Abstract
Despite the popular use of dietary supplements during conventional cancer treatments, their impacts on the efficacies of prevalent immunotherapies, including immune checkpoint therapy (ICT), are unknown. Surprisingly, our analyses of electronic health records revealed that ICT-treated cancer patients who took vitamin E (VitE) had significantly improved survival. In mouse models, VitE increased ICT antitumor efficacy, which depended on dendritic cells (DCs). VitE entered DCs via SCARB1 receptor and restored tumor-associated DCs’ functionality by directly binding to and inhibiting protein tyrosine phosphatase SHP1, a DC-intrinsic checkpoint. SHP1 inhibition, genetically, or by VitE treatment, enhanced tumor antigen cross-presentation by DCs and DC-derived extracellular vesicles (DC-EVs) triggering systemic antigen-specific T cell antitumor immunity. Combining VitE with DC-recruiting cancer vaccines, or immunogenic chemotherapies, greatly boosted ICT efficacy in animals. Therefore, combining VitE supplement, or SHP1-inhibited DCs/DC-EVs, with DCs-enrichment therapies could substantially augment T cell antitumor immunity and enhance the efficacies of cancer immunotherapies.
INTRODUCTION
Dietary supplements are commonly used in general populations (1) and cancer patients (2). The use of dietary supplements during conventional cancer therapy has brought growing attention as an eminently practical approach (3). However, little is known regarding the effects of dietary components and nutritional supplements on current prevalent immunotherapies. The link between nutritional status and immune homeostasis (4) suggests that dietary interventions may impact antitumor immunity and the efficacy of cancer immunotherapies. Thus, identifying beneficial dietary supplements and clarifying the specific mechanism of functions in enhancing cancer immunotherapy can guide clinical application in cancer patients.
T cell-based immune checkpoint therapies (ICT), such as anti-CTLA-4, anti-PD-1, and anti-PD-L1, have demonstrated striking clinical success in reviving dysfunctional tumor-infiltrating effector T cells and providing long-lasting protection in a subset of cancer patients (5). However, ICT is still limited by low clinical response rates and substantial side effects (6). Thus, maximizing anticancer therapeutic effects and minimizing the toxicities of ICT is an unmet clinical challenge (6). A successful anticancer immune response requires a series of stepwise events (7), of which immunogenic antigen presentation in tumors is a key determinant. Dendritic cells (DCs) uptake and cross-present tumor antigens to prime and activate cytotoxic T lymphocytes for antigen-specific T cell response (8). However, tumor-infiltrating DCs often become profoundly dysfunctional or tolerogenic for inducing a potent immune response in the suppressive tumor microenvironment (TME) (8,9), limiting the efficacy of T cell-based therapies. Activating DCs or overcoming DC-suppressive signals can potentiate T cell antitumor response and enhance the therapeutic effects of ICT and other immunotherapies. Unfortunately, there is no clinically effective strategy to reinvigorate dysfunctional DCs at present. Improved understanding of regulatory mechanisms of DCs function and maneuvering DCs accordingly may enable therapeutic development in various clinical settings.
In this study, combining analysis of electronic health records (EHR) of ICT-treated cancer patients and experimental data from various mouse cancer models, we uncovered that supplementary VitE increased ICT response. VitE is an abundantly used dietary supplement among adults (1,10) including cancer patients (11). As antioxidants, VitE supplements have been implicated in strengthening immunity with anti-inflammatory, anti-atherosclerotic, and antitumor effects in animal models (12). However, the benefits of VitE supplements on cancer patients are unclear since its clinical studies showed mixed efficacies (13,14). Importantly, the mechanisms underlying the disease-modifying activities of VitE remain obscure. Here, we identified that VitE boosted antitumor immunity by mainly enhancing the tumor antigen presentation of DCs and DC-EVs via inhibiting the Src homologous-region-2-domain-containing phosphatase-1 (SHP1), a DC intrinsic checkpoint. Compellingly, unleashing DCs and DC-EVs by VitE is an immediately applicable and advantageous strategy for inducing efficient antitumor immunity and enhancing immunotherapy response.
RESULT
Dietary VitE enhances ICT efficacy in humans and mice
To assess the impact of various dietary supplements on immunotherapy responses, we retrospectively analyzed EHR for the clinical outcomes of cancer patients receiving immunotherapies (anti-PD-1/PD-L1) at The University of Texas MD Anderson Cancer Center following a rational patient selection process (Supplementary Fig. S1). We found that melanoma patients who took VitE during anti-PD-1/PD-L1 treatments had a reduced death rate compared to patients taking any of the 14 other common dietary supplements (Supplementary Fig. S2A). Strikingly, melanoma patients who took VitE while receiving ICT showed a significantly (HR, 0.7; 95% CI, 0.53–0.92; P < 0.05) improved overall survival (OS) compared with those who didn’t take vitamins (Fig. 1A; Supplementary Table S1) or took multivitamins (Supplementary Fig. S2B) from univariate survival analyses. Also, patients who took VitE showed a trend of better responses to ICT treatment than those who didn’t take vitamins (Supplementary Fig. S2C). Multivariate analyses confirmed that VitE intake was significantly associated with OS independent of age, gender, and race (Supplementary Table S2). Likewise, significantly increased OS was found in a mixed cohort of ICT-treated cancer patients (breast, colon, and kidney cancers) who took VitE (HR, 0.74; 95% CI, 0.57–0.95; P < 0.05) (Fig. 1B; Supplementary Table S3–S6). Furthermore, in another independent mixed cohort of cancer patients with breast, lung, kidney, and bladder cancers from a different hospital, taking VitE while receiving ICT treatment was also associated with a reduced death rate compared to patients without taking VitE (Supplementary Table S7). In contrast, VitE had minimal or no effect on reducing the death rate of chemotherapy-treated (Taxol or Doxorubicin) cancer patients (Supplementary Fig. S2D). These clinical data suggest that VitE supplementation improved the clinical outcomes in cancer patients with ICT treatment.
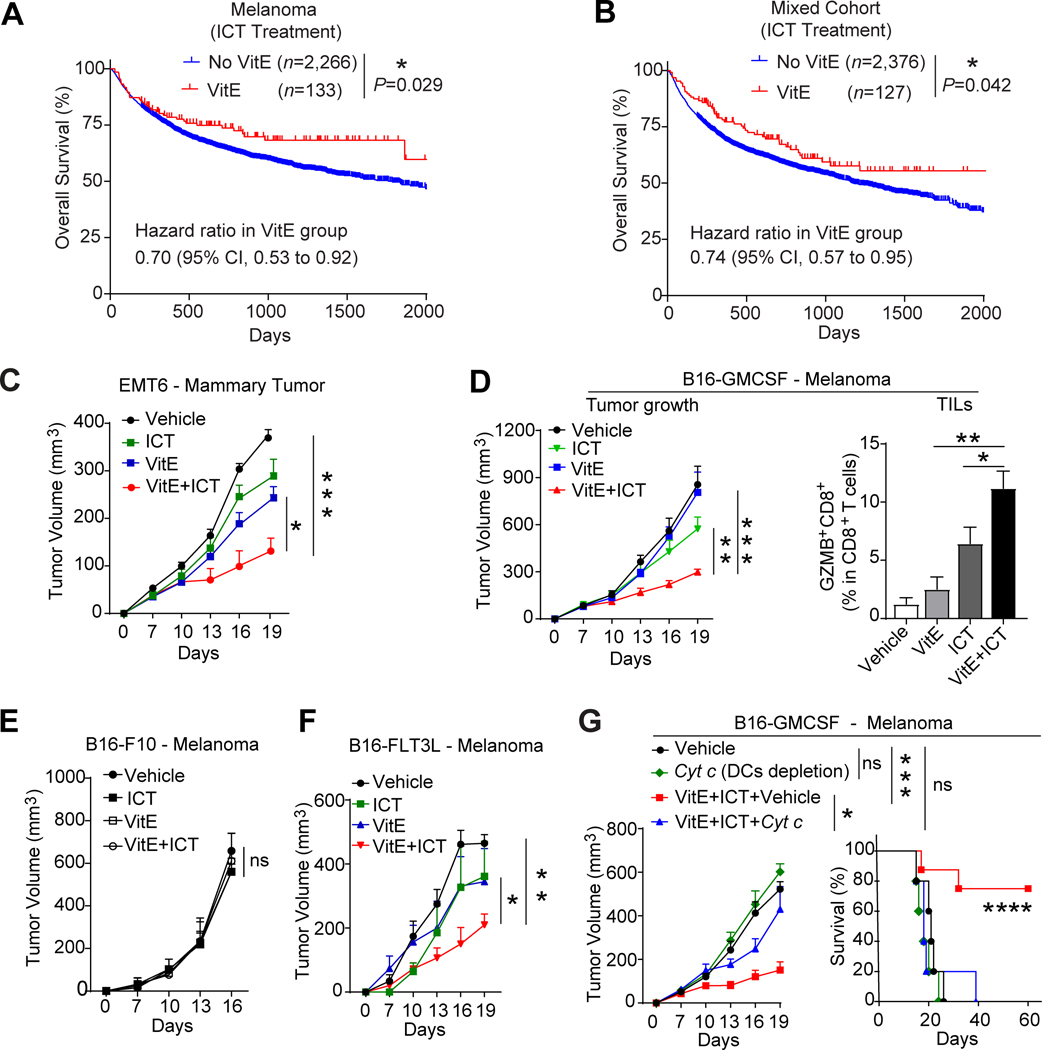
Figure 1. Dietary VitE augments ICT efficacy dependent on DCs.
A, Overall survival of melanoma patients who took dietary vitamin E supplement (VitE, n=133) during ICT treatment (Anti-PD-1/PDL1) compared with patients who didn’t take VitE and/or multivitamins (No VitE, n=2,266).
B, Overall survival of a mixed cohort of cancer patients (599 breast cancer, 914 colon cancer, and 990 kidney cancer) who took dietary vitamin E supplement (VitE, n=127) during ICT treatment compared with patients who didn’t take VitE and/or multivitamins (No VitE, n=2, 376).
C, Growth curve of EMT6 mammary tumors in mammary fat pads of BALB/c mice treated with vehicle, VitE (50mg/kg, oral gavage), ICT (anti-PD1, 200 mg/mouse, by i.p.) or VitE+ICT.
D-F, Growth curves of orthotopic B16-GMCSF (D, left), B16-F10 (E), B16-FLT3L (F) melanomas in C57BL/6 mice treated with vehicle, VitE (50mg/kg, oral gavage), ICT (anti-PD1, 200μg/mouse and anti-CTLA4, 100μg/mouse by i.p.), or VitE+ICT. Flow cytometry quantification of GranzymeB+CD8+ tumor-infiltrating lymphocytes (TILs) in B16-GMCSF tumor tissues collected 20 days post-implantation (D, right). (n=5/group).
G, Growth curves (left) and Kaplan–Meier survival curves (right) of C57BL/6 mice bearing B16-GMCSF melanomas treated with vehicle (n=4), Cyt c that depletes DCs (n=5), ICT (anti-PD1/anti-CTLA4) +VitE (n=8), or ICT+VitE+Cyt c (n=10).
Mean ± s.e.m (C, D, E, F, G). One-way analysis of variance (ANOVA) and Tukey’s test for multiple comparisons (C, D, E, F, G tumor growth), log-rank (Mantel-Cox) test survival comparison (A, B, G). The statistical significance is defined by P-value: ns, no significant. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
These clinical findings prompted us to test whether dietary VitE may potentiate ICT efficacy in animal models. Consistent with the clinical data, VitE and ICT combo-treatment achieved significantly improved ICT-response in mice bearing the EMT6 mammary tumors (Fig. 1C; Supplementary Fig. S2E). Similarly, VitE and ICT combo-treatment were significantly more effective in inhibiting the B16-GMCSF melanoma growth, reducing lung metastasis, increasing tumor infiltration of granzyme B (GZMB)+ and IFN-γ+ CD8+ cytolytic T cells compared to ICT alone (Fig. 1D; Supplementary Fig. S2F–S2J). Together, VitE supplementation correlated with better ICT response in cancer patients and enhanced ICT response in mouse tumor models.
VitE-boosted ICT response depends on DCs
When testing in mice bearing the B16-F10 melanoma, we were perplexed that VitE and ICT combo-treatments showed no therapeutic effect (Fig. 1E), unlike in the B16-GMCSF, a B16-F10 subline overexpressing the granulocyte-macrophage colony-stimulating factor (GM-CSF) that is positively associated with DCs infiltration in tumors (15) (Supplementary Fig. S3A–S3C). Likewise, growth factor FMS-like tyrosine kinase 3 ligand (FLT3L) promotes hematopoietic progenitor commitment to the DC lineage, thus increasing DC survival and proliferation (16). In mice bearing the B16-F10 subline overexpressing FLT3L (B16-FLT3L), VitE effectively enhanced their ICT responses (Fig. 1F). The futility in DCs-desert tumors (B16-F10) versus the strong activity in DCs-enriched tumors (B16-GMCSF and B16-FLT3L) (Supplementary Fig. S3A and S3B) suggested that the enhanced ICT-response by VitE depends on DCs infiltration. Indeed, depletion of DCs by cytochrome c (Cyt c) (17) (Supplementary Fig. S3D and S3E) abolished the VitE-augmented ICT-response in the B16-GMCSF model (Fig. 1G), but had no effect in the B16-F10 model (Supplementary Fig. S3F). Furthermore, DC-depletion by Cyt c also abolished the inhibition of EMT6 tumors by VitE (Supplementary Fig. S3G and S3H), confirming the DCs-dependency of VitE-mediated antitumor effect across several immunogenic tumor models.
VitE augments DC activation in vitro and in vivo
To directly assess VitE’s effect on DCs, we generated human monocyte-derived DCs (MoDCs) from healthy donors and treated MoDCs with VitE, other nutritional supplements, or vehicles. Compared to other common nutritional supplements, VitE most effectively activated DCs (Fig. 2A; Supplementary Fig. S4A), as indicated by the significantly increased expression of DC activation markers CD40, CD86, and HLA-DR (Fig. 2B). Notably, only natural VitE (DL-α-tocopherol) further enhanced DC activation upon Lipopolysaccharides (LPS) stimulation, but not synthesized VitE acetate (VEA) nor antioxidant N-acetylcysteine (NAC) (Supplementary Fig. S4B), suggesting that general antioxidant function probably does not contribute to VitE-induced MoDCs activation. VitE was also the most effective supplement that activated mouse bone marrow-derived DCs (BMDCs) (Supplementary Fig. S4C). Functionally, VitE-treated human MoDCs increased autologous IFN-γ+CD8+ cytotoxic T cells (Fig. 2C) and VitE-treated mouse BMDCs promoted the autologous T cells’ proliferation in co-cultures (Supplementary Fig. S4D). Furthermore, VitE-treated and ovalbumin (OVA)-loaded BMDCs increased the OVA-derived SIINFEKL antigen-specific OT-1 T cells in co-culture (Fig. 2D).
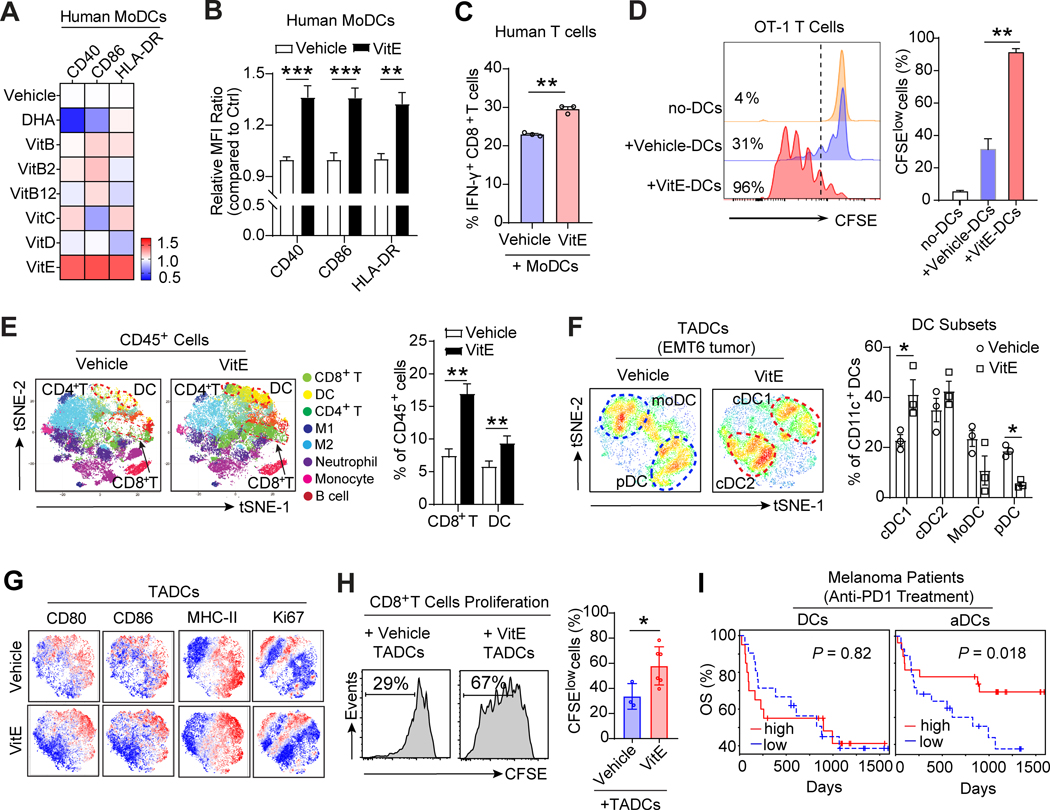
Figure 2. VitE enhances DCs activation in vitro and reinvigorates DCs function in vivo.
A, Heat-map depicting relative expressions of co-stimulatory molecules in human MoDCs treated with indicated dietary supplements, including VitE (50μg/ml), VitC (20μg/ml), VitD (100ng/ml), VitB (folate, 200ng/ml), VitB2 (2μg/ml), VitB12 (10ng/ml), and DHA (Omega-3, 100μM) for 48h, and then stimulated with LPS (20ng/ml) for 24h. The mean fluorescence intensity (MFI) of each molecule was normalized to the MFI of the vehicle-treated group measured by flow cytometry.
B, Quantification of the indicated DC activation marker expressions in human MoDCs treated with vehicle or VitE (50μg/ml) for 48h in the presence of LPS (20ng/ml), and MFI were calculated relative to vehicle treatment (defined as 1).
C, Percentage of human IFN-γ+CD8+ T cells out of total CD8+ T cells in coculture with the vehicle- or VitE-treated autologous MoDCs (n=3).
D, Representative flow cytometric analysis and quantification of antigen-specific OT-1 T cell proliferation cocultured with the vehicle- or VitE-treated and OVA-loaded mouse BMDCs at day3.
E, t-Distributed stochastic neighbor embedding (t-SNE) plot of tumor-infiltrating CD45+ immune cells overlaid with color-coded clusters from the vehicle- or VitE-treated EMT6 tumors. Dotted ellipses highlight clusters with significant differences between the two groups at day 15 post-implantation (n=6). The quantification of CD8+ T cells and DCs are shown (left panel, n=6).
F, The overlaid density plots of CD11c+ tumor-associated dendritic cells (TADCs) from vehicle- or VitE-treated EMT6 tumors in BALB/c mice. The quantification of distinct DCs subsets was shown (left panel, n=3). The distinct DCs subsets are labeled: cDC1, conventional type 1 DC; cDC2, conventional type 2 DC; pDC, plasmacytoid DC; MoDC, monocyte-derived DC.
G, t-SNE plots of TADCs overlaid with the expressions of indicated markers from the vehicle- versus VitE-treated EMT6 mammary tumors.
H, Representative histograms of CD8+ T cell proliferation. CD8+ T cells were cocultured with TADCs at 10:1 ratio (left panel) and quantified using CFSE dilution (right panel). TADCs were purified from EMT6 tumor-bearing mice at day 10 post-treatment with vehicle or VitE.
I, Overall survival of melanoma patients who had tumor-infiltrating total DCs high (n=20) vs. low (n=21) or activated DCs (aDCs) high (n=20) vs. low (n=21) before anti-PD-1 treatment. High and low were defined as higher or lower, respectively, than the median for the cohort (PRJEB23709).
Mean ± s.e.m (B, C, D, E, F, H, I). Two-sided Student’s t-test (B, C, E, F, H). One-way analysis of variance (ANOVA) and Tukey’s test for multiple comparisons (D), log-rank (Mantel-Cox) test survival comparison (I). The statistical significance is defined by P-value: *P < 0.05, **P < 0.01, ***P < 0.001.
To decipher VitE’s effect on DCs and antitumor immunity in vivo, we used the EMT6 orthotopic mouse model. VitE treatment reduced tumor growth compared with the vehicle-treated group in vivo (Supplementary Fig. S4E and S4F) without a direct effect on tumor cell proliferation in culture (Supplementary Fig. S4G). Cytometry by time-of-flight (CyTOF) analysis of CD45+ immune cells isolated from EMT6 tumors showed that VitE treatment increased the tumor infiltrations of CD11c+ DCs and CD8+ T cells (Fig. 2E). Further analyses of CD11c+ DCs from EMT6 tumors showed that VitE treatment induced a striking component shift of tumor-associated DCs (TADCs) from naïve monocyte-derived DCs (MoDCs) (CD14+CD206+) and plasmacytoid DCs (pDCs, CD11clowB220+) to conventional DCs (CD103+ cDC1 and CD11b+ cDC2) (Fig. 2F; Supplementary Fig. S4H–S4J), which are critical for tumor-reactive T cell responses (8), indicating that VitE treatment instigated a functional differentiation of the DC compartments and induced polarization to cross-presenting DCs. Consistently, the majority of VitE induced-DCs exhibited the characteristics of activated DCs (CD80+CD86+MHC-II+Ki67+) (Fig. 2G). Furthermore, expression of genes associated with DCs activation and effector function (Cd40, Ccr7, Ifnb1) were increased in TADCs isolated from VitE-treated EMT6 tumors versus controls, along with increased cytotoxic IFN-γ+CD8+ T cell infiltration (Supplementary Fig. S4K and S4L). Significantly, TADCs of VitE-treated tumors promoted T cell proliferation more than TADCs from vehicle-treated tumors (Fig. 2H). Therefore, VitE-induced enhancement of DCs function could adequately trigger antitumor T cell immunity in vivo.
DC activation is associated with patients’ ICT response
To examine the association between DCs activation and antitumor T cell immunity in patients, we performed a bioinformatics analysis of human breast cancer data in The Cancer Genome Atlas (TCGA). We discovered that activated DCs (aDCs), but not total DCs or other immune cells in the TME, were most tightly correlated with CD8+ T cell infiltration and intra-tumoral immune cytolytic activity (CYT) (Supplementary Fig. S5A and S5B). Higher aDCs, but not total DCs, also significantly correlated with longer patient survival in multiple cancer types (Supplementary Fig. S5C). Moreover, pembrolizumab (anti-PD1)-treated melanoma patients with higher tumor-infiltrating aDCs had markedly increased overall survival compared with patients with lower aDCs infiltration (PRJEB23709) (18) (Fig. 2I). These findings were corroborated in separate cohorts of melanoma (19) and bladder cancer (20) patients treated with anti-CTLA4 or anti-PD-L1 antibodies, respectively (Supplementary Fig. S5D and S5E). Together, the effect of VitE on DCs activation and the profound association of aDCs with patients’ ICT responses indicate that VitE-enhanced DCs activation likely contributes to augmented ICT efficacy.
VitE enhances DC activation by inhibiting the SHP1 checkpoint of DC
In investigating how VitE activates DCs, our reverse-phase protein array (RPPA) analyses on VitE-treated DCs without or with tumor-conditioned medium (TCM) revealed that VitE induced major changes in DCs: 1) increased phosphorylation of several kinases, e.g., p-P38(T180/Y182), p-ERK(T202/Y204), and p-c-JUN(S73); and 2) decreased phosphorylation at tyrosine 564 (Y564) of SHP1 (Fig. 3A; Supplementary Fig. S6A). SHP1 is a cellular tyrosine phosphatase encoded by the Ptpn6 gene and can reduce phosphorylation of Syk and its downstream MAPKs, e.g., p-ERK1/2 and p-c-JUN (21,22). SHP1 is a DC-intrinsic checkpoint (22) that regulates multiple Toll-like receptors (TLRs) and cytokine signaling responses (23). The Y564 phosphorylation on SHP1 is critical for its phosphatase activity (24). We validated the decrease of Y564-phosphorylated SHP1 (pY564-SHP1) in VitE-treated mouse BMDCs (without or with TCM) (Supplementary Fig. S6B and S6C) and human MoDCs (Supplementary Fig. S6D). The pY564-SHP1 levels were markedly higher in TADCs than in splenic DCs of EMT6 tumor-bearing mice (Fig. 3B), not in intra-tumoral myeloid cells and T cells (Supplementary Fig. S6E). There was no significant change in the pY542-SHP2 level between TADCs and splenic DCs (Supplementary Fig. S6E). Conversely, VitE treatment reduced the pY564-SHP1 level in TADCs (Fig. 3C) and increased MAPK pathway activation, correspondingly (Supplementary Fig. S6F). SHP1 expression was undetectable in tested tumor cells (Supplementary Fig. S6G). Silencing SHP1 expression in human MoDCs by PTPN6-siRNA (siPTPN6) led to increased DCs activation compared to control-siRNA (siCtrl)-transfected MoDCs (Fig. 3D). Mouse siPtpn6-BMDCs induced co-cultured OT-1 T cell proliferation significantly more than siCtrl-BMDCs (Fig. 3E), similar to VitE’s effect on DC-primed T cell proliferation (Fig. 2D). Indeed, VitE-treated siCtrl-BMDCs significantly induced co-cultured OT-1 cell proliferation compared to vehicle treatment, while siPtpn6-BMDCs didn’t further respond to VitE treatment (Fig. 3F), indicating that VitE treatment enhanced DCs function by inhibiting SHP1.
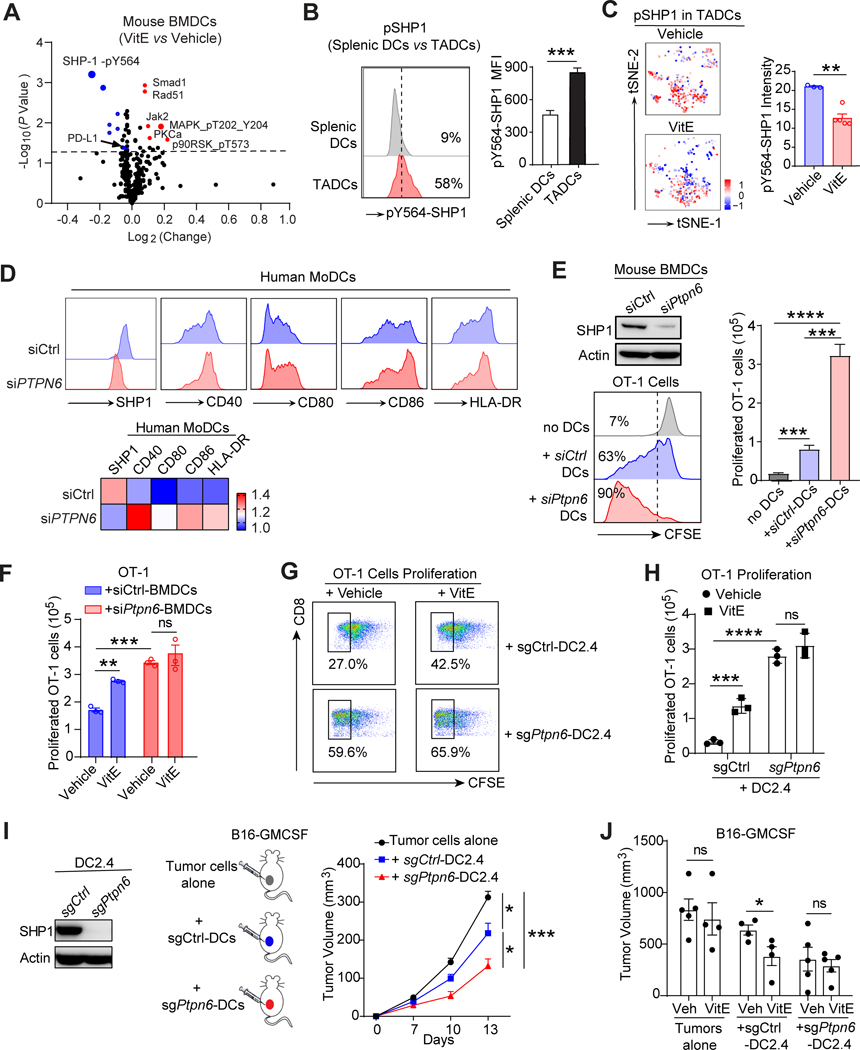
Figure 3. VitE restores TADCs function by inhibiting SHP1 and boosts DCs-induced antitumor immunity.
A, Volcano plot of reverse phase protein array (RPPA) data of vehicle- or VitE (50μg/ml)-treated mouse BMDCs growing in tumor conditioned media (TCM) for 48 hours. Differentially expressed proteins are labeled, and the top hits are marked in red (up-regulated proteins) or blue (down-regulated proteins). The grey dashed line indicates P-value of 0.05.
B, Flow cytometric analysis of the intensity of pY564-SHP1 in spleen DCs and TADCs from EMT6 mammary tumor-bearing mice (n=6).
C, t-SNEs by CyTOF and quantification comparing the intensity of pY564-SHP1 in TADCs from the vehicle- or VitE-treated EMT6 tumors at day 15 post-implantation.
D, Flow cytometry analysis (top) and heat-map (bottom) depicting relative expressions of SHP1 and co-stimulatory molecules on siCtrl or siPTPN6 transfected human MoDCs.
E, The effective SHP1 knockdown in BMDCs by siPtpn6 versus siCtrl (left, top). CFSE-dilution analysis (left, bottom) and quantification (right panel) of OT-1 T cells co-cultured with OVA-pulsed BMDCs (ratio 10:1) transfected with siPtpn6 versus with siCtrl as a control (n=3).
F, Quantified proliferation of OT-1 T cell cocultured with OVA-loaded vehicle- or VitE-treated siCtrl- or siPtpn6-mouse BMDCs (n = 3).
G, Representative flow cytometric CFSE-dilution analysis of OT-1 T cells co-cultured with OVA-pulsed sgCtrl-DC2.4 or sgPtpn6-DC2.4 cells treated with vehicle or VitE (ratio 10:1) (n=3).
H, Quantification (absolute numbers) of OT-1 T cells co-cultured with OVA-pulsed sgCtrl-DC2.4 or sgPtpn6-DC2.4 cells treated with vehicle or VitE (ratio 10:1) (n=3) as in G.
I, Growth curves of B16-GMCSF melanoma cells alone (n=6), versus co-implanted with sgCtrl-DC2.4 cells (n=10) or sgPtpn6-DC2.4 cells (n=10) in C57BL/6 mice.
J, The quantification of tumor size (Day 20) in C57BL/6 mice transplanted with B16-GMCSF tumor cells alone, or co-implanted with sgCtrl- or sgPtpn6-DC2.4 cells and under the vehicle- or VitE- (50mg/kg, oral gavage, daily) treatment (n=4–5).
Mean ± s.e.m (B, C, E, F, H, I, J). Two-sided Student’s t-test (B, C). One-way analysis of variance (ANOVA) and Tukey’s test for multiple comparisons (E, F, H, I, J). The statistical significance is defined by P-value: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Deleting the SHP1-encoding Ptpn6 gene by CRISPR-Cas9 in the murine DC cell line DC2.4 (sgPtpn6-DC2.4) also activated DC2.4 cells (Supplementary Fig. S7A and S7B) and increased phosphorylation of ERK and p38 MAPK following LPS stimulation compared to the control sgCtrl-DC2.4 cells (Supplementary Fig. S7C). Functionally, OVA antigen-loaded sgPtpn6-DC2.4 cells increased antigen-specific OT-1 T cell proliferation (without or with TCM) compared to sgCtrl-DC2.4 cells (Fig. 3G–H, Supplementary Fig. S7D). Analogous to VitE’s effect on BMDCs (Fig. 3F), VitE-treated sgCtrl-DC2.4 cells, but not sgPtpn6-DC2.4 cells, significantly induced co-cultured OT-1 T cell proliferation compared to vehicle-treated DCs (Fig. 3G and H), revalidating SHP1 inhibition was critical for VitE-mediated DC2.4 cell functional activation.
When the sgCtrl-DC2.4 or sgPtpn6-DC2.4 cells were subcutaneously co-transplanted with B16-GMCSF cells into mice, the sgCtrl-DC2.4 showed mild antitumor effects, whereas the sgPtpn6-DC2.4 highly significantly inhibited tumor growth (Fig. 3I; Supplementary Fig. S7E). Furthermore, B16-GMCSF cells were co-implanted with sgCtrl-DC2.4 into the right flank and with sgPtpn6-DC2.4 cells into the left flank of each mouse. Strikingly, tumors at both flanks regressed similarly (Supplementary Fig. S7F), indicating a systemic antitumor immune response triggered by Ptpn6-deleted DCs. Notably, VitE treatment further reduced the growth of sgCtrl-DC2.4 co-implanted B16-GMCSF tumors in mice but didn’t further inhibit sgPtpn6-DC2.4 co-implanted B16-GMCSF tumors (Fig. 3J; Supplementary Fig. S7G), indicating that VitE activated DCs by blocking SHP1 to enhance antitumor capacity.
VitE directly binds to and inhibits SHP1
Next, we examined whether VitE may interact with SHP1 in DCs by computational structure analyses. Indeed, molecular docking analysis showed that VitE preferentially docked in the central cavity, at an interface formed by SHP1’s N-terminal SH2 (N-SH2) and C-terminal SH2 (C-SH2) domains with the protein tyrosine phosphatase (PTP) domain (Fig. 4A). This region is critical for stabilizing SHP1 auto-inhibitory conformation through an N-SH2 domain steric blockade of the phosphatase activation site (25), and docking of VitE in this region indicates that VitE functions as an allosteric inhibitor of SHP1. Specifically, three types of molecular forces are implied in the interaction between folded VitE and all three domains of inactive SHP1: 1) hydrogen bonds with His112 (C-SH2) and Ser250 (PTP); 2) hydrophobic interactions with the sidechains of His112 (C-SH2), Tyr214 (PTP) and Glu247 (PTP); and 3) SHP1 intramolecular hydrogen bonds: etheric oxygen from the VitE chroman moiety receives a hydrogen bond from Ser 250 (PTP), which is engaged with Ser107 (N-SH2) (Fig. 4B).
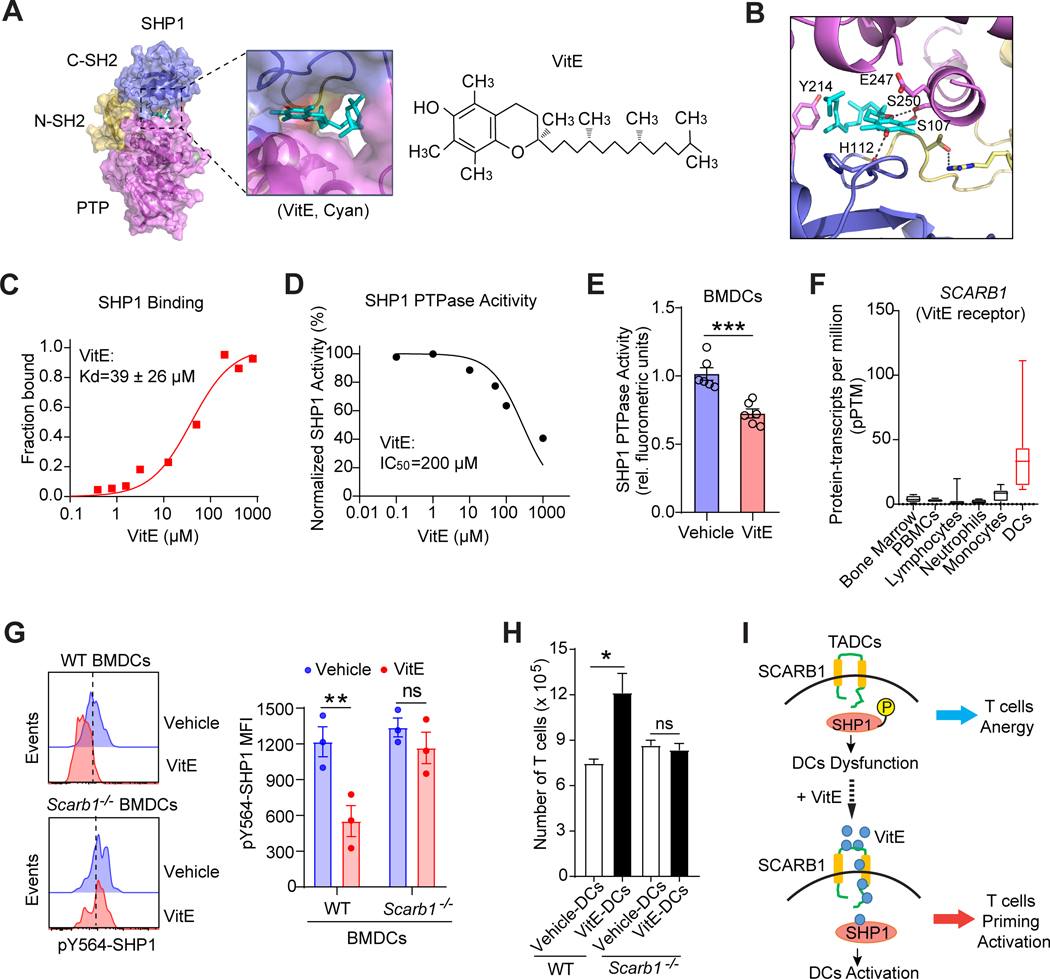
Figure 4. VitE directly binds and inhibits SHP1 activity after entering DCs via SCARB1.
A, Docking model of VitE (α-tocopherol) and auto-inhibitory SHP1 (PDB ID: 2B3O). Surface presentation of SHP1 in complex with VitE (cyan) bound in its central cavity, which is formed at the interface of all three domains of SHP1 (N-SH2, brown; C-SH2, blue; PTP, magenta).
B, Interactions of VitE with all three domains of SHP1 stabilized in its inhibitory conformation. Molecular forces implied in the interactions are hydrogen bonds with His112 (C-SH2) and Ser250 (PTP), hydrophobic interactions with His112 (C-SH2), Tyr214 (PTP) and Glu247 (PTP), and intramolecular hydrogen bonds between Ser 250 and Ser107.
C, The binding of VitE to purified SHP1 was analyzed by microscale thermophoresis (MST) binding assay. The apparent dissociation constant Kd is 39 ± 26 μM (mean of triplicates).
D, Relative tyrosine phosphatase activity of SHP1 immunoprecipitated from LPS (100ng/ml)-activated DC2.4 cells in the presence of indicated doses of VitE (mean of triplicates).
E, SHP1 tyrosine phosphatase activity in LPS (100ng/ml)-activated BMDCs (n=6) treated with vehicle or VitE (50μg/ml).
F, Normalized consensus protein-transcripts per million (pPTM) of SCARB1 in human immune cells from the Human Protein Atlas (HPA).
G, FACS analyses of pSHP1 expression in the vehicle- or VitE-treated BMDCs from wild type (WT, Scarb1+/+) or Scarb1 knockout (Scarb1−/−) C57BL/6 mice (n=3).
H, Quantification of T cell proliferation under coculture with WT or Scarb1−/− DCs (n=5).
I, Model of VitE restoring TADCs function via SCARB1-SHP1 axis, leading to enhanced antitumor T cell immunity.
Error bars, s.e.m. Two-sided Student’s t-test (E, G, H). The statistical significance is defined by P-value: ns, no significant. *P < 0.05, **P < 0.01, ***P < 0.001.
Biochemically, our in vitro binding assay demonstrated a direct interaction between purified SHP1 protein and VitE with a dissociation constant of 39 μM (Fig. 4C). Furthermore, we tested the inhibitory function of VitE on the tyrosine phosphatase activities of SHP1. Clearly, adding different doses of VitE to SHP1 that were immunoprecipitated from LPS-activated DC2.4 cells inhibited the SHP1 phosphatase function (Fig. 4D). Moreover, SHP1 tyrosine phosphatase activities in LPS-activated BMDCs were significantly inhibited by VitE treatment (Fig. 4E). Cumulatively, VitE directly binds SHP1 and inhibits SHP1 phosphatase activities.
VitE enters DCs through SCARB1 to inhibit SHP1
VitE can enter enterocytes via the SCARB1 receptor (26). SCARB1 facilitates the uptake of cholesteryl esters from high-density lipoproteins (27). Notably, DCs have the highest SCARB1 expressions among all major human immune cell populations (Fig. 4F; Supplementary Fig. S8A). Similarly, mouse DCs, particularly intra-tumoral conventional DCs (CD103+ cDC1 and CD11b+ cDC2) which are critical for priming antigen-specific antitumor T cell immune response, have the highest SCARB1 expressions (Supplementary Fig. S8B and S8C). To determine whether SCARB1 is required for VitE-induced DC activation, we generated BMDCs from Scarb1−/− versus wild-type mice. VitE treatment failed to inhibit pY564-SHP1 in Scarb1−/− DCs unlike in wild-type DCs (Fig. 4G; Supplementary Fig. S8D and S8E). Also, VitE didn’t enhance the DC activation in Scarb1−/− DCs upon TCM stimulation, distinct from VitE-induced activation of wild-type DCs (Supplementary Fig. S8F). Additionally, VitE-treated Scarb1−/− DCs did not induce proliferation of co-cultured T cells, contrasting VitE-treated wild-type DCs (Fig. 4H). Likewise, pretreatment of DCs with SCARB1-neutralizing antibody (anti-CD36L1) eliminated VitE-induced DCs activation (Supplementary Fig. S8G). Taken together, VitE enters DCs via SCARB1 and binds SHP1 to inhibit pSHP1 leading to DCs activation, which increases antitumor T cell immunity (Fig. 4I).
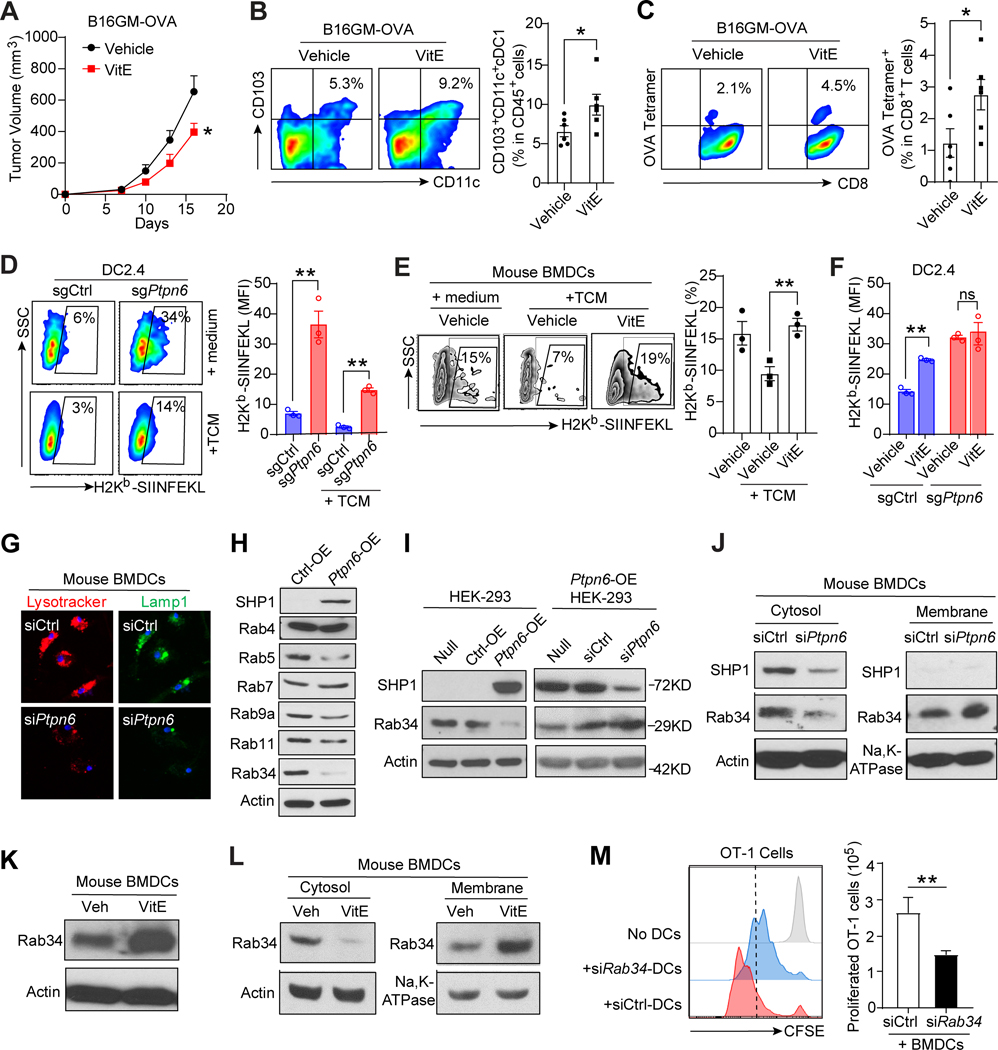
Silencing SHP1 increases antigen cross-presentation of DCs
Since VitE treatment of OVA-antigen-loaded DCs significantly increased co-cultured antigen-specific OT-1 T cell proliferation compared to vehicle treatment in vitro (Fig. 3F–H), we further assessed the effect of VitE on antigen-specific T cell response in vivo by using B16-GMCSF-OVA mouse model, in which immunogenic protein OVA was overexpressed in B16-GMCSF cells to investigate OVA antigen-specific immune responses. VitE treatment inhibited the growth of B16-GMCSF-OVA melanoma (Fig. 5A), decreased the pSHP1 expression in TADCs (Supplementary Fig. S9A), and increased tumor infiltration of cross-presenting CD103+ DCs (Fig. 5B) and OVA antigen-specific CD8+ T cells (Fig. 5C) compared to vehicle treatment. Given the strong antitumor immunity of DCs triggered by inhibiting SHP1 with VitE and by blocking SHP1 genetically (Fig. 3I and J), we reasoned that it could be caused by an increased ability of DCs cross-presentation of antigens, leading to better cross-priming of CD8+ T cells as antigen cross-presentation is the most critical function of DCs (28). To investigate whether VitE treatment or SHP1-knockout directly impact DCs’ cross-presentation, we measured the membrane expression level of OVA-derived peptide (SIINFEKL) bound to H-2Kb of MHC class I on OVA-loaded DCs after SHP1-knockout or VitE treatment. H-2Kb-SIINFEKL molecules were significantly increased on membranes of sgPtpn6-DC2.4 cells compared with sgCtrl-DC2.4 cells (Fig. 5D). VitE treatment of BMDCs significantly restored their H-2Kb-SIINFEKL level that was reduced by TCM (Fig. 5E; Supplementary Fig. S9B and S9C). Of Note, both VitE treatment and SHP1 knockout significantly increased H-2Kb-SIINFEKL molecules on DC2.4 cells compared with vehicle-treated sgCtrl-DC2.4 cells, but VitE treatment didn’t further increase H-2Kb-SIINFEKL molecules on sgPtpn6-DC2.4 cells (Fig. 5F). Thus, SHP1 inhibition in DCs by VitE treatment or Ptpn6-knockout enhances antigen cross-presentation that boosts CD8+ T cell antitumor immunity.
Figure 5. SHP1 inhibition enhances the antigen cross-presentation of DCs.
A, Growth curves of orthotopic B16-GMCSF-OVA (B16GM-OVA) melanomas in C57BL/6 mice treated with vehicle or 50mg/kg of VitE (n=5).
B, Flow cytometry analysis and quantification of CD103+CD11c+ cross-presenting DCs in B16GM-OVA tumor tissues collected at 16 days post-implantation from vehicle- or VitE-treated C57BL/6 mice (n=5).
C, Flow cytometry analysis and quantification of OVA antigen-specific OVA-tetramer+CD8+ T (SIINFEKL+CD8+) cells in tumor tissues as in B above (n=5).
D, Flow cytometric analysis and quantification of H-2Kb-SIINFEKL MFI in OVA-loaded sgCtrl or sgPtpn6-DC2.4 cells cultured in RPMI1640+10%FBS without (medium) or with TCM (n=3).
E, FCM analysis and quantification of H-2Kb-SIINFEKL (%) in BMDCs cultured in medium without or with TCM and treated with the vehicle- or VitE (n=3).
F, Quantification of H-2Kb-SIINFEKL MFI in the vehicle- or VitE-treated sgCtrl-DC2.4 or sgPtpn6-DC2.4 (n=3).
G, Confocal microscopy projections of staining of LysoTracker (red) and LAMP-1 (green) of scrambled siRNA (control) or Ptpn6 siRNA transfected BMDCs.
H, Immunoblot of indicated Rab GTPase proteins in Ptpn6-transfected versus control HEK-293 cells.
I, Immunoblot of Rab34 protein in HEK-293 cells with Ptpn6-overexpression (OE) or Ptpn6-OE-HEK-293 cells with Ptpn6 knockdown (KD).
J, Cytosol and membrane Rab34 levels in siCtrl- and siPtpn6-BMDCs.
K, Immunoblot of Rab34 proteins in BMDCs treated with vehicle (Veh) or VitE for 48h.
L, Cytosol and membrane Rab34 levels in BMDCs pre-treated with vehicle (Veh) or VitE for 48h.
M, CFSE-dilution analysis of OT-1 T cells co-cultured with OVA-pulsed BMDCs with knockdown of Rab34 by siRab34 versus control BMDCs transfected with siCtrl (n=3).
Error bars, s.e.m. Two-sided Student’s t-test (B, C, M). One-way ANOVA with Tukey’s multiple comparison post-test (D, E, F). The statistical significance is defined by P-value: ns, no significant. *P < 0.05, **P < 0.01.
When exploring the potential mechanism of enhanced cross-presentation in SHP1-inhibited DCs, we observed a remarkable reduction of phagolysosome fusion after silencing SHP1 in BMDCs, as indicated by reduced staining of lysotracker and Lamp1 (Fig. 5G). Reduced phagolysosome fusion indicates a less degradative phagosome which has been tightly linked with an enhanced cross-presentation efficiency (29). Multiple Rab GTPase proteins are reported to regulate phagolysosome trafficking and fusion (30). To examine the SHP1 downstream molecular pathways that are involved in regulating phagolysosome fusion and subsequent cross-presentation, we performed a Rab GTPase library mini-screen and identified that overexpression of SHP1 most dramatically decreased Rab34 expression (Fig. 5H), whereas knockdown of SHP1 increased Rab34 expression (Fig. 5I). Furthermore, knockdown SHP1 in mouse BMDCs reduced cytosolic Rab34 but increased membrane-accumulation of Rab34 (Fig. 5J), which was reported to decrease phagolysosome fusion resulting in increased cross-presentation efficiency of DCs (29,31). Consistently, VitE-treated BMDCs also had increased total Rab34 expression (Fig. 5K), as well as increased membrane Rab34 and decreased cytosolic Rab34 proteins (Fig. 5L). Moreover, compared with control BMDCs, silencing Rab34 in BMDCs (siRab34-DCs) reduced the proliferation of antigen-specific OT-1 T cells in co-culture (Fig. 5M), signifying the decreased cross-presentation efficiencies of siRab34-DCs. These data indicate that inhibiting SHP1 by VitE or genetic knockdown increases Rab34 membrane-accumulation and suppresses phagolysosome fusion, thereby promoting antigen cross-presentation and T cell activation.
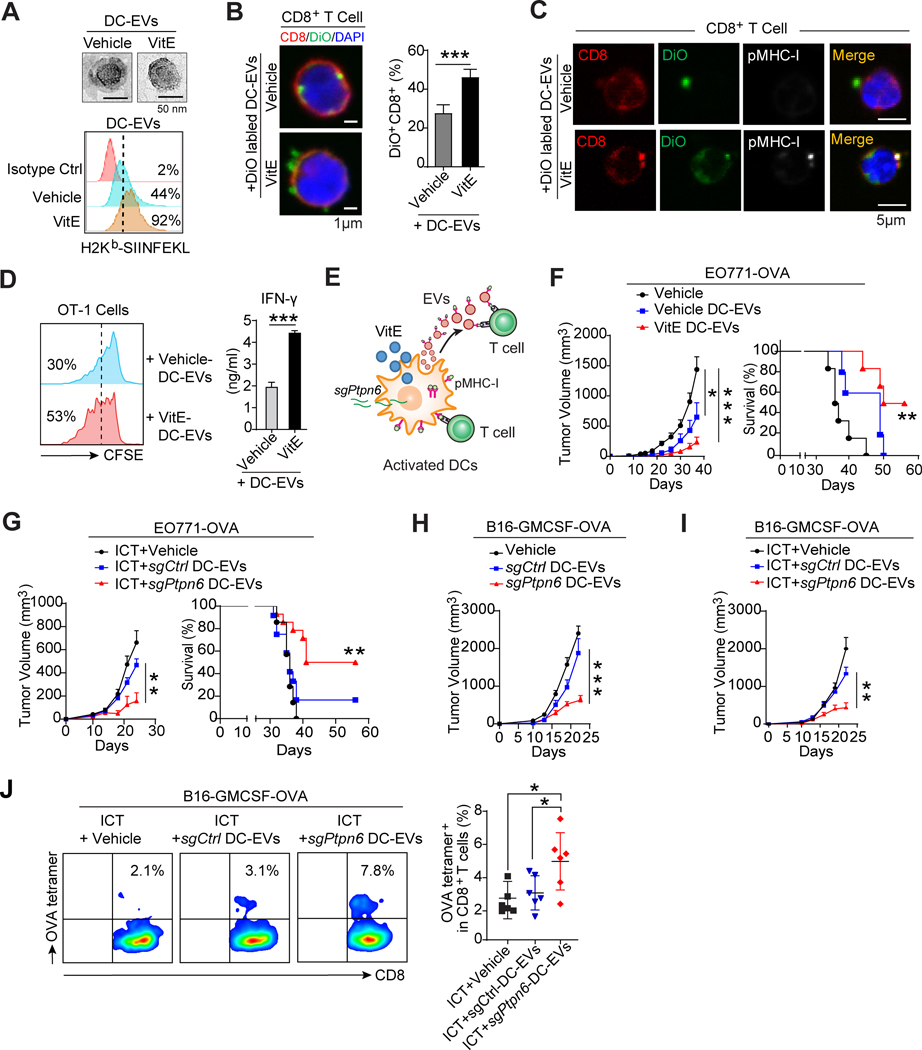
SHP1-inhibited DC-EVs boost system antitumor immunity
An efficient antitumor response by DCs requires multifaceted communication modes (32) between DCs and T cells, including direct local intercellular contact and indirect distant communication by extracellular vehicles (EVs). DC-derived extracellular vehicles (DC-EVs) carry biomolecules from DCs including membrane peptide-MHC-I (pMHC-I) complexes, which also function in antigen cross-presentation (32). Remarkably, VitE treatment of DCs also increased the level of H-2Kb-SIINFEKL complexes in DC-EVs compared to vehicle treatment (Fig. 6A). VitE-treated DC-EVs had increased binding to CD8+ T cells than vehicle-treated DC-EVs (Fig. 6B), and the pMHC-I complex was more detected in it (Fig. 6C). Moreover, compared to vehicle-treated DC-EVs, VitE-treated DC-EVs more effectively enhanced the proliferation and IFN-γ production of OT-1 T cells (Fig. 6D). Similarly, DC-EVs from sgPtpn6-DC2.4 also enhanced T cell proliferation (Supplementary Fig. S9D). Thus, similar to those observed in DCs, DC-EVs from VitE-treated DCs or sgPtpn6-DCs have a greater capacity of antigen-presentation relative to the control DC-EVs (Fig. 6E).
Figure 6. SHP1 inhibition enhances the antigen cross-presentation of DC-derived EVs to boost system antitumor immunity.
A, A representative transmission electron microscope (TEM) image of DC-EVs (top) and flow cytometric analysis of H-2Kb-SIINFEKL in DC-EVs (bottom) derived from OVA-loaded BMDCs treated with vehicle or VitE.
B, Confocal microscopy analysis (left) and quantification (right, n=5) of DiO+CD8+ T cells after T cells were incubated with vehicle- or VitE-treated DiO-labelled DC-EVs for 48 hrs.
C, Confocal microscopy analysis of pMHC-I+CD8+ T cells after incubating T cells with vehicle- or VitE-treated BMDCs-derived EVs and immunofluorescent staining with CD8 and pMHC-I antibodies. EVs were collected from DiO-labelled and OVA-loaded BMDCs.
D, Flow cytometry analysis of the proliferation of OT-1 T cells cultured with the addition of EVs derived from OVA-loaded vehicle-DC-EVs or VitE-DC-EVs (left panel). IFN-γ production by OT-1 T cells with the indicated treatment was measured by ELISA (right panel, n=5).
E, Schema of adjacent T cell activation by cell-cell contact with VitE-treated DCs or sgPtpn6-transfected DCs and of distal T cell activation via pMHC-I expressing DC-EVs.
F, Growth of EO771-OVA mammary tumors and survival of mice treated with PBS (Vehicle), or DC-EVs (30 μg/mouse) isolated from DCs pretreated with vehicle (vehicle-DC-EVs), or with VitE (VitE-DC-EVs) (n=6/group).
G, Growth of EO771-OVA mammary tumors and survival of mice treated with ICT (anti-PD1, 200μg/mouse by i.p. at day 7, 10, 13) in combination with PBS, or DC-EVs (30 μg/mouse) isolated from DC2.4 cells transfected with sgCtrl (sgCtrl-DC2.4-EVs), or with sgPtpn6 (sgPtpn6-DC2.4-EVs) by i.v. at day 2, 9, and 16 (n=6/group).
H and I, Growth curves of subcutaneous B16-GMCSF-OVA tumors in mice treated with vehicle, sgCtrl-DC2.4-EVs or sgPtpn6-DC2.4-EVs (30μg/mouse) by i.v. at day 2, 9, and 16 (H, n = 6/group) and treated with ICT (anti-PD1) in combination with vehicle, sgCtrl-DC2.4-EVs or sgPtpn6-DC2.4-EVs (I, n=6/group).
J, Flow cytometry analysis and quantification of OVA-tetramer+CD8+ T cells in B16-GMCSF-OVA tumors from the 3 mouse groups described in I (n=6/group).
Error bars, s.e.m. Two-sided Student’s t-test (B, D). One-way ANOVA with Tukey’s multiple comparison post-test (F tumor volume, G tumor volume, H, I, and J). Two-sided log-rank test (F, G, survival curves). The statistical significance is defined by P-value: ns, no significant. *P < 0.05, **P < 0.01, ***P < 0.001.
To test the effect of DC-EVs from VitE-treated DCs or sgPtpn6-DCs in antitumor immunity and tumor inhibition in vivo, we induced mammary tumors in mice by mammary fat pads (mfp) injection of OVA-expressing EO771 cells (EO771-OVA) and treated mice with DC-EVs from vehicle- versus VitE-treated BMDCs or from sgCtrl-DC2.4 versus sgPtpn6-DC2.4 cells loaded with OVA. VitE-treated DC-EVs significantly exceeded vehicle-treated DC-EVs in tumor inhibition and increased mouse survival time (Fig. 6F; Supplementary Fig. S9E) and induced a higher CD8+/regulatory T cell ratio and higher tumor infiltration of OVA-specific CD8+ T cells (Supplementary Fig. S9F), indicating that VitE-treated DC-EVs elicit a specific antitumor immune response. Similarly, sgPtpn6-DC-EVs significantly inhibited EO771-OVA tumor growth whereas sgCtrl-DC-EVs did not (Supplementary Fig. S9G). Moreover, combining sgPtpn6-DC-EVs with ICT yielded a strong therapeutic response in mice bearing the ICT-resistant EO771-OVA tumors, as indicated by delayed tumor growth, prolonged survival, and reduced lung metastasis (Fig. 6G; Supplementary Fig. S9H–S9K). Consistently, sgPtpn6-DC-EVs alone (Fig. 6H) or in combination with ICT (Fig. 6I) markedly inhibited tumor growth in mice bearing the B16-GMCSF-OVA melanoma (Supplementary Fig. S9L–S9N) and increased tumor infiltration of OVA antigen-specific CD8+ T cells (Fig. 6J). Together, EVs from SHP1-inhibited (by VitE treatment or Ptpn6-knockout) DCs offer a promising option to generate an antigen-specific immune response and empower immunotherapy.
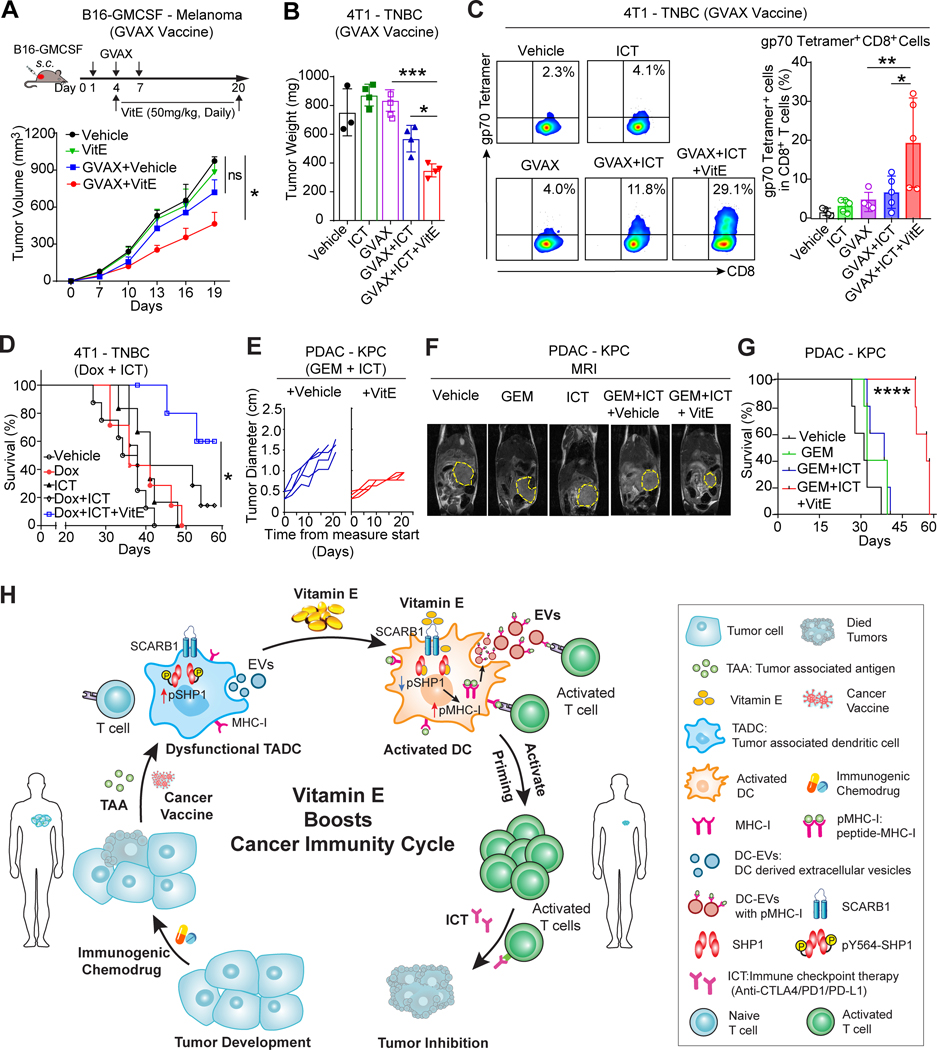
VitE augments immune induction therapies
Next, we further tested whether VitE may enhance the efficacies of anticancer therapies, especially, those that release tumor antigens and enrich DCs infiltration, i.e. tumor vaccines (33) or immunogenic chemotherapies (34). Mice were inoculated with the GM-CSF-secreting tumor cell vaccine (GVAX) post-injection of B16-GMCSF-tumor cells and treated with vehicle or VitE (Fig. 7A). Although GVAX+vehicle showed no therapeutic effect, GVAX+VitE led to a significant tumor inhibition (Fig. 7A) and increased tumor-infiltrating cytolytic GZMB+CD8+ T cells (Supplementary Fig. S10A). Similarly, aggressive 4T1 tumors of a mouse triple-negative breast cancer (TNBC) model are resistant to either GVAX or ICT, and only partly respond to the GVAX+ICT combination; still, VitE treatment combined with GVAX+ICT significantly enhanced the antitumor effect (Fig. 7B; Supplementary Fig. S10B), increased tumor infiltration of CD11c+CD40+ activated DCs with reduced pSHP1 expression (Supplementary Fig. S10C), along with the increased tumor-infiltrating antigen-experienced CD44+CD62L−CD8+ T cells and tumor-reactive gp70 tetramer-specific CD8+ T cells which recognize the 4T1 tumor-associated gp70 antigen (Fig. 7C; Supplementary Fig. S10D).
Figure 7. VitE enhances immune induction therapies to augment immunotherapy.
A, Diagram of the experimental paradigm (top) and growth curves of orthotopic B16-GMCSF tumors (bottom) in mice vaccinated with or without GVAX (s.c. at day 1, 4, 7), then treated with vehicle or VitE (50mg/kg/day) by oral gavage from day 4 to day 20 (n=6/group).
B, Tumor weight of 4T1 tumors from mice treated with vehicle, GVAX, ICT (anti-PD1/anti-CTLA4), or GVAX+ICT in combination with vehicle or VitE (50mg/kg/day, oral gavage from day 4 to day 25). Tumors were harvested on day 25 post-inoculation.
C, Flow cytometric analysis (left) and quantification (right) of gp70 tetramer+ CD8+ T cells in 4T1 tumors collected at day 14 post-implantation from mice under indicated treatments (n=3–5/group).
D, Kaplan-Meier survival curve of 4T1 tumor-bearing mice treated with vehicle, low dose of Doxorubicin (Dox, 5mg/kg by i.v.), ICT (anti-PD1/anti-CTLA4), or Dox+ICT plus vehicle- or VitE-administrations (n=5–10).
E, Measurement of maximal tumor diameter in HY1936 KPC tumor-bearing mice treated with GEM (gemcitabine, 25 mg/kg, i.p.) and ICT (anti-PD1/anti-CTLA4) plus vehicle- or VitE-administrations (n=4–5).
F, Representative magnetic resonance images (MRI) of mice with established HY19636KPC PDAC tumors (yellow outline) after indicated treatments for 20 days.
G, Kaplan-Meier survival curve of HY19636KPC tumor-bearing mice treated with vehicle, GEM (gemcitabine, 25 mg/kg, i.p.), GEM + ICT (anti-PD1/anti-CTLA4) plus vehicle or VitE administrations.
H, Model of VitE reinvigorating TADCs function and boosting antitumor immunity. Dendritic cells (DCs) are central regulators of the cancer-immunity cycle. Upon VitE treatment, VitE reinvigorates dysfunctional TADCs by inhibiting pSHP1/SHP1 to enhance cross-presentation of DCs and DC-EVs, which synergize with ICT and/or DC-enrichment immune induction therapies leading to effective antitumor immunity.
Error bars, s.e.m. One-way ANOVA with Tukey’s multiple comparison post-test (A, B, C). Two-sided log-rank test (D, G). The statistical significance is defined by P-value: ns, no significant. *P < 0.05, ***P < 0.001, ****P < 0.0001.
Clinically, cancer types with low mutation burden are resistant to ICT treatment and combination with certain immunogenic chemotherapeutics improves the ICT efficacy in late-stage cancer patients (35). Thus, we assessed whether VitE further enhances chemotherapy+ICT antitumor effects in 4T1 tumor-bearing mice treated with low-dose doxorubicin+ICT (Supplementary Fig. S11A). Strikingly, compared to the doxorubicin+ICT group, doxorubicin+ICT+VitE treatment substantially prolonged the survival of mice bearing the ICT-resistant 4T1 tumors (Fig. 7D), inhibited tumor growth and lung metastasis (Supplementary Fig. S11B–C). Moreover, adding VitE into the combinatorial treatment (doxorubicin+ICT) increased the infiltration of CD86+ activated DCs with reduced pSHP1 expression (Supplementary Fig. S11D and S11E), along with increased tumor-infiltrating gp70 tetramer-specific CD8+ T cells (Supplementary Fig. S11F).
Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive and lethal types of cancer that is resistant to all standard treatments such as gemcitabine (GEM) chemotherapy and immunotherapies. The HY19636KPC cells, which were derived from the P48-Cre; LSL-KrasG12D; Trp53fl/+ (KPC) mice, develop PDAC in mice (36) that recapitulate the extremely aggressive human PDAC phenotype with low immunogenicity and poor response to therapies, e.g., GEM or/and ICT (37). Notably, supplementing VitE to the GEM+ICT combinatorial regimen effectively inhibited the HY19636KPC PDAC tumor growth, reduced tumor-induced ascites, and prolonged the survival time of tumor-bearing mice compared to the GEM+ICT+vehicle group (Fig. 7E–G; Supplementary Fig. S12A–S12C). Moreover, GEM+ICT+VitE treatment increased tumor infiltration of CD86+ activated DCs with low pSHP1 expression (Supplementary Fig. S12D and S12E). Collectively, VitE greatly enhanced the antitumor effect of tumor vaccines or immunogenic chemotherapies that release tumor antigens and enrich DC infiltrations, and ultimately sensitized various highly ICT-resistant tumors to the ICT antitumor effect.
DISCUSSION
A determining step in the multi-step cancer-immunity cycle is eliciting immunogenic antigen presentation in tumors. DCs are critical for the cross-presentation of tumor antigens to activate the T cells. Here, we discovered that natural VitE, a commonly used dietary supplement, activates antigen-specific immune responses and enhances immunotherapy responses by increasing antigen presentation of DCs and DC-EVs via targeting a DC checkpoint, SHP1 (Fig. 7H).
VitE, a fat-soluble vitamin, is required for the proper function of many organs in the body (38). But, the potential general anticancer effect of VitE has been investigated for decades with controversial findings (39,40). Although some early preclinical studies indicated that VitE had antitumor activities (38,41), large-scale clinical intervention studies haven’t shown a significant effect (13,42,43). Our findings here reveal one possible reason why VitE lacked efficacy in these clinical intervention studies. We found that VitE increases antigen presentation of DCs and VitE-enhanced anticancer immunity is dependent on DC-induced antigen-specific immune responses. Therefore, VitE supplement may only produce a significant effect in patients with highly immunogenic microenvironments that are already DC-enriched but would typically be insufficient to induce antitumor immune responses without much tumor antigen release. However, when combined with cancer vaccines or immunogenic chemotherapies that induce antigen-release and enrich tumor-infiltrating DCs in non-immunogenic tumors, VitE can indeed potentiate anticancer immune responses to further enhance ICT response. Here, we unveiled the anticancer efficacy of therapies fostering co-operation among non-redundant mechanisms of anticancer immunity: i) releasing tumor antigens, ii) increasing antigen presentation of DCs, and iii) activating T cell-mediated adaptive immune response. It will be important to conduct randomized clinical trials with carefully controlled VitE dosages and other extraneous confounding factors to prospectively test the efficacy of VitE in combination with ICT and/or other immune induction therapies.
The key downstream target of VitE in DCs is SHP1, a DC-intrinsic checkpoint and master regulator of DCs (22). TADCs have markedly elevated SHP1 activity that limits tumor antigen cross-presentation, whereas VitE inhibits SHP1 activity and thus rescues the dysfunctional TADCs. Commonly, VitE is regarded as an antioxidant with anti-inflammatory properties (38). In this study, our computational structural analysis and biochemical assays revealed that VitE, after entering DCs via the SCARB1 receptor, directly binds to SHP1, and functions as an allosteric inhibitor of SHP1 to reinvigorate TADCs’ function. Targeting SHP1 is the key mechanism of VitE-induced DC activation as knockout of the SHP1 encoding gene Ptpn6 led to DC activation and blunted the effect of VitE on DCs. Thus, SHP1 is an attractive target to effectively activate DCs for the development of potent immunotherapy. The structural insight on the interaction between VitE and SHP1 will guide us to develop more specific allosteric SHP1 inhibitors to effectively target SHP1 for enhancing the functions of DCs.
We found that VitE promotes antitumor T cell immunity through both direct intercellular contact with DCs and via DC-EVs. DC-EVs also mediate the VitE effect of increasing cross-presentation of tumor antigens. DC-EVs are attractive candidates for drug development and delivery because of their safety, targeting capabilities, and clinical practicality (44). DC-EVs-based clinical trials have been conducted in advanced melanomas, yet showed limited efficacy (44). Realistically, the weak antigen presentation of in vitro generated MoDCs cannot effectively license adaptive T cells for antitumor immunity, which could result in the DC-EVs’ lack of efficacies. Our data showed that DC-EVs derived from SHP1-inhibited (i.e., VitE-treated or Ptpn6-knockout) DCs significantly enhanced their cross-presentation of tumor antigens triggering potent systemic antigen-specific T cell antitumor immunity, and facilitated immunotherapy compared to corresponding control DC-EVs, highlighting that SHP1 inhibition fundamentally alters the biological functions of DCs and DC-EVs. Given their unique effector function, adoptive transfer of SHP1-silenced DCs or DC-EVs can be developed as therapeutics for various diseases that demand boosted antigen cross-presentation and activate antigen-specific T cell immunity for effective treatment.
Based on our findings in this study, we envision that combining VitE with DC-enrichment therapies could be a new strategy to potentially enhance various anticancer immunotherapies. Certain immunogenic chemotherapeutics, radiation therapy, or targeted therapies for cancer treatment trigger immunogenic cell death that promotes antitumor immune responses (45), which depend on DCs functions to license cytotoxic T cells. Cancer vaccine therapies release tumor antigens to induce antitumor immune responses which also depend on DCs to elicit potent T cell immunity (33). Although these cancer therapies are the major pillars of the cancer treatment powerhouse, they frequently are ineffective. It is conceivable that activating DCs by VitE treatment or SHP1 inhibition and increasing DC-mediated antigen processing and presentation to T cells can yield more effective immune responses and might enhance the efficacies of these anticancer therapies.
In summary, we discovered that dietary VitE supplement enhances antitumor immunity and immunotherapy efficacy largely by a DC-dependent function via inhibiting SHP1, despite that VitE also has other possible antitumor functions (46,47). Our preclinical findings pave the way for clinical trials to test combinatorial therapies of VitE, or SHP1-depleted DCs/DC-EVs, with antigen-releasing therapies (vaccines, immunogenic chemotherapies) and ICT as promising and efficacious anticancer immunotherapies.
METHODS
Human Subjects
All procedures in studies involving human subjects were performed under the ethical standards of the institutional research committee and the Helsinki declaration or comparable ethical standards following the guidelines approved by the Institutional Review Board (IRB) at The University of Texas MD Anderson Cancer Center (MDACC) and the Houston Methodist Hospital. In this study, we performed retrospective reviews of the institutional medical databases fed from the electronic health record. To protect patient confidentiality, de-identified patient data was included, and no Protected Health Information (PHI) was collected. No treatments were given and no subjects were contacted. It did not involve more than minimal risk to the subjects. The informed consent from the patients was therefore waived and the request for Waiver of Informed Consent has been approved by the IRB committee. All patient-related data and unique identifiers were removed so that human information was anonymized before any further processing.
Electrical health records (EHR) analysis
Our clinical data mining study was conducted on cancer patients encountered from 2016 to 2021 at MDACC. The study population consisted of cancer patients with the International Classification of Diseases (ICD) code (10th version). Anonymized aggregate-level data were collected using the SlicerDicer function within MD Anderson Epic electronic medical records. Using the Epic SlicerDicer, we identified all the cancer patients with a visit diagnosis, billing diagnosis, or active problem list with malignant cancers. EPIC SlicerDicer was used to further identify patients who received anti-PD-1 antibody (pembrolizumab, nivolumab, or cemiplimab) or anti-PD-L1 antibody (durvalumab, atezolizumab, or avelumab) treatment during the study period. To analyze the impacts of common dietary components and nutritional supplements on patients’ outcomes, the 2,715 melanoma patients receiving anti-PD-1/PD-L1 treatment during 2016 to 2019 were further divided into 15 patient subgroups based on the supplements they took during ICT immunotherapeutic treatment. The deceased patient percentage of each subgroup was calculated based on the patient number of each subgroup and the average patient deceased rate. Patient information, including age, sex, disease stage, and survival (alive or dead), was extracted. No major selection bias is envisioned in these clinical data collections and analyses. Simultaneously, a similar analysis was performed in 3,967 breast cancer patients, 475 melanoma patients, and 323 prostate cancer patients receiving Taxol treatment and in 3,489 breast cancer patients, 460 melanoma patients, and 416 prostate cancer patients with Doxorubicin treatment encountered at MDACC during 2016–2019. The patient data from the Houston Methodist Hospital during 2016 to 2021 was collected, proceeded, and analyzed following the same standard. Due to limited patient numbers with the record of dietary supplements administration, only death rate data was collected and analyzed.
For overall survival analysis and anti-PD-1/PD-L1 therapeutic response analysis, patients encountered during 2016–2021 at MDACC were separated into two subgroups, patients who took VitE and patients who took neither VitE nor multivitamins. The survival data were updated to 2021 August 31st. In melanoma patients, patients who only took multivitamins were also separated as a subgroup for survival analysis. Patients who were treated with more than one round of ICTs were excluded from the analysis. Additionally, patients without the date information of starting ICTs treatment were also excluded from the survival analysis. To perform patient overall survival analysis, the patient death date and the date starting the ICT treatment were pulled out from Epic. The survival time was calculated as the days between the two dates. The univariate survival analysis and multivariate survival analysis (Cox proportional hazards regression analysis, CoxPH) were performed using the IBM SPSS Statistics version 23 (RRID: SCR_019096). Since patients with breast cancer, kidney cancer, or colon cancer shared similar median survival time with limited patient numbers separately, they were combined together as a mixed cohort for overall survival analysis. Therapeutic response to ICTs was defined as the event /Body Site (Onc Hx) recorded within 6 months after receiving the ICTs, including Progressed Disease (PD), Complete Remission/Response (CR), Partial Remission/Mixed Response (PR), and Stable Disease (SD). For therapeutic response analysis, the disease control rate (DCR) was calculated as the ratio of patients with CR, PR, or SD compared to total patients with available therapeutic response data (patients of CR, PR, SD, plus PD).
Mice
C57BL/6J (RRID:IMSR_JAX:000664), BALB/c (RRID:IMSR_JAX:000651), and B6;129S-Scarb1tm1Kri/J mice (Scarb1−/−, IMSR Cat# JAX:003379, RRID:IMSR_JAX:003379) were purchased from The Jackson Laboratory and allowed to acclimatize for at least one week before experimentation. OT-1 T cell receptor–transgenic mice (C57BL /6-Tg (TcraTcrb)1100Mjb/J, RRID:IMSR_JAX:003831) have a T cell receptor that recognizes OVA residues 257–264 in the context of H2Kb. For all experiments, 8–12-week-old mice were matched by age and sex and randomly assigned to specific treatment groups. Animals were bred and maintained in a specific pathogen-free animal facility, and all mouse protocols and experiments were conducted in accordance with an Institutional Animal Care and Use Committee (IACUC) approved protocol at The University of Texas MD Anderson Cancer Center (MDACC, protocol number 00000883) and in compliance with all relevant ethical regulations.
Cell lines
Mouse melanoma cell B16-F10 (ATCC Cat# CRL-6475, RRID: CVCL_0159) and mouse breast carcinoma cells: EMT6 (ATCC Cat# CRL-2755, RRID: CVCL_1923), EO771 (ATCC Cat# CRL-3461, RRID: CVCL_GR23), 4T1 (ATCC Cat# CRL-2539, RRID: CVCL_0125) were purchased from the American Type Culture Collection (ATCC; Manassas, VA). The murine melanoma cell lines B16-GMCSF (RCB Cat# RCB1158, RRID: CVCL_L292) (15) and B16-FLT3L (RRID: CVCL_IJ12) (48) were generated as described previously. The hybridoma RMP1–14 for αPD-1 production was provided by Dr. Hideo Yagita at the Juntendo University School of Medicine. HY19636 cells were established from KPC mice (p48-cre+KrasLSL-G12D/+Trp53lox/+, C57BL/6 background) (36). EO771-OVA and B16-GMCSF-OVA were derived from EO771 and B16-GMCSF respectively following the protocol previously described (49), in which tumor cells were transfected with the plasmid pCI-neo-mOVA (RRID: Addgene_25099). Mouse dendritic cell DC2.4 (Millipore Cat# SCC142, RRID: CVCL_J409) was cultured in DCs medium (RPMI 1640 containing 2 mM L-glutamine and 25 mM HEPES, supplemented with 10 mM Sodium Pyruvate, 1% MEM nonessential amino acids, 100 U/ml penicillin/streptomycin, 50 μM 2-mercaptoethanol, and 10% FBS). Details of cell line generation using CRISPR/Cas9 KO, shRNA, and siRNA knockdowns are included in the supplementary methods section, and gRNA and shRNA sequences are listed in Supplementary Table S8. These cell lines were authenticated by the Characterized Cell Line Core Facility at MDACC. All cells were used at low passage numbers and were found to be negative for Mycoplasma upon repeated testing every 2 months using the MycoAlert Mycoplasma Detection Kit (Lonza Cat# LT07–118).
Tumor challenge and treatment experiments
For the mammary tumor models, 2 × 105 EMT6, 4T1, or EO771 cells were orthotopically injected into the mammary fat pads of female BALB/c mice (4T1, EMT6) or C57BL/6J (EO771) on day 0. For the melanoma tumor challenges, 2 × 105 B16-F10, B16-GMCSF, or B16-FLT3L cells were subcutaneously (s.c.) injected into the right flank of C57BL/6 mice on day 0. Syngeneic orthotopic PDAC tumors were established by surgical implantation. Briefly, 2,000 HY19636 KPC cells in 50 μl PBS were injected into the pancreas of C57BL/6J mouse.
Treatments were given as single agents or in combinations with the indicated regimens for each group. VitE ((+)-α-Tocopherol, type VI, Sigma-Aldrich Cat# T1539) was formulated in the vehicle (10% ethanol in sterile water) and administered by oral gavage once a day at a dose of 50mg/kg body weight (100μl at 10mg/ml per day), corresponding to 400 to 600 mg/day in a human weighing 60kg, which is within the range of doses recommended for supplement and pharmacological use. VitE treatment was initiated on day 4 lasting until the ending point. For ICT experiments, mice were given by intraperitoneal (i.p.) injection of anti-mouse PD-1 antibody (200 μg/mouse, clone RMP1–14, the hybridoma RMP1–14 for αPD-1 production) and/or anti-mouse CTLA-4 antibody (100 μg/mouse, Bio X Cell Cat# BE0164, RRID: AB_10949609) on days 7, 10, 13 for the indicated tumor models unless otherwise specified. Rat IgG2a isotype control (200 μg, Bio X Cell Cat# BE0089, RRID: AB_1107769) and/or mouse IgG2b isotype control (100 μg, Bio X Cell Cat# BE0086, RRID: AB_1107791) respectively was used in control mice corresponding to the ICT treatment group. For in vivo DCs adoptive transfer experiments, 2 × 105 B16-GMCSF tumor cells mixed with sgCtrl-DC2.4 or sgPtpn6-DC2.4 cells at a ratio of 1:1 were injected subcutaneously into C57BL/6J mice, respectively. For DC-EVs treatment experiments, 30μg DC-EVs were administered intravenously (i.v.) on days 2, 9, and 16 after tumors transplantation.
Where indicated, mice were vaccinated subcutaneously on their contralateral flank with either PBS or 1.0 × 106 35 Gy-irradiated GM-CSF-secreting B16 cells or GM-CSF-secreting 4T1 cells (GVAX) on days 1, 4, and 7 post-tumor injections. For chemo combination treatment, HY19636 KPC tumor-bearing mice were treated with gemcitabine (GEM, LC laboratories Cat# G-4177) at 25mg/kg body weight by i.p. injection every 4 days from day 10, and 4T1 TNBC tumor-bearing mice were given with doxorubicin (Dox, 5mg/kg body weight, LC laboratories Cat# G-4000) by i.v. once a week from day 10.
Tumor size was measured with a digital caliper and tumor volume was calculated using the formula V = (L × W2)/2 and expressed as mm3, where V is tumor volume, L is the length of the tumor (longer diameter) and W is the width of the tumor (shorter diameter). For HY19636 KPC mice, KPC tumors were measured by gross tumor diameter using twice-weekly palpation and external caliper measurement. For magnetic resonance images (MRI), HY19636 KPC-bearing mice were imaged with Bruker 7T small animal Magnetic Resonance Imager (RRID:SCR_019759).
DCs supplementation with VitE and other supplements
A stock solution of VitE ((+)-α-Tocopherol, type VI, Sigma-Aldrich Cat# T1539) was prepared by dissolving VitE in absolute ethanol to a final concentration of 100 mg/ml. To optimize cellular uptake, the VitE stock solution was then mixed in FBS at 1 mg/ml and incubated in a 37°C incubator for 1h protected from the light with periodic vortexing. VitE was added to the culture media of mouse BMDCs, mouse TADCs, or human MoDCs at 50 μg/ml (116 μM) or at the indicated concentrations for 24–48h, in which vehicle (0.02% ethanol in medium) as control. This dose of VitE (50 μg/ml) is safe in humans and this treatment mimics the effects of in vivo feeding with VitE (50mg/kg) in mice and taking a daily VitE supplement in humans. Vehicle or VitE-treated DCs were washed twice with PBS before continuing to perform antigen presentation assay, coculture with T cells, or conduct the indicated staining assay.
Mass cytometry data acquisition
The single cells from mouse tumor tissues were prepared as described previously (50). The cells were incubated with metal-conjugated Ab cocktails against the surface and intracellular proteins (Supplementary Table S9). To analyze the phosphorylated signal proteins, single cells isolated from tumor tissues of EMT6-bearing mice with indicated vehicle or VitE treatment were stimulated by LPS (100ng/ml) for 15 min in vitro and then stained with distinct isotope-labeling phosphorylated Ab cocktails after permeabilization. Cell samples were then analyzed by Fluidigm Helios Cell Mass Cytometer (RRID:SCR_019917) in the Flow Cytometry & Cellular Imaging Core Facility, which is supported in part by the NIH through MDACC Support Grant (CA016672) and a Shared Instrumentation Award from the Cancer Prevention Research Institution of Texas (CPRIT) (RP121010). Detailed CyTOF samples collection and data analysis were described in the supplementary methods.
Flow cytometry and Immunoblotting
Detailed protocols for flow cytometry and immunoblotting are described in the Supplementary Methods, and antibodies used in these protocols are listed in Supplementary Tables S10 and S11, respectively.
Statistical analyses
Statistical analyses were performed with GraphPad Prism v.8.0 (RRID:SCR_002798). Unless mentioned otherwise, all data are presented as mean with s.e.m. Sample size, error bars, and statistical methods are reported in the figure legends. P values are shown in figures or associated legends. The statistical significance of differences between two experimental groups was assessed by unpaired two-tailed Student’s t-test, and one-way ANOVA was performed for comparisons between more than two groups unless indicated otherwise. Pearson correlation analysis was performed for correlation analysis. No statistical methods were used to predetermine sample size in the mouse studies, and mice with matched sex and age were randomized into different treatment groups and, where possible, mixed among cages. For the Kaplan–Meier analyses of clinical data, the log-rank test was used to compare groups. The significance of survival outcomes in each cohort was not affected when adjusted for race/ethnicity, age at the start of ICT in a multivariate analysis. Statistics on patient demographics were conducted by a two-sided Fisher exact test and related analyses were performed on the IBM SPSS Statistics version 23 (RRID: SCR_019096).
Data Availability
The human clinical data generated in this study are not publicly available due to patient privacy requirements of MD Anderson Cancer Center and the Houston Methodist Hospital. Other data generated in this study are available within the article and its supplementary data files.
Supplementary Material
SIGNIFICANCE:
The impacts of nutritional supplements on responses to immunotherapies remain unexplored. Our study revealed that dietary vitamin E binds to and inhibits DC checkpoint SHP1 to increase antigen presentation, prime antitumor T cell immunity, and enhance immunotherapy efficacy. Vitamin E-treated or SHP1-silenced DCs/DC-EVs could be developed as potent immunotherapies.
Acknowledgments
We thank the National Institutes of Health (NIH) Tetramer Core Facility for providing gp70 tetramer and OVA tetramer, Dr. Jedd Wolchok for the B16-GMCSF cell line, Dr. Hideo Yagita for the hybridoma RMP1–14, Drs. Stephanie Watowich, Chunru Lin, Jianjun Gao, and Florencia McAllister for critical comments on the manuscript. This work was supported by NIH grants R01CA184836 (D.Y.), R01CA208213 (D.Y.), R01CA231149 (D.Y.), METAVivor research grants #56675 and #58284 (D.Y.), MD Anderson Duncan Family Institute for Cancer Prevention and Risk Assessment (D.Y.), and NIH Cancer Center Support Grant P30CA016672 to MDACC (Flow Cytometry & Cellular Imaging Facility, Functional Genomics Core, Functional Proteomics Core, Research Histology Core, Characterized Cell Line Core, and Research Animal Support Facility-Houston). J.A. Tainer is supported by Cancer Prevention Research Institute of Texas (CPRIT) grant RP180813, NIH grants P01CA092584 and R35CA220430, and the Robert A. Welch Chemistry Chair. D. Yu. is the Hubert L. & Olive Stringer Distinguished Chair in Basic Science at MDACC.
Authors’ Disclosures: D. Yu. reports grants from NIH/NCI during the conduct of the study. D. Yu, X. Yuan, Y. Duan, Y. Xiao, Y. Qi have filed US Provisional Patent Application No. 63/252,721 (pending). Other authors had no disclosures.
REFERENCES
- 1.Kantor ED, Rehm CD, Du M, White E, Giovannucci EL. Trends in Dietary Supplement Use Among US Adults From 1999–2012. Jama 2016;316(14):1464–74 doi 10.1001/jama.2016.14403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Du M, Luo H, Blumberg JB, Rogers G, Chen F, Ruan M, et al. Dietary Supplement Use among Adult Cancer Survivors in the United States. The Journal of nutrition 2020;150(6):1499–508 doi 10.1093/jn/nxaa040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tajan M, Vousden KH. Dietary Approaches to Cancer Therapy. Cancer cell 2020;37(6):767–85 doi 10.1016/j.ccell.2020.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Jordan S, Tung N, Casanova-Acebes M, Chang C, Cantoni C, Zhang D, et al. Dietary Intake Regulates the Circulating Inflammatory Monocyte Pool. Cell 2019;178(5):1102–14 e17 doi 10.1016/j.cell.2019.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wei SC, Duffy CR, Allison JP. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer discovery 2018;8(9):1069–86 doi 10.1158/2159-8290.CD-18-0367. [DOI] [PubMed] [Google Scholar]
- 6.Sharma P, Siddiqui BA, Anandhan S, Yadav SS, Subudhi SK, Gao J, et al. The Next Decade of Immune Checkpoint Therapy. Cancer discovery 2021;11(4):838–57 doi 10.1158/2159-8290.CD-20-1680. [DOI] [PubMed] [Google Scholar]
- 7.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013;39(1):1–10 doi 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 8.Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nature reviews Immunology 2020;20(1):7–24 doi 10.1038/s41577-019-0210-z. [DOI] [PubMed] [Google Scholar]
- 9.Cubillos-Ruiz JR, Silberman PC, Rutkowski MR, Chopra S, Perales-Puchalt A, Song M, et al. ER Stress Sensor XBP1 Controls Anti-tumor Immunity by Disrupting Dendritic Cell Homeostasis. Cell 2015;161(7):1527–38 doi 10.1016/j.cell.2015.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gahche JJ, Bailey RL, Potischman N, Ershow AG, Herrick KA, Ahluwalia N, et al. Federal Monitoring of Dietary Supplement Use in the Resident, Civilian, Noninstitutionalized US Population, National Health and Nutrition Examination Survey(().). The Journal of nutrition 2018;148(Suppl 2):1436S-44S doi 10.1093/jn/nxy093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richardson MA. Research of complementary/alternative medicine therapies in oncology: promising but challenging. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 1999;17(11 Suppl):38–43. [PubMed] [Google Scholar]
- 12.Galli F, Azzi A, Birringer M, Cook-Mills JM, Eggersdorfer M, Frank J, et al. Vitamin E: Emerging aspects and new directions. Free radical biology & medicine 2017;102:16–36 doi 10.1016/j.freeradbiomed.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 13.Klein EA, Thompson IM Jr., Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). Jama 2011;306(14):1549–56 doi 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobs EJ, Henion AK, Briggs PJ, Connell CJ, McCullough ML, Jonas CR, et al. Vitamin C and vitamin E supplement use and bladder cancer mortality in a large cohort of US men and women. American journal of epidemiology 2002;156(11):1002–10 doi 10.1093/aje/kwf147. [DOI] [PubMed] [Google Scholar]
- 15.De Henau O, Rausch M, Winkler D, Campesato LF, Liu C, Cymerman DH, et al. Overcoming resistance to checkpoint blockade therapy by targeting PI3Kgamma in myeloid cells. Nature 2016;539(7629):443–7 doi 10.1038/nature20554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karsunky H, Merad M, Cozzio A, Weissman IL, Manz MG. Flt3 ligand regulates dendritic cell development from Flt3+ lymphoid and myeloid-committed progenitors to Flt3+ dendritic cells in vivo. The Journal of experimental medicine 2003;198(2):305–13 doi 10.1084/jem.20030323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin ML, Zhan Y, Proietto AI, Prato S, Wu L, Heath WR, et al. Selective suicide of cross-presenting CD8+ dendritic cells by cytochrome c injection shows functional heterogeneity within this subset. Proceedings of the National Academy of Sciences of the United States of America 2008;105(8):3029–34 doi 10.1073/pnas.0712394105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gide TN, Quek C, Menzies AM, Tasker AT, Shang P, Holst J, et al. Distinct Immune Cell Populations Define Response to Anti-PD-1 Monotherapy and Anti-PD-1/Anti-CTLA-4 Combined Therapy. Cancer cell 2019;35(2):238–55 e6 doi 10.1016/j.ccell.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science 2015;350(6257):207–11 doi 10.1126/science.aad0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, et al. TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018;554(7693):544–8 doi 10.1038/nature25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abram CL, Roberge GL, Pao LI, Neel BG, Lowell CA. Distinct roles for neutrophils and dendritic cells in inflammation and autoimmunity in motheaten mice. Immunity 2013;38(3):489–501 doi 10.1016/j.immuni.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carmi Y, Prestwood TR, Spitzer MH, Linde IL, Chabon J, Reticker-Flynn NE, et al. Akt and SHP-1 are DC-intrinsic checkpoints for tumor immunity. JCI insight 2016;1(18):e89020 doi 10.1172/jci.insight.89020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramachandran IR, Song W, Lapteva N, Seethammagari M, Slawin KM, Spencer DM, et al. The phosphatase SRC homology region 2 domain-containing phosphatase-1 is an intrinsic central regulator of dendritic cell function. Journal of immunology 2011;186(7):3934–45 doi 10.4049/jimmunol.1001675. [DOI] [PubMed] [Google Scholar]
- 24.Xiao W, Ando T, Wang HY, Kawakami Y, Kawakami T. Lyn- and PLC-beta3-dependent regulation of SHP-1 phosphorylation controls Stat5 activity and myelomonocytic leukemia-like disease. Blood 2010;116(26):6003–13 doi 10.1182/blood-2010-05-283937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang J, Liu L, He D, Song X, Liang X, Zhao ZJ, et al. Crystal structure of human protein-tyrosine phosphatase SHP-1. The Journal of biological chemistry 2003;278(8):6516–20 doi 10.1074/jbc.M210430200. [DOI] [PubMed] [Google Scholar]
- 26.Reboul E, Klein A, Bietrix F, Gleize B, Malezet-Desmoulins C, Schneider M, et al. Scavenger receptor class B type I (SR-BI) is involved in vitamin E transport across the enterocyte. The Journal of biological chemistry 2006;281(8):4739–45 doi 10.1074/jbc.M509042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santander N, Lizama C, Parga MJ, Quiroz A, Perez D, Echeverria G, et al. Deficient Vitamin E Uptake During Development Impairs Neural Tube Closure in Mice Lacking Lipoprotein Receptor SR-BI. Scientific reports 2017;7(1):5182 doi 10.1038/s41598-017-05422-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annual review of immunology 2002;20:621–67 doi 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- 29.Alloatti A, Kotsias F, Pauwels AM, Carpier JM, Jouve M, Timmerman E, et al. Toll-like Receptor 4 Engagement on Dendritic Cells Restrains Phago-Lysosome Fusion and Promotes Cross-Presentation of Antigens. Immunity 2015;43(6):1087–100 doi 10.1016/j.immuni.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Blander JM. Regulation of the Cell Biology of Antigen Cross-Presentation. Annual review of immunology 2018;36:717–53 doi 10.1146/annurev-immunol-041015-055523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kasmapour B, Gronow A, Bleck CK, Hong W, Gutierrez MG. Size-dependent mechanism of cargo sorting during lysosome-phagosome fusion is controlled by Rab34. Proceedings of the National Academy of Sciences of the United States of America 2012;109(50):20485–90 doi 10.1073/pnas.1206811109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindenbergh MFS, Stoorvogel W. Antigen Presentation by Extracellular Vesicles from Professional Antigen-Presenting Cells. Annual review of immunology 2018;36:435–59 doi 10.1146/annurev-immunol-041015-055700. [DOI] [PubMed] [Google Scholar]
- 33.Saxena M, van der Burg SH, Melief CJM, Bhardwaj N. Therapeutic cancer vaccines. Nature reviews Cancer 2021;21(6):360–78 doi 10.1038/s41568-021-00346-0. [DOI] [PubMed] [Google Scholar]
- 34.Pfirschke C, Engblom C, Rickelt S, Cortez-Retamozo V, Garris C, Pucci F, et al. Immunogenic Chemotherapy Sensitizes Tumors to Checkpoint Blockade Therapy. Immunity 2016;44(2):343–54 doi 10.1016/j.immuni.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. The New England journal of medicine 2018;379(22):2108–21 doi 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 36.Bardeesy N, Aguirre AJ, Chu GC, Cheng KH, Lopez LV, Hezel AF, et al. Both p16(Ink4a) and the p19(Arf)-p53 pathway constrain progression of pancreatic adenocarcinoma in the mouse. Proceedings of the National Academy of Sciences of the United States of America 2006;103(15):5947–52 doi 10.1073/pnas.0601273103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morrison AH, Byrne KT, Vonderheide RH. Immunotherapy and Prevention of Pancreatic Cancer. Trends in cancer 2018;4(6):418–28 doi 10.1016/j.trecan.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh U, Devaraj S, Jialal I. Vitamin E, oxidative stress, and inflammation. Annual review of nutrition 2005;25:151–74 doi 10.1146/annurev.nutr.24.012003.132446. [DOI] [PubMed] [Google Scholar]
- 39.Kristal AR, Darke AK, Morris JS, Tangen CM, Goodman PJ, Thompson IM, et al. Baseline selenium status and effects of selenium and vitamin e supplementation on prostate cancer risk. Journal of the National Cancer Institute 2014;106(3):djt456 doi 10.1093/jnci/djt456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hall KT, Buring JE, Mukamal KJ, Vinayaga Moorthy M, Wayne PM, Kaptchuk TJ, et al. COMT and Alpha-Tocopherol Effects in Cancer Prevention: Gene-Supplement Interactions in Two Randomized Clinical Trials. Journal of the National Cancer Institute 2019;111(7):684–94 doi 10.1093/jnci/djy204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee KY, Chiang YT, Hsu NY, Yang CY, Lo CL, Ku CA. Vitamin E containing polymer micelles for reducing normal cell cytotoxicity and enhancing chemotherapy efficacy. Acta biomaterialia 2015;24:286–96 doi 10.1016/j.actbio.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 42.Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women’s Health Study: a randomized controlled trial. Jama 2005;294(1):56–65 doi 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 43.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). Jama 2009;301(1):39–51 doi 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pitt JM, Andre F, Amigorena S, Soria JC, Eggermont A, Kroemer G, et al. Dendritic cell-derived exosomes for cancer therapy. The Journal of clinical investigation 2016;126(4):1224–32 doi 10.1172/JCI81137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nature reviews Immunology 2017;17(2):97–111 doi 10.1038/nri.2016.107. [DOI] [PubMed] [Google Scholar]
- 46.Jiang Q. Natural Forms of Vitamin E as Effective Agents for Cancer Prevention and Therapy. Adv Nutr 2017;8(6):850–67 doi 10.3945/an.117.016329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zingg JM. Vitamin E: A Role in Signal Transduction. Annual review of nutrition 2015;35:135–73 doi 10.1146/annurev-nutr-071714-034347. [DOI] [PubMed] [Google Scholar]
- 48.Mach N, Gillessen S, Wilson SB, Sheehan C, Mihm M, Dranoff G. Differences in dendritic cells stimulated in vivo by tumors engineered to secrete granulocyte-macrophage colony-stimulating factor or Flt3-ligand. Cancer research 2000;60(12):3239–46. [PubMed] [Google Scholar]
- 49.Yang J, Sanderson NS, Wawrowsky K, Puntel M, Castro MG, Lowenstein PR. Kupfer-type immunological synapse characteristics do not predict anti-brain tumor cytolytic T-cell function in vivo. Proceedings of the National Academy of Sciences of the United States of America 2010;107(10):4716–21 doi 10.1073/pnas.0911587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li H, Xiao Y, Li Q, Yao J, Yuan X, Zhang Y, et al. The allergy mediator histamine confers resistance to immunotherapy in cancer patients via activation of the macrophage histamine receptor H1. Cancer cell 2022;40(1):36–52 e9 doi 10.1016/j.ccell.2021.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The human clinical data generated in this study are not publicly available due to patient privacy requirements of MD Anderson Cancer Center and the Houston Methodist Hospital. Other data generated in this study are available within the article and its supplementary data files.