Summary
People with Down syndrome (DS) have increased risk of Alzheimer disease (AD) presumably conferred through genetic predispositions arising from trisomy 21. These predispositions necessarily include triplication of the amyloid precursor protein (APP), but also other Ch21 genes that confer risk directly or through interactions with genes on other chromosomes. We discuss evidence that multiple genes on chromosome 21 are associated with metabolic dysfunction in DS. The resulting dysregulated pathways involve the immune system, which leads to chronic inflammation, the cerebrovascular system leading to disruption of the BBB, and cellular energy metabolism promoting increased oxidative stress. In combination, these disruptions may produce a precarious biological milieu which, in the presence of accumulating amyloid, drives the pathophysiological cascade of AD in people with DS. Critically, mechanistic drivers of this dysfunction may be targetable in future clinical trials of pharmaceutical and/or lifestyle interventions.
Introduction
History
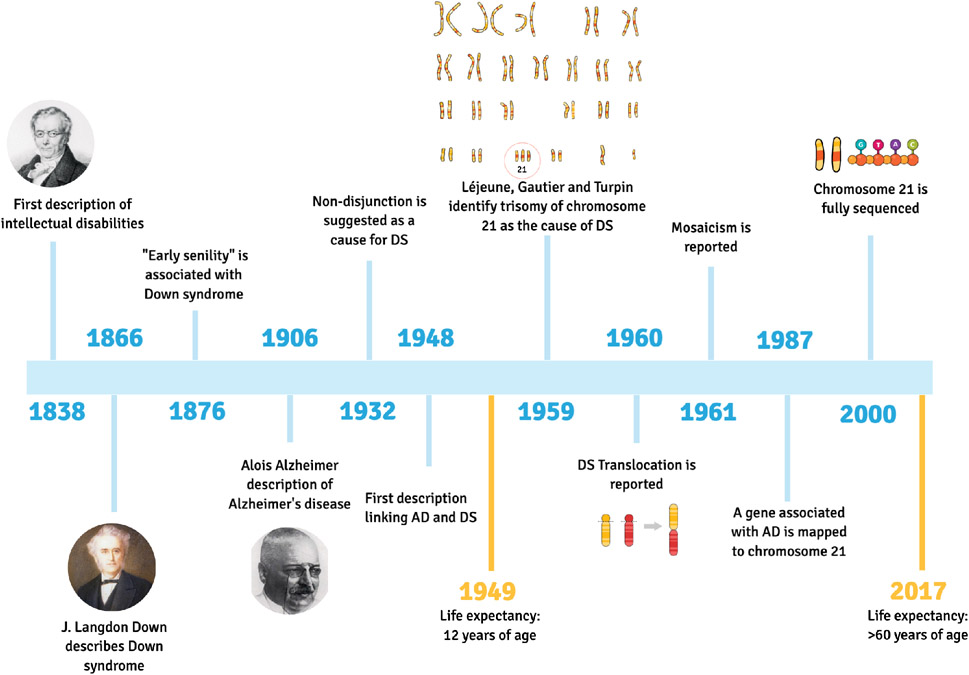
The first description of individuals with intellectual and developmental disabilities was published in 1838 by Jean-Étienne Esquirol; however, it was in 1866 that John Langdon Down identified a subcategory of people with cognitive impairments based on their phenotypic similarities, describing the clinical features of what we currently know as Down syndrome (DS). Depictions of the syndrome before 1838 are rare, but can be found in historical material (Starbuck, 2011). Ancient remains found in France dating back to the 5-6th century AD are consistent with the phenotype of DS and, interestingly, suggest that individuals with DS were not stigmatized by members of the community at that time (Rivollat et al., 2014). Notably, the first hint of an association between DS and Alzheimer disease (AD) was described in 1876 by John Fraser and Arthur Mitchell (Fraser, 1876), decades before Alois Alzheimer’s description of AD (Alzheimer, 1906). The evidence for this association became stronger with George Jervis reporting the presence of senile plaques in three young “mongoloid” adults (Jervis, 1948). In 1987, the description of a defective gene linked to familial AD development was mapped to chromosome 21 (Goldgaber et al., 1987; Glenner and Wong, 1984). Years before, in 1932, non-disjunction was first suggested as a cause for DS (Davenport, 1932); however, it was only in 1959 that trisomy of chromosome 21 was identified as the most common cause of DS by Drs. Léjeune, Gautier, and Turpin (Lejeune et al., 1959; Lejeune, 1959). In the following years, translocation (Carter et al., 1960; Polani et al., 1960) and mosaicism (Clarke et al., 1961; Clarke et al., 1963) were also described as forms of trisomy 21 (see Figure 1).
Figure 1. Down syndrome research and life expectancy.
Timeline of events from the first description of intellectual disabilities, in 1838, to the complete sequencing of Chromosome 21 in the year 2000. Life expectancy improved from 12 years of age in 1949, to more than 60 years of age in 2017. Created with MindtheGraph.com.
DS or Trisomy 21 is the most common cause of intellectual disability and prevalence in the United States is approximately 250,000 individuals (Presson et al., 2013). The United Nations estimates the prevalence of DS to be between 1 in 1,000 to 1 in 1,100 live births worldwide, but counts are not available in different parts of the world, making it harder to obtain a precise number (Nations, 2021). Meiotic nondisjunction causes the extra copy of chromosome 21 and accounts for 95% of cases. Partial trisomy (translocation) and somatic mosaicism represent 4% and less than 1% of all cases, respectively. Advances in genetic studies have continued at a rapid pace and in 1987 the characterization of a cDNA, encoding brain amyloid on chromosome 21, provided a strong explanation for the association of AD with DS (Goldgaber et al., 1987; St George-Hyslop et al., 1987). In 2000, the full sequencing of chromosome 21 was completed, and this dramatically accelerated DS research (Gardiner and Davisson, 2000; Hattori et al., 2000). Recent advances in medicine and our understanding of factors associated with the syndrome have significantly improved quality of life and extended life expectancy of people with DS, increasing from 12 in 1949 to over 60 years of age today (de Graaf et al., 2017b; Penrose, 1949). However, because increased age is the most potent known risk factor for AD, these advances have also resulted in increased prevalence of AD in people with DS (DSAD). The overexpression of the β-amyloid precursor protein gene (APP) on chromosome 21 leads to an enhanced production of beta-amyloid (Aβ) and is likely to be one of the main causes for increased risk of AD in this population (Doran et al., 2017; Prasher et al., 1998). People with DS show blood and cerebrospinal fluid (CSF) biomarker changes consistent with observations in both familial, autosomal dominant AD (ADAD) and sporadic, late-onset AD (LOAD). Studying AD in people with DS has the unique advantage of a distinct age-related pattern of emerging neuropathology commencing in childhood which allows us to investigate the earliest appearance of dysfunction in specific pathways that may contribute to AD pathogenesis. These changes could suggest comparisons in DSAD to the abnormal aging also observed in ADAD and LOAD. However, unlike these other clinical populations, people with DS demonstrate lifelong patterns of cognitive and neurobiological developmental differences compared to chromosomally typical individuals. Better appreciation of these unique, life-course trajectories of elevated risk in DSAD could substantially inform specific therapies in this population, if not suggest underappreciated attributes and drivers of AD pathobiology in all at-risk populations.
Aging and Lifespan
Statistics from studies in the late 1990’s suggested that after 35 years of age, mortality rates doubled every 6.4 years in people with DS as compared to every 9.6 years for people without DS (Strauss and Eyman, 1996). The mean and median age of death has significantly increased by 3.75-fold over the past 40 years (Presson et al., 2013). These statistics continue to improve with estimates from 2010 showing that 28% of people with DS are living past 40 years of age, compared to 4% in the 1950s (de Graaf et al., 2017b) More enriched lifestyles, management of co-occurring illnesses, and improvements in medical care for children and adults with DS led to this significant extension in lifespan and enhanced quality of life (Bittles et al., 2007; Glasson et al., 2002). Indeed, the number of people with DS over the age of 40 years is rapidly rising (de Graaf et al., 2017a; de Graaf et al., 2017b; 2020). Thus, in the coming decades there will be an increasing need to address age-associated health issues and, in particular, manage the development of AD in this vulnerable population.
Although all people with DS with full trisomy 21 develop AD neuropathology by the time they are 40 years of age, there is approximately a 10-year delay, on average, to when clinical features of dementia may be observed (between 53-55 years of age) (Fortea 2020; Sinai et al., 2018). Of note, a subset of people with DS, estimated to be between 10-15%, reach over 60 years of age without signs of dementia (Fortea 2020; Schupf and Sergievsky, 2002; Sinai et al., 2018). There is great interest in identifying factors that affect onset of clinical dementia because these modifiers may be targetable by either pharmacological or lifestyle interventions (Karmiloff-Smith et al., 2016). People with DS have a higher prevalence of several common conditions including hypothyroidism, high BMI/obesity, high cholesterol, sleep apnea and other sleep disturbances (Cody et al., 2020), and metabolic disorders such as diabetes, that are known to affect risk of AD and dementia in the general population (Capone et al., 2020; Capone et al., 2018; Tsou et al., 2020). Conversely, there are also protective factors that were historically underappreciated (Alić et al., 2020). For example, people with DS have low rates of hypertension and atherosclerosis (Draheim et al., 2010; Draheim et al., 2002; Murdoch et al., 1977), which are typically significant contributors to cerebrovascular dysfunction and resulting cognitive vulnerability in abnormal aging. We and others propose that multiple etiologies predispose people with DS to an elevated risk of AD that may be mediated, in part, by systemically extensive metabolic dysfunction linking immune system dyshomeostasis, inflammation, cerebrovascular pathology, and cellular metabolic deficits.
Contributions of immune system and inflammation
Immune dysregulation in people with DS is detected as early as during fetal development. Consequently, people with DS are highly susceptible to develop certain health conditions, including autoimmune diseases, hematological and autoimmune skin disorders, among others (Aversa et al., 2015; Ram and Chinen, 2011; Verstegen and Kusters, 2020). Importantly, individuals with DS are at high risk for infections, especially of the respiratory tract (Bloemers et al., 2010a; Chan et al., 2017), usually with increased rates of hospitalization and higher mortality (Beckhaus and Castro-Rodriguez, 2018; Perez-Padilla et al., 2010). Several immune-related genes, including ITGβ2, IFNAR1, IFNGR2, ICOSL, AIRE, etc., are found on chromosome 21, raising potential explanation for the altered expression and function of specialized immune cells and mediators in DS (Satgé and Seidel, 2018; Wilcock and Griffin, 2013), similar to that observed with aging in the general population.
Differences in immune parameters in people with DS may affect both innate and adaptive immunity (Aiello et al., 2019; Fulop et al., 2017; Gensous et al., 2020; Goronzy and Weyand, 2019; Huggard et al., 2020). The thymus gland is a specialized organ for T cell maturation. T lymphocytes, divided into CD4+ and CD8+ cells, are key components of the adaptive immune system (Germain, 2002; Laidlaw et al., 2016). In DS, the thymus is known to be smaller, hypocellular, and has a reduced number of mature thymocytes (Murphy and Epstein, 1990; 1992), while in the periphery a reduced number of lymphocytes and Regulatory T cells (Tregs) could increase the propensity to autoimmune disorders (de Hingh et al., 2005; Marcovecchio et al., 2019). Reductions in the number of circulating CD4+ T cells, inversion of CD4+/CD8+ ratio, and impairments of other T cell subpopulations are reported in DS (Barrena et al., 1993; Cossarizza et al., 1990; Guazzarotti et al., 2009; Pellegrini et al., 2012). More recently, higher expression of markers of activation and senescence in CD8+ T cells from adults with DS were described, along with increased differentiation of CD4+ cells and overproduction of cytokines related to autoimmunity (Araya et al., 2019; Schoch et al., 2017). Upon antigen presentation, B lymphocytes proliferate, differentiate, and produce defense antibodies, while also retaining memory to rapidly react to subsequent exposure to previous stimulating antigens. Adults with DS have higher levels of differentiated B cell subsets associated with inflammation and autoimmunity (Waugh et al., 2019). In general, there are reduced numbers of circulating B cells in people with DS, particularly at young ages; although the actual levels of immunoglobulins reported between different studies is variable and may be related to differences in study cohort sizes (Carsetti et al., 2015; Cetiner et al., 2010; Dieudonne et al., 2020; Farroni et al., 2018; Verstegen et al., 2014). Altogether, overall immune dysregulation, reduction in switched memory B cells, and changes in immunoglobulins may directly affect the response of individuals with DS to vaccinations (Carsetti et al., 2015; Valentini et al., 2015) and lead to worse outcomes due to viral infections including that caused by SARS-CoV-2 (COVID-19) (De Toma and Dierssen, 2021; Espinosa, 2020; Huls et al., 2021; Illouz et al., 2021).
Recently, disturbances in iron homeostasis linked to increased cytokine expression and the hepcidin hormone were described in people with DSAD, suggesting shared mechanisms between increased susceptibility to infections and neurodegeneration (Raha, 2021). Changes in the innate immune system are consistent with the overall immune dysregulation in DS. Recent studies suggested that people with DS have lower granulocyte, myeloid dendritic cell counts (Bloemers et al., 2010b), and increased inflammatory monocytes (Waugh et al., 2019). Studies on natural killer (NK) cells are contradictory, but tend to demonstrate higher numbers of NK cells in children and adolescents with DS (Cetiner et al., 2010; Schoch et al., 2017) accompanied by hyper-reactivity to interferon-α (IFN-α) in adults (Waugh et al., 2019), as well as inefficient and lower NK cell counts (Bloemers et al., 2010b; de Hingh et al., 2005).
Neuroinflammation is directly associated with aging and AD development and some of the mechanisms operating in people with DS involve the activation of the complement pathway, cytokine release, and glial cell alterations, among others. The inflammatory phenotype of people with DS differs from age-matched neurotypical controls and reflects an increase in pro-inflammatory gene expression (Wilcock et al., 2015). Cytokine expression across the lifespan is region specific within the brain and, with respect to some specific mediators, exacerbated before the occurrence of AD pathology (Flores-Aguilar et al., 2020; Iulita et al., 2016). Inflammatory markers measured in plasma show a striking upregulation of proinflammatory cytokines in people with DSAD compared to those without AD (Petersen et al., 2020). In the brains of people with DSAD, there is increased expression of C1q, the initial factor of the classical complement pathway, which is consistently associated with compact Aβ plaques (Head et al., 2001; Stoltzner et al., 2000). Interestingly, proteomic analysis of blood suggests an overall deficiency of complement factor in people with DS (Sullivan et al., 2017).
Glial cells, including astrocytes and microglia, are critically involved in AD development specifically playing a role in modulating neuroinflammatory processes. S100 calcium binding protein B (S100β) is primarily found in astrocytes and promotes the healthy development of the CNS, while also exhibiting cytokine-like activities. In people with DS, the overexpression of the S100B gene on chromosome 21, leading to increased numbers of S100β-positive astrocytes may have negative consequences related to age-associated cognitive decline (Griffin et al., 1989). When modeled in vitro, DS astrocytes generate higher levels of reactive oxygen species (ROS) with reduced synaptogenic molecules (Chen et al., 2014). DS iPSC-derived astroglia can also lead to structural and functional deficits in co-cultured neurons, specifically a decreased global excitability accompanied by increased amplitudes of post-synaptic activity and density (Mizuno et al., 2018). However, the dysregulation of astrocyte functions is known to cause hyper excitability or promote the development of epilepsy through multiple different mechanisms (Verhoog et al., 2020), a prevalent comorbidity in people with DS (Altuna et al., 2021). Abnormal astrocyte function in people with DS may also be reflected in metabolic imaging studies of the aging DS brain, where significantly lowered N-acetyl-aspartate (a marker of neuron health) to myo-inositol (a marker of inflammation) ratios (NAA/MI) characterize DSAD compared to both euploid controls and cognitively stable people with DS (Lin et al., 2016; Montal et al., 2021). The hexose sugar myo-inositol becomes increasingly abundant in the brains of aging individuals with DS and may indicate elevated levels of glial neuroinflammation (Huang et al., 1999). However, it is important to consider that the gene encoding the myo-inositol transporter protein (SLC5A3) is on chromosome 21 and due to its triplication in DS, myo-inositol may be overexpressed for this reason (Huang et al., 1999). Expression of several other genes not found on chromosome 21 has also been implicated in the neuroinflammatory response in people with DS. For example, reactive astrocytes surrounding Aβ plaques in the hippocampus of people with DS show increased expression of STARD1 that is involved in intracellular cholesterol trafficking (Arenas et al., 2020). Glial fibrillary acidic protein (GFAP), another important astrocyte protein, is stable in the plasma of people with DS until the late 40s and increases gradually with age (Hendrix et al., 2021). Taken together, these findings suggest that glial associated neuroinflammation in DS is related, at least in part, to the triplication and overexpression of genes associated with chromosome 21 and their trans gene interactions.
Single-nuclei analyses of the neurotypical human AD brain suggest a unique microglia-related transcriptomic signature with disease (Olah et al., 2020). Although similar studies have yet to be completed in the brains of people with DS, microglial morphological phenotypes in DS and DSAD overlap with observations in AD, but also may display unique features. For example, in DSAD, there is a shift towards the presence of higher numbers of dystrophic (dying) and rod-shaped (chronically inflamed) microglial cells suggesting that microglial changes progress gradually over time as people with DS age (Flores-Aguilar et al., 2020; Martini et al., 2020). Levels of the triggering receptor expressed on myeloid cells 2 (TREM2), which are also implicated in microglial function and homeostasis, are elevated in young adults with DS (Raha-Chowdhury et al., 2018) and decline with age (Weber et al., 2020). Polymorphisms on the gene encoding TREM2 (TREM2) are linked to a higher risk of AD (Ulland and Colonna, 2018). In combination, there is strong evidence of significant neuroinflammation in DS, that is exacerbated with AD pathology, and present both common and distinct patterns when compared to AD in the general population. A vast body of evidence suggests an intrinsic relationship between impaired neuronal energy metabolism, inflammation, and Aβ deposition (for complete review, please see (Kapogiannis and Mattson, 2011; Yan et al., 2020) ). In hippocampal neurons, hyperinsulinemia drives the upregulation of genes for inflammatory and immune pathways while downregulating insulin signaling genes. These changes result in decreased mitochondrial function and blockage of glucose utilization (Wu et al., 2008) (Blalock et al., 2010). Additionally, Aβ may also activate microglia cells and shift their metabolism to aerobic glycolysis in a transgenic mouse model (Baik et al., 2019). Age-associated changes in the immune system of people with DS occur in a relatively shorter time and at earlier ages than in the neurotypical population. These factors contribute to the unique neuroinflammatory signature in people with DS and are consistent with the notion of DS as a “segmental progeroid” condition of biologically accelerated aging. Because of their unique metabolism within the CNS and roles in driving neuroinflammation, “immunometabolic” changes involving reactive and activated glia could link immunological and biochemical perspectives in DSAD considered independently in this review (Bernier et al., 2020; Chausse et al., 2021; Muri and Kopf, 2021; Price et al., 2021). Such dysmetabolic dynamics could, however, extend distally from the CNS to also include peripheral immune cells and associated processes in AD (Gate et al., 2020; Runtsch et al., 2020; Town et al., 2005).
Neuroinflammation in people with DS may be driven in part by genetics, but also as we discuss next, may be a consequence of cerebrovascular pathology with subsequent leakage of serum proteins into the brain.
Cerebrovascular pathology in DS
Cerebral amyloid angiopathy (CAA), the progressive deposition of Aβ within the walls of leptomeningeal and cortical vessels, is a major contributor to AD pathogenesis and is found in nearly 50% of sporadic AD cases (Jakel et al., 2021). CAA can lead to both micro- and macro- hemorrhages (Thal et al., 2003; Thal et al., 2008; Vinters, 1987). Adults with DS, especially those older than 55 years of age, consistently present with significant CAA (Belza and Urich, 1986; Head et al., 2017; Mann et al., 2018). In this population, increasing age is strongly associated with increased CAA severity (Head et al., 2017), and possible consequences include vascular dysfunction and disruptions in the blood brain barrier, among others, which can contribute to an earlier age of dementia onset.
Interestingly, individuals with DS have a low prevalence of systemic vascular risk factors (e.g. hypertension) and low risk for intracerebral hemorrhage, despite the high prevalence of obesity and sleep apnea (which can cause vascular disease), suggesting that genes on chromosome 21 and other factors may be protective (Buss et al., 2016; Morrison et al., 1996; Rodrigues et al., 2011). Some of the comorbidities associated with cerebrovascular disease or protection in DS include atherosclerosis and Moyamoya disease, hypertension/hypotension, dyslipidemia, obesity and sleep apnea, among others that will be discussed shortly (for complete reviews see (Carmona-Iragui et al., 2019; Wilcock et al., 2016). Cerebrovascular pathology in the brains of people with DS may also drive neuroinflammation. In work by Wilcock and colleagues, levels of pro-inflammatory factors including CHI3L3, IL-1Ra, CD86 and TGF-β were significantly elevated (Wilcock et al., 2015). These mediators are typically associated with the formation of immune complexes (Edwards et al., 2006; Sudduth et al., 2013) and suggest that the vascular leakage results in extravasation of serum proteins into the brain causing microglial activation which, in turn, may be linked to metabolic stress. The neurovascular unit – the union of endothelial cells, pericytes, astrocytes, neurons, and vascular smooth muscle cells – require ongoing metabolic adaptations to accommodate the brain’s blood flow and metabolic supplies. With ongoing aging processes, both neurovascular coupling or unit and blood brain barrier may become progressively perturbed, directly affecting cerebrovascular health. These and other perturbations could contribute to an earlier age of dementia onset in the DS population, and similar lines of inquiry could be very productive for better understanding DSAD-associated pathobiology broadly and beyond amyloidosis itself. Importantly, substantial cortical amyloidosis and bioenergetics may both exist subject to metabolically dyshomeostatic constraints in emerging AD (Agrawal et al., 2020; Wilkins and Swerdlow, 2017).
“Type III Diabetes” and lipid metabolism in DS and AD
AD has recently been described as “Type III diabetes” and this moniker serves to highlight the importance of metabolic contributions to its histopathological, molecular, and biochemical dyshomeostases (de la Monte, 2014; 2019; de la Monte et al., 2019; Kandimalla et al., 2017; Stanley et al., 2016). Along with dysregulated glucose metabolism itself, recent studies also demonstrate abnormal insulin signaling early in the development of DSAD (Tramutola et al., 2020). This accompanies an early-onset, disproportionately elevated occurrence of type 1 diabetes in people with DS (Aitken et al., 2013; Gillespie et al., 2006; Mortimer and Gillespie, 2020). Interestingly, type 2 diabetes in people with DS appears to be lower than in the general population (Esbensen, 2010; Jørgensen et al., 2019) despite sometimes substantial, sedentary dysmetabolic and lifestyle risk in aging (Agiovlasitis et al., 2020; Pape et al., 2021).
One early feature of diabetes is insulin resistance (IR), broadly studied in muscle and other tissues. Recent studies have shown that IR in the brain of neurotypical individuals matched on diabetes status was associated with AD neuropathology and cognitive function (Arvanitakis et al., 2020). Given that diabetes may cause vascular complications in many organs, it is not surprising that brain infarcts may be associated with diabetes and IR, and that IR in the periphery is related to cerebrovascular disease (Lee et al., 2016). Recently, an association of IR with cerebrovascular disease was reported in postmortem neurotypical human brains, a finding that links brain metabolism and function (Arvanitakis et al., 2021). Furthermore, in AD patients, CAA-related microbleeds are found along with gray matter atrophy and glucose hypometabolism, in positive correlation with cognitive function (Samuraki et al., 2015). In DS, brain IR develops early, in association with mitochondrial defects and loss of synaptic proteins, all of which may promote the development of AD (Barone, 2022; Tramutola et al., 2020), but more evidence for involvement in cerebrovascular disease is still needed.
Most strikingly, dysmetabolic risk factors including elevated triglycerides, total cholesterol, and central adiposity occur more frequently in people with DS despite lower rates of cardiovascular pathologies such as atherosclerosis and hypertension (Draheim et al., 2010; Wiseman et al., 2015; Zigman et al., 2007). This disconnect of metabolic and vascular features in people with DS contrasts with the typical concordance of dysmetabolic and vascular risk observed in the neurotypical population, where these factors in the absence of AD neuropathology can contribute to cognitive decline. Infarcts and vascular dementia, are relatively rare in DSAD compared to LOAD (Wiseman et al., 2015).
Cerebral glucose metabolism – a measure of metabolic dysfunction
Cerebral glucose metabolism can provide important insights into AD-related brain metabolic dysfunction in people with DS. Numerous studies have reported higher levels of glucose metabolism measured by FDG-PET in younger non-demented adults with DS (Azari et al., 1994; Cutler, 1986; Haier et al., 2003; Lengyel et al., 2006; Matthews et al., 2016; Schwartz et al., 1983) compared to similarly aged neurotypical adults (Schapiro et al., 1992). Most of these studies show increased glucose metabolism in prefrontal, sensorimotor, and inferior temporal/entorhinal cortices, in addition to the thalamus. Haier and colleagues (2008) also show that increased glucose metabolism is linked to decreased gray matter volume in the temporal cortex (parahippocampus/hippocampus), suggesting that hypermetabolism non-demented adults with DS may be an early compensatory response to neuronal loss (Haier et al., 2008). However, as evidence of cognitive decline or dementia emerges, FDG-PET studies consistently indicate a loss of glucose metabolism, particularly in posterior brain regions including posterior cingulate cortex, hippocampus, parietal, and temporal cortex (Azari et al., 1994; Cutler, 1986; Head et al., 2018; Lao et al., 2018; Matthews et al., 2016; Neale et al., 2018; Rafii et al., 2017; Rafii et al., 2015; Sabbagh et al., 2015; Schapiro et al., 1992; Schwartz et al., 1983; Zammit et al., 2020).
Many studies have now shown a link between brain regions where glucose metabolism declines with age and the emergence of AD pathology. Specifically, reduced glucose metabolism in older adults with DS and dementia is associated with decreased cortical volumes (Matthews et al., 2016), increased amyloid PET binding (Lao et al., 2018; Matthews et al., 2016) and increased tau PET binding (Rafii et al., 2017). An exception to negative correlations between amyloid accumulation (amyloid PET) and metabolism (FDG-PET) is in the putamen, which is affected early during aging in DS where higher amyloid binding is linked to higher glucose metabolism (Zammit et al., 2020). This finding corresponds with recent amyloid PET studies in people with DS suggesting that striatal subcortical regions disproportionately accumulate early amyloid as in ADAD, but unlike LOAD (Cohen et al., 2018). Recent work by Fortea and colleagues (2021) further suggests that the presence of the APOE4 allele, one of the strongest risk factors for AD, shifts brain glucose hypometabolism to younger ages in people with DS (Bejanin et al., 2021).
Critically, disordered glucose metabolism observed in DSAD (Haier et al., 2003; Zammit et al., 2020) could reflect more than simply neurodegeneration alone, in contrast with some prevailing, common interpretations of glucose hypometabolism in sporadic AD (Jack et al., 2018; Jagust, 2018). This possibility remains to be examined in detail in DSAD; however, accumulating evidence suggests this to be the case in sporadic, euploid LOAD. Specifically, AD-associated changes in cortical glucose metabolism impact primate-specific (if not human-specific) neocortical metabolism via aerobic glycolysis and associated biosynthesis (Bauernfeind and Babbitt, 2014; Bauernfeind et al., 2014; Goyal et al., 2020; Terada et al., 2020; Terada et al., 2021; Vlassenko et al., 2018; Vlassenko et al., 2010). These AD-associated changes are also dynamically hypo- and hyper-metabolic in the sequence of advancing cognitive decline (Ashraf et al., 2015; Corriveau-Lecavalier et al., 2019; Dickerson et al., 2005), spare (if not favor) ketone body fuel metabolism (Castellano et al., 2019; Castellano et al., 2015; Croteau et al., 2018; Cunnane et al., 2020), and exist in white matter tracts apart from neuronal cell bodies ultimately subject to degeneration (Roy et al., 2020).
Recent imaging studies targeting the synaptic density/ neurodegenerative marker synaptic vesicle glycoprotein 2A (SV2A) most directly suggest that glucose hypometabolism in LOAD does not directly correspond to neurodegeneration. In one study, the neocortex, but not medial temporal lobes, demonstrated glucose metabolic deficits in excess of those accounted for by neurodegeneration alone (Chen et al., 2021). Further studies should address if similar interregional neurometabolic dissociations can be observed in emerging DSAD, especially as they relate to complex patterns of dynamic change and homoeostasis, rather than dysmetabolism associated with frank cortical atrophy itself. Importantly, this could include or be mediated by diffusible signaling molecules such as insulin.
How does trisomy 21 contribute to metabolic dysfunction in DSAD beyond the role of APP?
Genetic overexpression of the APP gene explains the substantial cortical amyloidosis observed in aging adults with DS. APP overexpression may also precipitate downstream proteopathies (i.e. neuritic Aβ plaques, neurofibrillary tangles), neurodegeneration, and cognitive deficits in these adults as they age (Doran et al., 2017; Head et al., 2012; Lott and Head, 2005; Teller et al., 1996). Supporting the importance of APP gene dosage in DSAD, two case studies have reported cognitive sparing in aging adults with DS who possessed otherwise euploid copy number for APP (Doran et al., 2017; Prasher et al., 1998). Interestingly, recent findings challenge whether overabundant APP production alone is sufficient or necessary to initiate and drive cognitive decline in DSAD (Ovchinnikov et al., 2018; Wiseman et al., 2018) based upon work in mouse models of DS and induced pluripotent stem cell (iPSC) derived from DS participants. Evidence for successful cognitive aging, hereby considered as a lack of decline in cognitive or functional capacities indicative of dementia, in humans with DS is limited, but one case with complete, non-mosaic trisomy 21 has been described based on cognitive monitoring, without biomarker assessment (Krinsky-McHale et al., 2008). Thus, although there is strong evidence that APP overexpression in aging people with DS drives AD, it will be important to consider additional mechanisms and genes (Table 1).
Table 1.
Selected Human Chromosome 21 Genes with Metabolic Ontologies
| Gene Name (HGNC ID) | Cytoband Coordinates |
DSCR* Locus? |
Metabolic Gene Ontologies |
|---|---|---|---|
| holocarboxylase synthetase (HLCS) | 21q22.13 | Yes | biotin metabolism lipid, protein, and carbohydrate carboxylation |
| phosphatidylinositol glycan anchor biosynthesis class P (PIGP) | 21q22.13 | Yes | lipid metabolism |
| cystathionine beta-synthase (CBS) | 21q22.3 | Yes | homocysteine, transulfuration, and folate metabolism |
| phosphofructokinase liver type (PFKL) | 21q22.3 | No | glucose metabolism |
| FAM3 metabolism regulating signaling molecule B (FAM3B) | 21q22.3 | No | glucose metabolism |
| Lipase I (LIPI) | 21q11.2 | No | lipid metabolism |
| 1-acylglycerol-3-phosphate O-acyltransferase 3 (AGPAT3) | 21q22.3 | No | lipid metabolism |
| superoxide dismutase (SOD1) | 21q22.11 | No | redox metabolism |
| glutathione peroxidase pseudogene 2 (GPX1P2) | 21q21.3 | No | redox metabolism |
| regulator of calcineurin 1 (RCAN1) | 21q22.12 | No | insulin homeostasis |
DSCR Down Syndrome Critical Region on Chromosome 21.
Of the approximately 284 protein coding, predicted, and pseudo-genes on chromosome 21 (Gardiner and Davisson, 2000; Hattori et al., 2000), several are directly and indirectly involved in metabolic processes. Within the Down Syndrome Critical Region (DSCR), these include the holocarboxylase synthetase (HLCS) gene involved in the activating conjugation of biotin to carboxylase enzymes which metabolize lipids, proteins, and carbohydrates. This region of chromosome 21 also encodes the gene phosphatidylinositol glycan anchor biosynthesis class P (PIGP), which participates in inositol-phospholipid anchor formation where its dysfunction has been linked to several blood disorders. Additionally, the gene encoding the enzyme cystathionine beta-synthase (CBS) localizes to this same genomic region of chromosome 21 and participates in homocysteine, transulfuration, and folate biochemical pathways (Iacobazzi et al., 2014). Other genes present on chromosome 21 outside the DSCR encode components of glucose metabolism (phosphofructokinase liver type, PFKL; FAM3 metabolism regulating signaling molecule B, FAM3B) and lipid metabolism (lipase 1, LIPI; 1-acylglycerol-3-phosphate O-acyltransferase 3, AGPAT3) in addition to redox-associated genes (superoxide dismutase, SOD1; glutathione peroxidase pseudogene 2, GPX1P2). Of particular interest, PFKL regulates the second of three enzymatically rate-limiting steps of glycolysis by catalyzing the phosphorylation of fructose-6-phosphate to fructose-1,6-bisphosphate. This liver-expressed isoenzyme of phosphofructokinase is also active in the brain. In DS, PFKL is overexpressed in a manner consistent with the anticipated 3:2 trisomic gene dosage ratio and has been associated with glucose dysmetabolism in AD broadly (Bigl et al., 1996; Elson et al., 1992; Elson et al., 1994; Sims et al., 1987).
Overproduction of phosphofructokinase resulting from triplication of PFKL may also explain the faster brain glucose metabolic rate seen in younger individuals with DS (Lengyel et al., 2006), but the reasons for glucose hypometabolism in older adults with DS described previously and especially those with LOAD remains unclear. These similarities in brain glucose dysmetabolism suggest a possible common final pathway despite and perhaps as a function of dissociable genetic contributions across predisposing etiologies in AD. Future investigations in this population might consider mechanistically how the development of cognitive decline in AD represents both the incidence of “progressive proteopathies with many metabolic spectators,” yet also a “progressive metabolic disorder with associated proteopathies” in aging. In this capacity, APP triplication could directly or indirectly modulate several interesting and metabolically relevant genes on chromosome 21 possibly important in AD pathogenesis.
Chromosome 21 also encodes several genes which may contribute to insulin dyshomeostasis observed in both LOAD and DSAD, specifically as these relate to the elevated risk of type 1 diabetes in DS. This may occur through their effect of indirectly increasing the genetic penetrance of human leukocyte antigen (HLA) risk haplotypes associated with type 1 diabetes overall (Aitken et al., 2013; Gillespie et al., 2006; Labudova et al., 1999; Mortimer and Gillespie, 2020). Specifically, the chromosome 21 gene Down Syndrome Critical Region Gene 1 (DSCR1, RCAN1), when overexpressed in rodent models, has been associated with pancreatic β-cell mitochondrial dysfunction, decreased ATP synthesis, and blunted glucose-stimulated insulin secretion like that seen in people with DS, who also demonstrate increased levels of DSCR1-mediated oxidative stress (Helguera et al., 2013; Peiris et al., 2016). It remains to be seen if these same risk genes or gene × gene interactions moderate cognitive trajectories in aging individuals with DS. This includes the consideration of insulin dyshomeostasis specifically as a driver of AD and DSAD.
Bioenergetic dyshomeostasis: cause or consequence of DS and AD?
Because DS itself is rarely heritable, it might be possible that those parental and sporadically heritable genetic factors leading to the birth of a person with DS might also relate to AD outcomes in parents (particularly mothers), but possibly more distant family relations. There is some support for this notion of joint overrepresentation of DS and LOAD within these families (Petronis, 1999) but see also: (Berr et al., 1989). Thus, the genetic architecture and associated systems biology underlying the shared risk of DS and AD remains incompletely considered across LOAD and DSAD and potentially dissociable from trisomy 21 gene dosage effects themselves. Aneuploidy itself almost certainly contributes directly to the cognitive decline in DS through the copy number expansion of genes such as APP. Overexpression of chromosome 21 genes consequent to trisomy and resulting in age-associated neurodegeneration may thus represent a second, de novo genomic insult following from or possibly recapitulating early developmental vulnerabilities to DS within gametes and fertilized, reproductive tissues undergoing active mitosis. Another compelling line of evidence linking DS and maternal biology shows there is an increased risk of AD in mothers of DS adults, particularly those who completed such pregnancies prior to age 35 (Kline et al., 2000; Schupf et al., 1994; Schupf et al., 2001). This represents a four to five-fold elevated risk of AD neurodegeneration specifically compared to mothers of neurotypical children, but not fathers (Schupf et al., 2001). Advancing maternal age has also been linked to impaired cognitive aging in women and their chromosomally typical offspring (Mosconi et al., 2017; Mosconi et al., 2007; Mosconi et al., 2012; Rahman et al., 2020; Scheyer et al., 2018).
One possible mechanism linking risk of AD in mothers and their children with DS could be mitochondrial dysfunction associated with abnormal aging. There is a rich literature indicating mitochondrial and reactive oxygen species (ROS) dyshomeostasis in DS and DSAD (Busciglio and Yankner, 1995; Coskun and Busciglio, 2012; Lott, 2012; Pagano and Castello, 2012; Perluigi and Butterfield, 2012), which implicates metabolic and bioenergetic processes as drivers of emerging cognitive vulnerability (Cunnane et al., 2020; Zilberter and Zilberter, 2017). Consistent with complex, sporadic heritability, these metabolic and bioenergetic processes demonstrate potential maternal imprinting effects (Mosconi et al., 2010; Mosconi et al., 2011; Schupf et al., 1994; Schupf et al., 2001). Schon and colleagues suggest the bioenergetically demanding process of typical euploid chromosomal segregation within developing oocytes may itself depend upon the integrity of mitochondria and mitochondrial genomes within these reproductive cells (Schon et al., 2000). This is consistent with the hypothesis that subsequently emerging AD may mechanistically recapitulate heritable, early developmental risk predisposing toward DS. These same mechanisms have been considered by Mosconi and colleagues in AD, where maternal, but not paternal, family history of AD predisposes individuals toward a diversity of dementia-associated biological risk (Mosconi et al., 2010; Mosconi et al., 2007; Mosconi et al., 2011; Mosconi et al., 2012). Critically, these processes in AD progression are frequently associated with mitochondrial and bioenergetic dysmetabolism within an abnormally aging brain-peripheral metabolic axis spanning multiple scales of systems biology (Astarita et al., 2010; Astarita and Piomelli, 2011; Bassendine et al., 2020; Camandola and Mattson, 2017; Demetrius and Driver, 2013; Folch et al., 2019; Ghosh et al., 2018; Kim and Mook-Jung, 2019; Morris et al., 2019; Neth and Craft, 2017; Peng et al., 2020; Qi et al., 2019; Raz and Daugherty, 2018; Wang et al., 2017). This metabolic axis may jointly mediate A) critical embryological events in DS development and B) sporadic genetic risk for abnormal aging relevant to LOAD or DSAD.
A similar pattern of complex heritable liability may exist in the overlap between DS, AD, and metabolic syndrome. Specifically, diabetic mothers may be at higher risk of giving birth to infants with DS (Narchi and Kulaylat, 1997) (although see Martinez-Frias and colleagues: (Martínez-Frías et al., 2002)). Consistent with mitochondrial and bioenergetic heritable risk in DS and DSAD, intergenerational findings may also suggest that dysmetabolism could initially follow from elevated sporadically heritable risk dissociable from, but predisposing toward trisomy 21 during early embryonic development. Were this the case, mothers, and particularly young mothers of children with DS might demonstrate biological differences compared to parents of euploid children. Furthermore, these differences might anticipate and resemble changes observed in the evolution of LOAD and DSAD.
Peripheral lymphocytes from young mothers of DS infants contain several markers of genome instability and premature aging, including a higher frequency of micronuclei, telomere attrition, and global changes in DNA methylation patterns (Albizua et al., 2015; Božović et al., 2015; Migliore et al., 2006). Deficits in maternal folate metabolism and its epigenetic regulation have been similarly reported in this population relative to control mothers (Coppedè, 2016; Coppedè et al., 2016). All of these biological systems which are implicated in the pathophysiological evolution of LOAD, ADAD, and DSAD represent metabolically intensive, anabolic processes maintained at substantially catabolic, bioenergetic cost in vulnerably aging adults (López-Otín et al., 2016; Mattson and Arumugam, 2018; Nitsch et al., 1992; Rijpma et al., 2017; Saez-Atienzar and Masliah, 2020; Vlassenko and Raichle, 2015; Wurtman, 2011; 2014; Wurtman et al., 1985; Zhang and Raichle, 2010). Critically, these dyshomeostatic processes might pathologically self-organize to a point of irreparable failure which is incompatible with successful cognitive aging in people with DS and leads to clinical and neuropathological AD.
Conclusions
Although Jerome Lejeune is best known for helping to establish the trisomic basis of DS, his later work focused on DS and DSAD cognition in metabolic terms (Lejeune, 1990; Lejeune et al., 1990). This perspective remains understudied in DSAD, but is seeing application now in recent perspectives in LOAD (Goyal et al., 2020; Pettegrew et al., 1988; Rijpma et al., 2018; Vlassenko et al., 2018). Metabolic deficits are a prevalent feature of the AD pathobiological process, including those changes specifically accompanying DSAD (Gross et al., 2019; Mapstone et al., 2020; Pecze et al., 2020; Pecze and Szabo, 2021). Importantly, these findings not only suggest read outs of emerging metabolic dysfunction, but might also advance specific therapeutic strategies to normalize and support vulnerable metabolic pathways in DS aging (Fortier et al., 2021; Soininen et al., 2021; Wurtman, 2011). Lejeune’s observations on metabolic dysfunction in DSAD parallel a longstanding literature investigating metabolic therapeutics in AD (Cunnane et al., 2011; Cunnane et al., 2020; Jennings et al., 2020; Nitsch et al., 1992; Rijpma et al., 2017; Wurtman, 2011).
The recent development of advanced metabolomics technologies has prompted the reconciliation of molecular and metabolic perspectives on multifactorial diseases of abnormal aging including AD and DSAD (McKnight, 2010). This accords with recently proposed translational policy and funding initiatives to advance the study and treatment of biologically complex, refractory human diseases through data-intensive technologies including -omics methods (Collins et al., 2021). Omics-scale measurement approaches including metabolomics have allowed researchers to explicitly pursue metabolic hypotheses of the kind suggested by Lejeune and others in DS and LOAD. These metabolic considerations have, however, only been recently considered in their specific contributions to the pathogenesis and dynamic course of DSAD.
More broadly in abnormal aging, the catabolic functions of metabolism could often belie the anabolically vital biosynthetic and signaling roles often served by these same biomolecules in support of prolonged cognitive resilience. Critically, all such metabolic programs may become limited as a function of DS, advancing AD, or their combination in pathological aging. This presents a particular challenge because the extent, scale, duration, and scope of these changes in the DSAD pathobiological process remain unclear. The current limits on this knowledge, however, also suggest many opportunities to identify novel therapeutic targets and biomarkers in LOAD and DSAD specifically.
Amyloid-attenuating pharmaceutical therapies have recently been approved by the FDA; however, their efficacy to robustly halt, reverse, or stabilize emerging cognitive deficits in prodromal LOAD remains to be clarified in coming years (Nisticò and Borg, 2021). This only encourages the pursuit of Lejeune’s assertion in DS that: “[…] victory over the neural disturbances resulting from the genetic overdose of trisomy 21 would very likely also lead to a cure or to a prevention of Alzheimer [sic] dementia” (Lejeune, 1990). For DS and other dissociable, AD-risk-imposing etiologies, these dynamics of biologically and metabolically “futile cycles” could accompany, if not drive dementia progression in which “compensations for failure” precipitate biologically non-random “failures of compensation.”
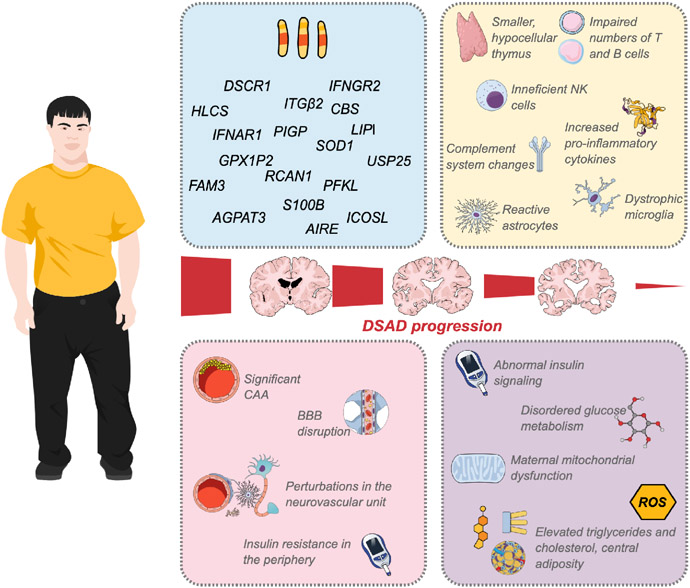
Figure 2. Systems and mechanisms altered in Down syndrome leading to DSAD.
Immune- and metabolism-related genes encoded on Chromosome 21 may lead to and potentially explain the altered expression and function of cells and mediators in DS. Disturbances in innate and adaptive immunity and inflammatory response may include a smaller and hypocellular thymus gland; impaired numbers of T and B lymphocytes, increasing the propensity to autoimmune disorders; changes in the function and counts of NK cells; overexpression of inflammatory cytokines; increased C1q in the brain and deficiency of complement factors in blood; and, ultimately, changes in the phenotypes of essential cells like microglia and astrocytes. In the vascular system, CAA is a critical component of AD pathogenesis, and frequently found in aged people with DS. Its consequences may include disruptions in the BBB and neurovascular unit. In the periphery, insulin resistance can also contribute to cerebrovascular disease. Dysregulated insulin signaling and glucose metabolism are observed early in the development of DSAD. Additionally, maternal mitochondrial dysfunction, ROS dyshomeostasis, and dysmetabolic risk factors represented by elevated triglycerides, cholesterol, and adiposity, may promote the development of AD in DS. Created with MindtheGraph.com.
Acknowledgements:
The authors are grateful for support from the National Institute on Aging (U19AG068054, U01AG051412, U01AG051406, P50AG016573) the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD064993), and the INCLUDE (INvestigation of Co-occurring conditions across the Lifespan to Understand Down syndromE) Project. Additional support was from the Brightfocus Foundation (CA2018010).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This review by Martini et al discusses evidence for immune, cerebrovascular, and metabolic contributions to Alzheimer’s disease risk in people with Down syndrome. The authors argue that these mechanisms may interact with characteristic amyloid over expression due to trisomy 21.
REFERENCES
- Agiovlasitis S, Choi P, Allred AT, Xu J, and Motl RW (2020). Systematic review of sedentary behaviour in people with Down syndrome across the lifespan: A clarion call. J Appl Res Intellect Disabil 33, 146–159. 10.1111/jar.12659. [DOI] [PubMed] [Google Scholar]
- Agrawal RR, Montesinos J, Larrea D, Area-Gomez E, and Pera M (2020). The silence of the fats: A MAM’s story about Alzheimer. Neurobiology of Disease 145, 105062. 10.1016/j.nbd.2020.105062. [DOI] [PubMed] [Google Scholar]
- Aiello A, Farzaneh F, Candore G, Caruso C, Davinelli S, Gambino CM, Ligotti ME, Zareian N, and Accardi G (2019). Immunosenescence and Its Hallmarks: How to Oppose Aging Strategically? A Review of Potential Options for Therapeutic Intervention. Front Immunol 10, 2247. 10.3389/fimmu.2019.02247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken RJ, Mehers KL, Williams AJ, Brown J, Bingley PJ, Holl RW, Rohrer TR, Schober E, Abdul-Rasoul MM, Shield JP, and Gillespie KM (2013). Early-onset, coexisting autoimmunity and decreased HLA-mediated susceptibility are the characteristics of diabetes in Down syndrome. Diabetes Care 36, 1181–1185. 10.2337/dc12-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albizua I, Rambo-Martin BL, Allen EG, He W, Amin AS, and Sherman SL (2015). Association between telomere length and chromosome 21 nondisjunction in the oocyte. Hum Genet 134, 1263–1270. 10.1007/s00439-015-1603-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alić I, Goh PA, Murray A, Portelius E, Gkanatsiou E, Gough G, Mok KY, Koschut D, Brunmeir R, Yeap YJ, et al. (2020). Patient-specific Alzheimer-like pathology in trisomy 21 cerebral organoids reveals BACE2 as a gene dose-sensitive AD suppressor in human brain. Mol Psychiatry. 10.1038/s41380-020-0806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altuna M, Gimenez S, and Fortea J (2021). Epilepsy in Down Syndrome: A Highly Prevalent Comorbidity. J Clin Med 10. 10.3390/jcm10132776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer A (1906). Über einen eigenartigen schweren Erkrankungsprozeβ der Hirnrincle. Neurol Central 25. [Google Scholar]
- Araya P, Waugh KA, Sullivan KD, Nunez NG, Roselli E, Smith KP, Granrath RE, Rachubinski AL, Enriquez Estrada B, Butcher ET, et al. (2019). Trisomy 21 dysregulates T cell lineages toward an autoimmunity-prone state associated with interferon hyperactivity. Proc Natl Acad Sci U S A 116, 24231–24241. 10.1073/pnas.1908129116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenas F, Castro F, Nunez S, Gay G, Garcia-Ruiz C, and Fernandez-Checa JC (2020). STARD1 and NPC1 expression as pathological markers associated with astrogliosis in post-mortem brains from patients with Alzheimer's disease and Down syndrome. Aging (Albany NY) 12, 571–592. 10.18632/aging.102641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitakis Z, Capuano AW, Wang HY, Schneider JA, Kapasi A, Bennett DA, Ahima RS, and Arnold SE (2021). Brain insulin signaling and cerebrovascular disease in human postmortem brain. Acta Neuropathol Commun 9, 71. 10.1186/s40478-021-01176-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitakis Z, Wang HY, Capuano AW, Khan A, Taib B, Anokye-Danso F, Schneider JA, Bennett DA, Ahima RS, and Arnold SE (2020). Brain Insulin Signaling, Alzheimer Disease Pathology, and Cognitive Function. Ann Neurol 88, 513–525. 10.1002/ana.25826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf A, Fan Z, Brooks DJ, and Edison P (2015). Cortical hypermetabolism in MCI subjects: a compensatory mechanism? Eur J Nucl Med Mol Imaging 42, 447–458. 10.1007/s00259-014-2919-z. [DOI] [PubMed] [Google Scholar]
- Astarita G, Jung KM, Berchtold NC, Nguyen VQ, Gillen DL, Head E, Cotman CW, and Piomelli D (2010). Deficient liver biosynthesis of docosahexaenoic acid correlates with cognitive impairment in Alzheimer's disease. PLoS One 5, e12538. 10.1371/journal.pone.0012538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astarita G, and Piomelli D (2011). Towards a whole-body systems [multi-organ] lipidomics in Alzheimer's disease. Prostaglandins Leukot Essent Fatty Acids 85, 197–203. 10.1016/j.plefa.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aversa T, Valenzise M, Salerno M, Corrias A, Iughetti L, Radetti G, De Luca F, and Wasniewska M (2015). Metamorphic thyroid autoimmunity in Down Syndrome: from Hashimoto's thyroiditis to Graves' disease and beyond. Ital J Pediatr 41, 87. 10.1186/s13052-015-0197-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azari NP, Horwitz B, Pettigrew KD, Grady CL, Haxby JV, Giacometti KR, and Schapiro MB (1994). Abnormal pattern of cerebral glucose metabolic rates involving language areas in young adults with Down syndrome. Brain and language 46, 1–20. 10.1006/brln.1994.1001. [DOI] [PubMed] [Google Scholar]
- Baik SH, Kang S, Lee W, Choi H, Chung S, Kim JI, and Mook-Jung I (2019). A Breakdown in Metabolic Reprogramming Causes Microglia Dysfunction in Alzheimer's Disease. Cell Metab 30, 493–507 e496. 10.1016/j.cmet.2019.06.005. [DOI] [PubMed] [Google Scholar]
- Barone E (2022). Brain insulin resistance: an early risk factor for Alzheimer's disease development in Down syndrome. Neural Regen Res 17, 333–335. 10.4103/1673-5374.317979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrena MJ, Echaniz P, Garcia-Serrano C, and Cuadrado E (1993). Imbalance of the CD4+ subpopulations expressing CD45RA and CD29 antigens in the peripheral blood of adults and children with Down syndrome. Scand J Immunol 38, 323–326. 10.1111/j.1365-3083.1993.tb01733.x. [DOI] [PubMed] [Google Scholar]
- Bassendine MF, Taylor-Robinson SD, Fertleman M, Khan M, and Neely D (2020). Is Alzheimer's Disease a Liver Disease of the Brain? J Alzheimers Dis 75, 1–14. 10.3233/JAD-190848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauernfeind AL, and Babbitt CC (2014). The appropriation of glucose through primate neurodevelopment. J Hum Evol 77, 132–140. 10.1016/j.jhevol.2014.05.016. [DOI] [PubMed] [Google Scholar]
- Bauernfeind AL, Barks SK, Duka T, Grossman LI, Hof PR, and Sherwood CC (2014). Aerobic glycolysis in the primate brain: reconsidering the implications for growth and maintenance. Brain Struct Funct 219, 1149–1167. 10.1007/s00429-013-0662-z. [DOI] [PubMed] [Google Scholar]
- Beckhaus AA, and Castro-Rodriguez JA (2018). Down Syndrome and the Risk of Severe RSV Infection: A Meta-analysis. Pediatrics 142. 10.1542/peds.2018-0225. [DOI] [PubMed] [Google Scholar]
- Bejanin A, Iulita MF, Vilaplana E, Carmona-Iragui M, Benejam B, Videla L, Barroeta I, Fernandez S, Altuna M, Pegueroles J, et al. (2021). Association of Apolipoprotein E varepsilon4 Allele With Clinical and Multimodal Biomarker Changes of Alzheimer Disease in Adults With Down Syndrome. JAMA Neurol. 10.1001/jamaneurol.2021.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belza MG, and Urich H (1986). Cerebral amyloid angiopathy in Down's syndrome. Clin Neuropathol 5, 257–260. [PubMed] [Google Scholar]
- Bernier LP, York EM, and MacVicar BA (2020). Immunometabolism in the Brain: How Metabolism Shapes Microglial Function. Trends Neurosci 43, 854–869. 10.1016/j.tins.2020.08.008. [DOI] [PubMed] [Google Scholar]
- Berr C, Borghi E, Rethoré MO, Lejeune J, and Alperovitch A (1989). Absence of familial association between dementia of Alzheimer type and Down syndrome. Am J Med Genet 33, 545–550. 10.1002/ajmg.1320330427. [DOI] [PubMed] [Google Scholar]
- Bigl M, Bleyl AD, Zedlick D, Arendt T, Bigl V, and Eschrich K (1996). Changes of activity and isozyme pattern of phosphofructokinase in the brains of patients with Alzheimer's disease. Journal of neurochemistry 67, 1164–1171. 10.1046/j.1471-4159.1996.67031164.x. [DOI] [PubMed] [Google Scholar]
- Bittles AH, Bower C, Hussain R, and Glasson EJ (2007). The four ages of Down syndrome. Eur J Public Health 17, 221–225. 10.1093/eurpub/ckl103. [DOI] [PubMed] [Google Scholar]
- Blalock EM, Grondin R, Chen KC, Thibault O, Thibault V, Pandya JD, Dowling A, Zhang Z, Sullivan P, Porter NM, and Landfield PW (2010). Aging-related gene expression in hippocampus proper compared with dentate gyrus is selectively associated with metabolic syndrome variables in rhesus monkeys. J Neurosci 30, 6058–6071. 10.1523/JNEUROSCI.3956-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloemers BL, Broers CJ, Bont L, Weijerman ME, Gemke RJ, and van Furth AM (2010a). Increased risk of respiratory tract infections in children with Down syndrome: the consequence of an altered immune system. Microbes Infect 12, 799–808. 10.1016/j.micinf.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Bloemers BL, van Bleek GM, Kimpen JL, and Bont L (2010b). Distinct abnormalities in the innate immune system of children with Down syndrome. J Pediatr 156, 804–809, 809 e801-809 e805. 10.1016/j.jpeds.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Božović IB, Stanković A, Živković M, Vraneković J, Kapović M, and Brajenović-Milić B (2015). Altered LINE-1 Methylation in Mothers of Children with Down Syndrome. PLoS One 10, e0127423. 10.1371/journal.pone.0127423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busciglio J, and Yankner BA (1995). Apoptosis and increased generation of reactive oxygen species in Down's syndrome neurons in vitro. Nature 378, 776–779. 10.1038/378776a0. [DOI] [PubMed] [Google Scholar]
- Buss L, Fisher E, Hardy J, Nizetic D, Groet J, Pulford L, and Strydom A (2016). Intracerebral haemorrhage in Down syndrome: protected or predisposed? F1000Res 5. 10.12688/f1000research.7819.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camandola S, and Mattson MP (2017). Brain metabolism in health, aging, and neurodegeneration. EMBO J 36, 1474–1492. 10.15252/embj.201695810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capone G, Stephens M, Santoro S, Chicoine B, Bulova P, Peterson M, Jasien J, Smith AJ, and Down Syndrome Medical Interest Group Adult Health, W. (2020). Co-occurring medical conditions in adults with Down syndrome: A systematic review toward the development of health care guidelines. Part II. Am J Med Genet A. 10.1002/ajmg.a.61604. [DOI] [PubMed] [Google Scholar]
- Capone GT, Chicoine B, Bulova P, Stephens M, Hart S, Crissman B, Videlefsky A, Myers K, Roizen N, Esbensen A, et al. (2018). Co-occurring medical conditions in adults with Down syndrome: A systematic review toward the development of health care guidelines. American Journal of Medical Genetics, Part A 176, 116–133. 10.1002/ajmg.a.38512. [DOI] [PubMed] [Google Scholar]
- Carmona-Iragui M, Videla L, Lleo A, and Fortea J (2019). Down syndrome, Alzheimer disease, and cerebral amyloid angiopathy: The complex triangle of brain amyloidosis. Dev Neurobiol 79, 716–737. 10.1002/dneu.22709. [DOI] [PubMed] [Google Scholar]
- Carsetti R, Valentini D, Marcellini V, Scarsella M, Marasco E, Giustini F, Bartuli A, Villani A, and Ugazio AG (2015). Reduced numbers of switched memory B cells with high terminal differentiation potential in Down syndrome. Eur J Immunol 45, 903–914. 10.1002/eji.201445049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CO, Hamerton JL, Polani PE, Gunalp A, and Weller SD (1960). Chromosome translocation as a cause of familial mongolism. Lancet 2, 678–680. 10.1016/s0140-6736(60)91749-9. [DOI] [PubMed] [Google Scholar]
- Castellano CA, Hudon C, Croteau E, Fortier M, St-Pierre V, Vandenberghe C, Nugent S, Tremblay S, Paquet N, Lepage M, et al. (2019). Links Between Metabolic and Structural Changes in the Brain of Cognitively Normal Older Adults: A 4-Year Longitudinal Follow-Up. Front Aging Neurosci 11, 15. 10.3389/fnagi.2019.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano CA, Nugent S, Paquet N, Tremblay S, Bocti C, Lacombe G, Imbeault H, Turcotte É, Fulop T, and Cunnane SC (2015). Lower brain 18F-fluorodeoxyglucose uptake but normal 11C-acetoacetate metabolism in mild Alzheimer's disease dementia. J Alzheimers Dis 43, 1343–1353. 10.3233/JAD-141074. [DOI] [PubMed] [Google Scholar]
- Cetiner S, Demirhan O, Inal TC, Tastemir D, and Sertdemir Y (2010). Analysis of peripheral blood T-cell subsets, natural killer cells and serum levels of cytokines in children with Down syndrome. Int J Immunogenet 37, 233–237. 10.1111/j.1744-313X.2010.00914.x. [DOI] [PubMed] [Google Scholar]
- Chan M, Park JJ, Shi T, Martinon-Torres F, Bont L, Nair H, and Respiratory Syncytial Virus, N. (2017). The burden of respiratory syncytial virus (RSV) associated acute lower respiratory infections in children with Down syndrome: A systematic review and meta-analysis. J Glob Health 7, 020413. 10.7189/jogh.07.020413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chausse B, Kakimoto PA, and Kann O (2021). Microglia and lipids: how metabolism controls brain innate immunity. Semin Cell Dev Biol 112, 137–144. 10.1016/j.semcdb.2020.08.001. [DOI] [PubMed] [Google Scholar]
- Chen C, Jiang P, Xue H, Peterson SE, Tran HT, McCann AE, Parast MM, Li S, Pleasure DE, Laurent LC, et al. (2014). Role of astroglia in Down's syndrome revealed by patient-derived human-induced pluripotent stem cells. Nature communications 5, 4430. 10.1038/ncomms5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M-K, Mecca AP, Naganawa M, Gallezot J-D, Toyonaga T, Mondal J, Finnema SJ, Lin S.-f., O’Dell RS, and McDonald JW (2021). Comparison of [11C] UCB-J and [18F] FDG PET in Alzheimer’s disease: A tracer kinetic modeling study. Journal of Cerebral Blood Flow & Metabolism, 0271678X211004312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke CM, Edwards JH, and Smallpeice V (1961). 21-trisomy/normal mosaicism in an intelligent child with some mongoloid characters. Lancet 1, 1028–1030. 10.1016/s0140-6736(61)91833-5. [DOI] [PubMed] [Google Scholar]
- Clarke CM, Ford CE, Edwards JH, and Smallpeice V (1963). 21 Trisomy/Normal Mosaicism in an Intelligent Child with Some Mongoloid Characters. Lancet 2, 1229. 10.1016/s0140-6736(63)92959-3. [DOI] [PubMed] [Google Scholar]
- Cody KA, Piro-Gambetti B, Zammit MD, Christian BT, Handen BL, Klunk WE, Zaman S, Johnson SC, Plante DT, and Hartley SL (2020). Association of sleep with cognition and beta amyloid accumulation in adults with Down syndrome. Neurobiol Aging 93, 44–51. 10.1016/j.neurobiolaging.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AD, McDade E, Christian B, Price J, Mathis C, Klunk W, and Handen BL (2018). Early striatal amyloid deposition distinguishes Down syndrome and autosomal dominant Alzheimer's disease from late-onset amyloid deposition. Alzheimers Dement 14, 743–750. 10.1016/j.jalz.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins FS, Schwetz TA, Tabak LA, and Lander ES (2021). ARPA-H: Accelerating biomedical breakthroughs. Science 373, 165–167. 10.1126/science.abj8547. [DOI] [PubMed] [Google Scholar]
- Coppedè F (2016). Risk factors for Down syndrome. Arch Toxicol 90, 2917–2929. 10.1007/s00204-016-1843-3. [DOI] [PubMed] [Google Scholar]
- Coppedè F, Denaro M, Tannorella P, and Migliore L (2016). Increased MTHFR promoter methylation in mothers of Down syndrome individuals. Mutat Res 787, 1–6. 10.1016/j.mrfmmm.2016.02.008. [DOI] [PubMed] [Google Scholar]
- Corriveau-Lecavalier N, Mellah S, Clément F, and Belleville S (2019). Evidence of parietal hyperactivation in individuals with mild cognitive impairment who progressed to dementia: A longitudinal fMRI study. NeuroImage. Clinical 24, 101958. 10.1016/j.nicl.2019.101958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskun PE, and Busciglio J (2012). Oxidative Stress and Mitochondrial Dysfunction in Down's Syndrome: Relevance to Aging and Dementia. Curr Gerontol Geriatr Res 2012, 383170. 10.1155/2012/383170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossarizza A, Monti D, Montagnani G, Ortolani C, Masi M, Zannotti M, and Franceschi C (1990). Precocious aging of the immune system in Down syndrome: alteration of B lymphocytes, T-lymphocyte subsets, and cells with natural killer markers. Am J Med Genet Suppl 7, 213–218. 10.1002/ajmg.1320370743. [DOI] [PubMed] [Google Scholar]
- Croteau E, Castellano CA, Fortier M, Bocti C, Fulop T, Paquet N, and Cunnane SC (2018). A cross-sectional comparison of brain glucose and ketone metabolism in cognitively healthy older adults, mild cognitive impairment and early Alzheimer's disease. Exp Gerontol 107, 18–26. 10.1016/j.exger.2017.07.004. [DOI] [PubMed] [Google Scholar]
- Cunnane S, Nugent S, Roy M, Courchesne-Loyer A, Croteau E, Tremblay S, Castellano A, Pifferi F, Bocti C, Paquet N, et al. (2011). Brain fuel metabolism, aging, and Alzheimer's disease. Nutrition 27, 3–20. 10.1016/j.nut.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnane SC, Trushina E, Morland C, Prigione A, Casadesus G, Andrews ZB, Beal MF, Bergersen LH, Brinton RD, de la Monte S, et al. (2020). Brain energy rescue: an emerging therapeutic concept for neurodegenerative disorders of ageing. Nat Rev Drug Discov 19, 609–633. 10.1038/s41573-020-0072-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler NR (1986). Cerebral metabolism as measured with positron emission tomography (PET) and [18F] 2-deoxy-D-glucose: healthy aging, Alzheimer's disease and Down syndrome. Prog Neuropsychopharmacol Biol Psychiatry 10, 309–321. [DOI] [PubMed] [Google Scholar]
- Davenport CB (1932). Sixth International Congress of Genetics 1, 135–140. [Google Scholar]
- de Graaf G, Buckley F, Dever J, and Skotko BG (2017a). Estimation of live birth and population prevalence of Down syndrome in nine U.S. states. Am J Med Genet A 173, 2710–2719. 10.1002/ajmg.a.38402. [DOI] [PubMed] [Google Scholar]
- de Graaf G, Buckley F, and Skotko BG (2017b). Estimation of the number of people with Down syndrome in the United States. Genet Med 19, 439–447. 10.1038/gim.2016.127. [DOI] [PubMed] [Google Scholar]
- de Graaf G, Buckley F, and Skotko BG (2020). Estimation of the number of people with Down syndrome in Europe. Eur J Hum Genet. 10.1038/s41431-020-00748-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hingh YC, van der Vossen PW, Gemen EF, Mulder AB, Hop WC, Brus F, and de Vries E (2005). Intrinsic abnormalities of lymphocyte counts in children with down syndrome. J Pediatr 147, 744–747. 10.1016/j.jpeds.2005.07.022. [DOI] [PubMed] [Google Scholar]
- de la Monte SM (2014). Type 3 diabetes is sporadic Alzheimer’s disease: mini-review. Eur Neuropsychopharmacol 24, 1954–1960. 10.1016/j.euroneuro.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM (2019). The Full Spectrum of Alzheimer's Disease Is Rooted in Metabolic Derangements That Drive Type 3 Diabetes. Adv Exp Med Biol 1128, 45–83. 10.1007/978-981-13-3540-2_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, Tong M, Daiello LA, and Ott BR (2019). Early-Stage Alzheimer's Disease Is Associated with Simultaneous Systemic and Central Nervous System Dysregulation of Insulin-Linked Metabolic Pathways. J Alzheimers Dis 68, 657–668. 10.3233/JAD-180906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Toma I, and Dierssen M (2021). Network analysis of Down syndrome and SARS-CoV-2 identifies risk and protective factors for COVID-19. Sci Rep 11, 1930. 10.1038/s41598-021-81451-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetrius LA, and Driver J (2013). Alzheimer's as a metabolic disease. Biogerontology 14, 641–649. 10.1007/s10522-013-9479-7. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Greve DN, Chua EF, Rand-Giovannetti E, Rentz DM, Bertram L, Mullin K, Tanzi RE, Blacker D, et al. (2005). Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology 65, 404–411. 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieudonne Y, Uring-Lambert B, Jeljeli MM, Gies V, Alembik Y, Korganow AS, and Guffroy A (2020). Immune Defect in Adults With Down Syndrome: Insights Into a Complex Issue. Front Immunol 11, 840. 10.3389/fimmu.2020.00840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran E, Keator D, Head E, Phelan MJ, Kim R, Totoiu M, Barrio JR, Small GW, Potkin SG, and Lott IT (2017). Down Syndrome, Partial Trisomy 21, and Absence of Alzheimer's Disease: The Role of APP. J Alzheimers Dis 56, 459–470. 10.3233/JAD-160836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draheim CC, Geijer JR, and Dengel DR (2010). Comparison of intima-media thickness of the carotid artery and cardiovascular disease risk factors in adults with versus without the Down syndrome. Am J Cardiol 106, 1512–1516. 10.1016/j.amjcard.2010.06.079. [DOI] [PubMed] [Google Scholar]
- Draheim CC, McCubbin JA, and Williams DP (2002). Differences in cardiovascular disease risk between nondiabetic adults with mental retardation with and without Down syndrome. Am J Ment Retard 107, 201–211. . [DOI] [PubMed] [Google Scholar]
- Edwards JP, Zhang X, Frauwirth KA, and Mosser DM (2006). Biochemical and functional characterization of three activated macrophage populations. J Leukoc Biol 80, 1298–1307. 10.1189/jlb.0406249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson A, Bernstein Y, Degani H, Levanon D, Ben-Hur H, and Groner Y (1992). Gene dosage and Down's syndrome: metabolic and enzymatic changes in PC12 cells overexpressing transfected human liver-type phosphofructokinase. Somat Cell Mol Genet 18, 143–161. 10.1007/BF01233161. [DOI] [PubMed] [Google Scholar]
- Elson A, Levanon D, Weiss Y, and Groner Y (1994). Overexpression of liver-type phosphofructokinase (PFKL) in transgenic-PFKL mice: implication for gene dosage in trisomy 21. Biochem J 299 ( Pt 2), 409–415. 10.1042/bj2990409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esbensen AJ (2010). Health conditions associated with aging and end of life of adults with Down syndrome. Int Rev Res Ment Retard 39, 107–126. 10.1016/S0074-7750(10)39004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa JM (2020). Down Syndrome and COVID-19: A Perfect Storm? Cell Rep Med 1, 100019. 10.1016/j.xcrm.2020.100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farroni C, Marasco E, Marcellini V, Giorda E, Valentini D, Petrini S, D'Oria V, Pezzullo M, Cascioli S, Scarsella M, et al. (2018). Dysregulated miR-155 and miR-125b Are Related to Impaired B-cell Responses in Down Syndrome. Front Immunol 9, 2683. 10.3389/fimmu.2018.02683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Aguilar L, Iulita MF, Kovecses O, Torres MD, Levi SM, Zhang Y, Askenazi M, Wisniewski T, Busciglio J, and Cuello AC (2020). Evolution of neuroinflammation across the lifespan of individuals with Down syndrome. Brain 143, 3653–3671. 10.1093/brain/awaa326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folch J, Olloquequi J, Ettcheto M, Busquets O, Sánchez-López E, Cano A, Espinosa-Jiménez T, García ML, Beas-Zarate C, Casadesús G, et al. (2019). The Involvement of Peripheral and Brain Insulin Resistance in Late Onset Alzheimer's Dementia. Front Aging Neurosci 11, 236. 10.3389/fnagi.2019.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortea J.e.a. (2020). Clinical and biomarker changes of Alzheimer’s Disease in adults with Down Syndrome: a cross-sectional study. Lancet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier M, Castellano CA, St-Pierre V, Myette-Côté É, Langlois F, Roy M, Morin MC, Bocti C, Fulop T, Godin JP, et al. (2021). A ketogenic drink improves cognition in mild cognitive impairment: Results of a 6-month RCT. Alzheimers Dement 17, 543–552. 10.1002/alz.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser JM,A (1876). Kalmuc Idiocy: Report of a Case with Autopsy. Journal of Mental Science 22, 169–179. [Google Scholar]
- Fulop T, Larbi A, Dupuis G, Le Page A, Frost EH, Cohen AA, Witkowski JM, and Franceschi C (2017). Immunosenescence and Inflamm-Aging As Two Sides of the Same Coin: Friends or Foes? Front Immunol 8, 1960. 10.3389/fimmu.2017.01960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner K, and Davisson M (2000). The sequence of human chromosome 21 and implications for research into Down syndrome. Genome Biol 1, REVIEWS0002. 10.1186/gb-2000-1-2-reviews0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gate D, Saligrama N, Leventhal O, Yang AC, Unger MS, Middeldorp J, Chen K, Lehallier B, Channappa D, De Los Santos MB, et al. (2020). Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer's disease. Nature 577, 399–404. 10.1038/s41586-019-1895-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensous N, Bacalini MG, Franceschi C, and Garagnani P (2020). Down syndrome, accelerated aging and immunosenescence. Semin Immunopathol 42, 635–645. 10.1007/s00281-020-00804-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain RN (2002). T-cell development and the CD4-CD8 lineage decision. Nat Rev Immunol 2, 309–322. 10.1038/nri798. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Castillo E, Frias ES, and Swanson RA (2018). Bioenergetic regulation of microglia. Glia 66, 1200–1212. 10.1002/glia.23271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie KM, Dix RJ, Williams AJ, Newton R, Robinson ZF, Bingley PJ, Gale EA, and Shield JP (2006). Islet autoimmunity in children with Down's syndrome. Diabetes 55, 3185–3188. 10.2337/db06-0856. [DOI] [PubMed] [Google Scholar]
- Glasson EJ, Sullivan SG, Hussain R, Petterson BA, Montgomery PD, and Bittles AH (2002). The changing survival profile of people with Down's syndrome: implications for genetic counselling. Clinical genetics 62, 390–393. [DOI] [PubMed] [Google Scholar]
- Goldgaber D, Lerman MI, McBride OW, Saffiotti U, and Gajdusek DC (1987). Characterization and chromosomal localization of a cDNA encoding brain amyloid of Alzheimer's disease. Science 235, 877–880. 10.1126/science.3810169. [DOI] [PubMed] [Google Scholar]
- Goronzy JJ, and Weyand CM (2019). Mechanisms underlying T cell ageing. Nat Rev Immunol 19, 573–583. 10.1038/s41577-019-0180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal MS, Gordon BA, Couture LE, Flores S, Xiong C, Morris JC, Raichle ME, Benzinger TL, and Vlassenko AG (2020). Spatiotemporal relationship between subthreshold amyloid accumulation and aerobic glycolysis in the human brain. Neurobiology of Aging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WS, Stanley LC, Ling C, White L, MacLeod V, Perrot LJ, White CL 3rd, and Araoz C (1989). Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci U S A 86, 7611–7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross TJ, Doran E, Cheema AK, Head E, Lott IT, and Mapstone M (2019). Plasma metabolites related to cellular energy metabolism are altered in adults with Down syndrome and Alzheimer's disease. Developmental Neurobiology 0. 10.1002/dneu.22716. [DOI] [PubMed] [Google Scholar]
- Guazzarotti L, Trabattoni D, Castelletti E, Boldrighini B, Piacentini L, Duca P, Beretta S, Pacei M, Caprio C, Vigan Ago A, et al. (2009). T lymphocyte maturation is impaired in healthy young individuals carrying trisomy 21 (Down syndrome). Am J Intellect Dev Disabil 114, 100–109. 10.1352/2009.114.100-109. [DOI] [PubMed] [Google Scholar]
- Haier RJ, Alkire MT, White NS, Uncapher MR, Head E, Lott IT, and Cotman CW (2003). Temporal cortex hypermetabolism in Down syndrome prior to the onset of dementia. Neurology 61, 1673–1679. [DOI] [PubMed] [Google Scholar]
- Haier RJ, Head K, Head E, and Lott IT (2008). Neuroimaging of individuals with Down's syndrome at-risk for dementia: evidence for possible compensatory events. Neuroimage 39, 1324–1332. 10.1016/j.neuroimage.2007.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M, Fujiyama A, Taylor TD, Watanabe H, Yada T, Park HS, Toyoda A, Ishii K, Totoki Y, Choi DK, et al. (2000). The DNA sequence of human chromosome 21. Nature 405, 311–319. 10.1038/35012518. [DOI] [PubMed] [Google Scholar]
- Head E, Azizeh BY, Lott IT, Tenner AJ, Cotman CW, and Cribbs DH (2001). Complement association with neurons and beta-amyloid deposition in the brains of aged individuals with Down Syndrome. Neurobiol Dis 8, 252–265. 10.1006/nbdi.2000.0380. [DOI] [PubMed] [Google Scholar]
- Head E, Phelan MJ, Doran E, Kim RC, Poon WW, Schmitt FA, and Lott IT (2017). Cerebrovascular pathology in Down syndrome and Alzheimer disease. Acta Neuropathol Commun 5, 93. 10.1186/s40478-017-0499-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head E, Powell DK, and Schmitt FA (2018). Metabolic and Vascular Imaging Biomarkers in Down Syndrome Provide Unique Insights Into Brain Aging and Alzheimer Disease Pathogenesis. Front Aging Neurosci 10, 191. 10.3389/fnagi.2018.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head E, Silverman W, Patterson D, and Lott IT (2012). Aging and down syndrome. Curr Gerontol Geriatr Res 2012, 412536. 10.1155/2012/412536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix JA, Airey DC, Britton A, Burke AD, Capone GT, Chavez R, Chen J, Chicoine B, Costa ACS, Dage JL, et al. (2021). Cross-Sectional Exploration of Plasma Biomarkers of Alzheimer's Disease in Down Syndrome: Early Data from the Longitudinal Investigation for Enhancing Down Syndrome Research (LIFE-DSR) Study. J Clin Med 10. 10.3390/jcm10091907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Alexander GE, Daly EM, Shetty HU, Krasuski JS, Rapoport SI, and Schapiro MB (1999). High brain myo-inositol levels in the predementia phase of Alzheimer's disease in adults with Down's syndrome: a 1H MRS study. Am J Psychiatry 156, 1879–1886. 10.1176/ajp.156.12.1879. [DOI] [PubMed] [Google Scholar]
- Huggard D, Doherty DG, and Molloy EJ (2020). Immune Dysregulation in Children With Down Syndrome. Front Pediatr 8, 73. 10.3389/fped.2020.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huls A, Costa ACS, Dierssen M, Baksh RA, Bargagna S, Baumer NT, Brandao AC, Carfi A, Carmona-Iragui M, Chicoine BA, et al. (2021). Medical vulnerability of individuals with Down syndrome to severe COVID-19-data from the Trisomy 21 Research Society and the UK ISARIC4C survey. EClinicalMedicine 33, 100769. 10.1016/j.eclinm.2021.100769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacobazzi V, Infantino V, Castegna A, and Andria G (2014). Hyperhomocysteinemia: Related genetic diseases and congenital defects, abnormal DNA methylation and newborn screening issues. Molecular Genetics and Metabolism 113, 27–33. 10.1016/j.ymgme.2014.07.016. [DOI] [PubMed] [Google Scholar]
- Illouz T, Biragyn A, Frenkel-Morgenstern M, Weissberg O, Gorohovski A, Merzon E, Green I, Iulita F, Flores-Aguilar L, Del Mar Dierssen Sotos M, et al. (2021). Specific Susceptibility to COVID-19 in Adults with Down Syndrome. Neuromolecular Med. 10.1007/s12017-021-08651-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iulita MF, Ower A, Barone C, Pentz R, Gubert P, Romano C, Cantarella RA, Elia F, Buono S, Recupero M, et al. (2016). An inflammatory and trophic disconnect biomarker profile revealed in Down syndrome plasma: Relation to cognitive decline and longitudinal evaluation. Alzheimers Dement 12, 1132–1148. 10.1016/j.jalz.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, et al. (2018). NIA-AA Research Framework: Toward a biological definition of Alzheimer's disease. Alzheimers Dement 14, 535–562. 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust W (2018). Imaging the evolution and pathophysiology of Alzheimer disease. Nature reviews. Neuroscience 19, 687–700. 10.1038/s41583-018-0067-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakel L, De Kort AM, Klijn CJM, Schreuder F, and Verbeek MM (2021). Prevalence of cerebral amyloid angiopathy: A systematic review and meta-analysis. Alzheimers Dement. 10.1002/alz.12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings A, Cunnane SC, and Minihane AM (2020). Can nutrition support healthy cognitive ageing and reduce dementia risk? BMJ 369, m2269. 10.1136/bmj.m2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jervis GA (1948). Early senile dementia in mongoloid idiocy. Am J Psychiatry 105, 102–106. 10.1176/ajp.105.2.102. [DOI] [PubMed] [Google Scholar]
- Joshi AU, Minhas PS, Liddelow SA, Haileselassie B, Andreasson KI, Dorn GW 2nd, and Mochly-Rosen D (2019). Fragmented mitochondria released from microglia trigger A1 astrocytic response and propagate inflammatory neurodegeneration. Nat Neurosci 22, 1635–1648. 10.1038/s41593-019-0486-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen IF, Russo F, Jensen AB, Westergaard D, Lademann M, Hu JX, Brunak S, and Belling K (2019). Comorbidity landscape of the Danish patient population affected by chromosome abnormalities. Genetics in Medicine 21, 2485–2495. 10.1038/s41436-019-0519-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandimalla R, Thirumala V, and Reddy PH (2017). Is Alzheimer's disease a Type 3 Diabetes? A critical appraisal. Biochim Biophys Acta Mol Basis Dis 1863, 1078–1089. 10.1016/j.bbadis.2016.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapogiannis D, and Mattson MP (2011). Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer's disease. Lancet Neurol 10, 187–198. 10.1016/S1474-4422(10)70277-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmiloff-Smith A, Al-Janabi T, D'Souza H, Groet J, Massand E, Mok K, Startin C, Fisher E, Hardy J, Nizetic D, et al. (2016). The importance of understanding individual differences in Down syndrome. F1000Res 5. 10.12688/f1000research.7506.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DK, and Mook-Jung I (2019). The role of cell type-specific mitochondrial dysfunction in the pathogenesis of Alzheimer's disease. BMB Rep 52, 679–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline J, Kinney A, Levin B, Mayeux R, Schupf N, and Warburton D (2000). Alzheimer's disease in the parents of women with trisomic spontaneous abortions. Neuroreport 11, 795–799. 10.1097/00001756-200003200-00028. [DOI] [PubMed] [Google Scholar]
- Krinsky-McHale SJ, Devenny DA, Gu H, Jenkins EC, Kittler P, Murty VV, Schupf N, Scotto L, Tycko B, Urv TK, et al. (2008). Successful aging in a 70-year-old man with down syndrome: a case study. Intellect Dev Disabil 46, 215–228. 10.1352/2008.46:215-228. [DOI] [PubMed] [Google Scholar]
- Labudova O, Cairns N, Kitzmüller E, and Lubec G (1999). Impaired brain glucose metabolism in patients with Down syndrome. J Neural Transm Suppl 57, 247–256. 10.1007/978-3-7091-6380-1_16. [DOI] [PubMed] [Google Scholar]
- Laidlaw BJ, Craft JE, and Kaech SM (2016). The multifaceted role of CD4(+) T cells in CD8(+) T cell memory. Nat Rev Immunol 16, 102–111. 10.1038/nri.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao PJ, Handen BL, Betthauser TJ, Mihaila I, Hartley SL, Cohen AD, Tudorascu DL, Bulova PD, Lopresti BJ, Tumuluru RV, et al. (2018). Alzheimer-Like Pattern of Hypometabolism Emerges with Elevated Amyloid-beta Burden in Down Syndrome. J Alzheimers Dis 61, 631–644. 10.3233/JAD-170720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Shin DW, Yun JM, Kim SH, Nam YS, Cho B, Lim JS, Jeong HY, Kwon HM, and Park JH (2016). Insulin Resistance Is a Risk Factor for Silent Lacunar Infarction. Stroke 47, 2938–2944. 10.1161/STROKEAHA.116.014097. [DOI] [PubMed] [Google Scholar]
- Lejeune J (1990). Pathogenesis of mental deficiency in trisomy 21. Am J Med Genet Suppl 7, 20–30. 10.1002/ajmg.1320370705. [DOI] [PubMed] [Google Scholar]
- Lejeune J, Gautier M, and Turpin R (1959). [Study of somatic chromosomes from 9 mongoloid children]. C R Hebd Seances Acad Sci 248, 1721–1722. [PubMed] [Google Scholar]
- Lejeune J, Peeters M, Rethore MO, de Blois MC, and Devaux JP (1990). Down syndrome and 3,3',5'-triiodothyronine. Am J Dis Child 144, 19. 10.1001/archpedi.1990.02150250021017. [DOI] [PubMed] [Google Scholar]
- Lejeune JT,R; Gautier M (1959). Le mongolisme premier exemple d'aberration autosomique humaine. Annales de Génétique 1 (2), 41–49. [Google Scholar]
- Lengyel Z, Balogh E, Emri M, Szikszai E, Kollar J, Sikula J, Esik O, Tron L, and Olah E (2006). Pattern of increased cerebral FDG uptake in Down syndrome patients. Pediatr Neurol 34, 270–275. 10.1016/j.pediatrneurol.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Lin AL, Powell D, Caban-Holt A, Jicha G, Robertson W, Gold BT, Davis R, Abner E, Wilcock DM, Schmitt FA, and Head E (2016). (1)H-MRS metabolites in adults with Down syndrome: Effects of dementia. NeuroImage. Clinical 11, 728–735. 10.1016/j.nicl.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Otín C, Galluzzi L, Freije JMP, Madeo F, and Kroemer G (2016). Metabolic Control of Longevity. Cell 166, 802–821. 10.1016/j.cell.2016.07.031. [DOI] [PubMed] [Google Scholar]
- Lott IT (2012). Antioxidants in Down syndrome. Biochimica et biophysica acta 1822, 657–663. 10.1016/j.bbadis.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lott IT, and Head E (2005). Alzheimer disease and Down syndrome: factors in pathogenesis. Neurobiol Aging 26, 383–389. 10.1016/j.neurobiolaging.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Mann DMA, Davidson YS, Robinson AC, Allen N, Hashimoto T, Richardson A, Jones M, Snowden JS, Pendleton N, Potier MC, et al. (2018). Patterns and severity of vascular amyloid in Alzheimer's disease associated with duplications and missense mutations in APP gene, Down syndrome and sporadic Alzheimer's disease. Acta Neuropathol 136, 569–587. 10.1007/s00401-018-1866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapstone M, Gross TJ, Macciardi F, Cheema AK, Petersen M, Head E, Handen BL, Klunk WE, Christian BT, Silverman W, et al. (2020). Metabolic correlates of prevalent mild cognitive impairment and Alzheimer's disease in adults with Down syndrome. Alzheimers Dement (Amst) 12, e12028. 10.1002/dad2.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcovecchio GE, Bortolomai I, Ferrua F, Fontana E, Imberti L, Conforti E, Amodio D, Bergante S, Macchiarulo G, D'Oria V, et al. (2019). Thymic Epithelium Abnormalities in DiGeorge and Down Syndrome Patients Contribute to Dysregulation in T Cell Development. Front Immunol 10, 447. 10.3389/fimmu.2019.00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Frías ML, Rodríguez-Pinilla E, Bermejo E, and Prieto L (2002). Epidemiological evidence that maternal diabetes does not appear to increase the risk for Down syndrome. Am J Med Genet 112, 335–337. 10.1002/ajmg.10706. [DOI] [PubMed] [Google Scholar]
- Martini AC, Helman AM, McCarty KL, Lott IT, Doran E, Schmitt FA, and Head E (2020). Distribution of microglial phenotypes as a function of age and Alzheimer's disease neuropathology in the brains of people with Down syndrome. Alzheimers Dement (Amst) 12, e12113. 10.1002/dad2.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DC, Lukic AS, Andrews RD, Marendic B, Brewer J, Rissman RA, Mosconi L, Strother SC, Wernick MN, Mobley WC, et al. (2016). Dissociation of Down syndrome and Alzheimer's disease effects with imaging. Alzheimers Dement (N Y) 2, 69–81. 10.1016/j.trci.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, and Arumugam TV (2018). Hallmarks of Brain Aging: Adaptive and Pathological Modification by Metabolic States. Cell Metab 27, 1176–1199. 10.1016/j.cmet.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight SL (2010). On getting there from here. Science 330, 1338–1339. 10.1126/science.1199908. [DOI] [PubMed] [Google Scholar]
- Migliore L, Boni G, Bernardini R, Trippi F, Colognato R, Fontana I, Coppedè F, and Sbrana I (2006). Susceptibility to chromosome malsegregation in lymphocytes of women who had a Down syndrome child in young age. Neurobiol Aging 27, 710–716. 10.1016/j.neurobiolaging.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Mizuno GO, Wang Y, Shi G, Wang Y, Sun J, Papadopoulos S, Broussard GJ, Unger EK, Deng W, Weick J, et al. (2018). Aberrant Calcium Signaling in Astrocytes Inhibits Neuronal Excitability in a Human Down Syndrome Stem Cell Model. Cell Rep 24, 355–365. 10.1016/j.celrep.2018.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montal V, Barroeta I, Bejanin A, Pegueroles J, Carmona-Iragui M, Altuna M, Benejam B, Videla L, Fernandez S, Padilla C, et al. (2021). Metabolite Signature of Alzheimer's Disease in Adults with Down Syndrome. Ann Neurol. 10.1002/ana.26178. [DOI] [PubMed] [Google Scholar]
- Morris G, Berk M, Maes M, and Puri BK (2019). Could Alzheimer's Disease Originate in the Periphery and If So How So? Mol Neurobiol 56, 406–434. 10.1007/s12035-018-1092-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison RA, McGrath A, Davidson G, Brown JJ, Murray GD, and Lever AF (1996). Low blood pressure in Down's syndrome, A link with Alzheimer's disease? Hypertension 28, 569–575. 10.1161/01.hyp.28.4.569. [DOI] [PubMed] [Google Scholar]
- Mortimer GL, and Gillespie KM (2020). Early Onset of Autoimmune Diabetes in Children with Down Syndrome-Two Separate Aetiologies or an Immune System Pre-Programmed for Autoimmunity? Curr Diab Rep 20, 47. 10.1007/s11892-020-01318-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Berti V, Quinn C, McHugh P, Petrongolo G, Osorio RS, Connaughty C, Pupi A, Vallabhajosula S, Isaacson RS, et al. (2017). Perimenopause and emergence of an Alzheimer's bioenergetic phenotype in brain and periphery. PLoS One 12, e0185926. 10.1371/journal.pone.0185926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Berti V, Swerdlow RH, Pupi A, Duara R, and de Leon M (2010). Maternal transmission of Alzheimer's disease: prodromal metabolic phenotype and the search for genes. Hum Genomics 4, 170–193. 10.1186/1479-7364-4-3-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Brys M, Switalski R, Mistur R, Glodzik L, Pirraglia E, Tsui W, De Santi S, and de Leon MJ (2007). Maternal family history of Alzheimer's disease predisposes to reduced brain glucose metabolism. Proc Natl Acad Sci U S A 104, 19067–19072. 10.1073/pnas.0705036104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, de Leon M, Murray J, E L, Lu J, Javier E, McHugh P, and Swerdlow RH (2011). Reduced mitochondria cytochrome oxidase activity in adult children of mothers with Alzheimer's disease. J Alzheimers Dis 27, 483–490. 10.3233/JAD-2011-110866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Tsui W, Murray J, McHugh P, Li Y, Williams S, Pirraglia E, Glodzik L, De Santi S, Vallabhajosula S, and de Leon MJ (2012). Maternal age affects brain metabolism in adult children of mothers affected by Alzheimer's disease. Neurobiol Aging 33, 624.e621–629. 10.1016/j.neurobiolaging.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch J, Rodger JC, Rao S, Fletcher C, and Dunnigan M (1977). Down's syndrome: an atheroma-free model? Br Med J 2, 226–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muri J, and Kopf M (2021). Redox regulation of immunometabolism. Nat Rev Immunol 21, 363–381. 10.1038/s41577-020-00478-8. [DOI] [PubMed] [Google Scholar]
- Murphy M, and Epstein LB (1990). Down syndrome (trisomy 21) thymuses have a decreased proportion of cells expressing high levels of TCR alpha, beta and CD3. A possible mechanism for diminished T cell function in Down syndrome. Clin Immunol Immunopathol 55, 453–467. 10.1016/0090-1229(90)90131-9. [DOI] [PubMed] [Google Scholar]
- Murphy M, and Epstein LB (1992). Down syndrome (DS) peripheral blood contains phenotypically mature CD3+TCR alpha, beta+ cells but abnormal proportions of TCR alpha, beta+, TCR gamma, delta+, and CD4+ CD45RA+ cells: evidence for an inefficient release of mature T cells by the DS thymus. Clin Immunol Immunopathol 62, 245–251. 10.1016/0090-1229(92)90079-4. [DOI] [PubMed] [Google Scholar]
- Narchi H, and Kulaylat N (1997). High incidence of Down's syndrome in infants of diabetic mothers. Arch Dis Child 77, 242–244. 10.1136/adc.77.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nations, U. (2021). Down Syndrome. https://www.un.org/en/observances/down-syndrome-day.
- Neale N, Padilla C, Fonseca LM, Holland T, and Zaman S (2018). Neuroimaging and other modalities to assess Alzheimer's disease in Down syndrome. NeuroImage. Clinical 17, 263–271. 10.1016/j.nicl.2017.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neth BJ, and Craft S (2017). Insulin Resistance and Alzheimer's Disease: Bioenergetic Linkages. Front Aging Neurosci 9, 345. 10.3389/fnagi.2017.00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisticó R, and Borg JJ (2021). Aducanumab for Alzheimer’s disease: A regulatory perspective. Pharmacological Research 171, 105754. 10.1016/j.phrs.2021.105754. [DOI] [PubMed] [Google Scholar]
- Nitsch RM, Blusztajn JK, Pittas AG, Slack BE, Growdon JH, and Wurtman RJ (1992). Evidence for a membrane defect in Alzheimer disease brain. Proc Natl Acad Sci U S A 89, 1671–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olah M, Menon V, Habib N, Taga MF, Ma Y, Yung CJ, Cimpean M, Khairallah A, Coronas-Samano G, Sankowski R, et al. (2020). Single cell RNA sequencing of human microglia uncovers a subset associated with Alzheimer's disease. Nature communications 11, 6129. 10.1038/s41467-020-19737-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovchinnikov DA, Korn O, Virshup I, Wells CA, and Wolvetang EJ (2018). The Impact of APP on Alzheimer-like Pathogenesis and Gene Expression in Down Syndrome iPSC-Derived Neurons. Stem cell reports 11, 32–42. 10.1016/j.stemcr.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano G, and Castello G (2012). Oxidative stress and mitochondrial dysfunction in Down syndrome. Adv Exp Med Biol 724, 291–299. 10.1007/978-1-4614-0653-2_22. [DOI] [PubMed] [Google Scholar]
- Pape SE, Baksh RA, Startin C, Hamburg S, Hithersay R, and Strydom A (2021). The Association between Physical Activity and CAMDEX-DS Changes Prior to the Onset of Alzheimer's Disease in Down Syndrome. J Clin Med 10. 10.3390/jcm10091882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecze L, Randi EB, and Szabo C (2020). Meta-analysis of metabolites involved in bioenergetic pathways reveals a pseudohypoxic state in Down syndrome. Mol Med 26, 102. 10.1186/s10020-020-00225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecze L, and Szabo C (2021). Meta-analysis of gene expression patterns in Down syndrome highlights significant alterations in mitochondrial and bioenergetic pathways. Mitochondrion 57, 163–172. 10.1016/j.mito.2020.12.017. [DOI] [PubMed] [Google Scholar]
- Pellegrini FP, Marinoni M, Frangione V, Tedeschi A, Gandini V, Ciglia F, Mortara L, Accolla RS, and Nespoli L (2012). Down syndrome, autoimmunity and T regulatory cells. Clin Exp Immunol 169, 238–243. 10.1111/j.1365-2249.2012.04610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Gao P, Shi L, Chen L, Liu J, and Long J (2020). Central and Peripheral Metabolic Defects Contribute to the Pathogenesis of Alzheimer's Disease: Targeting Mitochondria for Diagnosis and Prevention. Antioxid Redox Signal 32, 1188–1236. 10.1089/ars.2019.7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penrose LS (1949). The incidence of mongolism in the general population. J Ment Sci 95, 685–688. 10.1192/bjp.95.400.685. [DOI] [PubMed] [Google Scholar]
- Perez-Padilla R, Fernandez R, Garcia-Sancho C, Franco-Marina F, Aburto O, Lopez-Gatell H, and Bojorquez I (2010). Pandemic (H1N1) 2009 virus and Down syndrome patients. Emerg Infect Dis 16, 1312–1314. 10.3201/eid1608.091931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perluigi M, and Butterfield DA (2012). Oxidative Stress and Down Syndrome: A Route toward Alzheimer-Like Dementia. Curr Gerontol Geriatr Res 2012, 724904. 10.1155/2012/724904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen ME, Zhang F, Schupf N, Krinsky-McHale SJ, Hall J, Mapstone M, Cheema A, Silverman W, Lott I, Rafii MS, et al. (2020). Proteomic profiles for Alzheimer's disease and mild cognitive impairment among adults with Down syndrome spanning serum and plasma: An Alzheimer's Biomarker Consortium-Down Syndrome (ABC-DS) study. Alzheimers Dement (Amst) 12, e12039. 10.1002/dad2.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petronis A (1999). Alzheimer's disease and down syndrome: from meiosis to dementia. Exp Neurol 158, 403–413. 10.1006/exnr.1999.7128. [DOI] [PubMed] [Google Scholar]
- Pettegrew JW, Moossy J, Withers G, McKeag D, and Panchalingam K (1988). 31P nuclear magnetic resonance study of the brain in Alzheimer's disease. Journal of neuropathology and experimental neurology 47, 235–248. 10.1097/00005072-198805000-00004. [DOI] [PubMed] [Google Scholar]
- Polani PE, Briggs JH, Ford CE, Clarke CM, and Berg JM (1960). A Mongol girl with 46 chromosomes. Lancet 1, 721–724. 10.1016/s0140-6736(60)90614-0. [DOI] [PubMed] [Google Scholar]
- Prasher VP, Farrer MJ, Kessling AM, Fisher EM, West RJ, Barber PC, and Butler AC (1998). Molecular mapping of Alzheimer-type dementia in Down's syndrome. Ann Neurol 43, 380–383. 10.1002/ana.410430316. [DOI] [PubMed] [Google Scholar]
- Presson AP, Partyka G, Jensen KM, Devine OJ, Rasmussen SA, McCabe LL, and McCabe ER (2013). Current estimate of Down Syndrome population prevalence in the United States. J Pediatr 163, 1163–1168. 10.1016/j.jpeds.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price BR, Johnson LA, and Norris CM (2021). Reactive astrocytes: The nexus of pathological and clinical hallmarks of Alzheimer's disease. Ageing Res Rev 68, 101335. 10.1016/j.arr.2021.101335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi G, Mi Y, and Yin F (2019). Cellular Specificity and Inter-cellular Coordination in the Brain Bioenergetic System: Implications for Aging and Neurodegeneration. Front Physiol 10, 1531. 10.3389/fphys.2019.01531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafii MS, Lukic AS, Andrews RD, Brewer J, Rissman RA, Strother SC, Wernick MN, Pennington C, Mobley WC, Ness S, et al. (2017). PET Imaging of Tau Pathology and Relationship to Amyloid, Longitudinal MRI, and Cognitive Change in Down Syndrome: Results from the Down Syndrome Biomarker Initiative (DSBI). J Alzheimers Dis 60, 439–450. 10.3233/JAD-170390. [DOI] [PubMed] [Google Scholar]
- Rafii MS, Wishnek H, Brewer JB, Donohue MC, Ness S, Mobley WC, Aisen PS, and Rissman RA (2015). The down syndrome biomarker initiative (DSBI) pilot: proof of concept for deep phenotyping of Alzheimer's disease biomarkers in down syndrome. Front Behav Neurosci 9, 239. 10.3389/fnbeh.2015.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raha AA, Ghaffari SD, Henderson J, Chakraborty S, Allinson K, Friedland RP, Holland A, Zaman SH , Mukaetova-Ladinska EB, Raha-Chowdhury R (2021). Hepcidin Increases Cytokines in Alzheimer's Disease and Down's Syndrome Dementia: Implication of Impaired Iron Homeostasis in Neuroinflammation. Front Aging Neurosci. 10.3389/fnagi.2021.653591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raha-Chowdhury R, Henderson JW, Raha AA, Stott SRW, Vuono R, Foscarin S, Wilson L, Annus T, Fincham R, Allinson K, et al. (2018). Erythromyeloid-Derived TREM2: A Major Determinant of Alzheimer's Disease Pathology in Down Syndrome. J Alzheimers Dis 61, 1143–1162. 10.3233/JAD-170814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Schelbaum E, Hoffman K, Diaz I, Hristov H, Andrews R, Jett S, Jackson H, Lee A, Sarva H, et al. (2020). Sex-driven modifiers of Alzheimer risk: A multimodality brain imaging study. Neurology 95, e166–e178. 10.1212/WNL.0000000000009781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram G, and Chinen J (2011). Infections and immunodeficiency in Down syndrome. Clin Exp Immunol 164, 9–16. 10.1111/j.1365-2249.2011.04335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, and Daugherty AM (2018). Pathways to Brain Aging and Their Modifiers: Free-Radical-Induced Energetic and Neural Decline in Senescence (FRIENDS) Model - A Mini-Review. Gerontology 64, 49–57. 10.1159/000479508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijpma A, van der Graaf M, Lansbergen MM, Meulenbroek O, Cetinyurek-Yavuz A, Sijben JW, Heerschap A, and Olde Rikkert MGM (2017). The medical food Souvenaid affects brain phospholipid metabolism in mild Alzheimer's disease: results from a randomized controlled trial. Alzheimers Res Ther 9, 51. 10.1186/s13195-017-0286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijpma A, van der Graaf M, Meulenbroek O, Olde Rikkert MGM, and Heerschap A (2018). Altered brain high-energy phosphate metabolism in mild Alzheimer's disease: A 3-dimensional (31)P MR spectroscopic imaging study. NeuroImage. Clinical 18, 254–261. 10.1016/j.nicl.2018.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivollat M, Castex D, Hauret L, and Tillier AM (2014). Ancient Down syndrome: An osteological case from Saint-Jean-des-Vignes, northeastern France, from the 5-6th century AD. Int J Paleopathol 7, 8–14. 10.1016/j.ijpp.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Rodrigues AN, Coelho LC, Goncalves WL, Gouvea SA, Vasconcellos MJ, Cunha RS, and Abreu GR (2011). Stiffness of the large arteries in individuals with and without Down syndrome. Vasc Health Risk Manag 7, 375–381. 10.2147/VHRM.S21273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M, Rheault F, Croteau E, Castellano CA, Fortier M, St-Pierre V, Houde JC, Turcotte É, Bocti C, Fulop T, et al. (2020). Fascicle- and Glucose-Specific Deterioration in White Matter Energy Supply in Alzheimer's Disease. J Alzheimers Dis 76, 863–881. 10.3233/JAD-200213. [DOI] [PubMed] [Google Scholar]
- Runtsch MC, Ferrara G, and Angiari S (2020). Metabolic determinants of leukocyte pathogenicity in neurological diseases. Journal of neurochemistry. 10.1111/jnc.15169. [DOI] [PubMed] [Google Scholar]
- Sabbagh MN, Chen K, Rogers J, Fleisher AS, Liebsack C, Bandy D, Belden C, Protas H, Thiyyagura P, Liu X, et al. (2015). Florbetapir PET, FDG PET, and MRI in Down syndrome individuals with and without Alzheimer's dementia. Alzheimers Dement 11, 994–1004. 10.1016/j.jalz.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez-Atienzar S, and Masliah E (2020). Cellular senescence and Alzheimer disease: the egg and the chicken scenario. Nature reviews. Neuroscience 21, 433–444. 10.1038/s41583-020-0325-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuraki M, Matsunari I, Yoshita M, Shima K, Noguchi-Shinohara M, Hamaguchi T, Ono K, and Yamada M (2015). Cerebral Amyloid Angiopathy-Related Microbleeds Correlate with Glucose Metabolism and Brain Volume in Alzheimer's Disease. J Alzheimers Dis 48, 517–528. 10.3233/JAD-150274. [DOI] [PubMed] [Google Scholar]
- Satgé D, and Seidel MG (2018). The Pattern of Malignancies in Down Syndrome and Its Potential Context With the Immune System. Front Immunol 9, 3058. 10.3389/fimmu.2018.03058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapiro MB, Haxby JV, and Grady CL (1992). Nature of mental retardation and dementia in Down syndrome: study with PET, CT, and neuropsychology. Neurobiol Aging 13, 723–734. [DOI] [PubMed] [Google Scholar]
- Scheyer O, Rahman A, Hristov H, Berkowitz C, Isaacson RS, Diaz Brinton R, and Mosconi L (2018). Female Sex and Alzheimer's Risk: The Menopause Connection. J Prev Alzheimers Dis 5, 225–230. 10.14283/jpad.2018.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch J, Rohrer TR, Kaestner M, Abdul-Khaliq H, Gortner L, Sester U, Sester M, and Schmidt T (2017). Quantitative, Phenotypical, and Functional Characterization of Cellular Immunity in Children and Adolescents With Down Syndrome. J Infect Dis 215, 1619–1628. 10.1093/infdis/jix168. [DOI] [PubMed] [Google Scholar]
- Schon EA, Kim SH, Ferreira JC, Magalhães P, Grace M, Warburton D, and Gross SJ (2000). Chromosomal non-disjunction in human oocytes: is there a mitochondrial connection? Hum Reprod 15 Suppl 2, 160–172. 10.1093/humrep/15.suppl_2.160. [DOI] [PubMed] [Google Scholar]
- Schupf N, Kapell D, Lee JH, Ottman R, and Mayeux R (1994). Increased risk of Alzheimer's disease in mothers of adults with Down's syndrome. Lancet 344, 353–356. 10.1016/s0140-6736(94)91398-6. [DOI] [PubMed] [Google Scholar]
- Schupf N, Kapell D, Nightingale B, Lee JH, Mohlenhoff J, Bewley S, Ottman R, and Mayeux R (2001). Specificity of the fivefold increase in AD in mothers of adults with Down syndrome. Neurology 57, 979–984. 10.1212/wnl.57.6.979. [DOI] [PubMed] [Google Scholar]
- Schupf N, and Sergievsky GH (2002). Genetic and host factors for dementia in Down's syndrome. British journal of psychiatry 180, 405–410. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Duara R, Haxby J, Grady C, White BJ, Kessler RM, Kay AD, Cutler NR, and Rapoport SI (1983). Down's syndrome in adults: brain metabolism. Science 221, 781–783. [DOI] [PubMed] [Google Scholar]
- Sims NR, Blass JP, Murphy C, Bowen DM, and Neary D (1987). Phosphofructokinase activity in the brain in Alzheimer's disease. Annals of Neurology 21, 509–510. 10.1002/ana.410210517. [DOI] [PubMed] [Google Scholar]
- Sinai A, Mokrysz C, Bernal J, Bohnen I, Bonell S, Courtenay K, Dodd K, Gazizova D, Hassiotis A, Hillier R, et al. (2018). Predictors of Age of Diagnosis and Survival of Alzheimer's Disease in Down Syndrome. J Alzheimers Dis 61, 717–728. 10.3233/JAD-170624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soininen H, Solomon A, Visser PJ, Hendrix SB, Blennow K, Kivipelto M, Hartmann T, and group L.c.s. (2021). 36-month LipiDiDiet multinutrient clinical trial in prodromal Alzheimer's disease. Alzheimers Dement 17, 29–40. 10.1002/alz.12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St George-Hyslop PH, Tanzi RE, Polinsky RJ, Haines JL, Nee L, Watkins PC, Myers RH, Feldman RG, Pollen D, Drachman D, and et al. (1987). The genetic defect causing familial Alzheimer's disease maps on chromosome 21. Science 235, 885–890. 10.1126/science.2880399. [DOI] [PubMed] [Google Scholar]
- Stanley M, Macauley SL, and Holtzman DM (2016). Changes in insulin and insulin signaling in Alzheimer's disease: cause or consequence? J Exp Med 213, 1375–1385. 10.1084/jem.20160493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starbuck JM (2011). On the Antiquity of Trisomy 21: Moving Towards a Quantitative Diagnosis of Down Syndrome in Historic Material Culture. Journal of Contemporary Anthropology II. [Google Scholar]
- Stoltzner SE, Grenfell TJ, Mori C, Wisniewski KE, Wisniewski TM, Selkoe DJ, and Lemere CA (2000). Temporal accrual of complement proteins in amyloid plaques in Down's syndrome with Alzheimer's disease. Am J Pathol 156, 489–499. 10.1016/S0002-9440(10)64753-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss D, and Eyman RK (1996). Mortality of people with mental retardation in California with and without Down syndrome, 1986-1991. Am J Ment Retard 100, 643–653. [PubMed] [Google Scholar]
- Sudduth TL, Greenstein A, and Wilcock DM (2013). Intracranial injection of Gammagard, a human IVIg, modulates the inflammatory response of the brain and lowers Abeta in APP/PS1 mice along a different time course than anti-Abeta antibodies. J Neurosci 33, 9684–9692. 10.1523/JNEUROSCI.1220-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan KD, Evans D, Pandey A, Hraha TH, Smith KP, Markham N, Rachubinski AL, Wolter-Warmerdam K, Hickey F, Espinosa JM, and Blumenthal T (2017). Trisomy 21 causes changes in the circulating proteome indicative of chronic autoinflammation. Sci Rep 7, 14818. 10.1038/s41598-017-13858-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teller JK, Russo C, DeBusk LM, Angelini G, Zaccheo D, Dagna-Bricarelli F, Scartezzini P, Bertolini S, Mann DM, Tabaton M, and Gambetti P (1996). Presence of soluble amyloid beta-peptide precedes amyloid plaque formation in Down's syndrome. Nature medicine 2, 93–95. [DOI] [PubMed] [Google Scholar]
- Terada T, Obi T, Bunai T, Matsudaira T, Yoshikawa E, Ando I, Futatsubashi M, Tsukada H, and Ouchi Y (2020). In vivo mitochondrial and glycolytic impairments in patients with Alzheimer disease. Neurology 94, e1592–e1604. 10.1212/WNL.0000000000009249. [DOI] [PubMed] [Google Scholar]
- Terada T, Therriault J, Kang MSP, Savard M, Pascoal TA, Lussier F, Tissot C, Wang YT, Benedet A, Matsudaira T, et al. (2021). Mitochondrial complex I abnormalities is associated with tau and clinical symptoms in mild Alzheimer's disease. Mol Neurodegener 16, 28. 10.1186/s13024-021-00448-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal DR, Ghebremedhin E, Orantes M, and Wiestler OD (2003). Vascular pathology in Alzheimer disease: correlation of cerebral amyloid angiopathy and arteriosclerosis/lipohyalinosis with cognitive decline. Journal of neuropathology and experimental neurology 62, 1287–1301. [DOI] [PubMed] [Google Scholar]
- Thal DR, Griffin WS, de Vos RA, and Ghebremedhin E (2008). Cerebral amyloid angiopathy and its relationship to Alzheimer's disease. Acta Neuropathol 115, 599–609. 10.1007/s00401-008-0366-2. [DOI] [PubMed] [Google Scholar]
- Town T, Tan J, Flavell RA, and Mullan M (2005). T-cells in Alzheimer's disease. Neuromolecular Med 7, 255–264. 10.1385/NMM:7:3:255. [DOI] [PubMed] [Google Scholar]
- Tramutola A, Lanzillotta C, Di Domenico F, Head E, Butterfield DA, Perluigi M, and Barone E (2020). Brain insulin resistance triggers early onset Alzheimer disease in Down syndrome. Neurobiol Dis 137, 104772. 10.1016/j.nbd.2020.104772. [DOI] [PubMed] [Google Scholar]
- Tsou AY, Bulova P, Capone G, Chicoine B, Gelaro B, Harville TO, Martin BA, McGuire DE, McKelvey KD, Peterson M, et al. (2020). Medical Care of Adults With Down Syndrome: A Clinical Guideline. JAMA 324, 1543–1556. 10.1001/jama.2020.17024. [DOI] [PubMed] [Google Scholar]
- Ulland TK, and Colonna M (2018). TREM2 - a key player in microglial biology and Alzheimer disease. Nat Rev Neurol 14, 667–675. 10.1038/s41582-018-0072-1. [DOI] [PubMed] [Google Scholar]
- Valentini D, Marcellini V, Bianchi S, Villani A, Facchini M, Donatelli I, Castrucci MR, Marasco E, Farroni C, and Carsetti R (2015). Generation of switched memory B cells in response to vaccination in Down syndrome children and their siblings. Vaccine 33, 6689–6696. 10.1016/j.vaccine.2015.10.083. [DOI] [PubMed] [Google Scholar]
- Verhoog QP, Holtman L, Aronica E, and van Vliet EA (2020). Astrocytes as Guardians of Neuronal Excitability: Mechanisms Underlying Epileptogenesis. Front Neurol 11, 591690. 10.3389/fneur.2020.591690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstegen RHJ, Driessen GJ, Bartol SJW, van Noesel CJM, Boon L, van der Burg M, van Dongen JJM, de Vries E, and van Zelm MC (2014). Defective B-cell memory in patients with Down syndrome. J Allergy Clin Immunol 134, 1346–1353 e1349. 10.1016/j.jaci.2014.07.015. [DOI] [PubMed] [Google Scholar]
- Verstegen RHJ, and Kusters MAA (2020). Inborn Errors of Adaptive Immunity in Down Syndrome. J Clin Immunol 40, 791–806. 10.1007/s10875-020-00805-7. [DOI] [PubMed] [Google Scholar]
- Vinters HV (1987). Cerebral amyloid angiopathy. A critical review. Stroke 18, 311–324. 10.1161/01.str.18.2.311. [DOI] [PubMed] [Google Scholar]
- Vlassenko AG, Gordon BA, Goyal MS, Su Y, Blazey TM, Durbin TJ, Couture LE, Christensen JJ, Jafri H, Morris JC, et al. (2018). Aerobic glycolysis and tau deposition in preclinical Alzheimer's disease. Neurobiol Aging 67, 95–98. 10.1016/j.neurobiolaging.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassenko AG, and Raichle ME (2015). Brain aerobic glycolysis functions and Alzheimer's disease. Clinical and translational imaging 3, 27–37. 10.1007/s40336-014-0094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassenko AG, Vaishnavi SN, Couture L, Sacco D, Shannon BJ, Mach RH, Morris JC, Raichle ME, and Mintun MA (2010). Spatial correlation between brain aerobic glycolysis and amyloid-β (Aβ ) deposition. Proc Natl Acad Sci U S A 107, 17763–17767. 10.1073/pnas.1010461107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Gu BJ, Masters CL, and Wang YJ (2017). A systemic view of Alzheimer disease - insights from amyloid-β metabolism beyond the brain. Nat Rev Neurol 13, 612–623. 10.1038/nrneurol.2017.111. [DOI] [PubMed] [Google Scholar]
- Wang L, Pavlou S, Du X, Bhuckory M, Xu H, and Chen M (2019). Glucose transporter 1 critically controls microglial activation through facilitating glycolysis. Mol Neurodegener 14, 2. 10.1186/s13024-019-0305-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh KA, Araya P, Pandey A, Jordan KR, Smith KP, Granrath RE, Khanal S, Butcher ET, Estrada BE, Rachubinski AL, et al. (2019). Mass Cytometry Reveals Global Immune Remodeling with Multi-lineage Flypersensitivity to Type I Interferon in Down Syndrome. Cell Rep 29, 1893–1908 e1894. 10.1016/j.celrep.2019.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber GE, Koenig KA, Khrestian M, Shao Y, Tuason ED, Gramm M, Lal D, Leverenz JB, and Bekris LM (2020). An Altered Relationship between Soluble TREM2 and Inflammatory Markers in Young Adults with Down Syndrome: A Preliminary Report. J Immunol 204, 1111–1118. 10.4049/jimmunol.1901166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock DM, and Griffin WS (2013). Down's syndrome, neuroinflammation, and Alzheimer neuropathogenesis. Journal of neuroinflammation 10, 84. 10.1186/1742-2094-10-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock DM, Hurban J, Helman AM, Sudduth TL, McCarty KL, Beckett TL, Ferrell JC, Murphy MP, Abner EL, Schmitt FA, and Head E (2015). Down syndrome individuals with Alzheimer's disease have a distinct neuroinflammatory phenotype compared to sporadic Alzheimer's disease. Neurobiol Aging 36, 2468–2474. 10.1016/j.neurobiolaging.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock DM, Schmitt FA, and Head E (2016). Cerebrovascular contributions to aging and Alzheimer's disease in Down syndrome. Biochimica et biophysica acta 1862, 909–914. 10.1016/j.bbadis.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins HM, and Swerdlow RH (2017). Amyloid precursor protein processing and bioenergetics. Brain Res Bull 133, 71–79. 10.1016/j.brainresbull.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman FK, Al-Janabi T, Hardy J, Karmiloff-Smith A, Nizetic D, Tybulewicz VL, Fisher EM, and Strydom A (2015). A genetic cause of Alzheimer disease: mechanistic insights from Down syndrome. Nature reviews. Neuroscience 16, 564–574. 10.1038/nrn3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman FK, Pulford LJ, Barkus C, Liao F, Portelius E, Webb R, Chávez-Gutiérrez L, Cleverley K, Noy S, Sheppard O, et al. (2018). Trisomy of human chromosome 21 enhances amyloid-β deposition independently of an extra copy of APP. Brain 141, 2457–2474. 10.1093/brain/awy159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtman RJ (2011). Non-nutritional uses of nutrients. Eur J Pharmacol 668 Suppl 1, S10–15. 10.1016/j.ejphar.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Wu W, Brickman AM, Luchsinger J, Ferrazzano P, Pichiule P, Yoshita M, Brown T, DeCarli C, Barnes CA, Mayeux R, et al. (2008). The brain in the age of old: the hippocampal formation is targeted differentially by diseases of late life. Ann Neurol 64, 698–706. 10.1002/ana.21557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtman RJ (2014). A nutrient combination that can affect synapse formation. Nutrients 6, 1701–1710. 10.3390/nu6041701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtman RJ, Blusztajn JK, and Maire JC (1985). "Autocannibalism" of choline-containing membrane phospholipids in the pathogenesis of Alzheimer's disease-A hypothesis. Neurochem Int 7, 369–372. 10.1016/0197-0186(85)90127-5. [DOI] [PubMed] [Google Scholar]
- Zammit MD, Laymon CM, Tudorascu DL, Hartley SL, Piro-Gambetti B, Johnson SC, Stone CK, Mathis CA, Zaman SH, Klunk WE, et al. (2020). Patterns of glucose hypometabolism in Down syndrome resemble sporadic Alzheimer's disease except for the putamen. Alzheimers Dement (Amst) 12, e12138. 10.1002/dad2.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Hu Y, Wang B, Wang S, and Zhang X (2020). Metabolic Dysregulation Contributes to the Progression of Alzheimer's Disease. Front Neurosci 14, 530219. 10.3389/fnins.2020.530219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, and Raichle ME (2010). Disease and the brain's dark energy. Nat Rev Neurol 6, 15–28. 10.1038/nrneurol.2009.198. [DOI] [PubMed] [Google Scholar]
- Zigman WB, Schupf N, Jenkins EC, Urv TK, Tycko B, and Silverman W (2007). Cholesterol level, statin use and Alzheimer's disease in adults with Down syndrome. Neurosci Lett 416, 279–284. 10.1016/j.neulet.2007.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberter Y, and Zilberter M (2017). The vicious circle of hypometabolism in neurodegenerative diseases: Ways and mechanisms of metabolic correction. J Neurosci Res 95, 2217–2235. 10.1002/jnr.2406 [DOI] [PubMed] [Google Scholar]