Abstract
Poly(γ-d-glutamic acid) (PGA)-producing strains of Bacillus species were investigated to determine their ability to contribute to reducing the amount of ammonium nitrogen in liquid manures and their ability to convert some of the ammonium into this polyamino acid as a transient depot for nitrogen. Organisms that do these things should help solve the serious environmental problems which are caused by the use of large amounts of liquid manure resulting from intensified agriculture; these problems are mainly due to the high content of ammonium nitrogen. Bacillus licheniformis ATCC 9945 and Bacillus subtilis were able to grow in liquid manure and to produce PGA in the presence of sodium gluconate. On artificial liquid manure these two strains were able to produce 0.85 and 0.79 g of PGA per liter, respectively. Under conditions that are found in intensified farming situations the ammonia content was reduced within 48 h from 1.3 to 0.75 g/liter. One mutant of B. subtilis 1551 impaired in the catabolism of PGA was obtained after nitrosoguanidine mutagenesis. This mutant produced PGA at a final concentration of 4.8 g/liter, whereas the wild type produced only 3.7 g/liter.
As a result of intensified agriculture, huge amounts of liquid manure are produced. On a typical pig-fattening farm (700 animals) in northwest Germany 2.8 m3 is produced daily (European Communities website, http://europa.eu.int). Liquid manure is a highly heterogeneous liquid mixture of fodder, litter, and animal cells, and the composition is extremely dependent on the age of the pigs, the type of fodder, the age of the liquid manure, and the storage conditions (8). Average values for the major parameters of this manure have been shown to be follows (20): dry weight, 60 to 80 g/liter; chemical oxygen demand, 50 to 80, g/liter; biological oxygen demand, 15 to 32 g/liter; nitrogen concentration, 5.0 to 6.0 g/liter; ammonium nitrogen concentration, 2.5 to 4.5 g/liter; potassium concentration, 2.1 to 2.5 g/liter; calcium concentration, 1.8 to 2.1 g/liter; phosphorus concentration, 0.7 to 0.9 g/liter; and magnesium concentration, 0.5 to 0.6 g/liter.
The traditional and most widely used way to dispose of liquid manure is by spreading it onto agricultural fields. Up to 80% of the ammonia is released into the atmosphere, and up to 56% (wt/vol) of the remaining ammonia can be utilized by plants (12, 19). In addition, depending on the type of soil and the microflora, the ammonium ions are partially oxidized to nitrate. Up to 40% (wt/wt) of the nitrate is released into the groundwater or washed into the surface water, which leads to unacceptably high concentrations of nitrate and eutrophication (1, 6). This causes serious health and environmental problems and has forced legislators to restrict the amount of nitrogen in liquid manure that is spread onto fields; in Germany the amount is restricted to 210 kg per ha per year (3). Therefore, new methods for disposal of liquid manure from intensified animal-fattening farms are needed.
Several gram-positive bacteria, such as Bacillus licheniformis, Bacillus subtilis, Bacillus megaterium, Bacillus anthracis, Sporosarcina halophila, and Planococcus halophila, are able to synthesize the polyamino acid (PAA) poly(γ-d-glutamic acid) (PGA) as a capsular substance or as a water-soluble slime (4, 22). In the presence of excess amounts of a suitable carbon source and ammonia, B. licheniformis excretes up to 20 g of PGA per liter into the medium (2). As PGA is remarkably resistant to proteolytic attack, degradation of this polymer by a nonadapted microflora proceeds very slowly (13).
In this study, we investigated conversion of the ammonia in liquid manure into PAAs by known PAA-producing bacteria. The PAAs should function as a transient depot for ammonia. Slow microbial degradation of the PAAs should then result in only low concentrations of free ammonia as a nutrient for plants. In addition, PAAs have a fertilizing function. Due to their polyanionic character, divalent cationic ions (i.e., Ca2+ and Mg2+) bind to the polymer, and by this process they are obviously concentrated and more efficiently transferred to the rhizosphere of plants (7).
MATERIALS AND METHODS
Bacterial strains.
All bacteria investigated in this study are listed in Table 1.
TABLE 1.
Bacterial strains used in this study
| Bacterial strain | Other designationa | Reference |
|---|---|---|
| Bacillus licheniformis ATCC 9945 | SK7085 | 22 |
| Bacillus subtilis 1551 | SK5668 | |
| Bacillus subtilis b′5 | SK5665 | |
| Bacillus subtilis DSM10 | 5 | |
| Bacillus subtilis natto IFO3335 | 9 | |
| Bacillus subtilis GM273 | SK5295 | |
| Bacillus subtilis GM329 | SK5296 | |
| Bacillus subtilis S35 | SK5664 | |
| Bacillus subtilis MP5 | SK7889 | |
| Streptomyces albulus 346 | 17 |
Our strain collection designation.
Growth of bacteria.
Bacillus strains were cultivated at different temperatures in 0.8% (wt/vol) nutrient broth, in mineral salts medium (15), in liquid manure, in artificial liquid manure, or in particle-free liquid manure supplemented with carbon sources by using different rates of aeration as indicated below. Artificial liquid manure contained (per liter) 16.52 g of (NH4)2SO4, 0.23 g of Na2HPO4 · 2H2O, 0.71 g of MgSO4 · 7H2O, and 1.34 g of KCl (19). The pH was adjusted to 7.5 with 1.0 N H2SO4. Liquid manure was obtained from the Landwirtschafliche Untersuchungs- und Forschungsanstalt (Oldenburg, Germany) and was stored at 4°C. Prior to use the liquid manure was filtered, centrifuged for 10 min at 3,000 × g to remove solid particles, and autoclaved. The chemical composition of the liquid manure was as follows: 0.46% (wt/wt) nitrogen, 0.32% (wt/wt) ammonium N, 0.27% (wt/wt) P2O5, 0.29% (wt/wt) K2O, and 3.9% (wt/wt) dry matter. For PGA production medium E was also used (11). To obtain solid media, 1.5% (wt/vol) agar agar was added. Growth was monitored photometrically at 600 nm or with a Klett-Summerson photometer by using a green no. 54 filter (520 to 580 nm).
Nitrosoguanidine mutagenesis.
N-Methyl-N′-nitro-N-nitrosoguanidine mutagenesis of B. subtilis 1551 was performed as described previously by Reh and Schlegel (14).
Electrophoretic methods.
Proteins were separated under denaturing conditions in 11.5% (wt/vol) polyacrylamide gels by the method of Laemmli (10) and were stained with Serva Blue R.
Quantitative determination of ammonia.
The ammonia content was determined with a type 15 230 3000 gas-sensitive electrode (Mettler Toledo GmbH, Steinbach/Ts, Germany) according to the instructions provided by the manufacturer.
Qualitative and quantitative determination of PAAs.
The amount of isolated PAAs was analyzed after precolumn derivatization of PAA hydrolysates with ortho-phthaldialdehyde reagent on a reversed-phase high-performance liquid chromatography column as recommended by the manufacturer (Merck, Darmstadt, Germany) (21). Hydrolysis was carried out by adding 1 ml of 6 N HCl to 1 mg of lyophilized PAAs and incubating the mixture for 6 h at 105°C.
RESULTS
Search for suitable strains able to produce PAAs in liquid manure.
In order to identify organisms suitable for conversion of the ammonia that is present in liquid manure into PAAs, strains of bacterial species which had been found previously to excrete PGA, including B. licheniformis ATCC 9945 and B. subtilis natto IFO3335, as well as polylysine-producing Streptomyces albulus strain 346, were tested for PAA production under the appropriate optimal conditions (Table 2). Whereas B. licheniformis ATCC 9945 and B. subtilis (natto) IFO3335 showed the expected PGA production, S. albulus 346 did not excrete any detectable polylysine. In addition, six other Bacillus strains from the culture collection of our laboratory were analyzed for PAA production. These studies identified two of these six strains as PGA producers.
TABLE 2.
Production of PAA by bacteria on solid medium E
| Bacterial strain | PAA productiona |
|---|---|
| Bacillus licheniformis ATCC 9945 | +++ |
| Bacillus subtilis 1551 | +++ |
| Bacillus subtilis b′5 | + |
| Bacillus subtilis DSM10 | (+) |
| Bacillus subtilis(natto) IFO3335 | − |
| Bacillus subtilis GM273 | − |
| Bacillus subtilis GM329 | − |
| Bacillus subtilis S35 | ++ |
| Bacillus subtilis MP5 | ++ |
| Streptomyces albulus 346 | − |
Organisms were cultivated for 24 h at 35°C. PAA production: +++ > ++ > + > (+). −, no PAA production.
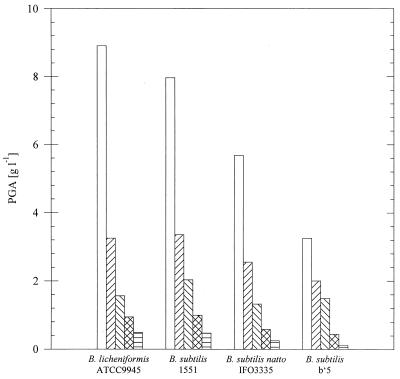
The PGA-producing strains B. licheniformis ATCC 9945, B. subtilis (natto) IFO3335, and B. subtilis 1551 and b′5 were chosen to investigate the influence of liquid manure on polymer production. To do this, the production medium was replaced stepwise by increasing the fraction (33, 50, 66, or 100%, [vol/vol]) of liquid manure. Although all strains produced reasonable amounts of PGA in the absence of liquid manure, the amounts of PGA were significantly reduced in the presence of increasing concentrations of manure, as shown in Fig. 1.
FIG. 1.
Cultivation of several Bacillus strains with
different concentrations of liquid manure. The strains were cultivated
at 35°C for 96 h in 500-ml conical flasks equipped with three
baffles containing a total volume of 100 ml of medium. Medium E was
replaced stepwise by increasing the fraction of liquid manure, as
follows: 0% (vol/vol) (□), 33% (vol/vol) (▨), 50% (vol/vol)
(▧), 66% (vol/vol) (▩), and 100% (vol/vol)
( ).
).
Effects of different carbon sources on PGA production.
In liquid manure, the nitrogen-to-carbon ratio is approximately 11:1 (16). In medium E, which provides the optimal amounts of carbon and nitrogen for PGA production by B. licheniformis ATCC 9945 (22), the nitrogen-to-carbon ratio is 1:13.8. Therefore, liquid manure was supplemented with different relatively inexpensive carbon sources to improve the conditions for PGA production.
As Table 3 shows, good growth and PGA production were obtained with both B. licheniformis ATCC 9945 and B. subtilis 1551 on liquid manure in the presence of sodium gluconate. Glucose and sucrose also had positive effects on growth and PGA production. Beet molasses stimulated growth only slightly and did not or almost did not result in production of PGA. Addition of whey also had a negligible effect. Since these carbon sources had no effect or only a negligible effect on growth of and PGA production by the other two strains of B. subtilis and also had no effect on growth of and polylysine production by S. albulus, B. licheniformis ATCC 9945 and B. subtilis 1551 were chosen for further detailed studies of the growth kinetics.
TABLE 3.
Growth of and PAA production by different bacteria on liquid manure plates with several carbon sources
| Strain | Glucosea
|
Sodium
gluconate
|
Sucrose
|
Beet
molasses
|
Whey
|
No carbon source
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Growth | PAA production | Growth | PAA production | Growth | PAA production | Growth | PAA production | Growth | PAA production | Growth | PAA production | |
| B. licheniformis ATCC 9945 | ++ | + | +++ | +++ | ++ | + | ++ | (+) | − | − | (+) | − |
| B. subtilis 1551 | ++ | + | +++ | +++ | ++ | + | + | − | (+) | − | (+) | − |
| B. subtilis b′5 | + | − | + | − | − | − | − | − | − | − | − | − |
| S. albulus 346 | − | − | − | − | − | − | − | − | − | − | − | − |
| B. subtilis (natto) IFO3335 | + | (+) | + | (+) | − | − | − | − | − | − | − | − |
The strains were cultivated for 72 h at 35°C on liquid manure plates containing a carbon source at a concentration of 0.5% (wt/vol). Growth and PAA production: +++ > ++ > + > (+). −, no growth or no PAA production.
Determination of growth kinetics by using artificial and particle-free liquid manure.
Differences in the composition of liquid manure made it impossible to obtain reproducible quantitative data for growth and conversion of ammonia into PGA. Furthermore, liquid manure contains up to 4.7% (wt/vol) solid material (e.g., hairs, straw, etc.), which prevented continuous measurement of growth and PGA content. Therefore, at the beginning of the experiments, artificial liquid manure was used as the medium for comparative studies of the growth kinetics of B. licheniformis ATCC 9945 and B. subtilis 1551. The composition of artificial liquid manure corresponded to the average values for the main soluble mineral constituents of liquid manure (19).
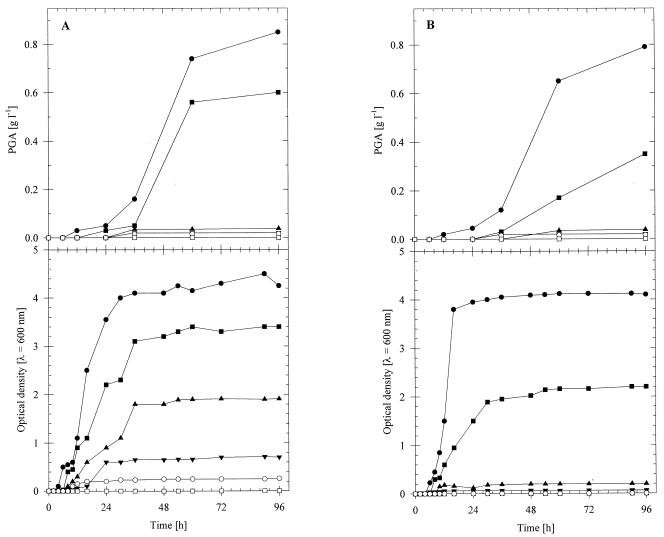
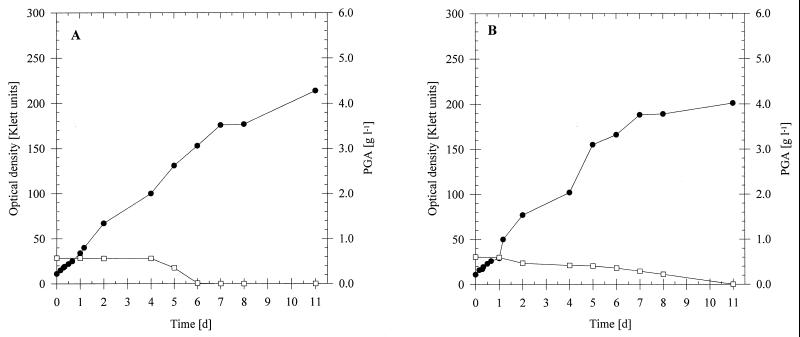
On artificial liquid manure containing 0.5% (wt/vol) sodium gluconate, both B. licheniformis ATCC 9945 (Fig. 2A) and B. subtilis 1551 (Fig. 2B) exhibited relatively fast growth, with doubling times of 3.2 and 2.4 h, respectively. PGA production started after the cells entered the stationary growth phase, and the concentrations reached maximum values of 0.85 and 0.79 g/liter, respectively, after 96 h of cultivation. Slower growth (doubling times, 13.0 and 10.8 h, respectively) and less PGA (0.58 and 0.39 g/liter, respectively) were obtained with these strains in the presence of 0.5% (wt/vol) glucose. No PGA production was detected during cultivation of either strain on sucrose, beet molasses, or whey. Whereas B. licheniformis ATCC 9945 exhibited weak growth on sucrose (doubling time, 6.6 h) and even less growth on molasses, B. subtilis 1551 was not able to use either substrate for growth. With both species, no growth occurred in the presence of whey.
FIG. 2.
PGA production and cultivation of B. licheniformis ATCC 9945 (A) and B. subtilis 1551 (B) in artificial liquid manure. The strains were cultivated at 35°C for 96 h in 500-ml conical flasks equipped with three baffles containing 100 ml of artificial liquid manure. The concentration of each carbon source was 0.5% (wt/vol). Symbols: ●, sodium gluconate; ■, glucose; ▴, sucrose; ▾, beet molasses; ○, whey; □, no carbon source.
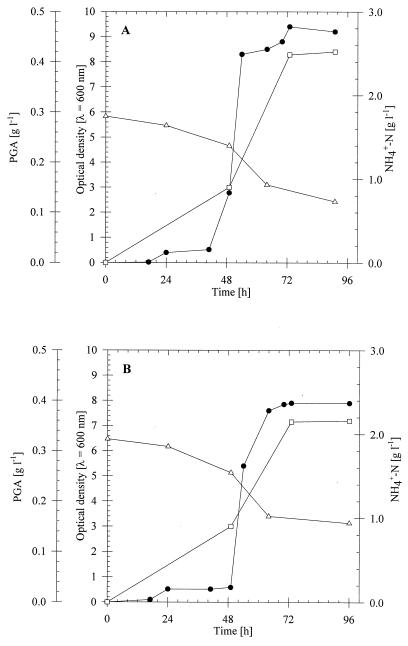
In order to verify these results under conditions more likely to occur in the field, natural liquid manure was used, but it was pretreated by filtration and centrifugation to obtain particle-free liquid manure. During cultivation of B. licheniformis ATCC 9945 and B. subtilis 1551 in particle-free liquid manure, which was supplemented with 0.5% (wt/vol) sodium gluconate, PGA production and reduction of ammonium occurred at rates which were 50% of those obtained with artificial liquid manure (Fig. 3). As revealed by comparison with a sterile control that was incubated under identical conditions, approximately 40% of the reduction was due to evaporation of NH3. Therefore, based on a PGA yield of approximately 0.4 g/liter, only 5% of the ammonia in each case was converted into PGA.
FIG. 3.
Cultivation of, ammonium reduction by, and PGA production by B. licheniformis ATCC 9945 (A) and B. subtilis 1551 (B) in particle-free liquid manure. The strains were cultivated at 35°C for 96 h in 500-ml conical flasks equipped with three baffles containing 100 ml of particle-free liquid manure. The concentration of sodium gluconate was 0.5% (wt/vol). Symbols: ●, optical density at 600 nm; ▵, ammonium concentration; □, PGA concentration.
Conversion of ammonia into PGA under conditions that occur in intensified farming situations.
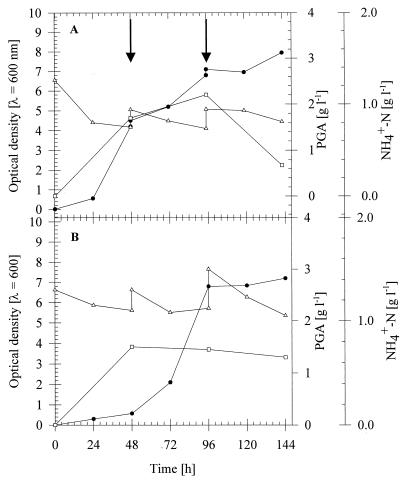
In order to test the ability of the PGA-producing bacteria to convert ammonia into the PGA polymer under conditions similar to those that occur in intensified agriculture situations, growth experiments were performed in a medium composed of 54% (vol/vol) tap water, 33% (vol/vol) medium E, and 13% (vol/vol) particle-free liquid manure. This composition resembled those that were used in a pilot plant at the Landwirtschaftliche Untersuchungs- und Forschungsanstalt. In this pilot plant, experiments to reduce the ammonia content of liquid manure by nitrification were done, and to 0.13 volume of liquid manure 0.33 volume of activated sludge and 0.54 volume of water were added. Once a week 0.13 volume of the culture fluid was replaced by fresh liquid manure.
As shown in Fig. 4, B. licheniformis ATCC 9945 and B. subtilis 1551 exhibited similar growth under the conditions mentioned above. After inoculation with B. licheniformis ATCC 9945, the ammonia content was reduced from 1.3 to 0.75 g/liter within 48 h. In two steps after 48 and 96 h of cultivation, 0.13 volume of the culture volume was replaced by fresh liquid manure. During the following 2 days of incubation, the ammonia concentration was reduced to 0.74 g/liter. Net production of PGA occurred only during the first 96 h, and the maximum value was 2.2 g/liter. During the following 2 days, the concentration of PGA decreased to 1.6 g/liter.
FIG. 4.
Cultivation of B. licheniformis ATCC 9945 (A) and B. subtilis 1551 (B) under fed batch conditions. The strains were cultivated at 35°C for 144 h in 500-ml conical flasks equipped with three baffles containing 100 ml of medium composed of 54% (vol/vol) tap water, 33% (vol/vol) medium E, and 13% (vol/vol) particle-free liquid manure. At the times indicated by arrows, 0.13 volume of particle-free liquid manure was added. Symbols: ●, optical density at 600 nm; ▵, ammonium concentration; □, PGA concentration.
The results obtained with B. subtilis 1551 differed from those obtained with B. licheniformis; both reduction of ammonia and production of PGA were lower (Fig. 4).
Isolation and characterization of a B. subtilis mutant affected in degradation of PGA.
PGA-producing bacteria are generally also able to utilize the excreted PGA polymer as a nutrient (13). Therefore, mutants of PGA-producing strains affected in degradation of PGA should give higher yields of the polymer during batch cultivation in liquid manure. After mutagenization of B. licheniformis ATCC 9945 and B. subtilis 1551 with N-methyl-N′-nitro-N-nitrosoguanidine, the cells were incubated in 50 ml of nutrient broth in the presence of penicillin (75 mg/ml) to enrich those mutants which were defective in degrading PGA. To identify and isolate the desired mutants, the cells were plated on medium E, on which slimy colonies indicated the wild type by the ability to excrete PGA. In parallel, the cells were also plated onto mineral medium with 0.5% (wt/vol) PGA as the sole carbon source, on which no or reduced growth indicated defective PGA degradation.
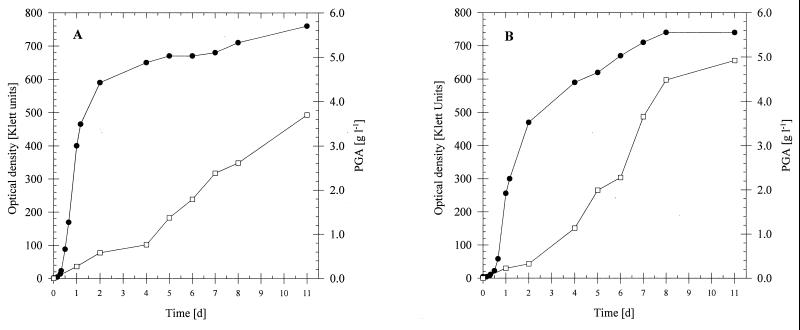
Although no mutant of B. licheniformis ATCC 9945 with the desired phenotype was obtained, one stable mutant of B. subtilis 1551 out of 1,500 candidates investigated showed reduced growth on PGA. Cultivation in liquid medium containing PGA as the sole carbon source revealed that this mutant, which was referred to as MP5, was able to grow on the polymer, but the rate of degradation was significantly lower than that of the wild type (Fig. 5). Thus, mutant MP5 exhibited the phenotype PGA leaky. This reduced capacity resulted in faster polymer production and a higher yield during cultivation in medium E, in which the mutant produced PGA at final concentrations up to 4.8 g/liter, whereas the final concentration of PGA obtained with the wild type was only 3.7 g/liter (Fig. 6).
FIG. 5.
Consumption of PGA by B. subtilis 1551 (A) and the PGA-degrading mutant B. subtilis MP5 (B) in mineral salts medium. The strains were cultivated at 35°C for 11 days in 500-ml conical flasks equipped with three baffles containing 100 ml of mineral salts medium and 0.5% (wt/vol) PGA as the sole carbon source. Symbols: ●, optical density as measured with a Klett-Summerson colorimeter; □, PGA concentration.
FIG. 6.
Production of PGA by B. subtilis 1551 (A) and the PGA-degrading mutant B. subtilis MP5 (B) in medium E. The strains were cultivated done at 35°C for 11 days in 500-ml conical flasks equipped with three baffles containing 100 ml of medium E. Symbols: ●, optical density as measured with a Klett-Summerson colorimeter; □, PGA concentration.
Polyacrylamide gel electrophoresis of denatured crude extracts of mutant MP5 and the wild type revealed a clear difference. Whereas the protein pattern for cells of the wild type grown on PGA had a weak but distinct band representing a protein with an apparent Mr of 35,000 ± 1,000, which was absent in wild-type cells after growth in PGA production medium, this protein was absent in the electropherograms of mutant MP5 cells in the early growth phase and in the late stationary growth phase.
DISCUSSION
The purpose of this study was to describe a process for conversion of the ammonia of liquid manure into PAAs. In this study, it was shown that PGA-producing species of the genus Bacillus are able to convert a significant fraction of the ammonium nitrogen present in liquid manure transiently into this PAA. By this process, the concentration of free ammonium in the manure could be reduced, and the PGA produced acted as a transient depot for ammonium. However, the conversion occurred only to a low extent. In the case of B. licheniformis ATCC 9945 and B. subtilis 1551 this was mainly due to the carbon-to-nitrogen ratio in liquid manure, which is the inverse of that in medium E, which is known to favor PGA production. Consequently, an increase in PGA production with a corresponding reduction in the amount of ammonia was achieved by adding a suitable carbon source or medium E to liquid manure. Another strategy involving a better nitrogen sink is application of organisms producing the proteinlike polymer cyanophycin, which consists of equimolar amounts of arginine and aspartic acid (nitrogen to carbon ratio, 1:2) (18). Cyanophycin is unique to cyanobacteria and has been reported to occur in many species of cyanobacteria. At present, the use of genetically engineered microorganisms would be necessary to produce such a nitrogen storage compound in manure. Further systematic optimization of the culture conditions would surely lead to improvement of the conversion rate. Whether such a strategy can be biotechnologically applied will depend on further optimization of the process and also on isolation of PGA-producing bacteria that are better adapted to the conditions prevailing at agricultural sites, including high concentrations of manure and relatively low temperatures.
However, any technical process for refinement of liquid manure must comply with the requirements of agriculture and consequently should be as simple and cheap as possible. Both treatment of liquid manure to improve the culture conditions and control of the process may be too costly and too sophisticated to be accepted by farmers.
ACKNOWLEDGMENTS
We are indebted to Landwirtschaftliche Untersuchungs- und Forschungsanstalt for providing the liquid manure and analytic data on the composition of the manure.
This study was supported by a grant from Bayer AG (Leverkusen, Germany).
REFERENCES
- 1.Archer J R, Nicholson R J. Liquid wastes from farm animal enterprises. In: Phillips C, Piggins D, editors. Farm animals and the environment. Wallingford, United Kingdom: C.A.B. International; 1992. pp. 325–343. [Google Scholar]
- 2.Birrer G A, Cromwick A M, Gross R A. γ-Poly(glutamic acid) formation by Bacillus licheniformisATCC9945a: physiological and biochemical studies. Int J Biol Macromol. 1994;16:265–275. doi: 10.1016/0141-8130(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 3.Bundesgesetzblatt. 1996. Verordnung über die Grundsätze der guten fachlichen Praxis beim Düngen (Düngerverordnung). 1(6):118–121.
- 4.Hara T, Ueda S. Regulation of polyglutamate production in Bacillus subtilis (natto): transformation of high γ-d-PGA productivity. Agric Biol Chem. 1982;46:2275–2281. [Google Scholar]
- 5.Heyndrickx M, Vandermeulebroecke K, Kersters K, De Vos P, Logan N A, Aziz M A, Ali N, Berkeley R C W. A polyphasic reassessment of the genus Paenibacillus, reclassification of Bacillus lautus as Paenibacillus lautus comb. nov. and of Bacillus peoriae as Paenibacillus peoriae comb. nov., and emended description of P. lautus and of P. peoriae. Int J Syst Bacteriol. 1996;46:988–1003. doi: 10.1099/00207713-46-4-988. [DOI] [PubMed] [Google Scholar]
- 6.Isermann K. Kuratorium für Technik und Bauwesen in der Landwirtschaft e.V. (KTBL), Verein Deutscher Ingenieure (VDI): Ammoniak in der Umwelt. Münster-Hiltrup, Germany: KTBL-Schriften-Vertrieb im Landwirtschaftsverlag GmbH; 1990. Ammoniakemission der Landwirtschaft als Bestandteil ihrer Stickstoffbilanz und Lösungsansätze zur hinreichenden Minderung; pp. 1.1–1.76. [Google Scholar]
- 7.Kinnersley, A., D. Strom, A. R. Y. Meah, and C. P. Koskan. May 1994. Composition and method for enhanced fertilizer uptake by plants. Patent WO94/09628
- 8.Kirchgessner M, Kreuzer M, Roth F X. Bestimmungsfaktoren der Güllecharakteristik beim Schwein. 1. Einfluß von Leistungsrichtung, -stadium und - intensität sowie Futteraufnahme und Geschlecht unter üblichen Fütterungsbedingungen. Agribiol Res. 1991;44:299–324. [Google Scholar]
- 9.Kunioka M, Goto A. Biosynthesis of poly(γ-glutamic acid) from l-glutamic acid, citric acid, and ammonium sulfate in Bacillus subtilisIFO3335. Appl Microbiol Biotechnol. 1994;40:867–872. [Google Scholar]
- 10.Laemmli U K. Cleavage of structural protein during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 11.Leonard C G, Housewright R D, Thorne C B. Effects of some metallic ions on glutamyl polypeptide synthesis by Bacillus subtilis. J Bacteriol. 1958;76:499–503. doi: 10.1128/jb.76.5.499-503.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miflin B J, Lea P J. Ammonia assimilation and amino acid metabolism. In: Boulter D, Parthier B, editors. Nucleic acids and proteins in plants. Vol. 1. Berlin, Germany: Springer-Verlag; 1982. pp. 5–64. [Google Scholar]
- 13.Opperman F B, Pickartz S, Steinbüchel A. Biodegradation of polyamides. Polym Degrad Stabil. 1998;59:337–344. [Google Scholar]
- 14.Reh M, Schlegel H G. Anreicherung und Isolierung auxotropher Mutanten von HydrogenomasH16. Arch Mikrobiol. 1969;67:99–109. [PubMed] [Google Scholar]
- 15.Schlegel H G, Kaltwasser H, Gottschalk G. Ein Submersverfahren zur Kultur wasserstoffoxidierender Bakterien: Wachstumsphysiologische Untersuchungen. Arch Mikrobiol. 1961;38:209–222. [PubMed] [Google Scholar]
- 16.Schulze-Rettmer R. Nitrogen in liquid manure. Wiss Umwelt. 1980;4:171–174. [Google Scholar]
- 17.Shima S, Sakai H. Polylysine produced by Streptomyces. Agric Biol Chem. 1977;41:1807–1809. [Google Scholar]
- 18.Simon R D, Weathers P. Determination of the structure of the novel polypeptide containing aspartic acid and arginine which is found in cyanobacteria. Biochim Biophys Acta. 1976;420:165–176. doi: 10.1016/0005-2795(76)90355-x. [DOI] [PubMed] [Google Scholar]
- 19.Syrett P J. Uptake and utilization of nitrogen compounds. In: Rogers L J, Gallon J R, editors. Biochemistry of the algae and cyanobacteria. Oxford, United Kingdom: Oxford Science Publications Clarendon Press; 1988. pp. 23–39. [Google Scholar]
- 20.Thomas P. Aerob mikrobielle Behandlung von Schweinegülle mit anschließender Hefebiomassebildung. Ph.D. thesis. Münster, Münster, Germany: Westfälische Wilhelms-Universität; 1984. [Google Scholar]
- 21.Tran H, Oppermann-Sanio F B, Steinbüchel A. Purification and characterization of cyanophycin synthetase from the thermophilic Synechococcussp. MA19. FEMS Microbiol Lett. 1999;181:229–236. doi: 10.1111/j.1574-6968.1999.tb08849.x. [DOI] [PubMed] [Google Scholar]
- 22.Troy F A. Chemistry and biosynthesis of the poly(γ-d-glutamyl) capsule in Bacillus licheniformis. I. Properties of the membrane-mediated biosynthetic reaction. J Biol Chem. 1973;248:305–315. [PubMed] [Google Scholar]