Abstract
Solid organ transplantation continues to be constrained by a lack of suitable donor organs. Advances in donor management and evaluation are needed to address this shortage, but performance of research studies in deceased donors is fraught with challenges. Here we discuss several of the major obstacles we faced in the conduct of the Donor Heart Study—a prospective, multi-site, observational study of donor management, evaluation, and acceptance for heart transplantation. These included recruitment and engagement of participating organ procurement organizations, ambiguities related to study oversight, obtaining authorization for donor research, logistical challenges encountered during donor management, sustaining study momentum, and challenges related to study data management. By highlighting these obstacles encountered, as well as the solutions implemented, we hope to stimulate further discussion and actions that will facilitate the design and execution of future donor research studies.
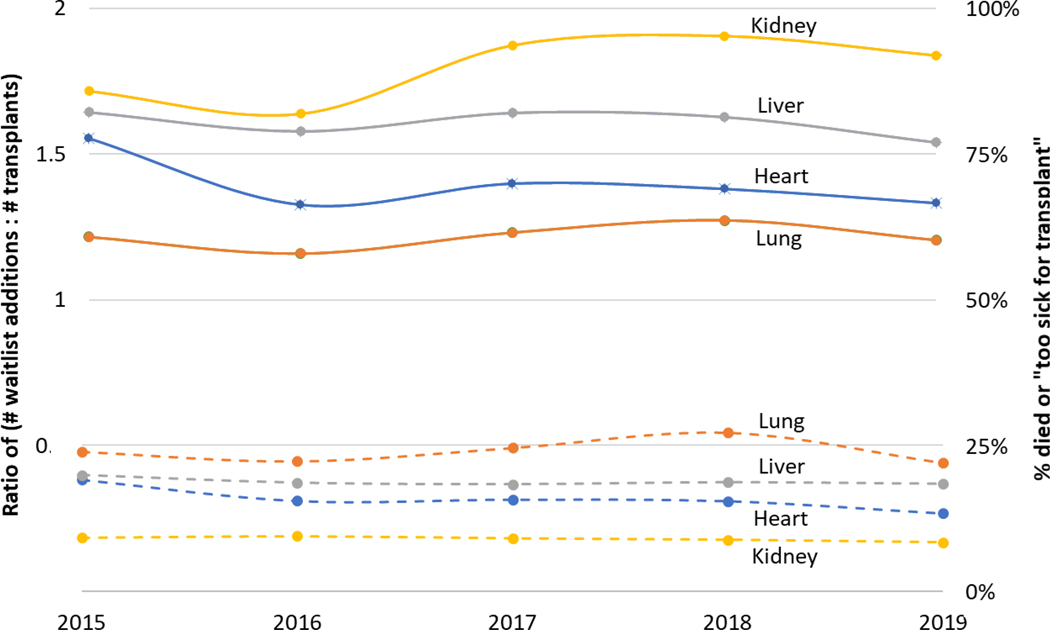
Due to ever-increasing disease burden and concomitant advances in the field, solid organ transplantation is the chosen therapy for a growing number and range of patients with end-stage organ diseases. Unfortunately, transplant volumes have not kept pace with this growing demand, primarily due to a scarcity of donor organs deemed suitable for transplant. The result is increasing wait times and persistently high waitlist morbidity for all solid organs (Figure 1). One solution is to identify and use more organs for transplant– however, doing so will require further research into the selection and management of viable donor organs.
Figure 1:
Supply-demand mismatch created by the shortage of suitable donor organs for solid organ transplantation. Solid lines represent the ratio of waitlist additions to the number of transplants performed per year. Dashed lines represent the percent of candidates who died or were too sick to be transplanted. Data shown for kidney, liver, heart, and lung transplants from 2015–2019.
Source:
2019 Annual Data Report. Scientific Registry of Transplant Recipients http://srtr.transplant.hrsa.gov/annual_reports/Default.aspx Accessed February 9, 2022
2017 Annual Data Report. Scientific Registry of Transplant Recipients http://srtr.transplant.hrsa.gov/annual_reports/Default.aspx Accessed February 9, 2022
Unfortunately, the field of donor research has been stymied by long-standing ethical and logistical challenges.(1–7) For example, the procedures necessary to authorize a donor’s inclusion in a research study remain unclear.(1) Notably, the federal Office for Human Research Protection – and by extension, the Institutional Review Boards (IRBs) it oversees - does not regulate research involving the deceased. The absence of these established regulatory channels creates confusion for investigators, organ procurement organizations (OPOs), donor hospitals, and transplant centers that wish to undertake deceased donor research. Such research entails potential risk and benefit to other parties beyond the deceased donors themselves; other affected parties include the families of the deceased and the transplant candidates waiting for and/or receiving the donor organs affected by any research interventions.(3) These are but a few of the many hurdles entailed in the design and conduct of research involving deceased donors.
The urgent need to address these hurdles was formally recognized in 2016, when the National Academy of Sciences (NAS) launched a study to “examine the ethical, policy, regulatory, and operational issues relevant to the conduct of research involving deceased organ donors”.(8) The NAS report, issued in 2018, called for a framework consisting of (1) a centrally administered and standing Donor-Research Oversight Committee, (2) a single national IRB for organ donor intervention research, and (3) study-specific data and safety monitoring boards (DSMBs), among other recommendations. This report included specific suggestions to enable coordination, implementation, tracking, analysis, and dissemination of organ donor intervention research.(8) Unfortunately, most of the NAS report recommendations have yet to be implemented.(3)
It was in this challenging environment that we designed and launched the Donor Heart Study (NIH R01 HL125303: Evidence Based Evaluation and Acceptance of Donor Hearts for Transplantation)—the largest prospective study of cardiac donor management, evaluation, and acceptance performed to-date. The Donor Heart Study was coordinated at Stanford University and conducted at 8 OPOs across the United States from 2015–2020, with 3 overarching aims: (1) to identify clinical correlates of cardiac function in potential donors being evaluated for heart transplantation (HT), (2) to prospectively study reasons for non- acceptance of hearts offered for transplantation, and (3) to develop clinical tools to assist transplant centers with real-time decisions regarding donor heart acceptance.
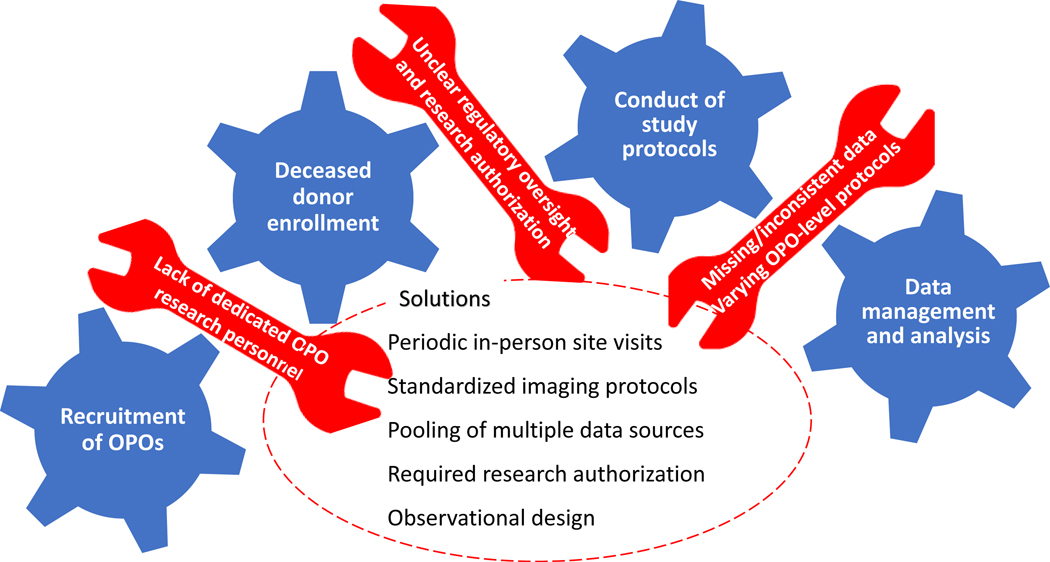
In this observational study, we enrolled 4,333 potential cardiac organ donors and collected detailed data on donor clinical characteristics, hemodynamic monitoring, administration of intravenous fluids and medications during donor management, laboratory testing, and invasive procedures. Serial cardiac biomarkers (troponin and B-type natriuretic peptide) were measured, and serial electrocardiograms and echocardiograms (TTEs) were performed, with core interpretation of all TTEs by a single, expert reviewer. Organ disposition was recorded, and real-time surveys were administered to transplant center personnel when organ offers were declined, in order to capture detailed data on reasons for donor heart non-acceptance in the current era. Herein, we attempt to describe the many challenges encountered in the implementation of this landmark study, in the hope that these experiences and lessons learned will inform the successful conduct of future donor- based research (Figure 2).
Figure 2:
Challenges encountered during conduct of the Donor Heart Study, and solutions implemented.
Recruitment and Engagement of Participating OPOs
We aimed to collaborate with large-volume OPOs across the United States, in order to obtain a nationally-representative sample of potential cardiac organ donors. In doing so, we faced significant challenges. While some OPOs had a well-established research infrastructure, with a dedicated director of research operations and research coordinators, others did not have dedicated research personnel. In these cases, we relied on the OPO medical director for study oversight, and on existing OPO personnel for study coordination and data entry. Even though the study provided financial reimbursement for part-time research staff effort, these tasks frequently fell to donor coordinators, nurses, administrative assistants, part-time employees, and other individuals who were already fulfilling multiple roles at the OPO. As such, completion of research-related tasks was not always the top priority for OPO site personnel. The lack of sufficient funding and resources for dedicated research personnel at the OPOs continues to limit their ability to participate in donor management studies.
The lack of dedicated OPO research personnel led to other challenges, including delays in data entry and the need to continually re-train study coordinators due to staff turnover. While we aimed for real-time data entry, in order to maximize the accuracy of the data entered into the online study databases, this task was often batched and performed retrospectively when staff were available. This practice likely resulted in more missing data than would have been the case if data were entered during the donor management period. Moreover, high staff turnover at the participating OPOs required frequent training on study orientation and procedures. This was performed by the main study research coordinator at Stanford University, who led monthly virtual research meetings and created online orientation materials to facilitate onboarding of new staff members.
Other barriers to OPO involvement included a lack of familiarity with federal research grants, which required establishment of contracts and subawards with each participating site, and the concern that the study would reduce the OPO’s donor heart acceptance rate, due to the requirement for serial cardiac testing (e.g. serial cardiac biomarkers and echocardiograms) that the OPO may not have performed as part of its routine clinical protocol. Of note, a subsequent review of donor heart utilization rates at the participating OPOs did not reveal a decline in utilization during the conduct of the Donor Heart Study.
Study Oversight
In the absence of a national, centralized IRB for organ donor research, we elected to submit the DHS study protocol to the Stanford University IRB (ID 31461), while recognizing that IRBs are responsible for ensuring the protection of living human research subjects and have no regulatory authority over deceased donors.(6) We concurrently submitted the protocol for approval at each of the 8 participating OPOs, which had different protocols for approval of donor research studies. For example, Donor Network West (San Ramon, California) has a team of internal staff members, led by the OPO Director of Research, who review research proposals. In some cases, investigators are invited to present their proposals, in person or virtually, to review and clarify study details. Once a research protocol is evaluated and receives passing scores for ethics, mission alignment, and potential impact, it is subject to a vote by members of the Medical Advisory Board Research Sub-Committee. Clinical trials involving donor interventions are also reviewed by the OPO’s governing board. At Gift of Life Michigan, prospective investigators submit a research application to the OPO Research Coordinator. The Research Coordinator vets the application against the OPO’s mission, the researcher’s specific needs, cost implications, and feasibility. The project summary and draft agreements are then submitted for executive leadership review and approval. Mechanisms for research project approval varied between participating OPOs.
Authorization for Donor Research
The mechanisms for designating oneself as an organ donor [First Person Authorization (FPA)] before death include authorization in an advanced directive or through a state registry, such as those maintained by the Department of Motor Vehicles. State registries vary in the extent to which they clarify the authorization for use of a donated organ for transplantation, research, or both. In states where registry designation solely authorizes transplantation, deceased donor research may require separate permission from a legal surrogate, as designated by the Uniform Anatomical Gift Act (UAGA).(1) Although the Donor Heart Study was an observational study that did not did not involve any donor- based interventions or procedures, and was therefore felt to pose minimal risk to the donor and potential recipients, we elected to obtain research authorization from the donor’s legal surrogates as a study inclusion criterion when the donor was not FPA. In cases where donors were not FPA and families had not authorized donor research studies in general, they were re-approached for authorization with the explanation that the Donor Heart Study was purely observational. Fortunately, of the 5965 donors who were eligible for enrollment, only 50 (<1%) were excluded due to lack of research authorization (an observation which suggests that potential donors and donor families want to support research, in addition to saving lives via organ donation).
Ideally, donor hospitals and transplant centers would also be notified when a donor is involved in a research study. Again, the lack of a centralized IRB to regulate deceased donor research has caused confusion and uncertainty about which entities are responsible for approving such protocols. In this case, as the Donor Heart Study was an observational study that posed minimal risk, and because the donor hospital does not have oversight of donor management after the OPO assumes responsibility, we did not seek approval from the donor hospitals. Donor Network West, one of the participating OPOs, gave a study overview at a United Network for Organ Sharing (UNOS) Region 5 research subcommittee meeting, which was followed by a written announcement through UNOS prior to the start of enrollment. We also provided real-time notification to the transplant centers during organ offers, similar to the process followed for a prior donor interventional trial conducted by one of the co-authors.(9) This consisted of a few sentences about the Donor Heart Study in the “donor highlights” section of the online electronic organ offer system, with a link to the study website (med.stanford.edu/donorheart.html).
Logistical Challenges During Donor Management
The Donor Heart Study was conducted at 8 OPOs and hundreds of donor hospitals across the United States. Each OPO had different donor management protocols, which made it impossible to standardize care of the donors (including hemodynamic monitoring, administration of medications, and laboratory and invasive testing) outside of the study- related testing. For example, many OPOs stopped placing pulmonary artery catheters during the study period, so we were unable to obtain detailed hemodynamic data, as originally planned. Protocols for administration of inotropes and vasopressors differed between OPOs, as did use of steroid and thyroid hormone supplementation. For example, half of participating OPOs treated all donors with thyroxine (T4), while the remaining OPOs administered T4 selectively to donors with left ventricular dysfunction or hemodynamic instability. Use of these medications can alter cardiac function, and had to be taken into consideration in the analysis phase of the study.
Another source of variability in study data resulted from the use of donor hospital laboratory testing. Different donor hospitals had different assays for performing certain laboratory tests, with different reference ranges. For example, cardiac troponins were measured as troponin I, troponin T, or high-sensitivity troponin, based on the donor hospital, and natriuretic peptides were measured as B-type natriuretic peptide (BNP) or N- terminal pro hormone BNP (NT-proBNP) levels, depending on local test availability. This posed challenges in data analysis and interpretation. For some variables, such as troponin values, we grouped lab test results into tertiles based on the observed ranges; in other cases we converted values of one assay into another (e.g. BNP to NT-proBNP) using published conversion formulas.(10)
Performance of imaging studies by donor hospital personnel also created inconsistencies in study data quality. Since the on-site OPO coordinators did not have the training and expertise required to perform echocardiograms on enrolled donors, we relied on donor hospital personnel to perform these studies. Ultrasound technicians were given a copy of the study imaging protocol that detailed the views and measurements required for comprehensive assessment of donor left ventricular function and wall motion. Overall, approximately 88% of studies were of adequate quality for core interpretation. In some cases, limited views were obtained or image quality was insufficient for performance of required measurements. Echocardiographic imaging of organ donors is notoriously challenging, due to donor supine positioning; mechanical ventilation; chest trauma; and placement of bandages, electrodes, and other devices on the chest. In addition, donor studies are often performed outside of standard daylight hours, by on-call personnel such as cardiology fellows who may not have the experience required to perform high quality imaging studies.
Finally, logistical constraints inherent to donor management limited our ability to collect some study-related data. For example, donor management often had to be accelerated or curtailed due to donor hemodynamic instability, competing timelines from the various procurement teams, and donor family wishes. As such, not all tests, such as serial echocardiograms and cardiac biomarkers, could be performed as planned.
Sustaining Study Momentum
Sustaining enthusiasm for a multi-year study is always difficult, particularly at sites that are removed from central study coordination and have competing priorities. The study principal investigator (KK) visited each site in-person at the time of study launch to present the study background and rationale, review procedures, and meet study staff. However, due to frequent staff turnover, and challenges encountered that were unique to each site, we found it necessary for the study research nurse (HL) to conduct periodic in-person site visits, in order to re-engage new staff members and troubleshoot any problems that may have arisen at the participating OPOs. We found that the site principal investigators (usually the OPO medical directors) were most effective when they (1) were personally committed to the project and regularly reviewed the need for and long-term goals of the project with their staff, (2) did an assessment of the time required for research project- related activities and ensured that the assigned OPO personnel (ideally dedicated research personnel) had sufficient protected time, and (3) offered incentives for staff to accomplish project-related milestones in a timely fashion.
Study engagement was also sustained via monthly newsletters that summarized progress towards enrollment and other milestones, protocol modifications, and study-related presentations. An in-person study investigator and staff meeting was held annually, in conjunction with the Association of Organ Procurement Organizations annual meeting. This annual meeting enabled us to summarize study progress to-date, brainstorm challenges encountered and potential solutions, and to re-connect with co-investigators and coordinators in person.
Challenges in Study Data Management
Several different sources and databases were used to collect data for the Donor Heart Study. Donor demographic data and selected test results were entered manually in real- time by OPO personnel into the study’s Research Electronic Data Capture (REDCap) database, hosted at Stanford University. Our study also employed the Donor Management Goals (DMG) Registry (https://dmginfo.nationaldmg.org/) - a secure, online prospective database that was developed in 2012 for donor management performance improvement, quality assurance, and research, and is now operated by UNOS. Donor data was either entered into the DMG database manually by the OPO study coordinator, or via an automated data upload from the donor electronic health record. Data entered into the DMG database included past medical history, vital signs and hemodynamic values, medications administered, laboratory test results, and donor organ disposition. Additional donor data, as well as transplant recipient data, were obtained from UNOS via Standard Transplant Analysis and Research (STAR) files. Finally, data from core interpretation of donor echocardiograms were recorded in a database created by Digisonics, Inc. (Houston, Texas). At study completion, the four study databases were linked and merged via the unique donor identification number (Donor ID) assigned to each potential organ donor in the United States. Merging multiple databases into a single, analytic data file invariably involves great effort to standardize naming and definition of variables, resolve inconsistencies, and identify missing and duplicated data. The creation of a centralized database that could serve as a resource and repository for donor research studies, with automatic links to national transplant registries, could greatly facilitate and streamline this process.
Conclusions
There continues to be a great need for innovation in the area of deceased donor research. Well-performed donor research studies are essential for identifying clinical practices that will improve organ utilization and transplant outcomes, and will require close collaborations between funding agencies, academic medical centers, OPOs, and other relevant organizations. At this time, most deceased donor research is performed on an ad hoc basis, driven by individual research groups, in the face of formidable barriers. Unless there is a concerted effort by the transplant community and regulatory agencies to resolve the legal, logistical, and ethical issues surrounding deceased donor research, it is unlikely that significant progress will be made. Ideally, dedicated funding (or at least an absence of financial disincentives) would also be available to support such work. Herein we have presented the Donor Heart Study as an example of the many challenges faced and obstacles that must be overcome when implementing large, multi-site donor research studies. The creation of a national framework to ensure centralized organizational and oversight mechanisms will greatly facilitate the performance of future studies, and will stimulate advances that will greatly benefit the field of transplantation.
Acknowledgments:
The investigators would like to acknowledge the thousands of donors enrolled in this study who selflessly gave the gift of life. We would also like to acknowledge the donor families, who agreed to research data collection, and the donor hospital staff who performed the required testing and procedures. Importantly, we would like to recognize the organ procurement organizations that participated in the Donor Heart Study [Donor Network West (San Ramon, California), Donor Network of Arizona (Tempe, Arizona), Gift of Hope (Itasca, Illinois), Gift of Life Michigan (Ann Arbor, Michigan), LifeGift (Houston, Fort Worth, and Lubbock, Texas), LifeLink (Norcross, Georgia), New England Donor Services (Waltham, Massachusetts), and LifeChoice Donor Services (Bloomfield, Connecticut)] and their staff who collected and entered study data. We finally would like to thank the coordinators and staff at the United Network for Organ Sharing, Oregon Health and Science University, Kaiser Permanente, and Stanford University for their contributions to this study.
Sources of Funding: This study was funded by the National Institutes of Health/National Heart Lung and Blood Institute (R01 HL125303).
Abbreviations
- BNP
B-type natriuretic peptide
- DMG
Donor Management Goals
- DSMB
Data Safety and Monitoring Board
- FPA
first person authorization
- IRB
Institutional Review Board
- NT-proBNP
N-terminal pro hormone of B-type natriuretic peptide
- NAS
National Academy of Sciences
- OPO
organ procurement organization
- REDCap
Research Electronic Data Capture
- STAR
Standard Transplant Analysis and Research
- TTE
transthoracic echocardiogram
- T4
thyroxine
- UAGA
Uniform Anatomical Gift Act
- UNOS
United Network for Organ Sharing
Footnotes
Disclosures: The authors have no conflicts of interest to disclose.
References
- 1.Abt PL, Marsh CL, Dunn TB, Hewitt WR, Rodrigue JR, Ham JM et al. Challenges to research and innovation to optimize deceased donor organ quality and quantity. Am J Transplant 2013;13(6):1400–1404. [DOI] [PubMed] [Google Scholar]
- 2.Gerber DA, Glazier A, Feng S. Research and innovation in the deceased donor. Am J Transplant 2014;14(3):505–506. [DOI] [PubMed] [Google Scholar]
- 3.Glazier AK, Heffernan KG. Addressing Barriers to Organ Donor Research-A Renewed Call for Regulatory Guidance. Kidney Int Rep 2021;6(9):2255–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glazier AK, Heffernan KG, Rodrigue JR. A Framework for Conducting Deceased Donor Research in the United States. Transplantation 2015;99(11):2252–2257. [DOI] [PubMed] [Google Scholar]
- 5.Mone T, Heldens J, Niemann CU. Deceased organ donor research: the last research frontier? Liver Transpl 2013;19(2):118–121. [DOI] [PubMed] [Google Scholar]
- 6.Rodrigue JR, Feng S, Johansson AC, Glazier AK, Abt PL. Deceased Donor Intervention Research: A Survey of Transplant Surgeons, Organ Procurement Professionals, and Institutional Review Board Members. Am J Transplant 2016;16(1):278–286. [DOI] [PubMed] [Google Scholar]
- 7.Ware LB, Koyama T, Billheimer D, Landeck M, Johnson E, Brady S et al. Advancing donor management research: design and implementation of a large, randomized, placebo- controlled trial. Ann Intensive Care 2011;1(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Academies of Sciences E, and Medicine. Opportunities for Organ Donor Intervention Research: Saving Lives by Improving the Quality and Quantity of Organs for Transplantation. Washington, DC: The National Academies Press, 2017. [PubMed] [Google Scholar]
- 9.Niemann CU, Feiner J, Swain S, Bunting S, Friedman M, Crutchfield M et al. Therapeutic Hypothermia in Deceased Organ Donors and Kidney-Graft Function. N Engl J Med 2015;373(5):405–414. [DOI] [PubMed] [Google Scholar]
- 10.Kasahara S, Sakata Y, Nochioka K, Miura M, Abe R, Sato M et al. Conversion formula from B-type natriuretic peptide to N-terminal proBNP values in patients with cardiovascular diseases. Int J Cardiol 2019;280:184–189. [DOI] [PubMed] [Google Scholar]