Trial registration
GA trial is registered under EudraCT#: 2013-001639-38.
Subject terms: Myelodysplastic syndrome, Phase III trials
Dear Editor,
Myelodysplastic syndromes (MDS) emerge as a disorder of stem cell differentiation and maturation, resulting in peripheral cytopenias and eventual progression to acute myeloid leukemia (AML). Numerous clonal genetic abnormalities together with varying degrees of cytopenias and myeloblast (MB) accumulation are the basis for the revised International Prognostic Scoring System (IPSS-R) [1]. Gradual disease progression worsens survival and is an indication for starting treatment with hypomethylating agents (HMAs) such as 5-azacytidine (AZA) or decitabine, in some cases as a bridge to transplantation or as continuous therapy until failure for patients who are not transplant candidates. Compared to conventional chemotherapy, AZA treatment prolongs survival in both higher-risk MDS and oligoblastic (20–30%) MDS/AML (24.5 vs 16 months) [2–4]. AZA induces more sustained hematologic responses, but does not lead to durable remissions and most patients eventually progress and fail therapy. To improve efficacy, new agents such as Venetoclax [5, 6], Pevonedistat [7] or Panobinostat [8] have been tested in combination with the standard AZA regimen, while others (Sabatolimab, Magrolimab, IDH1/IDH2 inhibitors) are being tested. G-CSF (granulocyte colony stimulating factor) activates myeloid gene transcription in stem cells if added prior to HMA [9, 10]. G-CSF is used in MDS for neutropenic complications. In older pre-treated patients with breast cancer or non-Hodgkin’s lymphoma, filgrastim administration is a risk for MDS development [11, 12]. G-CSF may act on MDS cells by activating their cell cycle and differentiation, leading to selection against G-CSF receptor signaling [13]. Our clinical retrospective data of 162 HR-MDS patients treated with AZA associated higher G-CSF consumption (N = 35) with a lower incidence of grade (Gr) 4 neutropenia, and consequently longer overall survival (OS, median 27.4 vs 18 Mo, p = 0.017, Supplementary Material SM1). We investigated the effect of G-CSF on AZA efficacy by academic prospective randomized trial (SM2).
A total of 76 patients with high risk MDS and MDS/AML bellow 30% myeloblasts ineligible for transplantation were enrolled in the GA study from February 6, 2017 to December 31, 2021. Patients were randomized into arm A (AZA monotherapy) and arm GA (G-CSF + AZA). Study objectives included response rate, OS, progression-free survival (PFS), duration of response and safety of administration (detailed in SM2-3). Three patients died early and were neither randomized nor started therapy and 3 randomized patients into arm A died during the first AZA cycle, thus 70 patients in GA (N = 39) and A (N = 31) arms were analyzed. The median age and male to female ratio in the GA arm were 73 years and 23:16 (59% males) versus 74 years and 15:16 (48%) in arm A. The data of the cohort tested including subtypes and IPSS-R are presented in SM4-5. Statistics is described in SM6-7. Patients in both arms had comparable hematology findings (SM5), but they were not perfectly balanced; for example, arm A had more bone marrow blasts and arm GA had a higher proportion of patients with t-MDS. Patients with EB2 and MDS/AML had a slightly higher mutational burden compared to other high-risk patients with MB counts below 10% (SM8-9).
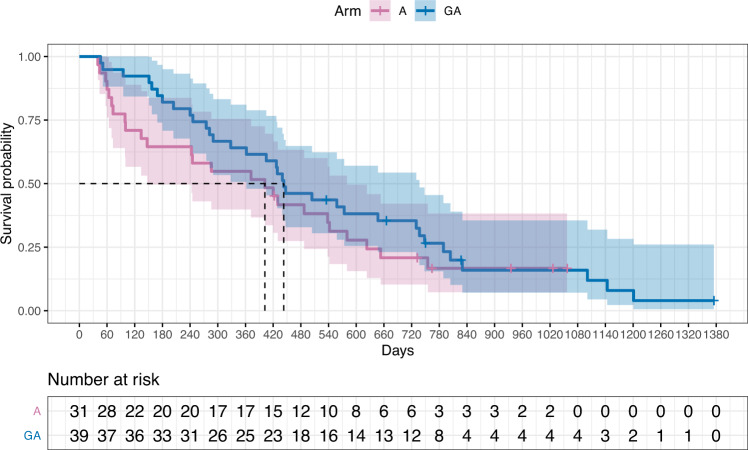
AZA administration was standard 7-day (75 mg/m2, SM2), G-CSF was administered 2 days prior the 1st dose of AZA and 2 days prior the 6th dose of AZA (SM2) at dose 5 μg/kg of body weight. The median number of AZA cycles was 8 (range 1–40). The efficacy of G-CSF administration was verified using the CD64 biomarker [14] on granulocytes (SM10) and by measuring plasma G-CSF levels (SM11) after the first cycle of therapy. OS and therapeutic response between the arms were assessed at multiple time points using longitudinal multivariate data analysis and a Joint model including time-constant (sex, input DNA variants, NGS analysis described in SM12) and time-varying (laboratory data) parameters. The Cox proportional hazards model containing time-varying covariates together with the ordinal multilevel logistic mixed model provide a plausible statistical framework for the aforementioned evaluation (Table 1). Although the Kaplan–Meier plot is crossed between the arms at the end of follow-up in terms of OS (Fig. 1), this view involves only univariate empirical analysis. For the designed arms, the median OS times are 443 days (14.8 months) in the GA arm and 402 days (13.4 months) in the A arm (95% CI: [362,737] and [147,580] days, respectively) (p = 0.300, Cochran-Mantel-Haenszel logrank test). However, there are confounding effects of G-CSF injections in particular, which stem from the fact that patients in both arms could receive G-CSF for ethical reasons in the event of febrile neutropenia (SM13). Thus, there are also patients in arm A who received G-CSF (N = 6, 19%). In addition, there is a trend towards more frequent use of G-CSF upon HMA failure in Year 2, and the difference between arms in terms of the number of G-CSF injections equalizes from a 4:1 to a 2:1 ratio. Thus, patients in the GA arm have a lower risk of death and GA treatment significantly prolongs OS (p = 0.0297). In contrast, for arm A, the risk of death is higher up to approximately 13 cycles of therapy, where the quadratic parabola of the relationship with G-CSF applications reaches its extreme. Such a declining-rising effect of the number of G-CSF cycles on survival is depicted in SM14. After roughly one year of HMA, when there is a gradual failure of therapy and an increase in infectious complications, G-CSF is a rather neutral parameter for survival. In addition to G-CSF, detected DNA variants also influence OS: negative predictors are DNMT3A mutations (p = 0.0131), ETV6 (p = 0.0012), EZH2 (p = 0.0044), positive: SF3B1 (p = 0.0005). Male patients tend to have a longer OS (p = 0.0041) while Gr4 neutropenia indicates a shorter OS (p = 0.0229). Predicted survival curves include SM15-16.
Table 1.
Fitted joint model for the overall survival on the GA vs A and the response to the G-CSF therapy of the GA vs A arm.
| Coefficient | SE | 95% CI for coefficient | Hazard ratioa/Odds ratiob | 95% CI for HR/OR | P value | |
|---|---|---|---|---|---|---|
| Cox PH model for OS timea | Hazard ratio | Score (logrank overall) test < 0.0001 | ||||
| Arm (GA vs A) | −0.4516 | 0.2078 | −0.8589, −0.0444 | 0.6366 | 0.4236, 0.9566 | 0.0297 |
| Number of G-CSF cycles (1 cycle increase) | 0.0885 | 0.0395 | 0.0110, 0.1660 | 1.0926 | 1.0111, 1.1806 | 0.0252 |
| (Number of G-CSF cycles)^2 | −0.0033 | 0.0013 | −0.0057, −0.0008 | 0.9967 | 0.9943, 0.9992 | 0.0100 |
| Gender (Male vs Female) | −0.5944 | 0.2073 | −1.0007, −0.1880 | 0.5519 | 0.3676, 0.8286 | 0.0041 |
| Neutropenia Gr4 in 4 cycles (Yes vs No) | 0.5639 | 0.2479 | 0.0780, 1.0498 | 1.7575 | 1.0811, 2.8570 | 0.0229 |
| DNMT3A (Mutated vs Unmutated) | 0.6185 | 0.2492 | 0.1299, 1.1069 | 1.8561 | 1.1388, 3.0251 | 0.0131 |
| ETV6 (Mutated vs Unmutated) | 1.2855 | 0.3961 | 0.5091, 2.0618 | 3.6164 | 1.6638, 7.8605 | 0.0012 |
| EZH2 (Mutated vs Unmutated) | 1.4236 | 0.5004 | 0.4429, 2.4043 | 4.1519 | 1.5572, 11.0701 | 0.0044 |
| SF3B1 (Mutated vs Unmutated) | −1.8870 | 0.5398 | −2.9450, −0.8290 | 0.1515 | 0.0526, 0.4365 | 0.0005 |
| Ordinal multivariate logistic mixed model for response to the therapyb | Odds ratio | Likelihood ratio (overall) test < 0.0001 | ||||
| Arm (GA vs A) | −1.3744 | 0.4139 | −2.1858, −0.5631 | 0.2530 | 0.1124, 0.5694 | 0.0009 |
| G-CSF injections / 4-cycle (1 inj. increase) | 0.3443 | 0.0659 | 0.2152, 0.4734 | 1.4110 | 1.2401, 1.6050 | <0.0001 |
| (Number of G-CSF inj. per 4-cycle)^2 | −0.0085 | 0.0022 | −0.0128, −0.0043 | 0.9915 | 0.9873, 0.9957 | <0.0001 |
| MB% PB | −0.1501 | 0.0445 | −0.2373, −0.0629 | 0.8606 | 0.7888, 0.9391 | 0.0007 |
| PLT | 0.0499 | 0.0087 | 0.0329, 0.0670 | 1.0513 | 1.0335, 1.0690 | <0.0001 |
| HB | 0.0073 | 0.0018 | 0.0038, 0.0108 | 1.0073 | 1.0038, 1.0110 | <0.0001 |
P values in bold (far right)
SE indicates standard error, CI confidence interval.
aA positive (negative) coefficient estimate in the time-varying Cox PH model indicates a higher (lower) risk of death and therefore a shorter (longer) OS.
bA positive coefficient estimate in the ordinal multivariate logistic mixed model indicates a remission response to treatment rather than progression.
Fig. 1. Kaplan–Meier plot.
Survival probability versus time (in days) for both treatment arms (GA vs A) of the clinical trial.
Response to treatment was assessed according to IWG criteria [15] (SM17, Table1). Overall response rate (ORR, GA vs A) was 77% vs 61% (p = 0.000899), CR 31% vs 23% (p = 0.575), PR 23% vs 23% (p = 0.554), SD with HI 18% vs 0% (p = 0.473), SD without HI 8% vs 13% (p = 0.739). Progression-free survival (PFS, GA vs A) was 9.7 vs 6.1 months (95% CI: [254,831] and [64,208] days, respectively) (p = 0.09, Cochran-Mantel-Haenszel logrank test). When in the first four cycles of AZA, many patients belonging to the GA arm responded with CR/PR/HI (N = 28, 72%), whereas twice as few in the A arm did (N = 14, 45%). This is particularly important for those patients, who are at increased risk of infectious and other complications associated with cytopenias during the initial cycles of AZA; on the other hand, achieving a better response gives the chance of a longer OS. Hemoglobin (HB) and platelets (PLT) have a positive expected effect on treatment response (p < 0.0001 for each), while peripheral blood MBs have a negative expected effect (p = 0.0007, SM18).
One very important parameter in this study was the rate of progression to AML during therapy in relation to G-CSF administration (SM19). Progression to AML was comparable as observed in 20 patients in the GA arm (52%) vs. 21 patients in the A arm (68%) (p = 0.968). Time to progression to AML was also comparable: 9.8 months in the GA arm vs. 8.9 months in the A arm (p = 0.450). This was comparably observed in both arms at each restaging (SM17). Thus, we found no effect of the addition of G-CSF on progression to AML throughout the HMA treatment period. There was also no difference between the arms in terms of treatment toxicity assessment (SM20). Regarding infectious complications, infection-related mortality in the GA arm during the first 4 cycles of therapy was lower compared to A arm (8 vs 29%, see SM21).
Clinical testing of G-CSF therapy, inspired by preclinical effects prior to the use of HMA [9, 10] has shown that G-CSF prior AZA is useful in the very early stages of therapy by inducing more durable responses and thus avoiding complications associated with cytopenia, and secondly by allowing the administration of AZA in the introduction at full dose and without prolonging the intervals between treatments, which is often caused by infectious complications. Both arms have experienced therapeutic failure of AZA at later time points comparably and thus the use of G-CSF is unable to prevent therapeutic failure of AZA. Interestingly, responses in the GA arm occurred relatively early in the first four cycles of AZA (31 in GA vs 18 in A; Fisher exact probability test gives p = 0.0260), which was not observed in the A arm, where a significant proportion of patients died due to infectious complications. Furthermore, the time to response is significantly lower in the GA arm compared to the A arm (p = 0.00184). Moreover, the positive effect of G-CSF is reinforced by the fact that the presence of Gr4 neutropenia is associated with significantly shorter OS (SM15-16).
Our primary objective of increasing treatment response and survival in the GA versus A arm was confirmed, particularly in patients with initial neutropenia in the first year of HMA treatment. We did not detect an effect of G-CSF on progression to AML, which is also significant. Thus, the administration of G-CSF prior to AZA represents an improvement to the standard AZA regimen in patients with high-risk MDS and oligoblastic AML.
Supplementary information
Acknowledgements
StopkaLab has been funded by Ministry of Health (AZV: NU21-08-00312, NU22-05-00374), EXCELES (National Institute for Cancer Research, LX22NPO5102 - Next Generation EU), and Charles University (UNCE/MED/016, ProgresQ26, SVV260521). MP was financed by GAČR 21-13323 S. MK and ZZ were supported by General Hospital Fund # RVO-VFN-64165. We appreciate kind help of Dr. G. Kislik in determining level of G-CSF in patient sera, and of Dr. Adel Schaffartzik who helped with NGS (all BIOCEV). We thank Drs. E.Cmunt, M. Šišková, J. Straub, E. Kolešková and H. Poláčková (all from General Faculty Hospital, Prague) for clinical care for patients.
Author contributions
TS study design, patient care, data review, writing, LM NGS and data review, ND patient data recording and CRF, VK NGS analysis, MP statistics, MŠ flow cytometry, ZZ cytogenetics, MK NGS, AJ study design, patient care, data review. Authors met all four criteria for authorship in the ICMJE Recommendations. TS, AJ, LM & ND verified the underlying data.
Data availability
Original data and protocols are available to other investigators upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Tomáš Stopka, Lubomír Minařík.
Contributor Information
Tomáš Stopka, Email: tstopka@lf1.cuni.cz.
Anna Jonášová, Email: Anna.Jonasova@vfn.cz.
Supplementary information
The online version contains supplementary material available at 10.1038/s41408-022-00698-2.
References
- 1.Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Sole F, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454–65. doi: 10.1182/blood-2012-03-420489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fenaux P, Gattermann N, Seymour JF, Hellstrom-Lindberg E, Mufti GJ, Duehrsen U, et al. Prolonged survival with improved tolerability in higher-risk myelodysplastic syndromes: azacitidine compared with low dose ara-C. Br J Haematol. 2010;149:244–9. doi: 10.1111/j.1365-2141.2010.08082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–32. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Gattermann N, Germing U, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010;28:562–9. doi: 10.1200/JCO.2009.23.8329. [DOI] [PubMed] [Google Scholar]
- 5.Garcia JS, Wei AH, Borate U, Fong CY, Baer MR, Nolte F, et al. Safety, efficacy, and patient-reported outcomes of venetoclax in combination with azacitidine for the treatment of patients with higher-risk myelodysplastic syndrome: a phase 1b study. Abstract #656 Presented at the 2020 ASH Annual Meeting 2020.
- 6.Zeidan AM, Gracia JS, Fenaux P, Platzbecker U, Miyazaki Y, Xiao Z, et al. Phase 3 VERONA study of venetoclax with azacitidine to assess change in complete remission and overall survival in treatment-naïve higher-risk myelodysplastic syndromes. Meeting Abstract. 2021 ASCO Annual Meeting I2021.
- 7.Sekeres MA, Watts J, Radinoff A, Sangerman MA, Cerrano M, Lopez PF, et al. Randomized phase 2 trial of pevonedistat plus azacitidine versus azacitidine for higher-risk MDS/CMML or low-blast AML. Leukemia. 2021. [DOI] [PMC free article] [PubMed]
- 8.Garcia-Manero G, Sekeres MA, Egyed M, Breccia M, Graux C, Cavenagh JD, et al. A phase 1b/2b multicenter study of oral panobinostat plus azacitidine in adults with MDS, CMML or AML with 30% blasts. Leukemia. 2017;31:2799–806.. doi: 10.1038/leu.2017.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu Z, Negrotto S, Gu X, Mahfouz R, Ng KP, Ebrahem Q, et al. Decitabine maintains hematopoietic precursor self-renewal by preventing repression of stem cell genes by a differentiation-inducing stimulus. Mol Cancer Ther. 2010;9:1536–43. doi: 10.1158/1535-7163.MCT-10-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curik N, Burda P, Vargova K, Pospisil V, Belickova M, Vlckova P, et al. 5-azacitidine in aggressive myelodysplastic syndromes regulates chromatin structure at PU.1 gene and cell differentiation capacity. Leukemia. 2012;26:1804–11. doi: 10.1038/leu.2012.47. [DOI] [PubMed] [Google Scholar]
- 11.Calip GS, Moran KM, Sweiss KI, Patel PR, Wu Z, Adimadhyam S, et al. Myelodysplastic syndrome and acute myeloid leukemia after receipt of granulocyte colony-stimulating factors in older patients with non-Hodgkin lymphoma. Cancer. 2019;125:1143–54.. doi: 10.1002/cncr.31914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calip GS, Malmgren JA, Lee WJ, Schwartz SM, Kaplan HG. Myelodysplastic syndrome and acute myeloid leukemia following adjuvant chemotherapy with and without granulocyte colony-stimulating factors for breast cancer. Breast Cancer Res Treat. 2015;154:133–43. doi: 10.1007/s10549-015-3590-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sapra A, Jaksik R, Mehta H, Biesiadny S, Kimmel M, Corey SJ. Effect of the unfolded protein response and oxidative stress on mutagenesis in CSF3R: a model for evolution of severe congenital neutropenia to myelodysplastic syndrome/acute myeloid leukemia. Mutagenesis. 2020;35:381–9. doi: 10.1093/mutage/geaa027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kakinoki Y, Kubota H, Yamamoto Y. CD64 surface expression on neutrophils and monocytes is significantly up-regulated after stimulation with granulocyte colony-stimulating factor during CHOP chemotherapy for patients with non-Hodgkin’s lymphoma. Int J Hematol. 2004;79:55–62. doi: 10.1007/BF02983535. [DOI] [PubMed] [Google Scholar]
- 15.Cheson BD, Greenberg PL, Bennett JM, Lowenberg B, Wijermans PW, Nimer SD, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108:419–25. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Original data and protocols are available to other investigators upon request.