Abstract
Background and Objective
The histologic variants of urothelial carcinoma (UC) are tumors arising from within the urothelium in which some component of the tumor morphology is other than urothelial. They are underdiagnosed, aggressive and have varying pathologic response rates to systemic chemotherapy. There are no consensus guidelines on the use of systemic chemotherapy in variant histology (VH) of UC. We performed a contemporary review on pathologic response rates to neoadjuvant systemic therapy and survival outcomes following radical cystectomy in order to provide a rationale for clinical practice recommendations on the management of UC with VH.
Methods
A PubMed literature search was conducted for all English articles from inception reporting either pathological response rates to neoadjuvant treatment or survival outcomes after radical cystectomy in non-metastatic VH of UC.
Key Content and Findings
Neoadjuvant chemotherapy (NAC) prior to radical cystectomy was shown to be a beneficial treatment strategy in UC with VH. The micropapillary, plasmacytoid, nested and sarcomatoid histologic variants were associated with worse survival outcomes compared to conventional UC and UC with squamous or glandular differentiation despite initial downstaging with chemotherapy. There is evidence of improved survival in patients with sarcomatoid differentiation receiving NAC compared to RC alone. The major prognostic factors that affect survival outcomes in VH of UC include histologic variant subtype, patient age, presence of lymphovascular invasion, hydronephrosis, nodal metastasis and advanced T stage at diagnosis. Recent studies demonstrate that VH of UC are heterogenous tumors and responsiveness to NAC may be a function of the molecular subtypes present.
Conclusions
Based on these findings, NAC to achieve pathologic downstaging prior to radical cystectomy is recommended for MIBC with VH. Biomarkers identified by molecular profiling with immunohistochemistry will need to be validated as predictors of response to NAC in future trials.
Keywords: Urothelial variants, squamous neoplasms, glandular neoplasms, radical cystectomy, neoadjuvant chemotherapy
Introduction
Urothelial carcinoma (UC) is the most common malignancy of the urinary tract. The American cancer society estimates there are over 83,000 new cases and 17,100 deaths annually (1). UC accounts for over 90% of bladder cancers (2).
The histologic variants of UC include tumors arising from within the urothelium in which some component of the tumor morphology is other than urothelial. When present, these tumors can occur in mixed form or with a predominant histologic subtype. The histologic variants of urothelial carcinoma include micropapillary, microcystic, nested, lymphoepithelioma-like, plasmacytoid, sarcomatoid, giant cell, poorly differentiated, lipid rich, clear cell and urothelial carcinoma with divergent differentiation (including squamous, glandular, trophoblastic differentiation, among others) (2).
These histologic variants pose a diagnostic and treatment challenge for urologists, oncologists and pathologists alike. They are often underdiagnosed but not uncommon in clinical practice as the incidence of divergent differentiation, for instance, can be as high as 33% in cystectomy specimen and interobserver variability by reviewing pathologists could account for differences in incidence reporting (2). They are traditionally believed to be more aggressive, often diagnosed as locally advanced disease, and may have varying pathologic response rates to systemic chemotherapy (3).
In recent years, advances in genomics have led to a better understanding of the molecular drivers of carcinogenesis in urothelial carcinoma. However, the histologic variants of urothelial carcinoma have not been subjected to the same amount of scrutiny.
The purpose of this article is to review the histopathologic features, treatment modalities and prognostic factors in treatment of variant histology (VH) of urothelial carcinoma by providing a concise and detailed review of the pathologic response rates to treatment in non-metastatic disease and survival outcomes following radical cystectomy. We will also briefly discuss areas of ongoing research such as immunotherapy and the genomics of histologic variant subtypes of urothelial carcinoma. Neuroendocrine tumors, squamous cell carcinoma (SCC), primary adenocarcinoma, urachal adenocarcinoma, mesenchymal and other types of non-urothelial neoplasms are beyond the scope of this review. We present the following article in accordance with the Narrative Review reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-43/rc).
Methods
Search strategy
PubMed database was utilized to conduct a literature search. Our search strategy featured two combinations of regular keywords, separate combinations of a MeSH term with individual keywords “micropapillary variant”, “plasmacytoid variant”, “nested variant”, “sarcomatoid variant”, “lymphoepithelioma-like”, “clear cell variant”, “microcystic variant”, “squamous differentiation”, “glandular differentiation” and a combination of MeSH terms. Table 1 is the search strategy summary. Details of the Search results from keyword and MeSH term combinations are in the Table S1. All articles published from inception, in English, were taken into consideration due to rarity of histologic variants of urothelial carcinoma in literature.
Table 1. The search strategy summary.
| Items | Specification |
|---|---|
| Date of search | 10/3/2021 |
| Databases and other sources searched | PubMed |
| Search terms used | “variant histology bladder cancer” AND “radical cystectomy” AND “neoadjuvant chemotherapy” (“Urothelial carcinoma” OR “urothelial cancer” OR “bladder cancer” OR “bladder carcinoma”) AND (“variant” OR “differentiation” OR “VH”) AND (“multivariate OR multivariable”) |
| “transitional cell, carcinoma” AND “micropapillary variant” | |
| “transitional cell, carcinoma” AND “plasmacytoid variant” | |
| “transitional cell, carcinoma” AND “nested variant” | |
| “transitional cell, carcinoma” AND “sarcomatoid variant” | |
| “transitional cell, carcinoma” AND “lymphoepithelioma-like” | |
| “transitional cell, carcinoma” AND “clear cell variant” | |
| “transitional cell, carcinoma” AND “microcystic variant” | |
| “transitional cell, carcinoma” AND “squamous differentiation” | |
| “transitional cell, carcinoma” AND “glandular differentiation” | |
| “Carcinoma, transitional cell/therapy” AND “neoadjuvant therapy/methods | |
| Timeframe | From inception till 10/3/2022 |
| Inclusion and exclusion criteria | Inclusion criteria: Randomized clinical control trials (RCTs) and institutional observational studies reporting pathologic outcomes of histologic variant subtypes of urothelial carcinoma (UC) in non-metastatic disease or survival outcomes after cystectomy with or without neoadjuvant chemotherapy (NAC) |
| Exclusion criteria: Studies that queried non institutional databases, review articles, case reports or series with a variant histology sample size <10 |
Inclusion and exclusion criteria
Studies within inclusion criteria were randomized clinical control trials (RCTs) and institutional observational studies reporting pathologic outcomes of histologic variant subtypes of UC in non-metastatic disease or survival outcomes after cystectomy with or without neoadjuvant chemotherapy (NAC). Studies that queried non institutional databases, review articles, case reports or series with a variant histology sample size <10 were excluded. The chosen studies were screened by two reviewers working independently.
Types of outcomes measured
❖ Pathologic outcomes, namely pathologic complete response (pCR) or pathologic downstaging after treatment at time of RC in non-metastatic disease.
❖ Survival outcomes following cystectomy regardless of treatment received. Survival outcomes of interest include disease recurrence, cancer specific survival (CSS), disease specific survival (DSS), overall survival (OS) and recurrence free survival (RFS).
Results
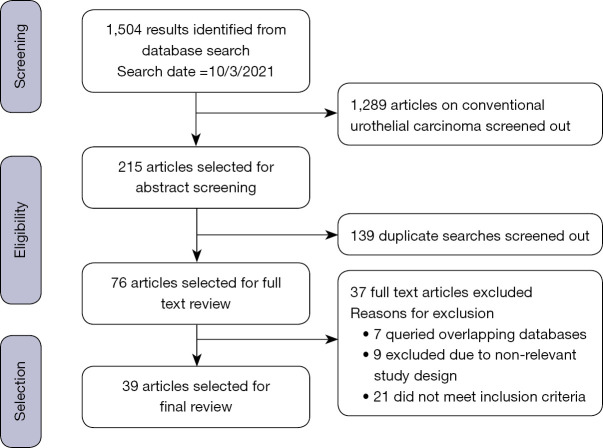
The composition of the 39 articles selected for final review included 1 clinical trial (4) and 38 observational studies. There were a total number n=24,775 patients in the studies chosen among whom n=5,553 (22.4%) were patients with VH of UC. Figure 1 is a flow chart depicting the process of identifying relevant studies.
Figure 1.
Selection of relevant studies.
Quality assessment
Quality assessment for the chosen studies was done using the Cochrane bias risk tool for clinical trials and the Newcastle-Ottawa scale tool for cohort studies.
Actual results
A total of twelve studies (4-15) meeting the inclusion criteria reported pathologic response to treatment. Ten discussed NAC, one (12) discussed trimodal bladder preserving therapy (TMT) whereas one (4) discussed neoadjuvant immunotherapy.
There were a total of 35 papers, 25 studies (5-7,9-11,14-32), then 10 studies (33-42) reporting survival outcomes after cystectomy. Five of these studies (18,21,24,38,40) reported survival after RC with adjuvant chemotherapy. One study (21) reported worse OS when adjuvant chemotherapy was not administered in glandular differentiation.
Pathologic complete response was reported in ten studies (4-6,8,10-14,36) while pathologic downstaging was reported in nine studies (4,5,7,8,10,11,13-15).
Survival benefit with NAC in VH of UC was demonstrated in two studies (5,14), and specifically with sarcomatoid differentiation (11) and squamous differentiation (6,9).
Nineteen studies (5,6,8-10,13,16,17,19,21,23,24,27,29-31,33,41,42) analyzed independent factors other than VH affecting either response to treatment or survival outcomes. Age, lymphovascular invasion, hydronephrosis, nodal metastasis and pathological/clinical stage were the most commonly reported factors. At least one of these factors was reported as having an impact of survival after RC in ten studies (10,17,21,23,24,26,30,31,41,42).
Other independent prognostic factors mentioned in the literature reviewed include circulating tumor cells in preoperatively collected blood samples prior to radical cystectomy (19) and neutrophil-lymphocyte ratio in squamous differentiation (9).
Tables 2-11 is a concise summary of the reviewed studies categorized by variant histology and type of treatment reported.
Table 2. Studies on micropapillary UC reporting outcomes with either neoadjuvant chemotherapy or upfront radical cystectomy.
| Author (year) | Pathologic complete response | Pathologic downstaging | Median follow up after cystectomy | Survival outcomes after cystectomy | Major independent factor(s) other than histologic phenotype affecting response/outcomes analyzed | Comments |
|---|---|---|---|---|---|---|
| Meeks (10) (2013), n=44 (VH n=44) | Comparing those who received NAC with those who did not, pCR occurred in 45% vs. 13% (P=0.049) | Comparing those who received NAC with those who did not, pathologic downstaging was achieved in 35% vs. 47% (P=0.4) | 24 months | Patients with p T0 disease had improved survival outcomes after cystectomy at 24 months follow up. Higher survival rates (25 vs. 92%) with a longer mean time to death [72 (95% CI: 55–90) vs. 29 (95% CI: 22–37) months, P=0.004] and lower rates of disease recurrence (21 vs. 79%) with longer time to recurrence [73 (95% CI: 56–90) vs. 26 (95% CI: 17–35) months, P=0.03] | Pathologic T stage was associated with worse survival outcomes | |
| Mitra (17) (2019), n=1,497 (VH n=151) | 10.7 years for conventional UC group, 7.8 years for micropapillary VH | Inferior 5-year RFS (70% vs. 44%; P<0.01) and OS (61% vs. 38%; P<0.01) compared to conventional UC. No association with risk of recurrence or mortality on multivariable analysis | Pathologic T stage, tumor grade and lymphovascular invasion were associated with worse survival outcomes | |||
| Kamat (15) (2007), n=100 (VH n=100) | 14 of 23 patients (61%) achieved pathologic downstaging with NAC | Patients alive at 5 yrs follow up were 32% for NAC group and 71% among those treated with initial cystectomy. Median OS was 43.2 for the 23 patients in NAC group. The median survival had not been reached at time of last follow up for the 32 patients treated with initial cystectomy | In this study, 55 patients underwent RC for surgically resectable disease-≤c T4A. In this subgroup, 23 received NAC and 32 had initial cystectomy. | |||
| Fairey (22) (2014), n=1,380 (VH n=33) | 10 years | Comparing patients with conventional UC and micropapillary UC, predicted five-year OS (61% vs. 67%, log rank P=0.96) and RFS (69% vs. 58%, log rank P=0.33). Micropapillary histologic subtype was not independently associated with OS on multivariable analysis (HR 0.91, 95% CI: 0.55–1.49, P=0.70) or RFS (HR 0.97,95% CI: 0.55–1.73, P=0.92) | Controlling for clinical and pathologic factors, survival outcomes of micropapillary histologic subtype were similar to conventional UC after radical cystectomy | |||
| Compérat (23) (2010), n=72 (VH n=72) 57 patients were treated with RC stratified into three groups based on % histologic variant on TURB1. <10%2. 10–50%3. >50% |
Comparison of groups stratified based on % of micropapillary variant, mean survival was 18 months, 16.8 months and 12.1 months for <10%, 10–50% and >50% groups respectively. | Percentage of histologic variant was analyzed. Tumors with a higher percentage of histologic variant had higher likelihood of disease recurrence and death (P=0.04 and P=0.009). Pathological T stage was predictive of disease specific survival both in univariate and multivariate analysis (P=0.01 and P=0.04, respectively) | The presence of micropapillary histologic subtype in any amount including CIS had impact on clinical outcomes | |||
| Wang (27) (2012), n=821 (VH n=73) | 9.6 years | Comparing UC with micropapillary VH with conventional UC, 10-year cancer specific survival was 31% vs. 53%; P=0.001. No significant difference was noted between groups after being stage matched for 10-year RFS (62% vs. 69%; P=0.87) or CSS (31% vs. 40%; P=0.41) | Percentage of histologic variant had no correlation with worse survival outcomes | When matched to persons with conventional UC, UC with micropapillary VH was observed not to have increased rates of local/distant disease recurrence or inferior CSS after RC |
UC, urothelial carcinoma; VH, variant histology; NAC, neoadjuvant chemotherapy; pCR, pathologic complete response; RFS, recurrence free survival; RC, radical cystectomy; CSS, cancer specific survival; OS, overall survival; CIS, carcinoma-in-situ; TURB, transurethral resection of bladder tumor.
Table 3. Studies on plasmacytoid UC reporting outcomes with either neoadjuvant chemotherapy or upfront radical cystectomy.
| Author (year) | Pathologic complete response | Pathologic downstaging | Median follow up after cystectomy | Survival outcomes after cystectomy | Major independent factor(s) other than histologic phenotype affecting response/outcomes analyzed | Comments |
|---|---|---|---|---|---|---|
| Li (7) (2019), n=1,410 (VH n=98) (45 patients with plasmacytoid variant received, NAC and 41 patients did not) | Regardless of receipt of NAC, 22% (95% CI: 14–32%) of patients who had a clinical stage ≥ cT2 were down staged to pT1 on RC | 5 years for plasmacytoid group. 7.6 years for conventional UC group | Median OS for plasmacytoid histology vs. conventional UC was 3.8 and 8 yr respectively. Plasmacytoid histology was associated with increased overall mortality on univariable analysis (HR=1.34, 95% CI: 1.02–1.78; P=0.039) but not on multivariable analysis | 17.6% of patients developed peritoneal carcinomatosis on median follow up of 4.6 years | ||
| Kaimakliotis (28) (2014), n=308 (VH n=30) | 30 months | UC with plasmacytoid VH had significantly inferior OS outcomes. Median OS for plasmacytoid VH patients was 19 months, median OS for conventional UC patients had not been reached at 68 months | Plasmacytoid VH at cystectomy was associated with increased adjusted risk of mortality (HR 2.1; 95% CI: 1.2-3.8; P=0.016) |
UC, urothelial carcinoma; VH, variant histology; NAC, neoadjuvant chemotherapy; RC, radical cystectomy; CSS, cancer specific survival; OS, overall survival.
Table 4. Studies on nested UC reporting outcomes with either neoadjuvant chemotherapy or upfront radical cystectomy.
| Author (year) | Pathologic complete response | Pathologic downstaging | Median follow up after cystectomy | Survival outcomes after cystectomy | Major independent factor(s) other than histologic phenotype affecting response/outcomes analyzed | Comments |
|---|---|---|---|---|---|---|
| Linder (36) (2013), n=52 (VH n=52) | None of the patients had achieved pCR with NAC | – | 10.8 years | When matched with a cohort of patients with conventional UC, there was no significant difference in 10-year local RFS (83% vs. 80%; P=0.46) or 10-year CSS (41% vs. 46%; P=0.75) | – | – |
UC, urothelial carcinoma; VH, variant histology; NAC, neoadjuvant chemotherapy; pCR, pathologic complete response; RFS, recurrence free survival; CSS, cancer specific survival.
Table 5. Studies on sarcomatoid differentiation reporting outcomes with either neoadjuvant chemotherapy or upfront radical cystectomy.
| Author (year) | Pathologic complete response | Pathologic downstaging | Median follow up after cystectomy | Survival outcomes after cystectomy | Major independent factor(s) other than histologic phenotype affecting response/outcomes analyzed | Comments |
|---|---|---|---|---|---|---|
| Almassi (11) (2021), n=1,853 (VH n=259) | Comparing UC with sarcomatoid differentiation with conventional UC, pCR rate 20% vs. 24% (P=0.6) | Pathologic downstaging to <T2 rate 24% vs. 31% (P=0.6), comparing between UC with sarcomatoid differentiation and conventional UC | 5.8 years | Sarcomatoid differentiation was associated with inferior survival outcomes compared to conventional UC: five-year RFS of 45% vs. 71%, CSS estimate at five years of 51% vs. 75%, OS estimate at five years of 45% vs. 63%. In the sarcomatoid differentiation group, comparing patients who received NAC vs. those who did not, five-year RFS after RC was 55% vs. 40% (P=0.1) | – | Pathologic response rates to NAC between UC with sarcomatoid differentiation and conventional UC was similar. UC with sarcomatoid differentiation was associated with inferior survival outcomes but greater survival among patients receiving NAC |
UC, urothelial carcinoma; VH, variant histology; NAC, neoadjuvant chemotherapy; pCR, pathologic complete response; RFS, recurrence free survival; CSS, cancer specific survival; OS, overall survival.
Table 6. Studies on glandular/squamous differentiation reporting outcomes with either neoadjuvant chemotherapy or upfront radical cystectomy.
| Author (year) | Pathologic complete response | Pathologic downstaging | Median follow up after cystectomy | Survival outcomes after cystectomy | Major independent factor(s) other than histologic phenotype affecting response/outcomes analyzed | Comments |
|---|---|---|---|---|---|---|
| Speir (6) (2021), n=71 (VH n=71) | NAC resulted in 12.4 times higher likelihood to achieve pCR, more than seen in patients who did not receive NAC [60% vs. 10.8%; odds ratio (OR), 12.4; 95% confidence interval (CI), 3.15–48.7; P<0.001] | 38.2 months | Comparing patients who received NAC and those who did not, there was improved OS, DSS and RFS in patients receiving NAC | % squamous differentiation was used to group patients into two categories (<50% vs. ≥50%), probability of pCR was higher in patients with % squamous differentiation <50% (26.7 vs. 8.7) and benefit in OS and RFS with NAC was lost when % SV was ≥50 | ||
| MIBC patients having Squamous differentiation who had Radical Cystectomy | ||||||
| Buisan (9) (2017), n=50 (VH n=50) | 29 months | Neutrophil-to-lymphocyte (NLR) ratio in squamous differentiation was predictive of positive response to NAC. NAC was of significant benefit in patients with NLR<5. CSS was 91.2 months vs. 38.1 months for those treated with upfront RC (P=0.009). There was no statistically significant difference in survival for patients with NLR ≥5 | Aim of this study was to determine impact of neutrophil-to-lymphocyte (NLR) ratio on treatment outcomes. Patients were stratified into two groups, 1. NLR <5; 2. NLR ≥5 | |||
| MIBC patients having squamous differentiation. 19 patients were treated with NAC | ||||||
| Zargar-Shoshtari (13) (2016), n=126 (VH n=20) | Comparing two patient groups receiving NAC - UC with divergent glandular or squamous differentiation vs. conventional UC, pCR were similar between the groups (25% vs. 21%; P=0.07) | Comparing two patient groups receiving NAC – UC with divergent differentiation vs. conventional UC, rates of pathological downstaging were significantly higher in divergent glandular or squamous differentiation subgroup receiving NAC (60 vs. 32%; P=0.02) | Only clinical stage was predictive of pathologic response to NAC | Divergent glandular or squamous differentiation were independent predictors of pathologic downstaging [odds ratio (OR), 4.01; 95% confidence interval (CI), 1.16-13.9] and clinical stage (OR, 2.91; 95% CI, 1.06-7.94) in a multivariable logistics regression model | ||
| Kim (29) (2012), n=1,023 (VH n=186) | 11.4 years | Comparing survival outcomes between UC with divergent squamous or glandular differentiation with conventional UC, 10-year CSS did not differ significantly between the two groups (52% vs. 51%; P=0.71) | Pathological stage, lymph node status and lymphovascular invasion were associated with an increased risk of mortality | UC with divergent squamous or glandular differentiation was not significantly associated with risk of death from bladder cancer after adjusting for clinicopathologic features (HR 0.79, P=0.10) | ||
| Antunes (30) (2007), n=133 (VH n=25) | 24 months | Comparing patients with tumors having squamous differentiation to those who did not, disease recurrence occurred in 64% vs. 34% (P=0.001), and death occurred in 40% vs. 16% of patients (P=0.002) | Univariate analysis revealed that pathological T stage, tumor size and lymph node involvement were predictors of CSS. Only tumor size was an independent predictor of outcome on multivariable analysis | |||
| Ehdie (31) (2012), n=145 (VH n=67) | 44 months | There was no statistically significant difference in survival outcomes between SCC and UC with squamous differentiation in terms of CSS and OS | Patients with squamous differentiation with lymph node involvement had worse OS and CSS | The aim of the study was to compare outcomes and determine predictors of CSS and OS between SCC cases and UC with squamous differentiation cases after RC | ||
| Minato (33) (2018), n=101 (VH n=20) | 31 months | Comparing UC with squamous differentiation and conventional UC, five-year OS and RFS rates were 41.1% vs. 69.7 % (P=0.002) and 51.8% vs. 59.5% (P=0.027) respectively | Percentage/extent of squamous differentiation had no effect on survival outcomes | Squamous differentiation was a significant independent predictor of OS on multivariate analysis (HR: 4.22; 95% CI: 1.20–14.8; P=0.024) |
UC, urothelial carcinoma; VH, variant histology; NAC, neoadjuvant chemotherapy; MIBC, muscle invasive bladder cancer; pCR, pathologic complete response; RFS, recurrence free survival; CSS, cancer specific survival; OS, overall survival; DSS, disease specific survival; NLR, neutrophil-lymphocyte ratio; OR, odds ratio; SCC, squamous cell carcinoma.
Table 7. Studies on multiple variant histologies reporting outcomes with either neoadjuvant chemotherapy or upfront radical cystectomy.
| Author (year) | Pathologic complete response | Pathologic downstaging | Median follow up after cystectomy | Survival outcomes after cystectomy | Major independent factor(s) other than histologic phenotype affecting response/outcomes analyzed | Comments |
|---|---|---|---|---|---|---|
| Kaimakliotis (5) (2016), n=919 (VH n=181) | Patients who received either regimen had higher incidence of pCR (29% vs. 8%, P=0.002) compared to those who did not. The pCR to NAC in squamous and glandular differentiation was similar to conventional UC (pCR 29 vs. 22%; P=0.443 | There was higher incidence of pathologic downstaging (52% vs. 14%; P<0.001), comparing those who received either regimen as NAC versus those who did not. Pathological downstaging to NAC in squamous and glandular differentiation was similar to conventional UC (52% vs. 43%; P=0.370) | 77 months | VH was not associated with an increased risk of mortality. Use of either MVAC or GC as NAC was associated with decreased the risk of mortality in the cox proportional hazards regression model | Regimen used had no impact on pathologic response as both MVAC and GC resulted in improved pathologic response rates. Comparing MVAC to GC, either resulted in decreased mortality in VH subgroup studied. Only MVAC reached statistical significance | – |
| Patients received either MVAC or GC as NAC regimen | ||||||
| Shen (8) (2015), n=90 (VH n=90) | pCR reported as 23.9% vs. 4.5% comparing recipients of NAC vs. recipients of upfront RC | Rate of downstaging to < pT2 was 32.6% vs. 9%. Comparing recipients of NAC vs. recipients of upfront RC | Notable changes both in percent variant differentiation and mitotic rate occurred with NAC use from Bx/TUR to RC but not in non-NAC treated tumors. Neither change was predictive of response to NAC | The aim of this study was to describe changes in bladder tumors during NAC use and to identify features at Bx/TUR that may predict response | ||
| Hajiran (14) (2021), n=768 (VH n=358) | pCR was achieved in 23% of conventional UC cases, 30% of UC with VH cases and 18% of UC with divergent squamous or glandular differentiation cases (P=0.30) | Partial response was achieved in 46% of conventional UC cases, 41% of UC with VH cases and 37% of UC with divergent squamous or glandular differentiation cases (P=0.40) | 21 months | Conventional UC cases had OS benefit (HR 0.71, 95% CI: 0.51–0.98, P=0.0013) with NAC. UC with VH cases has CSS benefit (HR 0.55, 95% CI: 0.30–0.99, P=0.0495) with NAC. UC with divergent glandular or squamous differentiation cases did not experience any survival benefit with NAC prior to RC | Rates of complete and partial pathologic response were similar between the three groups | |
| NAC Treatment response stratified into three groups1. Conventional UC2. UC with VH3. UC with divergent glandular or squamous differentiation | ||||||
| Soave (19) (2017), n=188 (VH n=47) | 25 months | UC with VH had worse survival outcomes compared to UC with divergent squamous differentiation. UC with VH and presence of circulating tumor cells (CTC) were associated with reduced RFS and CSS (P≤0.016) | In multivariable analysis, presence of CTC, not variant histology, was an independent predictor for recurrence of disease (HR 3.45; P≤0.001) and CSS (HR 2.62; P=0.002) | The primary aim of this study was to determine the effect of circulating tumor cells (CTC) on survival outcomes of in patients with VH after RC | ||
| Patients with UC treated with RC without NAC | ||||||
| Soave (16) (2015), n=485 (VH n=96) | 45 months | VH was associated with inferior CSS (P≤0.02) and disease recurrence (P=0.002). | Comparing survival outcomes based on extent of VH i.e., ≥70% or <70%, there was no statically significant difference between the two groups. Age, advanced tumor stage, lymph node involvement and a positive soft tissue margin were all associated with worse survival outcomes | |||
| Moschini (25) (2017), n=338 (VH n=338) | 6.5 years | On multivariable analysis, patients with a predominant VH had inferior survival outcomes compared to conventional UC (P<0.01). On multivariable cox regression analysis predicting recurrence, cancer specific mortality and overall mortality, there were no difference between mixed variant and conventional UC (all P>0.1) | Only the presence of a predominant VH was associated with inferior survival outcomes after RC compared the conventional UC. Same was not true for UC with mixed VH cases which had similar survival outcomes with conventional UC cases | |||
| Patients with non-metastatic UC treated with RC were stratified in three groups:1. UC with mixed VH cases2. UC with a predominant VH (micropapillary or small cell) cases3. Conventional UC cases | ||||||
| Martini (26) (2021), n=2,422 (VH=528) | Relative to conventional UC, patients with VH had higher rates of recurrence with RFS 30% vs. 51%; P<0.001 and shorter median time to disease recurrence (88 vs. 123 months; P<0.01) | The study concluded by Study recommending longer oncologic surveillance time for VH UC | ||||
| Sefik (32) (2018), n=146 (VH n=23) | No significant difference in survival was observed between VH and conventional UC | |||||
| Xylinas (34) (2013), n=1,983 (VH n=488) | 55 months | In univariable analysis, patients with conventional UC and squamous differentiation had similar risk for disease recurrence and cancer-specific mortality. UC with VH had significantly higher risk for disease recurrence and cancer specific mortality on univariable but not multivariable analysis | ||||
| Takemoto (35) (2020), n=102 (VH n=26) | 39.5 months | VH of UC was associated with significantly worse survival compared to conventional UC in RFS (P=0.018) and CSS (P=0.036) | ||||
| Naspro (37) (2021), n=525 (VH n=131) | 31 months | Presence of VH was an independent risk factor for cancer-specific mortality (P=0.001) with significantly higher risk for recurrence, cancer-specific mortality and overall mortality (all P ≤0.001) | ||||
| Stroman (39), n=403 (VH n=73) | 45 months | VH, specifically squamous differentiation was associated with worse survival outcomes | ||||
| Komina (41) (2021), n=185 (VH n=58) | 28.9 months | On multivariable analysis, positive soft tissue margin and lymphovascular invasion were associated with worse RFS and OS | ||||
| Marks (42) (2019), n=138 (VH n=96) | 45 months | Although VH was not associated with increased incidence of lymph node involvement, patients with positive lymph nodes or extra nodal extension undergoing RC had worse survival outcomes |
UC, urothelial carcinoma; VH, variant histology; NAC, neoadjuvant chemotherapy; pCR, pathologic complete response; RC, radical cystectomy; CSS, cancer specific survival; OS, overall survival; RFS, recurrence free survival; MVAC, methotrexate-vinblastine-doxorubicin-cisplatin chemotherapy; GC, gemcitabine-cisplatin chemotherapy; Bx/TUR, biopsy/transurethral resection; CTC, circulating tumor cells.
Table 8. Studies reporting outcomes with perioperative chemotherapy.
| Author (year) | Pathologic complete response | Pathologic downstaging | Median follow up after cystectomy | Survival outcomes after cystectomy | Major independent factor(s) other than histologic phenotype affecting response/outcomes analyzed | Comments |
|---|---|---|---|---|---|---|
| Ghoneim (20) (2011), n=38 (VH n=38) | – | – | 17 months | Median survival of the patients who had and had not received perioperative cisplatin-based chemotherapy was 23 months (95% CI: 9–32) and 46 months (95% CI: 12-69), respectively. All patients who received perioperative cisplatin-based chemotherapy died of metastatic disease. 5-year OS rate was 40% (95% CI: 16–64) | – | – |
| Patients with micropapillary UC treated with perioperative chemotherapy |
VH, variant histology; UC, urothelial carcinoma; OS, overall survival.
Table 9. Studies reporting outcomes with radical cystectomy + adjuvant chemotherapy (survival outcomes only).
| Author (year) | Pathologic complete response | Pathologic downstaging | Median follow up after cystectomy | Survival outcomes after cystectomy | Major independent factor(s) other than histologic phenotype affecting response/outcomes analyzed | Comments |
|---|---|---|---|---|---|---|
| Monn (18) (2015), n=411 (VH n=411) | 38 months | UC with VH cases at RC had 1.69-times increased risk of disease-specific mortality (P=0.030) and 1.57-times increased adjusted risk of all-cause mortality (P=0.027) compared to UC with divergent squamous differentiation | UC with VH was associated with worse survival outcomes regardless of pathologic stage, NAC or adjuvant chemotherapy compared to UC with divergent squamous differentiation | |||
| Mitra (21) (2014), n=1,762 (VH n=259) | 15.2 years for cases, 11.0 years for controls, 12.2 for independent cohort | No differences in survival outcomes between cases and controls were observed | Higher pathologic T stage, age and hydronephrosis were associated with increased mortality risk | Patients with squamous or glandular or both differentiation had survival outcomes similar to conventional UC after cystectomy. they however presented with a higher pathologic stage | ||
| This was a case-control analysis, cases were stratified into three groups:1. Squamous differentiation2. Glandular differentiation3. Squamous + glandular differentiation | Pathologic stage was predictive of outcome in cases with differentiation | In glandular differentiation, non-administration of adjuvant chemotherapy was associated with worse OS | ||||
| Controls were conventional UC patients matched 1:1 to cases and an independent cohort of 1,244 conventional UC | ||||||
| Masson-Lecomte (24) (2015), n=266 (VH n=31) | Comparing patients with micropapillary VH with conventional UC, median survival was 29 vs. 31 months. Five-year RFS (15% vs. 42%; P=0.007), five-year CSS (24% vs. 47%; P=0.058) | Positive tissue margin and high ASA score was associated with worse RFS in univariate and multivariate analysis. Age, lymph node positivity and a positive soft tissue margin were associated with CSS in univariate and multivariate analysis | Micropapillary histologic subtype was associated with higher disease recurrence rates after RC and platinum-based adjuvant chemotherapy compared to conventional UC | |||
| Patients with MIBC were treated with RC and adjuvant platinum based chemotherapy. | ||||||
| Keck (38) (2013), n=205 (VH n=27) | Plasmacytoid UC had significantly worse outcomes compared to micropapillary and conventional UC after RC and adjuvant cisplatin-based chemotherapy with a median OS of 27.4 months | |||||
| Zamboni (40) (2021), n=3,963 (VH n=906) | 32 months | Adjuvant chemotherapy failed to improve survival outcomes in any of histologic variants (P>0.05) | ||||
| 723 patients received RC and adjuvant chemotherapy |
VH, variant histology; UC, urothelial carcinoma; NAC, neoadjuvant chemotherapy; RC, radical cystectomy; MIBC, muscle invasive bladder cancer; OS, overall survival; ASA, American Society of Anesthesiologists; RFS, recurrence free survival; CSS, cancer specific survival.
Table 10. Studies reporting outcomes with trimodal bladder preserving therapy (TMT).
| Author (year) | Pathologic complete response | Pathologic downstaging | Median follow up after cystectomy | Survival outcomes after cystectomy | Major independent factor(s) other than histologic phenotype affecting response/outcomes analyzed | Comments |
|---|---|---|---|---|---|---|
| Nagumo (12) (2020), n=148 (VH n=11) 11 patients having VH MIBC were treated with TMT | pCR was achieved in 9 out of 11 (82%) patients treated with TMT | – | – | – | – | 6 out of 7 patients with squamous or glandular differentiation achieved pCR with TMT. |
VH, variant histology; MIBC, muscle invasive bladder cancer; TMT, trimodal bladder preserving therapy; pCR, pathologic complete response.
Table 11. Studies reporting outcomes with neoadjuvant immunotherapy (Pembrolizumab).
| Author (year) | Pathologic complete response | Pathologic downstaging | Median follow up after cystectomy | Survival outcomes after cystectomy | Major independent factor(s) other than histologic phenotype affecting response/outcomes analyzed | Comments |
|---|---|---|---|---|---|---|
| Neechi (4) (2020), n=114 (VH n=34) VH patients were stratified into two groups | pCR to neoadjuvant pembrolizumab compared between predominant VH, non-predominant VH and conventional UC was 16%, 53% and 39% respectively | Pathologic downstaging to pT ≤ 1N0 was 42%, 67% and 56% in predominant VH, non-predominant VH and conventional UC patients respectively | – | – | A high tumor mutational burden and combined positive score for PD-L1 expression was found to correlate with response to therapy | The efficacy of neoadjuvant pembrolizumab was significantly lower in patients with predominant VH. However, a subgroup analysis showed the response to be greatest among patients with the squamous differentiation and lymphoepithelioma-like VH |
| FGFR3 mutations had no correlation with response |
VH, variant histology; pCR, pathologic complete response; UC, urothelial carcinoma; PD-L1, programmed death ligand 1; FGFR3, fibroblast growth factor receptor 3.
Discussion
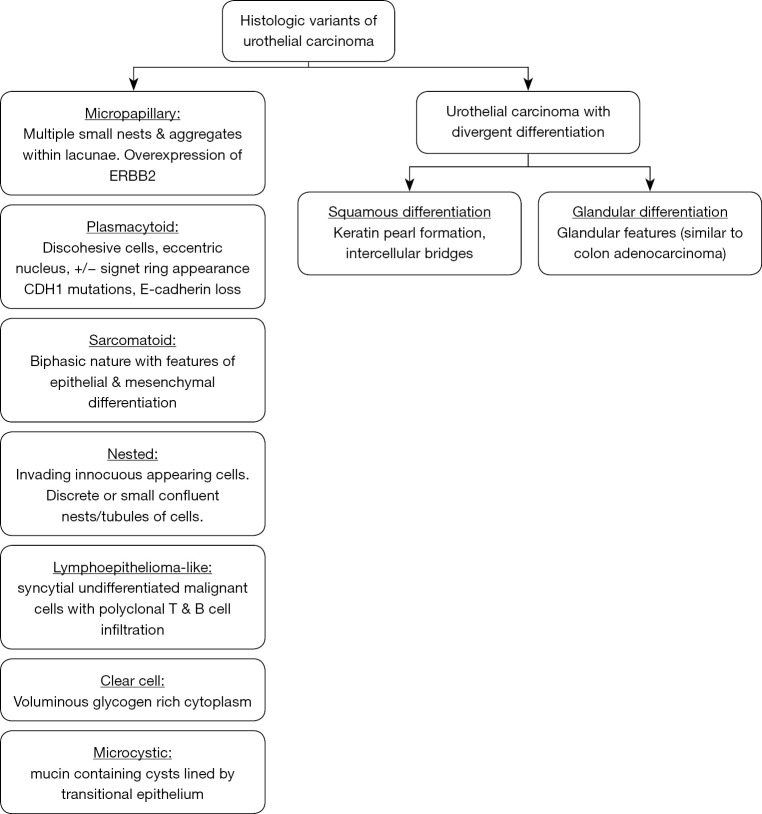
This review provides a concise summary of the 2016 WHO classification of urothelial tumors and presents the data on pathologic response with neoadjuvant systemic therapy including immunotherapy and survival outcomes in histologic variants of UC after RC regardless of treatment received. There are no consensus guidelines on the use of systemic chemotherapy in VH of UC. There are few randomized clinical trials involving VH of UC. This review provides an executive summary of most the studies that report either pathologic response in non-metastatic disease or survival outcome after RC. The histopathologic features of individual histologic variants included in this review are summarized in Figure 2 and are discussed briefly.
Figure 2.
Histopathologic features of histologic variants of urothelial carcinoma.
Histologic variants of urothelial carcinoma
Micropapillary
Micropapillary urothelial carcinoma has been described, morphologically, as tumor cells arranged in multiple small nests without fibrovascular cores aggregating within lacunae (43). The presence of multiple small nests within the same lacunar space is characteristic. Overexpression or amplification of ERBB2 at a molecular level has been reported in up to 42% of these tumors (44-46). The management of this variant has been disputed to date with conflicting reports of responsiveness to NAC and survival outcomes after RC. Some studies (15,47) have recommended expeditious RC. The earliest recommendation for expeditious RC over NAC in muscle-invasive bladder cancer (MIBC), was made by Kamat et al. in a series involving 55 patients at MD Anderson cancer center (15). This recommendation was made after a higher percentage of patients receiving NAC had ≥ pT3 disease than those who didn’t (69% vs. 35%, P=0.0016) at time of cystectomy (15). A more recent series by Meeks et al. with 44 patients showed that 45% of patients with micropapillary UC treated with NAC achieved downstaging prior to RC compared to 13% who did not (P=0.049) (10). The rate of ≥ pT3 disease was observed to be only 39% leading to the suggestion that this observed difference between studies may be indicative of improved pre-RC clinical staging and that NAC should be considered in these patients. Another study showed that despite presenting as a more locally advanced disease, patients with micropapillary UC had similar pathologic response rates to NAC compared to conventional UC (48).
A retrospective review of a single institutional bladder cancer database at Cleveland Clinic with 38 bladder cancer patients having the micropapillary UC concludes by recommending neoadjuvant chemotherapy alongside RC as an ideal treatment strategy (20). In this study, 26 (93%) in a group of 28 patients with localized disease, clinical stage ≤ T2N0 who did not receive NAC demonstrated pathologic upstaging at cystectomy. This study also reports poor outcomes in patients with stage cTa-T1 treated initially with bladder preservation approach using BCG as these patients all had pathologically advanced disease at cystectomy. All patients treated with perioperative chemotherapy died of metastatic disease (20).
To determine the impact of micropapillary UC on survival outcomes after RC, two studies (22,27) reported that controlling for clinical and pathologic factors, survival outcomes of micropapillary UC were similar to conventional UC after radical cystectomy. However, Mitra et al. (17) observed that micropapillary UC was associated with inferior 5-year RFS (70% vs. 44%; P<0.01) and OS (61% vs. 38%; P<0.01) compared to conventional UC but this association was lost on multivariable analysis. Masson-Lecomte et al. (24) studied patients who received adjuvant chemotherapy after RC and observed that micropapillary histologic subtype was associated with higher disease recurrence rates after RC and platinum-based adjuvant chemotherapy compared to conventional UC. It has been observed that micropapillary UC has a preponderance to metastasize to abdomen and lungs after RC (26).
Most of the data about treatment outcomes in micropapillary UC are from single-center retrospective studies or population-based registries. A cumulative summary of patient characteristics and outcomes was made by Abufaraj et al. in a systematic review (49). It included studies comprising of a total of 863,459 participants with urothelial carcinoma among whom 3,154 (0.4%) had the micropapillary UC. There was a notable male predominance (84%) and a higher prevalence of muscle-invasive disease at diagnosis (41%). Pooled results in that review showed that the use of neoadjuvant chemotherapy resulted in pathological downstaging and pathological complete response in 55% and 37% of patients respectively, but this did not translate into better survival outcomes (49).
Plasmacytoid
Plasmacytoid UC, a rare neoplasm, is one of the more aggressive variants that often presents as a locally advanced disease at the time of diagnosis (2). These tumors are comprised of discohesive cells with eccentric nuclei and abundant eosinophilic cytoplasm. Some of the tumor cells may have cytoplasmic vacuoles giving them a signet ring appearance. Of note, the 2016 WHO update classification describes signet ring cell carcinoma as belonging in same category as plasmacytoid UC (2). Loss of E-cadherin, which is encoded by CDH1 gene, has been described in a large cohort of patients with plasmacytoid urothelial carcinoma. Truncating mutations in CDH1 have been described in 87% of plasmacytoid UC, whereas no CDH1 mutations have been reported in conventional urothelial carcinoma. Loss of E-cadherin protein expression by immunohistochemistry has been reported in up to 70% of plasmacytoid carcinomas as opposed to 11% of conventional urothelial carcinomas (50-52). The loss of E-cadherin is thought to confer enhanced migratory capacity in tumor cells and may explain why this variant is frequently associated with peritoneal carcinomatosis (53). Like the micropapillary histologic variant, there are also conflicting findings in the literature regarding its responsiveness to NAC (53).
Regarding survival outcomes after RC, Kaimakliotis et al. (28) observed that plasmacytoid UC at cystectomy was associated with increased adjusted risk of mortality (HR 2.1; 95% CI: 1.2–3.8; P=0.016). Keck et al. observed these outcomes were worse when compared to micropapillary UC (38). The study by Li et al. (7) represents the largest single institutional series on plasmacytoid UC (n=98). In this study, median OS for plasmacytoid UC vs. conventional UC was 3.8 years and 8 years respectively. Plasmacytoid histology was associated with increased overall mortality on univariable analysis (HR 1.34, 95% CI: 1.02–1.78; P=0.039) but not on multivariable analysis and almost one out of every five patients with plasmacytoid UC developed peritoneal carcinomatosis at median follow up of 4.6 years (7).
Nested
Nested urothelial carcinoma is less commonly reported in the literature. Histologically it has been described as innocuous appearing and invading lamina propria either as discrete or small confluent nests/tubules of cells (2). It may resemble other benign proliferations such as von Brunn’s nests but foci of cytologically malignant cells are present in every case (54). A “large-nested” histologic variant has also been described in the literature (55). Nested UC is associated with advanced stage at time of diagnosis and its innocuous histologic appearance may delay definitive treatment. Linder et al. (36) studied 52 MIBC patients with nested UC and observed that when matched with a cohort of patients with conventional UC, there was no significant difference in 10-year local RFS (83% vs. 80%; P=0.46) or 10-year CSS (41% vs. 46%; P=0.75) at median follow up of 10.8 years.
Sarcomatoid
Sarcomatoid UC has a biphasic nature with features of epithelial and mesenchymal differentiation (both morphologically and on immunohistochemical staining). The epithelial component consists of urothelial carcinoma in most cases (56). Occurrence is associated with prior pelvic irradiation and intravesical cyclophosphamide therapy (57). Almassi et al. (11) assessed pathologic response to NAC and survival outcomes after RC in this histologic variant. Pathologic response rates to NAC were similar for sarcomatoid and conventional UC. Regarding survival outcomes after RC, sarcomatoid UC was independently associated with inferior survival outcomes, but greater survival was observed among patients receiving NAC.
Lymphoepithelioma-like carcinoma (LELC)
LELC, is so named due to its resemblance to lymphoepithelial carcinoma of the nasopharynx but unlike LELC in other organ systems, it is not associated with Epstein- Barr virus infection (58). It commonly presents as painless hematuria in older males (59). Microscopically, tumor cells have a syncytial pattern with prominent lymphoid infiltration which may be mistaken for chronic cystitis (58). A study by Monn et al. (18) showed that the lymphoepithelioma-like variant shares similar worse outcomes alongside other UC histologic variants when compared to conventional urothelial carcinoma, after adjusting for systemic chemotherapy and pathological stage. It is thought that this shared prognosis occurs when mixed with other variants. In its pure form, LELC has a low metastatic potential and has shown to be responsive to platinum-based chemotherapy (59).
Clear cell variant
The clear cell (glycogen-rich) variant of UC has been very rarely reported in literature. The tumor cells have clear (glycogen-rich) cytoplasm and can closely resemble clear cell adenocarcinoma of the bladder or female genital tract (2). Immunohistochemistry is revealing for CK7, GATA3, p40 and S100P staining, with some cases being positive for desmoglein 3 as well (60). They are aggressive tumors and can rapidly progress to muscle invasive and metastatic disease (61). The single largest series on this histologic variant did not specifically report pathologic response to treatment, however, there was an observed rapid progression to T3 disease in a majority and death in up to half of patients at 20 months of follow up (62).
Microcystic
Microcystic UC has a deceptively benign histologic appearance, with microcysts, macrocysts or tubular structures lined by transitional cells. The cysts and tubular structures may be empty or contain necrotic debris or mucin. It may mimic other benign growths such as cystitis cystica (and glandularis) or nephrogenic metaplasia. Focal high-grade conventional UC may also be identified in about 40% of cases. Microcystic urothelial carcinoma has been associated with asymptomatic colon cancer (63). There are no large series that describe pathologic response rate for this rare histologic variant. Aggressiveness is reportedly related to advanced stage at diagnosis (61).
Urothelial carcinoma with divergent differentiation
Squamous cell differentiation
Urothelial carcinoma with squamous differentiation is the most reported variant (21% of urothelial carcinomas) and is defined by the presence of squamous features such as intercellular bridges or keratinization (2). Strictly speaking, squamous cell carcinoma (SCC), which makes up about 3% of all bladder cancers, should be differentiated from UC with squamous differentiation. UC with squamous differentiation can be distinguished from SCC by the presence of associated urothelial carcinoma in the former entity. We discuss studies that report outcomes in divergent squamous differentiation to the exclusion of SCC. NAC resulted in higher likelihood to achieve pCR, more than seen in patients who did not receive NAC (5,6) and higher incidence of downstaging (5). Three studies (5,13,14) reported that in comparison, the pathologic complete response rates to NAC were similar in conventional UC and UC with squamous differentiation while rates of pathologic downstaging was higher in UC with squamous differentiation (5,13). A single institutional study showed excellent rates of pathologic complete response with trimodal bladder preserving therapy as well (12). Regarding survival outcomes after RC, statistically, patients who had NAC had better OS and RFS outcomes at median follow up of 38.2 months (6), and similar survival outcomes compared to conventional UC (20,29,31,34).
Glandular differentiation
Urothelial carcinoma with glandular differentiation is the second most reported histologic variant (about 6% of urothelial carcinoma) and is defined by the presence of glandular features (i.e., true glandular spaces) within the tumor (64). A tumor with mixed glandular and urothelial differentiation is classified as urothelial carcinoma with glandular differentiation, and the percentage of the glandular component should be reported. The presence of glandular differentiation if present, even focally, in association with urothelial carcinoma, portends poor prognosis. The diagnosis of adenocarcinoma is reserved for pure tumors (lacking an associated urothelial component). Primary bladder adenocarcinomas account for 0.5–2% of malignant bladder tumors. The 2016 WHO classification of bladder tumors divides adenocarcinomas into the following categories: enteric (colonic) type, mucinous type, mixed type and adenocarcinoma, not otherwise specified (NOS). These tumors can be impossible to distinguish from colonic adenocarcinoma based on microscopic and immunophenotypic features alone, and clinical and radiologic correlation is often necessary to determine the site of origin (2). A study in our review reports that it shares similar responsiveness to NAC as UC with squamous differentiation and conventional urothelial carcinoma (5). Two studies (20,29) highlight that survival outcomes after RC in conventional UC and UC with divergent squamous or glandular differentiation are similar with one (20) showing survival benefit with adjuvant chemotherapy after RC.
In summary, the data regarding response to systemic chemotherapy from single/multicenter observational studies is not robust and often conflicting. Other published literature such as a retrospective cohort of non-metastatic cases from the National Cancer Database (NCDB) show that use of NAC correlates with higher likelihood to achieve pathologic complete response or downstaging prior to RC, whereas patients who did not receive NAC had very low rates of pCR (<6%) and downstaging (<10%) for all histologic variants (65).
Prognostic factors that impact response to NAC or survival outcomes in histologic variants of urothelial carcinoma after RC
Certain histologic variants have been associated with worse survival outcomes after RC. Micropapillary, plasmacytoid, nested and sarcomatoid UC have been associated with worse survival outcomes compared to conventional UC and UC with squamous or glandular differentiation (16,18,19,35,37,38). Among these four histologic variants, survival benefit with NAC was demonstrated in sarcomatoid differentiation when controlling for other prognostic factors (11). It can be hypothesized that in mixed histology, the presence of urothelial histologic variants might be a driver for poorer outcomes. Moshcini et al. (25) demonstrated that only the presence of pure histologic variants of UC was associated with inferior survival outcomes after RC compared the conventional UC. The same was not true for comparisons between mixed variant histology with conventional UC. It is worth noting that some studies that reported no significant difference in survival outcomes between conventional UC and VH of UC had predominantly squamous and/glandular differentiation such as was reported in Sefik et al. (32). Hajiran et al. (14) was the only study to compare the survival benefit of NAC between the three groups of conventional UC, UC with VH and UC with variant differentiation (squamous or glandular); here survival benefit with NAC was only achieved in conventional UC and UC with VH. A possible explanation for this apparent lack of survival benefit for NAC in variant differentiation was offered by Chakiryan et al. (65). It was suggested that these patients may appear not benefit from NAC, despite significant pathological downstaging, because of generally more favorable outcomes.
The impact of the extent or percentage of variant subtype pattern is controversial. Shen et al. (8) investigated the change in variant differentiation during NAC in VH of UC between TUR and RC. An interesting finding was that the percent differentiation at TUR did not affect responsiveness to NAC. However, Speir et al. (6) demonstrated an inverse correlation between the percent of histologic variant involvement and likelihood to achieve a complete pathologic response with NAC prior to radical cystectomy in patients with squamous differentiation. The study also suggests that the survival benefit of NAC is lost in the subgroup of patients with variant differentiation ≥50%. Soave et al. (16) noted that the presence of variant histology particularly non-squamous cell differentiation and the extent were associated with cancer-specific mortality in univariable but not in multivariable analyses. In micropapillary UC for instance, one study (23), demonstrated that tumors with a higher percentage of micropapillary UC at TURBT, had a higher likelihood of death from disease on univariate (P=0.01) but not on multivariate analysis and the presence of CIS was predictive of recurrence on univariate and multivariate analysis (P=0.03 and P=0.003) while another study (27), found no association between variant percentage and survival outcomes. In summary, although the studies present differing views regarding the impact of the extent or percentage of variant histology, its presence and percentage should be reported because of potential impact on survival outcomes.
Molecular subtypes of urothelial carcinoma
There are several overlapping molecular subtyping nomenclatures that have been developed for MIBC. The Lund university approach, for instance, is based on mRNA profiling. It includes the following subtypes: urothelial-like, genomically unstable, SCC-like, mesenchymal-like and neuroendocrine-like (66).
The molecular subtypes of bladder tumors can be broadly classified into the following two groups:
Luminal subtype—demonstrates markers of urothelial differentiation
Basal-squamous subtype—demonstrates markers of basal cell or squamous differentiation.
The Cancer Genome Atlas (TCGA) bladder cancer group analysis further expanded the luminal subtype into luminal-papillary (enriched with FGFR3 overexpression) and luminal-infiltrated (distinguished by lymphocyte infiltration) (67).
The consensus molecular classification for MIBC, identified a set of six molecular classes namely- luminal papillary, luminal non-specified, luminal unstable, stroma-rich, basal/squamous and neuroendocrine-like (68).
Micropapillary and plasmacytoid UC express luminal biomarkers. The luminal papillary subtypes have better survival outcomes. Their high rates of FGFR3 mutations make them potentially susceptible to FGFR inhibitors; however, more study is needed (69).
LELC and sarcomatoid UC express basal biomarkers. The basal type appears more aggressive but is chemo sensitive and has improved outcomes with NAC (70).
Table 12 summarizes the molecular subtypes of VH of UC.
Table 12. Molecular subtypes in variant histology of urothelial carcinoma.
| Variant subtype | Molecular subtype |
|---|---|
| Micropapillary | Luminal |
| Plasmacytoid/signet ring | Luminal |
| Microcystic | Luminal/Basal |
| Nested | Luminal/Basal |
| Clear cell | Luminal/Basal |
| Lymphoepithelioma-like | Basal |
| Sarcomatoid | Basal |
Warrick et al. (70) studied the molecular subtypes of the histologic variants using the immunohistochemistry method developed at Lund University. This was performed on a consecutive retrospective series of cystectomy cases (n=83). 39% of these tumors with multiple VH of UC exhibited molecular heterogeneity. This heterogeneity was highest among basal-squamous tumors with almost 80% co-occurring with either urothelial-like or genomically unstable molecular subtypes. The following observations were made in this study:
Despite exhibiting molecular heterogeneity, these tumors had common markers of cell cycle regulation such as loss of P16 or RB1 expression. This suggests that CDK4/6 inhibitors are a potential effective treatment strategy since P16 or RB1 expression loss is an early occurrence in carcinogenesis shared by these tumors (70).
Tumors treated with NAC had a substantially lower basal-squamous cancer burden compared to tumors that were only treated with cystectomy (P=0.02). This gives credence to a prior study which suggested that platinum-based NAC would be most effective in patients with basal tumors (71).
The significance of these findings is that it presents pathologic response to neoadjuvant therapy as a function of the molecular subtypes of the variants rather than their histologic phenotypes. However, any future attempts at risk-stratifying tumors based on molecular subtypes will have to consider the molecular heterogeneity within any given tumor before being applicable in a clinical setting.
Neoadjuvant immunotherapy, predictors of response and directions for future research
Immune checkpoint inhibitors (ICIs) could potentially change the treatment outlook for VH of UC. Currently, there are five FDA approved immune checkpoint inhibitors for UC, namely atezolizumab, avelumab, durvalumab, nivolumab and pembrolizumab. In urothelial carcinoma, ICIs have only been approved for use after progression on platinum-based chemotherapy and in cisplatin ineligible patients with PDL1 positive tumors.
In VH of UC there are ongoing trials evaluating the efficacy of combination immunotherapy in VH patients with advanced disease (72,73), however, data on efficacy of immunotherapy in the neoadjuvant setting is lacking.
Majority of the clinical trials that have investigated pathologic response rates of UC to ICIs such as the KEYNOTE-057 (74) or ABACUS (75) trials, have the major limitation of excluding patients with a predominant VH. Therefore, the study of the efficacy of neoadjuvant immunotherapy in VH of UC constitutes an area of unmet medical need.
An attempt to address this need was made by the amended PURE-01 study (4). It is the largest clinical trial of a neoadjuvant checkpoint inhibitor in MIBC till date. The updated results (details in Table 11) allowed for the inclusion of patients with a predominant VH and was more representative of the heterogenous MIBC population. Future clinical trials will need to include more patients with VH of UC to better reflect the real-world population of UC.
The biomarkers that have been studied that may predict response to immunotherapy in VH of UC include PD1 and PDL1 expression, tumor inflammatory microenvironment and tumor mutational burden (TMB). Li et al. (76) stratified these biomarkers by histologic and molecular subtypes in an attempt to predict response to immunotherapy. The biomarkers (PD1, CD3, CD8, CD68 in tumor infiltrating immune cells and PDL1 expression in tumor microenvironment) were evaluated by immunohistochemistry and semi-quantitatively assigned a score (0-3). The major conclusion in this study was that the immunogenicity and thus likelihood to respond to immunotherapy correlates with tumor invasive status, histologic phenotype and molecular subtype. Regarding tumor invasive status, the highest and lowest scores were assigned to MIBC and non-invasive papillary UC, respectively. Therefore, CIS and NMIBC may require additional therapy to augment a predicted low response to immunotherapy. Invasive sarcomatoid differentiation had a significantly higher PD-L1 score while plasmacytoid, sarcomatoid and squamous histologies tended to have greater intra-tumoral lymphocyte infiltration. Based on the scoring system, these histologic phenotypes were considered as being “immunogenic”. Likewise, regarding molecular subtypes, the genomically unstable subtype was observed to be enriched with a high immune cluster (76).
The only caveat is due to intra-tumoral heterogeneity; hence caution is needed when attempting to extrapolate results from a limited tissue sample.
These findings seem to be supported by the results of the PURE-01 study wherein it was observed that responsiveness to pembrolizumab was dependent on tumor biomarkers (4).
Regardless of how promising these findings are, any proposed biomarkers to predict response to immunotherapy will also need to be validated in large prospective trials before being applicable in clinical decision making.
Limitations
The limitations of this review include the absence of detailed demographic data in some studies. Several studies do not specify the type and regimen (dosage and number of cycles) of chemotherapeutic agents used, thus comparisons between regimen could not be made. Some less commonly reported variants were categorized together under sub-heading ‘others’, thus limiting data that can be attributable to the rare variant histologies. In addition, most of the outcomes pertain to only MIBC with VH.
Clinical practice recommendations
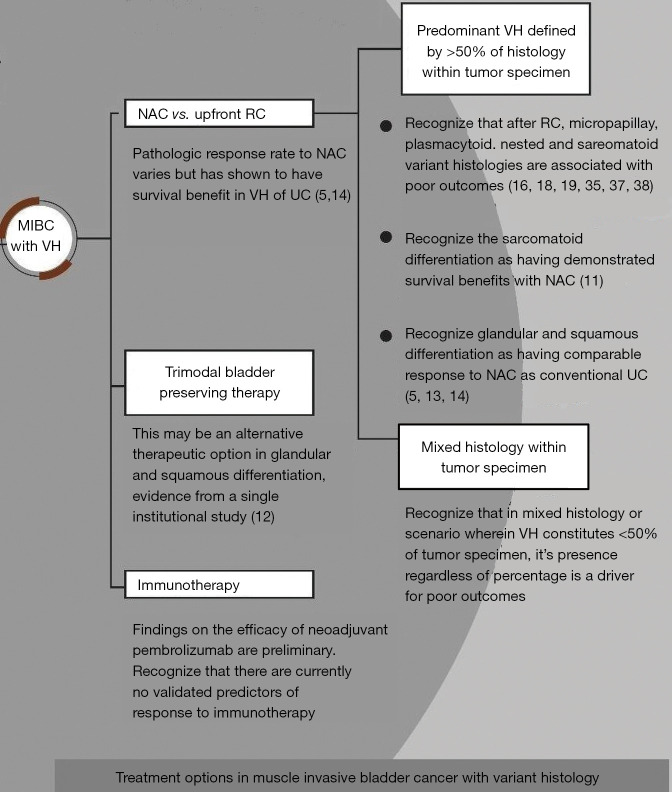
Based on the available evidence, a decision-making aid summarizing our findings pertaining to treatment options in non-metastatic MIBC with VH is presented in Figure 3 and VH specific recommendations are given in Table 13.
Figure 3.
Treatment options in muscle invasive bladder cancer with variant histology. MIBC, muscle invasive bladder cancer; VH, variant histology; NAC, neoadjuvant chemotherapy; RC, radical cystectomy; UC, urothelial carcinoma.
Table 13. Variant histology specific recommendations.
| Variant histology | Recommendation |
|---|---|
| Micropapillary, plasmacytoid, nested, clear cell and microcystic UC | In MIBC with VH, we recommend NAC to achieve pathologic downstaging prior to RC as a higher pathologic T stage at RC is associated with worse survival outcomes and there is evidence of survival benefit |
| A longer period of surveillance after RC is also warranted | |
| Sarcomatoid differentiation | We recommend NAC in MIBC due to evidence of survival benefit |
| Lymphoepithelioma-like UC | Where LELC constitutes the predominant histology in MIBC, we recommend NAC due to chemosensitivity and evidence of survival benefit in other variants |
| Squamous/glandular divergent differentiation | NAC is warranted in these tumors. Despite having similar pathologic response to NAC compared to conventional UC, they are more likely to present at a higher pathologic stage. Adjuvant chemotherapy should be considered as a therapeutic option due to evidence of worse OS in glandular differentiation when not administered |
UC, urothelial carcinoma; MIBC, muscle invasive bladder cancer; VH, variant histology; NAC, neoadjuvant chemotherapy; RC, radical cystectomy; LELC, lymphoepithelioma-like carcinoma; OS, overall survival.
Conclusion
VH of UC presents a diagnostic and treatment dilemma due to their rarity and aggressive nature. Despite somewhat conflicting reports in the literature, we present a comprehensive review of the available data on pathologic response rates to neoadjuvant systemic therapy and survival outcomes after radical cystectomy. NAC prior to radical cystectomy has been shown to be a beneficial treatment strategy with the responsiveness of urothelial carcinoma with squamous and glandular differentiation being comparable to conventional UC among studies reviewed. The micropapillary, plasmacytoid, nested and sarcomatoid histologic variants have been associated with worse survival outcomes compared to conventional UC and UC with squamous or glandular differentiation despite initial downstaging with chemotherapy. There is evidence of improved survival in patients with sarcomatoid differentiation receiving NAC compared to RC alone. The major prognostic factors that affect survival outcomes in VH of UC include histologic variant, patient age, presence of lymphovascular invasion, hydronephrosis, nodal metastasis and advanced T stage at diagnosis. Recent studies demonstrate that VH of UC are heterogenous tumors and responsiveness to NAC may be a function of the molecular subtypes present. Biomarkers identified by molecular profiling with immunohistochemistry will need to be validated as predictors of response to NAC in future trials.
Supplementary
The article’s supplementary files as
Acknowledgments
The authors would like to thank the medical librarian Irene Szentkiralyi at Cleveland Clinic Fairview hospital library for her contributions towards this paper.
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-43/rc
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-43/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-43/coif). The authors have no conflicts of interest to declare.
References
- 1.Key statistics for Bladder Cancer [Internet]. American Cancer Society. [cited 2021Aug28]. Available online: https://www.cancer.org/cancer/bladder-cancer/about/key-statistics.html
- 2.Moch H, Cubilla AL, Humphrey PA, et al. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur Urol 2016;70:93-105. 10.1016/j.eururo.2016.02.029 [DOI] [PubMed] [Google Scholar]
- 3.Vetterlein MW, Wankowicz SAM, Seisen T, et al. Neoadjuvant chemotherapy prior to radical cystectomy for muscle-invasive bladder cancer with variant histology. Cancer 2017;123:4346-55. 10.1002/cncr.30907 [DOI] [PubMed] [Google Scholar]
- 4.Necchi A, Raggi D, Gallina A, et al. Updated Results of PURE-01 with Preliminary Activity of Neoadjuvant Pembrolizumab in Patients with Muscle-invasive Bladder Carcinoma with Variant Histologies. Eur Urol 2020;77:439-46. 10.1016/j.eururo.2019.10.026 [DOI] [PubMed] [Google Scholar]
- 5.Kaimakliotis HZ, Monn MF, Cho JS, et al. Neoadjuvant chemotherapy in urothelial bladder cancer: impact of regimen and variant histology. Future Oncol 2016;12:1795-804. 10.2217/fon-2016-0056 [DOI] [PubMed] [Google Scholar]
- 6.Speir RW, Barboza MP, Calaway A, et al. Role of Neoadjuvant Chemotherapy in Squamous Variant Histology in Urothelial Bladder Cancer: Does Presence and Percentage Matter? Clin Genitourin Cancer 2021;19:47-52. 10.1016/j.clgc.2020.06.004 [DOI] [PubMed] [Google Scholar]
- 7.Li Q, Assel M, Benfante NE, et al. The Impact of Plasmacytoid Variant Histology on the Survival of Patients with Urothelial Carcinoma of Bladder after Radical Cystectomy. Eur Urol Focus 2019;5:104-8. 10.1016/j.euf.2017.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen HM, D'Souza AM, Green IF, et al. Do amount of variant differentiation and mitotic rate in bladder cancer change with neoadjuvant chemotherapy? Hum Pathol 2015;46:1367-75. 10.1016/j.humpath.2015.05.020 [DOI] [PubMed] [Google Scholar]
- 9.Buisan O, Orsola A, Oliveira M, et al. Role of Inflammation in the Perioperative Management of Urothelial Bladder Cancer With Squamous-Cell Features: Impact of Neutrophil-to-Lymphocyte Ratio on Outcomes and Response to Neoadjuvant Chemotherapy. Clin Genitourin Cancer 2017;15:e697-706. 10.1016/j.clgc.2017.01.024 [DOI] [PubMed] [Google Scholar]
- 10.Meeks JJ, Taylor JM, Matsushita K, et al. Pathological response to neoadjuvant chemotherapy for muscle-invasive micropapillary bladder cancer. BJU Int 2013;111:E325-30. 10.1111/j.1464-410X.2012.11751.x [DOI] [PubMed] [Google Scholar]
- 11.Almassi N, Vertosick EA, Sjoberg DD, et al. Pathological and oncological outcomes in patients with sarcomatoid differentiation undergoing cystectomy. BJU Int 2022;129:463-9. 10.1111/bju.15428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagumo Y, Kojima T, Shiga M, et al. A single-institute experience of trimodal bladder-preserving therapy for histologic variants of urothelial carcinoma. Int J Clin Oncol 2020;25:354-61. 10.1007/s10147-019-01553-4 [DOI] [PubMed] [Google Scholar]
- 13.Zargar-Shoshtari K, Sverrisson EF, Sharma P, et al. Clinical Outcomes After Neoadjuvant Chemotherapy and Radical Cystectomy in the Presence of Urothelial Carcinoma of the Bladder With Squamous or Glandular Differentiation. Clin Genitourin Cancer 2016;14:82-8. 10.1016/j.clgc.2015.08.006 [DOI] [PubMed] [Google Scholar]
- 14.Hajiran A, Azizi M, Aydin AM, et al. Pathological and Survival Outcomes Associated with Variant Histology Bladder Cancers Managed by Cystectomy with or without Neoadjuvant Chemotherapy. J Urol 2021;205:100-8. 10.1097/JU.0000000000001325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamat AM, Dinney CP, Gee JR, et al. Micropapillary bladder cancer: a review of the University of Texas MD Anderson Cancer Center experience with 100 consecutive patients. Cancer 2007;110:62-7. 10.1002/cncr.22756 [DOI] [PubMed] [Google Scholar]
- 16.Soave A, Schmidt S, Dahlem R, et al. Does the extent of variant histology affect oncological outcomes in patients with urothelial carcinoma of the bladder treated with radical cystectomy? Urol Oncol 2015;33:21.e1-9. 10.1016/j.urolonc.2014.10.013 [DOI] [PubMed] [Google Scholar]
- 17.Mitra AP, Fairey AS, Skinner EC, et al. Implications of micropapillary urothelial carcinoma variant on prognosis following radical cystectomy: A multi-institutional investigation. Urol Oncol 2019;37:48-56. 10.1016/j.urolonc.2018.10.013 [DOI] [PubMed] [Google Scholar]
- 18.Monn MF, Kaimakliotis HZ, Cary KC, et al. The changing reality of urothelial bladder cancer: should non-squamous variant histology be managed as a distinct clinical entity? BJU Int 2015;116:236-40. 10.1111/bju.12877 [DOI] [PubMed] [Google Scholar]
- 19.Soave A, Riethdorf S, Dahlem R, et al. Detection and oncological effect of circulating tumour cells in patients with variant urothelial carcinoma histology treated with radical cystectomy. BJU Int 2017;119:854-61. 10.1111/bju.13782 [DOI] [PubMed] [Google Scholar]
- 20.Ghoneim IA, Miocinovic R, Stephenson AJ, et al. Neoadjuvant systemic therapy or early cystectomy? Single-center analysis of outcomes after therapy for patients with clinically localized micropapillary urothelial carcinoma of the bladder. Urology 2011;77:867-70. 10.1016/j.urology.2010.11.043 [DOI] [PubMed] [Google Scholar]
- 21.Mitra AP, Bartsch CC, Bartsch G, Jr, et al. Does presence of squamous and glandular differentiation in urothelial carcinoma of the bladder at cystectomy portend poor prognosis? An intensive case-control analysis. Urol Oncol 2014;32:117-27. 10.1016/j.urolonc.2012.08.017 [DOI] [PubMed] [Google Scholar]
- 22.Fairey AS, Daneshmand S, Wang L, et al. Impact of micropapillary urothelial carcinoma variant histology on survival after radical cystectomy. Urol Oncol 2014;32:110-6. 10.1016/j.urolonc.2012.04.020 [DOI] [PubMed] [Google Scholar]
- 23.Compérat E, Roupret M, Yaxley J, et al. Micropapillary urothelial carcinoma of the urinary bladder: a clinicopathological analysis of 72 cases. Pathology 2010;42:650-4. 10.3109/00313025.2010.522173 [DOI] [PubMed] [Google Scholar]
- 24.Masson-Lecomte A, Xylinas E, Bouquot M, et al. Oncological outcomes of advanced muscle-invasive bladder cancer with a micropapillary variant after radical cystectomy and adjuvant platinum-based chemotherapy. World J Urol 2015;33:1087-93. 10.1007/s00345-014-1387-1 [DOI] [PubMed] [Google Scholar]
- 25.Moschini M, Dell’Oglio P, Luciano’ R, et al. Incidence and effect of variant histology on oncological outcomes in patients with bladder cancer treated with radical cystectomy. Urol Oncol 2017;35:335-41. 10.1016/j.urolonc.2016.12.006 [DOI] [PubMed] [Google Scholar]
- 26.Martini A, Afferi L, Zamboni S, et al. Oncologic Surveillance for Variant Histology Bladder Cancer after Radical Cystectomy. J Urol 2021;206:885-93. 10.1097/JU.0000000000001886 [DOI] [PubMed] [Google Scholar]
- 27.Wang JK, Boorjian SA, Cheville JC, et al. Outcomes following radical cystectomy for micropapillary bladder cancer versus pure urothelial carcinoma: a matched cohort analysis. World J Urol 2012;30:801-6. 10.1007/s00345-012-0976-0 [DOI] [PubMed] [Google Scholar]
- 28.Kaimakliotis HZ, Monn MF, Cary KC, et al. Plasmacytoid variant urothelial bladder cancer: is it time to update the treatment paradigm? Urol Oncol 2014;32:833-8. 10.1016/j.urolonc.2014.03.008 [DOI] [PubMed] [Google Scholar]
- 29.Kim SP, Frank I, Cheville JC, et al. The impact of squamous and glandular differentiation on survival after radical cystectomy for urothelial carcinoma. J Urol 2012;188:405-9. 10.1016/j.juro.2012.04.020 [DOI] [PubMed] [Google Scholar]
- 30.Antunes AA, Nesrallah LJ, Dall'Oglio MF, et al. The role of squamous differentiation in patients with transitional cell carcinoma of the bladder treated with radical cystectomy. Int Braz J Urol 2007;33:339-45; discussion 346. 10.1590/S1677-55382007000300006 [DOI] [PubMed] [Google Scholar]
- 31.Ehdaie B, Maschino A, Shariat SF, et al. Comparative outcomes of pure squamous cell carcinoma and urothelial carcinoma with squamous differentiation in patients treated with radical cystectomy. J Urol 2012;187:74-9. 10.1016/j.juro.2011.09.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sefik E, Celik S, Basmaci I, et al. Effect of variant histology presence and squamous differentiation on oncological results and patient's survival after radical cystectomy. Arch Ital Urol Androl 2018;90:172-5. 10.4081/aiua.2018.3.172 [DOI] [PubMed] [Google Scholar]
- 33.Minato A, Noguchi H, Tomisaki I, et al. Clinical significance of squamous differentiation in urothelial carcinoma of the bladder. Cancer Control 2018;25:1073274818800269. 10.1177/1073274818800269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xylinas E, Rink M, Robinson BD, et al. Impact of histological variants on oncological outcomes of patients with urothelial carcinoma of the bladder treated with radical cystectomy. Eur J Cancer 2013;49:1889-97. 10.1016/j.ejca.2013.02.001 [DOI] [PubMed] [Google Scholar]
- 35.Takemoto K, Teishima J, Kohada Y, et al. The Impact of Histological Variant on Oncological Outcomes in Patients With Urothelial Carcinoma of the Bladder Treated With Radical Cystectomy. Anticancer Res 2020;40:4787-93. 10.21873/anticanres.14481 [DOI] [PubMed] [Google Scholar]
- 36.Linder BJ, Frank I, Cheville JC, et al. Outcomes following radical cystectomy for nested variant of urothelial carcinoma: a matched cohort analysis. J Urol 2013;189:1670-5. 10.1016/j.juro.2012.11.006 [DOI] [PubMed] [Google Scholar]
- 37.Naspro R, Finati M, Roscigno M, et al. The impact of histological variants on outcomes after open radical cystectomy for muscle-invasive urothelial bladder cancer: results from a single tertiary referral centre. World J Urol 2021;39:1917-26. 10.1007/s00345-020-03364-z [DOI] [PubMed] [Google Scholar]
- 38.Keck B, Wach S, Stoehr R, et al. Plasmacytoid variant of bladder cancer defines patients with poor prognosis if treated with cystectomy and adjuvant cisplatin-based chemotherapy. BMC Cancer 2013;13:71. 10.1186/1471-2407-13-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stroman L, Nair R, Russell B, et al. The impact of non-urothelial variant histology on oncological outcomes following radical cystectomy. BJU Int 2019;124:418-23. 10.1111/bju.14704 [DOI] [PubMed] [Google Scholar]
- 40.Zamboni S, Afferi L, Soria F, et al. Adjuvant chemotherapy is ineffective in patients with bladder cancer and variant histology treated with radical cystectomy with curative intent. World J Urol 2021;39:1947-53. 10.1007/s00345-020-03362-1 [DOI] [PubMed] [Google Scholar]
- 41.Komina S, Petrusevska G, Janevska V, et al. Effect of bladder cancer variant histology on survival outcome in patients treated with radical cystectomy: A single-centre experience. Urol Ann 2021;13:288-95. 10.4103/UA.UA_95_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marks P, Gild P, Soave A, et al. The impact of variant histological differentiation on extranodal extension and survival in node positive bladder cancer treated with radical cystectomy. Surg Oncol 2019;28:208-13. 10.1016/j.suronc.2019.01.008 [DOI] [PubMed] [Google Scholar]
- 43.Mazzucchelli R, Marzioni D, Tossetta G, et al. Bladder Cancer Sample Handling and Reporting: Pathologist's Point of View. Front Surg 2021;8:754741. 10.3389/fsurg.2021.754741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang H, Jadallah S, Chen YB. Her2 expression in urothelial carcinoma with micropapillary morphology: Preferential overexpresssion by the micropapillary component. Modern Pathology 2013;26. [Google Scholar]
- 45.Ross JS, Wang K, Gay LM, et al. A high frequency of activating extracellular domain ERBB2 (HER2) mutation in micropapillary urothelial carcinoma. Clin Cancer Res 2014;20:68-75. 10.1158/1078-0432.CCR-13-1992 [DOI] [PubMed] [Google Scholar]
- 46.Schneider SA, Sukov WR, Frank I, et al. Outcome of patients with micropapillary urothelial carcinoma following radical cystectomy: ERBB2 (HER2) amplification identifies patients with poor outcome. Mod Pathol 2014;27:758-64. 10.1038/modpathol.2013.201 [DOI] [PubMed] [Google Scholar]
- 47.Willis DL, Fernandez MI, Dickstein RJ, et al. Clinical outcomes of cT1 micropapillary bladder cancer. J Urol 2015;193:1129-34. 10.1016/j.juro.2014.09.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diamantopoulos LN, Holt SK, Khaki AR, et al. Response to Neoadjuvant Chemotherapy and Survival in Micropapillary Urothelial Carcinoma: Data From a Tertiary Referral Center and the Surveillance, Epidemiology, and End Results (SEER) Program. Clin Genitourin Cancer 2021;19:144-54. 10.1016/j.clgc.2020.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abufaraj M, Foerster B, Schernhammer E, et al. Micropapillary Urothelial Carcinoma of the Bladder: A Systematic Review and Meta-analysis of Disease Characteristics and Treatment Outcomes. Eur Urol 2019;75:649-58. 10.1016/j.eururo.2018.11.052 [DOI] [PubMed] [Google Scholar]
- 50.Al-Ahmadie H, Iyer G, Mehra R, et al. E-cadherin (CDH1) is frequently mutated in urothelial carcinoma with signet-ring cell and plasmacytoid morphology. Modern Pathology 2013;26. [Google Scholar]
- 51.Sangoi AR, Chan E, Stohr BA, et al. Invasive plasmacytoid urothelial carcinoma: A comparative study of E-cadherin and P120 catenin. Hum Pathol 2020;102:54-9. 10.1016/j.humpath.2020.07.006 [DOI] [PubMed] [Google Scholar]
- 52.Keck B, Stoehr R, Wach S, et al. The plasmacytoid carcinoma of the bladder--rare variant of aggressive urothelial carcinoma. Int J Cancer 2011;129:346-54. 10.1002/ijc.25700 [DOI] [PubMed] [Google Scholar]
- 53.Dayyani F, Czerniak BA, Sircar K, et al. Plasmacytoid urothelial carcinoma, a chemosensitive cancer with poor prognosis, and peritoneal carcinomatosis. J Urol 2013;189:1656-61. 10.1016/j.juro.2012.11.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holmäng S, Johansson SL. The nested variant of transitional cell carcinoma--a rare neoplasm with poor prognosis. Scand J Urol Nephrol 2001;35:102-5. 10.1080/003655901750170425 [DOI] [PubMed] [Google Scholar]
- 55.Cox R, Epstein JI. Large nested variant of urothelial carcinoma: 23 cases mimicking von Brunn nests and inverted growth pattern of noninvasive papillary urothelial carcinoma. Am J Surg Pathol 2011;35:1337-42. 10.1097/PAS.0b013e318222a653 [DOI] [PubMed] [Google Scholar]
- 56.Eble JN, Sauter G, Epstein JI, et al. World Health Organization classification of tumours: pathology and genetics of tumours of the urinary system and male genital organs. Lyon, France: IARC Press; 2004. [Google Scholar]
- 57.Diamantopoulos LN, Korentzelos D, Alevizakos M, et al. Sarcomatoid Urothelial Carcinoma: A Population-Based Study of Clinicopathologic Characteristics and Survival Outcomes. Clin Genitourin Cancer 2022;20:139-47. 10.1016/j.clgc.2021.12.015 [DOI] [PubMed] [Google Scholar]
- 58.Yang AW, Pooli A, Lele SM, et al. Lymphoepithelioma-like, a variant of urothelial carcinoma of the urinary bladder: a case report and systematic review for optimal treatment modality for disease-free survival. BMC Urol 2017;17:34. 10.1186/s12894-017-0224-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lopez-Beltrán A, Luque RJ, Vicioso L, et al. Lymphoepithelioma-like carcinoma of the urinary bladder: a clinicopathologic study of 13 cases. Virchows Arch 2001;438:552-7. 10.1007/s004280000378 [DOI] [PubMed] [Google Scholar]
- 60.Knez VM, Barrow W, Lucia MS, et al. Clear cell urothelial carcinoma of the urinary bladder: a case report and review of the literature. J Med Case Rep 2014;8:275. 10.1186/1752-1947-8-275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lopez Beltran A, Montironi R, Cheng L. Microcystic urothelial carcinoma: morphology, immunohistochemistry and clinical behaviour. Histopathology 2014;64:872-9. 10.1111/his.12345 [DOI] [PubMed] [Google Scholar]
- 62.Mai KT, Bateman J, Djordjevic B, et al. Clear Cell Urothelial Carcinoma. Int J Surg Pathol 2017;25:18-25. 10.1177/1066896916660195 [DOI] [PubMed] [Google Scholar]
- 63.Kadouri Y, Zaoui Y, Derkaoui S, et al. Microcystic urothelial carcinoma of the bladder: A case report. Urol Case Rep 2020;33:101369. 10.1016/j.eucr.2020.101369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.El-Asmar JM, Moussally M, Abou Heidar N, et al. Differentiation of Urothelial Carcinoma into Two Distinct Subtypes, Glandular and Squamous, in Three Different Organs. Cureus 2020;12:e7280. 10.7759/cureus.7280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chakiryan NH, Jiang DD, Gillis KA, et al. Pathological Downstaging and Survival Outcomes Associated with Neoadjuvant Chemotherapy for Variant Histology Muscle Invasive Bladder Cancer. J Urol 2021;206:924-32. 10.1097/JU.0000000000001855 [DOI] [PubMed] [Google Scholar]
- 66.Sjödahl G, Eriksson P, Liedberg F, Höglund M. Molecular classification of urothelial carcinoma: global mRNA classification versus tumour-cell phenotype classification. J Pathol 2017;242:113-25. 10.1002/path.4886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Robertson AG, Kim J, Al-Ahmadie H, et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell 2017;171:540-556.e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kamoun A, de Reyniès A, Allory Y, et al. A Consensus Molecular Classification of Muscle-invasive Bladder Cancer. Eur Urol 2020;77:420-33. 10.1016/j.eururo.2019.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Processali T, Diminutto A, Cerruto MA, et al. The impact of histological variants on bladder cancer outcomes. AME Med J 2020;5:4. 10.21037/amj.2020.02.02 [DOI] [Google Scholar]
- 70.Warrick JI, Sjödahl G, Kaag M, et al. Intratumoral Heterogeneity of Bladder Cancer by Molecular Subtypes and Histologic Variants. Eur Urol 2019;75:18-22. 10.1016/j.eururo.2018.09.003 [DOI] [PubMed] [Google Scholar]
- 71.Seiler R, Ashab HAD, Erho N, et al. Impact of Molecular Subtypes in Muscle-invasive Bladder Cancer on Predicting Response and Survival after Neoadjuvant Chemotherapy. Eur Urol 2017;72:544-54. 10.1016/j.eururo.2017.03.030 [DOI] [PubMed] [Google Scholar]
- 72.McGregor BA, Campbell MT, Xie W, et al. Phase II study of nivolumab and ipilimumab for advanced bladder cancer of variant histologies (BCVH). J Clin Oncol 2019;37:4518. 10.1200/JCO.2019.37.15_suppl.4518 [DOI] [Google Scholar]
- 73.Marandino L, Raggi D, Calareso G, et al. Cabozantinib Plus Durvalumab in Patients With Advanced Urothelial Carcinoma After Platinum Chemotherapy: Safety and Preliminary Activity of the Open-Label, Single-Arm, Phase 2 ARCADIA Trial. Clin Genitourin Cancer 2021;19:457-65. 10.1016/j.clgc.2021.04.001 [DOI] [PubMed] [Google Scholar]
- 74.Balar AV, Kamat AM, Kulkarni GS, et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): an open-label, single-arm, multicentre, phase 2 study. Lancet Oncol 2021;22:919-30. 10.1016/S1470-2045(21)00147-9 [DOI] [PubMed] [Google Scholar]
- 75.Powles T, Kockx M, Rodriguez-Vida A, et al. Clinical efficacy and biomarker analysis of neoadjuvant atezolizumab in operable urothelial carcinoma in the ABACUS trial. Nat Med 2019;25:1706-14. 10.1038/s41591-019-0628-7 [DOI] [PubMed] [Google Scholar]
- 76.Li H, Zhang Q, Shuman L, et al. Evaluation of PD-L1 and other immune markers in bladder urothelial carcinoma stratified by histologic variants and molecular subtypes. Sci Rep 2020;10:1439. 10.1038/s41598-020-58351-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as