Abstract
Objective:
Uganda is HIV-endemic with a prevalence of 5.7%. Lack of epidemic control has been attributed to low engagement with HIV testing. Collaborating with informal healthcare providers, such as traditional healers, has been proposed as a strategy to increase testing uptake. We explored acceptability and implementation of an HIV testing program where traditional healers delivered point-of-care testing and counseling to adults of unknown serostatus (clinicaltrials.gov NCT#03718871).
Methods:
This study was conducted in rural, southwestern Uganda. We interviewed participating traditional healers (N=17) and a purposive sample of trial participants (N=107). Healers were practicing within 10 kilometers of Mbarara township, and 18+ years old. Participants were 18+ years old; sexually active; had received care from participating healers; self-reported not receiving an HIV test in prior 12 months; and not previously diagnosed with HIV-infection. Interviews explored perceptions of a healer-delivered HIV testing model and were analyzed following a content-analysis approach.
Results:
Most participants were female (N=68, 55%). Healer-delivered HIV testing overcame structural barriers such as underlying poverty and rural locations that limited use, as transportation was costly and often prohibitive. Additionally, healers were located in villages and communities, which made services more accessible compared with facility-based testing. Participants also considered healers trustworthy and “confidential.” These qualities explain some preference for healer-delivered HIV testing, in contrast to “stigmatizing” biomedical settings.
Conclusions:
Traditional healer-delivered HIV testing was considered more confidential and easily accessible compared to clinic-based testing. Offering services through traditional healers may improve uptake of HIV testing services in rural, medically pluralistic communities.
Keywords: HIV, Uganda, traditional healers, qualitative, community-based intervention
Introduction
In 2020, an estimated 37.5 million people were living with HIV globally[1]. While global trends show an increase in HIV prevalence and significant declines in AIDS-related deaths, largely due to the increase of antiretroviral treatment (ART), Sub-Saharan Africa still carries a disproportionate burden of HIV[2,3]. Sub-Saharan Africa is inhabited by 12% of the world’s population, yet accounts for over 70% of the global HIV burden[4]. Six percent of people living with HIV (PLWH) reside in Uganda alone, with a national prevalence of 5.7%[4,5]. HIV testing is essential to facilitate entry into HIV care and prevent onward transmission[6–9]. However, over 25% of HIV-infected Ugandans are unaware of their status and nearly one third of sexually active men have never been tested for HIV[5].
HIV testing services are frequently located at healthcare facilities. Provider-initiated HIV counseling and testing is the primary avenue for HIV testing at healthcare facilities in Sub-Saharan Africa, but is predicated on an individual seeking facility-based clinical care in the first place[10–12]. In Uganda, utilization of these clinic-based services is limited by several factors including cost of transportation, waiting time to access centralized services, HIV-related stigma, and lack of perceived need for testing[13–16]. Various community-based HIV testing strategies have been employed in Uganda to improve uptake of testing with varying degrees of success[17]. A hybrid approach using a combination of mobile clinics and community health worker-delivered home testing improved HIV testing uptake from 57% to 92% after one year[18]. Other home-based strategies have relied on partner- and peer-delivered HIV self-tests, and technology-assisted HIV self-testing. These strategies have achieved HIV testing uptake of 77%, 82%, and 83% uptake, respectively[19–21]. The effectiveness of these community-based strategies has been limited by low literacy, a desire for pre- and post-test counseling, and, in some cases, misinterpreting results[22–25]. As such, HIV testing rates among rural Ugandans remain far below UNAIDS benchmarks[26].
Another factor contributing to low uptake of HIV testing in Uganda, and across Sub-Saharan Africa, is that people rely on both facility-based care as well as informal healthcare providers. Informal providers, such as traditional healers, frequently serve as the first line of healthcare for many Ugandans[27–31]. However, healers typically do not routinely offer HIV counseling or HIV testing services. Prior research from rural Mozambique showed that many PLWH utilize traditional healers, and that visiting a traditional healer exponentially prolongs time to HIV diagnosis[32]. Some have suggested that traditional healers might be able to improve uptake of HIV services among their clients[33–36].
This qualitative study was conducted as part of a cluster randomized trial where traditional healers were trained to deliver HIV counseling and facilitate HIV self-testing among adults of unknown HIV serostatus (ClinicalTrials.gov NCT03718871). In the parent study, the primary outcome was receipt of an HIV test. Results from the trial showed that by training traditional healers to deliver HIV counseling and offer self-testing, uptake of HIV testing increased more than four-fold among adults of unknown HIV serostatus, compared with referral for facility-based testing. HIV testing uptake was 23% among participants in the control arm who were referred for facility-based HIV testing, compared with 100% in the intervention where HIV self-testing was offered at the traditional healer practice. Trial details and results are described elsewhere[37]. We conducted individual interviews with trial participants in order to evaluate the acceptability of the traditional healer-delivered intervention and characterize trial results in the context of participant experiences.
Methods
Study Setting and Design
This study was completed in Mbarara District, Southwestern Uganda, which has a disproportionately high HIV-prevalence of 7.9%, compared with the national prevalence of 5.7%[5,38]. In Mbarara, both provider- and patient-initiated HIV testing are available at both government-run and private health clinics. The Immune Suppression Syndrome clinic is the largest government-supported HIV clinic in the District, which provides free care to a catchment area of nearly 475,000 residents. HIV testing is also a central component of prenatal care. Mobile and community-based testing campaigns are sporadically present; people who receive care from traditional healers report having primarily used clinical facilities for prior HIV testing[39].
The World Health Organization estimates that nearly 80% of the population in Uganda utilizes indigenous traditional medicine[40]. A previous study from Mbarara showed that 98% of clients of traditional healers also utilize formal facility-based care, although healers are visited more four times more frequently[39]. In the parent trial, traditional healers were recruited as clusters, and as the unit of randomization. Seventeen traditional healers in Mbarara Township were randomized to an intervention or usual care (control) arm. In the intervention arm, nine healers offered supervised HIV self-testing (Oraquick©) at their practices with pre- and post-test counseling. In the control arm, eight healers delivered protocolized usual care with HIV education and a referral to existing clinic-based HIV testing services. 250 individuals were enrolled per study arm between August 2019 and February 2020 (overall N=500). The trial’s primary outcome was HIV testing within 90 days of study enrollment and secondary outcomes included new HIV diagnoses and linkage to care for those newly diagnosed as PLWH.
Sampling and Recruitment
All participants included in this qualitative study were originally recruited as part of the parent trial. Purposive sampling was used to identify a sample of trial participants representing the range of trial outcomes, recruitment sites, gender and age. Inclusion criteria from the parent study were as follows: 1) 18 years of age or older; 2) sought care from participating traditional healers during the trial enrollment period, 3) sexually active, defined as self-report of ever having intercourse; 4) self-report of not having received an HIV test in the prior 12 months; and 5) not previously diagnosed with HIV-infection. Interviews with traditional healer clients in both study arms were conducted following 90-day study follow up. Participating traditional healers from both study arms were invited to complete an interview after recruitment had been completed at their practice location. All participants provided written informed consent. Participants were contacted by co-authors PT and GN via telephone and invited to take part in an interview. Overall, 124 individuals were recruited into this qualitative study.
Data Collection
All interviews were conducted in-person between December 2019 and May 2020. Interviews were conducted in the local language (Runyankole) and translated/transcribed into English for analysis, by two co-authors (PT, GN) who are fluent in both languages. Interviews were conducted following an interview guide to maintain focus on certain key topics, while allowing for open-ended participant responses. Interviews for trial participants and traditional healers explored the following topics: 1) general experiences participating in the trial; and 2) perceptions of traditional healers being involved in HIV counseling and testing services. In addition, participant interviews explored factors influencing outcome of HIV testing or not testing. Interviews lasted approximately 60 minutes, and were audio recorded to facilitate verbatim transcription/translation.
Data Analysis
Following an inductive, content-analysis approach[41], all transcripts were reviewed to develop a coding scheme relevant to the trial outcomes, and to describe experiences of trial participants. Codes were independently developed by two authors (MP, RS) in vivo, through repeated engagement with the dataset. Coding schema were compared, with discrepancies in codes resolved through discussion until a consensus was reached. Using a framework approach[42], coded data was organized by topic, and entered into an analytical matrix by author MP. Authors MP and RS reviewed the matrix to identify larger concepts that could explain results of the parent trial.
Ethics Approvals
This study was approved by the Weill Cornell Medicine Institutional Review Board (Protocol 18–03019105), the Mbarara University of Science and Technology Institutional Review Board (Protocol 16-/01–17), and the Ugandan National Council on Science and Technology (Protocol SS-4338). All interviews were conducted in secure, private locations to maintain participant confidentiality.
Results
Characteristics of study participants:
A total of 107 adults receiving care from traditional healers and 17 traditional healers were included in this study. All potential participants who were invited agreed to complete an interview. A summary of characteristics of interview participants is shown in Table 1. Characteristics were largely similar between those recruited from the control and intervention arms. The majority of clients of traditional healers were female (N = 62, 58%). Out of 107 interviews, approximately ⅓ (N = 38) were conducted with intervention arm participants. Ages ranged from 19 to 80 years old, with mean age of 35 years. Most participants had completed primary school or less. Participants had not received an HIV test for an average of 30 months (SD = 21.2) Interviews were also conducted with the 17 participating traditional interviews. The mean age of healers was 44 years old, and 7 were female (41%). Healer specialties included spiritual healers (N = 6, 35%), herbalists (N = 5, 29%), bonesetters (N = 4, 24%) and traditional birth attendants (N = 2, 12%).
Table 1.
Participant demographics.
| Characteristic | Healer patients (n = 107) | Healers (n = 17) |
|---|---|---|
| Female, n (%) | 62 (58) | 6 (35) |
| Age (years), (mean, SD) | 35 (14.1) | 44 (11.5) |
| Intervention arm, n (%) | 38 (35.5) | 9 (52.9) |
| Highest level of education, n (%) | Primary school or less, 59 (55.1) Secondary school, 29 (27.1) Diploma or higher, 19 (17.8) |
Primary school or less, 5 (29.4) Secondary school, 7 (41.2) Diploma or higher, 5 (29.4) |
| Ever received an HIV test, n (%) | 97 (93%) | 16 (94%) |
| Months since last HIV test, mean (mean, SD) | 30 (21.2) | N/A |
Data for date of last HIV test was not collected for traditional healers
Qualitative results
This qualitative study explored issues related to receiving HIV testing, with special attention to provider and participant experiences. First, we present factors contributing towards low uptake of existing HIV testing services. Second, we explore variables that explain high uptake of HIV testing when self-testing was offered by the traditional healers. Third, we report other potential avenues for traditional healers to be incorporated into the HIV continuum of care. Finally, we present an overall framework that explains how a POC HIV testing program successfully expanded uptake of HIV testing among adults who received care from traditional healers. In Table 2, we present excerpts from interview transcripts to illustrate our findings, which are numbered for reference.
Table 2.
Summary of themes pertinent to trial results and quotes from exit interviews
| Trial Result | Theme | Representative Quotes |
|---|---|---|
| Low uptake of clinic-based testing in the control arm | Long distance to clinics and lack of motorized transportation |
|
| Prioritizing income generating activities over HIV testing |
|
|
| HIV-related stigma |
|
|
| Healers’ counseling was sometimes adequate to support uptake of HIV testing |
|
|
| Peer support improved uptake of HIV testing |
|
|
| High uptake of HIV testing delivered at traditional healer locations among intervention arm participants | Acceptability and ease of POC HIV testing technology |
|
| Close relationships between community members with perceived “confidentiality” of practices |
|
|
| Healer practices are accessible |
|
|
| Traditional healers could support clients through the HIV continuum of care | Healers spontaneously accompanied PLWH to HIV clinics to (re)initiate ART |
|
| Clients described a need for continued psychosocial and adherence support |
|
|
| Healers delivered effective post-test counseling |
|
Explaining uptake of clinic-based HIV testing at existing facilities
Study participants described three important barriers to receiving an HIV test at existing facilities. First, testing services were frequently located outside of a radius which could be reached on foot. Motorized transport was necessary to access these services, and many lacked funds for transportation (Table 2: Q1). Lack of money for transportation was described as a significant barrier to uptake of HIV testing, with competing household costs as a common reason for inability to pay for transportation.
Second, participants described difficulty finding time to travel to facilities. Perceived lack of time for testing, and prioritization of income generation, created barriers to uptake of facility-based HIV testing (Q2). Finally, HIV-related stigma was a significant deterrent from seeking testing at clinical facilities. By presenting for HIV testing, patients reported feeling stigmatized about potential serostatus or being engaged in high-risk activities (Q3). Even general health facilities maintained dedicated spaces and queues for HIV testing and services. By seeking care at existing clinical locations, patients describe feeling unable to maintain confidentiality regarding the purpose of their visit.
Despite aforementioned challenges, 23% of control arm participants presented for facility-based HIV testing. Among those receiving testing, they described HIV education and referrals from traditional healers as particularly impactful. Participants reported that they felt “encouraged” by healers to maintain their health, which motivated them to seek testing (Q4). Others reported that support from their peers was a critical component of their decision to receive HIV testing. Forms of peer support included visiting the HIV testing facility together and sharing transportation costs, which reduced each person’s financial burden (Q5). There was no observable trend in modality of peer support when considering age or sex.
Explaining high uptake of HIV testing delivered at traditional healer locations
We note three factors that explain the significant success of HIV testing delivered by traditional healers directly to their clients. First, although technology of oral swab HIV testing was new to many participants, the self-test results were viewed as trustworthy. After receiving an HIV test as part of the trial, participants did not generally feel the need to confirm the results. Some participants did, however, express that they sought verification from healthcare professionals regarding the validity of self-test results, particularly because the technology was unfamiliar. Healers similarly reported ease and confidence in use of the POC oral swab test (Q6). In addition, our participants report being pleased with the traditional healers’ role delivering HIV testing as part of the study. While some were surprised to see that their healer could offer such services, they generally welcomed the idea (Q7).
Second, participants expressed a preference for HIV testing at the traditional healer practice rather than at clinical facilities because many had pre-existing relationships with healers. Healers and their clients have the benefit of living in the same community, which was described as providing foundations of trust, interpersonal comfort, and familiarity (Q8). Additionally, receiving HIV testing at the healer location was described as more “confidential,” compared with presenting to facility-based testing sites. Specifically, healers treat many types of ailments and participants reported being less concerned with anticipated stigma of seeking HIV-testing when visiting a traditional healer (Q9). Therefore, healer-delivered HIV testing allowed clients to retain privacy regarding the reason for the visit. Finally, healers are accessible and have locations within their communities. These characteristics supported delivery and uptake of HIV testing at their locations, circumventing the financial barriers of accessing clinic-based services (Q10).
Taken together, these factors contributed towards high acceptability of traditional healer-delivered counseling and HIV testing. The overwhelming success of this intervention can be attributed to positive user and provider experience with HIV self-testing technology, and the strong sense of trust and confidentiality patients have with traditional healers. Given that healers are located in communities, HIV testing delivered at these locations were easier to access, compared with facility-based services.
Traditional healers could potentially support clients through the HIV continuum of care
Study participants explained how traditional healers could be further incorporated into the HIV continuum of care for PLWH. While we did not instruct healers to provide support for those newly diagnosed as PLWH, interviews with participants revealed that healers were spontaneously providing assistance for these clients. In some cases, traditional healers decided to accompany PLWH to the HIV clinic to either initiate or reinitiate ART (Q11). Clients of traditional healers who were newly diagnosed with HIV described the desire for psychosocial support from their healers (Q12), explaining that adherence support and counseling delivered by traditional healers would be impactful towards maximizing ART uptake and adherence. We also noted that healer-delivered post-test counseling motivated clients newly diagnosed with HIV to accept their diagnosis and adhere to treatment plans (Q13). In some cases, intervention arm healers’ post-test counseling also encouraged PLWH to disclose their status to sexual partners and provided education on partner testing (Q14).
Summary of study results explaining intervention success
We describe low uptake of existing HIV testing services as a function of three significant barriers: 1) lack of funds for transportation; 2) lack of time away from income-generating activities; and 3) HIV-related stigma. In contrast, HIV self-testing delivered by traditional healers had high uptake, which can be explained by 1) acceptability of healer-facilitated HIV counseling and self-testing; 2) preference for healers’ “confidential” practices; and 3) accessibility of healer locations. Taken together, we developed a framework to summarize user experiences with explanations of how the intervention processes supported HIV testing uptake (Figure 1).
Figure 1.
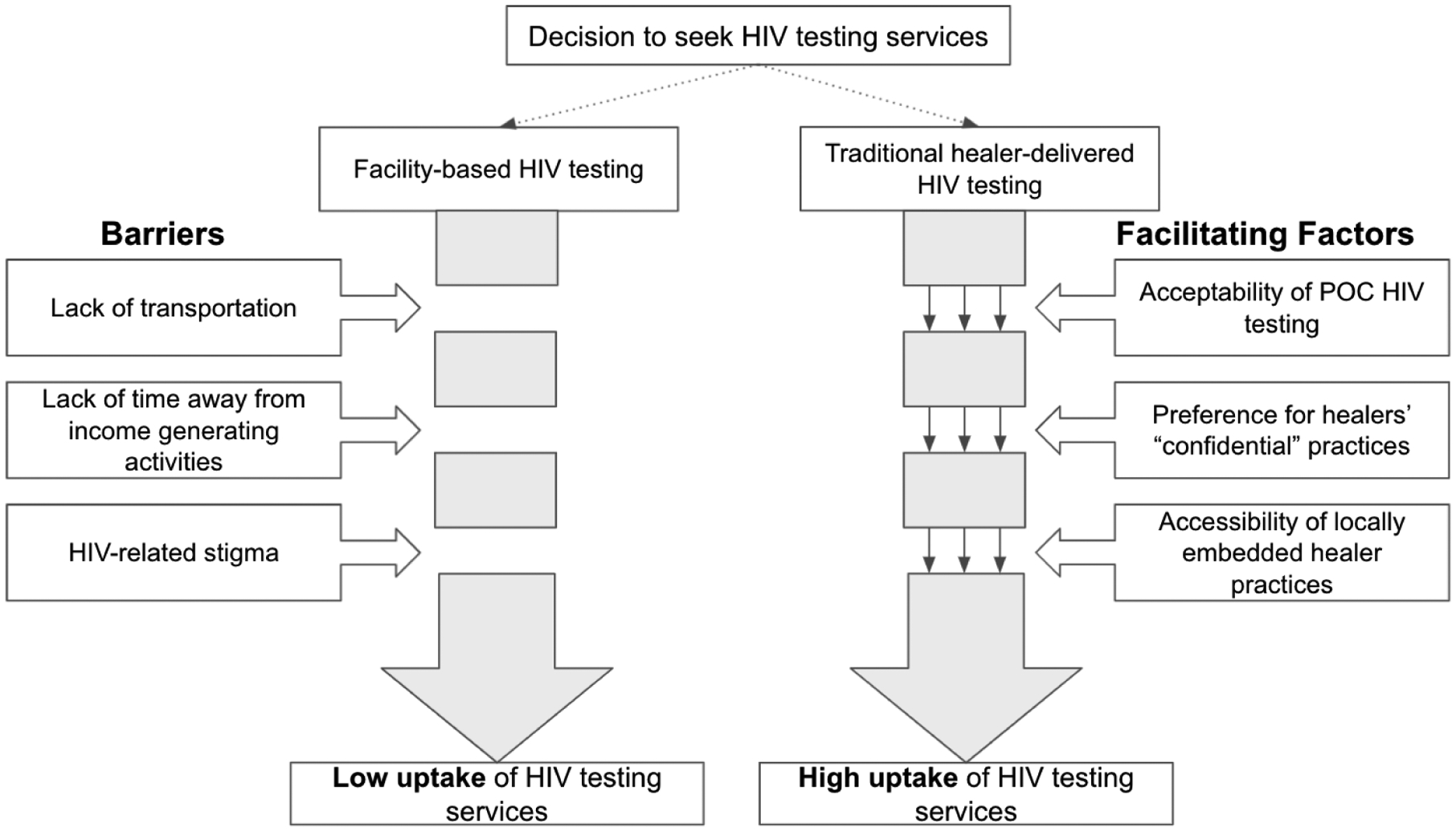
Summary of study results explaining high uptake of traditional healer-delivered HIV counseling and testing intervention, compared with usual clinic-based HIV testing.
Our findings indicate that delivery of HIV self-testing was successful in these contexts because traditional healers wield social capital, as demonstrated by preference for receiving testing from healers compared with clinical facilities, and participants’ confidence in healer-delivered HIV counseling and testing. This social capital could even overcome inhibitory social factors such as HIV-related stigma. Healers are also locally embedded providers, with practice locations close to the homes of their clients, circumventing economic and logistical challenges associated with transportation.
Discussion
Our qualitative data illustrate how an HIV counseling and testing program delivered by traditional healers significantly increased uptake of HIV testing among a sample of adults of unknown serostatus in rural Uganda. We noted that byproducts of poverty and HIV-related stigma created significant barriers to uptake of clinic-based testing. Our data explain some of the success of the healer-delivered testing program were because of perceived trust, confidentiality, and ease of access. We also note that traditional healers may be well-positioned to provide support for downstream components in the HIV continuum of care.
Our work illustrates how and why current clinic-based HIV testing programs may not effectively increase uptake of HIV testing in global, low-resource contexts. In rural settings, where communities are often geographically isolated, accessing and utilizing clinic-based facilities presents challenges that deter engagement with healthcare services. These economic and logistic barriers are not unique to HIV testing, and may reflect barriers to healthcare utilization in rural, impoverished settings more broadly. Transportation as a barrier to accessing HIV services have been previously described in other resource-poor settings[15,43–48]. Additionally, we found that many participants tended to prioritize income generating activities over HIV testing; this finding mirrors other work which illustrates similar emphasis on economic productivity over healthcare utilization, particularly among men[49–52]. Finally, our participants described anticipated stigma of being seen at HIV testing facilities, which is consistent with the literature demonstrating that stigma remains a pervasive barrier to engagement with HIV services in Sub-Saharan Africa[53–57].
Structural and social factors such as poverty and HIV-related stigma are not easily modifiable and must be accounted for during implementation of health-related interventions. Consequently, traditional healers present a local, culturally concordant solution with the potential to overcome many barriers associated with clinic-based facilities. Participant preference for traditional healers over facility-based clinical care is an important factor driving the success of this intervention. Healers are consulted for a myriad of health concerns; they are considered highly respected members of the community, and consequently wield enormous influence [28,29,58]. Our findings suggest that healers’ social capital plays an important role in overcoming social factors such as HIV-related stigma and lack of confidentiality at clinic-based facilities. Participants noted feeling more comfortable being tested by someone they know and trust, and preferred being tested in the privacy of their healer’s worksite as opposed to being identified at HIV testing clinics. Participant narratives also suggest that HIV self-testing technology was readily accepted due to trust and confidence participants had with their healers despite the technology being new to participants. From the provider’s perspective, our data demonstrate that traditional healers are both willing and able to provide HIV counseling and testing. Healers in other settings have similarly expressed interest in delivery of HIV testing[59]. Finally, traditional healers are locally embedded in their communities, and within walking distance of most of their clients, removing logistical and economic barriers associated with accessing clinical facilities.
Furthermore, given that 50% of African PLWH default from HIV care within two years, additional support measures are needed to improve retention in HIV care[60]. Use of community lay providers to deliver non-clinical support for PLWH, such as psychosocial and adherence support, has effectively increased entry and retention in the HIV continuum of care[34,61–66]. However, traditional healers remain an untapped resource as community lay-providers for non-clinical HIV care. Our data suggest they could play an important role in supporting linkage to care, ART initiation and adherence. Further research is needed to explore the potential of incorporating traditional healers into additional steps of the HIV continuum of care.
Our study has few limitations. The majority of participants were among control arm participants. These participants were intentionally over-sampled to explore experiences of receiving an HIV test at a clinical facility versus not testing in this sub-set of trial participants. Another limitation of this study is recruitment bias. All trial participants were either traditional healers or adults seeking care from traditional healers. The barriers to HIV testing described in this study may not reflect barriers to facility-based care among those who prefer or exclusively use formal clinical services. However, the vast majority of people in Sub-Saharan Africa receive care from traditional healers[67], which suggests our findings may be generalizable to other African contexts. The goal of qualitative research is to present highly detailed and contextual results. Additional work is therefore needed to evaluate if our findings are generalizable to other HIV endemic regions. Finally, further studies are needed to assess if use of traditional healers as health care extenders is a cost-effective strategy.
Failure to engage with HIV testing services remains a significant challenge for controlling the HIV endemic in Sub-Saharan Africa. We identified social and structural factors that deter engagement with existing clinic-based HIV testing services, and demonstrate how traditional healer-delivered services are a viable solution for improving uptake of HIV testing. Traditional healers were a culturally acceptable and effective way to improve uptake of HIV testing in a rural Ugandan community, and could be strong partners to improve HIV testing in other areas of Sub-Saharan Africa.
Acknowledgements
This study was funded by the US National Institutes of Health, National Institute of Mental Health (K23MH111409, PI: Sundararajan). We appreciate our participants and thank them for taking part in this study.
Footnotes
Conflicts of Interest Statement: The authors have no conflicts of interest to disclose.
References
- 1.Health TLP. HIV 40: inequalities fuel pandemics. The Lancet Public Health. 2021. Jul 1;6(7):e434. [DOI] [PubMed] [Google Scholar]
- 2.Zaidi J, Grapsa E, Tanser F, Newell M-L, Bärnighausen T. Dramatic increases in HIV prevalence after scale-up of antiretroviral treatment: a longitudinal population-based HIV surveillance study in rural kwazulu-natal. AIDS. 2013. Sep 10;27(14):2301–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sartorius B, VanderHeide JD, Yang M, Goosmann EA, Hon J, Haeuser E, et al. Subnational mapping of HIV incidence and mortality among individuals aged 15–49 years in sub-Saharan Africa, 2000–18: a modelling study. The Lancet HIV. 2021. Jun 1;8(6):e363–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kharsany ABM, Karim QA. HIV Infection and AIDS in Sub-Saharan Africa: Current Status, Challenges and Opportunities. Open AIDS J. 2016. Apr 8;10:34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ugandan Ministry of Health. Uganda Population-based HIV Impact Assessment (UPHIA) 2016–2017. https://www.afro.who.int/sites/default/files/2017-08/UPHIA%20Uganda%20factsheet.pdf. 2017. Accessed April 30, 2021.
- 6.Prabhu S, Harwell JI, Kumarasamy N. Advanced HIV: diagnosis, treatment, and prevention. Lancet HIV. 2019. Aug;6(8):e540–51. [DOI] [PubMed] [Google Scholar]
- 7.May MT. Better to know: the importance of early HIV diagnosis. Lancet Public Health. 2017. Jan;2(1):e6–7. [DOI] [PubMed] [Google Scholar]
- 8.Thomas R, Probert WJM, Sauter R, Mwenge L, Singh S, Kanema S, et al. Cost and cost-effectiveness of a universal HIV testing and treatment intervention in Zambia and South Africa: evidence and projections from the HPTN 071 (PopART) trial. Lancet Glob Health. 2021. May;9(5):e668–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kapadia F, Landers S. Ending the HIV Epidemic: Getting to Zero AND Staying at Zero. Am J Public Health. 2020. Jan 1;110(1):15–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wanyenze RK, Kamya MR, Fatch R, Mayanja-Kizza H, Baveewo S, Sawires S, et al. Missed Opportunities for HIV Testing and Late-Stage Diagnosis among HIV-Infected Patients in Uganda. PLOS ONE. 2011. Jul 5;6(7):e21794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Creek TL, Ntumy R, Seipone K, Smith M, Mogodi M, Smit M, et al. Successful introduction of routine opt-out HIV testing in antenatal care in Botswana. J Acquir Immune Defic Syndr. 2007. May 1;45(1):102–7. [DOI] [PubMed] [Google Scholar]
- 12.De Cock KM, Barker JL, Baggaley R, El Sadr WM. Where are the positives? HIV testing in sub-Saharan Africa in the era of test and treat. AIDS. 2019. Feb 1;33(2):349–52. [DOI] [PubMed] [Google Scholar]
- 13.Mulogo EM, Abdulaziz AS, Guerra R, Baine SO. Facility and home based HIV Counseling and Testing: a comparative analysis of uptake of services by rural communities in southwestern Uganda. BMC Health Services Research. 2011. Mar 4;11(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bogart LM, Naigino R, Maistrellis E, Wagner GJ, Musoke W, Mukasa B, et al. Barriers to Linkage to HIV Care in Ugandan Fisherfolk Communities: A Qualitative Analysis. AIDS Behav. 2016. Oct;20(10):2464–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tuller DM, Bangsberg DR, Senkungu J, Ware NC, Emenyonu N, Weiser SD. Transportation Costs Impede Sustained Adherence and Access to HAART in a Clinic Population in Southwestern Uganda: A Qualitative Study. AIDS Behav. 2010. Aug;14(4):778–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mugisha E, van Rensburg GH, Potgieter E. Strategic Framework for Increasing Accessibility and Utilization of Voluntary Counseling and Testing Services in Uganda. AIDS Res Treat;2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma M, Ying R, Tarr G, Barnabas R. Systematic review and meta-analysis of community and facility-based HIV testing to address linkage to care gaps in sub-Saharan Africa. Nature. 2015. Dec 3;528(7580):S77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Havlir DV, Balzer LB, Charlebois ED, Clark TD, Kwarisiima D, Ayieko J, et al. HIV Testing and Treatment with the Use of a Community Health Approach in Rural Africa. New England Journal of Medicine. 2019. Jul 18;381(3):219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korte JE, Kisa R, Vrana-Diaz CJ, Malek AM, Buregyeya E, Matovu JKB, et al. HIV Oral Self-Testing for Male Partners of Women Attending Antenatal Care in Central Uganda: Uptake of Testing and Linkage to Care in a Randomized Trial. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2020. Jul 1;84(3):271–9. [DOI] [PubMed] [Google Scholar]
- 20.Choko AT, Nanfuka M, Birungi J, Taasi G, Kisembo P, Helleringer S. A pilot trial of the peer-based distribution of HIV self-test kits among fishermen in Bulisa, Uganda. PLoS One. 2018;13(11):e0208191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horvath KJ, Bwanika JM, Lammert S, Banonya J, Atuhaire J, Banturaki G, et al. HiSTEP: A Single-Arm Pilot Study of a Technology-Assisted HIV Self-testing Intervention in Kampala, Uganda. AIDS Behav. 2021. Aug 28. [DOI] [PubMed] [Google Scholar]
- 22.Burke VM, Nakyanjo N, Ddaaki W, Payne C, Hutchinson N, Wawer MJ, et al. HIV self-testing values and preferences among sex workers, fishermen, and mainland community members in Rakai, Uganda: A qualitative study. PLOS ONE. 2017. Aug 16;12(8):e0183280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matovu JKB, Nambuusi A, Wanyenze RK, Serwadda D. Peer-leaders’ experiences and challenges in distributing HIV self-test kits in a rural fishing community, Rakai, Uganda. BMC Public Health. 2021. Apr 12;21(1):708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ortblad KF, Musoke DK, Ngabirano T, Nakitende A, Haberer JE, McConnell M, et al. Female Sex Workers Often Incorrectly Interpret HIV Self-Test Results in Uganda. J Acquir Immune Defic Syndr. 2018. Sep 1;79(1):e42–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okoboi S, Twimukye A, Lazarus O, Castelnuovo B, Agaba C, Immaculate M, et al. Acceptability, perceived reliability and challenges associated with distributing HIV self-test kits to young MSM in Uganda: a qualitative study. J Int AIDS Soc. 2019. Mar;22(3):e25269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perkins JM, Nyakato VN, Kakuhikire B, Mbabazi PK, Perkins HW, Tsai AC, et al. Actual Versus Perceived HIV Testing Norms, and Personal HIV Testing Uptake: A Cross-Sectional, Population-Based Study in Rural Uganda. AIDS Behav. 2018. Feb;22(2):616–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wanyama JN, Tsui S, Kwok C, Wanyenze RK, Denison JA, Koole O, et al. Persons living with HIV infection on antiretroviral therapy also consulting traditional healers: a study in three African countries. Int J STD AIDS. 2017. Sep 1;28(10):1018–27. [DOI] [PubMed] [Google Scholar]
- 28.Sundararajan R, Mwanga-Amumpaire J, King R, Ware NC. Conceptual model for pluralistic healthcare behaviour: results from a qualitative study in southwestern Uganda. BMJ Open. 2020. 20;10(4):e033410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hooft A, Nabukalu D, Mwanga-Amumpaire J, Gardiner MA, Sundararajan R. Factors Motivating Traditional Healer versus Biomedical Facility Use for Treatment of Pediatric Febrile Illness: Results from a Qualitative Study in Southwestern Uganda. Am J Trop Med Hyg. 2020. Jul;103(1):501–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atwine F, Hultsjö S, Albin B, Hjelm K. Health-care seeking behaviour and the use of traditional medicine among persons with type 2 diabetes in south-western Uganda: a study of focus group interviews. Pan Afr Med J. 2015;20:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abbo C. Profiles and outcome of traditional healing practices for severe mental illnesses in two districts ofEastern Uganda. Glob Health Action. 2011;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Audet CM, Blevins M, Rosenberg C, Farnsworth S, Salato J, Fernandez J, et al. Symptomatic HIV-positive persons in rural Mozambique who first consult a traditional healer have delays in HIV testing: a cross-sectional study. J Acquir Immune Defic Syndr. 2014. Aug 1;66(4):e80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Homsy J, King R, Balaba D, Kabatesi D. Traditional health practitioners are key to scaling up comprehensive care for HIV/AIDS in sub-Saharan Africa. AIDS. 2004. Aug 20;18(12):1723–5. [DOI] [PubMed] [Google Scholar]
- 34.King R, Kaboru Balaba D. The Role of Traditional Healers in Comprehensive HIV/AIDS AIDS AIDS AIDS Prevention and Care in Africa: Untapped Opportunities. In: From The Ground Up. Washington D.C., USA: Elizabeth Glaser Pediatric AIDS Foundation; 2009. p. 301–32. [Google Scholar]
- 35.Ponticiello M, Mwanga-Amumpaire J, Nansera D, King R, Sundararajan R. Could use of informal healthcare providers increase uptake of HIV testing? Qualitative results from southwestern Uganda. The Lancet Global Health. 2021. Mar 1;9:S25. [Google Scholar]
- 36.Broderick K, Ponticiello M, Nabukalu D, Tushemereirwe P, Nuwagaba G, King R, et al. Shortening “the Road” to Improve Engagement with HIV Testing Resources: A Qualitative Study Among Stakeholders in Rural Uganda. AIDS Patient Care and STDs. 2021. Jan 20;apc.2020.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sundararajan R, Ponticiello M, Lee MH, Strathdee SA, Muyindike W, Nansera D, et al. Traditional healer-delivered point-of-care HIV testing versus referral to clinical facilities for adults of unknown serostatus in rural Uganda: a mixed-methods, cluster-randomised trial. The Lancet Global Health. 2021. Nov 1;9(11):e1579–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joint United Nations Program on HIV/AIDS (UNAIDS). Uganda: Country Fact Sheet. http://www.unaids.org/en/regionscountries/countries/uganda. 2019. Accessed May 6, 2021.
- 39.Nabukalu D, Ponticiello M, Bennett T, Clark S, King R, Mwanga-Amumpaire J, et al. Factors associated with HIV testing among traditional healers and their clients in rural Uganda: Results from a cross-sectional study. Int J STD AIDS. 2021. May 12;9564624211015028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.WHO global report on traditional and complementary medicine 2019. Geneva: World Health Organization; 2019. https://apps.who.int/iris/bitstream/handle/10665/312342/9789241515436-eng.pdf?sequence=1&isAllowed=y. Accessed February 24, 2022. [Google Scholar]
- 41.Hsieh H-F, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005. Nov;15(9):1277–88. [DOI] [PubMed] [Google Scholar]
- 42.Gale NK, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Medical Research Methodology. 2013. Sep 18;13(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hardon AP, Akurut D, Comoro C, Ekezie C, Irunde HF, Gerrits T, et al. Hunger, waiting time and transport costs: time to confront challenges to ART adherence in Africa. AIDS Care. 2007. May;19(5):658–65. [DOI] [PubMed] [Google Scholar]
- 44.Siedner MJ, Lankowski A, Tsai AC, Muzoora C, Martin JN, Hunt PW, et al. GPS-measured distance to clinic, but not self-reported transportation factors, are associated with missed HIV clinic visits in rural Uganda. AIDS. 2013. Jun 1;27(9):1503–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boender TS, Sigaloff KCE, Kayiwa J, Musiime V, Calis JCJ, Hamers RL, et al. Barriers to Initiation of Pediatric HIV Treatment in Uganda: A Mixed-Method Study. AIDS Research and Treatment. 2012. Feb 6;2012:e817506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marum E, Taegtmeyer M, Chebet K. Scale-up of Voluntary HIV Counseling and Testing in Kenya. JAMA. 2006. Aug 16;296(7):859–62. [DOI] [PubMed] [Google Scholar]
- 47.Buor D Analysing the primacy of distance in the utilization of health services in the Ahafo-Ano South district, Ghana. Int J Health Plann Manage. 2003. Dec;18(4):293–311. [DOI] [PubMed] [Google Scholar]
- 48.Lankowski AJ, Siedner MJ, Bangsberg DR, Tsai AC. Impact of geographic and transportation-related barriers on HIV outcomes in sub-Saharan Africa: a systematic review. AIDS Behav. 2014. Jul;18(7):1199–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ponticiello M, Mwanga-Amumpaire J, Tushemereirwe P, Nuwagaba G, King R, Sundararajan R. “Everything is a Mess”: How COVID-19 is Impacting Engagement with HIV Testing Services in Rural Southwestern Uganda. AIDS Behav. 2020. May 25;1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Camlin CS, Ssemmondo E, Chamie G, El Ayadi AM, Kwarisiima D, Sang N, et al. Men “missing” from population-based HIV testing: insights from qualitative research. AIDS Care. 2016;28 Suppl 3:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsai AC, Siedner MJ. The Missing Men: HIV Treatment Scale-Up and Life Expectancy in Sub-Saharan Africa. PLoS Med. 2015. Nov 24;12(11):e1001906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chikovore J, Gillespie N, McGrath N, Orne-Gliemann J, Zuma T, ANRS 12249 TasP Study Group. Men, masculinity, and engagement with treatment as prevention in KwaZulu-Natal, South Africa. AIDS Care. 2016;28 Suppl 3:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nabunya P, Byansi W, Sensoy Bahar O, McKay M, Ssewamala FM, Damulira C. Factors Associated With HIV Disclosure and HIV-Related Stigma Among Adolescents Living With HIV in Southwestern Uganda. Front Psychiatry [Internet]. 2020. [cited 2021 Apr 16];11. Available from: https://www.frontiersin.org/articles/10.3389/fpsyt.2020.00772/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tshabalala J, Visser M. Developing a Cognitive Behavioural Therapy Model to Assist Women to Deal with HIV and Stigma. South African Journal of Psychology. 2011. Mar 1;41(1):17–28. [Google Scholar]
- 55.Kalichman S, Simbayi L. HIV testing attitudes, AIDS stigma, and voluntary HIV counselling and testing in a black township in Cape Town, South Africa. Sex Transm Infect. 2003. Dec;79(6):442–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steward WT, Bharat S, Ramakrishna J, Heylen E, Ekstrand ML. Stigma is associated with delays in seeking care among HIV-infected people in India. J Int Assoc Provid AIDS Care. 2013. Apr;12(2):103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kitara DL, Aloyo J. HIV/AIDS Stigmatization, the Reason for Poor Access to HIV Counseling and Testing (HCT) Among the Youths in Gulu (Uganda). African Journal of Infectious Diseases. 2012;6(1):12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Green EC. Sexually transmitted disease, ethnomedicine and health policy in Africa. Social Science & Medicine. 1992. Jul 1;35(2):121–30. [DOI] [PubMed] [Google Scholar]
- 59.Audet CM, Clemens EM, Ngobeni S, Mkansi M, Sack DE, Wagner RG. Throwing the bones to diagnose HIV: Views of rural South African traditional healers on undertaking HIV counselling and testing. AIDS Care. 2020. Aug 17;0(0):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rosen S, Fox MP, Gill CJ. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS Med. 2007. Oct 16;4(10):e298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fatti G, Mothibi E, Shaikh N, Grimwood A. Improved long-term antiretroviral treatment outcomes amongst patients receiving community-based adherence support in South Africa. AIDS Care. 2016. Nov;28(11):1365–72. [DOI] [PubMed] [Google Scholar]
- 62.Jopling R, Nyamayaro P, Andersen LS, Kagee A, Haberer JE, Abas MA. A Cascade of Interventions to Promote Adherence to Antiretroviral Therapy in African Countries. Curr HIV/AIDS Rep. 2020. Oct;17(5):529–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wouters E, Van Damme W, van Rensburg D, Masquillier C, Meulemans H. Impact of community-based support services on antiretroviral treatment programme delivery and outcomes in resource-limited countries: a synthetic review. BMC Health Serv Res. 2012. Jul 9;12:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bogart LM, Wagner GJ, Mutchler MG, Risley B, McDavitt BW, McKay T, et al. Community HIV treatment advocacy programs may support treatment adherence. AIDS Educ Prev. 2012. Feb;24(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.SEARCH Collaboration. Evaluating the feasibility and uptake of a community-led HIV testing and multi-disease health campaign in rural Uganda. J Int AIDS Soc. 2017. Mar 30;20(1):21514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Asiimwe S, Ross JM, Arinaitwe A, Tumusiime O, Turyamureeba B, Roberts DA, et al. Expanding HIV testing and linkage to care in southwestern Uganda with community health extension workers. J Int AIDS Soc. 2017. Jul 21;20(Suppl 4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.World Health Organization. WHO traditional medicine strategy: 2014–2023. http://www.who.int/medicines/publications/traditional/trm_strategy14_23/en/. 2015. Accessed May 3, 2021.


