Abstract
Purpose
To investigate the role of pre- and post-stereotactic body radiation therapy (SBRT) neutrophil-to-lymphocyte ratio (NLR) in patients with localized pancreatic cancer treated with anti-PD-1 (programmed cell death protein-1) antibody and SBRT.
Materials and Methods
This was a retrospective review of 68 patients with borderline resectable or locally advanced pancreatic cancer treated with anti-PD-1 antibody and SBRT after multi-agent chemotherapy. Immunotherapy was administered with 5-fraction SBRT in the neoadjuvant, concurrent, or adjuvant/maintenance setting. Clinical outcomes included overall survival (OS), local progression-free survival, distant metastasis-free survival, and progression-free survival. Median pre- and post-SBRT peripheral blood markers were compared with the Mann-Whitney U test. Univariate and multivariable analyses (UVA and MVA) were performed to identify variables associated with clinical outcomes. Linear regression was performed to determine correlations between variables and peripheral blood markers.
Results
A total of 68 patients were included in the study. The percent change between median pre- and post-SBRT absolute lymphocyte count (ALC), absolute neutrophil count, and NLR were -36.0% (p < 0.001), -5.6% (p = 0.190), and +35.7% (p = 0.003), respectively. Median OS after SBRT was 22.4 months. On UVA, pre-SBRT CA19-9 (hazard ratio [HR] = 1.001; 95% confidence interval [CI], 1.000–1.001; p = 0.031), post-SBRT ALC (HR = 0.33; 95% CI, 0.11–0.91; p = 0.031), and post-SBRT NLR (HR = 1.13; 95% CI, 1.04–1.22; p = 0.009) were associated with OS. On MVA, induction chemotherapy duration (HR = 0.75; 95% CI, 0.57–0.99; p = 0.048) and post-SBRT NLR (HR = 1.14; 95% CI, 1.04–1.23; p = 0.002) predicted for OS. Patients with post-SBRT NLR ≥3.2 had a median OS of 15.6 months versus 27.6 months in patients with post-SBRT NLR <3.2 (p = 0.009). On MVA linear regression, log10CTV had a negative correlation with post-SBRT ALC (regression coefficient = -0.314; 95% CI, -0.626 to -0.003; p = 0.048).
Conclusion
Elevated NLR after SBRT is primarily due to depletion of lymphocytes and associated with worse survival outcomes in localized pancreatic cancer treated with anti-PD-1 antibody. Larger CTVs were associated with decreased post-SBRT ALC.
Keywords: Stereotactic body radiotherapy; Immunotherapy; Pancreatic cancer; PD-1 inhibitor; Immune checkpoint inhibitor; Neutrophil, Lymphocyte
Introduction
Pancreatic cancer is currently the third most common cause of cancer related deaths in the United States, responsible for over 48,000 deaths each year [1]. In fact, it is projected to be the second most common cause by the year 2030 [2]. Treatment of pancreatic cancer usually involves a combination of chemotherapy, radiation therapy, and surgery [3]. Even with aggressive therapy, prognosis is poor with 5-year overall survival (OS) rates of less than 15% for borderline resectable and locally advanced pancreatic adenocarcinoma (BRPC/LAPC) [4]. Novel therapies are needed to improve outcomes.
One area of investigation is the use of immune checkpoint inhibitors (ICIs), such as programmed cell death-1 (PD-1) inhibitors, which have shown great promise in a wide range of malignancies including non-small cell lung cancer, melanoma, and esophageal cancer [5-7]. However, the role of ICIs in pancreatic cancer is tenuous. The current literature is sparse, with some reports demonstrating that there is limited benefit of ICI monotherapy in pancreatic cancer [8,9]. This is thought to be due to the immunosuppressive and stroma-rich microenvironment of pancreatic cancer [10]. The combination of ICIs with other immunotherapeutic agents and stereotactic body radiation therapy (SBRT) may increase the immunogenicity of the pancreatic tumor microenvironment (TME) and is currently being investigated [11-16].
One of the hallmarks of cancer is inflammation which is an immunologic response mediated by a variety of cells and signaling molecules [17]. The neutrophil-to-lymphocyte ratio (NLR) is a readily available marker of inflammation and immunogenicity, with elevated levels associated with poor outcomes in many solid tumors [18]. Although studies have demonstrated the prognostic and predictive value of NLR in various cancers treated with ICIs, there have been no studies investigating the role of NLR in localized pancreatic cancer treated with ICIs and SBRT [19,20]. Such information may inform decisions regarding radiation planning (dose, target volume, etc.) in the setting of immunotherapy for pancreatic cancer. At our institution, we have been exploring the combination of ICI therapy and SBRT in localized pancreatic cancer in the setting of several clinical trials. As such, the purpose of this study was to investigate the impact of pre- and post-SBRT NLR on clinical outcomes in a cohort of localized pancreatic cancer patients treated with anti-PD-1 antibody and SBRT as well as to identify factors associated with NLR dynamics.
Materials and Methods
1. Study design
This study was was a single institution retrospective review of patients with BRPC/LAPC who were treated with multi-agent induction chemotherapy followed by anti-PD-1 antibody and SBRT between August 2016 and May 2021. Patients were included in the study if they met the following criteria: (1) biopsy proven diagnosis of pancreatic adenocarcinoma, (2) locally advanced or borderline resectable disease per the National Comprehensive Cancer Network (NCCN) guidelines [3], (3) treatment with induction chemotherapy, anti-PD-1 antibody, and SBRT, (4) peripheral blood markers available for review pre- and post-SBRT, and (5) regular follow-up with post-treatment diagnostic imaging. All data including patient information, treatment details, clinical outcomes, and peripheral blood markers were retrospectively collected. Initial induction chemotherapy regimens consisted of FOLFIRINOX (FFX) and gemcitabine/nab-paclitaxel (GnP). Immunotherapy consisted of various experimental immunologic agents in combination with anti-PD-1 antibody. Immunotherapy was administered with SBRT in the neoadjuvant, concurrent, and/or adjuvant/maintenance setting. When given in the concurrent setting, anti-PD-1 antibody was administered a few hours after fraction one of SBRT. The study was conducted in accordance with the Declaration of Helsinki, and was approved by our institutional review board of Johns Hopkins University (No. 00285919). Given the retrospective nature of the study, written informed consent was waived.
2. SBRT details
Patients were treated with 5-fraction SBRT on 5 consecutive business days. Prior to simulation, endoscopic ultrasound guided placement of fiducials was performed to be used for daily image guidance. At time of simulation, thin sliced computed tomography (CT) scans with intravenous contrast were obtained, with patients positioned supine and arms above head in a Vac-lok device (CIVCO Medical Solutions, Coralville, IA, USA) for immobilization. Active breathing control (ABC; Elekta, Stockholm, Sweden) was utilized for respiratory motion management. In patients who could not tolerate ABC, a four-dimensional CT scan was acquired, and an internal target volume (ITV) was generated from the maximum inspiratory and expiratory phases. Pinnacle treatment planning system (Phillips Radiation Oncology Systems, Fitchburg, WI, USA) was used for target and organ-at-risk delineation. The clinical target volume (CTV) included the gross tumor plus regions at risk for microscopic disease, such as full circumference of involved and/or adjacent vasculature. The planning target volume was generated by adding a 2–5 mm isotropic margin to the CTV in breath-hold cases and to the ITV in free-breathing cases. Radiation prescription goals were as follows: (1) dose coverage—prescription dose to cover ≥98% of CTV and ≥90% of PTV, 25 Gy to cover 100% of the CTV and ≥99% of PTV, (2) gastrointestinal organs (stomach, duodenum, bowel)—V33 <1 mL, V20 <20 mL, max dose to planning organ-at-risk volume (3 mm margin around organs) <40 Gy. Pre-treatment and intrafractional cone-beam CTs were acquired to verify patient positioning. Patients were aligned to spine and then shifted to align to fiducials. All patients were treated on an Elekta linear accelerator unit.
3. Peripheral blood markers
Absolute lymphocyte counts (ALC) and absolute neutrophil count (ANC) were collected pre- and post-SBRT. Values were recorded within 4-weeks prior to SBRT and 1–6 weeks after completion of SBRT. If multiple values existed, the values closest to start of SBRT and closest to 4-weeks after SBRT were recorded. The NLR was calculated by dividing the ANC by ALC for each patient.
4. Clinical outcomes
Clinical outcomes included OS, local progression-free survival (LPFS), distant metastasis-free survival (DMFS), and progression-free survival (PFS). OS was defined as time from SBRT to death. LPFS and DMFS were defined as time from SBRT to radiographic evidence of locoregional progression and distant progression, respectively. PFS was defined as time from SBRT to any radiographic evidence of progression or death.
5. Statistical analysis
Descriptive statistics was used to record patient demographic, treatment, and disease characteristics, including age, sex, Eastern Cooperative Oncology Group status, disease extent, disease grade, chemotherapy duration and regimen, immunotherapy, radiation therapy, resection status, and peripheral blood values. Differences in median pre- and post-SBRT ALC, ANC, and NLR values were assessed using the Mann-Whitney U test. To account for multicollinearity, continuous variables were selected with backwards elimination such that variables with a variance inflation factor (VIF) >2.5 were eliminated. Univariate Cox analysis was performed to identify associations between the aforementioned variables with OS, LPFS, DMFS, and PFS. Variables with p < 0.1 on univariate Cox analysis were entered in multivariable Cox analysis and subsequently removed if significance rose to p > 0.1. Receiver operative characteristics analysis was performed to identify the optimal post-SBRT NLR cut-off value in predicting OS. Kaplan-Meier method was used for survival analysis and log-rank test was performed to assess significance between groups. Correlations between peripheral blood markers and CTV were assessed with Spearman coefficient. Univariate and multivariable linear regression were performed to determine the association between variables and post-SBRT lymphocyte counts. The CTV values were log transformed (log10CTV) to generate a normal distribution. Variables with p < 0.1 on univariate linear regression were entered into multivariable linear regression and subsequently removed if significance rose to p > 0.1. All p-values were two-sided and statistical significance was considered p < 0.05. All statistical analysis was performed with JMP version 15.0 (SAS Institute, Cary, NC, USA).
Results
1. Cohort characteristics
Patient, treatment, and disease characteristics are shown in Table 1. From August 2016 to May 2021, 68 patients were treated with multi-agent induction chemotherapy followed by anti-PD-1 antibody and SBRT. The median age was 64.5 years (range, 41.7 to 84.1 years) and 57.3% were male. Borderline resectable and locally advanced disease was present in nine patients (13.2%) and 59 patients (87.8%), respectively. Baseline and pre-SBRT CA19-9 values were 224.5 U/mL (range, <1 to 8094.0 U/mL) and 40.0 U/mL (range, <1 to 3264.4 U/mL), respectively. Initial induction chemotherapy consisted of FFX (61/68; 89.7%) and GnP (7/68; 10.3%). Of note, five patients were transitioned from FFX to GnP, one patient from FFX to capecitabine, and one patient from GnP to gemcitabine due to intolerance of initial chemotherapy. Anti-PD-1 antibody therapy was delivered with SBRT in the neoadjuvant/concurrent (30/68; 44.1%), neoadjuvant/concurrent/adjuvant/maintenance (20/68; 29.4%), adjuvant alone (15/68; 22.1%), neoadjuvant alone (2/68; 2.9%), and concurrent alone (1/68; 1.5%) setting. Nearly all patients received SBRT to 33 Gy in 5 fractions (67/68; 98.5%), with one patient receiving 30.5 Gy in 5 fractions. Surgical resection was performed in 61.8% of patients (42/68), with the specific types of surgical procedure including the Whipple procedure (26/42; 61.9%), distal pancreatectomy (13/42; 30.9%), and total pancreatectomy (3/42; 7.2%).
Table 1.
Patient, treatment, and disease characteristics (n = 68)
| Characteristic | Value |
|---|---|
| Age (yr) | 64.5 (41.7–84.1) |
| Sex | |
| Male | 39 (57.3) |
| Female | 29 (42.7) |
| ECOG performance status | |
| 0 | 24 (35.3) |
| 1 | 44 (64.7) |
| Location of primary tumor | |
| Head | 37 (54.4) |
| Other | 31 (45.6) |
| Disease extent | |
| Borderline resectable | 9 (13.2) |
| Locally advanced | 59 (87.8) |
| Baseline CA19-9 (U/mL) | 224.5 (<1.0–8094.0) |
| Pre-SBRT CA19-9 (U/mL) | 40.0 (<1.0–3264.4) |
| Induction chemotherapy duration (mo) | 5 (1–8) |
| Initial induction chemotherapy | |
| FFX | 61 (89.7) |
| GnP | 7 (10.3) |
| Anti-PD-1 antibody duration (cycles) | 2 (1–18) |
| Anti-PD-1 antibody timing with SBRT | |
| Neoadjuvant/concurrent | 30 (44.1) |
| Neoadjuvant/concurrent/adjuvant/maintenance | 20 (29.4) |
| Adjuvant/maintenance | 15 (22.1) |
| Neoadjuvant | 2 (2.9) |
| Concurrent | 1 (1.5) |
| SBRT dose and fractionation | |
| 33 Gy in 5 fractions | 67 (98.5) |
| 30.5 Gy in 5 fractions | 1 (1.5) |
| CTV (cm3) | 88.2 (19.2–288.5) |
| Surgically resected | 42 (61.8) |
| Whipple | 26 (61.9) |
| Distal | 13 (30.9) |
| Total pancreatectomy | 3 (7.2) |
Values are presented as median (range) or number of patients (%).
ECOG, Eastern Cooperative Oncology Group; CA19-9, cancer antigen 19-9; FFX, FOLFIRINOX; GnP, gemcitabine/nab-paclitaxel; PD-1, programmed cell death protein 1; SBRT, stereotactic body radiation therapy; CTV, clinical target volume.
2. Increase in NLR is a result of depletion of lymphocytes
Table 2 shows pre- and post-SBRT ALC, ANC, and NLR values for the entire cohort. The median pre-SBRT ALC, ANC, and NLR values were 1.50 ×103/μL (range, 0.33 to 3.73 ×103/μL), 3.19 ×103/μL (range, 1.36 to 17.9 ×103/μL), and 2.38 (range, 0.52 to 44.1), respectively. The median post-SBRT ALC, ANC, and NLR values were 0.84 ×103/μL (range, 0.32 to 1.93 ×103/μL), 2.87 ×103/μL (range, 1.24 to 9.64 ×103/μL), and 3.41 (range, 1.32 to 16.9), with a change of -36.0% (p < 0.001), -5.6% (p = 0.190), and +35.7% (p = 0.003), respectively. To determine if timing of blood draws after SBRT influenced NLR, we performed Mann-Whitney U test between patients who had labs drawn 1–4 weeks versus 4–6 weeks after SBRT, with no significant difference in NLR between the two groups (p = 0.994). We did the same analysis to determine if chemotherapy regimen (FFX vs. GnP) influenced NLR, with no significant difference detected (p = 0.703).
Table 2.
Pre- and post-SBRT lymphocyte, neutrophil, and NLR values
| Variable | Pre-SBRT | Post-SBRT | % Change | p-value |
|---|---|---|---|---|
| ALC (×103/μL) | 1.50 (0.33–3.73) | 0.84 (0.32–1.93) | -36.0 | <0.001 |
| ANC (×103/μL) | 3.19 (1.36–17.9) | 2.87 (1.24–9.64) | -5.6 | 0.190 |
| NLR | 2.38 (0.52–44.1) | 3.41 (1.32–16.9) | +35.7 | 0.003 |
Values are presented as median (range).
SBRT, stereotactic body radiation therapy; ALC, absolute lymphocyte count; ANC, absolute neutrophil count; NLR, neutrophil to lymphocyte ratio.
3. Clinical outcomes: post-SBRT NLR is associated with OS
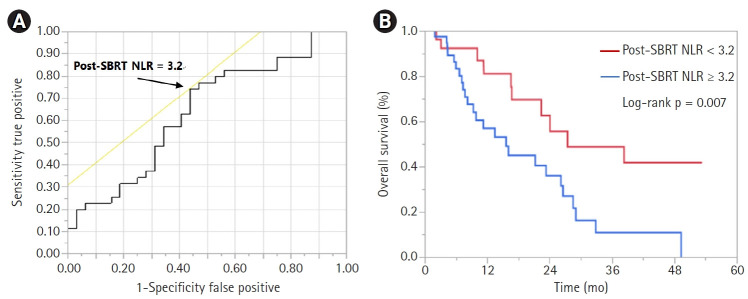
The median follow-up time for the entire cohort was 10.7 months (range, 1.9 to 53.3 months). Of the 68 patients, 33 (48.5%) had died and 35 (51.5%) were alive. Among patients who were alive, median follow-up time was 8.3 months (range, 1.9 to 53.3 months). Median OS from time of SBRT was 22.4 months, with 1-year, 2-year, and 3-year OS rates of 66.9%, 47.3%, and 28.2%, respectively. On UVA, pre-SBRT CA19-9 (hazard ratio [HR] = 1.00; 95% confidence interval [CI], 1.000–1.001; p = 0.031), post-SBRT ALC (HR = 0.33; 95% CI, 0.11–0.91; p = 0.031), and post-SBRT NLR (HR = 1.13; 95% CI, 1.04–1.22; p = 0.009) were associated with OS, with a trend towards significance for induction chemotherapy duration and CTV volume (Table 3). On stepwise MVA, only induction chemotherapy duration (HR = 0.75; 95% CI, 0.57–0.99; p = 0.048) and post-SBRT NLR (HR = 1.14; 95% CI, 1.04–1.23; p = 0.002) were associated with OS (Table 3). Receiver operator characteristic analysis identified the optimal post-SBRT NLR cut-off value of 3.2 in predicting OS (area under the curve = 0.639, sensitivity = 74.3%, specificity = 56.3%) (Fig. 1A). Patients with post-SBRT NLR ≥3.2 had a median OS of 15.6 months versus 27.6 months in patients with post-SBRT NLR <3.2 (p = 0.009) (Fig. 1B).
Table 3.
Univariate and multivariable analyses of overall survival
| Univariate | Multivariable | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age (yr) | 1.02 | 0.99–1.06 | 0.182 | |||
| Sex (male vs. female) | 0.79 | 0.40–1.53 | 0.467 | |||
| ECOG performance status (1 vs. 0) | 1.56 | 0.76–3.20 | 0.225 | |||
| Disease extent (BRPC vs. LAPC) | 1.20 | 0.37–3.96 | 0.761 | |||
| Tumor location (head vs. other) | 1.30 | 0.67–2.54 | 0.442 | |||
| Induction CT duration (mo) | 0.79 | 0.60–1.04 | 0.094 | 0.75 | 0.57–0.99 | 0.048 |
| Induction CT (FFX vs. GnP) | 0.67 | 0.26–1.76 | 0.417 | |||
| Grade (I/II vs. III) | 0.92 | 0.44–1.93 | 0.831 | |||
| Resected (yes vs. no) | 0.66 | 0.34–1.29 | 0.229 | |||
| CTV (cm3) | 1.01 | 0.99–1.01 | 0.051 | |||
| Baseline CA19-9 (U/mL) | 1.00 | 0.99–1.00 | 0.309 | |||
| Pre-SBRT CA19-9 (U/mL) | 1.001 | 1.000–1.001 | 0.031 | |||
| Baseline total bilirubin (mg/dL) | 1.08 | 0.96–1.17 | 0.173 | |||
| Pre-SBRT | ||||||
| ALC (×103/μL) | 0.66 | 0.34–1.13 | 0.140 | |||
| NLR | 1.04 | 0.94–1.10 | 0.354 | |||
| Post-SBRT | ||||||
| ALC (×103/μL) | 0.33 | 0.11–0.91 | 0.031 | |||
| NLR | 1.13 | 1.04–1.22 | 0.009 | 1.14 | 1.04–1.23 | 0.002 |
ECOG, Eastern Cooperative Oncology Group; BRPC, borderline resectable pancreatic cancer; LAPC, locally advanced pancreatic cancer; CT, chemotherapy; FFX, FOLFIRINOX; GnP, gemcitabine/nab-paclitaxel; CTV, Clinical target volume; CA19-9, cancer antigen 19-9; SBRT, stereotactic body radiation therapy; ALC, absolute lymphocyte count; ANC, absolute neutrophil count; NLR, neutrophil to lymphocyte ratio; HR, hazard ratio; CI, confidence interval.
Fig. 1.
Receiver operating characteristic (ROC) curve showing (A) optimal post-SBRT NLR value predicting overall survival and (B) Kaplan-Meier curve showing overall survival stratified by post-SBRT NLR of 3.2. Intersection of yellow line and ROC curve in Fig. 1A corresponds to the optimal NLR cut-off value of 3.2. SBRT, stereotactic body radiation therapy; NLR, neutrophil to lymphocyte ratio.
Supplementary Tables S1–S3 show UVA and MVA for LPFS, DMFS, and PFS. On UVA of LPFS, only larger CTV volume was associated with inferior outcome (HR = 1.01; 95% CI, 1.00–1.02; p = 0.019). As such, MVA was not performed for LPFS. On UVA of DMFS, elevated pre-SBRT CA19-9 (HR = 1.01; 95% CI, 1.000–1.001; p < 0.001) was associated with worse outcome. As such, MVA was not performed for DMFS. On MVA of PFS, only elevated pre-SBRT CA19-9 was associated with inferior outcome (HR = 1.01; 95% CI, 1.00–1.01; p < 0.001).
4. CTV is associated post-SBRT lymphocyte counts
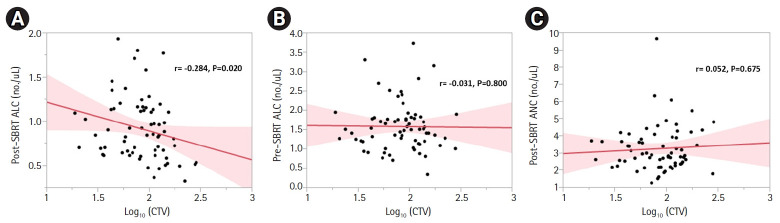
Given that the increase in NLR was primarily due to depletion of lymphocytes (Table 2), we wanted to determine if there were any variables, including modifiable treatment characteristics, associated with post-SBRT ALC. There was a negative correlation between log10CTV and post-SBRT ALC (r = -0.284, p = 0.020) (Fig. 2A). To determine if this association existed prior to radiation or was radiation induced, log10CTV was plotted against pre-SBRT ALC, with no correlation found (r = -0.031, p = 0.800) (Fig. 2B). Similarly, there was no correlation between log10CTV and post-SBRT ANC (r = 0.052, p = 0.675) (Fig. 2C). Table 4 shows univariate and multivariable linear regression of variables associated with post-SBRT ALC. On MVA, when accounting for age, sex, ECOG, disease extent, induction chemotherapy duration and regimen, and disease grade, log10CTV (regression coefficient = -0.314; 95% CI, -0.626 to -0.003; p = 0.048) had a significant negative correlation with post-SBRT ALC, while pre-SBRT ALC (regression coefficient = 0.217; 95% CI, 0.057 to 0.344; p = 0.001) had significant positive correlation with post-SBRT ALC.
Fig. 2.
Correlations among (A) post-SBRT ALC, (B) pre-SBRT ALC, and (C) post-SBRT ANC with log10(CTV). Spearman correlation coefficients (r) and p-values are displayed. SBRT, stereotactic body radiation therapy; ALC, absolute lymphocyte count; ANC, absolute neutrophil count; CTV, clinical target volume.
Table 4.
Univariate and multivariable linear regression of post-SBRT ALC
| Univariate | Multivariable | |||||
|---|---|---|---|---|---|---|
| Regression coefficient | 95% CI | p-value | Regression coefficient | 95% CI | p-value | |
| Age (yr) | -0.005 | -0.014 to 0.004 | 0.297 | |||
| Sex (male vs. female) | -0.035 | -0.124 to 0.054 | 0.439 | |||
| ECOG performance status (1 vs. 0) | -0.005 | 0.098 to 0.088 | 0.914 | |||
| Disease extent (BRPC vs. LAPC) | 0.080 | -0.055 to 0.214 | 0.242 | |||
| Tumor location (head vs. other) | 0.056 | -0.031 to 0.143 | 0.203 | |||
| Induction CT duration (mo) | -0.016 | -0.083 to 0.051 | 0.643 | |||
| Induction CT (FFX vs. GnP) | 0.056 | -0.102 to 0.213 | 0.483 | |||
| Grade (I/II vs. III) | 0.003 | -0.010 to 0.103 | 0.959 | |||
| Log10CTV | -0.326 | -0.661 to 0.010 | 0.057 | -0.314 | -0.626 to -0.003 | 0.048 |
| Pre-SBRT ALC (×103/μL) | 0.220 | 0.090 to 0.349 | 0.001 | 0.217 | 0.057 to 0.344 | 0.001 |
SBRT, stereotactic body radiation therapy; ALC, absolute lymphocyte count; ECOG, Eastern Cooperative Oncology Group; BRPC, borderline resectable pancreatic cancer; LAPC, locally advanced pancreatic cancer; CT, chemotherapy; FFX, FOLFIRINOX; GnP, gemcitabine/nab-paclitaxel; CTV, clinical target volume; CI, confidence interval.
Discussion and Conclusion
In this report, we show that elevated NLR after SBRT is a result of depletion of lymphocytes and associated with inferior survival outcomes in localized pancreatic cancer treated with anti-PD-1 antibody. Furthermore, larger CTVs was associated with decreased post-SBRT lymphocyte counts. These findings may have implications on radiation field design for localized pancreatic cancer, particularly in the setting of combination therapy with immunotherapeutic agents.
The role of ICIs in pancreatic cancer is still under investigation. Only a handful of studies exist, which suggest that ICI monotherapy may have limited benefit [8,9,21,22]. This is likely due to immunosuppressive and hypoxic environment of the pancreatic TME [10]. Additionally, pancreatic adenocarcinoma is associated with low mutational burden, affecting neoantigen production and immune recognition [23,24]. To increase tumor immunogenicity, trials are investigating combination therapy of ICI with vaccines, chemokine inhibitors, oncolytic viruses, and SBRT [11-16,25]. Prognostic markers that take into account immunogenicity may aid in selection of these personalized therapies.
It is widely known that inflammation and cancer are inextricably linked [26]. The NLR is a marker of inflammation and immunogenicity, with elevated levels associated with poor outcomes in various malignancies [18]. The NLR is practical measure since it can be calculated from routine blood counts. Although the exact mechanism is unknown, it is thought that the NLR takes into account both the pro-tumorigenic and anti-tumorigenic activity of neutrophils and lymphocytes, respectively. Neutrophils have been shown to secrete signaling molecules which promote tumor angiogenesis and evasion while suppressing cytotoxic T lymphocytes [27,28]. Lymphocytes, however, are associated with direct killing of cancerous cells [29]. The NLR has been validated as a prognostic and predictive factor in a wide range of cancers including pancreatic adenocarcinoma [18,30]. Recently, the prognostic value of NLR has been confirmed in various cancers treated with immunotherapy, including ICIs [19]. A report by Li et al. [31] showed that baseline and on-treatment NLR ≥5 predicted for worse OS in advanced cancers treated with ICIs. However, there have been no reports investigating the role of NLR in localized pancreatic cancer treated with ICIs and SBRT.
We show that elevated post-radiation NLR is predictive of worse OS in BRPC/LAPC treated with anti-PD-1 antibody and SBRT. The mechanism by which elevated post-radiation NLR is associated with worse OS in these patients is unknown. A focus on lymphocyte counts could provide some insight. Our data suggest that the increase in NLR after SBRT is largely due to depletion of lymphocytes, which is in agreement with a recent study by Wolfe et al. [32]. Radiation induced lymphopenia is associated with worse survival outcomes in both resected and locally advanced pancreatic cancer [33,34]. Furthermore, anti-PD-1 antibody induce immunogenic cell death through direct activation of cytotoxic T lymphocytes. Therefore, a possible explanation is that elevated post-radiation NLR is associated with inferior survival as a result of radiation-induced depletion of lymphocytes, with decreased efficacy of ICI therapy as a result. However, lymphocytes alone cannot fully explain this interaction, as ALC was not associated with outcomes in our study, suggesting that neutrophils may play a role as well. Indeed, a recent report showed that neutrophilia was associated with inferior outcomes in LAPC treated with chemoradiation [35]. Therefore, further studies are needed to further elucidate the roles of neutrophils and lymphocytes in the pancreatic cancer TME and how they interact with SBRT and ICIs.
Our data also demonstrate that larger CTVs correlated with decreased lymphocyte counts, which is in agreement with findings from others [36,37]. This suggests that field design is crucial in the radiation planning process for the treatment of pancreatic cancer. Currently, there is varying consensus on the appropriate target volumes for the treatment of intact pancreatic cancer. Some advocate for coverage of smaller volumes including gross disease and adjacent vasculature, while others suggest that treating larger volumes with elective nodal irradiation may improve outcomes [38-41]. A prior analysis from our institution (results not published), for example, showed that nearly all local failures mapped to a “triangle volume” bordered by peri-pancreatic vasculature, which supports irradiation of a larger volume beyond gross disease that contains perineural tracts and lymphatic channels at risk of microscopic residual disease. However, the immunologic implications of targeting such a volume should be explored. One important consideration is whether radiation-induced lymphopenia is a result of irradiation to circulating blood/lymphatic channels or hematopoietic organs such as the spleen and vertebral bodies or a combination of both. If post-radiation lymphopenia is primarily due to irradiation of hematopoietic organs, then treating larger volumes such as the aforementioned “triangle volume” with optimization of splenic and vertebral body dosimetry may be appropriate. Prior reports do suggest that splenic and vertebral body dose is an important contributor to lymphopenia and that meeting specific dose constraints can mitigate this effect [42,43].
The current study has several limitations, including its retrospective nature. Additionally, patients were treated with heterogeneous induction chemotherapy regimens and different experimental immunotherapy agents in combination with anti-PD-1 antibody, which in turn, could influence peripheral blood markers, tumor inflammation, interaction with SBRT, and clinical outcomes. Similarly, the duration and timing of immunotherapy were also variable. Finally, post-SBRT peripheral blood markers were collected anywhere from 1–6 weeks after completion of treatment. It is possible that these values may have fluctuated during this time. The strengths of this study include its large sample size, homogeneity in SBRT dose/fractionation, and long follow-up time. Despite the limitations, this is the first report on this subject and provides new information regarding the role of NLR in pancreatic cancer treated with anti-PD-1 antibody and SBRT.
In summary, we demonstrate that elevated NLR after SBRT is primarily due to depletion of lymphocytes and is associated with inferior survival in localized pancreatic adenocarcinoma treated with anti-PD-1 antibody. Additionally, larger CTVs were associated with decreased post-SBRT lymphocyte counts. Further investigation into the complex relationship between SBRT, NLR, and ICI activity is warranted.
Footnotes
Conflict of Interest
Dr. Joseph M Herman is a former employee of PANCAN and current employee of 1440 Foundation. Dr. Jeffrey Meyer receives royalties from Uptodate and Springer and honorarium from Springer. No other conflicts of interest to disclose.
Author Contribution
Conceptualization: AVR, AKN; Investigation and methodology: AVR, AKN; Resources: AKN; Supervision: AKN, LZ, DAL, ADJ, JMH, JM; Writing of the original draft: AVR; Writing of the review and editing: AVR, AKN, CSH, SS, LZ, DAL, ADJ, JMH, JM; Formal analysis: AVR; Data curation: AVR, CSH, SS. All the authors have proofread the final version.
Supplementary Materials
Supplementary materials can be found via https://doi.org/10.3857/roj.2021.01060.
Univariate and multivariable analyses of local progression-free survival
Univariate and multivariable analyses of distant metastasis-free survival
Univariate and multivariable analyses of progression-free survival
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–21. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network . Plymouth Meeting, PA: National Comprehensive Cancer Network; Pancreatic adenocarcinoma version 2.2021 [Internet] c2022 [cited 2022 Apr 25]. Available from: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1455. [Google Scholar]
- 4.Rawla P, Sunkara T, Gaduputi V. Epidemiology of pancreatic cancer: global trends, etiology and risk factors. World J Oncol. 2019;10:10–27. doi: 10.14740/wjon1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antonia SJ, Villegas A, Daniel D, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379:2342–50. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 6.Kelly RJ, Ajani JA, Kuzdzal J, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med. 2021;384:1191–203. doi: 10.1056/NEJMoa2032125. [DOI] [PubMed] [Google Scholar]
- 7.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377:1345–56. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henriksen A, Dyhl-Polk A, Chen I, Nielsen D. Checkpoint inhibitors in pancreatic cancer. Cancer Treat Rev. 2019;78:17–30. doi: 10.1016/j.ctrv.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erkan M, Hausmann S, Michalski CW, et al. The role of stroma in pancreatic cancer: diagnostic and therapeutic implications. Nat Rev Gastroenterol Hepatol. 2012;9:454–67. doi: 10.1038/nrgastro.2012.115. [DOI] [PubMed] [Google Scholar]
- 11.Nevala-Plagemann C, Hidalgo M, Garrido-Laguna I. From state-of-the-art treatments to novel therapies for advanced-stage pancreatic cancer. Nat Rev Clin Oncol. 2020;17:108–23. doi: 10.1038/s41571-019-0281-6. [DOI] [PubMed] [Google Scholar]
- 12.Bethesda, MD: ClinicalTrials.gov; Study with CY, pembrolizumab, GVAX, and SBRT in patients with locally advanced pancreatic cancer (ClinicalTrials.gov Identifier: NCT02648282) [Internet] 2016 [cited 2022 Apr 25]. Available from: https://clinicaltrials.gov/ct2/show/NCT02648282. [Google Scholar]
- 13.Bethesda, MD: ClinicalTrials.gov; Trial of neoadjuvant and adjuvant nivolumab and BMS-813160 with or without GVAX for locally advanced pancreatic ductal adenocarcinomas (ClinicalTrials.gov Identifier: NCT03767582) [Internet] 2018 [cited 2022 Apr 25]. Available from: https://clinicaltrials.gov/ct2/show/NCT03767582. [Google Scholar]
- 14.Bethesda, MD: ClinicalTrials.gov; GVAX pancreas vaccine (with CY) in combination with nivolumab and SBRT for patients with borderline resectable pancreatic cancer (ClinicalTrials.gov Identifier: NCT03161379) [Internet] 2017 [cited 2022 Apr 25]. Available from: https://clinicaltrials.gov/ct2/show/NCT03161379. [Google Scholar]
- 15.Bethesda, MD: ClinicalTrials.gov; Pilot study with CY, pembrolizumab, GVAX, and IMC-CS4 (LY3022855) in patients with borderline resectable adenocarcinoma of the pancreas (ClinicalTrials.gov Identifier: NCT03153410) [Internet] 2017 [cited 2022 Apr 25]. Available from: https://clinicaltrials.gov/ct2/show/NCT03153410. [Google Scholar]
- 16.Bethesda, MD: ClinicalTrials.gov; Losartan and nivolumab in combination with FOLFIRINOX and SBRT in localized pancreatic cancer (ClinicalTrials.gov Identifier: NCT03563248) [Internet] 2018 [cited 2022 Apr 25]. Available from: https://clinicaltrials.gov/ct2/show/NCT03563248. [Google Scholar]
- 17.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 19.Faget J, Peters S, Quantin X, Meylan E, Bonnefoy N. Neutrophils in the era of immune checkpoint blockade. J Immunother Cancer. 2021;9:e002242. doi: 10.1136/jitc-2020-002242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suh KJ, Kim SH, Kim YJ, et al. Post-treatment neutrophil-to-lymphocyte ratio at week 6 is prognostic in patients with advanced non-small cell lung cancers treated with anti-PD-1 antibody. Cancer Immunol Immunother. 2018;67:459–70. doi: 10.1007/s00262-017-2092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Royal RE, Levy C, Turner K, et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33:828–33. doi: 10.1097/CJI.0b013e3181eec14c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patnaik A, Kang SP, Rasco D, et al. Phase I study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in patients with advanced solid tumors. Clin Cancer Res. 2015;21:4286–93. doi: 10.1158/1078-0432.CCR-14-2607. [DOI] [PubMed] [Google Scholar]
- 23.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA Jr, Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546–58. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hilmi M, Bartholin L, Neuzillet C. Immune therapies in pancreatic ductal adenocarcinoma: Where are we now? World J Gastroenterol. 2018;24:2137–51. doi: 10.3748/wjg.v24.i20.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katayama ES, Hue JJ, Bajor DL, et al. A comprehensive analysis of clinical trials in pancreatic cancer: what is coming down the pike? Oncotarget. 2020;11:3489–501. doi: 10.18632/oncotarget.27727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murata M. Inflammation and cancer. Environ Health Prev Med. 2018;23:50. doi: 10.1186/s12199-018-0740-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masucci MT, Minopoli M, Carriero MV. Tumor associated neutrophils: their role in tumorigenesis, metastasis, prognosis and therapy. Front Oncol. 2019;9:1146. doi: 10.3389/fonc.2019.01146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aarts CE, Hiemstra IH, Beguin EP, et al. Activated neutrophils exert myeloid-derived suppressor cell activity damaging T cells beyond repair. Blood Adv. 2019;3:3562–74. doi: 10.1182/bloodadvances.2019031609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martínez-Lostao L, Anel A, Pardo J. How do cytotoxic lymphocytes kill cancer cells? Clin Cancer Res. 2015;21:5047–56. doi: 10.1158/1078-0432.CCR-15-0685. [DOI] [PubMed] [Google Scholar]
- 30.Zhou Y, Wei Q, Fan J, Cheng S, Ding W, Hua Z. Prognostic role of the neutrophil-to-lymphocyte ratio in pancreatic cancer: a meta-analysis containing 8252 patients. Clin Chim Acta. 2018;479:181–9. doi: 10.1016/j.cca.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 31.Li M, Spakowicz D, Burkart J, et al. Change in neutrophil to lymphocyte ratio during immunotherapy treatment is a non-linear predictor of patient outcomes in advanced cancers. J Cancer Res Clin Oncol. 2019;145:2541–6. doi: 10.1007/s00432-019-02982-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolfe AR, Siedow M, Nalin A, et al. Increasing neutrophil-to-lymphocyte ratio following radiation is a poor prognostic factor and directly correlates with splenic radiation dose in pancreatic cancer. Radiother Oncol. 2021;158:207–14. doi: 10.1016/j.radonc.2021.02.035. [DOI] [PubMed] [Google Scholar]
- 33.Wild AT, Ye X, Ellsworth SG, et al. The association between chemoradiation-related lymphopenia and clinical outcomes in patients with locally advanced pancreatic adenocarcinoma. Am J Clin Oncol. 2015;38:259–65. doi: 10.1097/COC.0b013e3182940ff9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balmanoukian A, Ye X, Herman J, Laheru D, Grossman SA. The association between treatment-related lymphopenia and survival in newly diagnosed patients with resected adenocarcinoma of the pancreas. Cancer Invest. 2012;30:571–6. doi: 10.3109/07357907.2012.700987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schernberg A, Vernerey D, Goldstein D, et al. Predictive value of neutrophils count for local tumor control after chemoradiotherapy in patients with locally advanced pancreatic carcinoma. Int J Radiat Oncol Biol Phys. 2021;110:1022–31. doi: 10.1016/j.ijrobp.2021.01.052. [DOI] [PubMed] [Google Scholar]
- 36.Zhang HG, Yang P, Jiang T, et al. Lymphopenia is associated with gross target volumes and fractions in hepatocellular carcinoma patients treated with external beam radiation therapy and also indicates worse overall survival. Can J Gastroenterol Hepatol. 2019;2019:9691067. doi: 10.1155/2019/9691067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang C, Liao Z, Gomez D, et al. Lymphopenia association with gross tumor volume and lung V5 and its effects on non-small cell lung cancer patient outcomes. Int J Radiat Oncol Biol Phys. 2014;89:1084–91. doi: 10.1016/j.ijrobp.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 38.Brunner TB, Haustermans K, Huguet F, et al. ESTRO ACROP guidelines for target volume definition in pancreatic cancer. Radiother Oncol. 2021;154:60–9. doi: 10.1016/j.radonc.2020.07.052. [DOI] [PubMed] [Google Scholar]
- 39.Oar A, Lee M, Le H, et al. Australasian Gastrointestinal Trials Group (AGITG) and Trans-Tasman Radiation Oncology Group (TROG) guideline for pancreatic stereotactic body radiation therapy (SBRT) Pract Radiat Oncol. 2020;10:e136–46. doi: 10.1016/j.prro.2019.07.018. [DOI] [PubMed] [Google Scholar]
- 40.Caravatta L, Sallustio G, Pacelli F, et al. Clinical target volume delineation including elective nodal irradiation in preoperative and definitive radiotherapy of pancreatic cancer. Radiat Oncol. 2012;7:86. doi: 10.1186/1748-717X-7-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller JA, Toesca DA, Baclay JR, et al. Pancreatic stereotactic body radiation therapy with or without hypofractionated elective nodal irradiation. Int J Radiat Oncol Biol Phys. 2022;112:131–42. doi: 10.1016/j.ijrobp.2021.07.1698. [DOI] [PubMed] [Google Scholar]
- 42.Chadha AS, Liu G, Chen HC, et al. Does unintentional splenic radiation predict outcomes after pancreatic cancer radiation therapy? Int J Radiat Oncol Biol Phys. 2017;97:323–32. doi: 10.1016/j.ijrobp.2016.10.046. [DOI] [PubMed] [Google Scholar]
- 43.Deek MP, Benenati B, Kim S, et al. Thoracic vertebral body irradiation contributes to acute hematologic toxicity during chemoradiation therapy for non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2016;94:147–54. doi: 10.1016/j.ijrobp.2015.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Univariate and multivariable analyses of local progression-free survival
Univariate and multivariable analyses of distant metastasis-free survival
Univariate and multivariable analyses of progression-free survival