Abstract
Objective:
Sudden cardiac death (SCD) is a common initial manifestation of coronary heart disease (CHD); however, SCD risk prediction remains elusive.
Background:
Coronary artery calcium (CAC) is a marker of plaque burden. Whether CAC improves risk stratification for incident SCD beyond atherosclerotic cardiovascular disease (ASCVD) risk factors is unknown.
Methods:
We studied 66,636 primary prevention patients from the CAC Consortium. Multivariable competing risks regression and C-statistics were used to assess the association between CAC and SCD, adjusting for demographics and traditional risk factors.
Results:
The mean age was 54.4 years old, 33% were female, 11% were of non-white ethnicity, and 55% had CAC >0. A total of 211 SCD events (0.3%) were observed during a median follow-up of 10.6 years, 91% occurring among those with baseline CAC>0. Compared to CAC=0, there was a stepwise higher risk (p-trend <0.001) in SCD for CAC 100-399 (SHR =2.8, 95% CI: 1.6-5.0), CAC 400-999 (SHR =4.0, 95% CI: 22-13), and CAC >1000 (SHR =4.9, 95% CI: 2.6-9.9). CAC provided incremental improvements in the C-statistic for the prediction of SCD among individuals with a 10-year risk <7.5% (ΔC-statistic=+0.046, p=0.02) and 7.5-20% (ΔC-statistic=+0.069, p=0.003), which were larger when compared to persons with a 10-year risk >20% (ΔC-statistic=+0.01, p=0.54).
Conclusions:
Higher CAC burden strongly associates with incident SCD beyond traditional risk factors, particularly among primary prevention patients with low-intermediate risk. SCD risk stratification can be useful in the early stages of CHD through the measurement of CAC, identifying patients most likely to benefit from further downstream testing.
Keywords: coronary artery calcium, multidetector computed tomography, sudden cardiac death, cardiovascular diseases
Introduction
Sudden cardiac death (SCD) is characterized as an unexpected death within one hour of a change in clinical status among persons with or without preexisting atherosclerotic cardiovascular disease (ASCVD)(1). Overall, SCD contributes to between 180,000 and 450,000 deaths (7-18%) annually, and accordingly is one of the leading causes of death in the United States(2). Coronary heart disease (CHD) secondary to atherosclerosis is responsible for up to 80% of all SCD events(3) and less than 15% of cases occur among individuals with a known arrhythmia or heart failure(4). Despite significant improvements to the management of subclinical CHD in primary prevention patients, prediction of SCD remains elusive and is largely only initiated in secondary prevention populations after individuals have already experienced an initial ASCVD event(5). Low-cost and readily available subclinical atherosclerosis imaging has emerged as an important modality for ASCVD risk assessment in primary prevention patients and may have the potential to refine SCD risk assessment much earlier in the causal disease pathway.
Coronary artery calcium (CAC) is the most commonly employed marker of subclinical coronary atherosclerosis and is non-invasively measured on cardiac-gated non-contrast computed tomography (CT) via the Agatston method(6). CAC is strongly associated with incident ASCVD events and is a recommended diagnostic imaging modality for adults >40 years old when there is uncertainty in the 10-year predicted risk and initiation of primary prevention pharmacotherapy(7, 8). Although the role of CAC scoring is increasing for ASCVD risk stratification and the prediction of all-cause mortality, the utility of CAC for predicting SCD remains unknown.
The documented potential for discordance between measured traditional ASCVD risk factors and subclinical atherosclerosis(9, 10) supports the need for the testing of novel strategies to predict SCD in the general population. It has been previously shown that the presence and extent of CHD is a predictor of SCD(11); however, the use of a noninvasive subclinical atherosclerosis detection method for predicting SCD has not been pursued, especially in asymptomatic primary prevention patients. Here, we sought to assess the prospective association of CAC burden with SCD among persons without clinical ASCVD at baseline, and to determine how age and sex modify the relationship between CAC and future SCD.
Methods
Study Population
The CAC Consortium is a multicenter cohort study that includes four high-volume centers the United States, Cedars-Sinai Medical Center (Los Angeles, CA), PrevaHealth Wellness Diagnostic Center (Columbus, OH), Harbor-UCLA Medical Center (Torrance, CA), and Minneapolis Heart Institute (Minneapolis, MN). The multicenter retrospective cohort study aimed to assess the association between CAC and long-term, disease-specific mortality, and the study design and methods have been previously described in detail elsewhere(12). In brief, investigators included individuals >18 years of age who were primarily free of clinical ASCVD or cardiovascular symptoms at the time of CAC scanning. For the current study, we included all 66,636 participants in the CAC Consortium who were referred to undergo CAC scanning (1991-2010) due to the presence of underlying ASCVD risk factor(s) and uncertainty in long-term ASCVD risk. Written informed consent for participation in research was collected at each respective field center at the time of CAC scanning at baseline, and Institutional Review Board approval for coordinating center actions was by the Johns Hopkins University School of Medicine
Measurement of Coronary Artery Calcium
A standard protocol was used to quantify CAC using non-contrast, ECG-gated cardiac computed tomography (CT) at all participating medical centers(12). Electron beam and multidetector CT were used for imaging protocols, and earlier assessments have demonstrated that there are no clinically significant differences in CAC quantification between the two different scanning methods. Calcium scores were computed using the Agatston method. Participants were categorized into 5 CAC score groups: CAC=0, CAC 1 to 99, CAC 100 to 399, and CAC 400-999, CAC >1000.
Outcome Ascertainment
Mortality in the CAC Consortium was assessed by linking patient records with the Social Security Administration Death Master File using a previously validated algorithm. Similar to previous studies involving SCD, death certificates were obtained from the National Death Index and underlying cause of death was categorized into common causes of death using International Classification of Diseases (ICD), 9th and 10th Revision codes(13-15). When compared to known deaths identified via the electronic medical record in a subsample of the CAC Consortium, there was up to 90% specificity and sensitivity for the identification of deaths(12). Death rates in the CAC Consortium are similar when compared to US Census Bureau data and relevant population-based cohorts(12).
Similar to previously conducted epidemiological studies(13-15), SCD was defined as mortality due to cardiac dysrhythmia (ICD-9: 427), cardiac arrest (ICD-10: I46), paroxysmal tachycardia (ICD-10: I47), and/or other cardiac arrythmias (ICD-10: I49). For the current analysis, an SCD event was considered if a participant had one of the specific SCD ICD codes in the underlying cause position on the death certificate or if the SCD ICD code was in the first position of the death certificate when CHD was the underlying cause of death.
Evaluation of ASCVD Risk Factors
Assessment of ASCVD risk factors occurred contemporaneously with CAC testing. Diabetes and hypertension were defined by a previous clinical diagnosis or reported antihypertensive or glucose-lowering medication utilization. There was no information regarding the differentiation between type 1 versus type 2 diabetes. Dyslipidemia (low-density lipoprotein-cholesterol >160 mg/dL), hypertriglyceridemia (triglycerides >150 mg/dL), and low high-density lipoprotein-cholesterol (<40 mg/dL in men, <50 mg/dL in women) were defined by a previous clinical diagnosis or utilization of lipid-lowering therapy. Information on smoking and family history of CHD (first-degree relative with history of CHD at any age) were obtained through self-report data. The 10-year risk for ASCVD was calculated using the pooled-cohort equations (PCE) (16), including using the raw equations to extrapolate risk for those less than 40 years of age. Multiple imputation was performed in the case of limited missing data by utilizing non-missing data on age, sex, race, ASCVD risk factors, and CAC data (28% of participants in the overall CAC Consortium cohort). Of those participants with missing data, the majority (>72%) were lacking information for only one demographic or risk factor variable.
As noted in the original CAC Consortium methodology paper, multiple imputation was performed on 28% of individuals who missing data on ASCVD risk factors(12). Missing observations were treated as missing at random and the standard STATA command, ‘MI impute’, was used in the imputation algorithm which involved 10 imputations by default. The missing at random imputation method was utilized because the assumption was made that the probability of missingness of traditional ASCVD risk factors was not dependent on other missing values or other patterns of risk factors prevalence. Multiple imputation was performed controlling for observed non-missing values of all non-missing adjacent traditional ASCVD factors. As described previously(12), mean and median 10-year ASCVD risk scores have had strong agreement when using non-imputed versus imputed CAC Consortium data.
Statistical Analysis
Study population characteristics were stratified according to CAC burden (CAC=0, CAC 1-99, CAC 100-399, CAC 400-999, and CAC >1000). Given previous data suggesting that very-high CAC (>1000) in primary prevention patients confers an equivalent risk for an incident ASCVD event similar to that of stable secondary prevention patients(17), an upper limit of CAC >1000 was used in analyses. Continuous variables were presented as means and standard deviations, while percentages are used for categorical variables for categorical variables. Due to a non-normal distribution of CAC, the median was used to represent the central tendency of CAC scores. The Student’s t-test and Wilcoxon signed-rank test were used to assess differences in normally and non-normally distributed continuous variables, respectively. Differences between categorical variables were evaluated through the Chi-square test.
For associative analyses, we compared the number of SCD events across CAC burden categories (CAC=0, CAC 1-99, CAC 100-399, CAC 400-999, CAC >1000). While the original ASCVD risk groups consist of four categories (0-5%, 5-7.5%, 7.5-20%, >20%), we analyzed results across three guideline-based ASCVD risk groups (<7.5%, 7.5-20%, >20%) to optimize statistical power in the setting of a limited number of SCD events. Overall, SCD events were expressed as absolute numbers and proportions for the overall study sample and according to CAC category. The total number of events was divided by person-years of follow-up to calculate SCD event rates (per 1,000-year follow-up). Kaplan-Meier survival curves were computed for SCD events for individuals with CAC=0, CAC 1-99, CAC 100-399, CAC 400-999, and CAC>1000. Differences in survival among CAC burden groups were assessed through the log-rank test.
The associations between CAC burden and SCD was assessed through competing risks regression, with non-ASCVD mortality considered the competing risk. Similar to previously conducted analyses using SCD as an outcome(18), we performed competing risk regression to account for non-SCD deaths. The base model was adjusted for age and sex. A subsequent model was additionally adjusted for diabetes, current cigarette smoking, hypertension, hyperlipidemia, and a family history of CHD. Statistical testing for a significant linear trend across increasing CAC burden categories was assessed by reporting the p-value for the five-level CAC term when evaluated as a continuous independent variable in a linear regression model.
The predictive value of CAC for future SCD events was evaluated through Harrel’s C-statistic for all individuals and also according to ASCVD risk groups (low <7.5%, intermediate 7.5-20%, high >20%). C-statistics were compared by testing the equality of ROC areas using approaches derived by Weiand et al. for evaluating differences among nonparametric and parametric ROC curves(19). Given the sex-specific distribution of CAC burden(20) and association between CAC with left ventricular function(21), we performed two sensitivity analyses assessing 1) the association between CAC and incident SCD separately in men versus women, and 2) the association between CAC and incident SCD after excluding individuals who had a diagnosis of HF-associated mortality.
Statistical analyses were conducted using STATA 17 (Stata Corp., College Station, Texas). Statistical significance was defined as a p-value <0.05 on a 2-tailed test.
Results
The average age of participants was 54.4 years old, 33% were women, 11% were nonwhite, and there were 36,879 persons (55.3%) with CAC >0. More than one half of persons (55%) had a 10-year ASCVD less than 5%, although hypertension (31%), dyslipidemia (54.4%), and a family history of CHD (46.1%) were particularly prevalent among study participants (Table 1). Except for current cigarette smoking and a family history of CHD, there was a consistently higher prevalence of ASCVD risk factors across increasing CAC burden categories.
Table 1.
Baseline Characteristics of Study Participants in the CAC Consortium by CAC Score Categories
| Variable | Overall N=66,636 |
CAC=0 n=29,757 |
CAC 1–99 n=20,534 |
CAC 100–399 n=9,067 |
CAC 400-999 n=4,409 |
CAC >1000 N=2,869 |
|---|---|---|---|---|---|---|
| Age, mean ± SD, years | 54.4 ± 10.6 | 49.9 ± 9.21 | 54.9 ± 9.5 | 60.0 ± 9.5 | 63.0 ± 9.4 | 66.3 ± 9.7 |
| Women, % | 33.0 | 44.5 | 27.2 | 22.4 | 17.5 | 13.7 |
| Race, % | ||||||
| White | 89.0 | 88.7 | 88.9 | 90.4 | 90.7 | 87.6 |
| Asian | 3.8 | 4.2 | 3.5 | 3.4 | 3.2 | 4.0 |
| Black | 2.3 | 2.3 | 2.4 | 2.1 | 1.5 | 3.1 |
| Hispanic | 3.1 | 3.3 | 3.3 | 2.6 | 2.8 | 3.4 |
| Other | 1.7 | 1.6 | 1.9 | 1.6 | 1.8 | 1.9 |
| CAC Score, mean ± SD, AU | 164 ± 480 | 0 ± 0 | 28 ± 27 | 211 ± 85 | 638 ± 169 | 1953 ± 1152 |
| CAC Score, median (Q1, Q3), AU | 3 (0, 95) | 0 (0, 0) | 18 (5, 45) | 193 (138, 275) | 610 (489, 771) | 1572 (1225, 2256) |
| 10-year ASCVD risk, mean ± SD | 7.4 ± 9.2 | 3.8 ± 4.7 | 7.3 ± 7.5 | 11.5 ± 10.3 | 15.1 ± 12.0 | 20.2 ± 14.9 |
| 10-year ASCVD risk category | ||||||
| <7.5% | 68.6 | 87.6 | 67.2 | 44.5 | 30.0 | 17.9 |
| 7.5-20% | 23.5 | 11.0 | 26.9 | 40.5 | 45.0 | 42.8 |
| >20% | 7.9 | 1.4 | 5.9 | 15.1 | 25.0 | 39.3 |
| Hypertension, % | 31.0 | 22.8 | 31.9 | 40.2 | 46.5 | 55.4 |
| Dyslipidemia, % | 54.4 | 48.0 | 56.6 | 60.9 | 65.1 | 67.3 |
| Current smoker, % | 9.6 | 8.9 | 9.7 | 11.0 | 11.0 | 10.2 |
| Family history of CHD, % | 46.1 | 45.6 | 46.4 | 46.2 | 46.5 | 48.5 |
| Diabetes mellitus, % | 6.8 | 3.9 | 6.6 | 9.6 | 12.8 | 19.5 |
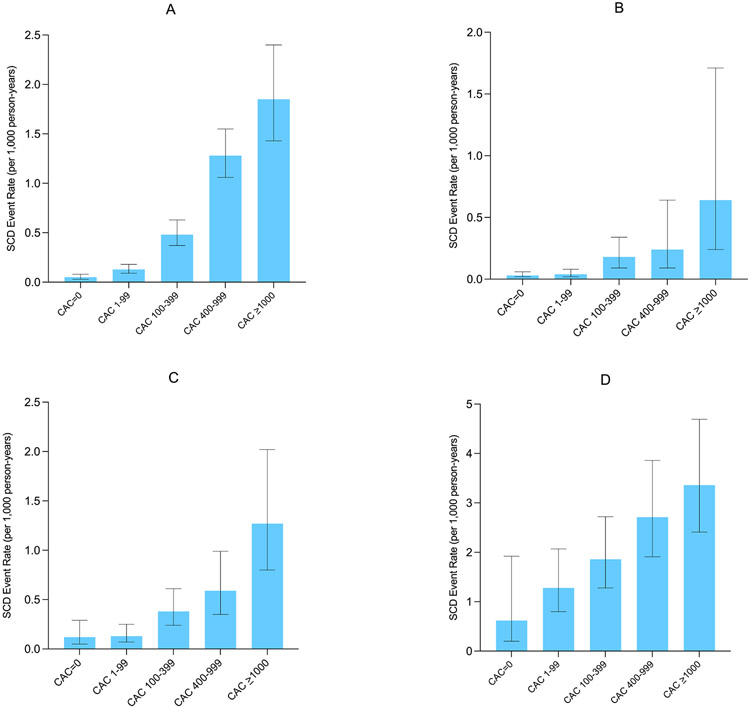
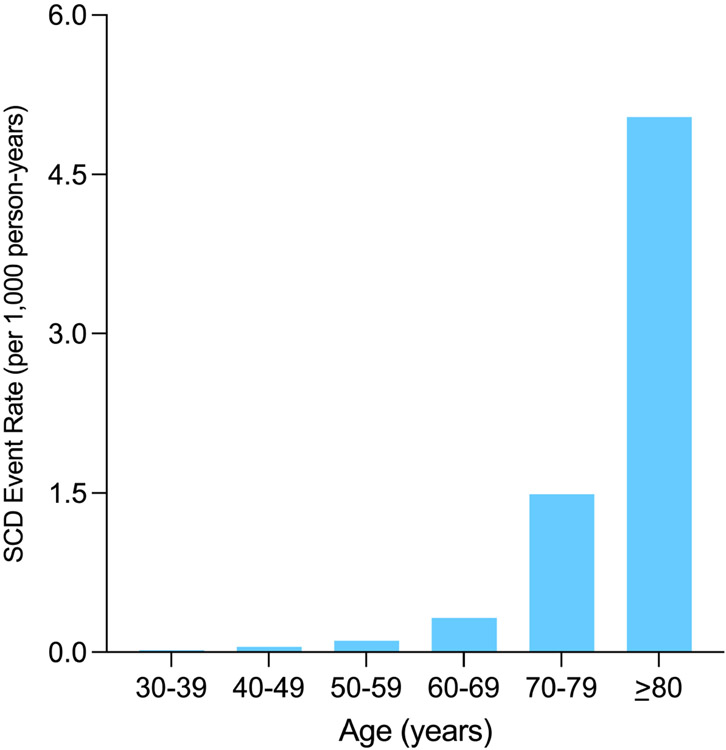
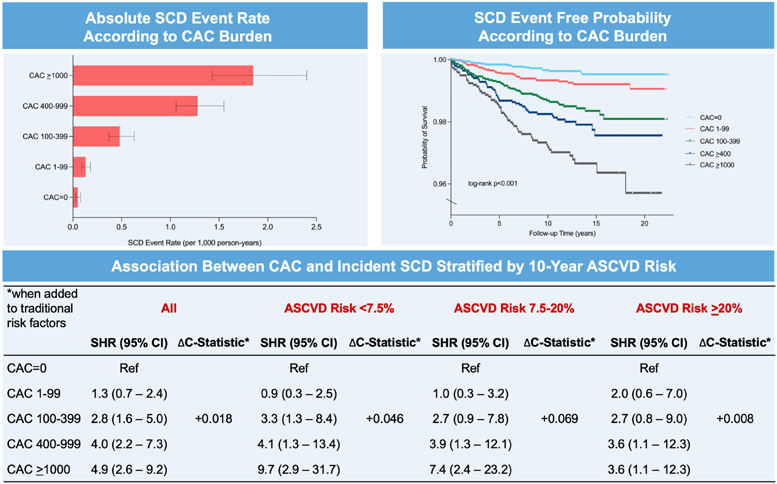
There were 211 SCD deaths over a median follow-up of 10.6 years, and a total of 91% of all SCD events occurred in patients with CAC>0. The SCD rate was less than 0.50 per 1,000 person years through 60 years of age, though individuals aged between 70-79 and those >80 years old had SCD event rates of 1.49 and 5.04 per 1,000 person-years, respectively (Figure 1). The SCD event rate was consistently higher across increasing CAC burden categories in the overall sample and when stratifying by baseline ASCVD risk (Figures 2A-2D). Compared to individuals with CAC=0 (0.05 per 1,000 person-years), individuals with CAC 1-99 had a more than 2-fold higher SCD event rate (0.13 per 1,000 person-years), whereas this difference was considerably larger for those CAC 100-399 (0.48 per 1,000 person-years), CAC 400-999 (0.94 per 1,000 person-years), and CAC >1000 (1.85 per 1,000 person-years).
Figure 1. Absolute SCD event rates in the overall sample (A), individuals with a 10-year ASCVD risk <7.5% (B), 7.5-20% (C), and >20% (D).
The SCD event rate was consistently higher across increasing CAC burden categories in the overall sample and when stratifying by baseline ASCVD risk.
Figure 2. Absolute SCD event rates, stratified by age.
SCD has a logarithmic association with age, strongly increasing in magnitude past 60 years old
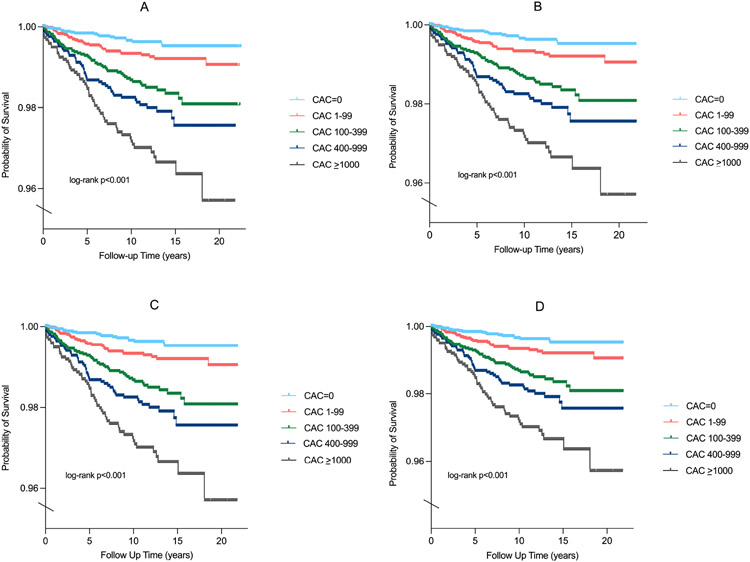
Kaplan Meier curves showed divergence in SCD event rates as early as 2 years of follow-up, particularly for persons with CAC 100-399, CAC 400-999, and CAC >1000 compared to those with CAC=0 and CAC 1-99 (Figures 3A). Significant differences in SCD survival were consistently observed after stratifying by baseline ASCVD risk (Figures 3B-3D). In fully adjusted models, there was a sequentially higher risk (p-trend <0.001) of SCD for increasing CAC burden (Table 2). Compared to individuals with CAC=0, persons with CAC 1-399 were 2.8 times more likely to experience incident SCD (SHR=2.8, 95% CI: 1.6-5.0), whereas CAC 400-999 (HR=4.0, 95% CI: 2.2-7.3) and CAC >1000 conferred a 4-fold and 4.9-fold higher risk for SCD (HR=4.9, 95% CI: 2.6-9.2), respectively.
Figure 3. Kaplan Meier plots for SCD survival probability in the overall sample (A), individuals with a 10-year ASCVD risk <7.5% (B), 7.5-20% (C), and >20% (D).
Kaplan Meier curves showed divergence in SCD event rates as early as 2 years of follow-up across CAC burden categories.
Table 2:
Multivariable-Adjusted SHRs (95% CIs) for CAC burden with SCD
| CAC Score Group | Events (n=211) |
Unadjusted SHR (95% Cl) |
P-trend | Model 1 SHR (95% CI)A |
P-trend | Model 2 SHR (95% CI)B |
P-trend |
|---|---|---|---|---|---|---|---|
| CAC=0 | 19 | - | - | - | |||
| CAC 1-99 | 33 | 2.5 (1.4 - 4.4) | 1.4 (0.8 - 2.5) | 1.3 (0.7 - 2.4) | |||
| CAC 100-399 | 53 | 9.2 (5.5 - 15.6) | <0.001 | 3.2 (1.8 - 5.6) | <0.001 | 2.8 (1.6 - 5.0) | <0.001 |
| CAC 400-999 | 49 | 17.8 (10.5 – 30.2) | 4.7 (2.6 – 8.5) | 4.0 (2.2 – 7.3) | |||
| CAC >1000 | 57 | 33.1 (19.7 – 55.7) | 6.3 (3.4–11.8) | 4.9 (2.6 – 9.2) |
adjusted for age and sex
adjusted for age, sex, current cigarette smoking, diabetes, hypertension, hyperlipidemia, and a family history of CHD
Although baseline 10-year ASCVD did not significantly modify the association between CAC and SCD (p-interaction=0.21), CAC scores 400-999 and >1000 appeared nominally more strongly associated with SCD among persons with a risk <7.5% (SHR=4.1, 95% CI: 1.3-13.4; SHR=9.7, 95% CI: 2.9-31.7) and 7.5-20% (SHR=3.9, 95% CI: 1.3-12.1; SHR=7.4, 95% CI: 2.4-23.2) compared to those with a 10-year risk >20% (SHR=3.6, 95% CI: 1.1-12.3; SHR=3.6, 95% CI: 1.1-12.3). The difference in SCD risk between individuals with CAC 400-999 and those with CAC >1000 was most notably appreciated among persons with low and intermediate 10-year ASCVD risk.
There was a similar SCD risk within a given CAC burden category for men versus women, and sex did not modify the association between CAC burden and SCD (Supplemental Table 1). Similar strengths of association between CAC burden and incident SCD were observed after excluding individuals who experienced HF-associated mortality (Supplemental Table 2, Supplemental Table 3).
CAC scoring improved discrimination of SCD when added to traditional risk factors (Table 4). In addition to improving SCD prediction in the overall sample (C-statistic=0.876 versus C-statistic=0.858, Δ+0.018, P <0.001), CAC scoring significantly improved SCD discrimination among persons spanning low, borderline, and intermediate 10-year ASCVD risks. CAC provided incremental improvements in the C-statistic for the prediction of SCD among individuals with a 10-year risk <7.5% (ΔC-statistic=+0.046, p=0.02) and 7.5-20% (ΔC-statistic=+0.069, p=0.003), which were considerably larger when compared to persons with a 10-year risk >20% (ΔC-statistic=+0.01, p=0.54). Overall, the addition of CAC burden categories to traditional ASCVD risk factors provided a similar magnitude improvement in the C-statistic for incident SCD prediction in men and women (Supplemental Table 4). Significant improvements for incident SCD prediction were consistently noted for individuals with a 10-year ASCVD risk <7.5% and between 7.5-20% after excluding individuals who experienced HF-associated mortality (Supplemental Table 5).
Table 4:
Discrimination statistics of Coronary Artery Calcium for SCD events overall and stratified by 10-year ASCVD risk.
| C-Statistic | Risk factorsA | CAC + Risk factors | P value |
|---|---|---|---|
| All | 0.858 | 0.876 | <0.001 |
| ASCVD Risk <7.5% | 0.765 | 0.811 | 0.02 |
| ASCVD Risk 7.5-20% | 0.708 | 0.777 | 0.003 |
| ASCVD Risk >20% | 0.682 | 0.690 | 0.54 |
age, sex, current cigarette smoking, diabetes, hypertension, hyperlipidemia, and a family history of CHD
Discussion
Despite significant advancements in the management of subclinical CHD in primary prevention patients, the prediction of SCD remains elusive. Here, in a large sample of primarily asymptomatic patients undergoing CAC testing for risk stratification in primary prevention, we observed a stepwise increase in the SCD event rate across increasing CAC burden, such that middle-aged individuals with CAC scores 100-399, 400-999, and >1000 had between a 2.8 to 4.9-fold higher risk for SCD compared to those with CAC=0, independent of traditional risk factors. Contrary to the current clinical paradigm, SCD risk stratification may be most useful in the very early stages of CHD through the quantification of calcified atherosclerotic plaque burden, which may help to inform additional downstream genetic testing and imaging.
A large majority of SCD prevention efforts have focused on the burden of ventricular arrhythmias and/or SCD risk stratification after individuals have suffered an initial myocardial infarction(5)(22). These strategies will undoubtedly continue to remain important; however, the novelty of our study is that we demonstrate how an early approach focused on quantifying the spectrum of plaque burden among primary prevention patients can help to refine SCD risk stratification very early in the atherosclerotic process. Compared to electrophysiological predictors from prior studies, such as QT prolongation, QRS duration, and Cornell voltage criteria,(23-25) prevalent CAC >100 appeared more strongly associated with incident SCD in our primary prevention cohort. Approximately four out five of SCD deaths are found to result from atherosclerotic CHD(3), thus our study fulfills an unmet need to support identifying potential anatomical predictors for SCD, especially early in the disease process.
With respect to cardiac function, several previous studies have observed that left ventricular ejection fraction predicts incident SCD, and current guidelines recommend implantable cardiac defibrillator (ICD) placement for 1) persons with a prior myocardial infarction at least 40 days ago who have an ejection fraction <30%, and 2) persons with New York Heart Association defined heart failure stage II and III who have an ejection fraction <35%(5). Our findings are unique in that all participants were free of clinical ASCVD at baseline, and further confirm that there exists a continuum of risk between primary and secondary prevention, which may be most effectively assessed through CAC scoring(17, 26). A potential clinical question arising from of our findings is whether CAC plus ejection fraction may be a better predictor of SCD than either alone, and whether those who have CAC >400 and especially CAC >1000 would potentially benefit from selective further downstream primary prevention screening such as assessment of left ventricular function and/or specific treatment for heightened SCD risk. Although, high CAC burden is not an established risk factor for abnormal left ventricular ejection fraction and/or vice-versa, individuals with very-high CAC (≥1000) for example may potentially benefit from an assessment of ventricular function. Such considerations may be most important amongst patients in high-risk occupations or who are newly initiating an intensive exercise program. Alternatively, CAC scoring may also help to refine SCD risk after a newly abnormal echocardiogram in persons with no known ischemic etiology, as it is known that the long-term mortality benefit of ICD placement in non-ischemic vs. potentially ischemic cardiomyopathy patients appears to differ(27).
Although the addition of CAC to traditional risk factors yielded the largest magnitude C-statistic improvements for SCD prediction among persons with low, borderline, and intermediate 10-year ASCVD risk compared to those with a high 10-year risk, the overall strength of association between CAC and SCD is perhaps the more important novel finding of our study. Whereas the C-statistic is a statistical tool to assess global discrimination, the stepwise increase in SCD risk across higher CAC burden suggests an underlying pathophysiological mechanism linking total calcified plaque burden and SCD, which could be mediated by tertiary variables such as left ventricular function, myocardial scar, and exercise-induced ischemia.
We identified a logarithmic association between age and incident SCD events, such that there was a large increase in SCD burden beginning at an age of 60 years old. More than 80% of Westernized adults >65 years old have CAC(28), therefore leveraging the differences in CAC burden in this demographic group may be especially useful to predict and prevent incident SCD events. Given the age-specific pathobiology of SCD(29), our results linking CAC testing to SCD may thus be most generalizable in middle-aged and older persons compared to younger individuals. The absolute risk of SCD due to coronary atherosclerosis, including progressive vascular calcification, increases in middle-aged and older persons versus younger individuals because the development of CAC occurs over several decades(30). Thus, evaluation of SCD risk should expectedly look different as individuals age.
Although very low in magnitude, a total of 19 SCD events (0.06%) occurred among persons with CAC=0, and the potential mechanisms underlying SCD in persons with CAC=0 should be discussed. The prevalence of non-calcified atherosclerotic plaque burden has been reported to be as high as 11% in persons with CAC=0(31), which may increase the risk for potential plaque rupture or thrombosis leading to SCD. Alternatively, undiagnosed structural heart disease, coronary dissection or spasm, or other electrophysiologic predispositions could be potential mechanisms that explains the low residual SCD risk for individuals with CAC=0.
The major strengths of this study include the measurement of CAC among more than 65,000 individuals who were enriched in ASCVD risk factors and a family history of CHD. As the cost of non-contrast computed tomography decreases and the imaging modality becomes more readily available, further investigation regarding the association of CAC with downstream ASCVD outcomes will be increasingly important for refining risk assessment. Furthermore, we conducted one of the first associative analyses between CAC and SCD, and here we demonstrate that anatomical-based testing may be important for SCD risk stratification and prevention even among persons without overt clinical ASCVD who have an intermediate 10-year risk. Future research that assesses the association between CAC burden and SCD may extend upon our findings by further phenotyping survival among sudden cardiac arrests and also utilizing autopsy studies as a mechanism to assess validity in SCD ascertainment.
Our study should be considered in the setting of certain limitations. Given that the CAC Consortium is a primary prevention population, there was an overall low number of SCD events, and our study was underpowered for less represented lower risk groups including women. Similar to all studies ascertaining SCD as an outcome from death certificates and ICD codes, misclassification bias(32) and generalizability are potential limitations. For example, one of the main challenges that continues to affect all studies of SCD is that there is no universal standardized definition of SCD, including the duration of time from clinical onset of symptoms to cardiac arrest(33). Such standardization will undoubtedly be necessary to improve precision in the prevention and risk assessment of SCD, particularly for identifying the subgroup of low ASCVD risk patients that contribute to the majority of SCD cases. Our findings relate specifically to the risk of SCD in screening populations and should not be misconstrued to represent the relationship of CAC scores to SCD among patients presenting with symptoms that are suggestive of CAD or in established secondary prevention. We used a validated method for SCD event ascertainment using National Death Index records to overcome this potential bias, and our results should only be generalized to middle-aged individuals who may have prevalent CAC and not used to guide SCD risk stratification in young adults. Furthermore, the CAC Consortium consists of predominantly participants of white ethnicity, and future studies involving CAC burden and SCD should incorporate greater ethnic diversity, specifically African American, Hispanic, and other non-white populations. Lastly, our statistical modeling did not account for multi-modality testing and/or information on cardiac arrhythmias as the CAC Consortium did not collect electrocardiogram, echocardiogram, stress testing data, or potential genetic channelopathies among patients.
In conclusion, there was a stepwise increase in SCD event rates with increasing CAC burden. Among persons with low-to-intermediate 10-year ASCVD risk, CAC score categories from 100 through 1000 independently conferred up to an approximate 5-fold higher risk for incident SCD compared to CAC=0, and the addition of CAC improved incident SCD prediction when added to traditional risk factors. Overall, these results suggest that SCD risk stratification may be most useful in the very early stages of CHD through the quantification of calcified atherosclerotic plaque burden. Future studies that assess the role of CAC scoring as an initial modality to support the selective use of downstream testing, such as echocardiogram, and/or exercise treadmill testing with or without imaging or coronary CT angiography, may be useful to help further guide primary prevention strategies for SCD risk assessment.
Supplementary Material
Central Illustration. Coronary artery calcium burden and incident sudden cardiac death.
There is a stepwise increase in SCD event rates across increasing CAC burden, and SCD survival probability significantly differed as early as 2 years of follow up across CAC burden categories. When added to traditional risk factors in the overall sample, CAC significantly improved the prediction of incident SCD.
Table 3:
Multivariable-Adjusted SHRs (95% CIs) for CAC burden with SCD, stratified by 10-year ASCVD Risk.
| CAC Score Group | N | Unadjusted SHR (95% CI) |
Model 1 SHR (95% CI)A |
Model 2 HR (95% CI)B |
|---|---|---|---|---|
| ASCVD Risk <7.5% | 35 | |||
| CAC=0 | 1 | 1 | 1 | |
| CAC 1-99 | 1.2 (0.5 - 3.1) | 1.1 (0.4 - 2.9) | 0.9 (0.3 - 2.5) | |
| CAC 100-399 | 5.3 (2.2 - 12.6) | 4.1 (1.6 - 10.5) | 3.3 (1.3 - 8.4) | |
| CAC 400-999 | 7.1 (2.3 – 22.3) | 5.4 (1.6 – 18.6) | 4.1 (1.3 – 13.4) | |
| CAC >1000 | 18.5 (5.9 – 58.0) | 13.8 (4.1 – 46.9) | 9.7 (2.9 – 31.7) | |
| ASCVD Risk ≥7.5–20% | 63 | |||
| CAC=0 | 1 | 1 | 1 | |
| CAC 1-99 | 1.1 (0.4 - 3.2) | 1.1 (0.3 - 3.3) | 1.0 (0.3 - 3.2) | |
| CAC 100 - 399 | 3.1 (1.1 - 8.4) | 2.9 (1.0 - 8.5) | 2.7 (0.9 - 7.8) | |
| CAC 400-999 | 4.8 (1.7 – 13.2) | 4.5 (1.5 – 13.7) | 3.9 (1.3 – 12.1) | |
| CAC >1000 | 10.0 (3.7 – 26.9) | 9.3 (3.0 – 28.4) | 7.4 (2.4 – 23.2) | |
| ASCVD Risk ≥20% | 113 | |||
| CAC=0 | 1 | 1 | 1 | |
| CAC 1-99 | 2.0 (0.6 - 6.9) | 2.1 (0.6 - 7.2) | 2.0 (0.6 - 7.0) | |
| CAC 100-399 | 2.9 (0.9 - 9.5) | 2.7 (0.8 - 9.2) | 2.7 (0.8 - 9.0) | |
| CAC 400-999 | 4.1 (1.3 – 13.6) | 3.8 (1.1 – 12.8) | 3.6 (1.1 – 12.3) | |
| CAC >1000 | 4.8 (1.5 – 15.8) | 4.0 (1.2 – 13.3) | 3.6 (1.1 – 12.3) |
adjusted for age and sex
adjusted for age, sex, current cigarette smoking, diabetes, hypertension, hyperlipidemia, and a family history of CHD
Clinical Perspectives.
Competency in Medical Knowledge:
Coronary artery calcium (CAC) burden, particularly CAC >100, confers an increased risk for incident sudden cardiac death independent of traditional atherosclerotic cardiovascular disease (ASCVD) risk factors.
Translational Outlook:
Future studies are required to assess the use of CAC scoring via non-contrast computed tomography as an initial gatekeeper test guiding limited and selective use of additional noninvasive cardiac imaging/testing when advanced SCD risk stratification is required.
Acknowledgements
The authors thank the other investigators, the staff, and the participants of the CAC Consortium for their valuable contributions.
Sources of Funding
This project was supported in part by a research grant from the National Institutes of Health (NIH)-National Heart, Lung, and Blood Institute (NHLBI) [L30 HL110027].
Abbreviations:
- AU
Agatston units
- ASCVD
atherosclerotic cardiovascular disease
- CAC
coronary artery calcification
- CHD
coronary heart disease
- PCE
pooled cohort equations
- SCD
Sudden cardiac death
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Michael Blaha reports grants from the National Institutes of Health, US Food and Drug Administration, American Heart Association, and Aetna Foundation; grants and personal fees from Amgen; and personal fees from Sanofi, Regeneron, Novartis, Bayer, and Novo Nordisk outside the submitted work. No other disclosure for the other authors are reported. None of the other authors have any conflict of interest.
References
- 1.Zipes DP, Rubart M. Neural modulation of cardiac arrhythmias and sudden cardiac death. Hear. Rhythm 2006;3:108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kong M, Fonarow GC, Peterson ED, et al. Systematic review of the incidence of sudden cardiac death in the United States. J. Am. Coll. Cardiol 2011;57:794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Myerburg RJ. Sudden cardiac death: Exploring the limits of our knowledge. J. Cardiovasc. Electrophysiol 2001;12:369–381. [DOI] [PubMed] [Google Scholar]
- 4.Myerburg RJ, Reddy V, Castellanos A. Indications for Implantable Cardioverter-Defibrillators Based on Evidence and Judgment. J. Am. Coll. Cardiol 2009;54:747–763. [DOI] [PubMed] [Google Scholar]
- 5.Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death. Circulation 2018;138:f249–f253. [Google Scholar]
- 6.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol 1990;15:827–32. [DOI] [PubMed] [Google Scholar]
- 7.Detrano R, Guerci AD, Carr JJ, et al. Coronary Calcium as a Predictor of Coronary Events in Four Racial or Ethnic Groups. N. Engl. J. Med 2008;358:1336–1345. [DOI] [PubMed] [Google Scholar]
- 8.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease. J. Am. Coll. Cardiol 2019;74:e177–e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al Rifai M, Cainzos-Achirica M, Kanaya AM, et al. Discordance between 10-year cardiovascular risk estimates using the ACC/AHA 2013 estimator and coronary artery calcium in individuals from 5 racial/ethnic groups: Comparing MASALA and MESA. Atherosclerosis 2018;279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Razavi AC, Wong N, Budoff M, et al. Predicting Long-Term Absence of Coronary Artery Calcium in Metabolic Syndrome and Diabetes. JACC Cardiovasc. Imaging 2020;14:219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myerburg RJ, Junttila MJ. Sudden cardiac death caused by coronary heart disease. Circulation 2012;125:1043–1052. [DOI] [PubMed] [Google Scholar]
- 12.Blaha MJ, Whelton SP, Al Rifai M, et al. Rationale and design of the coronary artery calcium consortium: A multicenter cohort study. J. Cardiovasc. Comput. Tomogr 2017;11:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Narasimhan B, Patel N, Ho K, et al. Incidence and Predictors of Sudden Cardiac Arrest in Sarcoidosis: A Nationwide Analysis. JACC Clin. Electrophysiol 2021. [DOI] [PubMed] [Google Scholar]
- 14.Cheng CY, Hsu CY, Wang TC, Liu CY, Yang YH, Yang WH. Risk of Cardiac Morbidities and Sudden Death in Patients With Epilepsy and No History of Cardiac Disease: A Population-Based Nationwide Study. Mayo Clin. Proc 2021;96:964–974. [DOI] [PubMed] [Google Scholar]
- 15.Ha FJ, Han HC, Sanders P, et al. Sudden cardiac death in the young: Incidence, trends, and risk factors in a nationwide study. Circ. Cardiovasc. Qual. Outcomes 2020;13:e006470. [DOI] [PubMed] [Google Scholar]
- 16.Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American college of cardiology/American heart association task force on practice guidelines. Circulation 2014;125:S49–S73. [DOI] [PubMed] [Google Scholar]
- 17.Peng AW, Dardari ZA, Blumenthal RS, et al. Very High Coronary Artery Calcium (≥1000) and Association with Cardiovascular Disease Events, Non-Cardiovascular Disease Outcomes, and Mortality: Results from MESA. Circulation 2021;143:1571–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaduganathan M, Claggett BL, Chatteijee NA, et al. Sudden Death in Heart Failure With Preserved Ejection Fraction: A Competing Risks Analysis From the TOPCAT Trial. JACC Hear. Fail 2018;6:653–661. [DOI] [PubMed] [Google Scholar]
- 19.Wieand S, Gail MH, James BR, James KL. A family of nonparametric statistics for comparing diagnostic markers with paired or unpaired data. Biometrika 1989;76. [Google Scholar]
- 20.McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: Results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2006;113:30–37. [DOI] [PubMed] [Google Scholar]
- 21.Yared GS, Moreira HT, Ambale-Venkatesh B, et al. Coronary Artery Calcium From Early Adulthood to Middle Age and Left Ventricular Structure and Function. Circ. Cardiovasc. Imaging 2019;12:e009228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bezzina CR, Walsh R, Lahrouchi N. Predicting Risk for Adult-Onset Sudden Cardiac Death in the Population. J. Am. Coll. Cardiol 2019;74:2635–2637. [DOI] [PubMed] [Google Scholar]
- 23.Kurl S, Mäkikallio TH, Rautaharju P, Kiviniemi V, Laukkanen JA. Duration of QRS complex in resting electrocardiogram is a predictor of sudden cardiac death in men. Circulation 2012;125:2588–2594. [DOI] [PubMed] [Google Scholar]
- 24.Howell SJ, German D, Bender A, et al. Does sex modify an association of electrophysiological substrate with sudden cardiac death? The Atherosclerosis Risk in Communities (ARIC) study. Cardiovasc. Digit. Heal. J 2020;1:80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Neal WT, Singleton MJ, Roberts JD, et al. Association between QT-interval components and sudden cardiac death: The ARIC study (Atherosclerosis Risk in Communities). Circ. Arrhythmia Electrophysiol 2017;10:e005485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mortensen MB, Dzaye O, Steffensen FH, et al. Impact of Plaque Burden Versus Stenosis on Ischemic Events in Patients With Coronary Atherosclerosis. J. Am. Coll. Cardiol 2020;76:2803–2813. [DOI] [PubMed] [Google Scholar]
- 27.Poole JE, Olshansky B, Mark DB, et al. Long-Term Outcomes of Implantable Cardioverter-Defibrillator Therapy in the SCD-HeFT. J. Am. Coll. Cardiol 2020;76:405–415. [DOI] [PubMed] [Google Scholar]
- 28.Newman AB, Naydeck BL, Sutton-Tyrrell K, Feldman A, Edmundowicz D, Kuller LH. Coronary artery calcification in older adults to age 99 prevalence and risk factors. Circulation 2001;104:2679–2684. [DOI] [PubMed] [Google Scholar]
- 29.Kaltman JR, Thompson PD, Lantos J, et al. Screening for sudden cardiac death in the young Report from a national heart, lung, and blood institute working group. Circulation 2011;123:1911–1918. [DOI] [PubMed] [Google Scholar]
- 30.Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary Calcium Score and Cardiovascular Risk. J. Am. Coll. Cardiol 2018;72:434–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iwasaki K, Matsumoto T, Aono H, Furukawa H, Samukawa M. Prevalence of noncalcified coronary plaque on 64-slice computed tomography in asymptomatic patients with zero and low coronary artery calcium. Can. J. Cardiol 2010;26:377–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huikuri HV., Mäkikallio TH, Raatikainen MJP, Perkiömaki J, Castellanos A, Myerburg RJ. Prediction of sudden cardiac death: Appraisal of the studies and methods assessing the risk of sudden arrhythmic death. Circulation 2003;108:110–115. [DOI] [PubMed] [Google Scholar]
- 33.Carter-Monroe N, Virmani R. Current trends in the classification of sudden cardiac death based on autopsy derived data: A review of investigations into the etiology of sudden cardiac death. Rev. Esp. Cardiol 2011;64:10–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.