Abstract
Background
Peripheral blood leukocyte telomere length (PBL-TL) is associated with outcomes in idiopathic pulmonary fibrosis patients. Whether PBL-TL is associated with progression of systemic sclerosis-associated interstitial lung disease (SSc-ILD) is unknown.
Methods
A retrospective observational cohort study was performed using prospectively collected data from 213 SSc patients followed at the University of California San Francisco (UCSF) Scleroderma Center. PBL-TL was measured by qPCR of DNA isolated from peripheral blood. Associations between PBL-TL and PFT trends in patients with SSc-ILD were assessed by longitudinal analysis using Generalized Linear Mixed Models. Findings were validated in a cohort of 61 SSc-ILD patients enrolled in the Stanford University (SU) Scleroderma Center database.
Results
UCSF SSc patients with ILD were found to have shorter PBL-TL compared to those without ILD (6554± 671 base pairs (bp) vs 6782 ± 698 bp, p=0.01). Shorter PBL-TL was associated with the presence of ILD (adjusted odds ratio [OR] 2.1 per 1000 bp TL decrease, 95%CI [1.25–3.70], p=0.006). PBL-TL was shorter in SSc-ILD patients lacking SSc-specific autoantibodies compared to seropositive subjects (6237± 647 bp vs 6651± 653 bp, p=0.004). Shorter PBL-TL was associated with increased risk for lung function deterioration with an average of 67 ml greater loss in FVC per year for every 1000 bp decrease in PBL-TL in the combined SSc-ILD cohorts (longitudinal analysis, adjusted model: 95% CI −104ml to −33ml, p<0.001).
Conclusions
These findings suggest that telomere dysfunction may be associated with SSc-ILD progression and that PBL-TL measurement may be useful for stratifying risk for SSc-ILD progression.
Keywords: Interstitial Lung Disease, pulmonary fibrosis, scleroderma, DNA damage
Introduction
Interstitial lung disease (ILD) affects up to 90% of patients with systemic sclerosis (SSc) and is the leading cause of death1. Although in many patients with SSc-ILD lung function worsens over time, identifying subjects who are at risk for progression is challenging. Currently, effective management of SSc-ILD revolves around early detection of lung fibrosis and assessment of its severity. Pulmonary function tests (PFTs) play a key role in monitoring and quantifying the progression of SSc-ILD. Disease severity at baseline and pulmonary function trends, such as decline in forced vital capacity (FVC) are associated with mortality in SSc-ILD2.
Serum biomarkers have been associated with outcomes in SSc-ILD. These are circulating proteins made by epithelial cells (e.g. Surfactant protein D: SP-D3, 4, Krebs von den Lungen-6: KL-6 4, Tissue inhibitor of matrix metalloproteinases-1: TIMP-1 5, Growth differentiation factor 15: GDF-15 6), immune cells (e.g. CC chemokine ligand 18: CCL183, 7, serum interleukin 15: IL-15, Chitinase-1 8, Pentraxin-3 9), and oxidative stress pathways 10. While studies have shown that levels of these biomarkers6, 11, 12 correlate with pulmonary ventilation function (FVC, diffusing capacity for carbon monoxide: DLCO) at baseline in SSc-ILD, most are not prognostic other than IL-6, which appears to be prognostic early in SSc-ILD 13. Recently the SADL model—which incorporates age, ever smoking history, and percent predicted DLCO—was developed to predict mortality risk for SSc-ILD patients14. However, this model does not predict change in lung function, and can not be used in patients who are unable to perform the DLCO maneuver.
Telomeres are nucleoprotein structures that protect the ends of chromosomes from degradation, and progressively shorten with cell division and aging. Shorter peripheral blood leukocyte telomere length (PBL-TL) is associated with loss of FVC and worse survival in patients with idiopathic pulmonary fibrosis (IPF)15, chronic hypersensitivity pneumonitis (cHP)16, unclassifiable ILD17, and interstitial pneumonia with autoimmune features18 and is increasingly recognized as a molecular driver of these diseases 19. Previous studies of telomere length (TL) in patients with SSc have reported shorter TLs20 in SSc patients, especially in lymphocytes of SSc-ILD patients21. Because there may be shared pathobiology between various clinical subtypes of pulmonary fibrosis, and there have been no studies investigating the relationship between TL and outcomes of SSc-ILD patients, in this study we tested whether PBL-TL is associated with progression of SSc-ILD.
Methods
Study design and patient populations
This is a retrospective study analyzing two observational cohorts of 213 SSc patients (including both ILD and non-ILD patients) prospectively enrolled at the University of California, San Francisco, CA, (UCSF) ILD and Scleroderma Centers from January 2005 to February 2017, and a separate validation cohort of 61 SSc-ILD patients seen at the Stanford University (SU) Scleroderma Center clinic from October 2008 to July 2018. All patients met the 2013 American College of Rheumatology/EUropean League Against Rheumatism criteria for systemic sclerosis 22. Patients were considered to have ILD based on high resolution computed tomography (HRCT) of the chest at the time of first visit. The study was approved by the Institutional Review Boards at UCSF and SU. Written informed consent was obtained from all subjects.
Clinical data
Socio-demographic and clinical data including age, sex, body mass index (BMI), ever smoking history, ethnicity, disease subtype classified as either limited cutaneous SSc (including sine sclerosis) or diffuse cutaneous SSc, the presence of autoantibodies and medication use were recorded. Patients were categorized as nonsmokers, current smokers, or ex-smokers (defined as ≥ 1 cigarette per day for at least 1 year, stopping at least 6 months before enrollment). Pulmonary hypertension (PH) was defined based on echocardiographic evidence of an estimated pulmonary arterial systolic pressure greater than or equal to 40 mmHg or a mean pulmonary artery pressure of > 25 mmHg at right heart catheterization 23.
Pulmonary function data
PFT tests obtained at baseline (PFT closest to enrollment; within 6 months of first clinic visit date) and during the subsequent 24 months were analyzed. Patients were included in longitudinal analysis if they had at least 2 PFTs within 2 years following diagnosis. PFT trends were analyzed as continuous change and categorical change in separate models. Categorical deterioration of lung function at 12 months was defined as a relative decline in FVC (% predicted) greater than 10% or relative decline in DLCO (% predicted) greater than 15%.
Radiographic assessments
Chest HRCT images were reviewed by the same expert chest radiologist for consistency in both cohorts. The pattern of HRCT abnormality was defined as definite usual interstitial pneumonia (UIP), probable UIP, indeterminant for UIP, or suggestive of an alternative diagnosis24. For the UCSF SSc-ILD cohort, HRCTs were also scored for presence or absence of parenchymal fibrosis, groundglass opacities (GGO), consolidation, airway changes (mosaic perfusion, air trapping), nodules, emphysema, cysts, interlobular septal thickening or subpleural sparing and extent of honeycombing as the percentage of total lung volume involved (none, mild [<10%], moderate [10–50%], or severe [>50%]) as previously described25.
PBL-TL measurements
PBL genomic DNA was isolated from UCSF patients using the Gentra Puregene cell kit (from Qiagen, Valencia, CA, USA), and from the SU cohort using the PAXgene blood DNA kit (BD biosciences, Franklin Lakes, NJ, USA). DNA was visualized on agarose gel to determine quality, and degraded DNA samples were excluded from the analysis. Average PBL TLs were measured while blinded to the study endpoints using quantitative uniplex qPCR in triplicate with the acidic ribosomal phosphoprotein 36B4 gene as a reference housekeeping gene, as previously described 16, 26–28. Average relative TLs were determined by subtracting telomere and reference median cycle threshold (CT) values. TLs were calculated by comparison to reference samples29. Using these methods, the intra-assay coefficient of variation (CV) was <1%, the interassay CV was <3% and the intraclass correlation coefficient for triplicate measurements was 0.98 (95% CI 0.97–0.99) for both cohorts.
MUC5B Genotyping
MUC5B rs35705950 single-nucleotide polymorphism (SNP) was measured with the Taqman SNP Genotyping Assays (Applied Biosystems, Foster City, CA, USA).
Statistical analysis
All analyses were performed using the R software package, v 3.0.1 (http://www.R-project.org/). Clinical characteristics of the study cohorts were compared using Student’s t-test or Kruskal-Wallis rank sum test for continuous variables and χ2 or Fisher’s exact tests for categorical variables. Telomere length was modeled as a continuous variable in univariate and multivariable analyses. To evaluate the independent association of PBL-TL and the presence of ILD in patients with SSc, a multivariable logistic regression model was conducted. For the SNP genotype association analyses, minor allele frequency (MAF) was calculated in each population as the total number of minor alleles divided by the total number of alles in the population. Longitudinal PFT analysis was performed using Generalized Linear Mixed Models for the UCSF cohort, the SU cohort, and the combined SSc-ILD cohort. Associations between telomere length and binary clinical and radiographic variables were assessed with Student’s t-test, and between telomere length and continuous clinical variables with Pearson’s correlation coefficient. The baseline PFT measure variables were log transformed to correct the nonlinearity of the relationships and to stabilize the variance when Pearson’s correlation coefficient were evaluated. A multivariable model was built adjusting for age, male sex, body mass index (BMI), ethnicity, smoking status, antitopoisomerase antibodies, and disease subtype. Missing data were omitted and managed using the default ‘na.omit’ setting under the glm function in R. P-values <0.05 were considered statistically significant.
Results
UCSF Cohort characteristics
Demographic data, clinical characteristics and baseline PFT variables for the UCSF cohort are summarized in Table 1. UCSF SSc patients (n=213) were mostly middle age (mean 55.6 ±13.0 years) women (85%) with ILD present in 134 (63%) subjects. ILD was associated with anti-topoisomerase I positivity (37.3% vs 13.9%, p-value <0.001). PBL-TL showed a normal distribution (Supplement Figure 1) and, as expected, exhibited a linear decrement with age (r= −0.28, p-value<0.001). The MUC5B rs35705950 minor allele frequency did not differ significantly between SSc patients with and without ILD (Table 1). However, PBL-TL was shorter in patients carrying a MUC5B minor allele (6245± 354 bp) compared to homozygous wild-type (6609± 699 bp, p<0.001).
Table 1:
Demographic and clinical characteristics of the UCSF SSc cohort
| UCSF cohort (n=213) |
|||
|---|---|---|---|
| SSc-ILD (n=134) |
SSc no ILD (n=79) |
P-value | |
| Age, mean (SD) | 55.5 (29.5) | 55.9 (13.7) | 0.84 |
| Female, n (%) | 107 (79.9) | 74 (93.7) | 0.002 |
| BMI, mean (SD) | 25.2 (5.3) | 24.3 (5.1) | 0.23 |
| Race, n (%) | 0.60 | ||
| White, non-Hispanic/Latino | 81 (60.4) | 56 (70.9) | |
| Hispanic or Latino | 6 (4.5) | 3 (3.8) | |
| African American | 13 (9.7) | 7 (8.9) | |
| Asian | 21 (15.7) | 9 (11.4) | |
| Other/unknown | 13 (9.7) | 4 (5.0) | |
| Smoking status, n (%) | 0.13 | ||
| Never smoker | 83 (61.9) | 56 (70.9) | |
| Current smoker | 3 (2.2) | 3 (3.8) | |
| Former smoker | 48 (35.8) | 20 (25.3) | |
| Pulmonary function tests, mean (SD) | |||
| FVC, %Predicted | 70.3 (19.4) | 92.5 (18.0) | <0.001 |
| FEV1, %Predicted | 71.4 (19.2) | 91.3 (19.6) | <0.001 |
| DLCO, %Predicted | 52.4 (19.5) | 71.9 (19.3) | <0.001 |
| Anti-topoisomerase antibody, n (%) | <0.001 | ||
| Positive | 50 (37.3) | 11 (13.9) | |
| Unknown | 6 (4.5) | 2 (2.6) | |
| SSc subtype, n (%) | 0.37 | ||
| Limited | 73 (54.5) | 50 (63.3) | |
| Diffuse | 38 (28.4) | 22 (27.8) | |
| Pulmonary hypertension*, n (%) | 32 (23.9) | 15 (19.0) | 0.40 |
| High-resolution CT available for scoring, n (%) | 99 (73.9) | ND | |
| Fibrosis | 81 (81.8) | ||
| Diffuse ground-glass opacities | 27 (27.3) | ||
| Consolidation | 9 (9.1) | ||
| Mosaic perfusion, air-trapping, or both | 18 (18.2) | ||
| Nodules | 11 (11.1) | ||
| Emphysema | 8 (8.1) | ||
| Cysts | 11 (11.1) | ||
| Subpleural sparing | 49 (49.5) | ||
| UIP pattern | |||
| Definite | 6 (6.1) | ||
| Probable | 15 (15.2) | ||
| Indeterminate | 5 (5.1) | ||
| Alternative diagnosis | 73 (73.7) | ||
| Death, n (%) | 9 (6.7) | 4 (5.1) | 0.85 |
| MUC5B rs35705950 genotype | 0.69 | ||
| GG, n (%) | 114 (85.1) | 69 (87.3) | |
| GT, n (%) | 20 (14.9) | 10 (12.7) | |
| TT, n (%) | 0 | 0 | |
| MAF | 7.5% | 6.3% | |
Pulmonary hypertension was defined based on echocardiographic evidence of an estimated pulmonary arterial systolic pressure greater than or equal to 40 mmHg or a mean pulmonary artery pressure of > 25 mmHg at right heart catheterization.
UCSF, University of California San Francisco; SSc, systemic sclerosis; ILD, interstitial lung disease; BMI, body mass index; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; DLCO, diffusing capacity for carbon monoxide; CT, computed tomography; ND, not done; UIP, usual interstitial pneumonia; MAF, minor allele frequency.
PBL-TL is associated with the presence of ILD in the UCSF SSc cohort
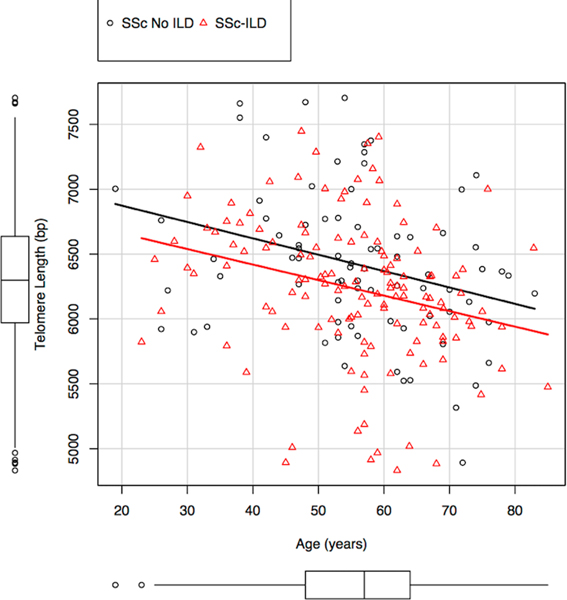
SSc patients with ILD had shorter PBL-TL than those without ILD (6554± 671 vs 6782 ± 698 base pairs (bp), p-value=0.01, adjusted by age, Figure 1). Shorter PBL-TL remained associated with ILD after controlling for age, male sex, BMI, ethnicity, smoking history, anti-topoisomerase status, and disease subtype in a multiple logistic regression analysis (OR 2.11 per 1000 bp TL decrease, 95% CI 1.25–3.70, p-value=0.006, Table 2). PBL-TL was shorter in patients with pulmonary hypertension (PAH) (47 patients had PAH by echo and 43 by right heart catheterization) compared to controls (6295± 420 bp vs 6897± 703 bp, p-value=0.0001, Supplement Figure 2). Finally, PBL-TL was shorter in patients with limited SSc (6491± 687 bp) compared to those with diffuse SSc (6787± 648 bp, p-value =0.005).
Figure 1-.
Scatterplot of PBL-TL versus age in UCSF SSc patients with or without ILD.png
Table 2:
Shorter PBL-TL is a risk factor for ILD in UCSF SSc patients in a multivariable logistic regression model
| OR | 95%CI | P-value | |
|---|---|---|---|
| Age | 0.99 | 0.97–1.02 | 0.60 |
| Male gender | 0.42 | 0.12–1.21 | 0.12 |
| BMI | 1.05 | 0.98–1.13 | 0.19 |
| Hispanic ethnicity | 1.97 | 0.73–5.78 | 0.20 |
| Smoking history | 1.16 | 0.80–1.68 | 0.43 |
| Anti-topoisomerase antibody | 4.61 | 2.05–11.23 | <0.001 |
| SSc subtype, diffuse | 1.49 | 0.74–3.08 | 0.27 |
| TL (per 1kb decrease) | 2.11 | 1.25–3.70 | 0.006 |
PBL, peripheral blood leukocyte; TL, telomere length; ILD: interstitial lung disease; UCSF, University of California San Francisco; SSc, systemic sclerosis; OR, odds ratio; CI, confidence interval; BMI, body mass index.
PBL-TL was shorter in SSc-ILD patients lacking SSc-specific autoantibodies compared to seropositive subjects in the UCSF cohort
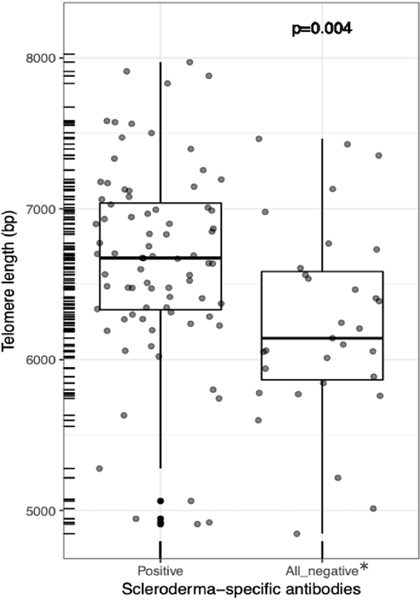
At least one SSc-associated autoantibody (anti-topoisomerase, anti-centromere, anti-RNA polymerase III, or U1-ribonucleoprotein) was detected in 59.7% of SSc-ILD patients. Subjects positive for anti-topoisomerase had a greater rate of decline in DLCO than those negative (−4.0%/year vs 0.5%/year, p-value=0.04) for this autoantibody, and a trend for more rapid FVC decline ( −3.3%/year vs −0.6%/year, p-value=0.07). Interestingly, PBL-TL was shorter in SSc-ILD patients lacking any of the common SSc-specific autoantibody compared to seropositive subjects (6237± 647 bp vs 6651± 653 bp, Figure 2, p-value=0.004, adjusted by age).
Figure 2-.
PBL-TLs are shorter in UCSF SSc-ILD patients lacking SSc-specific autoantibodies.png
Longer PBL TL was associated with ground glass opacities on chest HRCT in UCSF SSc-ILD patients
HRCTs were available for visual scoring in 99 (73.9%) UCSF SSc-ILD patients. The vast majority (73.7%) had a radiographic pattern consistent with nonspecific interstitial pneumonia (NSIP), 15.2% of patients had a pattern consistent with probable UIP and 6.1% of patients had an HRCT pattern consistent with UIP (Table 1). Radiographic signs of fibrosis were detected in 81.8% of patients. Among the radiographic abnormalities evaluated, the only significant association was between the presence of diffuse ground glass opacities (GGO) and longer PBL-TL (6815± 665 bp vs 6460± 650 bp, p-value=0.02, Supplement Figure 3; Supplement Table 1).
Shorter PBL-TL was associated with increased risk for FVC deterioration in UCSF SSc-ILD patients
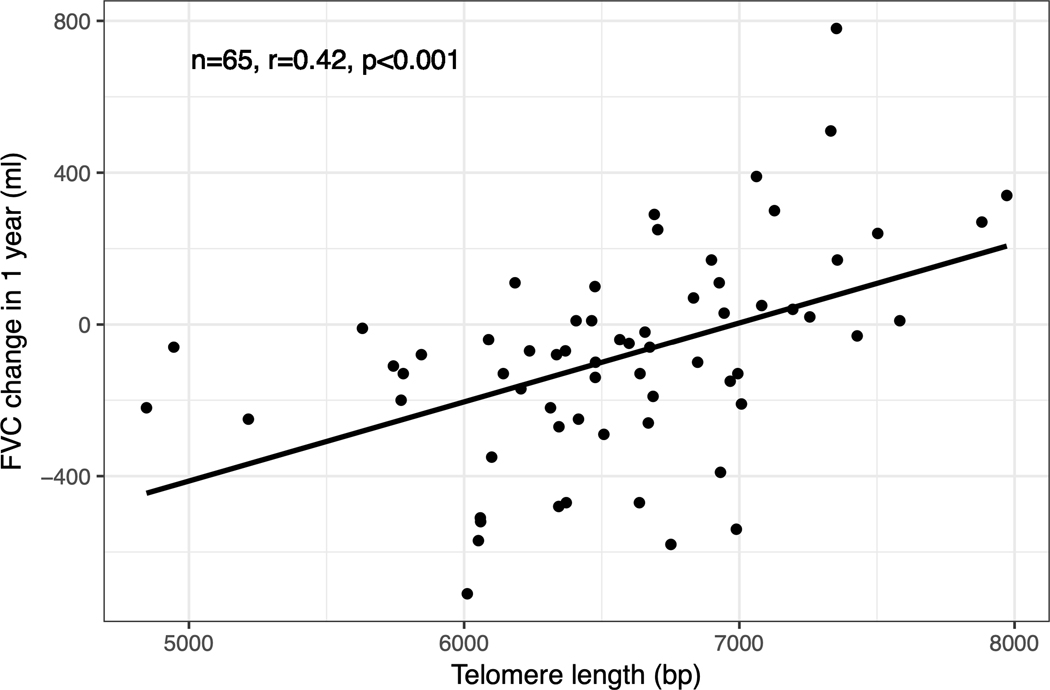
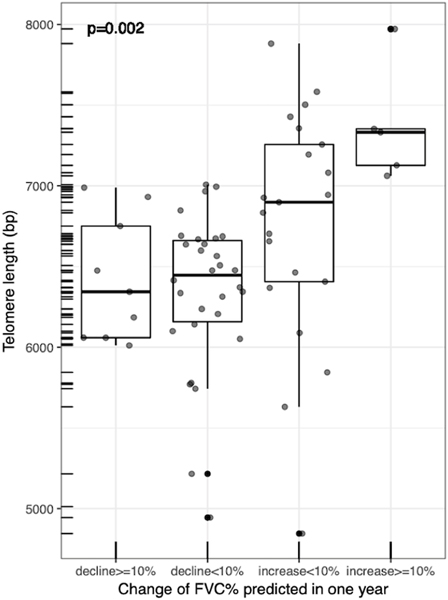
To investigate the primary objective of this study, we assessed the relationship between PBL-TL and SSc-ILD progression. PBL-TL was not associated with any baseline PFT measures including FVC (% predicted; r=−0.02, p-value=0.80), FEV1(% predicted; r=−0.07, p-value=0.44), and DLCO (% predicted; r=0.03, p-value=0.77). Associations between telomere length and log transformed baseline PFT measures in patients with SSc-ILD were assessed with Pearson’s correlation coefficient. There were 72 patients who had longitudinal PFT data available in the UCSF cohort (comparison of baseline data in patients with vs. without longitudinal PFTs is shown in Supplement Table 2). There were 65 patients who had an observation period of PFT close to 12 months in the UCSF cohort. There was a significant association between PBL-TL and one-year change of FVC in SSc-ILD (r=0.42, p-value=0.0001, Figure 3). Specifically, patients with more rapid progression (defined as relative decline in FVC% ≥10% at 12 months) had shorter PBL-TL (p-value=0.002, Figure 4). The median follow up time for the UCSF cohort was 281 days. On adjusted longitudinal analysis, shorter PBL-TL was associated with increased risk for worsening FVC with an average of 136 ml greater loss in FVC per year for every 1000 base pair decrease in PBL-TL (95% CI −239ml to −40ml, p=0.012, Table 3).
Figure 3.
Correlation between PBL TL and one-year FVC change in patients with SSc-ILD at the UCSF cohort.png
Figure 4-.
Boxplot comparing PBL-TL in UCSF SSc-ILD patients with different rates of progression after one-year based on percent FVC predicted.png
Table 3:
Association between PBL-TL and FVC trends in UCSF SSc-ILD patients using Generalized Linear Mixed Models
| UCSF Cohort (n=72, 203 PFTs) |
Telomere Length (Per 1000 base pair decrease) |
||
|---|---|---|---|
| Coefficient | 95%CI | P-value | |
| Unadjusted model | −0.134 | −0.235– −0.024 | 0.012 |
| Adjusted model* | −0.136 | −0.239– −0.040 | 0.012 |
Adjusted by age, male sex, body mass index (BMI), ethnicity, smoking status, anti-topoisomerase antibodies, and classification of disease.
PBL, peripheral blood leukocyte; TL, telomere length; FVC, forced vital capacity; UCSF, University of California San Francisco; SSc-ILD, systemic sclerosis associated interstitial lung disease; CI, confidence interval.
Validation cohort
To validate findings from the primary endpoint, an independent cohort of 61 SSc-ILD patients with longitudinal PFT data from the SU scleroderma center was analyzed. The median follow up time for the SU cohort was 561 days. Baseline characteristics of the two SSc-ILD patient cohorts with longitudinal PFT trends are listed in Supplement Table 3. SSc-ILD patients in the SU cohort had on average higher FVC (79.8%) and DLCO (65.8%) measures at baseline and more frequently presented ground glass opacities (80.3%) on HRCT scans. There were no significant differences in anti-topoisomerase antibody positive rate, or prevalence of pulmonary hypertension between the UCSF and SU cohorts.
There were 39 patients who had an observation period of PFT close to 12 months in the SU cohort. Consistent with findings in the UCSF cohort, PBL-TL in SU SSc-ILD patients was negatively associated with one-year FVC decline (r=0.33, p-value=0.03). In addition, PBL-TL was shorter in subjects exhibiting more rapid ILD progression (Figure 4). Finally, SU SSc-ILD patients exhibited a greater loss in FVC per year (41 ml) for every 1000 base pair decrease in PBL-TL (longitudinal analysis, adjusted model: 95% CI −74ml to −9ml, p-value=0.01, Table 4). When both cohorts of SSc-ILD patients were combined, shorter PBL-TL was also associated with an increased risk for FVC decline with an average of 67 ml greater loss in FVC per year for every 1000 base pair decrease in PBL-TL (longitudinal analysis, adjusted model: 95% CI −104ml to −33ml, p<0.001, Table 4).
Table 4:
Association between PBL-TL and FVC trends in SU SSc-ILD patients and combined cohorts using Generalized Linear Mixed Model
| Telomere Length (Per 1000 base pair decrease) |
||||
|---|---|---|---|---|
| Coefficient | 95%CI | P-value | ||
| SU Cohort (n=61, 329 PFTs) | ||||
| Unadjusted model | −0.035 | −0.061– −0.005 | 0.018 | |
| Adjusted model* | −0.041 | −0.074– −0.009 | 0.013 | |
| Combined Cohorts (n=133, 532 PFTs) | ||||
| Unadjusted model | −0.075 | −0.106– −0.045 | <0.001 | |
| Adjusted model* | −0.067 | −0.104– −0.033 | <0.001 | |
Adjusted by age, male sex, body mass index (BMI), ethnicity, smoking status, anti-topoisomerase antibodies, and classification of disease.
PBL, peripheral blood leukocyte; TL, telomere length; FVC, forced vital capacity; SU, Stanford University; SSc-ILD, systemic sclerosis associated interstitial lung disease; CI, confidence interval.
Discussion
This study investigated the relationship between PBL-TL and ILD progression in two large cohorts of SSc patients. We found that short PBL-TL is associated with the presence of SSc-ILD and the rate of decline of FVC. This evidence suggests the possibility that telomere dysfunction may contribute to ILD progression in a subset of SSc-ILD patients and that PBL-TL may be used to identify SSc-ILD patients more prone to progressive loss of lung function.
Previous studies have shown that shorter PBL-TL is associated with worse survival in patients with idiopathic pulmonary fibrosis, chronic hypersensitivity pneumonitis (cHP), unclassifiable ILD, or IPAF15–18, 30. In addition, IPF, IPAF and connective tissue disease associated ILD patients with shorter PBL telomeres have been reported to have more rapid loss of FVC18. These findings and the detection that telomere shortening in alveolar epithelial cells (AEC2 cells) is sufficient to cause fibrotic lung remodeling in mice 31 suggest that the progressive loss of FVC in these conditions may in part be driven by telomere dysfunction. This study reports that shorter PBL-TL is associated with the presence and progression of ILD in SSc patients. In broad terms, IPF and SSc-ILD appear to be initiated by different mechanisms (e.g. aging, AEC2 cell failure vs inflammation, autoimmunity). Nevertheless, the findings that short PBL-TL in both SSc-ILD and IPF patients is associated with FVC decline indicates that shared pathways involving telomere and telomerase dysfunction may contribute to both disease processes. Future studies addressing whether alveolar cells or other lung cellular subtypes exhibit telomere dysfunction in SSc-ILD patients will help clarifying this possibility. It will also be important to investigate the relevance of genetic predisposition, environmental exposure, or accentuated cellular replication driven by autoimmunity is driving telomere shortening in SSc-ILD. In addition, whether telomere dysfunction in PBLs contribute to inflammation and target tissue damage (i.e lungs) in SSc-ILD, similarly to what has been reported in rheumatoid arthritis32, it remains to be determined.
There is robust evidence that autoimmunity is a driver of SSc pathogenesis and that SSc-specific autoantibodies (anti-centromere, RNA polymerase III, and anti-topoisomerase I antibodies) are associated with unique clinical pehnotypes. The finding that SSc-ILD patients negative for these autoantibodies have shorter PBL-TL than seropositive subjects is intriguing. One could speculate that in these subjects the contribution of immune activation towards lung fibrosis is less relevant or has waned, with a pathobiology that has transitioned to one more similar to IPF. An alternative possibility is that unknown autoantibodies with the ability to interfere with telomere/telomerase function may be present. Overall, these findings suggest that telomere dysfunction may play a greater role in SSc-ILD progression in patients lacking common SSc specific autoantibodies.
Studies using genome-wide transcriptome analysis of SSc lungs has shown a robust and consistent pro-inflammatory signature, highlighting the role of immune-driven mechanisms in the pathogenesis of SSc-ILD33. In this study, longer PBL-TL was associated with GGO on chest HRCT. In the setting of ILD, GGO suggests the presence of active parenchymal inflammation (though it may not exclusively represent inflammation)34. An increased rate of cell replication of leukocytes may occur in autoimmune diseases leading to accelerated telomere attrition and immune cellular senescence35. Conversely, and consistent with our findings, it is possible that longer PBL-TL may sustain the activity of leukocytes enabling lung inflammation and GGO on HRCT. This possibility needs to be confirmed in independent studies.
A limitation of this study is that survival analysis could not be performed due to the small number of non-survivors in the investigated patient populations. There are other limitations, including the use of manual radiology scoring. We identified an association only between PBL-TL and GGO on HRCT. It is possible that automated radiologic scoring methods may be more sensitive to radiographic changes. Longitudinal PFT trends could be studied only in a subset of UCSF SSc-ILD patients as data were missing. This is unlikely to have biased the results as the findings were replicated in a separate cohort. We also could not determine the impact of PBL-TL on treatment response as the majority of SSc-ILD patients received therapy. Prospective studies conducted in large cohort of patients should be designed to further validate our findings and define whether telomere length measurement can reliably predict SSc-ILD progression as well as long term outcome and survival. Future studies should also define which cellular subtypes in the peripheral blood and within the lung tissues of SSc patients exhibit significant telomere shortening and how this contributes to unique molecular mechanisms involved with pulmonary fibrosis and SSc-ILD progression.
In conclusion, this study reports an association between shorter PBL-TL and lung function decline (FVC) over time in SSc-ILD, supporting the hypothesis that telomere dysfunction may contribute to ILD progression in a subset of SSc patients and that measuring PBL-TL may prove to be a useful tool to identify SSc-ILD subjects at risk for SSc-ILD progression. Measures of PBL-TL may provide valuable information for the prognostic evaluation of SSc-ILD and improve our understanding of disease pathogenesis.
Supplementary Material
What is the key question?
Is there a relationship between peripheral blood leukocyte telomere length (PBL-TL) and systemic sclerosis-associated interstitial lung disease (SSc-ILD) progression?
What is the bottom line?
Short PBL-TL is associated with the presence of SSc-ILD, and with increased risk for deterioration in lung function (forced vital capacity, FVC) over time in SSc-ILD patients.
Why read on?
Our data provide evidence supporting the idea that PBL-TL may be useful to identify SSc-ILD patients more prone to FVC decline and improve our understanding of disease pathogenesis.
Acknowledgments
Sources of support: Study was funded in part by National Natural Science Foundation of China (81700063), Scleroderma Research Foundation, the Nina Ireland Program for Lung Health, and an investigator initiated proposal from Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI; BIPI had no role in the design, analysis or interpretation of the results in this study; BIPI was given the opportunity to review the manuscript for medical and scientific accuracy as it relates to BIPI substances, as well as intellectual property considerations). MC was funded by the Training Program in Adult and Pediatric Rheumatology 5T32AR050942-13.
References
- 1.White B. Interstitial lung disease in scleroderma. Rheum Dis Clin North Am. May 2003;29(2):371–90. doi: 10.1016/s0889-857x(03)00025-5 [DOI] [PubMed] [Google Scholar]
- 2.Goh NS, Hoyles RK, Denton CP, et al. Short-Term Pulmonary Function Trends Are Predictive of Mortality in Interstitial Lung Disease Associated With Systemic Sclerosis. Arthritis Rheumatol. 08 2017;69(8):1670–1678. doi: 10.1002/art.40130 [DOI] [PubMed] [Google Scholar]
- 3.Elhai M, Hoffmann-Vold AM, Avouac J, et al. Performance of Candidate Serum Biomarkers for Systemic Sclerosis-Associated Interstitial Lung Disease. Arthritis Rheumatol. 06 2019;71(6):972–982. doi: 10.1002/art.40815 [DOI] [PubMed] [Google Scholar]
- 4.Hant FN, Ludwicka-Bradley A, Wang HJ, et al. Surfactant protein D and KL-6 as serum biomarkers of interstitial lung disease in patients with scleroderma. J Rheumatol. Apr 2009;36(4):773–80. doi: 10.3899/jrheum.080633 [DOI] [PubMed] [Google Scholar]
- 5.Kikuchi K, Kubo M, Sato S, Fujimoto M, Tamaki K. Serum tissue inhibitor of metalloproteinases in patients with systemic sclerosis. J Am Acad Dermatol. Dec 1995;33(6):973–8. [DOI] [PubMed] [Google Scholar]
- 6.Lambrecht S, Smith V, De Wilde K, et al. Growth differentiation factor 15, a marker of lung involvement in systemic sclerosis, is involved in fibrosis development but is not indispensable for fibrosis development. Arthritis Rheumatol. Feb 2014;66(2):418–27. doi: 10.1002/art.38241 [DOI] [PubMed] [Google Scholar]
- 7.Prasse A, Pechkovsky DV, Toews GB, et al. CCL18 as an indicator of pulmonary fibrotic activity in idiopathic interstitial pneumonias and systemic sclerosis. Arthritis Rheum. May 2007;56(5):1685–93. doi: 10.1002/art.22559 [DOI] [PubMed] [Google Scholar]
- 8.Lee CG, Herzog EL, Ahangari F, et al. Chitinase 1 is a biomarker for and therapeutic target in scleroderma-associated interstitial lung disease that augments TGF-β1 signaling. J Immunol. Sep 2012;189(5):2635–44. doi: 10.4049/jimmunol.1201115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwata Y, Yoshizaki A, Ogawa F, et al. Increased serum pentraxin 3 in patients with systemic sclerosis. J Rheumatol. May 2009;36(5):976–83. doi: 10.3899/jrheum.080343 [DOI] [PubMed] [Google Scholar]
- 10.Ogawa F, Shimizu K, Muroi E, et al. Serum levels of 8-isoprostane, a marker of oxidative stress, are elevated in patients with systemic sclerosis. Rheumatology (Oxford). Jul 2006;45(7):815–8. doi: 10.1093/rheumatology/kel012 [DOI] [PubMed] [Google Scholar]
- 11.Kodera M, Hasegawa M, Komura K, Yanaba K, Takehara K, Sato S. Serum pulmonary and activation-regulated chemokine/CCL18 levels in patients with systemic sclerosis: a sensitive indicator of active pulmonary fibrosis. Arthritis Rheum. Sep 2005;52(9):2889–96. doi: 10.1002/art.21257 [DOI] [PubMed] [Google Scholar]
- 12.Distler O, Assassi S, Cottin V, et al. Predictors of progression in systemic sclerosis patients with interstitial lung disease. Eur Respir J. May 2020;55(5)doi: 10.1183/13993003.02026-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Lauretis A, Sestini P, Pantelidis P, et al. Serum interleukin 6 is predictive of early functional decline and mortality in interstitial lung disease associated with systemic sclerosis. J Rheumatol. Apr 2013;40(4):435–46. doi: 10.3899/jrheum.120725 [DOI] [PubMed] [Google Scholar]
- 14.Morisset J, Vittinghoff E, Elicker BM, et al. Mortality Risk Prediction in Scleroderma-Related Interstitial Lung Disease: The SADL Model. Chest. 11 2017;152(5):999–1007. doi: 10.1016/j.chest.2017.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stuart BD, Lee JS, Kozlitina J, et al. Effect of telomere length on survival in patients with idiopathic pulmonary fibrosis: an observational cohort study with independent validation. Lancet Respir Med. Jul 2014;2(7):557–65. doi: 10.1016/S2213-2600(14)70124-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ley B, Newton CA, Arnould I, et al. The MUC5B promoter polymorphism and telomere length in patients with chronic hypersensitivity pneumonitis: an observational cohort-control study. Lancet Respir Med. Aug 2017;5(8):639–647. doi: 10.1016/S2213-2600(17)30216-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ley B, Liu S, Elicker BM, et al. Telomere length in patients with unclassifiable interstitial lung disease: a cohort study. Eur Respir J. Apr 2020;doi: 10.1183/13993003.00268-2020 [DOI] [PubMed] [Google Scholar]
- 18.Newton CA, Oldham JM, Ley B, et al. Telomere length and genetic variant associations with interstitial lung disease progression and survival. Eur Respir J. Apr 2019;53(4)doi: 10.1183/13993003.01641-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolters PJ, Blackwell TS, Eickelberg O, et al. Time for a change: is idiopathic pulmonary fibrosis still idiopathic and only fibrotic? Lancet Respir Med. Feb 2018;6(2):154–160. doi: 10.1016/S2213-2600(18)30007-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Artlett CM, Black CM, Briggs DC, Stevens CO, Welsh KI. Telomere reduction in scleroderma patients: a possible cause for chromosomal instability. Br J Rheumatol. Aug 1996;35(8):732–7. doi: 10.1093/rheumatology/35.8.732 [DOI] [PubMed] [Google Scholar]
- 21.Lakota K, Hanumanthu VS, Agrawal R, Carns M, Armanios M, Varga J. Short lymphocyte, but not granulocyte, telomere length in a subset of patients with systemic sclerosis. Ann Rheum Dis. 08 2019;78(8):1142–1144. doi: 10.1136/annrheumdis-2018-214499 [DOI] [PubMed] [Google Scholar]
- 22.van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis. Nov 2013;72(11):1747–55. doi: 10.1136/annrheumdis-2013-204424 [DOI] [PubMed] [Google Scholar]
- 23.Plastiras SC, Karadimitrakis SP, Kampolis C, Moutsopoulos HM, Tzelepis GE. Determinants of pulmonary arterial hypertension in scleroderma. Semin Arthritis Rheum. Jun 2007;36(6):392–6. doi: 10.1016/j.semarthrit.2006.10.004 [DOI] [PubMed] [Google Scholar]
- 24.Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med. 09 2018;198(5):e44–e68. doi: 10.1164/rccm.201807-1255ST [DOI] [PubMed] [Google Scholar]
- 25.Mooney JJ, Elicker BM, Urbania TH, et al. Radiographic fibrosis score predicts survival in hypersensitivity pneumonitis. Chest. Aug 2013;144(2):586–592. doi: 10.1378/chest.12-2623 [DOI] [PubMed] [Google Scholar]
- 26.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. May 2002;30(10):e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faust HE, Golden JA, Rajalingam R, et al. Short lung transplant donor telomere length is associated with decreased CLAD-free survival. Thorax. Apr 2017;doi: 10.1136/thoraxjnl-2016-209897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu S, Wang C, Green G, et al. Peripheral Blood Leukocyte Telomere Length is Associated with Survival of Sepsis Patients. Eur Respir J. Oct 2019;doi: 10.1183/13993003.01044-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Listerman I, Sun J, Gazzaniga FS, Lukas JL, Blackburn EH. The major reverse transcriptase-incompetent splice variant of the human telomerase protein inhibits telomerase activity but protects from apoptosis. Cancer Res. May 2013;73(9):2817–28. doi: 10.1158/0008-5472.CAN-12-3082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ley B, Torgerson DG, Oldham JM, et al. Rare Protein-Altering Telomere-related Gene Variants in Patients with Chronic Hypersensitivity Pneumonitis. Am J Respir Crit Care Med. Nov 2019;200(9):1154–1163. doi: 10.1164/rccm.201902-0360OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naikawadi RP, Disayabutr S, Mallavia B, et al. Telomere dysfunction in alveolar epithelial cells causes lung remodeling and fibrosis. JCI Insight. Sep 2016;1(14):e86704. doi: 10.1172/jci.insight.86704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y, Shen Y, Hohensinner P, et al. Deficient Activity of the Nuclease MRE11A Induces T Cell Aging and Promotes Arthritogenic Effector Functions in Patients with Rheumatoid Arthritis. Immunity. 10 2016;45(4):903–916. doi: 10.1016/j.immuni.2016.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greenblatt MB, Aliprantis AO. The immune pathogenesis of scleroderma: context is everything. Curr Rheumatol Rep. Jan 2013;15(1):297. doi: 10.1007/s11926-012-0297-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu H, Liu S, Zhang C, et al. Computed tomography and pathology evaluation of lung ground-glass opacity. Exp Ther Med. Dec 2018;16(6):5305–5309. doi: 10.3892/etm.2018.6886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wagner CL, Hanumanthu VS, Talbot CC, et al. Short telomere syndromes cause a primary T cell immunodeficiency. J Clin Invest. Oct 2018;128(12):5222–5234. doi: 10.1172/JCI120216 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.