Abstract
There is emerging evidence of microvascular thrombosis and thrombotic microangiopathy (TMA) induced by COVID-19, presumably from endothelial injury. Thrombomodulin (TM) is an endothelial glycoprotein that plays a dual role in maintaining healthy endothelium-as a natural anticoagulant by binding thrombin to activate protein C (APC) and a negative regulator of the alternate complement pathway (AP). TM is shed into the plasma as soluble TM (sTM) during endothelial injury.
We hypothesize that SARS-CoV-2 spike proteins cause direct microvascular endothelial injury, leading to TM shedding, decreased activation of PC, and consequently, microvascular thrombosis in COVID-19. We conducted this study twofold: 1) in vivo, we assessed endothelial injury (by measuring sTM) and AP activation by quantifying Ba (cleavage product of AP component Factor B) in a cohort of critically ill COVID-19 pediatric patients and the implications on clinical outcomes; and 2)in vitro, we investigated endothelial injury (TM shedding) by SARS-COV-2 spike proteins and the subsequent functional consequence in activated PC (APC) levels and Ba levels.
sTM and Ba in plasma samples from SARS-CoV-2 positive patients admitted to Texas Children's Hospital Pediatric Intensive Care Unit (n = 33) and from healthy controls (n = 38) were measured by ELISA. In vitro, confluent glomerular microvascular endothelial cells (GMVECs) were incubated for 48 h in the presence or absence (control) of purified SARS-CoV-2 spike proteins, S1 and S2. TM from the cell lysates while Ba and APC from the cell supernatants were measured by ELISA. sTM and Ba levels were significantly higher in the COVID-19 pediatric patients compared to healthy controls (p < 0.01 and p < 0.001, respectively). Among the COVID-19 patients, elevated sTM was associated with increased vasopressor use (p = 0.01) and elevated Ba was associated with increased duration of mechanical ventilation (p = 0.04). In vitro, surface bound TM and soluble APC were significantly lower in GMVECs after addition of spike proteins (p < 0.05), while Ba was undetectable in both control and spike proteins exposed GMVECs.
In conclusion, we provide evidence of endothelial injury in COVID-19 pediatric patients and demonstrate a potential pathway of SARS-CoV-2 induced thrombosis. Decreased surface-bound TM results in lower amount of thrombin-TM complex, hence lesser activation of PC, likely leading to a pro-thrombotic state. These findings in GMVECs could explain the vulnerability of kidneys to COVID-19-induced TMA.
Keywords: Endothelial injury, Microvascular thrombosis, COVID-19 and thrombosis, Thrombomodulin, Alternate complement pathway activation
1. Introduction
Coronavirus disease-19 (COVID-19) is an acute illness caused by the SARS-CoV-2 virus. COVID-19 is now increasingly recognized as a disease of the vasculature [1], thereby leading to systemic and multi-organ pathology. Patients can present with macro- or microvascular thrombosis [[2], [3], [4]] in the setting of inflammation (thromboinflammation), which has been reported as a major contributor to morbidity and mortality related to COVID-19 [5,6]. While lungs are the most frequently involved organ [7], other organs including kidneys [8], heart [9], and brain [10] can also be affected. In critically sick patients with COVID-19, kidneys are the second most involved organ, and abnormal renal function is one of the significant risk factors for death in the ICU setting [11]. A systematic review further showed that severe acute kidney injury is a worrying clinical predictor and associated with high mortality [12]. An autopsy study of deceased COVID-19 patients has shown detectable SARS-CoV-2 viral load in all kidney compartments, with preferential targeting of glomerular cells [13]. In kidneys, microvascular thrombosis can result from thrombotic microangiopathy (TMA) induced by COVID-19 [14,15]. While most of the studies so far have focused on the pulmonary vasculature and thrombosis, the relevance of microvascular thrombosis in kidneys remains a matter of investigation. Hence, the focus of this study is to investigate mechanisms of microvascular thrombosis in the kidneys and add to the growing literature on COVID-19 and thrombosis in order to improve outcomes.
SARS-CoV-2 is a type of coronavirus comprised of four main proteins, namely spike (S), membrane (M), nucleocapsid (N) and envelope (E) [16]. S protein, consisting of S1 and S2 subunits, is present on the cell surface and has been reported to be responsible for virus induced cell injury [17]. Studies have shown the S1 component binds to the ACE2 receptor on the cell surface and the S2 subunit releases the viral mRNA component into the host cell causing cellular injury [18]. Endothelial cells express ACE2, making them a target for SARS-CoV-2 virus [19].
Thrombomodulin (TM) is an endothelial glycoprotein that is ubiquitously expressed on endothelial cells and plays two crucial roles in maintaining a healthy endothelium – a natural anticoagulant and a negative regulator of the alternate complement pathway (AP) [20]. TM controls the coagulation pathway by binding to thrombin, and the resulting thrombin-TM complex converts protein C to activated protein C (APC). APC in the presence of protein S, inactivates factors Va and VIIIa, thereby limiting coagulation [21]. AP is one of the three complement pathways within the innate immune system that is activated during times of inflammation and/or infection. Upon activation, through a series of steps involving C3, factor B (FB), factor D (FD) and factor P (FP), the C3 and C5 convertases are generated (C3bBb and C3bBbC3b, respectively), and activation products C3a, Ba, and C5a are released [22]. In a normal physiologic/uninflamed state, TM attenuates AP activation at the endothelial surface by binding to AP regulators factor H (FH) and factor I (FI). This complex inactivates C3b, limiting the amplification of the AP [23]. During times of endothelial injury, TM is shed into plasma as soluble TM (sTM) [24,25], in turn affecting TM's role as a gatekeeper of coagulation and AP regulation.
One proposed mechanism of thrombosis in COVID-19 is from endothelial dysfunction, or “endotheliopathy”. A retrospective clinical study showed elevated plasma sTM in adult patients with COVID-19, suggesting the shedding of endothelial TM following injury [26]. In an in vitro study, Yu et al. demonstrated the spike protein subunit of SARS-CoV-2 acts as a potent activator of the AP using TF1PIGAnull cells [27]. Satyam et al. reported the deposition of complement components on lung tissue of patients who succumbed to COVID-19, consistent with activation of classical and alternate pathways [28]. These studies suggest TM shedding and complement dysregulation lead to endotheliopathy in acute COVID-19. Whether endothelial injury is a result of direct viral invasion or secondary to inflammatory response to the virus, however, remains unclear. Some autopsy findings suggest the former as the culprit [1,28], but there is no mechanistic evidence to support the hypothesis. Furthermore, Rotoli et al. elegantly demonstrated TM gene (THBD) downregulation by spike proteins in lung microvascular endothelial cells [29], however the mechanism of thrombosis in kidney microvasculature remains unknown, and the functional consequence of endothelial TM downregulation/shedding is yet to be clarified.
The objectives of our study were twofold. One, to determine endothelial injury and AP activation in children with COVID-19 and subsequent impact on clinical outcomes; and two, to investigate cellular mechanism of SARS-CoV-2 induced endotheliopathy in vitro. We hypothesized that endothelial injury and AP activation is associated with poor clinical outcomes in children with COVID-19; and that spike proteins (S1 and S2) cause direct microvascular endothelial injury in vitro resulting in TM shedding from the endothelium. We further hypothesized that loss of TM results in AP overactivation and decreased production of the natural anticoagulant APC, thereby creating a prothrombotic state.
2. Methods
2.1. Clinical study
Study population: This was a retrospective cohort study utilizing residual plasma samples from disseminated intravascular coagulation (DIC) panels that were obtained from patients admitted to the Intensive care unit (ICU) for a diagnosis of COVID-19 between Dec 2, 2020, and Jan 22, 2021 at Texas Children's Hospital. DIC panels were obtained as standard of care for all patients with COVID-19 admitted to the ICU at our center. All patients less than or equal to 21 years of age with a positive SARS-CoV-2 RT-PCR on admission to the ICU were included. Data regarding demographics (age, gender, race, ethnicity), length of ICU stay, and clinical indicators of end organ damage (mechanical ventilation, dialysis, use of vasopressors) were collected via retrospective chart review. All patients were on thromoboprophylaxis as per institutional standard of care for patients with COVID-19 admitted to the ICU.
For controls, residual plasma samples from DIC panels obtained from pediatric patients who came in for pre-op clearance during the study period, all presumed to be at their baseline state of health, were collected. Control patients with known autoimmune, inflammatory, or complement-mediated disorders were excluded. Pregnant females and anyone above 21 years of age were excluded from both experimental and control groups. Institutional IRB approval and waiver of consent were obtained.
2.1.1. Quantification of sTM and Ba levels
From patient plasma samples, sTM, as a marker of endothelial injury and Ba, as a marker of AP activation, were quantified by commercially available ELISA kits (Abcam, ab46508 and Quidel, A033, respectively) according to the manufacturer's instructions.
2.2. In vitro studies
Glomerular microvascular endothelial cells (GMVECs). Pooled primary human GMVECs were purchased from Cell Systems (CBRI-128). GMVECs were grown in complete media (CM131, MCDB-131 medium [Sigma-Aldrich, M8537], supplemented with penicillin/streptomycin/l-glutamine [Life Technologies], plus microvascular growth supplement [Life Technologies]). GMVECs (passage 4–7) were seeded in T-25 flasks and used when confluent, after 7–9 days of growth, for all experiments.
2.2.1. Measurement of TM from GMVEC lysates
Since TM is present on or within the cells, we quantified TM from cell lysates to get an accurate measurement of TM. Confluent GMVECs were washed with PBS and exposed to SARS-CoV-2 spike proteins (0.5 μg/ml each of S1 and S2) in complete media for 24 h, followed by exposure to an additional 0.5 μg/ml of each spike protein in serum-free media for another 24 h. These concentrations and exposure time were based on the initial experiment we performed to identify the optimal concentration of spike proteins (alone or together) for TM shedding (Supplemental Fig. 1). Control GMVECs were washed with PBS and exposed to an equal volume of serum-free media as the experimental GMVECs for 24 h. Subsequently, all cells were lysed with CelLytic M (Sigma-Aldrich, C-2978) containing Halt protease/phosphatase inhibitor cocktail (Thermo Scientific, 78,430). Lysed cells were collected and centrifuged, and the resultant supernatant was used for analysis. TM levels were measured in spike protein-exposed and control GMVEC lysates using a TM ELISA kit (Abcam, ab46508).
2.2.2. Measurement of APC from GMVEC supernatant
Spike proteins (0.5 μg/ml each of S1 and S2) were added to experimental GMVECs for 48 h, and serum-free media alone was added to control GMVECs, as described above. The GMVECs were then washed with PBS and supplemented with 0.2 mM human PC (Haematologic Technologies, HCPC-0070) and 10 nM human α-thrombin (Haematologic Technologies, HCT-0020) in “APC buffer” (0.1% BSA, 3 mM CaCl2, 0.6 mM MgCl2 in PBS) and incubated at 37 °C for 60 min [30]. Further activation of PC to APC was inhibited with the addition of 10 nM hirudin (1.5 U/ml, Sigma Aldrich, H7016). Supernatants were collected and APC levels were measured from cell supernatants using an APC ELISA kit (Cloud-Clone, SEA738Hu).
2.2.3. Measurement of Ba from GMVEC supernatant
Spike proteins were added to experimental GMVECs for 48 h, and an equal volume of serum-free media was added to control cells, as described above. Cell supernatants were subsequently collected, and Ba levels were measured from cell supernatants using a commercially available Ba ELISA kit (Quidel, A033).
Each of the above-described experiments were performed on 4–6 flasks to ensure reproducibility.
2.3. Statistical analysis
For comparing clinical outcomes within the COVID-19 patients' cohort, Pearson's Chi-squared, Fischer exact and Wilcoxon rank sum tests were used to assess the relationships between Ba or sTM and the clinical outcomes as appropriate. For the in vitro studies, unpaired t-tests with Welch's correction were used to test the difference in means between the treatment and the control groups in GraphPad Prism9. A p-value <0.05 was considered statistically significant.
3. Results and discussion
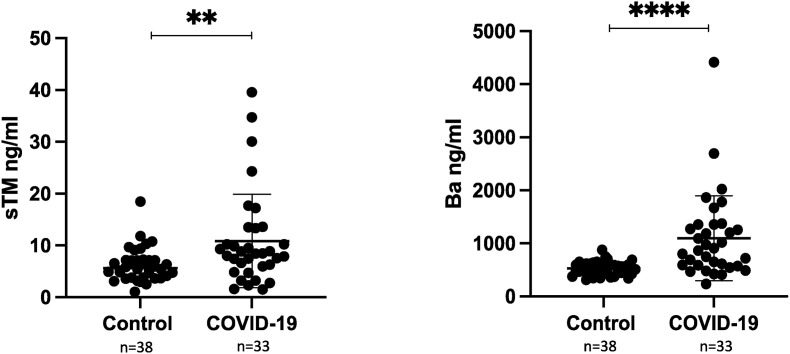
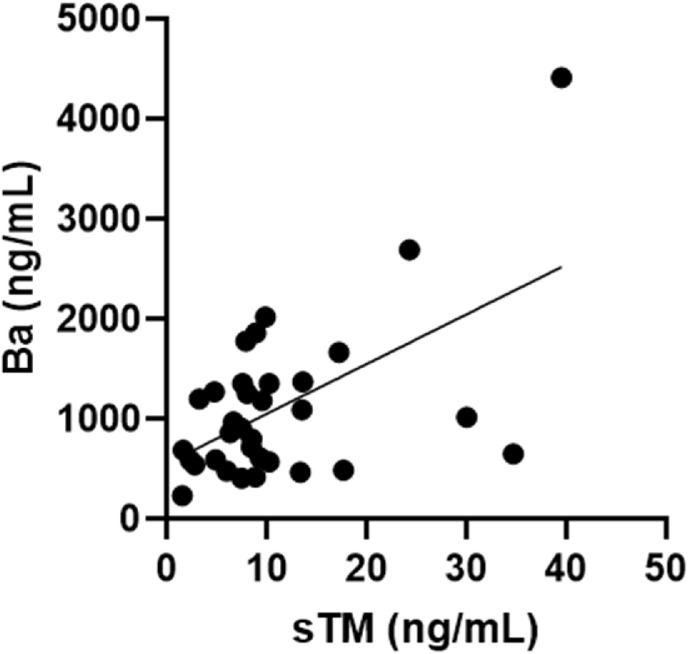
A total of 33 patients with COVID-19 and 38 controls were included in the final analysis. sTM and Ba levels were both significantly higher in the COVID-19 pediatric patients compared to the controls (mean sTM 6.2 ng/ml in controls and 10.9 ng/ml in COVID-19 patients, p < 0.01 and mean Ba 526.7 ng/ml in controls and 1098.0 ng/ml in COVID-19 patients, p < 0.001) (Fig. 1 ). Furthermore, sTM levels positively correlated with Ba in our patient cohort (Pearson correlation coefficient = 0.55, p < 0.001) (Fig. 2 ). In normal healthy individuals, sTM is released during physiologic cleavage and shedding of membrane-bound TM but in very low amounts (less than 10 ng/ml) [31]. However, sTM is released in increased amounts during certain pathologic conditions like sepsis, DIC and thrombotic thrombocytopenic purpura, as a result of endothelial cell damage [24,25,32]. Our findings of elevated sTM in pediatric patients with COVID-19 suggests endothelial injury in children with SARS-CoV-2 infection. Our results are in accordance with the previously published data in adults [26]. Additionally, our findings of elevated Ba are reflective of AP activation in children with COVID-19. This is the first study showing elevation of AP activation product in pediatric COVID-19 and are supportive of findings in a previously published study in critically ill, COVID-19-infected adults by Leatherdale et al. [33]. These findings further explain the potential role of anti-complement agents in the treatment of critically ill patients with COVID-19 [34,35].
Fig. 1.
Mean sTM and Ba levels in pediatric controls and COVID-19 patients. sTM and Ba were quantified in plasma samples using commercially available TM and Ba ELISA kits, respectively.
Error bar = standard deviation. **p < 0.01 and ****p < 0.001. Normal sTM levels were considered <7.6 ng/ml and normal Ba levels <1080 ng/ml.
Fig. 2.
Correlation between sTM and Ba in patient cohort. Moderately positive correlation between sTM and Ba levels. Pearson correlation coefficient = 0.55, p < 0.001.
We also analyzed the utility of sTM and Ba in predicting clinical outcomes (Table 1 ). Elevated sTM was associated with increased vasopressor use (p = 0.011). Goshua et al. had reported sTM as a predictor of mortality in adults with COVID-19 [26]. Although other clinical outcome variables, including mortality, did not reach statistical significance in our cohort, likely owing to small numbers, overall, the trend indicated worse outcomes in patients with elevated sTM. Additionally, COVID-19 related mortality in pediatrics is much lower than adults [36], which could also explain the variability of our findings from the adult study. Amongst the intubated patients, higher Ba levels were associated with an increased duration of mechanical ventilation (p = 0.039), which could suggest increased lung endothelial damage and subsequent respiratory failure. Regarding thrombosis, two patients had an acute thrombotic event (1 venous and 2 arterial), which could be the result of endothelial injury and TM shedding. It is possible though that the number of thrombotic events were low in our cohort despite the presence of endothelial injury because all patients were on thromboprophylaxis; however, it is difficult to draw any conclusion based on small sample size. Overall our findings suggest that the severity of endotheliopathy may predict clinical outcomes, however, large prospective studies are indicated to validate the use of sTM and Ba as prognostic markers in pediatric COVID-19 infection.
Table 1.
Association of sTM and Ba with clinical outcomes in critically ill pediatric COVID-19 patients.
| Clinical Indicators | sTM |
Ba |
||||
|---|---|---|---|---|---|---|
| Elevated sTMc n = 20a | Normal sTM n = 13a | p valueb | Elevated Bad n = 14a | Normal Ba n = 19a | p valueb | |
| Length of ICU stay | 8 (4,16) | 5 (3,7) | 0.15 | 6 (4,13) | 7 (4,13) | 0.80 |
| Intubation | 10 (50%) | 4 (31%) | 0.30 | 4 (29%) | 10 (53%) | 0.20 |
| Length of mechanical ventilation | 8 (3,13) | 3 (3,8) | 0.50 | 25 (12,40) | 3 (2,9) | 0.04 |
| Vasopressors | 12 (60%) | 2 (15%) | 0.01 | 5 (36%) | 9 (47%) | 0.50 |
| Dialysis | 4 (20%) | 0 (0%) | 0.14 | 3 (21%) | 1 (5%) | 0.30 |
| ECMO | 2 (10%) | 1 (8%) | 1.00 | 1 (7%) | 2 (11%) | >0.90 |
| Mortality | 3 (15%) | 0 (0%) | 0.30 | 1 (7%) | 2 (11%) | >0.90 |
| Thrombus (arterial and/or venous) | 2 (10%) | 0 (0%) | 0.51 | 2 (14%) | 0 (0%) | 0.17 |
Abbreviations used: ICU- Intensive care unit, ECMO- Extracorporeal membrane oxygenation
n (%), Median (IQR).
Fisher's exact test; Wilcoxon rank sum test; Pearson's chi-squared test.
Elevated sTM: sTM >7.6 ng/ml.
Elevated Ba: Ba > 1080 ng/ml.
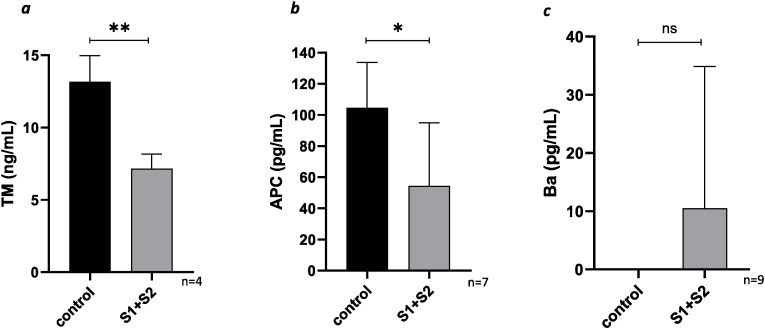
Our in vitro data further corroborates our in vivo findings. Since COVID-19 is associated with microvascular thrombosis and TMA resulting in kidney injury, we utilized GMVECs for our in vitro experiments. We hypothesized SARS-CoV-2 spike proteins cause direct microvascular endothelial cell injury resulting in loss of cell surface bound TM. Our data demonstrated endothelial-bound TM is approximately half as much on GMVECs exposed to spike proteins compared to GMVECs without exposure (13.2 ± 1.8 ng/ml on control lysates and 7.2 ± 1 ng/ml on spike protein exposed cells, p < 0.05, Fig. 3 a). These findings may explain the vulnerability of kidneys to SARS-CoV-2 infection and the subsequent risk of TMA. The high concentration of TM on the surface of the endothelial cells allowed us to better quantify TM from the cell lysates, providing us with the information needed to assess TM shedding. We believe the mechanism behind TM shedding is from disruption of cell membranes upon exposure to spike proteins, however, a study led by Rotoli et al. showed downregulation of the gene encoding for TM (THBD) upon exposure to spike proteins. Therefore, decreased TM in cell lysate could result from a combination of shedding from the cell surface as well as decreased transcription at the gene level on exposure to spike proteins. The mechanism of TM shedding from ECs is likely multifactorial. It is possible that the spike proteins cause a response in the GMVECs that leads to cell injury – this response could be secondary to up regulation of inflammatory cytokines and/or increased generation of cleaving proteases such as HGMB1 (high mobility group box 1) that cleave TM from the cell surface, but further work is warranted to determine the exact mechanism of TM shedding.
Fig. 3.
Fig. 3aMean TM levels from GMVEC lysates. Spike proteins or serum-free media alone (as a control) were added to GMVECs for 48 h. Cell lysates were then collected, and TM was quantified by ELISA.
Fig. 3b. Mean APC levels in GMVEC supernatant. Spike proteins or serum-free media alone (as a control) were added to the cells for 48 h. Then PC and thrombin were added to the cells for 1 h, after which further reaction was prevented by addition of hirudin. Supernatant was collected for measurement of APC by ELISA. Fig. 3c. Mean Ba levels in GMVEC supernatant. Spike proteins or serum-free media alone (as a control) were added to the cells for 48 h. Supernatant was collected for measurement of Ba by ELISA.
Error bar = standard deviation, **p < 0.01, *p < 0.05, ns = not significant, p value > 0.05.
While we have shown the effects of spike proteins on the glomerular microvasculature, we do acknowledge that lungs are the most common site of involvement in COVID-19. Most of the thrombotic phenomenon in the lungs is macrovascular (i.e. pulmonary embolism), although some studies have shown microvascular thrombosis [1,37] to occur as well. Therefore, we also evaluated TM shedding from lung microvascular ECs exposed to spike proteins and found similar results to those in GMVECs (Supplemental Fig. 2).
TM not only serves as a marker of endothelial injury, but also plays a crucial role in the anticoagulation pathway by converting protein C to its activated form (APC). Our in vitro data demonstrates that the lower amount of surface bound TM on cells exposed to spike protein leads to less generation of APC compared to cells without spike protein exposure (104.6 ± 29.2 pg/ml in controls and 54.4 ± 40.5 pg/ml in spike protein exposed cells, p < 0.05, Fig. 3b). As APC is a natural anticoagulant that inactivates coagulation factors Va and VIIIa, these findings suggest a mechanism in vivo for SARS-CoV-2-mediated hypercoagulability and microvascular thrombosis. While we did not assess FV or FVa levels in our patient cohort, a study by Stefely et al. assessed FV activity levels in 102 consecutive inpatients with COVID-19 [38]. They found significantly elevated FV activity in COVID-19 patients compared to healthy controls [38]. This data is further supportive of our hypothesis that in COVID-19 infection, less activation of PC leads to decreased inactivation of FVa, hence higher circulating amounts of active FV. Additionally, lower amounts of APC may also impact its interaction with endothelial PC receptor (EPCR), and thereby, affect its role as an anti-inflammatory agent. PC and EPCR attenuate inflammation by inhibiting the release of proinflammatory cytokines [39,40], and preventing TNF-α-mediated neutrophil adhesion to ECs [41]. Some studies have shown sEPCR levels are higher in plasma of patients with COVID-19 [42,43], suggesting shedding of EPCR from the endothelial cell surface. Lesser amounts of APC and EPCR imply less APC-EPCR pathway signaling, rendering the endothelium more susceptible to proinflammatory factors, but further studies are justified to understand the APC-EPCR pathway in COVID-19 infection.
In addition to its role in the coagulation system, TM plays an important role in AP regulation. We therefore analyzed the impact of TM downregulation on AP activation. In contrast to the observations in patient plasma samples, we did not observe significant differences in Ba levels between spike protein-exposed GMVECs and controls (10.5 ± 24.3 pg/ml in spike protein-exposed cells and 0 ± 0.002 pg/ml in controls, p > 0.05, n = 9, Fig. 3c). One explanation could be that the amount of Ba released from the cells was below the lower limit of detection of the Ba ELISA, hence was not measured accurately. Alternatively, it is possible that spike proteins alone may not cause enough TM loss for AP overactivation and may need other inflammatory mediators for additive effect. Yu et al. have previously demonstrated activation of AP by SARS-CoV-2, but the spike proteins in those experiments were produced in E. coli, whereas the spike proteins used in this study were produced in human cells [27]. E. coli, in contrast to human cells, naturally produce endotoxin. This endotoxin may be a contaminant in the E. coli-produced spike protein as it is difficult to remove [44,45]. Consequently, AP activation observed by Yu et al. may have been induced by endotoxin rather than the spike proteins themselves [27].
In conclusion, our study provides further insight into the complex pathogenesis of thrombo-inflammation seen with SARS-CoV-2 infection. We provide evidence of endothelial injury and AP overactivation in pediatric patients with COVID-19 associated with worse clinical outcomes. Additionally, our in vitro data support the hypothesis that the spike proteins cause direct microvascular endothelial cell injury, resulting in loss of TM, and consequently less activation of the natural anticoagulant PC and uninhibited coagulation. Further studies are warranted to understand if TM is also downregulated transcriptionally upon exposure to spike proteins. Future studies should also further investigate AP activation in COVID-19 infection and methods to block spike protein interaction with endothelial cells to prevent cell injury.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
Authors would like to thank Ms Karen Brudzowski at Texas Children's Hospital for her help with collecting pediatric patient plasma samples. We would also like to thank Mary R. Gibson Foundation for providing us with funding for this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tru.2022.100116.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Ackermann M., Verleden S.E., Kuehnel M., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19. N. Engl. J. Med. 2020;383(2):120–128. doi: 10.1056/nejmoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Minno A., Ambrosino P., Calcaterra I., Di Minno M.N.D. COVID-19 and venous thromboembolism: a meta-analysis of literature studies. Semin. Thromb. Hemost. 2020;46(7):763–771. doi: 10.1055/s-0040-1715456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lodigiani C., Iapichino G., Carenzo L., et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magro C., Mulvey J.J., Berlin D., et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl. Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemostasis. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu C., Chen X., Cai Y., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in wuhan, China. JAMA Intern. Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet (London, England) 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirsch J.S., Ng J.H., Ross D.W., et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tajbakhsh A., Gheibi Hayat S.M., Taghizadeh H., et al. COVID-19 and cardiac injury: clinical manifestations, biomarkers, mechanisms, diagnosis, treatment, and follow up. Expert Rev. Anti Infect. Ther. 2021;19(3):345–357. doi: 10.1080/14787210.2020.1822737. [DOI] [PubMed] [Google Scholar]
- 10.Hoyer C., Ebert A., Huttner H.B., et al. Acute stroke in times of the COVID-19 pandemic: a multicenter study. Stroke. 2020;51(7):2224–2227. doi: 10.1161/STROKEAHA.120.030395. [DOI] [PubMed] [Google Scholar]
- 11.Cheng Y., Luo R., Wang K., et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ali H., Daoud A., Mohamed M.M., et al. Survival rate in acute kidney injury superimposed COVID-19 patients: a systematic review and meta-analysis. Ren. Fail. 2020;42(1):393–397. doi: 10.1080/0886022X.2020.1756323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puelles V.G., Lütgehetmann M., Lindenmeyer M.T., et al. Multiorgan and renal tropism of SARS-CoV-2. N. Engl. J. Med. 2020;383(6):590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tiwari N.R., Phatak S., Sharma V.R., Agarwal S.K. COVID-19 and thrombotic microangiopathies. Thromb. Res. 2021;202:191–198. doi: 10.1016/j.thromres.2021.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X., Sahu K.K., Cerny J. Coagulopathy, endothelial dysfunction, thrombotic microangiopathy and complement activation: potential role of complement system inhibition in COVID-19. J. Thromb. Thrombolysis. 2021;51(3):657–662. doi: 10.1007/s11239-020-02297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol. Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaimes J.A., Bidon M.K., Straus M.R., Daniel S., Whittaker G.R. 2021. Proteolytic Activation of SARS-CoV - 2 Spike at the S1/S2 Boundary: Potential Role of Proteases beyond Furin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ke Z., Oton J., Qu K., et al. Structures and distributions of SARS-CoV-2 spike proteins on intact virions. Nature. 2020;588(7838):498–502. doi: 10.1038/s41586-020-2665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loghmani H., Conway E.M. Exploring traditional and nontraditional roles for thrombomodulin. Blood. 2018;132(2):148–158. doi: 10.1182/blood-2017-12-768994. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki K., Kusumoto H., Deyashiki Y., et al. Structure and expression of human thrombomodulin, a thrombin receptor on endothelium acting as a cofactor for protein C activation. EMBO J. 1987;6(7):1891–1897. doi: 10.1002/j.1460-2075.1987.tb02448.x. http://www.ncbi.nlm.nih.gov/pubmed/2820710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schreiber R.D., Pangburn M.K., Lesavre P.H., Mueller Eberhard H.J. Initiation of the alternative pathway of complement. Recognition of activators by bound C3b and assembly of the entire pathway from six isolated proteins. Proc. Natl. Acad. Sci. U. S. A. 1978;75(8):3948–3952. doi: 10.1073/pnas.75.8.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delvaeye M., Noris M., De Vriese A., et al. Thrombomodulin mutations in atypical hemolytic-uremic syndrome. N. Engl. J. Med. 2009;361(4):345–357. doi: 10.1056/NEJMoa0810739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhainaut J.-F., Yan S.B., Cariou A., Mira J.-P. Soluble thrombomodulin, plasma-derived unactivated protein C, and recombinant human activated protein C in sepsis. Crit. Care Med. 2002;30(5 Suppl):S318–S324. doi: 10.1097/00003246-200205001-00023. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J., Xue M., Chen Y., et al. Identification of soluble thrombomodulin and tissue plasminogen activator-inhibitor complex as biomarkers for prognosis and early evaluation of septic shock and sepsis-induced disseminated intravascular coagulation. Ann. Palliat. Med. 2021;10(10):10170–10184. doi: 10.21037/apm-21-2222. [DOI] [PubMed] [Google Scholar]
- 26.Goshua G., Pine A.B., Meizlish M.L., et al. Endotheliopathy in COVID-19-associated coagulopathy : evidence from a single-centre , cross-sectional study. Lancet Haematol. 2020;7(8):e575–e582. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu J., Yuan X., Chen H., Chaturvedi S., Braunstein E.M., Brodsky R.A. vol. 136. 2020. (Direct Activation of the Alternative Complement Pathway by SARS-CoV-2 Spike Proteins Is Blocked by Factor D Inhibition). 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Satyam A., Tsokos M.G., Brook O.R., Hecht J.L., Moulton V.R., Tsokos G.C. Activation of classical and alternative complement pathways in the pathogenesis of lung injury in COVID-19. Clin. Immunol. 2021;226(March) doi: 10.1016/j.clim.2021.108716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rotoli B.M., Barilli A., Visigalli R., Ferrari F., Dall'Asta V. Endothelial cell activation by SARS-CoV-2 spike S1 protein: a crosstalk between endothelium and innate immune cells. Biomedicines. 2021;9(9) doi: 10.3390/biomedicines9091220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sartain S.E., Turner N.A., Moake J.L. TNF regulates essential alternative complement pathway components and impairs activation of protein C in human glomerular endothelial cells. J. Immunol. 2016;196(2):832–845. doi: 10.4049/jimmunol.1500960. [DOI] [PubMed] [Google Scholar]
- 31.Ohlin A.-K., Larsson K., Hansson M. Soluble thrombomodulin activity and soluble thrombomodulin antigen in plasma. J. Thromb. Haemostasis. 2005;3(5):976–982. doi: 10.1111/j.1538-7836.2005.01267.x. [DOI] [PubMed] [Google Scholar]
- 32.Lu R., Sui J., Zheng X.L. Elevated plasma levels of syndecan-1 and soluble thrombomodulin predict adverse outcomes in thrombotic thrombocytopenic purpura. Blood Adv. 2020;4(21):5378–5388. doi: 10.1182/bloodadvances.2020003065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leatherdale A., Stukas S., Lei V., et al. Persistently elevated complement alternative pathway biomarkers in COVID-19 correlate with hypoxemia and predict in-hospital mortality. Med. Microbiol. Immunol. 2022;211(1):37–48. doi: 10.1007/s00430-021-00725-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laurence J., Mulvey J.J., Seshadri M., et al. Anti-complement C5 therapy with eculizumab in three cases of critical COVID-19. Clin. Immunol. 2020;219 doi: 10.1016/j.clim.2020.108555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Afzali B., Noris M., Lambrecht B.N., Kemper C. The state of complement in COVID-19. Nat. Rev. Immunol. 2022;22(2):77–84. doi: 10.1038/s41577-021-00665-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cristiani L., Mancino E., Matera L., et al. Will children reveal their secret? The coronavirus dilemma. Eur. Respir. J. 2020;55(4) doi: 10.1183/13993003.00749-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bradley B.T., Maioli H., Johnston R., et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet (London, England) 2020;396(10247):320–332. doi: 10.1016/S0140-6736(20)31305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stefely J.A., Christensen B.B., Gogakos T., et al. Marked factor V activity elevation in severe COVID-19 is associated with venous thromboembolism. Am. J. Hematol. 2020;95(12):1522–1530. doi: 10.1002/ajh.25979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grey S.T., Tsuchida A., Hau H., Orthner C.L., Salem H.H., Hancock W.W. Selective inhibitory effects of the anticoagulant activated protein C on the responses of human mononuclear phagocytes to LPS, IFN-gamma, or phorbol ester. J. Immunol. 1994;153(8):3664–3672. http://www.ncbi.nlm.nih.gov/pubmed/7523500 [PubMed] [Google Scholar]
- 40.Galligan L., Livingstone W., Volkov Y., et al. Characterization of protein C receptor expression in monocytes. Br. J. Haematol. 2001;115(2):408–414. doi: 10.1046/j.1365-2141.2001.03187.x. [DOI] [PubMed] [Google Scholar]
- 41.Bae J.-S., Yang L., Manithody C., Rezaie A.R. Engineering a disulfide bond to stabilize the calcium-binding loop of activated protein C eliminates its anticoagulant but not its protective signaling properties. J. Biol. Chem. 2007;282(12):9251–9259. doi: 10.1074/jbc.M610547200. [DOI] [PubMed] [Google Scholar]
- 42.Jacob G., Aharon A., Brenner B. COVID-19-Associated hyper-fibrinolysis: mechanism and implementations. Front. Physiol. 2020:1–10. doi: 10.3389/fphys.2020.596057. 11(December) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bayrakci N., Ozkan G., Mutlu L.C., et al. Relationship between serum soluble endothelial protein C receptor level and COVID-19 findings. Blood Coagul. Fibrinolysis. 2021;32(8):550–555. doi: 10.1097/MBC.0000000000001070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wakelin S.J., Sabroe I., Gregory C.D., et al. Dirty little secrets”--endotoxin contamination of recombinant proteins. Immunol. Lett. 2006;106(1):1–7. doi: 10.1016/j.imlet.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 45.Schwarz H., Schmittner M., Duschl A., Horejs-Hoeck J. Residual endotoxin contaminations in recombinant proteins are sufficient to activate human CD1c+ dendritic cells. PLoS One. 2014;9(12) doi: 10.1371/journal.pone.0113840. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.