Abstract
Using a newly developed 16S rRNA gene (rDNA)-targeted PCR assay with proposed group specificity for planctomycetes, we examined anoxic bulk soil of flooded rice microcosms for the presence of novel planctomycete-like diversity. For comparison, oxic rice roots were included as an additional sample in this investigation. The bacterial diversity detectable by this PCR assay was assessed by using a combined approach that included terminal restriction fragment length polymorphism (T-RFLP) analysis and comparative sequence analysis of cloned 16S rDNA. T-RFLP fingerprint patterns generated from rice roots contained 12 distinct terminal restriction fragments (T-RFs). In contrast, the T-RFLP fingerprint patterns obtained from the anoxic bulk soil contained 33 distinct T-RFs, a clearly higher level of complexity. A survey of 176 bulk soil 16S rDNA clone sequences permitted correlation of 20 T-RFs with phylogenetic information. The other 13 T-RFs remained unidentified. The predominant T-RFs obtained from rice roots could be assigned to members of the genus Pirellula within the Planctomycetales, while most of the T-RFs obtained from the bulk soil corresponded to novel lines of bacterial descent. Using a level of 16S rDNA sequence dissimilarity to cultured microorganisms of approximately 20% as a threshold value, we detected 11 distinct bacterial lineages for which pure-culture representatives are not known. Four of these lineages could be assigned to the order Planctomycetales, while one lineage was affiliated with the division Verrucomicrobia and one lineage was affiliated with the spirochetes. The other five lineages either could not be assigned to any of the main lines of bacterial descent or clearly expanded the known diversity of division level lineages WS3 and OP3. Our results indicate the presence of bacterial diversity at a subdivision and/or division level that has not been detected previously by the so-called universal 16S rDNA PCR assays.
It is widely accepted that only a minor fraction of bacterial and archaeal diversity has been isolated in pure culture (25, 49). Reasonable assumptions have been used to estimate that this fraction accounts for less than 1% of the naturally occurring diversity (1, 48). Consequently, cultivation-independent methods, based mainly on molecular retrieval of 16S rRNA gene (rDNA) sequences, have become the most important tools for exploration of microbial diversity (13, 24, 35). Use of these methods during the last decade has significantly increased our knowledge about the phylogenetic diversity of microorganisms and their evolutionary relationships. For instance, 12 novel division level lineages not represented by cultured microorganisms were recently detected in Obsidian Pool, a hot spring located in Yellowstone National Park (17). These lineages were regarded as candidate divisions. This term was created in order to characterize unaffiliated lineages in multiple treeing analyses of data sets with different types and numbers of taxa whose sequences exhibited <85% identity to previously reported sequences. Based on the currently available 16S rDNA sequence data, the domain Bacteria is thought to comprise about 40 or even more division level lineages, of which only 26 are represented by pure cultures to date (7, 17).
The planctomycetes represent one of the main lines of bacterial descent. Common characteristics of this intriguing phylum, which is represented by the genera Planctomyces, Pirellula, Gemmata, and Isosphaera, are the presence of a protein cell wall without peptidoglycan, budding cell division, and utilization of N-acetylglucosamine as a sole carbon and nitrogen source for growth. These bacteria were originally thought to inhabit only aquatic habitats (12, 42). However, retrieval and comparative sequence analysis of environmental 16S rDNA have revealed the occurrence of planctomycetes also in upland soils (24, 53). To date, all planctomycetes obtained in pure culture are chemoorganotrophs and either obligate or facultative aerobes; none of them is an obligate anaerobe (12). However, considering the intralineage phylogenetic depth of the order Planctomycetales, which is equivalent to the intralineage phylogenetic depths of other main lines of bacterial descent (e.g., the Proteobacteria), greater metabolic versatility of planctomycetes has been assumed. For instance, the existence of obligate anaerobes, autotrophs, and phototrophs was proposed (12). The hypothesis that (obligate) anaerobes occur among planctomycetes was supported by molecular retrieval of planctomycete-like 16S rDNA from an anoxic bioreactor (14), from an anoxic sediment (32), and from a microbial consortium highly enriched for novel planctomycetes and capable of anaerobic ammonia oxidation and chemolithoautotrophic growth (44).
Our investigation was aimed at obtaining molecular evidence for the existence of planctomycetes indigenous to anoxic soils. Signature nucleotides which were previously described as indicative of planctomycete-like 16S rDNA (24, 52) were used for development of a PCR assay which exhibited high group specificity for planctomycetes. However, some of these signature nucleotides are shared by Chlamydia spp. and members of the division Verrucomicrobia. These common signature nucleotides led to the hypothesis that planctomycetes, chlamydiae, and members of the Verrucomicrobia may share a common ancestry (24, 50, 52). The newly developed PCR detection system was designated the PV assay (Planctomycetales-Verrucomicrobia assay). Using the PV assay, we examined anoxic bulk soil of flooded rice microcosms for the presence of novel planctomycete-like diversity. For comparison, oxic rice roots from the same rice microcosms were also included in this investigation. The molecular survey, based on coordinated use of the terminal restriction fragment length polymorphism (T-RFLP) technique (26, 29) and comparative sequence analysis of cloned 16S rDNA, resulted in detection in the anoxic bulk soil of several novel sublineages within the Planctomycetales and, unexpectedly, in detection of several novel lines of bacterial descent for which pure-culture representatives are not known.
MATERIALS AND METHODS
Samples.
Rice (Oryza sativa var. Roma, type japonica) was grown in the laboratory as described by Frenzel et al. (11) by using plastic containers containing flooded soil obtained from wetland rice fields at the Italian Rice Research Institute in Vercelli, Italy. Samples of rice roots and anoxic bulk soil were obtained from laboratory rice cultures in which the plants were 90 days old. Cores of anoxic bulk soil were taken by pressing a plastic tube into the soil to a depth of about 15 cm. Only the lower (non-root-containing) 10-cm portions of the cores were used. Rice root samples were washed with careful shaking in distilled water to remove adhering soil particles. The root material was lyophilized and stored at −80°C until it was used.
Microbial strains.
Pirellula marina ATCC 49069 and Verrucomicrobium spinosum ATCC 43997 were purchased from the American Type Culture Collection (Manassas, Va.) and were grown aerobically at 25°C by using media described previously (41, 43).
DNA extraction.
Genomic DNA was extracted from pure cultures as described previously (14). For extraction and purification of total community DNA from rice roots and bulk soil of flooded rice microcosms we used protocols described elsewhere (15, 16). The final DNA pellets were resuspended in 200 μl of TE buffer (10 mM Tris, 1 mM EDTA; pH 8.0). The amount of DNA extracted from the bulk soil or rice roots was estimated by electrophoresing 5-μl aliquots on a 0.8% agarose gel and comparing the results to the results obtained with an HindIII digest of λ DNA. The gel was stained with ethidium bromide.
PV PCR assay.
Group specificity of the PV assay for planctomycetes should be achieved with forward primer PLA-40F, which targets a region corresponding to positions 40 to 57 of Escherichia coli 16S rRNA (3). Previously, this region was described as containing signature nucleotide sequence positions highly indicative of 16S rRNA genes of planctomycetes (24, 52). A detailed examination of primer PLA-40F with the probe match tool of the ARB program package (version 2.5b; developed by O. Strunk and W. Ludwig, Technische Universität München [http://www.mikro.biologie.tu-muenchen.de/pub/ARB/]) for variations in its target site for members of the domains Bacteria and Archaea revealed no mismatches for almost all planctomycetes, a minimum of one mismatch for some Chlamydia spp., and two mismatches for members of the Verrucomicrobia (e.g., V. spinosum) and for “Rubrobacter wessexii” (Table 1). All other bacterial and archaeal 16S rDNA sequences exhibited three or more mismatches in the target site of PLA-40F. For PCR, primer PLA-40F was combined with reverse primer 1492R, which is considered universal for members of the domain Bacteria (and Archaea) (51). For T-RFLP analysis, primer PLA-40F was labeled with the dye carboxyfluorescein. Each reaction mixture contained 1 μl of DNA solution, 10 μl of 10× reaction buffer (PCR buffer II; PE Applied Biosystems, Foster City, Calif.), 1.5 mM MgCl2, each deoxynucleoside triphosphate (U.S. Biochemical Corp., Cleveland, Ohio) at a concentration of 200 μM, each primer (MWG-Biotech, Ebersberg, Germany) at a concentration of 0.3 μM, and 2.5 U of Taq DNA polymerase (AmpliTaq; PE Applied Biosystems). The thermal PCR profile was as follows: initial denaturation step consisting of 5 min at 94°C; and 35 cycles (for total DNA extracted from bulk soil and rice roots) or 30 cycles (for DNA extracted from pure cultures and cloned 16S rDNA inserts) consisting of denaturation at 94°C for 30 s, primer annealing at 60°C for 60 s, and elongation at 72°C for 120 s. The length of the final elongation step was extended to 7 min. Amplification was performed with a total volume of 100 μl in 0.2-ml reaction tubes and a DNA thermal cycler (model 2400; PE Applied Biosystems). Ten-microliter aliquots of the 16S rDNA amplicons were checked by electrophoresis on a 1% agarose gel.
TABLE 1.
Alignment of the 16S rDNA target region of primer PLA-40F
| Oligonucleotide or organism(s) | Sequence |
|---|---|
| Primera | 5′-CGGCRTGGATTAGGCATG-3′ |
| Target | 3′-GCCGYACCTAATCCGTAC-5′ |
| Organisms | |
| Planctomyces limnophilus and other planctomycetes | 3′-==================-5′ |
| Chlamydia psittaci | 3′-==========C=======-5′ |
| Verrucomicrobium spinosum | 3′-==========T==T====-5′ |
| “Rubrobacter wessexii” | 3′-=======GA=========-5′ |
| Spirochaeta thermophila | 3′-=====G=AG=========-5′ |
| Rhodoferax fermentans | 3′-=======GG===GT====-5′ |
| Streptomyces griseus | 3′-=======GA=T=GT====-5′ |
PLA-40F targets a stretch corresponding to positions 40 to 57 of the E. coli 16S rRNA (3). Nucleotide positions indicative of planctomycete-like 16S rDNA sequence types are underlined. R = A or G; Y = C or T.
Cloning and sequencing.
The 16S rDNA PCR products recovered from the rice roots and bulk soil were cloned with a TA cloning kit (Invitrogen Corp., San Diego, Calif.) used in accordance with the manufacturer's instructions. Clones were randomly selected for further analysis. For preparation of cloned 16S rDNA, colonies grown on agar plates were picked, and the material was resuspended in 100 μl of TE buffer. Cells were lysed by boiling, the lysate was centrifuged, the supernatant was transferred to a new tube, and 1-μl aliquots were directly used for PCR. Inserts of cloned 16S rDNA were amplified by the PV assay. Sequencing of 16S rDNA clones was carried out as described previously (16). In addition to standard 16S rDNA sequencing primers (21), PLA-40F was also used for sequencing.
T-RFLP analysis.
16S rDNA was amplified from both total community DNA and individual 16S rDNA clones by the PV assay as described above. The amplicons were purified with a Prep-A-Gene purification kit (Bio-Rad, Munich, Germany) or Qiaquick spin columns (Qiagen, Hilden, Germany) and were resuspended in 30 μl of elution buffer. For generation of T-RFLP fingerprint patterns from PCR products we used a protocol described by Lukow et al. (29). Briefly, after purification with Qiaquick spin columns (Qiagen), approximately 100 ng of amplicons was digested with restriction endonuclease MspI (Promega, Mannheim, Germany). Aliquots (2.5 μl each) of the digested 16S rDNA fragment mixtures were size separated in relation to the internal lane standard (GeneScan-1000 ROX; PE Applied Biosystems) in the GeneScan mode of an automated DNA sequencer (model 373; PE Applied Biosystems). Only the 5′-terminal restriction fragments (T-RFs) were detected and analyzed further. The accuracy of determination of fragment sizes of individual T-RFs has been demonstrated previously (26, 31, 54). The number of distinct T-RFs in a given T-RFLP pattern was determined by taking into account only those T-RFs which showed a relative abundance of more than 1%. Relative abundance was determined by dividing the intensity of the fluorescence emission signal (= signal intensity) of a T-RF by the total signal intensity of all T-RFs detected in a fragment size range from 50 to 928 bp.
Phylogenetic placement.
The phylogenetic analysis (i.e., data processing and construction of trees) was done by using the ARB program package. The 16S rDNA clone sequences, each of which was between 1,100 and 1,400 bp long, were added to a database consisting of about 13,900 complete or partial bacterial 16S rRNA sequences. This database was part of the ARB program package. The 16S rDNA sequences were integrated into the database with the automatic alignment tool of the ARB program package. The resulting alignments were manually checked and corrected if necessary. Phylogenetic placement was done in comparison to reference sequences representing the main lines of descent in the domain Bacteria. The overall tree topology was evaluated by applying neighbor-joining analyses (40) and a 50% invariance criterion for inclusion of individual nucleotide sequence positions in the treeing analyses (10, 27). The base frequencies of the alignment positions were determined by using the complete data set consisting of 10,700 (almost full-length) bacterial sequences or by using subsets of these sequences and the appropriate tool of the ARB program package. We generated several trees, which differed in (i) the reference sequences used and (ii) the set of alignment positions used for tree reconstruction. In addition, trees were reconstructed by using maximum-parsimony (ARB and PHYLIP [9]) and maximum-likelihood (ARB and fastDNAml [30]) methods. The statistical significance levels of interior nodes were determined by performing bootstrap analyses by the neighbor-joining method.
To exclude obviously chimeric rDNA primary structures (20, 23, 47) prior to the phylogenetic analysis, the terminal 500 to 650 nucleotide sequence positions of the 5′ and 3′ ends of the environmental rDNA clone sequences recovered were used in separate treeing analyses. Chimeric sequences were also identified by secondary-structure anomalies, which are reported by the automatic alignment tool of the ARB program package. Overall 16S rDNA sequence similarities were determined by using the distance matrix tool of the ARB program package. A representative set of the 16S rDNA clone sequences recovered by using the PV assay was compared with the complete EMBL, GenBank, and DDBJ nucleotide sequence databases (2) (last checked on 12 December 2000). This was done to ensure that the public nucleotide sequence databases did not contain any previously published reference sequences that were more closely related to our environmental rDNA sequences than the reference sequences used for the treeing analyses were.
RESULTS AND DISCUSSION
We examined two distinct locations in flooded rice microcosms to determine the presence and diversity of planctomycetes by the PV assay. One of the characteristics of submerged rice plants is the diffusive flux of atmospheric oxygen into the roots via the plant vascular system. Thus, the narrow region surrounding rice roots can be considered oxic, whereas the bulk soil is completely anoxic (11). A major aim of our research was to determine with molecular tools whether certain planctomycetes occur preferentially in anoxic soil environments. This would be true if novel planctomycete-like sublineages were detectable in the anoxic bulk soil but not on the rice roots from the flooded rice microcosms examined. Since the T-RFLP diversity patterns obtained from samples of different flooded rice microcosms were very similar (data not shown), a detailed analysis in which cloning of PCR products and comparative sequence analysis of cloned 16S rDNA were used was carried out for only one of the flooded rice microcosms.
Assessment of the target specificity of the PV assay.
The proposed group specificity of the PV assay for planctomycetes was based on forward primer PLA-40F (Table 1). The optimal thermal profile was adjusted by PCR amplification of the target stretch by using genomic DNA of P. marina, V. spinosum, and other bacterial reference organisms. When the optimized thermal profile was used, 16S rDNA of V. spinosum was targeted, while 16S rDNA with more than two mismatches in the target site of PLA-40F were not amplified. This observation indicated that in addition to planctomycete-like 16S rDNA, the 16S rDNA of members of the Chlamydia and Verrucomicrobia lineages might also be targeted by the PV assay in environmental studies.
T-RFLP diversity patterns.
Altogether, 12 distinct T-RFs were identified in the T-RFLP diversity patterns obtained from the rice roots, while 33 T-RFs were detected in the anoxic bulk soil (Fig. 1). The T-RFs obtained from the rice roots were present in the bulk soil T-RFLP diversity patterns either at clearly lower relative levels or not at all. This observation suggested that the microbial diversity detectable by the PV assay on rice roots was less complex than the diversity detectable in the anoxic bulk soil and also that different kinds of diversity were recovered from these two distinct locations. Comparative sequence analysis of 16 randomly selected rice root 16S rDNA clones allowed correlation of three T-RFs with phylogenetic information. These three T-RFs exhibited the highest signal intensities in the T-RFLP diversity patterns obtained from the rice roots (Fig. 1A and 2). Eight minor T-RFs remained unidentified. For the anoxic bulk soil partial sequencing of 38 randomly selected rDNA clones allowed correlation of 11 T-RFs with phylogenetic information. To obtain additional information about the bulk soil bacterial diversity targeted by the PV assay, we compared the soil T-RFLP diversity pattern with the defined T-RFs of 138 randomly selected 16S rDNA clones. Only those 16S rDNA clones whose T-RFs corresponded to one of the bulk soil T-RFs not identified by the random approach were analyzed further by comparative sequence analysis. This directed approach resulted in correlation of nine additional T-RFs with phylogenetic information. The 20 T-RFs identified accounted for 77% of the total signal intensity of the 33 T-RFs detected in the anoxic bulk soil. However, although 176 bulk soil rDNA clones were surveyed, 13 bulk soil T-RFs could not be linked with phylogenetic information. Thus, a considerable portion of the microbial diversity recovered by the PV assay remained unidentified. Conversely, the T-RFs of some of the 16S rDNA clone sequences analyzed in detail (see below) could not be assigned to any of the 12 and 33 T-RFs which showed relative abundance of more than 1% in the T-RFLP diversity patterns obtained from the rice roots and anoxic bulk soil, respectively (e.g., clones PBS-II-13, PBS-114, and PBS-71 [Fig. 2], as well as clone PRR-1D [Fig. 3]).
FIG. 1.
16S rDNA-based T-RFLP fingerprint patterns obtained with the PV assay by using total DNA extracted from rice roots (A) and anoxic bulk soil (B) of a flooded rice microcosm. The numbers indicate the sizes of the T-RFs which could be correlated with phylogenetic information (Fig. 2 and 3). T-RFs for which no correspondence to cloned 16S rDNA could be found are indicated by arrows.
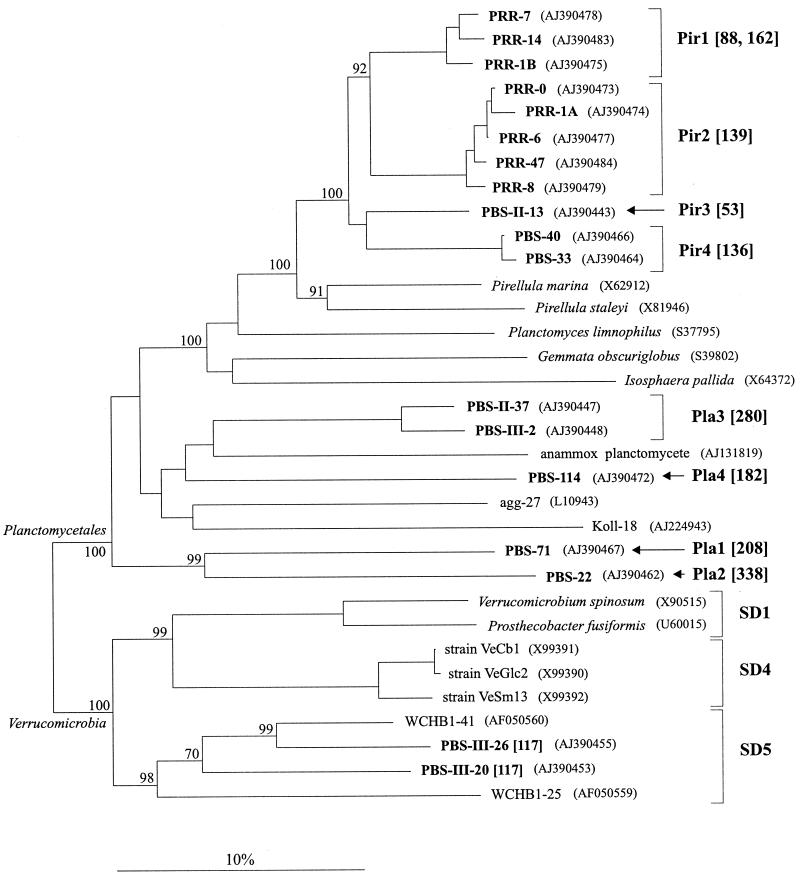
FIG. 2.
Evolutionary distance dendrogram showing the positions of environmental 16S rDNA clone sequences recovered from rice roots (PRR clones) and anoxic bulk soil (PBS clones) of a flooded rice microcosm in relation to representative members of the order Planctomycetales and the division Verrucomicrobia. In addition to previously described taxa, the reference sequences included the Verrucomicrobia-like ultramicrobacteria VeGlc2, VeCb1, and VeSm13 (19) and environmental 16S rDNA sequences retrieved from marine aggregates (agg-27) (5), a trickling filter biofilm (Koll-18; GenBank accession no. AJ224943), a highly enriched consortium capable of anaerobic ammonia oxidation (anammox planctomycete) (44), and the anaerobic zone of a contaminated aquifer (WCHB sequences) (6). The individual lineages are designated as follows: Pir1 to Pir4 (Pir = Pirellula-like); Pla1 to Pla4 (Pla = planctomycete-like); and SD1, SD4, and SD5 (SD = recently proposed subdivisions of the division Verrucomicrobia [18]). The dendrogram was reconstructed by using the neighbor-joining method (40) in combination with a 50% consensus filter for the domain Bacteria. The numbers at the nodes indicate the percentages of recovery in 1,000 bootstrap resamplings. Only relevant bootstrap values of 70% or more are shown. The sizes of the T-RFs (in base pairs) indicated in brackets for the PRR and PBS clone sequences correspond to sizes in the T-RFLP diversity patterns shown in Fig. 1. The EMBL, GenBank, and DDBJ accession numbers are given in parentheses. Scale bar = 10% estimated difference in nucleotide sequence positions.
FIG. 3.
Evolutionary distance dendrogram showing the positions of environmental 16S rDNA clone sequences recovered from rice roots (PRR clones) and anoxic bulk soil (PBS clones) of a flooded rice microcosm in relation to representative members of the Bacteria. In addition to previously described taxa, the reference sequences included environmental 16S rDNA sequences recovered from a hot spring in Yellowstone National Park (OPB-2) (17), a deep-sea sediment (BD4-9) (22), and the anaerobic zone of a contaminated aquifer (WCHA1-56) (6). The individual lineages are designated as follows: candidate divisions OP3 (17), WS3 (6), and BRC1 (= bacterial rice cluster); SP (= spirochete-like lineage); and NBL-UPA1 and NBL-UPA2 (NBL-UPA = novel bacterial lineage of uncertain phylogenetic affiliation). For WS3 and BRC1, the most divergent intralineage 16S rDNA clone sequences were used for the phylogenetic analysis. The dendrogram was reconstructed by using the neighbor-joining method (40) in combination with a 50% consensus filter for the domain Bacteria. The numbers at the nodes indicate the percentages of recovery of relevant branch points in 1,000 bootstrap resamplings. Branch points which were not supported by the confidence areas indicated around the nodes (bootstrap circle tool of the ARB program package) were at the division level collapsed back to the next significant branch point. For the most part such a lack of support corresponded to bootstrap values less than 50%. The root was determined by using archaeal 16S rRNA sequences as the outgroup reference. The sizes of the T-RFs (in base pairs) indicated in brackets for the PRR and PBS clone sequences correspond to the T-RFLP diversity patterns shown in Fig. 1. The EMBL, GenBank, and DDBJ accession numbers are given in parentheses. The only exception is the 16S rDNA sequence of Thermus thermarum LA3T, for which the identity codes in the Ribosomal Database Project (30) and the ARB software package (http://www.biol.chemie.tu-muenchen.de/pub/ARB/) are indicated. Scale bar = 10% estimated difference in nucleotide sequence positions.
Phylogenetic placement of 16S rDNA clone sequences recovered by the PV assay. (i) General comments.
Altogether, 12 and 36 16S rDNA clone sequences retrieved from the rice roots and anoxic bulk soil, respectively, were analyzed in detail by comparative sequence analysis. Between 1,100 and 1,400 nucleotide sequence positions were determined for each of these 16S rDNA clones; for most of them between 1,300 and 1,400 base pairs were analyzed. One of these 16S rDNA clone sequences was identified as a chimera (20, 23, 47), and thus this clone was excluded from further analysis. Using an overall level of 16S rDNA sequence dissimilarity to cultured bacteria of approximately 20% as a threshold value, 11 distinct lineages in the Bacteria were identified for which pure-culture representatives are not known. This threshold value was chosen because the typical level of interdivisional rRNA sequence difference is 20 to 25% (18). Unless indicated otherwise, all treeing analyses, including those performed by distance matrix, maximum-likelihood, and maximum-parsimony methods, produced consistent results with respect to the branching of these 11 lines of bacterial descent. Therefore, the following data and discussion are based mainly on the results obtained by the neighbor-joining analyses. Four of the 11 lineages could be assigned to the order Planctomycetales, while one lineage was affiliated with the Verrucomicrobia and one lineage was affiliated with the spirochetes (Fig. 2 and 3). The other five lineages either could not be assigned to any of the main lines of bacterial descent or clearly extended the currently known diversity of division level lineages WS3 and OP3 (Fig. 3).
(ii) 16S rDNA sequences recovered from oxic rice roots.
Of the 16S rDNA clone sequences randomly selected for analysis, 12 belonged to the planctomycetes and were related to members of the genus Pirellula. Phylogenetic placement of 8 of these 12 16S rDNA clones revealed two distinct clusters (Pir1 and Pir2 [Fig. 2]). The level of sequence dissimilarity between 16S rDNA clones belonging to these two clusters was 9%, while the two clusters were separated from cultured members of the genus Pirellula by levels of sequence dissimilarity of 14 to 16%. Sequence types belonging to Pir2 corresponded to the predominant 139-bp T-RF (Fig. 1A) and thus represented the major component of the 16S rDNA PCR products retrieved from the rice roots. The clearly minor importance of the 139-bp T-RF in the T-RFLP diversity patterns obtained from the bulk soil (Fig. 1B) may indicate that these Pirellula-like organisms can be considered typical root-associated bacteria.
One of the four 16S rDNA clone sequences which could not be assigned to the planctomycetes was clone PRR-1D. Independent of the treeing method used, this clone branched clearly distinctly from representatives of all known main lines of bacterial descent, including those hitherto represented only by environmental 16S rDNA. Based on the 1,105 nucleotide sequence positions determined, the lowest overall level of 16S rDNA sequence dissimilarity to cultured bacteria was 23%, which was obtained with representatives of different bacterial phyla, including Bacillus subtilis, Acidobacterium capsulatum, and Synechococcus elongatus. Thus, clone PRR-1D fulfilled the definition of a novel candidate division (17). However, this lineage is represented by only a single environmental 16S rDNA clone sequence. A reliable decision whether a bacterial clade may be considered a novel division level lineage is possible only when enough sequence types encompassing its phylogenetic diversity are available (27, 28). Therefore, instead of candidate division, we prefer to characterize this line as a “novel bacterial lineage of uncertain phylogenetic affiliation” (NBL-UPA1) (Fig. 3). The other three rice root 16S rDNA clones belonged to lineages WS3 and BRC1 (Fig. 3). These lineages are discussed below.
(iii) 16S rDNA sequences recovered from anoxic bulk soil.
Only 5 of the 20 identified bulk soil T-RFs could be assigned to planctomycete-like sequences. The other 15 T-RFs corresponded to members of the division Verrucomicrobia (3 T-RFs), to spirochetes (1 T-RF), or to lines of bacterial descent for which no pure-culture representatives are known (13 T-RFs). Some of the T-RFs corresponded to phylotypes belonging to more than one major group.
16S rDNA clones PBS-II-13, PBS-33, and PBS-40 (branches Pir3 and Pir4 [Fig. 2]) had a common interior node with the rice root 16S rDNA clones of branches Pir1 and Pir2. However, within the phylogenetic radiation of the genus Pirellula the 16S rDNA sequences retrieved from the bulk soil (PBS clones) were clearly distinct from the PRR clones of branches Pir1 and Pir2 (sequence dissimilarity, 9 to 11%) (Fig. 2).
In addition, five 16S rDNA clones which formed four distinct, deeply branching lineages within the planctomycetes were identified (Pla1 to Pla4 [Fig. 2]). Although lineages Pla1 to Pla4 were separated by overall levels of 16S rDNA dissimilarity to each other and to all cultured members of the planctomycetes of more than 20%, bootstrap values greater than 90% clearly supported assignment of these lineages to the Planctomycetales (Fig. 2). Based on the T-RFLP diversity patterns, we concluded that members of lineage Pla2, which corresponded to the 338-bp T-RF, were detectable only in the bulk soil. Members of lineage Pla3, corresponding to the 280-bp T-RF, seemed to be present as a minor component in the 16S rDNA PCR products obtained from rice roots (Fig. 1) (the relative levels of the T-RFs which corresponded to lineages Pla1 and Pla4 in the soil T-RFLP diversity patterns were below the 1% threshold). These data as a whole provide support for the existence of planctomycetes which are indigenous to rice paddy soil. This adds a new environment to the anoxic habitats in which novel major sublineages of planctomycetes have been detected. These habitats include an anaerobic bioreactor (14) and a consortium enriched from wastewater and capable of anaerobic ammonia oxidation (44). Moreover, Pirellula-like 16S rRNA has been retrieved from anoxic freshwater sediment (32), and the occurrence of planctomycetes in an anoxic zone of a wastewater treatment plant was shown by fluorescent in situ hybridization (34).
The planctomycete-like 16S rDNA sequences recovered from the anaerobic bioreactor and the ammonia-oxidizing consortium showed only distant relationships (dissimilarity values, 16 to 20 and more than 19.5%, respectively) to the PBS clones retrieved from the anoxic paddy soil (16S rDNA clones retrieved from the anoxic bioreactor were not included in the treeing analyses because of the limited sequence length of approximately 500 nucleotides) (Fig. 2). Similarly, the PBS clone sequences of lineages Pla1 to Pla4 branched in all treeing analyses clearly distinctly from the planctomycete-like 16S rDNA recovered from marine aggregates (clone agg-27) (5) and a trickling filter biofilm (clone Koll-18; GenBank accession no. AJ224943) (dissimilarity values, 19.6 to 28.1%) (Fig. 2). This distinctness clearly underlines the great intradivisional phylogenetic depth and diversity of the Planctomycetales.
The majority of the 16S rDNA sequences recovered by the PV assay belonged to bacterial lineages BRC1, WS3, and OP3 (Fig. 3). Lineages WS3 and OP3, represented previously by only one (WCHA1-56) or two (OPB-2, BD4-9) environmental 16S rDNA clone sequences, were proposed previously as candidate divisions (6, 17). The intracluster diversity of WS3 was characterized by three distinct branches (clones PBS-III-9 and WCHA1-56, clone PBS-II-5, and clones PRR-11 and PBS-III-4), which were separated by overall 16S rDNA sequence dissimilarity values of 16.5 to 17.5%. Similarly, the treeing analyses divided OP3 into three distinct sublineages (clones PBS-37 and PBS-87, clone OPB-2, and clone BD4-9 [Fig. 3]), which were separated at the subdivision level (i.e., by overall 16S rDNA sequence dissimilarity values of 20.2 to 24.1%). WS3 and OP3 branched in all treeing analyses clearly distinctly from the other main lines of bacterial descent, which provided support for considering these major lineages candidate divisions.
Furthermore, the PBS and PRR clones, which could be assigned to lineages WS3 and OP3, extended the kinds of environments in which members of these two candidate divisions have been detected. Clone WCHA1-56 has been recovered from the methanogenic zone of a contaminated aquifer (6), and thus the common characteristic of the known 16S rDNA sequence types belonging to WS3 is recovery from methanogenic environments. Detection of members of OP3 in sediment of a hot spring (clone OPB-2, from Obsidian Pool in Yellowstone National Park) (17), in deep-sea sediment (clone BD4-9) (22), and in rice paddy soil suggests wide geographical distribution of this division level clade and also the occurrence of its members in cold, moderate-temperature, and hot environments, presumably with anoxia as the common characteristic.
The 12 16S rDNA sequence types belonging to lineage BRC1 formed four distinct branches (Fig. 3), which were separated by overall 16S rDNA sequence dissimilarity values of 15.1 to 18.2%. Because of the range of levels of sequence dissimilarity between the BRC1 16S rDNA clones retrieved and because, like WS3 and OP3, this cluster branched in all treeing analyses clearly distinctly from the other main lines of bacterial descent, lineage BRC1 may be considered a novel candidate division.
The treeing analyses characterized clone PBS-25 as a novel bacterial lineage of uncertain phylogenetic affiliation (NBL-UPA2 [Fig. 3]). However, in some of the reconstructed trees NBL-UPA2 had a common interior branch point with OP3, which was supported by bootstrap values of 40 to 60%. In the future, availability of novel 16S rDNA sequence types belonging to OP3 might result in stable branching of NBL-UPA2 within the phylogenetic radiation of candidate division OP3. Finally, based on the set of reference sequences used to reconstruct the tree shown in Fig. 3, lineage SP (clones PBS-18 and PBS-21 [Fig. 3]) showed distinct affiliation with the spirochetes (bootstrap value, 93%), although the overall levels of 16S rDNA dissimilarity of the two PBS clones to spirochete representatives ranged from 23 to 27%. However, if Leptospira spp., which form a deeply branching sublineage of spirochetes, were included in the phylogenetic analysis, the bootstrap support for assignment of lineage SP to the spirochetes dropped to 40 to 60% (data not shown).
Concluding remarks.
In addition to the intended detection of novel planctomycete-like lineages, use of the PV assay led to unexpected recovery of distinct 16S rDNA sequence types which could not be assigned to the Planctomycetales or the Verrucomicrobia. As deduced from the T-RFLP diversity patterns, the bacterial diversity detected by the PV assay in the anoxic bulk soil was clearly different from that detectable on the rice roots. This allowed us to assume that a major portion of the novel bulk soil bacterial groups is highly adapted to anoxic soil conditions. Detection of these groups also indicates that signature nucleotides which have been considered indicative of planctomycetes, chlamydiae, and members of the Verrucomicrobia are more widely distributed among members of the domain Bacteria than previously thought. Additional support for this view is provided by the recently reported environmental 16S rDNA clone OPB-2 (17) (Fig. 3), which exhibits in the region of primer PLA-40F only one mismatch. Consequently, this raises some doubts about the value of these signature nucleotides as indicators for considering Planctomycetales, Chlamydia spp., and Verrucomicrobia bacterial sister clades, as originally proposed by Woese (52) and others (24, 50).
The discovery of novel bacterial diversity by the PV assay also raises the question of why members of the novel lineages have not been detected at all or have been detected very rarely by one of the various PCR-based environmental studies which used primers considered universal for 16S rDNA of Bacteria. For instance, none of 57 randomly selected 16S rDNA clones recovered from the same type of anoxic rice paddy soil by using universal primers 27F and 1492R (16) were affiliated with any of the bacterial lineages detected by the PV assay. Thus, either members of these novel lineages occur in the anoxic rice paddy soil at lower levels or their 16S rDNA might be subject to biased retrieval when universal primers are used (8, 33, 37–39, 46). One intriguing reason for biased retrieval might be mismatches in the target sites of the 16S rDNA primers considered universal for the domain Bacteria. This view is supported by a single base substitution in the region corresponding to bacterial primer 27F (21), which has been reported for planctomycetes (45) and some spirochetes (4, 36) among other organisms. Such mismatches in the target site could lead to competition with the 16S rDNA having perfectly matching target sites and to less efficient PCR amplification. Based on these considerations, it can be speculated that a wide range of novel bacterial diversity, which may be subject to this type of biased retrieval and thus (almost) undetectable by the so-called universal 16S rDNA PCR assays, still awaits detection.
ACKNOWLEDGMENTS
We thank Sonja Fleissner for excellent technical assistance.
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (SFB 395) to W.L.
REFERENCES
- 1.Amann R I, Ludwig W, Schleifer K H. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev. 1995;59:143–169. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker W, van den Broek A, Camon E, Hingamp P, Sterk P, Stoesser G, Tuli M A. The EMBL Nucleotide Sequence Database. Nucleic Acids Res. 2000;28:19–23. doi: 10.1093/nar/28.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brosius J, Palmer M L, Kennedy P J, Noller H R. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci USA. 1978;75:4801–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Defosse D L, Johnson R C, Paster B J, Dewhirst F E, Fraser G J. Brevinema andersonii gen. nov., sp. nov., an infectious spirochete isolated from the short-tailed shrew (Blarina brevicauda) and the white-footed mouse (Peromyscus leucopus) Int J Syst Bacteriol. 1995;45:78–84. doi: 10.1099/00207713-45-1-78. [DOI] [PubMed] [Google Scholar]
- 5.DeLong E F, Franks D G, Alldredge A L. Phylogenetic diversity of aggregate-attached vs. free-living marine bacterial assemblages. Limnol Oceanogr. 1993;38:924–934. [Google Scholar]
- 6.Dojka M A, Hugenholtz P, Haack S K, Pace N R. Microbial diversity in a hydrocarbon- and chlorinated-solvent-contaminated aquifer undergoing intrinsic bioremediation. Appl Environ Microbiol. 1998;64:3869–3877. doi: 10.1128/aem.64.10.3869-3877.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dojka M A, Harris J K, Pace N R. Expanding the known diversity and environmental distribution of an uncultured phylogenetic division of Bacteria. Appl Environ Microbiol. 2000;66:1617–1621. doi: 10.1128/aem.66.4.1617-1621.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farrelly V, Rainey F A, Stackebrandt E. Effect of genome size and rrn gene copy number on PCR amplification of 16S rRNA genes from a mixture of bacterial species. Appl Environ Microbiol. 1995;61:2798–2801. doi: 10.1128/aem.61.7.2798-2801.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Felsenstein J. PHYLIP—phylogeny inference package (version 3.2) Cladistics. 1989;5:164–166. [Google Scholar]
- 10.Finster K, Liesack W, Thamdrup B. Elemental sulfur and thiosulfate disproportionation by Desulfocapsa sulfoexigens sp. nov., a new anaerobic bacterium isolated from marine surface sediment. Appl Environ Microbiol. 1998;64:119–125. doi: 10.1128/aem.64.1.119-125.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frenzel P, Rothfuss F, Conrad R. Oxygen profiles and turnover in a flooded rice microcosm. Biol Fertil Soils. 1992;14:84–89. [Google Scholar]
- 12.Fuerst J A. The planctomycetes—emerging models for microbial ecology, evolution and cell biology. Microbiology. 1995;141:1493–1506. doi: 10.1099/13500872-141-7-1493. [DOI] [PubMed] [Google Scholar]
- 13.Giovannoni S J, Britschgi T B, Moyer C L, Field K G. Genetic diversity in Sargasso Sea North Atlantic Ocean bacterioplankton. Nature. 1990;345:60–63. doi: 10.1038/345060a0. [DOI] [PubMed] [Google Scholar]
- 14.Godon J J, Zumstein E, Dabert P, Habouzit F, Moletta R. Molecular microbial diversity of an anaerobic digestor as determined by small-subunit rDNA sequence analysis. Appl Environ Microbiol. 1997;63:2802–2813. doi: 10.1128/aem.63.7.2802-2813.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Großkopf R, Janssen P H, Liesack W. Diversity and structure of the methanogenic community in anoxic rice paddy soil microcosms as examined by cultivation and direct 16S rRNA sequence retrieval. Appl Environ Microbiol. 1998;64:960–969. doi: 10.1128/aem.64.3.960-969.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hengstmann U, Chin K-J, Janssen P H, Liesack W. Comparative phylogenetic assignment of environmental sequences of genes encoding 16S rRNA and numerically abundant culturable bacteria from an anoxic rice paddy soil. Appl Environ Microbiol. 1999;65:5050–5058. doi: 10.1128/aem.65.11.5050-5058.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hugenholtz P, Pitulle C, Hershberger K L, Pace N R. Novel division level bacterial diversity in a Yellowstone hot spring. J Bacteriol. 1998;180:366–376. doi: 10.1128/jb.180.2.366-376.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hugenholtz P, Goebel B M, Pace N R. Impact of culture-independent studies on the emerging phylogenetic view of bacterial diversity. J Bacteriol. 1998;180:4765–4774. doi: 10.1128/jb.180.18.4765-4774.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janssen P H, Schuhmann A, Mörschel E, Rainey F A. Novel anaerobic ultramicrobacteria belonging to the Verrucomicrobiales lineage of bacterial descent isolated by dilution culture from anoxic rice paddy soil. Appl Environ Microbiol. 1997;63:1382–1388. doi: 10.1128/aem.63.4.1382-1388.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kopczynski E D, Bateson M M, Ward D M. Recognition of chimeric small-subunit ribosomal DNAs composed of genes from uncultivated microorganisms. Appl Environ Microbiol. 1994;60:746–748. doi: 10.1128/aem.60.2.746-748.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lane D J. 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M, editors. Nucleic acid techniques in bacterial systematics. New York, N.Y: John Wiley & Sons, Inc.; 1991. pp. 115–175. [Google Scholar]
- 22.Li L N, Kato C, Horikoshi K. Bacterial diversity in deep-sea sediments from different depths. Biodivers Conserv. 1999;8:659–677. [Google Scholar]
- 23.Liesack W, Weyland H, Stackebrandt E. Potential risk of gene amplification by PCR as determined by 16S rDNA analysis of a mixed-culture of strict barophilic bacteria. Microb Ecol. 1991;21:191–198. doi: 10.1007/BF02539153. [DOI] [PubMed] [Google Scholar]
- 24.Liesack W, Stackebrandt E. Occurrence of novel groups of the domain Bacteria as revealed by analysis of genetic material isolated from an Australian terrestrial environment. J Bacteriol. 1992;174:5072–5078. doi: 10.1128/jb.174.15.5072-5078.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liesack W, Janssen P H, Rainey F A, Ward-Rainey N L, Stackebrandt E. Microbial diversity in soil: the need for a combined approach using molecular and cultivation techniques. In: van Elsas J D, Trevors J T, Wellington E M, editors. Modern soil microbiology. New York, N.Y: Marcel Dekker Inc.; 1997. pp. 375–439. [Google Scholar]
- 26.Liu W-T, Marsh T L, Cheng H, Forney L J. Characterization of microbial diversity by determining terminal restriction fragment length polymorphisms of genes encoding 16S rRNA. Appl Environ Microbiol. 1997;63:4516–4522. doi: 10.1128/aem.63.11.4516-4522.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ludwig W, Strunk O, Klugbauer S, Klugbauer N, Weizenegger M, Neumaier J, Bachleitner M, Schleifer K H. Bacterial phylogeny based on comparative sequence analysis. Electrophoresis. 1998;19:554–568. doi: 10.1002/elps.1150190416. [DOI] [PubMed] [Google Scholar]
- 28.Ludwig W, Schleifer K-H. Phylogeny of Bacteria beyond the 16S rRNA standard. ASM News. 1999;65:752–757. [Google Scholar]
- 29.Lukow T, Dunfield P F, Liesack W. Use of the T-RFLP technique to assess spatial and temporal changes in the bacterial community structure within an agricultural soil planted with transgenic and non-transgenic potato plants. FEMS Microbiol Ecol. 2000;32:241–247. doi: 10.1111/j.1574-6941.2000.tb00717.x. [DOI] [PubMed] [Google Scholar]
- 30.Maidak B L, Cole J R, Parker C T, Jr, Garrity G M, Larsen N, Li B, Lilburn T G, McCaughey M J, Olsen G J, Overbeek R, Pramanik S, Schmidt T M, Tiedje J M, Woese C R. A new version of the RDP (Ribosomal Database Project) Nucleic Acids Res. 1999;27:171–173. doi: 10.1093/nar/27.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mayrand P E, Koran K P, Ziegle J S, Robertson J M, Hoff L B, Kroninck M N. The use of fluorescence detection and internal lane standards to size PCR products automatically. Appl Theor Electrophor. 1992;3:1–11. [PubMed] [Google Scholar]
- 32.Miskin I P, Farrimond P, Head I M. Identification of novel bacterial lineages as active members of microbial populations in a freshwater sediment using a rapid RNA extraction protocol and RT-PCR. Microbiology. 1999;145:1977–1987. doi: 10.1099/13500872-145-8-1977. [DOI] [PubMed] [Google Scholar]
- 33.Morrison C, Gannon F. The impact of PCR plateau phase on quantitative PCR. Biochim Biophys Acta. 1994;1219:493–498. doi: 10.1016/0167-4781(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 34.Neef A, Amann R, Schlesner H, Schleifer K H. Monitoring a widespread bacterial group: in situ detection of planctomycetes with 16S rRNA-targeted probes. Microbiology. 1998;144:3257–3266. doi: 10.1099/00221287-144-12-3257. [DOI] [PubMed] [Google Scholar]
- 35.Pace N R. A molecular view of microbial diversity and the biosphere. Science. 1997;276:734–740. doi: 10.1126/science.276.5313.734. [DOI] [PubMed] [Google Scholar]
- 36.Paster B J, Dewhirst F E, Weisburg W G, Tordoff L A, Fraser G J, Hespell R B, Stanton T B, Zablen L, Mandelco L, Woese C R. Phylogenetic analysis of the spirochetes. J Bacteriol. 1991;173:6106–6109. doi: 10.1128/jb.173.19.6101-6109.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raeymaekers L. Quantitative PCR: theoretical considerations with practical implications. Anal Biochem. 1993;214:582–585. doi: 10.1006/abio.1993.1542. [DOI] [PubMed] [Google Scholar]
- 38.Raeymaekers L. A commentary on the practical applications of competitive PCR. Genome Res. 1995;5:91–94. doi: 10.1101/gr.5.1.91. [DOI] [PubMed] [Google Scholar]
- 39.Reysenbach A L, Giver L J, Wickham G S, Pace N R. Differential amplification of rRNA genes by polymerase chain reaction. Appl Environ Microbiol. 1992;58:3417–3418. doi: 10.1128/aem.58.10.3417-3418.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saitou N, Nei M. The neighbor-joining method: a new method for reconstruction of phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 41.Schlesner H. Pirellula marina sp. nov., a budding, peptidoglycanless bacterium from brackish water. Syst Appl Microbiol. 1986;8:177–180. [Google Scholar]
- 42.Schlesner H. The development of media suitable for the microorganisms morphologically resembling Planctomyces spp., Pirellula spp., and other Planctomycetales from various aquatic habitats using dilute media. Syst Appl Microbiol. 1994;17:135–145. [Google Scholar]
- 43.Staley J T, Fuerst J A, Giovannoni S, Schlesner H. The order Planctomycetales and the genera Planctomyces, Pirellula, Gemmata, and Isosphaera. In: Balows A, Dworkin M, Harder W, Schleifer K H, Trüper H G, editors. The prokaryotes. New York, N.Y: Springer Verlag; 1992. pp. 3710–3731. [Google Scholar]
- 44.Strous M, Fuerst J A, Kramer E H M, Logemann S, Muyzer G, van de Pas Schoonen K T, Webb R, Kuenen J G, Jetten M S M. Missing lithotroph identified as new planctomycete. Nature. 1999;400:446–449. doi: 10.1038/22749. [DOI] [PubMed] [Google Scholar]
- 45.Vergin K L, Urbach E, Stein J L, DeLong E F, Lanoil B D, Giovannoni S J. Screening of a fosmid library of marine environmental genomic DNA fragments reveals four clones related to members of the order planctomycetales. Appl Environ Microbiol. 1998;64:3075–3078. doi: 10.1128/aem.64.8.3075-3078.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.von Wintzingerode F, Göbel U, Stackebrandt E. Determination of microbial diversity in environmental samples: pitfalls of PCR-based rRNA analysis. FEMS Microbiol Rev. 1997;21:213–229. doi: 10.1111/j.1574-6976.1997.tb00351.x. [DOI] [PubMed] [Google Scholar]
- 47.Wang G C Y, Wang Y. The frequency of chimeric molecules as a consequence of PCR co-amplification of 16S rRNA genes from different bacterial species. Microbiology. 1996;142:1107–1114. doi: 10.1099/13500872-142-5-1107. [DOI] [PubMed] [Google Scholar]
- 48.Ward D M, Bateson M M, Weller R, Ruff-Roberts A L. Ribosomal RNA analysis of microorganisms as they occur in nature. Adv Microb Ecol. 1992;12:219–286. [Google Scholar]
- 49.Ward N, Rainey F A, Goebel B, Stackebrandt E. Identifying and culturing the ‘unculturables’: a challenge for microbiologists. In: Allsopp D, Colwell R R, Hawksworth D L, editors. Microbial diversity and ecosystem function. Wallingford, Oxon, United Kingdom: CAB International; 1995. pp. 89–110. [Google Scholar]
- 50.Ward-Rainey N, Rainey F A, Schlesner H, Stackebrandt E. Assignment of hitherto unidentified 16S rDNA species to a main line of descent within the domain Bacteria. Microbiology. 1995;141:3247–3250. [Google Scholar]
- 51.Weisburg W G, Barns S M, Pelletier D A, Lane D J. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:679–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woese C R. Bacterial evolution. Microbiol Rev. 1987;51:221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zarda B, Hahn D, Chatzinotas A, Schönhuber W, Neef A, Amann R I, Zeyer J. Analysis of bacterial community structure in bulk soil by in situ hybridization. Arch Microbiol. 1997;168:185–192. [Google Scholar]
- 54.Ziegle J S, Su Y, Corcoran K P, Nie L, Mayrand P E, Hoff L B, McBridge L J, Kroninck M N, Diehl S R. Application of automated DNA sizing technology for genotyping microsatellite loci. Genomics. 1992;14:1026–1031. doi: 10.1016/s0888-7543(05)80126-0. [DOI] [PubMed] [Google Scholar]