Abstract
The development of donor-specific antibodies (DSA) after lung transplantation is common and results in adverse outcomes. In kidney transplantation, Belatacept has been associated with a lower incidence of DSA, but experience with Belatacept in lung transplantation is limited. We conducted a 2-center pilot randomized controlled trial of de novo immunosuppression with Belatacept after lung transplantation to assess the feasibility of conducting a pivotal trial. Twenty-seven participants were randomized to Control (Tacrolimus, Mycophenolate Mofetil, and prednisone, n = 14) or Belatacept-based immunosuppression (Tacrolimus, Belatacept, and prednisone until day 89 followed by Belatacept, Mycophenolate Mofetil, and prednisone, n = 13). All participants were treated with rabbit anti-thymocyte globulin for induction immunosuppression. We permanently stopped randomization and treatment with Belatacept after 3 participants in the Belatacept arm died compared to none in the Control arm. Subsequently, 2 additional participants in the Belatacept arm died for a total of 5 deaths compared to none in the Control arm (log rank p = 0.016). We did not detect a significant difference in DSA development, acute cellular rejection, or infection between the 2 groups. We conclude that the investigational regimen used in this study is associated with increased mortality after lung transplantation.
INTRODUCTION
Lung transplantation is the ultimate treatment for patients with advanced lung disease, and approximately 4,500 patients undergo lung transplantation annually worldwide (1). The median survival after lung transplantation is 6 years, and the leading cause of death beyond the first year is chronic lung allograft dysfunction (CLAD) (1). Although the exact pathogenesis of CLAD is unknown, clinical risk factors have been identified. These include acute cellular rejection (ACR), lymphocytic bronchiolitis (LB), antibody-mediated rejection (AMR), and the development of donor-specific antibodies (DSA) to mismatched human leukocyte antigens (HLA) (2–7). The development of DSA is an independent risk factor for ACR, LB, and DSA directly cause AMR (4, 5). Furthermore, the development of DSA has been identified as an independent risk factor for death (6).
Cellular immune responses were recognized early in the history of transplantation as the primary barrier to organ acceptance. Therapeutic suppression of T-cell function and proliferation made organ transplantation clinically feasible. However, rejection remains a persistent obstacle to better long-term outcomes, especially among lung transplant recipients. Furthermore, a significant proportion of lung transplant recipients treated with conventional immunosuppression develop DSA early after transplantation. In a prospective multicenter observational study, 36% of lung recipients developed DSA within 120 days of transplantation (8). In other studies, the incidence of DSA in the first year after transplantation has been as high as 61% (6). This illustrates that conventional immunosuppression does not sufficiently suppress alloantibody production or its effects on the allograft.
T-cell activation requires 2 synergistic signals (9–11). The first is recognition of antigen bound by MHC molecules on antigen presenting cells by the T-cell receptor. The second signal comprises engagement of co-stimulatory molecules CD28 and CTLA4 expressed on T-cells to their cognate ligands CD80 and CD86 expressed on antigen presenting cells to modulate T-cell activity. In the absence of co-stimulatory signals, T-cells become anergic or undergo apoptosis (12, 13). Belatacept, a CTLA4-Ig fusion protein that binds CD80 and CD86 thereby blocking CD28 co-stimulatory signals, is approved for the prevention of kidney transplant rejection. In a multicenter randomized controlled trial (RCT), kidney recipients treated with Belatacept had significantly better patient and allograft survival than those treated with Cyclosporine, and there was no difference in the incidence of serious adverse events (SAE) or serious infections between the groups (14). Furthermore, recipients treated with Belatacept were significantly less likely to develop DSA (14). Based on these data, we hypothesized that Belatacept would inhibit the development of DSA after lung transplantation, and that this would result in better freedom from CLAD and survival. We performed a pilot RCT to assess the feasibility of conducting a large scale RCT because experience with Belatacept in lung transplantation is limited (15–18).
METHODS
Study Design and Medical Regimen
We performed a pilot 2-center open label phase II RCT to assess the feasibility of conducting a phase III multicenter RCT examining the efficacy and safety of Belatacept in lung transplantation. The study protocol and additional supporting information may be found online in the Supporting Information section. The study protocol was approved by the sites’ Institutional Review Boards (IRBs). The primary endpoint of this pilot study was the feasibility metric of randomizing 80% of eligible patients within 4 hours of completion of transplantation (Table 1). Secondary endpoints are listed in Table 1. We also sought to estimate the effect size of Belatacept on different clinical endpoints in this pilot study to inform the design and power calculations of a future clinical trial. We enrolled patients after listing for transplantation and randomized eligible participants after transplantation using a computer-generated block randomization method with a 1:1 ratio. Participants were randomized to either the Belatacept arm or the Control arm. In the Belatacept arm, participants were treated with Belatacept, Tacrolimus, and prednisone starting on day 0 through day 89. On day 90, Tacrolimus was replaced by Mycophenolate Mofetil (MMF), and Belatacept and prednisone were continued through day 365. In the Control arm, participants were treated with Tacrolimus, MMF, and prednisone from day 0 through day 365. In both study arms, Tacrolimus was initiated enterally or sublingually within the first 48 hours after transplantation and dosed to target a trough blood level of 8–15 ng/mL if kidney function was normal or 4–8 ng/mL if kidney function was impaired. MMF was dosed at 1 g twice daily. The first dose of Belatacept was given after transplant on day 0 at 10 mg/kg and subsequent doses were given at 10 mg/kg on days 7, 14, 28, 56, 84, then at 5 mg/kg on days 112, 140, 168, 196, 224, 252, 280, 308, 336 and 364. All participants were treated with rabbit anti-thymocyte globulin (ATG) 1 mg/kg on days 0, 1, and 2 for induction immunosuppression. The first dose of Belatacept was given 12 hours after the first dose of ATG in participants randomized to Belatacept to avoid thrombotic complications. All participants were treated with methylprednisolone 500 mg intravenously before perfusion of the allograft during the transplant surgery. After transplant, participants were treated with methylprednisolone 0.5 mg/kg intravenously twice daily for 6 doses, then prednisone 0.5 mg/kg orally daily through day 14, then 0.2 mg/kg daily through day 30, then 0.1 mg/kg daily through day 180, then 5 mg daily through day 365. CMV seronegative recipients of seropositive donors or CMV seropositive recipients were treated with valganciclovir for CMV prophylaxis through day 365. CMV seronegative recipients of seronegative donors were treated with acyclovir for herpes and varicella prophylaxis. All participants received prophylaxis against Pneumocystis jirovecii. Antifungal prophylaxis was tailored according to culture results from bronchoscopy specimens.
Table 1.
Study endpoints.
| Primary Endpoint |
| Feasibility metric of randomizing 80% of eligible patients within 4 hours of completion of transplantation |
| Secondary Endpoints |
| Enrollment of 50% of eligible patients at the 2 centers |
| Retention of 75% of randomized patients on the protocol |
| Development of DSA* with mean fluorescence intensity (MFI) ≥ 2000 |
| Death |
| Re-transplantation |
| Acute cellular rejection grade ≥ A1 |
| Acute cellular rejection grade ≥ A2 |
| Lymphocytic bronchiolitis grade ≥ B1R |
| DSA with mean fluorescence intensity (MFI) ≥ 4000 |
| DSA immunoglobulin G (IgG) subclasses |
| Complement-binding (C1q-positive) and activating (C3d) DSA |
| Definite antibody-mediated rejection based on the ISHLT definition |
| Probable antibody-mediated rejection based on the ISHLT definition |
| Chronic lung allograft dysfunction (CLAD) defined as Bronchiolitis Obliterans Syndrome stage 1 or Restrictive Allograft Syndrome |
| CLAD-free survival |
| Confirmed bacterial infection requiring antibiotic treatment |
| Cytomegalovirus (CMV) infection requiring antiviral treatment |
| Confirmed community-acquired respiratory viral (CARV) infection |
| Chronic kidney disease stage 3 as estimated by the Cockcroft-Gault equation |
| Kidney function as estimated by the Cockcroft-Gault equation at 6 and 12 months after randomization |
| Malignancy excluding squamous cell and basal cell skin cancer |
| Post-transplant lymphoproliferative disease (PTLD) |
| Progressive multifocal leukoencephalopathy (PML) |
| Diabetes mellitus requiring medical treatment |
| Systemic hypertension requiring medical treatment |
| Hypercholesterolemia requiring medical treatment |
DSA: donor-specific antibodies to mismatched human leukocyte antigens.
Details of safety monitoring and study oversight are provided online in the Supporting Information section and the study protocol. The study was registered on ClinicalTrials.gov (NCT03388008).
Testing
All participants underwent HLA typing using next generation sequencing (NGS) or reverse sequence specific oligonucleotide probes. Participants were tested for HLA antibodies using the single antigen bead assay before listing for transplantation. Donors were accepted only if the virtual crossmatch was negative, and a direct flow cytometry crossmatch was performed at the time of transplantation. Donors also underwent HLA typing at the sites after transplantation using NGS. Participants were followed at the sites’ clinics every 1–2 weeks in the first 3 months after transplantation then monthly for the duration of the study. Bronchoscopy with bronchoalveolar lavage (BAL) and transbronchial lung biopsies was performed on days 28, 84, 112, 168, 252, and 365 and if subjects developed signs or symptoms of allograft dysfunction. The study used a core HLA lab at Baylor University Medical Center for all post-transplant HLA antibody tests, and the study HLA investigator and technicians were blinded to participants’ study arm assignment. Samples were tested using the single antigen bead assay, and DSA was defined as reactivity with a mean fluorescence intensity (MFI) ≥ 2,000. Participants were tested for DSA on days 0, 10, 28, 56, 84, 112, 168, 252, and 365 and if they developed signs or symptoms of allograft dysfunction.
Statistical Analysis
This pilot study was designed to assess the feasibility of conducting a large-scale phase III RCT. We planned to assess the randomization rate to inform the design of a future trial and aimed to randomize 40 subjects as this would generate 95% confidence intervals of ± 12% for the randomization rate. We did not expect that this pilot study would detect a statistically significant difference in clinical outcomes between the 2 groups because of the small sample size. We compared baseline characteristics between the 2 groups using t-tests (or Wilcoxon-Rank sum tests if the data were not normally distributed) and chi-square tests. We used the Kaplan-Meier method to report freedom from DSA, ACR, and survival and compared the outcomes between the 2 groups using the log rank test. We conducted the statistical analyses using SPSS and Prism and considered p < 0.05 statistically significant. We conducted all analyses according to the intention to treat principle.
RESULTS
We began enrollment on 12/1/2019, and the first participant was randomized on 1/2/2020. As of 5/30/2021, 49 participants were enrolled, and 27 were randomized: 13 were randomized to Belatacept and 14 were randomized to Control (Figure 1). All subjects who were eligible for randomization were randomized within 4 hours of completion of transplantation. Baseline characteristics of the randomized participants are shown in Table 2. Between 1/2/2020 and 5/30/2021, 3 participants randomized to Belatacept died compared to none of the participants randomized to Control. After the 3rd death occurred on 5/30/2021, we halted enrollment and randomization and notified the Data Safety Monitoring Board (DSMB) and the Food and Drug Administration (FDA). The DSMB convened a meeting on 6/3/2021 to discuss study status. During the meeting, we proposed permanently terminating enrollment, randomization, and treatment and proposed ongoing follow-up of study participants for safety and efficacy reasons. The DSMB agreed that enrollment, randomization, and treatments should be permanently terminated, and ongoing follow-up was appropriate. Participants who were in the Belatacept arm were then converted to standard of care immunosuppression with Tacrolimus, MMF, and prednisone. Between 6/3/2021 and 7/29/2021, 2 additional subjects randomized to Belatacept died. As of 8/30/2021, 5 of the 13 subjects randomized to Belatacept died compared to none of the 14 randomized to Control (Figure 2, log rank p = 0.016). The first 3 deaths were due to COVID-19, restrictive allograft syndrome (RAS), and hemothorax. The subject who died of RAS developed rhinovirus/enterovirus infection with acute hypoxemic respiratory failure 6 weeks prior to the diagnosis of RAS. The subject who died of a hemothorax received the third dose of Belatacept hours before the onset of the hemothorax. The final 2 deaths were due to suspected pulmonary embolism and post-transplant lymphoproliferative disease (PTLD) 6 days and 52 days after conversion to standard of care immunosuppression, respectively. The subject who developed PTLD was seropositive for Epstein Barr Virus before transplantation. The pathology was monomorphic B-cell PTLD which was positive for Epstein Barr Encoding Region (EBER) by in situ hybridization. The subject who died of a suspected pulmonary embolism had suffered a pelvic fracture and died suddenly at home. All deaths were deemed to be possibly related to the investigational regimen meaning that there was a reasonable possibility that death may have been caused by the investigational regimen.
Figure 1.
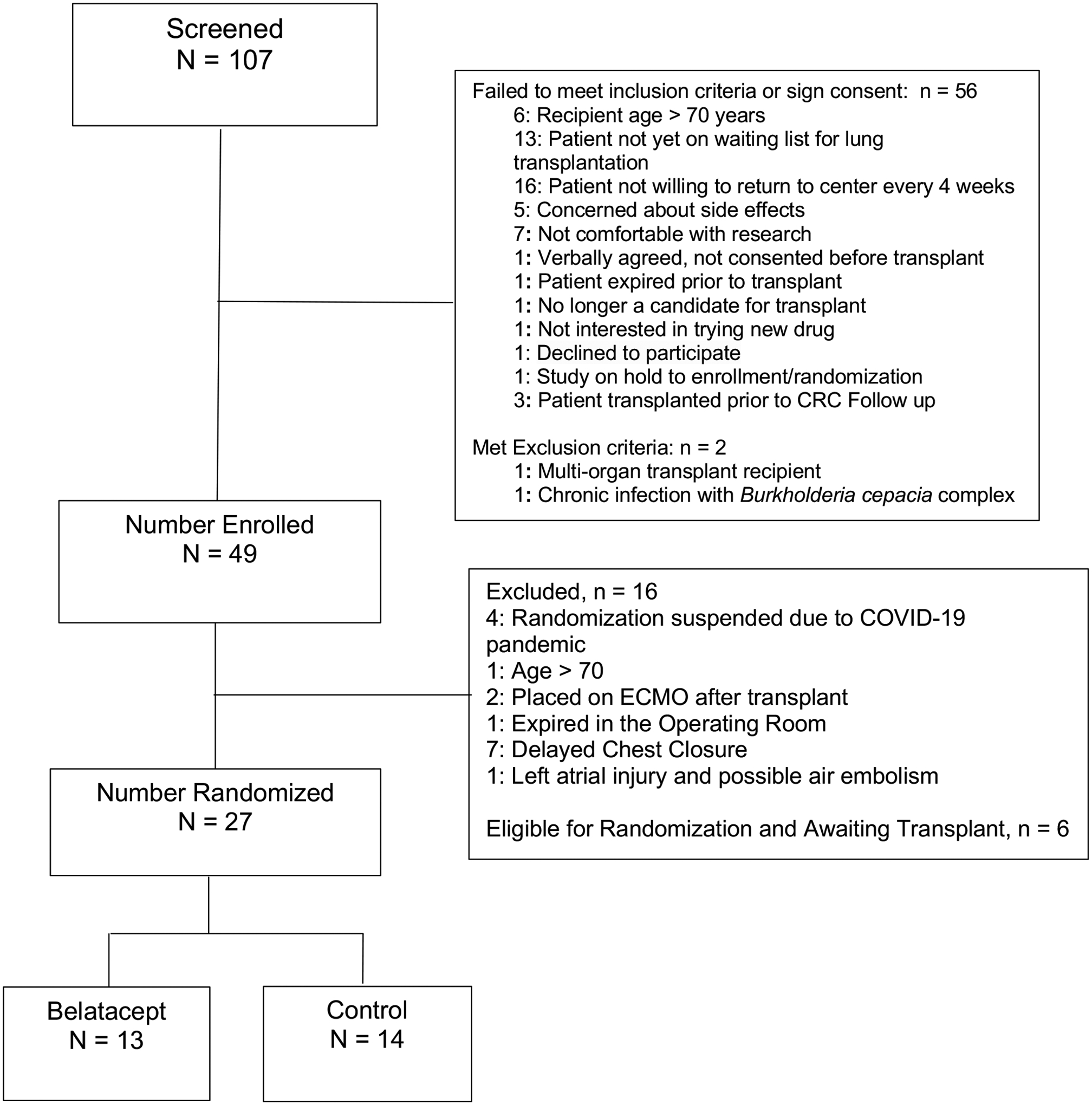
Study consort diagram.
Table 2.
Baseline characteristics of randomized participants.
| Belatacept N = 13 |
Control N = 14 |
p value | |
|---|---|---|---|
| Recipient age, mean ± SD | 57.8 ± 7.5 | 59.5 ± 11.8 | 0.655 |
| Recipient gender | 0.785 | ||
| Female, n (%) | 4 (31%) | 5 (36%) | |
| Male, n (%) | 9 (69%) | 9 (64%) | |
| Recipient race | 0.586 | ||
| Black, n (%) | 1 (8%) | 2 (14%) | |
| White, n (%) | 12 (92%) | 12 (86%) | |
| Recipient ethnicity | 0.957 | ||
| Hispanic, n (%) | 1 (8%) | 1 (7%) | |
| Not Hispanic, n (%) | 12 (92%) | 13 (93%) | |
| Diagnosis leading to transplantation | 0.202 | ||
| Interstitial lung disease, n (%) | 6 (46%) | 8 (57%) | |
| Chronic obstructive pulmonary disease, n (%) | 4 (31%) | 0 | |
| Pulmonary arterial hypertension, n (%) | 0 | 2 (14%) | |
| Bronchiectasis, n (%) | 1 (8%) | 1 (7%) | |
| Sarcoidosis, n (%) | 0 | 1 (7%) | |
| Other, n (%) | 2 (15%) | 2 (14%) | |
| Operation | 0.315 | ||
| Single lung transplant, n (%) | 1 (8%) | 3 (21%) | |
| Bilateral lung transplant, n (%) | 12 (92%) | 11 (79%) |
Figure 2.
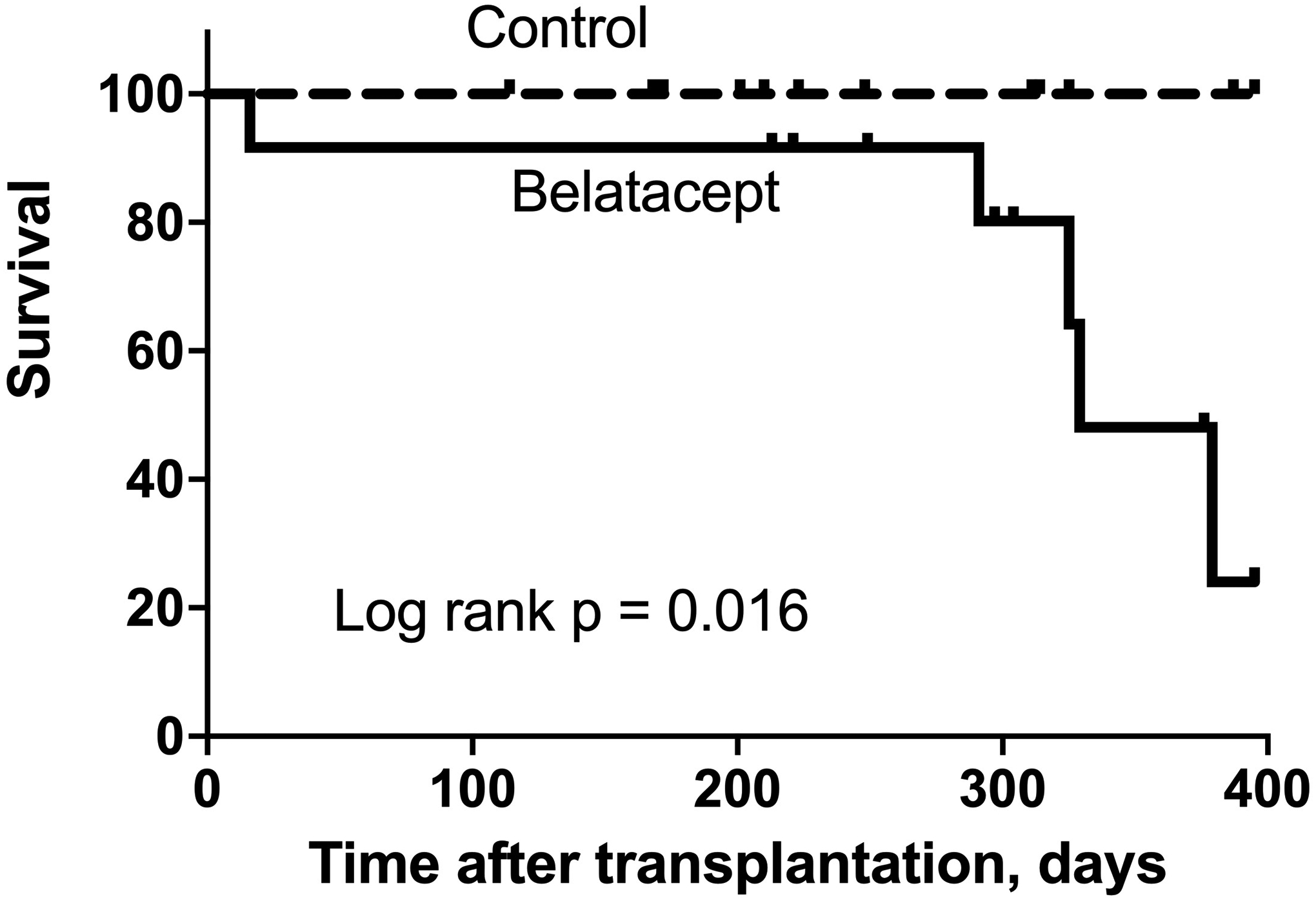
Patient survival. There was a significant survival difference between the Control group and the Belatacept group (log rank p = 0.016).
Using an MFI threshold ≥ 2,000 to identify DSA, 6 of the 13 participants randomized to Belatacept and 7 of the 14 randomized to Control developed DSA (p= 0.842), and there was no difference in freedom from DSA between the 2 groups (Figure 3A, log rank p = 0.908). There was also no significant difference in the DSA class between the 2 groups (p = 0.131): among those in the Belatacept arm, 3 developed DSA only to class I HLA, 1 developed DSA only to class II HLA, 2 developed DSA to class I and II HLA, and 3 developed DSA to HLA-DQ; among those in the Control arm, 5 developed DSA only to class II HLA, 2 developed DSA to class I and II HLA, and 5 developed DSA to HLA-DQ (Figure 3B). Using an MFI threshold ≥ 4,000 to identify DSA, 3 of the 13 participants randomized to Belatacept and 4 of the 14 randomized to Control developed DSA (p = 0.745), and there was no difference in freedom from DSA between the 2 groups (Figure 3C, log rank = 0.803). There was also no significant difference in the DSA class between the 2 groups (p = 0.745): among those in the Belatacept arm, 3 developed DSA only to class II HLA, and 3 developed DSA to HLA-DQ; among those in the Control arm, 4 developed DSA only to class II HLA, and 3 developed DSA to HLA-DQ (Figure 3D). Finally, 3 participants randomized to Belatacept and 4 randomized to Control developed C1q-positive DSA (p = 0.745).
Figure 3.
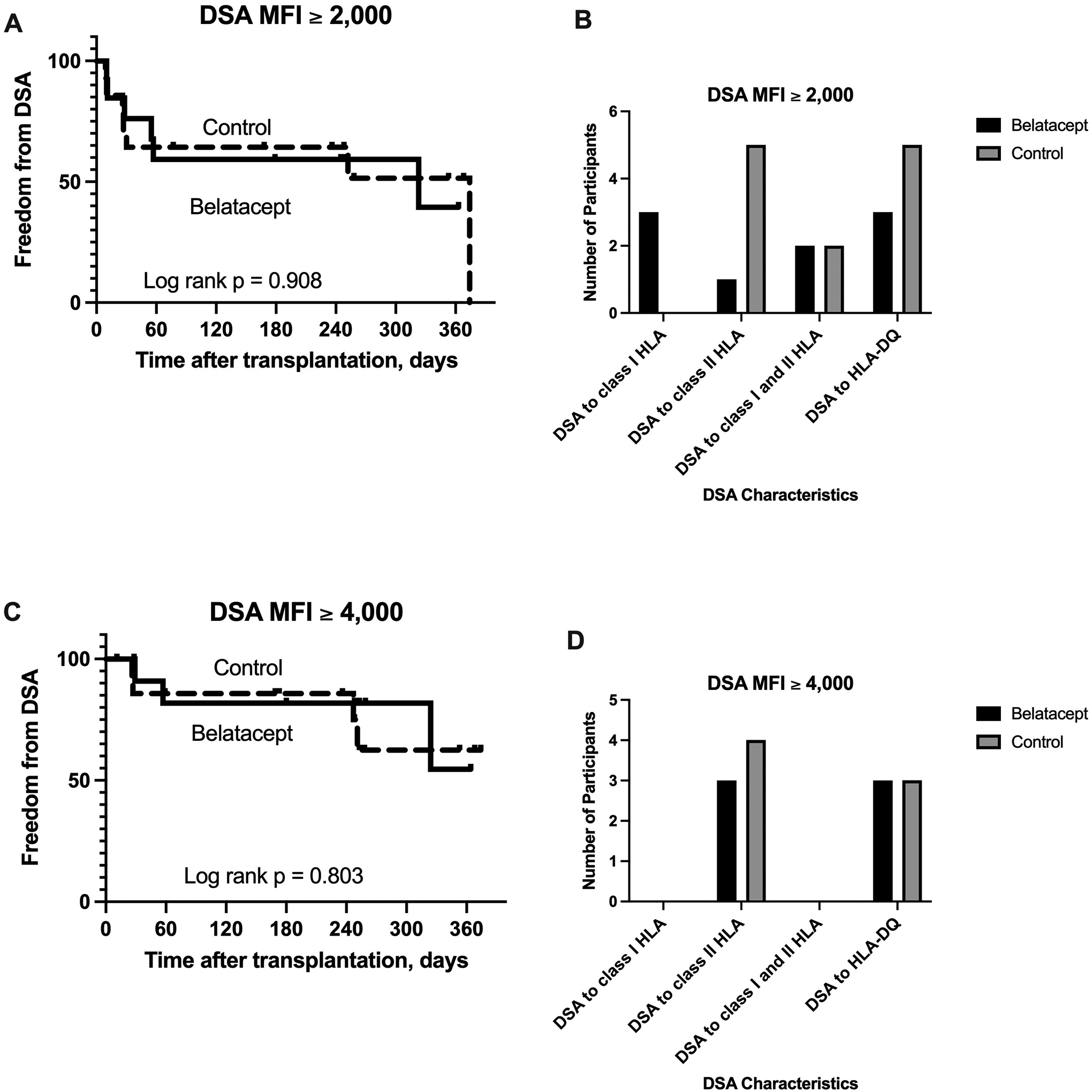
The development of donor-specific antibodies (DSA) to mismatched human leukocyte antigens (HLA). There was no significant difference in freedom from the development of DSA between the 2 groups.
During the follow-up period, there was no significant difference in the number of transbronchial lung biopsies performed in each group (p = 0.676). Those in the Belatacept arm underwent a median 4 transbronchial lung biopsies (mean ± SD = 3.9 ± 2.3), and those in the Control arm underwent a median 4 transbronchial lung biopsies (mean ± SD = 4.3 ± 2.1). There were no episodes of ACR grade A2 or higher during the follow-up period in either study arm. Four participants randomized to Belatacept developed at least 1 episode of ACR grade A1 compared to 5 participants randomized to Control (p = 0.785), and there was no significant difference in freedom from ACR grade A1 between the 2 groups (Figure 4A, log rank p = 0.702). Two participants in each group had more than 1 episode of ACR grade A1 during the follow-up period (p = 0.936). One participant in the Belatacept arm and 2 in the Control arm developed lymphocytic bronchiolitis grade B1R (p = 0.586), and there was no significant difference in freedom from lymphocytic bronchiolitis between the 2 groups (Figure 4B, log rank p = 0.634). There were no episodes of AMR in either group during the study period.
Figure 4.
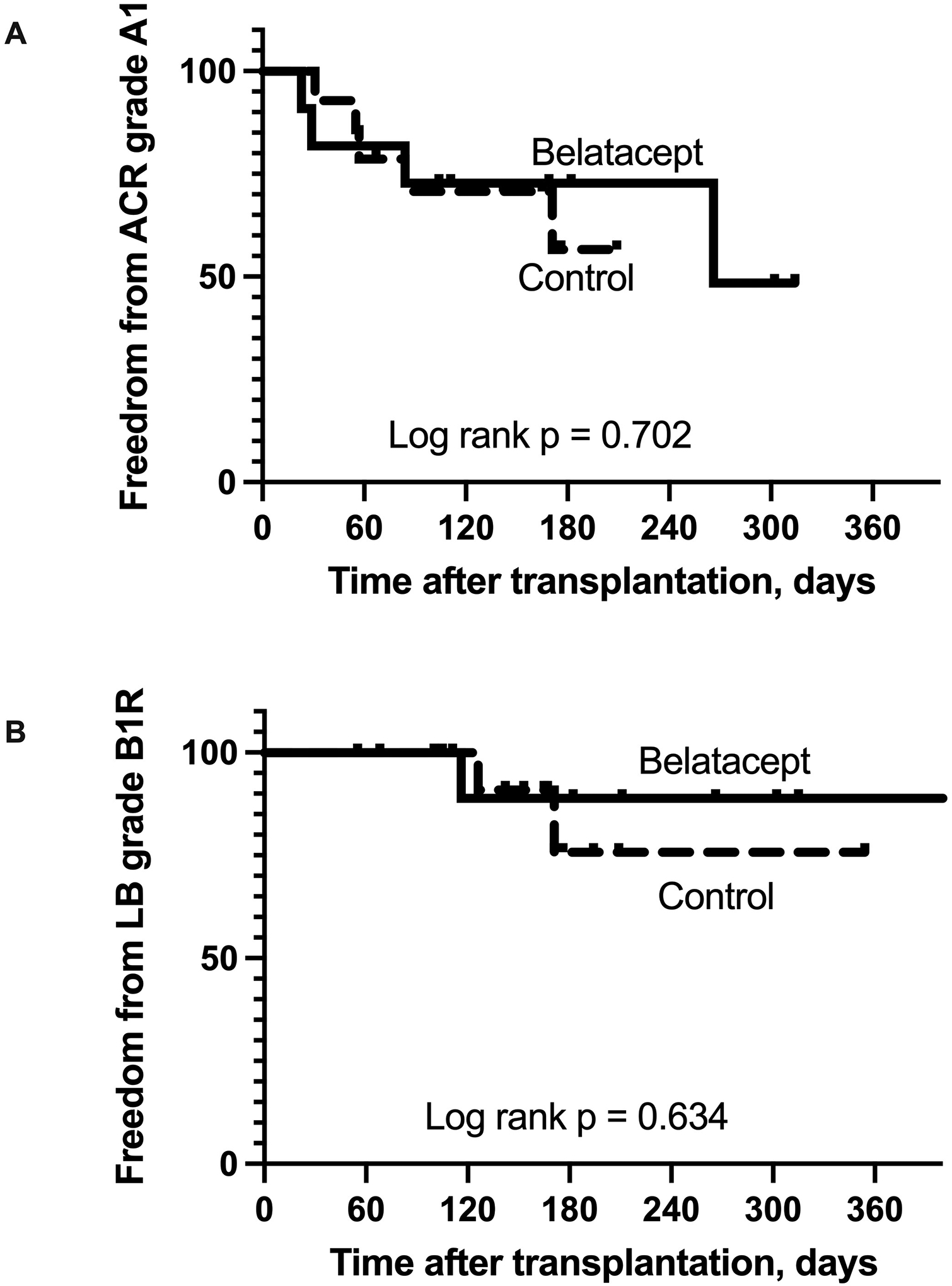
Acute cellular rejection and lymphocytic bronchiolitis. There was no significant difference in freedom from acute cellular rejection (ACR) or lymphocytic bronchiolitis (LB) between the 2 groups.
Eleven participants randomized to Belatacept and 9 randomized to Control had at least 1 infection during the follow-up period (Table 3, p = 0.228). In total, there were 24 infections in the Belatacept group and 26 in the Control group. Bacterial respiratory tract infections were most common; 5 participants in the Belatacept arm had 8 bacterial infections, and 6 participants in the Control arm had 9 bacterial infections (Table 3, p = 0.816). Two participants randomized to Belatacept and 3 randomized to Control developed COVID-19 infection (p = 0.686). This was fatal in 1 participant randomized to Belatacept whereas the remaining participants recovered without apparent sequelae.
Table 3.
Infections.
| Belatacept N = 13 |
Control N = 14 |
p value* | |||
|---|---|---|---|---|---|
| N affected | N events | N affected | N events | ||
| Any infection | 11 | 24 | 9 | 26 | 0.228 |
| Cytomegalovirus viremia | 4 | 5 | 2 | 3 | 0.303 |
| Community-acquired respiratory virus | 3 | 3 | 4 | 5 | 0.745 |
| COVID-19 | 2 | 2 | 3 | 3 | 0.686 |
| Bacterial respiratory tract infection | 5 | 8 | 6 | 9 | 0.816 |
| Bacterial urinary tract infection | 1 | 1 | 0 | 0 | 0.290 |
| Fungal respiratory tract infection | 3 | 4 | 6 | 6 | 0.276 |
the number of affected participants is compared between the 2 groups.
During the follow-up period, 10 participants randomized to Belatacept and 10 randomized to Control experienced at least 1 SAE (p = 0.745). The most common SAE was acute hypoxemic respiratory failure. Six participants randomized to Belatacept experienced 12 episodes of acute hypoxemic respiratory failure; 1 participant experienced 6 episodes of respiratory failure. Four participants randomized to Control experienced 4 episodes of respiratory failure (Table 4). Common causes of respiratory failure in both arms included respiratory infection, early post-operative respiratory failure, and airway complications. Other SAE are listed in Table 4; only events that met the definition of SAE are listed here.
Table 4.
Serious adverse events.
| Belatacept N = 13 |
Control N = 14 |
|||
|---|---|---|---|---|
| N affected | N events | N affected | N events | |
| Acute hypoxemic respiratory failure | 6 | 12* | 4 | 4 |
| Infection | ||||
| COVID-19 | 2 | 2 | 3 | 3 |
| Cytomegalovirus viremia | 2 | 2 | 1 | 1 |
| Parainfluenza virus infection | 0 | 0 | 1 | 1 |
| Pneumonia | 1 | 2 | 0 | 0 |
| Surgical site infection | 1 | 1 | 1 | 1 |
| Hemoptysis | 1 | 1 | 0 | 0 |
| Hemothorax | 1 | 1 | 0 | 0 |
| Atrial fibrillation | 0 | 0 | 2 | 2 |
| Dysphagia | 0 | 0 | 1 | 1 |
| Nausea/vomiting | 2 | 2 | 0 | 0 |
| Acute rejection | 0 | 0 | 1 | 1 |
| Polytrauma and fractures | 1 | 1 | 0 | 0 |
| Post-transplant lymphoproliferative disease | 1 | 1 | 0 | 0 |
| Headache | 1 | 1 | 0 | 0 |
| Syncope | 0 | 0 | 1 | 1 |
| Acute kidney injury | 0 | 0 | 2 | 2 |
| Limb ischemia | 0 | 0 | 1 | 1 |
| Venous thromboembolism | 2 | 2 | 0 | 0 |
1 subject in the Belatacept arm experienced 6 episodes of acute hypoxemic respiratory failure.
DISCUSSION
This pilot RCT is the first to examine the role of Belatacept in lung transplantation. The results demonstrate a significantly higher mortality among participants randomized to the investigational regimen. It is difficult to understand why these subjects had a higher mortality because causes of death were variable, and the sample size was small. Viruses may have contributed to 3 deaths suggesting that the investigational regimen may significantly impair immune responses to viral infection although this is speculative. It is important to note that our data are limited to the investigational regimen we used comprising ATG induction, Tacrolimus withdrawal, and this Belatacept dosing schedule. In designing this trial, we were concerned about the risk of early ACR that was seen in kidney transplantation. Therefore, we included ATG induction to ameliorate the risk of ACR, and in fact, we did not detect an increased risk of ACR. Obviously, we are unable to discern whether this was because of ATG induction or the maintenance immunosuppressive regimen. In addition, we did not identify a difference in the development of DSA or infections between the 2 groups. The lack of a clear association between Belatacept and ACR, DSA, or infection makes it challenging to determine whether this regimen resulted in “over-immunosuppression” or “under-immunosuppression.” Indeed, such a conclusion may be overly simplistic as lung transplant recipients are inherently complex with diverse pre- and post-transplant co-morbidities that may impact outcomes.
Immunosuppression after lung transplantation has typically been based on findings from clinical studies in kidney transplantation, and Tacrolimus, the only FDA-approved drug in lung transplantation, was approved in 2021. Single-center retrospective studies of Belatacept in lung transplantation have generally supported its use as a rescue treatment for calcineurin inhibitor toxicity although there are case reports of fulminant allograft failure and severe ACR after conversion to Belatacept (15–18). There are numerous differences between these studies and ours including study design and eligibility criteria. We used Belatacept as de novo immunosuppression immediately after transplantation and used ATG induction for all subjects. In contrast, many patients included in previous studies had undergone transplantation months or years earlier, and the risk of serious complications of immunosuppression is likely related to the timepoint after transplantation. Nonetheless, it is noteworthy that patients included in previous reports are inherently at higher risk of complications because of the toxicities that led to the initiation of Belatacept. Our results are similar to those reported by a multicenter RCT in liver transplantation which demonstrated increased mortality and allograft loss in 2 of 3 Belatacept groups compared to standard of care (19). In this RCT of Belatacept in liver transplantation, all Belatacept groups had higher rates of ACR and viral and fungal infections (19). We did not observe an increased risk of ACR or infection in our study, but it is possible that our data underestimate the true incidence of these events in the Belatacept arm because of the excess mortality and shorter duration of follow-up among subjects who died. The early termination of randomization in our study is similar to other complex clinical trials using Belatacept after kidney or kidney-pancreas transplantation that stopped randomization because of high rates of ACR or allograft thrombosis (20–22).
This study has important limitations including the possibility of alpha error given the small sample size and short duration of follow-up. The study was not powered to detect differences in clinical outcomes. Thus, the lack of differences in ACR, DSA, and infection between the 2 groups may be due to the small sample size. Furthermore, the findings are limited to this investigational regimen with Tacrolimus withdrawal and this Belatacept dosing schedule in combination with ATG induction. Nevertheless, we conclude that this regimen is associated with increased mortality after lung transplantation. These findings underscore the need for carefully designed and monitored clinical trials in lung transplantation.
Supplementary Material
ACKNOWLEDGMENTS
This study was funded by a grant from the National Heart, Lung, and Blood Institute (HL138186) and Bristol Myers Squibb through an Investigator Sponsored Research program (IM103-387).
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. MA is a member of the Scientific Advisory Board and has received an honorarium from One Lambda, Inc and has received grant funding from NHLBI and Bristol Myers Squibb; CB has received grant funding from NHLBI; PD has received grant funding from NHLBI; RRH has received grant funding from NHLBI and Bristol Myers Squibb and Mallinckrodt, RRH has received consulting fees from CareDx, Natera, and Transmedics; HJH has received grant funding from NHLBI, Bristol Myers Squibb, CareDx, CSL Behring, consulting fees from Atara Biotherapeutics and Regeneron Pharmaceuticals, speaker fees from Boehringer Ingelheim and serves on an advisory board for CareDx; DK has received grant funding and consulting fees from Compass Therapeutics and has pending patent application number 15/611,557; KS has received grant funding from NHLBI; BM has received grant funding from NHLBI; AG has received grant funding from NIAID and NHLBI, Royalties from UCLA School of Medicine and Quark Biopharmaceuticals. The other authors have no conflicts of interest to disclose.
ABBREVIATIONS
- ACR
acute cellular rejection
- AMR
antibody-mediated rejection
- ATG
anti-thymocyte globulin
- BAL
bronchoalveolar lavage
- CLAD
chronic lung allograft dysfunction
- DSA
donor-specific antibodies
- DSMB
Data Safety Monitoring Board
- FDA
Food and Drug Administration
- HLA
human leukocyte antigens
- LB
lymphocytic bronchiolitis
- MHC
major histocompatibility complex
- MMF
mycophenolate mofetil
- RAS
restrictive allograft syndrome
- RCT
randomized-controlled trial
- SAE
serious adverse event
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Chambers DC, Cherikh WS, Harhay MO, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-sixth adult lung and heart-lung transplantation report – 2019; Focus theme: Donor and recipient size match. J Heart Lung Transplant 2019; 38: 1042–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burton CM, Iversen M, Carlsen J, et al. Acute cellular rejection is a risk factor for bronchiolitis obliterans syndrome independent of post-transplant baseline FEV1. J Heart Lung Transplant 2009; 28: 888–93. [DOI] [PubMed] [Google Scholar]
- 3.Glanville AR, Aboyoun CL, Havryk A, et al. Severity of lymphocytic bronchiolitis predicts long-term outcome after lung transplantation. Am J Respir Crit Care Med 2008; 177: 1033–40. [DOI] [PubMed] [Google Scholar]
- 4.Witt CA, Gaut JP, Yusen RD, et al. Acute antibody-mediated rejection after lung transplantation. J Heart Lung Transplant 2013; 32: 1034–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Girnita AL, Duquesnoy R, Yousem SA, et al. HLA-specific antibodies are risk factors for lymphocytic bronchiolitis and chronic lung allograft dysfunction. Am J Transplant 2005; 5: 131–8. [DOI] [PubMed] [Google Scholar]
- 6.Le Pavec J, Suberbielle C, Lamrani L, et al. De-novo donor-specific anti-HLA antibodies 30 days after lung transplantation are associated with a worse outcome. J Heart Lung Transplant 2016; 35: 1067–77. [DOI] [PubMed] [Google Scholar]
- 7.Tikkanen JM, Singer LG, Kim SJ, et al. De novo DQ donor-specific are associated with chronic lung allograft dysfunction after lung transplantation. Am J Respir Crit Care Med 2016; 194: 596–606. [DOI] [PubMed] [Google Scholar]
- 8.Hachem RR, Kamoun M, Budev M, et al. Human leukocyte antigens antibodies after lung transplantation: Primary results of the HALT study. Am J Transplant 2018; 18: 2285–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janeway CA, Bottomly K. Signals and signs for lymphocyte responses. Cell 1994; 76: 275–85. [DOI] [PubMed] [Google Scholar]
- 10.Sayegh MH, Turka LA. The role of T-cell costimulatory activation pathways in transplant rejection. N Engl J Med 1998; 338: 1813–21. [DOI] [PubMed] [Google Scholar]
- 11.Ford ML, Adams AB, Pearson TC. Targeting co-stimulatory pathways: Transplantation and autoimmunity. Nat Rev Nephrol 2014; 10: 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gimmi CD, Freeman GJ, Gribben JG, Gray G, Nadler LM. Human T-cell clonal anergy is induced by antigen presentation in the absence of B7 costimulation. Proc Natl Acad Sci USA 1993; 90: 6586–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noel PJ, Boise LH, Green JM, Thompson CB. CD28 costimulation prevents cell death during primary T cell activation. J Immunol 1996; 157: 636–42. [PubMed] [Google Scholar]
- 14.Vincenti F, Rostaing L, Grinyo J, et al. Belatacept and long-term outcomes in kidney transplantation. N Engl J Med 2016; 374: 333–43. [DOI] [PubMed] [Google Scholar]
- 15.Timofte I, Terrin M, Barr E, et al. Belatacept for renal rescue in lung transplant patients. Transplant Int 2016; 29: 453–63. [DOI] [PubMed] [Google Scholar]
- 16.Iasella CJ, Winstead RJ, Moore CA, et al. Maintenance Belatacept-based immunosuppression in lung transplantation recipients who failed calcineurin inhibitors. Transplantation 2018; 102: 171–77. [DOI] [PubMed] [Google Scholar]
- 17.Brugière O, Cazes A, Champion L, et al. Fulminant acute respiratory distress syndrome after calcineurin inhibitor-Belatacept conversion in a lung transplant recipient. Transplantation 2018; 102: e255–56. [DOI] [PubMed] [Google Scholar]
- 18.Nachiappan A, Fallah T, Willert R, Chojnowski D, Deshpande C, Courtwright A. Severe acute cellular rejection with high-grade lymphocytic bronchiolitis following transition from Tacrolimus to Belatacept in a lung transplantation recipient: A case report. Transplant Proc 2021, In Press. [DOI] [PubMed] [Google Scholar]
- 19.Klintmalm GB, Feng S, Lake JR, et al. Belatacept-based immunosuppression in de novo liver transplant recipients: 1-year experience from a phase II randomized study. Am J Transplant 2014; 14: 1817–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newell KA, Mehta AK, Larsen CP, et al. Lessons learned: Early termination of a randomized trial of calcineurin inhibitor and corticosteroid avoidance using belatacept. Am J Transplant 2017; 17: 2712–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stock PG, Mannon RB, Armstrong B, et al. Challenges of calcineurin inhibitor withdrawal following combined pancreas and kidney transplantation: Results of a prospective, randomized clinical trial. Am J Transplant 2020; 20: 1668–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mannon RB, Armstrong B, Stock PG, et al. Avoidance of CNI and steroids using belatacept – Results of the Clinical Trials in Organ Transplantation 16 Trial. Am J Transplant 2020; 20: 3599–3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


