Abstract
Objective.
We investigated dynamics of inflammatory biomarkers in children with perinatally-acquired HIV (PHIV) who started antiretrovirals at age <3 years and achieved sustained virologic control (HIV plasma RNA<400 copies/mL).
Design.
This was a retrospective analysis of inflammatory biomarkers in children enrolled in a randomized trial of early (<3 years of age) PI-based versus NNRTI-based regimens (P1060), who achieved sustained virologic control and participated in a neurodevelopmental follow-up study (P1104s) between ages 5–11 years.
Methods.
We measured 20 inflammatory biomarkers using ELISA or chemiluminescence at onset of sustained virologic control (Tc) and at P1104s entry (Te).
Results.
The 213 participants had median ages of 1.2, 1.9, and 7.0 years at antiretroviral initiation, Tc, and Te, respectively, with 138 on PI-based and 74 on NNRTI-based regimens at Tc. Eighteen markers decreased and two increased from Tc to Te (Te-Tc). Biomarker subsets, particularly cytokines, the chemokine IP-10, and adhesion molecules sICAM-1 and sVCAM-1, correlated at Tc, Te, and Te-Tc. At Tc, higher biomarker levels were associated with younger age, female sex, HIV plasma RNA ≥750,000 copies/mL, lower nadir CD4+%, lower nadir weight z-scores, and NNRTI-based treatment. Greater Te-Tc biomarker declines were associated with younger age, male sex, higher Tc biomarker levels, lower nadir CD4+%, and NNRTI-based treatment. Duration of controlled viremia and nadir height Z-scores showed mixed associations.
Conclusions.
Biomarker expression showed substantial coordination. Most markers decreased after virologic control. Demographic and clinical variables associated with biomarker patterns were identified. Mechanistic studies of these biomarker patterns are needed to inform interventions to control inflammation.
Keywords: perinatal HIV infection, inflammatory biomarkers, children, antiretroviral therapy
Introduction
In recent years, persistent inflammation has become the major risk factor for morbidity and mortality in adults with human immunodeficiency virus (HIV)[1, 2]. High levels of soluble inflammatory markers have been associated with cardiovascular, renal, hepatic, metabolic and neurologic dysfunction, and decreased responses to vaccines[1–4]. Both acute and chronic HIV infection are characterized by increased inflammation that generally decreases after initiation of antiretroviral treatment (ART) without, however, declining to levels comparable to those observed in uninfected, age-matched controls[5–9]. The identity of the cells producing the inflammatory markers and the underlying mechanism(s) leading to persistent inflammation are not well understood. Although there are many conflicting reports on this subject, an emerging consensus is that both HIV replication and microbial translocation contribute to increased inflammation[10, 11], and early initiation of ART does not seem to mitigate the development of chronic inflammation[12, 13].
Little is known about the kinetics and health consequences of inflammation in children with perinatal HIV (PHIV). Previous studies demonstrated that older children and adolescents with PHIV have higher levels of inflammatory markers compared with age-matched healthy controls[11, 14, 15]. Studies also showed an association of inflammation with impaired neurocognitive development and mood disorders in children with PHIV[16–19].
We previously described an adverse association between inflammation and neurocognitive development in a population of virologically controlled children with PHIV[20]. To improve our understanding of inflammation in the context of PHIV, we conducted a new analysis, presented in this report, to investigate in greater depth the pattern of associations among biomarkers cross-sectionally and longitudinally and to assess the relationship of inflammatory biomarkers with demographic and HIV disease characteristics. The goal of this analysis was to improve our understanding of HIV pathogenesis in children with PHIV.
Participants and Methods
Participants and Study Design
Participants were children with PHIV enrolled in the P1060 (NCT00307151) ART study and P1104s (NCT02140255) neurodevelopmental follow-up sub-study in South Africa, Malawi, Uganda, and Zimbabwe[21, 22]. Children initiated nevirapine-based or lopinavir/ritonavir-based ART at age 2 months to 3 years per the P1060 protocol. Enrollment in P1104s occurred at age 5–11 years. During this interval, there were no study interventions, but ART could be adjusted per standard of care. Children were eligible for this analysis if they had at least one HIV-1 plasma RNA <400 copies/mL between 6–12 months prior to P1104s study entry (Te). Onset of sustained controlled viremia (Tc) was the date of the earliest plasma HIV-1 RNA <400 copies/mL with no subsequent consecutive measurements ≥400 copies/mL and no rolling 12-month period with more than one plasma HIV-1 RNA ≥1000 copies/mL prior to Te. P1104s entry. The parent study specified that VL should be measured every 6 months. A plasma HIV-1 RNA cutoff of 400 copies/mL was chosen because this was the lower limit of detection (LLOD) for the assay in use in 2005 when P1060 opened for enrollment. IRBs at participating sites approved the protocol; signed informed consent was obtained from participants’ parents/caregivers.
Biomarkers
Cryopreserved plasma samples collected during P1060 closest to and within six months of each of the two time-points of interest were identified. Levels of 20 biomarkers were included in this analysis: acute phase reactants (CRP); adhesion factors (sICAM-1, sVCAM-1); anti-inflammatory cytokines (IL-10); chemoattractants (fractalkine, MCP-1, MIP-1β, IP-10); pro-inflammatory cytokines (IFNγ, IFNα2, IL-1β, IL-6, TNFα); and markers of endothelial function (sE-selectin, sP-selectin, VEGF-A), matrix digestion (MMP9), monocyte activation (CD14, CD163), and pro-coagulant state (fibrinogen). Analytes were measured as per manufacturers’ instructions using the following commercial kits: MMP-9, IFNα2, IFNγ, IL-10, IL-1β, IL-6, IP-10, MCP-1, MIP-1β, TNFα, VEGF-A, CRP (LLOD = 1.33 pg/mL), sICAM-1, sVCAM-1, sE-selectin, sP-selectin (Mesoscale Discovery, chemiluminescence microarrays); fibrinogen, fractalkine (Millipore; ELISA); sCD14, sCD163 (R&D, ELISA).
Statistical methods
Biomarker levels at the two time points of interest were log10-transformed. For each biomarker, the change between time points was calculated as the difference in log10-transformed biomarker values.
Spearman rank-based correlations were calculated to assess correlations of each biomarker’s levels over time and correlations between biomarkers at a given time. To assess the correlation between biomarkers with respect to their change in values (Te-Tc), Spearman correlations were calculated based on the difference in log10-transformed biomarker values between time points. Based on the absolute value of the correlation coefficient (ρ), correlations were classified as strong (|ρ| ≥0.60), moderate (0.40 ≤|ρ| <0.60), weak (0.20 ≤|ρ| <0.40), or very weak (0 ≤|ρ| <0.20) [24]. All correlations described in the Results were positive, unless otherwise indicated.
Based on previous studies, we postulated that the level of biomarkers at Tc reflected peak inflammation and immune activation[23, 24]. We evaluated the potential role of demographic and clinical parameters on the peak level of inflammation and immune activation and on the difference between Tc and Te. For each biomarker, we fit separate multivariable parametric censored regression models with a normal distribution to estimate associations between participant characteristics and outcome measures corresponding to (a) log10-transformed biomarker levels at Tc and (b) the difference in log10-transformed biomarker levels between Tc and Te. This interval-censored regression approach was chosen to account for uncertainty in biomarker values when a measurement was above the upper limit of detection or below LLOD. For each regression model, covariates of interest included binary variables corresponding to sex, peak plasma HIV-1 RNA VL prior to Tc above 750,000 copies/mL, and ART regimen at Tc; and continuous variables corresponding to age at Tc, nadir CD4+% prior to Tc, and nadir height and weight World Health Organization (WHO) Z-scores prior to Tc. Covariates measured prior to Tc were obtained in P1060. For models involving changes in biomarker value from Tc to Te, covariates for biomarker concentration at Tc and duration of controlled viremia between Tc and Te were also included. For outcome measures corresponding to biomarker values at Tc, we estimated the difference (and 95% confidence interval) in log10 biomarker concentration values for a one-unit difference in the covariate value. For outcome measures corresponding to changes in biomarker values from Tc to Te, we estimated the relative increase (and 95% confidence interval) in the geometric mean of the ratio between biomarkers at Te and Tc for a one-unit difference in the covariate value. Associations with a p-value <0.05 are highlighted in the text. As this was a hypothesis-generating analysis, no adjustments were made for multiple comparisons.
Results
Participant characteristics
Of 246 study participants co-enrolled in P1060 and P1104s, 213 met inclusion criteria for this study and had ≥1 plasma sample available at Tc or Te for testing (Fig S1). Table 1 shows the demographic and HIV disease characteristics of the children included in the analysis. The study included similar numbers of boys and girls from sub-Saharan Africa. The median age was 1.2 years at ART initiation. Although most children achieved relatively rapid control of viremia following P1060 enrollment (median age = 1.6 years), approximately 25% of participants subsequently lost virologic control. The median age at Tc, defined as the onset of sustained controlled viremia prior to P1104s entry, was 1.9 years. The median duration of controlled viremia prior to Te was 5 years. At Tc, 138 children (65%) were on PI-containing ART, and all but one of the 74 remaining were on NNRTI-containing ART.
Table 1. Demographic and HIV disease characteristics of 213 children with PHIV.
|
Value* |
|
|---|---|
| Sex | |
| Male | 92 (43%) |
| Female | 121 (57%) |
| Age (yrs) at initiation of ARV | 1.2 (0.5, 2.6) |
| Age(yrs) at first controlled viremia onset | 1.6 (0.7, 2.9) |
| Age(yrs) at Tc | 1.9 (0.8, 3.7) |
| Age(yrs) at Te | 7.0 (5.7, 8.7) |
| Years of sustained controlled viremia before Te | 5.0 (3.6, 6.5) |
| Highest WHO stage prior to Tc | |
| WHO stage 1 | 22 (10%) |
| WHO stage 2 | 53 (25%) |
| WHO stage 3 | 123 (58%) |
| WHO stage 4 | 15 (7%) |
| Nadir weight WHO Z score prior to Tc** | −2.5 (−5.3, −0.6) |
| Nadir height WHO Z score prior to Tc | −1.5 (−4.0, 0.2) |
| Nadir CD4% prior to Tc | 15.1 (7.7, 25.3) |
| Peak VL (log-10) prior to Tc | 5.9 (5.2, 6.0) |
| <750,000 copies/mL | 85 (40%) |
| ≥750,000 copies/mL | 128 (60%) |
| ART regimen at time of Tc | |
| PI-based regimen | 138 (65%) |
| NNRTI-based regimen | 74 (35%) |
| Other | 1 (<1%) |
Value corresponds to median (10th percentile, 90th percentile) for continuous variables and frequency (percentage) for categorical variables.
Two participants are missing a weight Z-score measurement prior to controlled viremia onset.
Abbreviations: ARV=antiretrovirals; NNRTI= non-nucleoside reverse transcriptase inhibitor; PHIV = perinatal HIV; PI = protease inhibitor; Tc = onset of sustained controlled viremia; Te = time of P1104s entry; WHO = World Health Organization.
Kinetics of the plasma biomarkers
There were 184 participants with plasma samples available at Tc and 185 participants with plasma samples available at Te; 156 participants had plasma samples available at both time points. Median plasma concentrations at Te were lower than corresponding concentrations at Tc for 18 of the 20 cytokines, chemokines, growth factors, and other inflammatory biomarkers studied (Fig 1). Two biomarkers (sCD14 and fibrinogen) increased between Tc and Te. Differences between levels at Tc and Te were statistically significant for all biomarkers, with all p values <0.03. A description of biomarker levels at each time point is provided in Table S1.
Figure 1. Kinetics of plasma biomarkers in children with PHIV on effective ART.
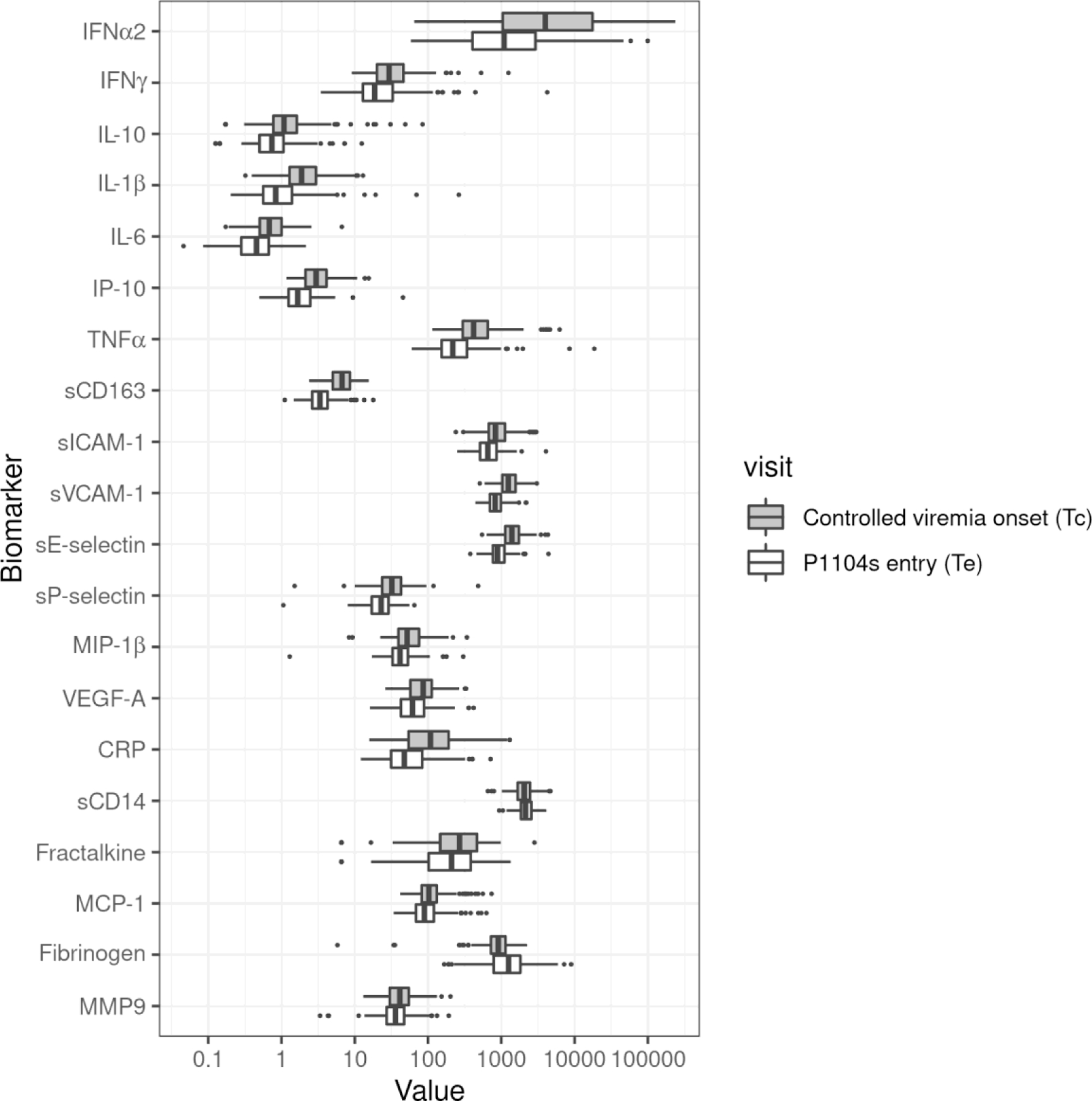
Data were derived from a total of 213 children with PHIV and HIV-1 plasma RNA (viral load; VL) <400 copies/mL for ≥9 months prior to P1104s entry, including 28 with samples at Tc only, 156 at Tc and Te, and 29 at Te only. Dark box plots show plasma concentrations at the time when children reached sustained controlled viremia (Tc) and light box plots at time of P1104s entry (Te). The order in which the analytes are listed is based on a previously published factor analysis[16]. Data are expressed in ng/ml of plasma for sCD14, sCD163, sICAM-1, sVCAM-1, sE-selectin, sP-selectin, CRP, fibrinogen and MMP9, and pg/ml for all other markers. Fibrinogen and sCD14 significantly increased from Tc to Te and all other markers significantly decreased.
Coordination of biomarker production
At Tc, Te, and Te-Tc, all of the cytokines, including IFNα2, IFNγ, IL-1β, IL-6, IL-10 and TNFα, showed moderate to strong pairwise correlations (Fig 2). An IFNγ-induced chemokine, IP-10, was strongly correlated with the cytokines at all time points and was thus included in the cytokine group in an extended cytokine group.
Figure 2. Plasma biomarker correlations in children with PHIV on effective ART.
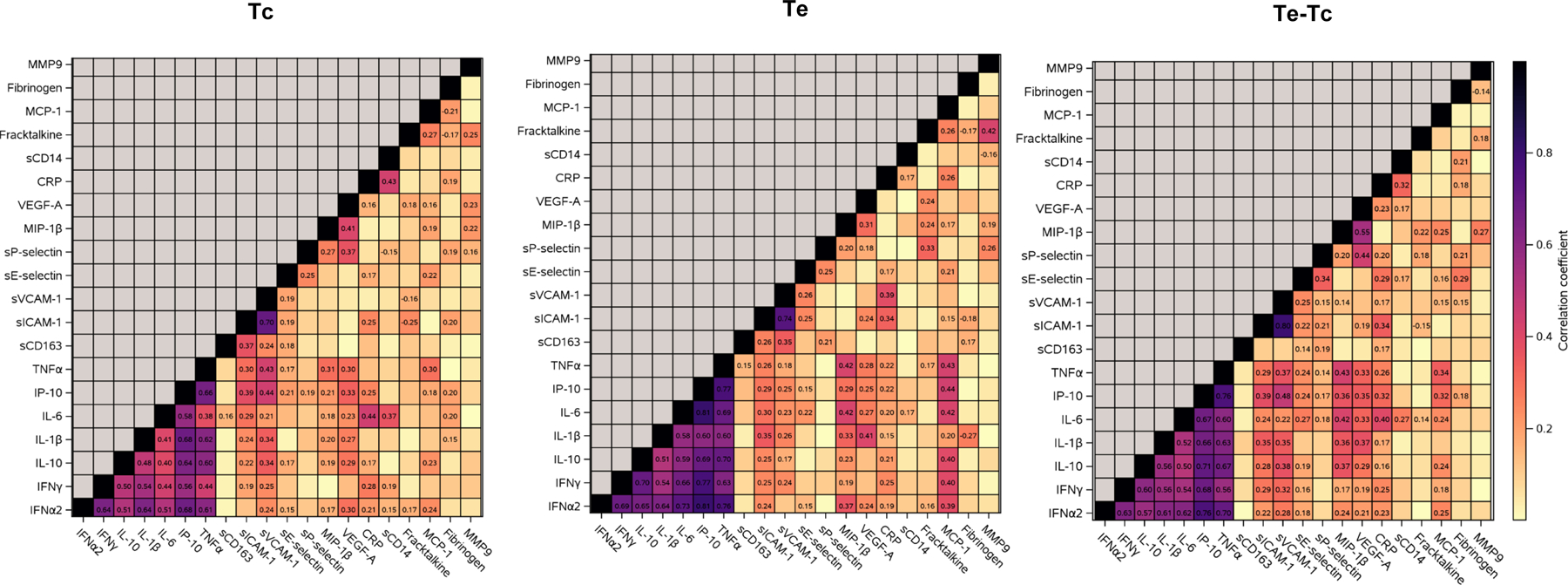
Data were derived from 213 children. Graphs represent heatmaps of pairwise Spearman correlations between the markers indicated on the abscissa and ordinate at the timepoints indicated on the graphs. Coefficients of correlation are displayed on the graphs for correlations with p<0.05. Tc indicates beginning of sustained controlled viremia; Te indicates entry in P1104s when the follow up plasma sample was analyzed.
The adhesion molecules sICAM-1 and sVCAM-1 were strongly correlated with each other and moderately to weakly correlated with members of the extended cytokine group at all time points. Both sICAM-1 and sVCAM-1 weakly correlated with the myeloid cell scavenger receptor sCD163 at Tc and Te. sICAM-1 also weakly correlated with the endothelial activation marker sE-selectin and the endothelial and platelet activation marker sP-selectin at various time points. sE-selectin and sP-selectin weakly correlated with each other at all time points.
At various time points, MIP-1β moderately to weakly correlated with analytes in the extended cytokine group, with VEGF-A, and with other chemokines, including fractalkine and MCP-1. CRP moderately to weakly correlated with markers in the extended cytokine group, with sCD14, and with adhesion factors and endothelial activation markers.
Associations between demographic and HIV disease characteristics and biomarker levels at Tc
Figure 3 shows associations between demographic and HIV disease characteristics and each biomarker at Tc from multivariable regression analyses. Female sex was associated with higher levels of four biomarkers in the extended cytokine group (IFNα2, IL-1β, IP-10, and TNFα) and two adhesion factors (sICAM-1 and sVCAM-1). Younger age at Tc was associated with higher levels of six of seven biomarkers in the extended cytokine group and with higher sVCAM-1, sE-selectin, and sP-selectin. Other notable associations included lower nadir CD4+% with higher levels of IL-6, sCD163, and sICAM-1; plasma HIV-1 RNA ≥750,000 copies/mL with higher IL-10, TNFα and sE-selectin; (3) NNRTI-containing ART with higher IFNγ, CRP and sCD14 and lower MIP-1β; and (4) lower nadir weight Z-score with higher IL-6, CRP, sICAM-1 and fibrinogen.
Figure 3. Association of demographic and HIV disease characteristics with biomarker levels at Tc.
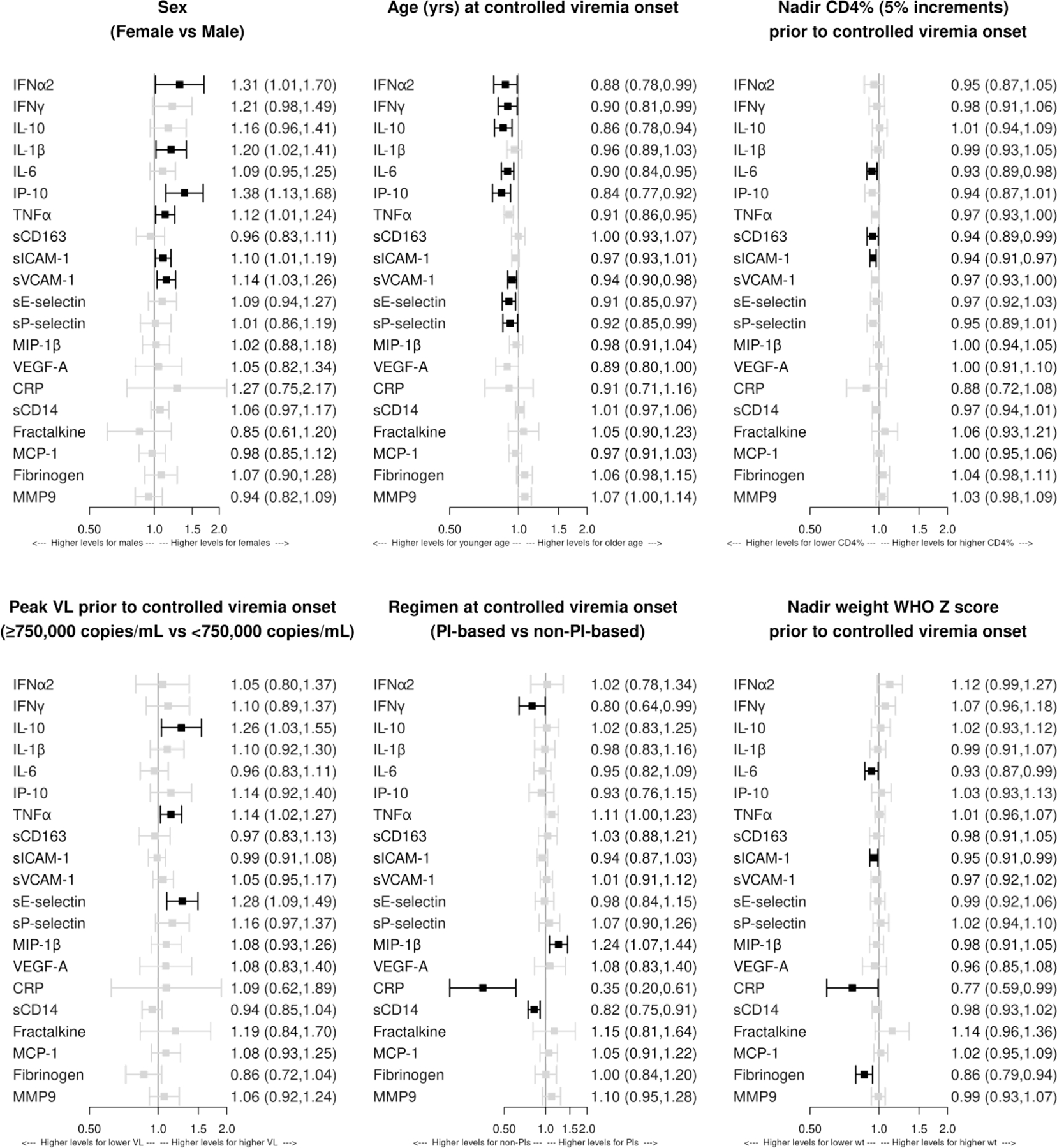
Effect sizes greater than 1.0 indicate higher biomarker concentrations at Tc (initiation of controlled viremia), on average, in the comparison group relative to the referent group. Separate multivariable models were fit for each biomarker. The following covariates were included in each model: sex, age at Tc, nadir CD4%, peak plasma HIV RNA copies/ml, antiretroviral regimen at Tc, and nadir height and weight WHO Z score. There were no associations with p<0.05 of biomarker levels at Tc with nadir WHO Z scores for height and, therefore, the panel was omitted. Dark boxes indicate associations with p<0.05.
Associations between demographic and HIV disease characteristics and the change in inflammation and activation markers during controlled viremia
Higher biomarker concentrations at Tc were associated with greater relative declines in biomarker concentrations from Tc to Te, except for fibrinogen and sCD14 for which higher concentrations at Tc were associated with smaller increases from Tc to Te (Fig 4). Additional notable associations included: (1) male sex with greater relative decreases in IFNα2, IFNγ, IL-1β, and IP-10; (2) younger age at Tc with greater relative decreases in IFNγ, sVCAM-1, fractalkine, and MCP-1; (3) lower nadir CD4+% with greater decrease in CRP; (4) non-PI-based regimen at Tc with greater relative decreases in TNFα, sE-selectin, sP-selectin, VEGF-A and fractalkine; (5) higher nadir height Z-score with greater relative decrease in sVCAM-1 and greater relative increase in fibrinogen; and (6) longer duration of controlled viremia between Tc and Te with greater relative decrease in sCD163, sICAM-1, and fractalkine. The peak plasma HIV-1 RNA pre-Tc and nadir weight Z-score were not associated with any Te-Tc changes in inflammatory biomarkers.
Figure 4. Association of demographic and HIV disease characteristics with changes in biomarker levels from Tc to Te.
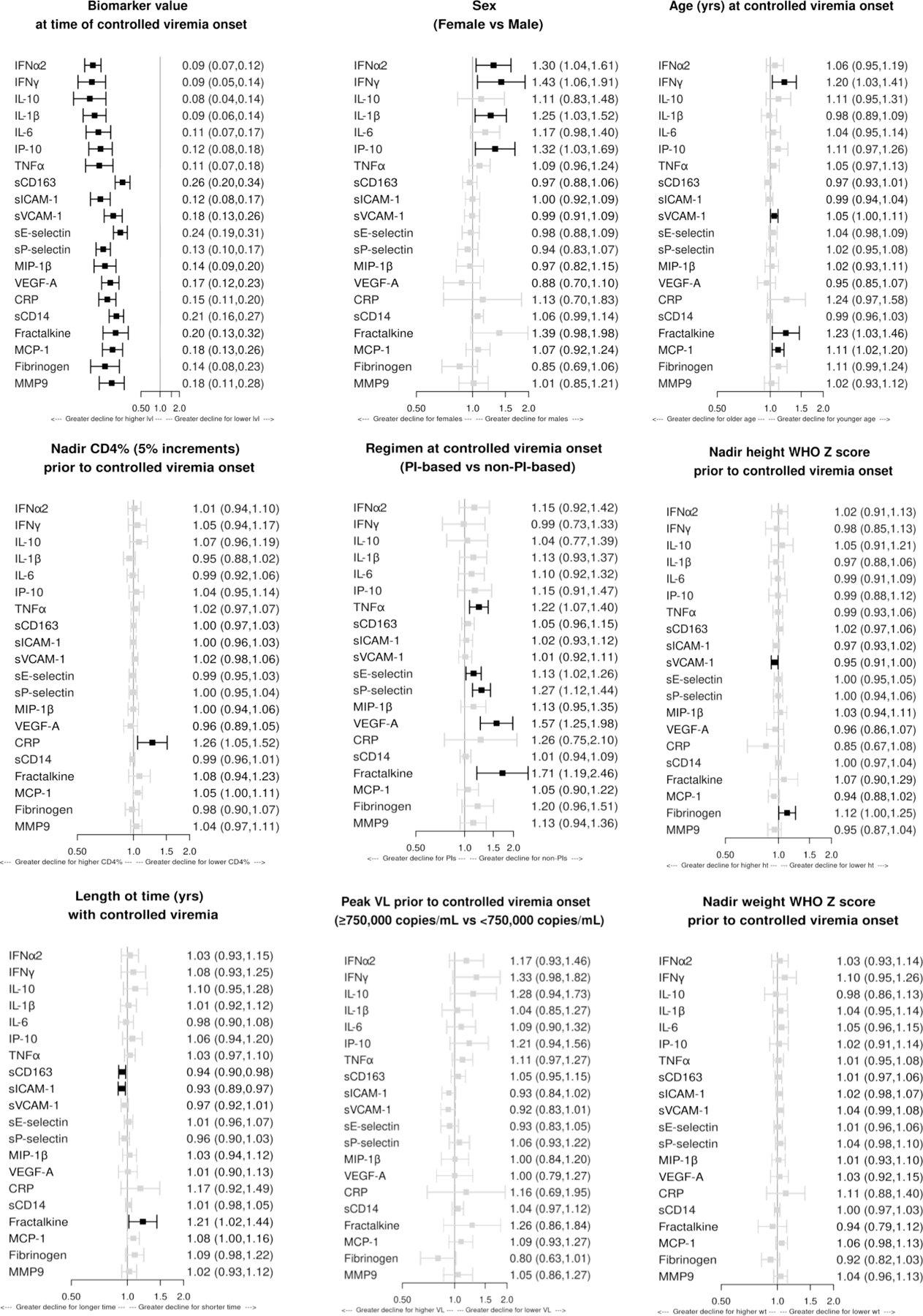
Changes were defined by the differences in biomarker concentrations from Tc to Te. Effect sizes greater than 1.0 indicate a lesser relative decline (or greater relative increase) in biomarker concentrations between Tc (initiation of controlled viremia) and Te (entry in P1104s) when follow up sample was collected, on average, in the comparison group relative to the referent group. Separate multivariable models were fit for each biomarker. The following covariates were included in each model: sex, age, antiretroviral regimen and biomarker concentration at Tc; nadir CD4%, height WHO Z score and weight WHO Z score; peak plasma HIV RNA copies/ml; and duration of controlled viremia from Tc to Te. Dark boxes indicate associations with p<0.05.
Discussion
We observed an almost universal decline in inflammatory biomarkers from Tc to Te in children with PHIV after control of viremia. This finding is in agreement with previous studies in adults with HIV[5–8]. While most studies have been conducted in adults, a few studies in children and adolescents, including participants with PHIV from sub-Saharan Africa, showed similar patterns[11, 14, 15]. Although our study did not include HIV-uninfected controls, it is reasonable to interpret the collective decrease in inflammatory biomarkers as representing a shift towards age- and sex-appropriate levels. Median levels of only two biomarkers, sCD14 and fibrinogen, increased from Tc to Te. sCD14 has been considered a marker of microbial translocation, but recent data have challenged this concept[25]. Thus, it is unclear if the increase in sCD14 concentration from Tc to Te indicates increased microbial translocation over time or some other process. Fibrinogen concentrations increased by approximately 5% from Tc to Te, which may reflect age-appropriate physiological changes[26, 27].
We observed patterns of correlations among cytokines, chemokines, and other inflammatory markers in children with PHIV that were generally consistent at the two time points of interest and the change between timepoints. The kinetic synchrony of markers that grouped themselves together suggests that secretion of these markers may be explained by a single or a few interconnected mechanisms. Notably, cytokines had very high correlation coefficients, suggesting that a single underlying process, such as viral replication in T-cells and macrophages, might account for their production. The extended cytokine group also had weaker but consistent correlations with integrins and chemokines (including sICAM-1, sVCAM-1, MIP-1β, and MCP-1, as well as VEGF-A and CRP) at Tc, Te, and Te-Tc. This is not surprising, since IL-1β, IL-6, IFNγ and/or TNFα stimulate the expression of these inflammatory markers.
sICAM-1, sVCAM-1, sE-selectin, and sP-selectin are expressed primarily by endothelial cells, although sP-selectin is also found on platelets, sICAM-1 on monocytes and lymphocytes, and sVCAM-1 on follicular dendritic cells. The coordinated decrease of sICAM-1 with s-VCAM-1, sE-selectin, and sP-selectin from Tc to Te suggests that reduction in endothelial cell activation contributed to the decrease. Notably, at both time points, sICAM-1 and sVCAM-1 also weakly correlated with sCD163 levels, suggesting an association of endothelial cell and macrophage activation consistent with previously reported findings in vitro and in adults with HIV[28]. At Tc and Te, sE-selectin weakly correlated with MCP-1 and sP-selectin weakly correlated with MIP-1β, fractalkine, VEGF-A, and/or MMP-9. In adults with HIV, increased levels of these markers indicate formation of atherosclerotic plaques, which have been recognized as significant contributors to morbidity and mortality in adults with HIV[29, 30]. Endothelial cell dysfunction has not been studied previously in children with PHIV and its clinical implications are not known. The decrease in inflammatory markers associated with endothelial dysfunction after effective ART suggests an improvement in endothelial function with virologic control, which is encouraging. Nevertheless, studies are needed to understand the clinical consequences of endothelial dysfunction in children with PHIV.
As expected, markers of greater severity of HIV disease before Tc were associated with higher inflammatory biomarker concentrations at Tc, including associations between lower nadir CD4+%, higher peak HIV plasma HIV-1 RNA, and lower nadir weight Z-scores with higher concentrations of IL-6, sCD163, sICAM-1, IL-10, TNFα, sE-selectin, CRP, sICAM-1, and/or fibrinogen. Perhaps more important was the finding that HIV disease characteristics before Tc had very limited association with the decay of inflammatory biomarkers. The corollary of this observation is that the severity of HIV disease before Tc might not impair the ability of children with PHIV to achieve lower levels of inflammation in the context of controlled HIV infection.
Older age at Tc was associated with lower levels of multiple cytokines and inflammatory biomarkers and with smaller relative decreases in IFNγ, sVCAM-1, fractalkine, and MCP-1. As these associations were opposite to the associations between markers of HIV disease severity and inflammatory biomarkers, the association between older age and lower biomarker levels was not likely explained by longer duration of uncontrolled HIV infection. The few pediatric studies that investigated changes in cytokine production with increasing age primarily focused on whole blood or mononuclear cell production of cytokines. These studies indicate that among the cytokines and chemokines included in our study, the production of TNFα in resting mononuclear cells decreases from 1 to 5 years of age, whereas the production of IFNγ and IP-10 does not change[31]. In the absence of normative age-specific data of plasma cytokines and chemokines, it is difficult to interpret the associations we observed between age and inflammatory biomarker levels.
Female sex was associated with higher cytokine and chemokine levels at Tc and smaller relative decreases from Tc to Te. Differences in immune responses between sexes at early, pre-pubertal ages have been previously described, including higher responses to childhood vaccination in females compared with males[32, 33]. This information is consistent with our observations since inflammatory responses to vaccines are the first step in the genesis of adaptive responses to vaccine antigens. The mechanism underlying the effect of sex on inflammation is incompletely understood. The X chromosome includes multiple genes and microRNAs associated with immune responses [34]. Recent studies have shown that 15–23% of X-linked human genes escape 2nd X chromosome inactivation, which may explain differences in inflammatory and immune responses between male and female children [33, 35].
Lopinavir/ritonavir-based and nevirapine-based ART were prescribed for 99% of the children in our study, reflecting the time when P1060 was conducted[21]. The use of PI-based ART was associated with lower concentrations of a few inflammatory markers at Tc. This might be explained by the fact that use of PI-based ART was associated with better virologic control compared with the use of NNRTI-based ART in P1060[21]. Since Tc in our study was defined by onset of sustained plasma HIV-1 RNA <400 copies/mL, it is conceivable that children on PI-based ART had greater virologic suppression compared to children on NNRTI-based ART, in turn leading to lower inflammation at Tc. In adults, suboptimal adherence to ART, even with HIV-1 plasma RNA maintained at <50 copies/mL, was associated with higher measures of inflammation, suggesting that even low levels of viral replication are sufficient to induce inflammation [36]. Conversely, after attainment of virologic control, use of PI-based ART was associated with smaller relative decreases from Tc to Te in several biomarkers of endothelial dysfunction. Importantly, the comparison of the Tc-Te decline in inflammatory markers between PI- and NNRTI-based ART was adjusted for marker levels at Tc, indicating that differences would be present even if the two groups had similar marker levels at Tc. This suggests that after achieving control of viral replication, PI-based ART may be associated with higher residual inflammation and/or endothelial dysfunction than NNRTI-based ART. To our knowledge, this is the first study investigating the differential effect of specific ARV regimens on inflammatory biomarkers in children with PHIV. Similar studies in adults with HIV have generated conflicting information [37–40]; however, a few studies showed that compared with NNRTI-based ART, PI-based therapy was associated with persistence of higher levels of inflammation, which is consistent with our findings [38, 41]. Moreover, there is a well-recognized association of PIs with insulin resistance and dyslipidemia resulting in increased inflammation and cardiovascular adverse events in adults with HIV[42–44]. Our results suggest that PIs may have a similar effect in children with PHIV. The association of PI-based ART with higher levels of residual inflammation after virologic control is achieved in children with PHIV is novel and underscores the importance of studying the effect of specific classes of ARV in this population.
The association between higher biomarker concentrations at Tc and greater relative decline in levels between Tc and Te needs to be interpreted with caution. This association may reflect the statistical phenomenon of regression to the mean, whereby more extreme values at one time point are more likely to be closer to the mean at later time points. However, we cannot rule out that a biological mechanism may contribute to this observation, e.g., higher levels of inflammatory markers at Tc may reflect higher HIV replication and more advanced HIV disease prior to Tc and greater potential for relative improvement in HIV replication and ongoing inflammation after initiation of sustained effective therapy. Future studies including higher precision measurements of viral replication and biomarker concentrations may provide further clarification.
Our study has limitations, most importantly the absence of an age-matched comparison group of children without HIV from the same geographic area. All children came from the same geographic area, where people with HIV generally have few comorbidities. The threshold for virologic control used in this study, HIV-1 plasma RNA <400 copies/mL, may have obscured low levels of persistent viral replication that might have confounded the patterns of inflammatory biomarkers and associations with demographic and clinical characteristics that we observed. Missing HIV-1 plasma RNA measurements after Tc were not taken into consideration in the analysis. Finally, due to the exploratory nature of this study, we did not correct for multiple comparisons, making it likely that some associations with p-values <0.05 may have been due to chance with no biological significance.
Our study provides the first comprehensive analysis of inflammatory factors in children with PHIV and how they relate to demographic and HIV disease characteristics. We showed a decrease in inflammation after control of viral replication, highlighted a high degree of coordination among subsets of biomarkers, and identified demographic and clinical variables associated with biomarker patterns. High levels of inflammation have been associated with specific pathologies in adults with HIV. While the significance of inflammation in children with PHIV is incompletely understood, previous studies have documented associations between inflammatory biomarkers and lowered performance on neurodevelopmental tasks [17–20]. Additional studies are needed to determine the potential effect of ongoing inflammation on neurologic, cardiovascular, renal, hepatic, and other outcomes in children with PHIV. The present study provides a first step toward future research into biological mechanisms underlying persistent inflammation and potential clinical interventions to control inflammation and improve long-term outcomes for children with HIV.
Supplementary Material
Acknowledgments:
We thank the study participants, parents and caregivers, and study personnel at each of the six sites for their invaluable contribution to this research. Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH), all components of the National Institutes of Health (NIH), under Award Numbers UM1 AI068616 (IMPAACT LOC), UM1 AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Source of Funding
This study was funded by the National Institute of Allergy and Infectious Diseases (NIAID) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH) under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), and by NICHD contract number HHSN275201800001I. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
Conflict of Interest
AW receives research grants from NIH, GSK, Merck and Janssen, and personal fees from Merck and Seqirus. Other authors do not have anything to declare.
References
- 1.Hunt PW, Lee SA, Siedner MJ. Immunologic Biomarkers, Morbidity, and Mortality in Treated HIV Infection. J Infect Dis 2016; 214 Suppl 2:S44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wada NI, Bream JH, Martinez-Maza O, Macatangay B, Galvin SR, Margolick JB, et al. Inflammatory Biomarkers and Mortality Risk Among HIV-Suppressed Men: A Multisite Prospective Cohort Study. Clin Infect Dis 2016; 63(7):984–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Letizia A, Eller MA, Polyak C, Eller LA, Creegan M, Dawson P, et al. Biomarkers of Inflammation Correlate With Clinical Scoring Indices in Human Immunodeficiency Virus-Infected Kenyans. J Infect Dis 2019; 219(2):284–294. [DOI] [PubMed] [Google Scholar]
- 4.Parmigiani A, Alcaide ML, Freguja R, Pallikkuth S, Frasca D, Fischl MA, et al. Impaired antibody response to influenza vaccine in HIV-infected and uninfected aging women is associated with immune activation and inflammation. PLoS One 2013; 8(11):e79816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castillo-Mancilla JR, Brown TT, Palella FJ Jr., Macatangay BJC, Breen EC, Jacobson LP, et al. Partial Normalization of Biomarkers of Inflammation and Immune Activation Among Virally Suppressed Men With HIV Infection and High ART Adherence. Open Forum Infect Dis 2020; 7(4):ofaa099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brochado-Kith O, Martinez I, Berenguer J, Medrano LM, Gonzalez-Garcia J, Garcia-Broncano P, et al. Near normalization of peripheral blood markers in HIV-infected patients on long-term suppressive antiretroviral therapy: a case-control study. AIDS 2020; 34(13):1891–1897. [DOI] [PubMed] [Google Scholar]
- 7.Wada NI, Jacobson LP, Margolick JB, Breen EC, Macatangay B, Penugonda S, et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS 2015; 29(4):463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiefer EM, Hoover DR, Shi Q, Dusingize JC, Sinayobye JD, Anastos K. Longitudinal evaluation of markers of inflammation in HIV-positive and HIV-negative Rwandan women. HIV Med 2018; 19(10):734–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burdo TH, Lentz MR, Autissier P, Krishnan A, Halpern E, Letendre S, et al. Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J Infect Dis 2011; 204(1):154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Younas M, Psomas C, Reynes C, Cezar R, Kundura L, Portales P, et al. Microbial Translocation Is Linked to a Specific Immune Activation Profile in HIV-1-Infected Adults With Suppressed Viremia. Front Immunol 2019; 10:2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dirajlal-Fargo S, Yu J, Albar Z, Sattar A, Mahtab S, Jao J, et al. Monocyte activation and gut barrier dysfunction in South African youth on antiretroviral therapy and their associations with endothelial dysfunction. AIDS 2020; 34(11):1615–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hellmuth J, Slike BM, Sacdalan C, Best J, Kroon E, Phanuphak N, et al. Very Early Initiation of Antiretroviral Therapy During Acute HIV Infection Is Associated With Normalized Levels of Immune Activation Markers in Cerebrospinal Fluid but Not in Plasma. J Infect Dis 2019; 220(12):1885–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sereti I, Krebs SJ, Phanuphak N, Fletcher JL, Slike B, Pinyakorn S, et al. Persistent, Albeit Reduced, Chronic Inflammation in Persons Starting Antiretroviral Therapy in Acute HIV Infection. Clin Infect Dis 2017; 64(2):124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eckard AR, Rosebush JC, Lee ST, O’Riordan MA, Habib JG, Daniels JE, et al. Increased Immune Activation and Exhaustion in HIV-infected Youth. Pediatr Infect Dis J 2016; 35(12):e370–e377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Generoso M, Alvarez P, Kravietz A, Mwamzuka M, Marshed F, Ahmed A, et al. High soluble CD163 levels correlate with disease progression and inflammation in Kenyan children with perinatal HIV-infection. AIDS 2020; 34(1):33–38. [DOI] [PubMed] [Google Scholar]
- 16.Kapetanovic S, Giganti MJ, Abzug MJ, Lindsey JC, Sirois PA, Montepiedra G, et al. Plasma biomarker factors associated with neurodevelopmental outcomes in children with perinatal HIV infection and controlled viremia. AIDS 2021; 35(9):1375–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ananworanich J, Kerr SJ, Jaimulwong T, Vibol U, Hansudewechakul R, Kosalaraksa P, et al. Soluble CD163 and monocyte populations in response to antiretroviral therapy and in relationship with neuropsychological testing among HIV-infected children. J Virus Erad 2015; 1(3):196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benki-Nugent SF, Martopullo I, Laboso T, Tamasha N, Wamalwa DC, Tapia K, et al. High Plasma Soluble CD163 During Infancy Is a Marker for Neurocognitive Outcomes in Early-Treated HIV-Infected Children. J Acquir Immune Defic Syndr 2019; 81(1):102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kapetanovic S, Leister E, Nichols S, Miller T, Tassiopoulos K, Hazra R, et al. Relationships between markers of vascular dysfunction and neurodevelopmental outcomes in perinatally HIV-infected youth. AIDS (London, England) 2010; 24(10):1481–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kapetanovic S, Giganti MJ, Abzug MJ, Lindsey JC, Sirois PA, Montepiedra G, et al. Plasma biomarker factors associated with neurodevelopmental outcomes in children with perinatal HIV infection and controlled viremia. AIDS 9000; Publish Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Violari A, Lindsey JC, Hughes MD, Mujuru HA, Barlow-Mosha L, Kamthunzi P, et al. Nevirapine versus Ritonavir-Boosted Lopinavir for HIV-Infected Children. New England Journal of Medicine 2012; 366(25):2380–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boivin MJ, Chernoff M, Fairlie L, Laughton B, Zimmer B, Joyce C, et al. African Multi-Site 2-Year Neuropsychological Study of School-Age Children Perinatally Infected, Exposed, and Unexposed to Human Immunodeficiency Virus. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2020; 71(7):e105–e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baker JV, Neuhaus J, Duprez D, Kuller LH, Tracy R, Belloso WH, et al. Changes in inflammatory and coagulation biomarkers: a randomized comparison of immediate versus deferred antiretroviral therapy in patients with HIV infection. Journal of acquired immune deficiency syndromes (1999) 2011; 56(1):36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pastor L, Urrea V, Carrillo J, Parker E, Fuente-Soro L, Jairoce C, et al. Dynamics of CD4 and CD8 T-Cell Subsets and Inflammatory Biomarkers during Early and Chronic HIV Infection in Mozambican Adults. Frontiers in Immunology 2018; 8(1925). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Voeght A, Maes N, Moutschen M. sCD14 is not a bona-fide biomarker of microbial translocation in HIV-1-infected Africans living in Belgium. AIDS 2016; 30(6):921–924. [DOI] [PubMed] [Google Scholar]
- 26.APPEL IM, GRIMMINCK B, GEERTS J, STIGTER R, CNOSSEN MH, BEISHUIZEN A. Age dependency of coagulation parameters during childhood and puberty. Journal of Thrombosis and Haemostasis 2012; 10(11):2254–2263. [DOI] [PubMed] [Google Scholar]
- 27.Ignjatovic V, Ilhan A, Monagle P. Evidence for age-related differences in human fibrinogen. Blood Coagulation & Fibrinolysis 2011; 22(2). [DOI] [PubMed] [Google Scholar]
- 28.Temu TM, Polyak SJ, Zifodya JS, Wanjalla CN, Koethe JR, Masyuko S, et al. Endothelial Dysfunction Is Related to Monocyte Activation in Antiretroviral-Treated People With HIV and HIV-Negative Adults in Kenya. Open Forum Infect Dis 2020; 7(10):ofaa425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Camaré C, Pucelle M, Nègre-Salvayre A, Salvayre R. Angiogenesis in the atherosclerotic plaque. Redox biology 2017; 12:18–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Apostolakis S, Spandidos D. Chemokines and atherosclerosis: focus on the CX3CL1/CX3CR1 pathway. Acta pharmacologica Sinica 2013; 34(10):1251–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Decker M-L, Gotta V, Wellmann S, Ritz N. Cytokine profiling in healthy children shows association of age with cytokine concentrations. Scientific Reports 2017; 7(1):17842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flanagan KL, Fink AL, Plebanski M, Klein SL. Sex and Gender Differences in the Outcomes of Vaccination over the Life Course. Annu Rev Cell Dev Biol 2017; 33:577–599. [DOI] [PubMed] [Google Scholar]
- 33.Fathi A, Addo MM, Dahlke C. Sex Differences in Immunity: Implications for the Development of Novel Vaccines Against Emerging Pathogens. Front Immunol 2020; 11:601170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghorai A, Ghosh U. miRNA gene counts in chromosomes vary widely in a species and biogenesis of miRNA largely depends on transcription or post-transcriptional processing of coding genes. Front Genet 2014; 5:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Libert C, Dejager L, Pinheiro I. The X chromosome in immune functions: when a chromosome makes the difference. Nat Rev Immunol 2010; 10(8):594–604. [DOI] [PubMed] [Google Scholar]
- 36.Castillo-Mancilla JR, Brown TT, Erlandson KM, Palella FJ Jr., Gardner EM, Macatangay BJ, et al. Suboptimal Adherence to Combination Antiretroviral Therapy Is Associated With Higher Levels of Inflammation Despite HIV Suppression. Clin Infect Dis 2016; 63(12):1661–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelesidis T, Tran TT, Stein JH, Brown TT, Moser C, Ribaudo HJ, et al. Changes in Inflammation and Immune Activation With Atazanavir-, Raltegravir-, Darunavir-Based Initial Antiviral Therapy: ACTG 5260s. Clin Infect Dis 2015; 61(4):651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Massanella M, Ouchi D, Marfil S, Llibre JM, Puertas MC, Buzon MJ, et al. Different plasma markers of inflammation are influenced by immune recovery and cART composition or intensification in treated HIV infected individuals. PLoS One 2014; 9(12):e114142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McComsey GA, Kitch D, Daar ES, Tierney C, Jahed NC, Melbourne K, et al. Inflammation markers after randomization to abacavir/lamivudine or tenofovir/emtricitabine with efavirenz or atazanavir/ritonavir. AIDS (London, England) 2012; 26(11):1371–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hileman CO, Kinley B, Scharen-Guivel V, Melbourne K, Szwarcberg J, Robinson J, et al. Differential Reduction in Monocyte Activation and Vascular Inflammation With Integrase Inhibitor-Based Initial Antiretroviral Therapy Among HIV-Infected Individuals. J Infect Dis 2015; 212(3):345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borges AH, O’Connor JL, Phillips AN, Ronsholt FF, Pett S, Vjecha MJ, et al. Factors Associated With Plasma IL-6 Levels During HIV Infection. J Infect Dis 2015; 212(4):585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holmberg SD, Moorman AC, Williamson JM, Tong TC, Ward DJ, Wood KC, et al. Protease inhibitors and cardiovascular outcomes in patients with HIV-1. Lancet 2002; 360(9347):1747–1748. [DOI] [PubMed] [Google Scholar]
- 43.Tsiodras S, Mantzoros C, Hammer S, Samore M. Effects of protease inhibitors on hyperglycemia, hyperlipidemia, and lipodystrophy: a 5-year cohort study. Arch Intern Med 2000; 160(13):2050–2056. [DOI] [PubMed] [Google Scholar]
- 44.Moyle G, Carr A. HIV-associated lipodystrophy, metabolic complications, and antiretroviral toxicities. HIV clinical trials 2002; 3(1):89–98. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


