.
Fig. 1. Decreased heme-binding protein 1 (Hebp1) expression under high glucose (HG) conditions. (A) Representative western blots for Hebp1 in mouse corpus cavernosum endothelial cells (MCECs) and mouse corpus cavernosum pericytes (MCPs). (B) Normalized band intensity values (n=4) were quantified by use of ImageJ software (n=4). ***p<0.001 vs. MCEC group. The relative ratio of the MCEC group was arbitrarily set to 1. (C) Immunofluorescent staining of MCPs with antibodies against Hebp1 (green) and PDGFRβ (pericyte marker). Nuclei were labeled with the DNA dye DAPI. Scale bar indicates 100 µm. (D) The Hebp1-immunopositive area in PDGFRβ-expressing cells was quantified by use of ImageJ software (n=4). (E) Representative western blots for Hebp1 in MCPs exposed to normal glucose (NG) and HG conditions. (F) Normalized band intensity values were quantified by ImageJ software (n=4). ***p<0.001 vs. NG group. The relative ratio of the NG group was arbitrarily set to 1. IF, immunofluorescent; WB, Western blots.
3. Pericyte-derived Hebp1 induces endothelial cell angiogenesis under high-glucose conditions
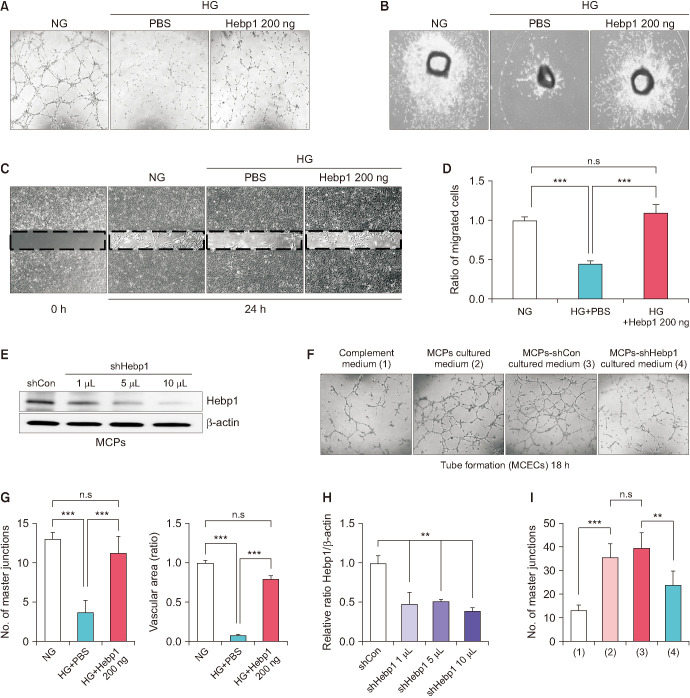
To determine whether Hebp1 can induce angiogenesis under HG conditions, particularly pericyte-derived Hebp1, we first performed tube formation assays (Fig. 2A, G, left), microvessel sprouting aortic ring assays (Fig. 2B, G, right), and migration assays (Fig. 2C, D) using MCECs under NG and HG conditions. We found that well-organized capillary-like structures (as shown in the tube formation assay), microvessel sprouting from aortic rings, and cell migration were severely reduced under HG conditions. However, Hebp1 treatment significantly ameliorated these adverse effects (Fig. 2A-D, G). Next, to assess whether Hebp1 derived from MCPs had a direct effect on MCECs, the MCPs were infected with scrambled shRNA control lentivirus (shCon) and shHebp1 (Hebp1 knockdown) lentivirus (Fig. 2E, H), and the conditioned medium derived from cultured MCPs, such as complement medium (negative control), MCP culture medium, MCP-shCon culture medium, and MCP-shHebp1 culture medium, was collected for the treatment of MCECs. We observed a significant increase in tube formation in MCECs treated with the MCP culture medium and MCP-shCon culture medium compared with that in MCECs treated with the complement medium. However, MCECs treated with the MCP-shHebp1 culture medium showed reduced tube formation compared with those treated with MCP culture medium and MCP-shCon culture medium (Fig. 2F, I). Overall, these results indicate that the exogenous delivery of Hebp1 can induce angiogenesis under HG conditions, and Hebp1 derived from MCPs may also affect the angiogenesis of MCECs.
Fig. 2. Pericyte-derived heme-binding protein 1 (Hebp1) induces angiogenesis under high glucose (HG) conditions. (A-C) Tube formation assays (A), aortic ring assays (B), and migration assays (C) of cells treated with phosphate-buffered saline (PBS) or Hebp1 (200 ng/mL) under normal glucose (NG) and HG conditions. ×40 magnification. (D) Number of migrated endothelial cells was quantified by using ImageJ software (n=4). ***p<0.001 vs. HG group. (E) Representative western blots for Hebp1 in mouse corpus cavernosum pericytes (MCPs) infected with shCon or shHebp1 lentivirus at three doses (1 µL, 1×104 TU; 5 µL, 5×104 TU; 10 µL, 1×105 TU/mL culture medium). (F) Tube formation assay of mouse corpus cavernosum endothelial cells (MCECs) exposed to MCP culture media at indicated conditions. ×40 magnification. (G) The number of master junctions (n=4, left) and intensity of area of microvessels sprouting from aortic rings were quantified by use of ImageJ software (n=4, right). ***p<0.001 vs. HG group. (H) Normalized band intensity values were quantified by use of ImageJ software (n=4). **p<0.01 vs. shCon group. (I) Number of master junctions was quantified by use of ImageJ software (n=4). ***p<0.001 vs. complement medium group, **p<0.01 vs. MCPs-shCon cultured medium group. The relative ratio of the NG, shCon, or complement medium group was arbitrarily set to 1. shCon, small hairpin RNA scramble control; shHebp1, shRNA heme-binding protein 1.
4. Intracavernous injection of Hebp1 improves erectile function in diabetic mice
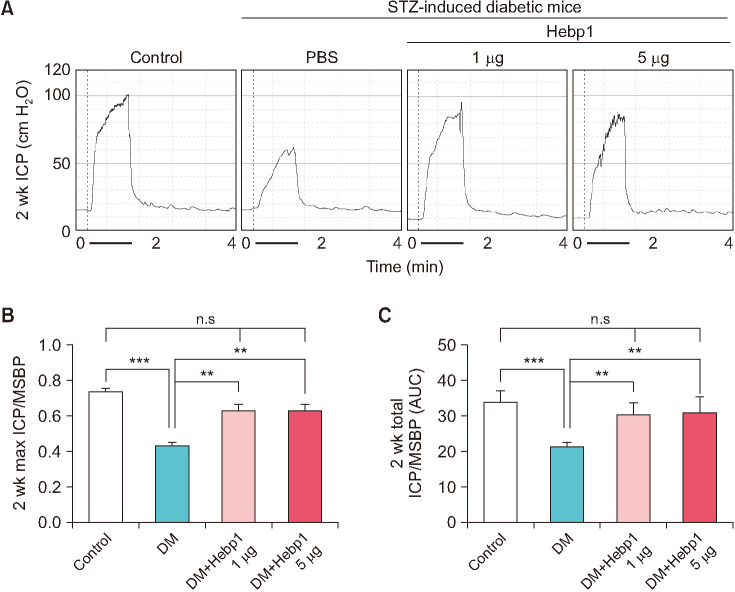
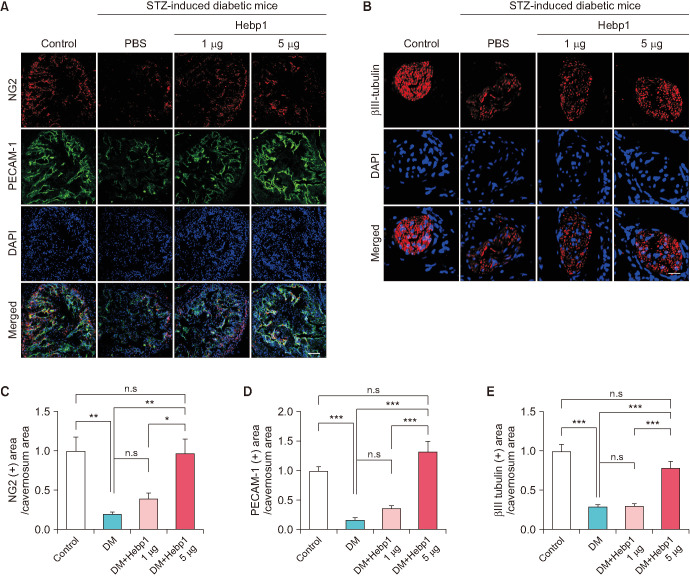
To observe whether Hebp1 has beneficial effects on erectile function in diabetic mice, we observed erectile function 2 weeks after intracavernous injection of PBS or a relative concentration of Hebp1 in age-matched control or diabetic mice (Fig. 3A). During electrical stimulation, the ratios of maximal and total ICP to MSBP were significantly lower in PBS-treated diabetic mice than in age-matched controls. However, after double intracavernous injections of Hebp1, the erection parameters significantly improved in diabetic mice treated with low and high doses of Hebp1 (1 µg/20 µL) and reached up to 94% of control values (Fig. 3). No detectable differences in MSBP were observed between the experimental groups. Immunofluorescence staining for PECAM-1 (an endothelial cell marker), NG2 (a pericyte marker), and βIII-tubulin (a neuronal cell marker) in cavernosum tissue demonstrated that Hebp1 significantly improved the endothelial cell, pericyte, and neuronal cell content in diabetic mice (Fig. 4). These results suggest that Hebp1 rescues MCEC and neuronal cell content, thereby ameliorating ED in diabetic mice.
Fig. 3. Heme-binding protein 1 (Hebp1) improves erectile function under diabetic conditions in mice with streptozotocin (STZ)-induced diabetes. (A) Representative intracavernous pressure (ICP) responses for age-matched control mice and mice with STZ-induced diabetes stimulated at 2 weeks after intracavernous injection of phosphate-buffered saline (PBS) (P; 20 µL) or Hebp1 (1 µg/20 µL and 5 µg/20 µL). The stimulus interval is indicated by a solid bar (1 minute). (B, C) Ratios of mean maximal ICP (B) and total ICP (C) (area under the curve) to mean systemic blood pressure (MSBP) were calculated for each group (n=5, **p<0.01, ***p<0.001). DM, diabetes mellitus; ns, not significant.
Fig. 4. Heme-binding protein 1 (Hebp1) increases corpus cavernosum endothelial cell, pericyte, and neuronal cell content. (A, B) Corpus cavernosum pericyte (A, NG2, red), endothelial cell (A, PECAM-1, green), and nerve (B, βIII-tubulin, red) staining in corpus cavernosum tissue from age-matched control mice and mice with streptozotocin (STZ)-induced diabetes stimulated at 2 weeks after intracavernous injection of PBS (20 µL) or Hebp1 (1 µg/20 µL and 5 µg/20 µL). Nuclei were labeled with DAPI (blue). Scale bars, 100 µm (A) or 25 µm (B). (C-E) Quantification of the NG2, PECAM-1, and βIII-tubulin immunopositive areas in corpus cavernosum tissue using ImageJ software. *p<0.05, **p<0.01, ***p<0.001. The relative ratio of the control group was arbitrarily set to 1. DM, diabetes mellitus; ns, not significant.
5. Hebp1 improves erectile function by reducing reactive oxygen species production and accelerating the PI3K/AKT/eNOS pathway in diabetic mice
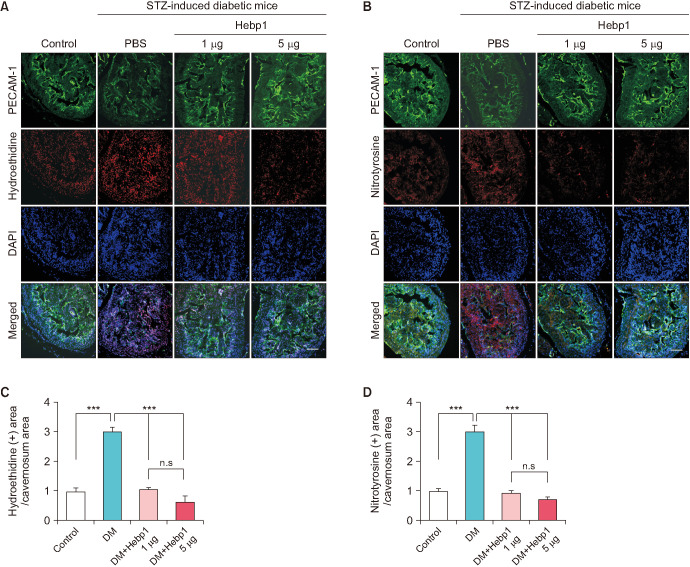
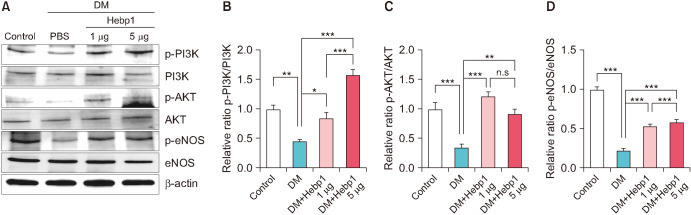
In diabetes, high levels of ROS induce apoptosis and cell death [18]. Therefore, we measured the generation of superoxide anions and peroxynitrite using hydroethidine and nitrotyrosine immunofluorescence staining, respectively. Oxidized hydroethidine and nitrotyrosine expression in MCECs was significantly increased in the PBS-treated diabetic mice compared with that in the age-matched controls. However, double intracavernous injections of Hebp1 significantly decreased the generation of superoxide anions (Fig. 5A, C) and peroxynitrite (Fig. 5B, D). In addition, because the PI3K/AKT/eNOS pathway plays an important role in preserving erectile function in diabetic conditions [19], we evaluated the effect of Hebp1 on the PI3K/AKT/eNOS pathway in diabetic mice. Interestingly, the phosphorylation of PI3K, AKT, and eNOS increased significantly after Hebp1 treatment in diabetic mice (Fig. 6). These results indicate that ROS levels and the PI3K/AKT/eNOS pathway constitute an important signaling pathway for Hebp1-mediated erectile function.
Fig. 5. Heme-binding protein 1 (Hebp1) decreases corpus cavernosum reactive oxygen species production in mice with streptozotocin (STZ)-induced diabetes. (A, B) In situ detection of superoxide anion (A, hydroethidine, red) and nitrotyrosine (B, red) production in corpus cavernosum endothelial cells (A and B, green) from age-matched control mice and mice with STZ-induced diabetes stimulated at 2 weeks after intracavernous injection of PBS (20 µL) or Hebp1 (1 µg/20 µL and 5 µg/20 µL). Nuclei were labeled with DAPI (blue). Scale bars, 100 µm. (C, D) The ethidium bromide fluorescence-immunopositive cavernosum area and nitrotyrosine-immunopositive cavernosum area were quantified by use of ImageJ software (n=4). ***p<0.001. The relative ratio of the control group was arbitrarily set to 1. DM, diabetes mellitus; ns, not significant.
Fig. 6. Heme-binding protein 1 (Hebp1) increases PI3K/AKT/eNOS signaling in mice with streptozotocin (STZ)-induced diabetes. (A) Representative western blots for p-PI3K/PI3K, p-AKT/AKT, and p-eNOS/eNOS in corpus cavernosum tissue from age-matched control mice and mice with STZ-induced diabetes stimulated at 2 weeks after intracavernous injection of PBS (20 µL) or Hebp1 (1 µg/20 µL and 5 µg/20 µL). (B-D) Normalized band intensity values were quantified by use of ImageJ software (n=4). *p<0.05, **p<0.01, ***p<0.001. The relative ratio of the control group was arbitrarily set to 1. DM, diabetes mellitus; ns, not significant.
DISCUSSION
Recently, several studies have shown that pericyte dysfunction plays an important role in diabetes-induced ED [13,15]. However, little is known about the detailed role of pericytes in diabetic ED or other pathological conditions. Previous global gene expression data showed that the expression of Hebp1 changes significantly in MCPs under HG conditions [5]. Consistently, in our current research we found that compared with that in MCECs, Hebp1 expression was increased in MCPs and significantly reduced under HG conditions. Therefore, we hypothesized that the reduced Hebp1 expression may be related to angiogenesis under HG conditions and that deciphering this function of Hebp1 may provide important clues for the treatment of diabetic ED.
To test our hypothesis, we performed tube formation and migration arrays using MCECs, which were isolated from mouse corpus cavernosum tissues and exposed to HG conditions to mimic diabetes-induced angiopathy. We found that exogenous application of mouse Hebp1 recombinant proteins significantly improved the reduced capillary-like structures and reduction in cell migration observed under HG conditions. We also obtained consistent results from the aortic ring microvessel sprouting assay. Hebp1 is a 22-kDa intracellular tetrapyrrole-binding protein that can produce extracellular chemokines [8]. However, whether Hebp1 can be secreted or released from cells remains unclear. Therefore, to assess whether Hebp1 could be released from MCPs and induce angiogenesis in MCECs, we collected conditioned medium from MCPs and used it to treat the MCECs. Our results clearly showed that the group treated with MCP-shHebp1 (Hebp1 knockdown) culture medium showed reduced tube formation compared with the group treated with normal MCP culture medium. These findings suggest that Hebp1 can be released from MCPs and affects the angiogenesis of MCECs. Nonetheless, the mechanisms by which Hebp1 is secreted by MCPs should be investigated further.
The pathophysiological manifestations of diabetic ED include microangiopathy and neuropathy [20,21]. Therefore, we used a diabetes-induced ED model [16] to evaluate the angiogenesis effect of Hebp1 under diabetic conditions. The production of superoxide anions and peroxynitrite in the penis was significantly higher in diabetic mice than in the control group [22]. Although ROS are associated with nearly all human diseases, few ROS modulators have been translated to the clinic to date [23] because the results of several clinical studies have shown that systemic antioxidant therapy can lead to adverse events. Selectively inhibiting only the disease-relevant ROS sources and leaving all other sources intact may effectively reduce these adverse events [24]. Interestingly, previous studies have shown that the processing of Hebp1 by cathepsin D can produce F2L, which can block the production of superoxide in human neutrophils [9]. Therefore, we hypothesized that exogenous delivery of Hebp1 inhibits the increased ROS levels and ultimately improves the erectile function of diabetic mice. Our results also verified our hypothesis that Hebp1 significantly reduces the production of superoxide anions and peroxynitrite in the MCECs of diabetic mice. It has also been reported that the PI3K/Akt pathway regulates eNOS phosphorylation, thereby enhancing the biological activity of endogenous nitric oxide [25,26]. Eventually, erectile function is induced [27]. In this study, repeated intracavernous injections of Hebp1 significantly induced the activation of the PI3K/AKT/eNOS pathway. These effects synergistically alleviated the abnormalities in blood vessels and nerves in the tissues of mice with diabetes-induced ED and ultimately led to improved erectile function. Furthermore, although the neurovascular contents (NG2-positive pericyte, PECAM-1-positive endothelial cell, and βIII-tubulin-neuronal cell) were mildly increased in diabetic mice treated with 1 µg of Hebp1 compared with untreated diabetic mice, this difference was not statistically significant. However, 1 µg and 5 µg of Hebp1 significantly restored erectile function to 94% of the control group. This may be because we used a somewhat high voltage (5 V) to measure nerve stimulation-induced erectile function. Using submaximal voltage (e.g., 0.5 or 1 V) with between 1 µg and 5 µg of Hebp1 may exhibit different erectile function. These findings indicate that the regulation of ROS levels and PI3K/AKT/eNOS signaling activity by Hebp1 may play an important role in the restoration of erectile function in diabetic ED.
To the best of our knowledge, this study is the first to report that Hebp1 exerts an angiogenic effect in diabetic conditions. However, the present study has a few limitations. First, the HG conditions did not fully reflect the complexity of ED caused by diabetes, and we did not use L-glucose for osmolality control. Second, we demonstrated that Hebp1 may be derived from MCPs and induce angiogenesis in MCECs but could not elucidate the detailed mechanism by which MCPs secrete Hebp1, which may be mediated via unconventional secretion pathways or delivery by extracellular vesicles. Further research is warranted to understand the detailed mechanisms and functions of Hebp1 in neural regeneration.
CONCLUSIONS
We assessed the angiogenesis effect of Hebp1 in the treatment of diabetes-induced ED. Our findings demonstrate that Hebp1 can rescue corpus cavernosum endothelial cell and neuronal cell content, modulate ROS levels and PI3K/AKT/eNOS signaling activity, and ultimately lead to the improvement of erectile function in diabetic mice.
Footnotes
CONFLICTS OF INTEREST: The author has nothing to disclose.
FUNDING: This research was supported by Inha University Research Grant (Guo Nan Yin).
References
- 1.Bivalacqua TJ, Usta MF, Champion HC, Kadowitz PJ, Hellstrom WJ. Endothelial dysfunction in erectile dysfunction: role of the endothelium in erectile physiology and disease. J Androl. 2003;24(6 Suppl):S17–S37. doi: 10.1002/j.1939-4640.2003.tb02743.x. [DOI] [PubMed] [Google Scholar]
- 2.Tang Z, Song J, Yu Z, Cui K, Ruan Y, Liu Y, et al. Inhibition of microRNA-92a improved erectile dysfunction in streptozotocin-induced diabetic rats via suppressing oxidative stress and endothelial dysfunction. World J Mens Health. 2022 Feb 24; doi: 10.5534/wjmh.210177. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kam SC, Lee SH, Jeon JH, So I, Chae MR, Park JK, et al. Gene expression profile comparison in the penile tissue of diabetes and cavernous nerve injury-induced erectile dysfunction rat model. Investig Clin Urol. 2016;57:286–297. doi: 10.4111/icu.2016.57.4.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yin GN, Ock J, Choi MJ, Limanjaya A, Ghatak K, Song KM, et al. Gene expression profiling of mouse cavernous endothelial cells for diagnostic targets in diabetes-induced erectile dysfunction. Investig Clin Urol. 2021;62:90–99. doi: 10.4111/icu.20200119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin GN, Wu J, Cui Y, Lin C, Shi L, Gao ZL, et al. Transcriptional profiling of mouse cavernous pericytes under high-glucose conditions: implications for diabetic angiopathy. Investig Clin Urol. 2021;62:100–110. doi: 10.4111/icu.20200272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Tell D, Armulik A, Betsholtz C. Pericytes and vascular stability. Exp Cell Res. 2006;312:623–629. doi: 10.1016/j.yexcr.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 7.Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Taketani S, Adachi Y, Kohno H, Ikehara S, Tokunaga R, Ishii T. Molecular characterization of a newly identified heme-binding protein induced during differentiation of urine erythroleukemia cells. J Biol Chem. 1998;273:31388–31394. doi: 10.1074/jbc.273.47.31388. [DOI] [PubMed] [Google Scholar]
- 9.Lee HY, Lee SY, Shin EH, Kim SD, Kim JM, Lee MS, et al. F2L, a peptide derived from heme-binding protein, inhibits formyl peptide receptor-mediated signaling. Biochem Biophys Res Commun. 2007;359:985–990. doi: 10.1016/j.bbrc.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal A, Nandipati KC, Sharma RK, Zippe CD, Raina R. Role of oxidative stress in the pathophysiological mechanism of erectile dysfunction. J Androl. 2006;27:335–347. doi: 10.2164/jandrol.05136. [DOI] [PubMed] [Google Scholar]
- 11.Jin HR, Kim WJ, Song JS, Piao S, Choi MJ, Tumurbaatar M, et al. Intracavernous delivery of a designed angiopoietin-1 variant rescues erectile function by enhancing endothelial regeneration in the streptozotocin-induced diabetic mouse. Diabetes. 2011;60:969–980. doi: 10.2337/db10-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yin GN, Ryu JK, Kwon MH, Shin SH, Jin HR, Song KM, et al. Matrigel-based sprouting endothelial cell culture system from mouse corpus cavernosum is potentially useful for the study of endothelial and erectile dysfunction related to high-glucose exposure. J Sex Med. 2012;9:1760–1772. doi: 10.1111/j.1743-6109.2012.02752.x. [DOI] [PubMed] [Google Scholar]
- 13.Yin GN, Das ND, Choi MJ, Song KM, Kwon MH, Ock J, et al. The pericyte as a cellular regulator of penile erection and a novel therapeutic target for erectile dysfunction. Sci Rep. 2015;5:10891. doi: 10.1038/srep10891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Detaille D, Guigas B, Chauvin C, Batandier C, Fontaine E, Wiernsperger N, et al. Metformin prevents high-glucose-induced endothelial cell death through a mitochondrial permeability transition-dependent process. Diabetes. 2005;54:2179–2187. doi: 10.2337/diabetes.54.7.2179. [DOI] [PubMed] [Google Scholar]
- 15.Yin GN, Ock J, Choi MJ, Song KM, Ghatak K, Minh NN, et al. A simple and nonenzymatic method to isolate human corpus cavernosum endothelial cells and pericytes for the study of erectile dysfunction. World J Mens Health. 2020;38:123–131. doi: 10.5534/wjmh.180091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin HR, Kim WJ, Song JS, Choi MJ, Piao S, Shin SH, et al. Functional and morphologic characterizations of the diabetic mouse corpus cavernosum: comparison of a multiple low-dose and a single high-dose streptozotocin protocols. J Sex Med. 2009;6:3289–3304. doi: 10.1111/j.1743-6109.2009.01464.x. [DOI] [PubMed] [Google Scholar]
- 17.Bivalacqua TJ, Usta MF, Kendirci M, Pradhan L, Alvarez X, Champion HC, et al. Superoxide anion production in the rat penis impairs erectile function in diabetes: influence of in vivo extracellular superoxide dismutase gene therapy. J Sex Med. 2005;2:187–197. doi: 10.1111/j.1743-6109.2005.20228_1.x. discussion 197-8. [DOI] [PubMed] [Google Scholar]
- 18.Yin GN, Choi MJ, Kim WJ, Kwon MH, Song KM, Park JM, et al. Inhibition of Ninjurin 1 restores erectile function through dual angiogenic and neurotrophic effects in the diabetic mouse. Proc Natl Acad Sci U S A. 2014;111:E2731–E2740. doi: 10.1073/pnas.1403471111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luan Y, Cui K, Tang Z, Ruan Y, Liu K, Wang T, et al. Human tissue kallikrein 1 Improves erectile dysfunction of streptozotocin-induced diabetic rats by inhibition of excessive oxidative stress and activation of the PI3K/AKT/eNOS pathway. Oxid Med Cell Longev. 2020;2020:6834236. doi: 10.1155/2020/6834236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kouidrat Y, Pizzol D, Cosco T, Thompson T, Carnaghi M, Bertoldo A, et al. High prevalence of erectile dysfunction in diabetes: a systematic review and meta-analysis of 145 studies. Diabet Med. 2017;34:1185–1192. doi: 10.1111/dme.13403. [DOI] [PubMed] [Google Scholar]
- 21.Kolluru GK, Bir SC, Kevil CG. Endothelial dysfunction and diabetes: effects on angiogenesis, vascular remodeling, and wound healing. Int J Vasc Med. 2012;2012:918267. doi: 10.1155/2012/918267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Volpe CMO, Villar-Delfino PH, Dos Anjos PMF, Nogueira-Machado JA. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018;9:119. doi: 10.1038/s41419-017-0135-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casas AI, Nogales C, Mucke HAM, Petraina A, Cuadrado A, Rojo AI, et al. On the clinical pharmacology of reactive oxygen species. Pharmacol Rev. 2020;72:801–828. doi: 10.1124/pr.120.019422. [DOI] [PubMed] [Google Scholar]
- 24.Kandhare AD, Mukherjee A, Bodhankar SL. Antioxidant for treatment of diabetic nephropathy: a systematic review and meta-analysis. Chem Biol Interact. 2017;278:212–221. doi: 10.1016/j.cbi.2017.10.031. [DOI] [PubMed] [Google Scholar]
- 25.Jeong HC, Bae WJ, Zhu GQ, Jeon SH, Choi SW, Kim SJ, et al. Synergistic effects of extracorporeal shockwave therapy and modified Ojayeonjonghwan on erectile dysfunction in an animal model of diabetes. Investig Clin Urol. 2019;60:285–294. doi: 10.4111/icu.2019.60.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawasaki K, Smith RS, Jr, Hsieh CM, Sun J, Chao J, Liao JK. Activation of the phosphatidylinositol 3-kinase/protein kinase Akt pathway mediates nitric oxide-induced endothelial cell migration and angiogenesis. Mol Cell Biol. 2003;23:5726–5737. doi: 10.1128/MCB.23.16.5726-5737.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davies KP. Development and therapeutic applications of nitric oxide releasing materials to treat erectile dysfunction. Future Sci OA. 2015;1:FSO53. doi: 10.4155/fso.15.53. [DOI] [PMC free article] [PubMed] [Google Scholar]