Abstract
A sensitive and specific detection method was developed for Xanthomonas hyacinthi; this method was based on amplification of a subsequence of the type IV fimbrial-subunit gene fimA from strain S148. The fimA gene was amplified by PCR with degenerate DNA primers designed by using the N-terminal and C-terminal amino acid sequences of trypsin fragments of FimA. The nucleotide sequence of fimA was determined and compared with the nucleotide sequences coding for the fimbrial subunits in other type IV fimbria-producing bacteria, such as Xanthomonas campestris pv. vesicatoria, Neisseria gonorrhoeae, and Moraxella bovis. In a PCR internal primers JAAN and JARA, designed by using the nucleotide sequences of the variable central and C-terminal region of fimA, amplified a 226-bp DNA fragment in all X. hyacinthi isolates. This PCR was shown to be pathovar specific, as assessed by testing 71 Xanthomonas pathovars and bacterial isolates belonging to other genera, such as Erwinia and Pseudomonas. Southern hybridization experiments performed with the labelled 226-bp DNA amplicon as a probe suggested that there is only one structural type IV fimbrial-gene cluster in X. hyacinthi. Only two Xanthomonas translucens pathovars cross-reacted weakly in PCR. Primers amplifying a subsequence of the fimA gene of X. campestris pv. vesicatoria (T. Ojanen-Reuhs, N. Kalkkinen, B. Westerlund-Wikström, J. van Doorn, K. Haahtela, E.-L. Nurmiaho-Lassila, K. Wengelink, U. Bonas, and T. K. Korhonen, J. Bacteriol. 179: 1280–1290, 1997) were shown to be pathovar specific, indicating that the fimbrial-subunit sequences are more generally applicable in xanthomonads for detection purposes. Under laboratory conditions, approximately 1,000 CFU of X. hyacinthi per ml could be detected. In inoculated leaves of hyacinths the threshold was 5,000 CFU/ml. The results indicated that infected hyacinths with early symptoms could be successfully screened for X. hyacinthi with PCR.
Xanthomonas belongs to the phytopathogenic bacterial family Pseudomonaceae. Xanthomonas species are subdivided into pathovars, and many of these infect economically important crop plants. Xanthomonas hyacinthi causes yellow disease in Hyacinthus (55) and in related members of the Liliaceae, such as Scilla, Muscari, and Puschkinia (28). X. hyacinthi is easily spread in the field from the focus of infection by wind and rain or by wounding of bulbs during mechanical sorting in the presence of diseased bulbs. Therefore, the development of a fast and specific test to ascertain whether symptoms are caused by this yellow-pigmented bacterium is of utmost importance to hyacinth growers.
Many techniques to classify or identify Xanthomonas species and their pathovars are available. Techniques based on unique biochemical features (10), membrane protein profiles as determined by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) (9), and immunoassays (2, 5, 6) are now being replaced by DNA techniques. The DNA assays are based mainly on fingerprinting methods, such as 16S ribosomal DNA amplification (35), ribosomal DNA gene restriction pattern analysis (8), and analysis of restriction fragment length polymorphisms of DNA (33, 53), which sometimes are combined with SDS-PAGE of membrane proteins (43), nucleic acid probe analysis (14, 22, 59), and genomic fingerprinting with repetitive sequences (7, 34) or with random amplified polymorphic DNA PCR (41). However, these methods are not suitable for fast and specific detection, as they require in most cases time-consuming isolation and cultivation of the bacteria in question. In the case of X. hyacinthi, immediate action is needed when field samples of hyacinth plants with symptoms are positive. Instant destruction of plants growing in the area surrounding an infection spot prevents further spread of this contagious disease.
Recently, X. hyacinthi-specific monoclonal antibodies were developed (57). One group of these monoclonal antibodies recognizes the O-antigen of the lipopolysaccharide of X. hyacinthi and is now used by the Dutch Bulb Inspection Service in an enzyme-linked immunosorbent assay (ELISA) format to detect yellow disease in hyacinths. The threshold for the number of bacteria that can be detected in samples is 5 × 105 CFU (57). In practice, this is sufficient to detect X. hyacinthi in most samples. However, during early stages of yellow disease, fewer bacteria can be present. Therefore, a more sensitive diagnostic test to ascertain whether the first lesions are caused by X. hyacinthi rather than by physical causes or plant stress is needed.
Recently, it has been found that X. hyacinthi and other Xanthomonas species and pathovars express type IV fimbriae (56). This type of fimbriae has been found in numerous bacterial species that infect animal and human hosts. Many aspects of the structure of these fimbriae and their role in pathogenesis (for instance, their role in attachment and motility), as well as the organization of the corresponding genes, have been studied extensively (40, 51, 54). However, very little is known about the function of the type IV fimbriae in the plant-pathogenic bacterium Xanthomonas. These extracellular polymers consist of identical protein subunits with molecular masses of 15.5 to 18 kDa (39, 56). In different pathovars these subunits have different molecular masses. This fact, together with the finding that most of the antifimbrial monoclonal antibodies that have been developed are pathovar specific (57), supports the theory that the type IV fimbria antigens of different xanthomonads contain unique, variable, and immunodominant regions. This has also been found for type IV fimbriae expressed by Neisseria gonorrhoeae (19), Moraxella bovis (20), and Dichelobacter nodosus (17). Thus, one practical use of the corresponding variable DNA sequences of the X. hyacinthi fimbrial-subunit gene could be in the design of specific primers for a sensitive PCR assay.
In this study we developed primers that were designed by using the type IV structural fimbrial-subunit gene. We found that even a very low number of X. hyacinthi cells can be detected with a PCR assay based upon specific amplification with these primers of part of the variable region of the structural fimbrial-subunit gene coding for the 17-kDa protein. Also, another xanthomonad, Xanthomonas campestris pv. vesicatoria, could be detected by specific amplification with nested primers located in the X. campestris pv. vesicatoria fimA gene, revealing a promising strategy for universal detection of Xanthomonas pathovars.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The bacterial strains used in this study are listed in Table 1. Xanthomonas species were cultured at 28°C on nutrient agar (Oxoid, Basingstoke, Hampshire, United Kingdom). PCR-amplified DNA fragments were cloned by direct ligation into commercially prepared, linearized pCRII vectors as described by the manufacturer (Invitrogen Corporation, San Diego, Calif.) and were used for transformation of competent Escherichia coli INVα cells (Invitrogen). Liquid cultures of Xanthomonas species and pathovars for DNA extraction were grown in nutrient broth (Difco Laboratories, Detroit, Mich.) supplemented with 2 g of yeast extract (Oxoid) per liter at 28°C to the early stationary phase. Bacterial strains were stored on beads at −80°C in vials with cryopreservative fluid (Protect; STC Limited, Heywood, Lancashire, United Kingdom). E. coli was cultivated in Luria broth at 37°C (47). Ampicillin was used to maintain selection for resistance at a final concentration of 50 μg/ml. Shear fractions of bacterial cells were obtained as previously described (56). For in vitro expression of amplicons cloned into vector pCRII, E. coli INVα containing this plasmid was cultured in the presence of 50 μg of ampicillin per ml.
TABLE 1.
Bacterial strains used in this study
| Species, pathovar, or subspecies | Strain(s) | Sourcea |
|---|---|---|
| Xanthomonas albilineans | LMG887 | LMG |
| Xanthomonas arboricola pv. pruni | LMG852 | LMG |
| Xanthomonas axonopodis pv. begoniae | NCPPB241 | NCPPB |
| Xanthomonas axonopodis pv. citri | LMG409 | LMG |
| Xanthomonas axonopodis pv. dieffenbachiae | IPO-DLO1104 | IPO-DLO |
| LMG761, LMG996 | LMG | |
| Xanthomonas axonopodis pv. manihotis | LMG784 | LMG |
| Xanthomonas axonopodis pv. phaseoli | LMG7488, LMG7455 | LMG |
| Xanthomonas axonopodis pv. vasculorum | LMG901 | LMG |
| Xanthomonas axonopodis pv. vesicatoria | LMG905, LMG910, LMG913, LMG922, LMG929, LMG668 | LMG |
| Xanthomonas axonopodis pv. vignicola | IPO-DLO381 | IPO-DLO |
| Xanthomonas campestris pv. campestris | LMG568 | LMG |
| Xanthomonas campestris pv. fici | LMG701 | LMG |
| Xanthomonas campestris pv. gummisudans | LMG732 | LMG |
| Xanthomonas fragariae | LMG708 | LMG |
| Xanthomonas hortorum pv. pelargonii | LMG7314 | LMG |
| Xanthomonas hyacinthi | S148, S133, S171, S172, NN1, NAD55, TV43, HK60 | LBO |
| LMG742 | LMG | |
| Xanthomonas oryzae pv. oryzae | LMG630 | LMG |
| Xanthomonas oryzae pv. oryzicola | LMG797 | LMG |
| Xanthomonas populi | PD889 | PD |
| Xanthomonas translucens pv. cerealis | LMG3212, LMG3213, LMG890, LMG679 | LMG |
| Xanthomonas translucens pv. graminis | LMG726 | LMG |
| Xanthomonas translucens pv. hordei | LMG737 | LMG |
| Xanthomonas translucens pv. phlei | NCPPB 3231 | NCPPB |
| LMG730 | LMG | |
| Xanthomonas translucens pv. poae | NCPPB3230 | NCPPB |
| Xanthomonas translucens pv. translucens | NCPPB2904, NCPPB2389, NCPPB920, NCPPB3215, NCPPB3170, NCPPB3176 | NCPPB |
| LMG876 | LMG | |
| Xanthomonas vasicola pv. holcicola | LMG736 | LMG |
| Xanthomonas vesicatoria | LMG911, LMG917, LMG920, LMG925 | LMG |
| NCPPB3240 | NCCPB | |
| ATCC 11551, ATCC 35937 | ATCC | |
| Erwinia amylovora | LMG2024 | LMG |
| Erwinia carotovora subsp. carotovora | 550 | LBO |
| LMG2417 | LMG | |
| Erwinia chrysanthemi | LMG2488 | LMG |
| Escherichia coli | INVα | Invitrogen |
| Erwinia rhapontici | 164 | LBO |
| Pseudomonas aeruginosa | LMG1242 | LMG |
| Pseudomonas fluorescens | PD2434 | PD |
| Pseudomonas marginata | 570 | LBO |
| Pseudomonas syringae pv. syringae | LMG1247 | LMG |
| Stenotrophomonas maltophilia | LMG958 | LMG |
| Xylophilus ampelinus | LMG523 | LMG |
Abbreviations: LMG, Bacterial Collection, Laboratorium voor Microbiologie, Universiteit Gent, Ghent, Belgium; NCPPB, National Collection of Plant Pathogenic Bacteria, Harpenden Laboratory, Harpenden, Hertsfordshire, United Kingdom; IPO-DLO, Research Institute for Plant Protection, Wageningen, The Netherlands; ATCC, American Type Culture Collection, Manassas, Va.; LBO, Bulb Research Centre, Lisse, The Netherlands; PD, Plant Protection Service, Wageningen, The Netherlands.
Fimbrial-subunit purification and amino acid sequencing of trypsin fragments.
Fimbriae of X. hyacinthi S148 were isolated and purified by preparative gel electrophoresis as described previously (56). Approximately 250 pmol of the 17-kDa fimbrial-subunit protein was digested twice with 5% (wt/wt) sequencing grade modified trypsin (Promega, Madison, Wis.) for 2 h at 37°C. Trypsin cleaves the peptide bond C terminus to arginine or lysine. Peptides present in the digest were separated by preparative reversed-phase high-performance liquid chromatography on a Nucleosil 10 C18 column (2.1 by 150 mm). Sequencing of selected peptides was done with a semiautomated model 477A Sequenator (Applied Biosystems, Foster City, Calif.) by Eurosequence, Groningen, The Netherlands.
Immunological methods and pathogenicity tests.
For controls, polyclonal rabbit antisera raised against X. hyacinthi S148 and against purified fimbriae, as well as fimbrial monoclonal antisera, were used in ELISA and immunoblotting experiments as described previously (57). Immunogold labelling of bacterial cells for electron microscopic studies was carried out as described previously (57). Hyacinth cultivars Pink Pearl and Delfts Blue were used for pathogenicity tests. The cultivars were maintained in a greenhouse with a day-night regimen of 12 h of light (25°C; relative humidity, 70%) and 12 h of darkness (10°C; relative humidity, 90%). The X. hyacinthi isolates used for inoculation were grown on agar plates for 48 h at 28°C, harvested and washed in phosphate-buffered saline, and diluted (107 CFU/ml) in sterile tap water (57). Leaves were spray inoculated with the diluted bacterial preparation or with phosphate-buffered saline as a control. After 2 weeks, the first lesions became visible (55), and leaf material was then collected for experimental use.
DNA amplification.
In vitro amplification of DNA was carried out with an Omnigene thermal cycler (Hybaid, Teddington, Middlesex, United Kingdom). Optimization of the PCR was performed by using a PCR Optimizer kit (Invitrogen). The nucleotide analog 7-deaza-2′-deoxyguanosine 5′-triphosphate (27) at an analog-to-GTP ratio of 1:3 was added to the nucleotide mixture because of the high G+C content (69%) of X. hyacinthi DNA (58). The reaction mixture contained 10 μl of a mixture containing 300 mM Tris-HCl (pH 9.0 at 20°C), 75 mM (NH4)2SO4, and 10.0 mM MgCl2, each deoxynucleoside triphosphate (HT Biotechnologies Ltd., Cambridge, United Kingdom) at a concentration of 200 μM, 50 pmol of each primer, and 1.5 U of AmpliTaq DNA polymerase (Roche Molecular Systems, Inc., Branchburg, N.J.). For amplification with degenerate primers, a touchdown PCR cycle protocol (15) was used, in which the annealing temperature was decreased by 1°C in each cycle for the first 10 cycles. After an initial denaturation step of 5 min at 96°C, the first 10 cycles consisted of denaturation for 30 s at 95°C, annealing for 1 min at 60 to 51°C, and extension for 1 min at 72°C. Subsequently, another 30 cycles of 30 s at 95°C, 1 min at 51°C, and 1 min at 72°C were performed, followed by a final 5-min extension step at 72°C. For amplification of X. hyacinthi fimA with primers designed by using the nucleotide sequence of fimA (see below), the PCR buffer system of HT Biotechnologies was used with 0.5 U of SuperTaq (HT Biotechnologies). Amplifications used to clone amplicons were carried out with 1.0 U of Taq DNA polymerase (Gibco BRL Life Technologies, Breda, The Netherlands).
For PCR analysis of X. hyacinthi in plant extracts or bacterial whole-cell preparations, SuperTaq and the corresponding buffer system (HT Biotechnologies) were used. Amplified DNA fragments were analyzed on 1.4% agarose gels by standard gel electrophoresis procedures (47).
To obtain the 5′-terminal nucleotides and the flanking sequences of fimA, an inverse PCR was carried out as previously described (26). Total DNA from X. hyacinthi S148 was digested with DraII, purified by phenol-chloroform extraction, and ligated with T4 DNA ligase (Pharmacia LKB, Uppsala, Sweden) as previously described (47). This fraction was used as the template in a PCR with nested inverse primers located in fimA (see below). For amplification in inverse PCR, a High Fidelity kit (Boehringer GmbH, Mannheim, Germany) was used; the reaction conditions were 35 cycles consisting of 30 s at 96°C, annealing at 60°C for 45 s, and extension at 68°C for 2 min, followed by final extension for 10 min at 72°C.
DNA manipulations and hybridization.
Bacterial genomic DNA was isolated as described by Chen and Kuo. (12). For Southern hybridization, approximately 2 μg of bacterial genomic DNA was digested with PvuII, subjected to electrophoresis in 0.9% agarose gels, and transferred to a positively charged nylon membrane (Boehringer) by standard procedures (47). The hybridized DNA was detected according to the instructions of the manufacturer (Boehringer) by using the digoxigenin (DIG) nonradioactive nucleic acid labelling and detection system. Amplicons were labelled during PCR with DIG-dUTP by using a PCR DIG probe synthesis kit (Boehringer). DIG-labelled amplicons were used as probes for experiments after they were made single stranded by boiling for 10 min, followed by chilling in ice. Blots were incubated with the labelled probes for 16 h at 65°C in hybridization solution (Boehringer). The membranes were prewashed twice at room temperature with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) containing 1% (wt/vol) SDS for 5 min, and this was followed by two stringency washes with 0.1× SSC–0.1% SDS for 15 min at 65°C. Chemiluminescent detection of the hybridized probes was carried out by using the instructions of the manufacturer (Boehringer) and CPD-Star as the detection reagent. Emitted light was recorded on X-ray film (Kodak Biomax MS-1; Eastman Kodak Co., Rochester, N.Y.).
Sequencing analysis, computer programs, and nucleotide sequence accession number.
The nucleotide sequences of PCR fragments cloned into plasmid pCRII were determined by using a Thermo Sequenase cycle sequencing kit (Amersham Pharmacia Biotech, Rainham, United Kingdom) and the M13 universal forward and reverse primers. The sequencing reaction mixtures were analyzed with a LiCor model 4000 automated sequencer (BaseClear, Leiden, The Netherlands). The PC/Gene 6.7 package (IntelliGenetics, Inc., Mountain View, Calif.) was used for comparing DNA sequences (CLUSTAL) and for designing specific primer sequences (PCRPLAN). To search for homologies, the nucleotide and amino acid sequences were compared with sequences in the GenBank databases by using BLAST (1).
Processing of plants for PCR-mediated detection of X. hyacinthi in lesions.
To evaluate leaf symptoms on hyacinths, leaf surfaces were cleansed with 70% ethanol. A 1- to 2-cm2 area with symptoms was excised from each leaf and macerated. The homogenized leaf material was then incubated in 4 ml of 0.05 M Tris-HCl (pH 7.0) with 0.5% (vol/vol) Triton X-100 for 1 h in a rotary shaker (100 rpm). Subsequently, the bacteria in 1 ml of the sample were pelleted by centrifugation for 10 min at 13,000 rpm (Eppendorf); the pelleted bacteria were resuspended in 100 μl of Tris-HCl buffer (pH 7.0). For PCR, 1 and 5 μl of the suspension were used as templates; in some cases 50 μl was used in an ELISA as previously described (57).
Sensitivity of the PCR.
To determine the detection limits of the X. hyacinthi- and Xanthomonas vesicatoria-specific primers, 10-fold dilutions of X. hyacinthi S148 and X. vesicatoria NCPPB3240 harvested in the exponential phase of growth were prepared. Five microliters of each of the dilutions was used in a PCR as the template. The corresponding viable counts were determined by plating 50 μl of each dilution on nutrient agar plates in triplicate and incubating the plates at 28°C for 2 days.
Nucleotide sequence accession numbers.
The nucleotide sequence of fimA with flanking sequences has been deposited in the GenBank nucleotide sequence database under accession number AF281159; the partial fimbrial sequences of Xanthomonas translucens pv. cerealis and X. translucens pv. translucens have been deposited under accession numbers AF282629 and AF282630, respectively.
RESULTS
Design of primers.
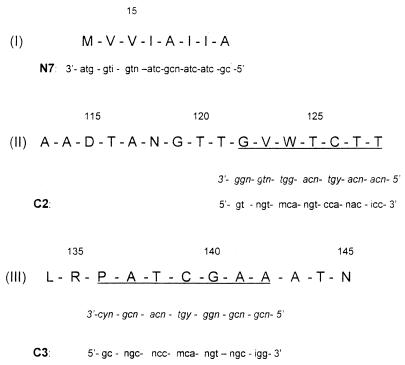
To identify the fimA gene of X. hyacinthi, the amino acid sequence was required. The conserved N-terminal amino acid sequence of FimA was already known (56). Degenerate primer N7 was designed on the basis of residues 7 to 14 of this sequence (Fig. 1, peptide I). Internal and more C-terminal peptides were obtained by incubation of the purified 17-kDa fimbrial-subunit protein with trypsin. By using several isolated peptides a partial amino acid sequence was determined. Two peptides (Fig. 1, peptides II and III) were selected, and their complete sequences were determined. An internal arginine residue was present in peptide III, indicating that it was only partially digested. Peptide III appeared to be the C-terminal fragment of FimA, as no amino acid was found after the final asparagine residue of this peptide.
FIG. 1.
Amino acid sequences of the N terminus of the 17-kDa fimbrial subunit (peptide I) and internal and C-terminal peptides II and III obtained after digestion with trypsin. The sequences of degenerate primers N7, C2, and C3, which were designed by using the codons of the corresponding underlined amino acid sequences (in italics), are shown below the peptide sequences. The amino acid sequences are numbered based on the protein sequence of X. hyacinthi shown in Fig. 5. i = inosine residues; n = A, G, C, or T; m = C or A; y = T or C; and r = G or A.
In order to amplify the corresponding fimbrial-subunit gene fimA, degenerate oligonucleotides C2 and C3 were developed as reverse primers on the basis of the amino acid sequences of peptides II and III (Fig. 1). For the variable bases of the coding triplet, a C/G/A/T wobble or, at the 3′ side, an inosine was introduced into the DNA sequence. The N7-C2 and N7-C3 primer pairs were used in PCR.
Amplification of the fimA sequence.
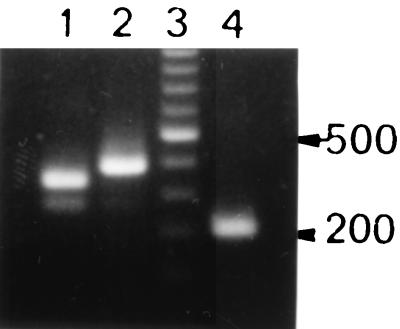
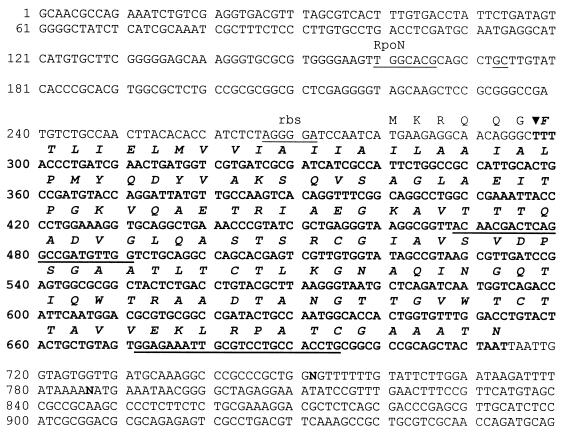
A PCR was carried out with purified, diluted total DNA of X. hyacinthi S148 as a template by using a touchdown PCR protocol (15). With primers N7 and C2 an approximately 345-bp amplicon was obtained (Fig. 2, lane 1). Primers N7 and C3 amplified an approximately 390-bp DNA fragment (Fig. 2, lane 2). When other strains of X. hyacinthi (Table 1) were used in similar PCR experiments, the same results were obtained (data not shown). The largest amplicon, obtained with primers N7 and C3, was cloned into vector pCRII and sequenced. As expected, the sequence coded for the fimbrial-subunit gene (fimA) without the most N-terminal sequences (nucleotides 297 to 314) and the 12 most C-terminal bases (nucleotides 702 to 713). To obtain the most N- and C-terminal DNA base pairs, inverse nested primers B (nucleotides 470 to 494) and F (nucleotides 515 to 539) were designed on the basis of the internal sequence of fimA (Fig. 3). Inverse PCR of circularized X. hyacinthi S148 chromosomal DraII fragments with the inverse nested primers amplified a 2,088-bp DNA fragment. This amplicon was cloned into vector pCRII, giving plasmid pCJO2, and was sequenced. After rearrangement of the sequence, we found that it contained the complete fimA gene together with a 1,317-bp upstream flanking sequence and a 354-bp downstream flanking sequence (GenBank nucleotide sequence database accession number AF281159). A subsequence of this 2,088-bp DNA fragment is shown in Fig. 3. Characteristics of a typical type IV fimbrial gene were confirmed (51). The fimA gene of X. hyacinthi S148 encoded a 139-amino-acid polypeptide with a calculated molecular weight of 14,339, which is somewhat less than the estimated molecular mass of FimA (approximately 17 kDa) (56). The most N-terminal amino acid of the mature FimA peptide was a phenylalanine, which is normally found in type IV fimbriae. Four cysteine residues were present in the C-terminal half of the subunit protein, indicating that disulfide bridges were present. The leader sequence of FimA (MKRQQG) showed strong similarity to the leader sequences normally found for type IV fimbrial subunits in other bacteria (54). A putative ribosome-binding site (3) was found 7 bases upstream from the translational initiation codon ATG. No other coding regions were found in this cloned DNA fragment, except for a putative protein coding region located 741 bp upstream from fimA (52 amino acids) (data not shown).
FIG. 2.
Electrophoretic analysis of PCR-amplified DNA from X. hyacinthi S148. Lane 1, amplicon obtained by using degenerate primers N7 and C2; lane 2, amplicon obtained by using primers N7 and C3; lane 3, 100-bp ladder (Promega) with a spiked 500-bp DNA fragment; lane 4, 226-bp amplicon obtained with nested primers JAAN and JARA located in the fimA gene.
FIG. 3.
The 959-bp subsequence of the 2,088-bp DraII DNA fragment of X. hyacinthi S148 (GenBank accession number AF281159), as obtained by inverse PCR with primers B and F. The translated coding region of fimA is located between nucleotides 297 and 713 (boldface type). Inverse nested primers B (positions 470 to 494) and F (positions 515 to 539) are located in the coding region; primers JAAN (positions 468 to 490) and JARA (positions 671 to 694), which were used for detection, are underlined. rbs, ribosome-binding site; RpoN, activator-regulated promoter sequence; N, unidentified nucleotide.
Expression of fimA in E. coli.
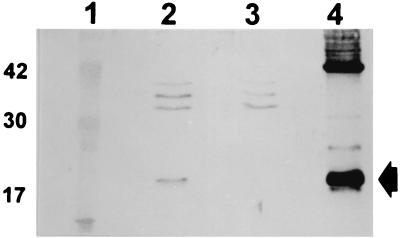
To confirm that fimA encoded the fimbrial subunit of X. hyacinthi, E. coli INVα (Invitrogen) with plasmid pCJO2 was cultured in Luria-Bertani medium supplemented with ampicillin. Bacterial cells were harvested and, after SDS-PAGE, subjected to immunoblotting. After incubation of the membrane with the transferred bacterial components with antifimbrial polyclonal rabbit serum (56) and further developing of the immunoblot, a protein band at an apparent molecular mass of 17 kDa was visible (Fig. 4, lane 2). This indicated that the fimA gene contained in pCJO2 was expressed in the E. coli K-12 strain; no FimA protein was detected in E. coli cells containing empty vector pCRII (Fig. 4, lane 3). Immunogold labelling with antisera against X. hyacinthi fimbriae and gold-tagged conjugate revealed no fimbrial strands on the surface of the bacterial cells during electron microscopic studies of E. coli cells harboring the pCJO2 plasmid. Also, no fimbrial subunits were found in the shear fraction of E. coli(pCJO2), as determined by SDS-PAGE and subsequent immunoblotting experiments. These findings indicated that the type IV fimbrial subunits of X. hyacinthi were not secreted to the cell surface and assembled into native fimbriae.
FIG. 4.
Immunoblot analysis of whole-cell extracts of E. coli INVα and crude fimbriae of X. hyacinthi. Lane 1, molecular size markers (sizes [in kilodaltons] are indicated on the left); lane 2, E. coli INVα containing pCJOII; lane 3, E. coli INVα with plasmid pCRII; lane 4, X. hyacinthi S148 crude fimbrial preparation. For developing the immunoblot, rabbit antiserum (2 μl/ml) raised against purified fimbriae from X. hyacinthi S148 was used. The arrow indicates the 17-kDa fimbrial-subunit protein.
Comparison of the fimbrial sequence of X. hyacinthi with the sequences of other type IV fimbriae.
The codons corresponding to the N-terminal amino acid residues and the internal and C-terminal trypsin peptide fragments (Fig. 1) were found in the DNA sequence of fimA, which confirmed that the fimbrial-subunit gene of X. hyacinthi S148 was cloned. The amino acid sequence of the fimbrial subunit was compared with other type IV sequences by searching the GenBank database using BLAST (1) (Fig. 5). The highest levels of homology were found with the fimA-encoded fimbrillin of X. campestris pv. vesicatoria (39) and the pilin of Xanthomonas axonopodis pv. citri (52) (levels of identity, 47 and 48%, respectively). Levels of identity between 39 and 35% were found with type IV fimbrial-subunit sequences (Fig. 5) from Pseudomonas stutzeri (GenBank accession number AJ132364), Pseudomonas putida (13), M. bovis (20), Pseudomonas aeruginosa (11), and Vibrio cholerae (21). As is characteristic for type IV fimbrial-subunit sequences, the highest level of homology was obtained for the first 30 N-terminal amino acid residues of the mature subunit protein (51).
FIG. 5.
Alignment of the primary structures of type IV fimbrial-subunit sequences. The BLAST program was used for computer analysis. Sequences from X. hyacinthi, X. campestris pv. vesicatoria (39), X. axonopodis pv. citri (GenBank accession number AJ132364). P. aeruginosa (11), and M. bovis (20) were compared. An asterisk indicates that a position in the alignment is perfectly conserved; a dot indicates that a position is well conserved.
Development of nested primers for fimA.
The N-terminal amino acid sequence of X. hyacinthi FimA shows high levels of homology not only with other fimbrial sequences but also with sequences of proteins of bacterial protein secretion systems and DNA uptake systems of gram-positive bacteria (16, 45). To develop a specific PCR for detection of X. hyacinthi and to minimize possible cross-reactions with secretion genes of Xanthomonas and other bacteria (45), we developed primers in the variable part of the fimA gene (Fig. 3). The 23-mer oligonucleotide JAAN (nucleotides 468 to 490) and the 24-mer oligonucleotide JARA (nucleotides 671 to 694) were tested with X. hyacinthi isolates. As expected, a 226-bp DNA fragment was amplified with X. hyacinthi S148 (Fig. 2, lane 4) and other X. hyacinthi strains (data not shown). To check the specificity of the primers, a large collection of plant-pathogenic bacterial strains, including strains of Xanthomonas spp. and their pathovars (Table 1), were tested in PCR performed with primers JAAN and JARA. None of the strains reacted with the primers; only X. translucens pv. cerealis LMG679 (data not shown) and X. translucens pv. translucens LMG876 (Fig. 6, lane 18) showed weak amplification. To assess the level of homology, the X. translucens pv. translucens fimA gene was amplified with primers N7 and C2. Degenerate primers N7 and C3 did not give any amplification, which reflected differences in the DNA sequence of the X. translucens fimA gene at least on the 3′ side of C3. The approximately 350-bp fragment was cloned in pCRII and sequenced. A comparison with the DNA sequence of X. hyacinthi revealed that the internal 348-bp fimA sequence from X. translucens pv. translucens showed high homology (90% identity) to the X. hyacinthi sequence. The corresponding FimA amino acid sequence showed that only five amino acids (GenBank accession number AF282630) were different; the translated internal fimA sequence (390 bp) of X. translucens pv. cerealis differed at 10 amino acids (GenBank accession number AF282629).
FIG. 6.
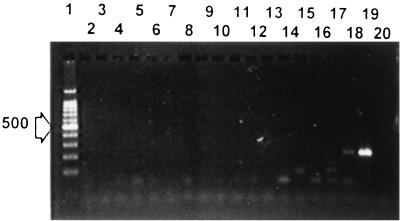
Specificity of the PCR with nested primers JAAN and JARA amplifying part of fimA. Lane 1, molecular size ladder spiked with a 500-bp DNA fragment (indicated by the arrow on the left); lane 2, Xanthomonas populi LMG889; lane 3, X. axonopodis pv. citri LMG409; lane 4, X. campestris pv. fici LMG701; lane 5, X. campestris pv. gummisudans S131; lane 6, X. translucens pv. phlei LMG730; lane 7, Xanthomonas oryzae pv. oryzicola LMG797; lane 8, X. axonopodis pv. vignicola IPO381; lane 9, Xanthomonas (Stenotrophomonas) maltophilia LMG958; lane 10, X. translucens pv. graminis LMG726; lane 11, Xanthomonas fragariae LMG708; lane 12, X. vesicatoria NCPPB3240; lane 13, X. axonopodis pv. manihotis LMG784; lane 14, Xanthomonas arboricola pv. pruni LMG852; lane 15, X. campestris pv. campestris LMG568; lane 16, X. translucens pv. cerealis LMG890; lane 17, Xanthomonas albilineans LMG887; lane 18, X. translucens pv. translucens LMG876; lane 19, X. hyacinthi S148; lane 20, Erwinia chrysanthemi LMG2488.
Hybridization of a fimA fragment with X. hyacinthi strains and other Xanthomonas spp. and pathovars.
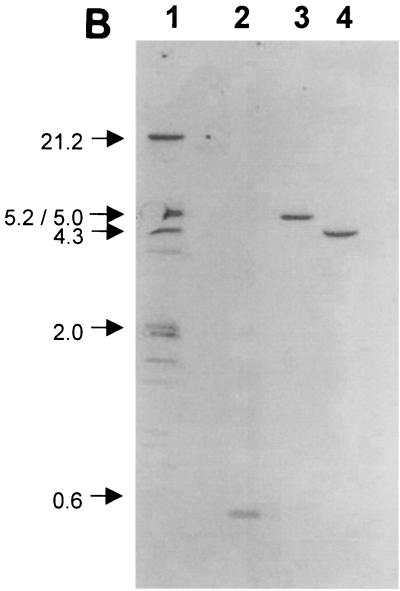
To determine whether a single copy or multiple copies of the structural gene coding for the fimbrial subunit are present in the chromosome, as found for some other type IV-producing bacteria, such as X. campestris pv. vesicatoria (39), and to make sure that no other sequences are recognized, DNA hybridization experiments were carried out. PvuII-digested genomic DNA of X. hyacinthi strains and Xanthomonas spp. and pathovars and DNA from other plant-pathogenic bacteria were used in these experiments. The membranes were probed with the DIG-labelled 226-bp internal fimA DNA fragment amplified with primers JAAN and JARA. The probe hybridized with an approximately 4.5-kb PvuII DNA fragment in X. hyacinthi S148 and with a 0.48-kb AluI DNA fragment (Fig. 7B). The latter DNA fragment corresponded with the DNA sequence at positions 227 to 707 in Fig. 3. The other X. hyacinthi isolates showed the same hybridization patterns with the 226-bp probe as strain S148 (data not shown); no signal was obtained for other bacteria tested (Fig. 7A), except for one strain of X. translucens pv. translucens; a 2.0-kb PvuII DNA fragment from strain LMG876 hybridized weakly with the fimA probe (data not shown). Surprisingly, other X. translucens pv. translucens strains, as well as X. translucens pv. cerealis LMG 679, did not hybridize with the probe under the hybridization conditions used (data not shown).
FIG. 7.
Southern blot showing hybridization of the DIG-labelled 226-bp fimA probe with PvuII-digested genomic DNA from isolates of Xanthomonas, Pseudomonas, and Erwinia (A) and genomic DNA from X. hyacinthi S148 (B) to evaluate the presence of fimA homologs. (A) Lane 1, Pseudomonas syringae pv. syringae LMG1247; lane 2, DIG-labelled DNA marker (sizes [in kilobases] are indicated on the left); lane 3, Xanthomonas hortorum pv. pelargonii LMG7314; lane 4, X. axonopodis pv. begoniae NCPPB241; lane 5, X. hyacinthi S148; lane 6, X. translucens pv. phlei UH3231; lane 7, X. translucens pv. translucens LMG876; lane 8, X. translucens pv. graminis LMG726; lane 9, X. translucens pv. poae NCPPB3230; lane 10, Xanthomonas albilineans LMG887; lane 11, Xanthomonas fragariae LMG708; lane 12, Xanthomonas oryzicola LMG797; lane 13, Stenotrophomonas maltophilia LMG958; lane 14, P. aeruginosa LMG1242; lane 15, Erwinia carotovora subsp. carotovora LMG2417; lane 16, Erwinia amylovora LMG2024; lane 17, X. vesicatoria LMG920; lane 18, Pseudomonas fluorescens PD2434. (B) Lane 1, DIG-labelled DNA marker (sizes [in kilobases] are indicated on the left); lanes 2 to 4, X. hyacinthi digested with AluI (lane 2), BamHI (lane 3), or PvuII (lane 4).
Sensitivity of PCR-mediated detection of X. hyacinthi.
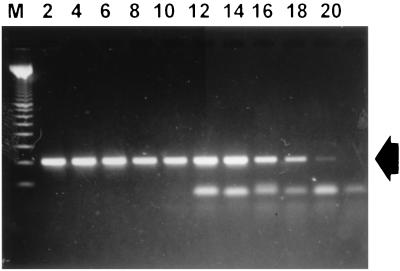
The sensitivity of amplification of the specific DNA fragment of X. hyacinthi isolates was determined by using 10-fold dilutions of a bacterial suspension of strain S148. After PCR with primers JAAN and JARA, as few as 5 cells (1,000 CFU/ml), as estimated by viable counting of corresponding dilutions, could be detected in agarose gels (Fig. 8, lane 18).
FIG. 8.
Sensitivity of the PCR assay performed with nested primers JAAN and JARA. A 5- μl portion of each serially diluted sample taken from an exponentially grown culture (approximately 3 × 108 CFU/ml) of X. hyacinthi S148 was used as a template in PCR. The detection limit was about 5 CFU/5 μl (lane 18). The arrow indicates the 229-bp amplicon from fimA. Lane M contained a 100-bp DNA ladder (Promega); the other lane designations indicate the reciprocal serial dilutions of the culture used in the 1.4% agarose gel.
The sensitivity was also tested for detection of X. hyacinthi in hyacinth leaves with symptoms. Leaf samples from hyacinths with early symptoms of yellow disease were homogenized, diluted, and used in PCR as templates. The sensitivity of the PCR with primers JAAN and JARA appeared to be 5,000 CFU/ml (data not shown). This PCR assay proved to be about 100 times more sensitive, than the direct antibody sandwich (DAS)-ELISA (57) performed with monoclonal antibody 2E5 specific for X. hyacinthi (detection limit with leaf samples, about 500,000 CFU/ml [57]).
General application of type IV fimbrial sequences: identification and detection of X. vesicatoria.
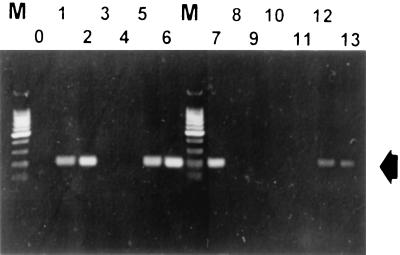
The structural gene coding for the (major) subunit of type IV fimbriae might be used for PCR-mediated detection of other Xanthomonas species at the pathovar level. To test this hypothesis, we developed primers for the recently published fimA sequence of X. campestris pv. vesicatoria NCPPB3240 (39). Oligonucleotide 5′-GCCTCGCTGAGATCAATCCTGG-3′ at nucleotides 382 to 403 and oligonucleotide 5′-TGTCACCTTCTTGCCCACAACC-3′ at nucleotides 563 to 584 amplified a 202-bp DNA fragment in the fimA coding region of X. campestris pv. vesicatoria NCPPB3240. However, not all X. campestris pv. vesicatoria strains showed amplification products (Fig. 9A). Further studies showed that only group A strains (50) were recognized by these primers. This group, including X. campestris pv. vesicatoria NCPPB 3240, has recently been reclassified as X. vesicatoria, and the nonreacting strains (formerly group B) have now been classified as X. axonopodis pv. vesicatoria (30). The other Xanthomonas isolates listed in Table 1 did not cross-react with the primers developed in this study (data not shown). This confirmed the specificity of the X. vesicatoria fimA sequence. The sensitivity limit of this PCR, as determined under laboratory conditions, was approximately 400 CFU/ml (data not shown).
FIG. 9.
Specificity of the PCR with nested primers developed by using the internal sequence of fimA from X. campestris pv. vesicatoria NCPPB3240: analysis of isolates of X. vesicatoria and X. axonopodis pv. vesicatoria. Lane O, negative control; lane 1, X. vesicatoria LMG911; lane 2, X. vesicatoria LMG917; lane 3, X. axonopodis pv. vesicatoria LMG668; lane 4, X. axonopodis pv. vesicatoria LMG905; lane 5, X. vesicatoria LMG920; lane 6, X. vesicatoria LMG925; lane 7, X. vesicatoria NCCPB3240; lane 8, X. axonopodis pv. vesicatoria LMG910; lane 9, X. axonopodis pv. vesicatoria LMG913; lane 10, X. axonopodis pv. vesicatoria LMG922; lane 11, X. axonopodis pv. vesicatoria LMG929; lane 12, X. vesicatoria ATCC 35937; lane 13, X. vesicatoria ATCC 11551; lanes M, 100-bp ladder (Promega). The arrow indicates 202-bp X. vesicatoria-specific amplicons.
DISCUSSION
In this study we characterized the fimA structural fimbrial-subunit gene of X. hyacinthi and developed a detection assay based on amplification of the hypervariable central and C-terminal region of this fimA gene. The presence of fimA homologs in X. hyacinthi was examined by performing hybridization studies (Fig. 7A). When AluI-digested X. hyacinthi DNA was probed with the labelled 226-bp fimA fragment, only a 0.48-kb DNA fragment hybridized (Fig. 7B), indicating the presence of one fimA homolog. Other type IV fimbria-producing bacterial species, such as P. aeruginosa, class I D. nodosus and V. cholerae, also possess a single structural subunit gene (54). However, this does not eliminate the possibility that there are more subunit gene homologs, as found in X. campestris pv. vesicatoria (39) and the bacterial species M. bovis and Eikenella corrodens (51). In X. hyacinthi, another subunit gene might be present downstream of fimA or show too little homology to fimA to be detected. Interestingly, the G+C content of the fimA gene (56%) is significantly lower than the overall G+C content of the Xanthomonas genome (69%). There are indications that horizontal transfer of the genes could have occurred (49).
Translation of the nucleotide sequence of part of the 2,088-bp fragment showed that FimA was preceded by a putative 6-amino-acid leader sequence. The antigenic area of type IV fimbriae is located predominantly in the disulfide loop at the carboxy terminus which is exposed at the tip of the fimbriae (40); for X. hyacinthi FimA this is the peptide sequence EKLRPA. An RpoN-dependent promoter (ς54) was located upstream from fimA (Fig. 3, nucleotides 160 to 174) and had the consensus sequence −27 TGGCAC-N5-TTGCA −11. The ς54 factor has also been found to be a transcriptional regulator of other type IV fimbrial-subunit genes, including the pilin genes of Moraxella spp. (20), P. aeruginosa (51, 54), and X. campestris pv. vesicatoria (39). It is possible that RpoN is required for expression of the fimbrial-subunit gene. However, in contrast to some other type IV-producing bacteria, such as Neisseria meningitidis and M. bovis (51), until now no antigenic or phase variation of fimbriae has been found in X. hyacinthi isolates. As only one fimbrial-subunit gene was found, the presence of antigenic variation is unlikely.
Expression of pCJO containing fimA in E. coli resulted in production of the 17-kDa subunit protein, confirming that transcription of fimA resulted in production of the X. hyacinthi fimbrial subunit. The E. coli K-12 strain used in this experiment does not produce type IV fimbriae although it possesses a number of chromosomal genes that are involved in exoprotein secretion or in the formation of type IV fimbriae (42) and can produce plasmid-encoded type IV pili (32). There have been several reports describing proper expression of native type IV fimbriae of bacterial species, such as D. nodosus, M. bovis, and N. gonorrhoeae (4, 18, 25), on the cell surface of P. aeruginosa and, recently, also in E. coli (48). However, no native X. hyacinthi fimbriae seemed to be assembled or secreted to the surface of E. coli(pCJO2) as no fimbriae were found in shear fractions or were labelled in immunogold experiments (van Doorn, unpublished data).
Nested primers JAAN and JARA amplified a 226-bp fragment in all X. hyacinthi strains. No cross-reactions were found in any of the bacterial species tested, except for weak cross-reactions in X. translucens pv. translucens LMG837 and X. translucens pv. cerealis LMG679. For X. translucens pv. translucens this is in agreement with what was found in previous immunological studies, as strain LMG837 was also recognized by antifimbrial antisera (57). The (incomplete) amino acid sequence of fimA of X. translucens pv. translucens LMG837 differed only at five amino acids, one of which lies in the region where primer JAAN is located in the corresponding DNA sequence. Surprisingly, X. translucens pv. hordei LMG737, which reacted with some of the antifimbrial monoclonal antibodies (57), was not recognized in the PCR. This might reflect the importance of the tertiary structure for immunological recognition. X. translucens pathovars and X. hyacinthi are pathogens of monocotylendonous plants; the G+C contents of their DNA are almost identical and higher than those of the other Xanthomonas species and their pathovars (36, 58). However, no cross-reactions with the other X. translucens pv. translucens isolates (Table 1) were found when they were probed in Southern blots with the labelled 226-bp fimA DNA fragment. This finding might reflect the existence of genetic variation among the X. translucens pv. translucens isolates.
To evaluate the use of fimbrial sequences for development of pathovar-specific PCR assays, X. campestris pv. vesicatoria was selected as its fimA sequence was available (39) and specific primers were designed. Only X. vesicatoria strains were detected with the primers developed, and isolates of X. axonopodis pv. vesicatoria were not detected. This showed that these Xanthomonas species, both of which infect tomatoes (50), differed at least in the fimA nucleotide sequence and that the variability in the type IV fimbrial-subunit composition seemed to reflect the taxonomic differences (30, 31). The sensitivity of PCR-mediated detection of X. campestris pv. vesicatoria NCPPB3240 under laboratory conditions was much higher than the sensitivity obtained with immunological methods (29).
For detection of the first symptoms of yellow disease in hyacinth, amplified sensitivity combined with high specificity was observed during PCR-mediated detection performed with the variable part of the fimA gene as the target. With nested primers JAAN and JARA the sensitivity was approximately 5,000 CFU/ml, which is sufficient to monitor even the first stages of infection by X. hyacinthi. Under routine conditions, the detection limit in hyacinth leaf extract is close to 500,000 CFU/ml (57). PCR detection of X. hyacinthi in leaves with symptoms was successfully conducted without prior DNA extraction and purification. The appeal of this PCR lies not only in more sensitive detection of yellow disease but also in confirmation of ELISA data and in large-scale screening of hyacinth tissue culture material to be used for propagation of (new) hyacinth cultivars, which should be absolutely free of yellow disease. Also, fast, sensitive, specific monitoring of hydrocultures of hyacinths and equipment used for handling, sorting, or rinsing hyacinth bulbs for the presence of this xanthomonad might be possible by applying the PCR assay to samples taken from these materials.
Most of the previously described methods for PCR-mediated detection of Xanthomonas pathovars are based on amplification of unknown sequences (23, 24, 33, 60). Ribosomal sequences are frequently used but are not pathovar specific (36, 37). Amplified sequences of Xanthomonas genes involved in the hypersensitive reaction and pathogenicity (hrp genes) are pathovar specific only after restriction fragment length polymorphism analysis of the amplicon (34). A PCR based on a characterized DNA sequence such as the type IV fimbrial subunit has certain advantages. As shown for X. hyacinthi and X. vesicatoria, the variable C-terminal sequence of fimA might be unique for Xanthomonas at the pathovar level. The variation in the molecular masses of the fimbrial subunits in different Xanthomonas pathovars (56) supports this theory. As a general strategy for the development of a specific detection assay, the fimA sequence of a certain Xanthomonas pathovar could be determined and used to develop primers for a nested PCR. A similar PCR has been described for V. cholerae; in this PCR the nucleotide sequence of type IV fimbrial-subunit tcpA of V. cholerae is used (46).
The type IV fimbriae of plant-pathogenic bacteria might be involved in several functions: the formation of microfilms on the leaf surface, attachment to stomates and hydathodes, and twitching motility. The type IV fimbriae of X. campestris pv. vesicatoria are associated with aggregation (39). Recently, another type of fimbriae was found in Xanthomonas (38). These hrp fimbriae, originally characterized in Pseudomonas syringae (44), are excreted by the type III export system and are not related to type IV fimbriae.
In conclusion, a PCR assay which can be used for highly sensitive detection of yellow disease in hyacinth plants has been developed.
ACKNOWLEDGMENT
We thank M. Romantschuk for critical reading of the manuscript.
REFERENCES
- 1.Altschuk S F, Madden T L, Schaffer A A, Zhang J, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez A M, Benedict A A, Mizumoto C Y, Pollard L W, Civerolo E L. Analysis of Xanthomonas campestris pv. citri and Xanthomonas campestris pv. citrumelo with monoclonal antibodies. Phytopathology. 1991;81:857–865. [Google Scholar]
- 3.Barrios H, Valderrama B, Morret E. Complementation and analysis of ς54 - dependent promoter sequences. Nucleic Acids Res. 1999;27:4305–4313. doi: 10.1093/nar/27.22.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beard M K M, Mattick J S, Moore L J, Mott M R, Marrs C F, Egerton J R. Morphogenetic expression of Moraxella bovis fimbriae (pili) in Pseudomonas aeruginosa. J Bacteriol. 1990;172:2601–2607. doi: 10.1128/jb.172.5.2601-2607.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benedict A A, Alvarez A M, Berestecky J, Imanaka W, Mizumoto C Y, Pollard L W, Mew T W, Gonzalez C F. Pathovar-specific monoclonal antibodies for Xanthomonas campestris pv. oryzae and for Xanthomonas campestris pv. oryzicola. Phytopathology. 1989;79:322–328. [Google Scholar]
- 6.Benedict A A, Alvarez A M, Pollard L W. Pathovar-specific antigens of Xanthomonas campestris pv. begoniae and X. campestris pv. pelargonii detected with monoclonal antibodies. Appl Environ Microbiol. 1990;56:572–574. doi: 10.1128/aem.56.2.572-574.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berthier Y, Thierry D, Lemattre M, Guesdon J-L. Isolation of an insertion sequence (IS1051) from Xanthomonas campestris pv. dieffenbachiae with potential use for strain identification and characterization. Appl Environ Microbiol. 1994;60:377–384. doi: 10.1128/aem.60.1.377-384.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berthier Y, Verdier V, Guesdou J-L, Chevrier D, Denis J-B, DeCoux G, Lemattre M. Characterization of Xanthomonas campestris pathovars by rRNA gene restriction patterns. Appl Environ Microbiol. 1993;59:851–859. doi: 10.1128/aem.59.3.851-859.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouzar H, Jones J B, Minsavage G V, Stall R E, Scott J W. Proteins unique to phenotypically distinct groups of Xanthomonas campestris pv. vesicatoria revealed by silver staining. Phytopathology. 1994;84:39–44. [Google Scholar]
- 10.Bouzar H, Jones J B, Stall R E, Hodge N C, Minsavage G V, Benedict A A, Alvarez A M. Physiological, chemical, serological, and pathogenic analyses of a worldwide collection of Xanthomonas campestris pv. vesicatoria strains. Phytopathology. 1994;84:663–671. [Google Scholar]
- 11.Castric P A, Deal C D. Differentiation of Pseudomonas aeruginosa pili based on sequence and B-cell epitope analyses. Infect Immun. 1994;62:371–376. doi: 10.1128/iai.62.2.371-376.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen W-P, Kuo T-T. A simple and rapid method for the preparation of gram-negative bacterial genomic DNA. Nucleic Acids Res. 1993;21:2260. doi: 10.1093/nar/21.9.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Groot A, Heijnen I, de Cock H, Filloux A, Tommassen J. Characterization of type IV pilus genes in plant-growth promoting Pseudomonas putida WCS358. J Bacteriol. 1994;176:642–650. doi: 10.1128/jb.176.3.642-650.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeParasis J, Roth D A. Nucleic acid probes for identification of phytobacteria: identification of genus-specific 16S rRNA sequences. Phytopathology. 1990;80:618–621. [Google Scholar]
- 15.Don R H, Cox P T, Wainright B J, Baker K, Mattick J S. Touchdown PCR to circumvent spurious priming during gene amplification. Nucleic Acids Res. 1991;19:4008. doi: 10.1093/nar/19.14.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dums F, Dow J M, Daniels M J. Structural characterization of protein secretion genes of the bacterial phytopathogen Xanthomonas campestris pv. campestris; relatedness to secretion systems of other gram negative bacteria. Mol Gen Genet. 1991;229:357–364. doi: 10.1007/BF00267456. [DOI] [PubMed] [Google Scholar]
- 17.Elleman T C. Pilins of Bacteroides nodosus: molecular basis of serotypic variation and relationships to other bacterial pilins. Microbiol Rev. 1988;52:233–247. doi: 10.1128/mr.52.2.233-247.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elleman T C, Hoyne P A, Stewart D J, McKern N M, Peterson J E. Expression of pili from Bacteroides nodosus in Pseudomonas aeruginosa. J Bacteriol. 1986;168:574–580. doi: 10.1128/jb.168.2.574-580.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forest K T, Bernstein S L, Getzoff E D, So M, Tribbick G, Geysen H M, Deal C D, Tainer J A. Assembly and antigenicity of the Neisseria gonorrhoeae pilus mapped with antibodies. Infect Immun. 1996;64:644–658. doi: 10.1128/iai.64.2.644-652.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fulks K A, Marrs C F, Stevens S P, Green M R. Sequence analysis of the inversion region containing the pilin genes of Moraxella bovis. J Bacteriol. 1990;172:310–316. doi: 10.1128/jb.172.1.310-316.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fullner K J, Mekalanos J J. Genetic characterization of a new type IV-A pilus gene cluster found in both classical and El Tor biotypes of Vibrio cholerae. Infect Immun. 1999;67:1393–1404. doi: 10.1128/iai.67.3.1393-1404.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hartung J S. Plasmid-based hybridization probes for detection and identification of Xanthomonas campestris pv. citri. Plant Dis. 1992;76:889–893. [Google Scholar]
- 23.Hartung J S, Daniel J F, Pruvost O P. Detection of Xanthomonas campestris pv. citri by the polymerase chain reaction method. Appl Environ Microbiol. 1993;59:1143–1148. doi: 10.1128/aem.59.4.1143-1148.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartung J S, Pruvost O P, Villemot I, Alvarez A M. Rapid and sensitive colorimetric detection of Xanthomonas axonopodis pv. citri by immunocapture and a nested-polymerase chain reaction assay. Phytopathology. 1996;86:95–101. [Google Scholar]
- 25.Hoyne P A, Haas R, Meyer T F, Davies J K, Elleman T C. Production of Neisseria gonorrhoeae pili (fimbriae) in Pseudomonas aeruginosa. J Bacteriol. 1992;174:7321–7327. doi: 10.1128/jb.174.22.7321-7327.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang S-H, Wu C-H, Cai B, Holcenberg C. cDNA cloning by inverse polymerase chain reaction. Methods Mol Biol. 1993;15:349–356. doi: 10.1385/0-89603-244-2:349. [DOI] [PubMed] [Google Scholar]
- 27.Innes M A. PCR with 7-deaza-2′-deoxyguanosine triphosphate. In: Innis M A, Gelfland D H, Sninsky J J, White T J, editors. PCR protocols: a guide to methods and applications. San Diego, Calif: Academic Press, Inc.; 1990. pp. 54–59. [Google Scholar]
- 28.Janse J D, Miller H J. Yellow disease in Scilla tubergeniana and related bulbs caused by Xanthomonas campestris pv. hyacinthi. Neth J Plant Pathol. 1983;89:203–206. [Google Scholar]
- 29.Jones J B, Sodomi G C, Scott J W. Increased ELISA sensitivity using a modified extraction buffer for detection of Xanthomonas campestris pv. vesicatoria in leaf tissue. J App Microbiol. 1997;83:397–401. doi: 10.1046/j.1365-2672.1997.00251.x. [DOI] [PubMed] [Google Scholar]
- 30.Jones J B, Stall R E, Bouzar H. Diversity among xanthomonads pathogenic on pepper and tomato. Annu Rev Phytopathol. 1998;36:41–58. doi: 10.1146/annurev.phyto.36.1.41. [DOI] [PubMed] [Google Scholar]
- 31.Jones J B, Stall R E, Scott J W, Somodi G C, Bouzar H, Hodge N C. A third tomato race of Xanthomonas campestris pv. vesicatoria. Plant Dis. 1995;79:395–398. [Google Scholar]
- 32.Kim S-R, Komano T. The plasmid R64 thin pilus identified as a type IV pilus. J Bacteriol. 1997;179:3594–3603. doi: 10.1128/jb.179.11.3594-3603.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leite R P, Jr, Jones J B, Somodi G V, Stall R E. Detection of Xanthomonas campestris pv. vesicatoria associated with pepper and tomato seeds by DNA amplification. Plant Dis. 1995;79:917–922. [Google Scholar]
- 34.Leite R P, Minsavage G V, Bonas U, Stall R E. Detection and identification of phytopathogenic Xanthomonas strains by amplification of DNA sequences related to the hrp genes of Xanthomonas campestris pv. vesicatoria. Appl Environ Microbiol. 1994;60:1068–1077. doi: 10.1128/aem.60.4.1068-1077.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Louws F J, Fulbright D W, Taylor Stephens C, de Bruijn F J. Specific genomic fingerprints of phytopathogenic Xanthomonas and Pseudomonas pathovars and strains generated with repetitive sequences and PCR. Appl Environ Microbiol. 1994;60:2286–2295. doi: 10.1128/aem.60.7.2286-2295.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maes M, Garbeva P, Kamoen O. Recognition and detection in seed of the Xanthomonas pathogens that cause cereal leaf streak using rDNA spacer sequences and polymerase chain reaction. Phytopathology. 1996;86:63–69. [Google Scholar]
- 37.Maes M. Fast classification of plant associated bacteria in the Xanthomonas genus. FEMS Microbiol Lett. 1993;113:161–166. [Google Scholar]
- 38.Ojanen-Reuhs T. Xanthomonas cell surface: molecular variation and role in pathogenesis. Ph.D. thesis. Finland: University of Helsinki; 2000. [Google Scholar]
- 39.Ojanen-Reuhs T, Kalkkinen N, Westerlund- Wikstrom B, van Doorn J, Haahtela K, Nurmiaho-Lassila E-L, Wengelink K, Bonas U, Korhonen T K. Characterization of the fimA gene encoding bundle-forming fimbriae of the plant pathogen Xanthomonas campestris pv. vesicatoria. J Bacteriol. 1997;179:1280–1290. doi: 10.1128/jb.179.4.1280-1290.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parge H E, Forest K T, Hickey M J, Christensen D A, Getzoff E D, Tainer J A. Structure of the fibre-forming protein pilin at 2.6 Å resolution. Nature. 1995;378:32–38. doi: 10.1038/378032a0. [DOI] [PubMed] [Google Scholar]
- 41.Pooler M R, Ritchie D F, Hartung J S. Genetic relationships among strains of Xanthomonas fragariae based upon random amplified polymorphic DNA PCR, repetitive extragenic palindromic PCR, and enterobacterial repetitive intergenic consensus PCR data and generation of multiplexed PCR primers useful for the identification of this phytopathogen. Appl Environ Microbiol. 1996;62:3121–3127. doi: 10.1128/aem.62.9.3121-3127.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pugsley A P, Francetic O. Protein secretion in Escherichia coli K-12: dead or alive? Cell Mol Life Sci. 1998;54:347–352. doi: 10.1007/s000180050162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qhobela Q, Claflin L E. Eastern and Southern African strains of Xanthomonas campestris pv. vasculorum are distinguishable by restriction fragment length polymorphism of DNA and polyacrylamide gel electrophoresis of membrane proteins. Plant Pathol. 1992;41:113–121. [Google Scholar]
- 44.Roine E, Wei W, Yuan J, Nurmiaho-Lassila E-L, Kalkkinen N, Romantschuk M, He S Y. Hrp pilus: an hrp-dependent bacterial surface appendage produced by Pseudomonas syringae pv. tomato DC3000. Proc Natl Acad Sci USA. 1997;94:3459–3464. doi: 10.1073/pnas.94.7.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Russel M. Macromolecular assembly and secretion across the bacterial cell envelope: type II protein secretion systems. J Mol Biol. 1998;279:485–499. doi: 10.1006/jmbi.1998.1791. [DOI] [PubMed] [Google Scholar]
- 46.Said B, Smith H R, Scotland S M, Rowe B. Detection and differentiation of the gene for toxin co-regulated pili (tcpA) in Vibrio cholerae non-O1 using the polymerase chain reaction. FEMS Microbiol Lett. 1995;125:205–210. doi: 10.1111/j.1574-6968.1995.tb07359.x. [DOI] [PubMed] [Google Scholar]
- 47.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Press; 1989. [Google Scholar]
- 48.Sauvonnet N, Gounou P, Pugsley A P. PpdD type IV pilin of Escherichia coli K-12 can be assembled into pili in Pseudomonas aeruginosa. J Bacteriol. 2000;182:848–854. doi: 10.1128/jb.182.3.848-854.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spangenberg C, Fisley R, Römling U, Tümmler B. Disrespectful type IV pilins. Mol Microbiol. 1997;25:203–204. [PubMed] [Google Scholar]
- 50.Stall R E, Beaulieu C, Egel D, Hodge N C, Leite R P, Minsavage G V, Bouzar H, Jones J B, Alvarez A M, Benedict A A. Two genetically diverse groups of strains are included in Xanthomonas campestris pv. vesicatoria. Int J Syst Bacteriol. 1994;44:47–53. [Google Scholar]
- 51.Strom M S, Lory S. Structure-function and biogenesis of the type IV pili. Annu Rev Microbiol. 1993;47:565–596. doi: 10.1146/annurev.mi.47.100193.003025. [DOI] [PubMed] [Google Scholar]
- 52.Su W-C, Tung S-Y, Yang M-K, Kuo T-T. The pilA gene of Xanthomonas campestris pv. citri is required for infection by the filamentous phage cf. Mol Gen Genet. 1999;262:22–26. doi: 10.1007/s004380051055. [DOI] [PubMed] [Google Scholar]
- 53.Sulzinski M A, Moorman G W, Schlagnhaufer G W, Romaine C P. Fingerprinting of Xanthomonas campestris pv. pelargonii and related pathovars using random-primed PCR. J Phytopathol. 1995;143:429–433. [Google Scholar]
- 54.Tennant J M, Mattick J S. Type 4 fimbriae. In: Klemm P, editor. Fimbriae, adhesion, genetics, biogenesis, and vaccines. Boca Raton, Fla: CRC Press; 1994. pp. 127–146. [Google Scholar]
- 55.van Doorn J, Roebroeck E J A. Xanthomonas campestris pv. hyacinthi: cause of yellow disease in Hyacinthus. In: Swings J G, Civerolo E L, editors. Xanthomonas. London, United Kingdom: Chapman & Hall; 1993. pp. 83–91. [Google Scholar]
- 56.van Doorn J, Boonekamp P M, Oudega B. Partial characterization of fimbriae of Xanthomonas campestris pv. hyacinthi. Mol Plant-Microbe Interact. 1994;7:334–344. doi: 10.1094/mpmi-7-0334. [DOI] [PubMed] [Google Scholar]
- 57.van Doorn J, Ojanen-Reuhs T, Hollinger T C, Reuhs B L, Schots A, Boonekamp P M, Oudega B. Development and application of pathovar-specific monoclonal antibodies that recognize the lipopolysaccharide O antigen and the type IV fimbriae of Xanthomonas hyacinthi. Appl Environ Microbiol. 1999;65:4171–4180. doi: 10.1128/aem.65.9.4171-4180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vauterin L, Hoste B, Kerstens K, Swings J. Reclassification of Xanthomonas. Int Syst Bacteriol. 1995;45:472–489. [Google Scholar]
- 59.Verdier V, Mosquera G. Specific detection of Xanthomonas axonopodis pv. manihotis with a DNA hybridization probe. J Phytopathol. 1999;147:417–423. [Google Scholar]
- 60.Verdier V, Mosquera G, Assigbétsé K. Detection of the cassava bacterial blight pathogen, Xanthomonas axonopodis pv. manihotis, by polymerase chain reaction. Plant Dis. 1998;82:79–83. doi: 10.1094/PDIS.1998.82.1.79. [DOI] [PubMed] [Google Scholar]