Abstract
Both type 2 diabetes and immune-mediated inflammatory diseases (IMIDs), such as Crohn’s disease (CD), ulcerative colitis, rheumatoid arthritis (RA), ankylosing spondylitis (AS), and psoriasis (PsO) are risk factors of cardiovascular disease. Whether presence of IMIDs in patients with type 2 diabetes increases their cardiovascular risk remains unclear. We aimed to investigate the risk of cardiovascular morbidity and mortality in patients with type 2 diabetes and IMIDs. Patients with type 2 diabetes without cardiovascular disease were retrospectively enrolled from nationwide data provided by the Korean National Health Insurance Service. The primary outcome was cardiovascular mortality, and the secondary outcomes were myocardial infarction (MI), stroke, and all-cause mortality. Inverse probability of treatment weighting (IPTW)-adjusted Cox proportional hazard regression analysis was performed to estimate the hazard ratios (HRs) and 95% confidence intervals (95% CIs) for each IMID. Overall 2,263,853 patients with type 2 diabetes were analyzed. CD was associated with a significantly higher risk of stroke (IPTW-adjusted HR: 1.877 [95%CI 1.046, 3.367]). UC was associated with a significantly higher risk of MI (1.462 [1.051, 2.032]). RA was associated with a significantly higher risk of cardiovascular mortality (2.156 [1.769, 2.627]), MI (1.958 [1.683, 2.278]), stroke (1.605 [1.396, 1.845]), and all-cause mortality (2.013 [1.849, 2.192]). AS was associated with a significantly higher risk of MI (1.624 [1.164, 2.266]), stroke (2.266 [1.782, 2.882]), and all-cause mortality (1.344 [1.089, 1.658]). PsO was associated with a significantly higher risk of MI (1.146 [1.055, 1.246]), stroke (1.123 [1.046, 1.205]) and all-cause mortality (1.115 [1.062, 1.171]). In patients with type 2 diabetes, concomitant IMIDs increase the risk of cardiovascular morbidity and mortality. Vigilant surveillance for cardiovascular disease is needed in patients with type 2 diabetes and IMIDs.
Subject terms: Medical research, Outcomes research, Type 2 diabetes
Introduction
Type 2 diabetes, a metabolic disease with an increasing global prevalence, is associated with an increased risk of cardiovascular disease1–3. Importantly, cardiovascular disease is a major cause of increased mortality in patients with type 2 diabetes4,5. Accurate cardiovascular risk stratification in these patients may result in stricter monitoring and better outcomes in high-risk patients6.
Immune-mediated inflammatory diseases (IMIDs) are a heterogeneous group of chronic inflammatory diseases that affect the inner barrier (gut and joints) and/or outer barrier (skin) of the body7. Crohn’s disease (CD) and ulcerative colitis (UC) are IMIDs that mainly affect the gut, rheumatoid arthritis (RA) and ankylosing spondylitis (AS) are IMIDs that mainly affect the joints, and psoriasis (PsO) is an IMID that mainly affects the skin8–12. A large body of evidence suggests that IMIDs are associated with an increased risk of cardiovascular disease13–16. A population-based study has demonstrated that inflammatory bowel disease (IBD, i.e., CD and UC) confers a higher risk of myocardial infarction (MI) (odds ratio [OR] 1.25)13. Similarly, a higher risk of cardiovascular disease has also been reported in IMIDs that mainly affect the joints; the reported pooled relative risk for cardiovascular disease was 1.48 in patients with RA14, and the OR for MI was 1.6 in patients with AS15. A higher risk of MI (OR 1.25) was also observed in patients with PsO16.
Although IMIDs are well-known to increase the risk of cardiovascular disease in the general population13–16, it remains unclear whether the presence of IMIDs also increases the risk of cardiovascular disease in patients with type 2 diabetes who are already at a higher risk of cardiovascular disease. A comprehensive understanding of the influence of IMIDs on the cardiovascular disease in patients with type 2 diabetes can aid in better cardiovascular risk stratification in these patients. Considering that the prevalence of IMIDs is relatively low (approximately 3%)5, a population-based study is needed to sufficiently assess the influence of IMIDs on the risk of cardiovascular disease in patients with type 2 diabetes. In this study, we used nationwide population data to evaluate the risk of cardiovascular morbidity and mortality in patients with type 2 diabetes and IMIDs.
Methods
Data source
The nationwide population data from the Korean National Health Insurance Service (NHIS) claims database were used in this study. The NHIS provides comprehensive data that includes the demographics, socioeconomic status, medical treatments and procedures, disease diagnoses according to the International Classification of Diseases, Tenth Revision (ICD-10), and rare intractable disease (RID) registration information17. In the Korean RID system, a diagnosis is based on the uniform diagnostic criteria provided by the NHI and is carefully reviewed by the corresponding healthcare institution as well as the NHI before registration. The profile of the data source has been described previously18. All participants registered in the NHIS database are advised to undergo national health check-ups every 2 years. The categories of health check-up data include the anthropometric data, blood pressure, and laboratory data, such as serum fasting glucose levels, cholesterol levels, and creatinine levels. Using standardized self-reporting questionnaires, data regarding previous medical history and lifestyle factors, including smoking, alcohol consumption, and physical activity, were collected. This study was approved by the Institutional Review Board (IRB) of Gangnam Severance Hospital (IRB No: 3-2020-0269). Owing to the retrospective nature of this study, the requirement for informed consent was waived and approved by the IRB of Gangnam Severance Hospital. The study was performed in accordance with the Declaration of Helsinki, the relevant guidelines and regulations.
Study cohort
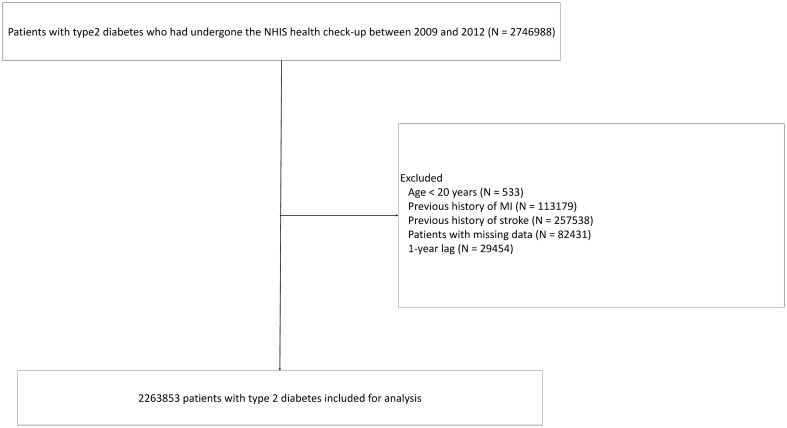
We initially selected 2,746,988 patients with type 2 diabetes who underwent the NHIS health check-up between January 1, 2009 and December 31, 2012. Patients with type 2 diabetes were defined as those with ICD-10 codes E11–14 and at least one annual claim of a prescription of anti-diabetic medications19. The following patients were excluded (Fig. 1): (i) younger than 20 years (n = 533); (ii) previous history of MI (n = 113,179); (iii) previous history of stroke (n = 257,538); (iv) missing data regarding any of the following: regular physical activity (n = 1364), height (n = 713), weight (1160), body mass index (n = 1207), systolic blood pressure (n = 677), diastolic blood pressure (n = 683), proteinuria (n = 13,511), hemoglobin (n = 688), fasting glucose (n = 481), total cholesterol (n = 464), aspartate aminotransferase (n = 474), alanine aminotransferase (n = 504), gamma-glutamyl transferase (n = 488), waist circumference (n = 977), triglyceride (n = 573), high density lipoprotein cholesterol (n = 624), low density lipoprotein cholesterol (n = 31,339), creatinine (n = 517), smoking (n = 8600), and alcohol consumption (n = 23,876); and (v) MI, stroke, or death occurred within 1 year from the baseline (i.e., the date of the first health check-up between January 1, 2009 and December 31, 2012) (n = 29,454). The remaining 2,263,853 patients with type 2 diabetes were included in the analysis (Fig. 1).
Figure 1.
Flowchart depicting inclusion of patients. NHIS, National Health Insurance Service; MI, myocardial infarction.
Patients were classified according to the presence of each IMID (CD, UC, RA, AS, and PsO). The prevalence of each IMID and its effect on cardiovascular morbidity and mortality were assessed. Patients were followed up from the baseline till the date of a cardiovascular event, death, or December 31, 2020, whichever was earlier.
Definition of IMIDs, comorbidities, and study outcomes
CD was defined as ICD-10 code K50 with RID code V13020,21; UC was defined as ICD-10 code K51 with RID code V13120,21; RA was defined as ICD-10 code M05 with RID code V223; AS was defined as ICD-10 code M45 with RID code V14022; and PsO was defined as ICD-10 code L4023. Baseline comorbidities were defined as follows: hypertension was defined as ICD-10 codes I10‒I13 and I15 with prescriptions for antihypertensive agents or systolic or diastolic blood pressure ≥ 140 mmHg or ≥ 90 mmHg, respectively24; and dyslipidemia was defined as ICD-10 code E78 with prescriptions for lipid-lowering agents or serum total cholesterol ≥ 240 mg/dL24. The primary outcome was cardiovascular mortality (death due to MI and/or stroke). The secondary outcomes included the incidence of MI and stroke, and all-cause mortality. MI was defined as ICD-10 codes I21 or I22 during hospitalization or these codes being recorded at least twice during the study period19. Stroke was defined as ICD-10 codes I63 or I64 during hospitalization with claims for brain magnetic resonance imaging or computed tomography19.
Statistical analysis
Continuous variables of parametric and non-parametric distributions are expressed as mean (± standard deviation) and median (interquartile range), respectively. Categorical variables are expressed as numbers (%). Continuous variables were compared using independent Student’s t test or Mann–Whitney U test. Categorical variables were compared using χ2 test. The incidence of outcomes was expressed as the number of events per 1000 person-years. The cumulative incidences of outcomes were analyzed using the Kaplan–Meier method and compared using log-rank test according to the presence of each IMID. Hazard ratios (HRs) and the corresponding 95% confidence intervals (CI) for the outcomes in each IMID were estimated using Cox proportional hazard models. Model 1 was adjusted for none of the covariates (univariable analysis). Multivariable models were first adjusted for age and sex (model 2) and additional adjustments for other confounders in the following models (models 3–6). Bonferroni correction was performed for multiple comparisons. To balance the baseline characteristics between patients with and without each IMID, an inverse probability of treatment weighting (IPTW)-adjusted model was used. The propensity score for each IMID used in the IPTW-adjusted model was estimated by multiple logistic regression analysis including all variables in Table 1. All p-values were two-sided, and a p-value < 0.05 was considered statistically significant. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA), and Stata statistical software v12 (StataCorp, College Station, TX, USA).
Table 1.
Baseline characteristics of patients with type 2 diabetes according to the presence of immune-mediated inflammatory diseases.
| Total population | No CD | CD | p | No UC | UC | p | No RA | RA | p | No AS | AS | p | No PsO | PsO | p | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients | 2,263,853 | 2,263,692 | 161 | 2,262,964 | 889 | 2,259,950 | 3903 | 2,263,050 | 803 | 2,244,425 | 19,428 | |||||
| Male sex, n (%) | 1,388,001 (61.31) | 1,387,888 (61.31) | 113 (70.19) | 0.0208 | 1,387,380 (61.31) | 621 (69.85) | < .0001 | 1,386,986 (61.37) | 1015 (26.01) | < .0001 | 1,387,327 (61.3) | 674 (83.94) | < .0001 | 1,374,944 (61.26) | 13,057 (67.21) | < .0001 |
| Age, years, Mean ± SD | 55.65 ± 12.31 | 55.65 ± 12.31 | 51.39 ± 13.43 | < .0001 | 55.65 ± 12.31 | 56.61 ± 12.15 | 0.0204 | 55.64 ± 12.32 | 61.71 ± 9.27 | < .0001 | 55.65 ± 12.31 | 50.05 ± 12.64 | < .0001 | 55.64 ± 12.32 | 57.37 ± 11.72 | < .0001 |
| BMI, kg/m2, Mean ± SD | 25.07 ± 3.69 | 25.07 ± 3.69 | 23.53 ± 3.45 | < .0001 | 25.07 ± 3.69 | 24.38 ± 3.03 | < .0001 | 25.07 ± 3.69 | 23.76 ± 3.45 | < .0001 | 25.07 ± 3.69 | 25.33 ± 3.57 | 0.0427 | 25.07 ± 3.7 | 25.07 ± 3.41 | 0.745 |
| Waist circumference, cm, Mean ± SD | 85.21 ± 8.85 | 85.21 ± 8.85 | 82.15 ± 8.83 | < .0001 | 85.21 ± 8.85 | 84.4 ± 8.06 | 0.0065 | 85.22 ± 8.85 | 81.83 ± 8.91 | < .0001 | 85.21 ± 8.85 | 86.94 ± 9.15 | < .0001 | 85.2 ± 8.85 | 86.21 ± 8.63 | < .0001 |
| Smoking, n (%) | 0.2004 | < .0001 | < .0001 | < .0001 | < .0001 | |||||||||||
| Non-smoker | 1,227,012 (54.2) | 1,226,927 (54.2) | 85 (52.8) | 1,226,565 (54.2) | 447 (50.28) | 1,223,989 (54.16) | 3023 (77.45) | 1,226,724 (54.21) | 288 (35.87) | 1,217,815 (54.26) | 9197 (47.34) | |||||
| Ex-smoker | 404,394 (17.86) | 404,357 (17.86) | 37 (22.98) | 404,088 (17.86) | 306 (34.42) | 403,957 (17.87) | 437 (11.2) | 404,197 (17.86) | 197 (24.53) | 400,092 (17.83) | 4302 (22.14) | |||||
| Current smoker | 632,447 (27.94) | 632,408 (27.94) | 39 (24.22) | 632,311 (27.94) | 136 (15.3) | 632,004 (27.97) | 443 (11.35) | 632,129 (27.93) | 318 (39.6) | 626,518 (27.91) | 5929 (30.52) | |||||
| Alcohol consumption, n (%) | 0.0202 | < .0001 | < .0001 | 0.2546 | 0.0139 | |||||||||||
| Never | 1,234,060 (54.51) | 1,233,958 (54.51) | 102 (63.35) | 1,233,468 (54.51) | 592 (66.59) | 1,230,759 (54.46) | 3301 (84.58) | 1,233,644 (54.51) | 416 (51.81) | 1,223,294 (54.5) | 10,766 (55.41) | |||||
| Mild drinker | 785,224 (34.69) | 785,173 (34.69) | 51 (31.68) | 784,981 (34.69) | 243 (27.33) | 784,702 (34.72) | 522 (13.37) | 784,924 (34.68) | 300 (37.36) | 778,677 (34.69) | 6547 (33.7) | |||||
| Heavy drinker | 244,569 (10.8) | 244,561 (10.8) | 8 (4.97) | 244,515 (10.81) | 54 (6.07) | 244,489 (10.82) | 80 (2.05) | 244,482 (10.8) | 87 (10.83) | 242,454 (10.8) | 2115 (10.89) | |||||
| Regular physical activity, n (%) | 469,505 (20.74) | 469,475 (20.74) | 30 (18.63) | 0.5099 | 469,299 (20.74) | 206 (23.17) | 0.0735 | 468,799 (20.74) | 706 (18.09) | < .0001 | 469,361 (20.74) | 144 (17.93) | 0.0498 | 465,297 (20.73) | 4208 (21.66) | 0.0015 |
| Low income, n (%) | 433,815 (19.16) | 433,784 (19.16) | 31 (19.25) | 0.9763 | 433,673 (19.16) | 142 (15.97) | 0.0157 | 433,109 (19.16) | 706 (18.09) | 0.088 | 433,690 (19.16) | 125 (15.57) | 0.0096 | 429,979 (19.16) | 3836 (19.74) | 0.0384 |
| Hypertension, n (%) | 1,175,667 (51.93) | 1,175,605 (51.93) | 62 (38.51) | 0.0007 | 1,175,288 (51.94) | 379 (42.63) | < .0001 | 1,173,325 (51.92) | 2342 (60.01) | < .0001 | 1,175,262 (51.93) | 405 (50.44) | 0.396 | 1,164,849 (51.9) | 10,818 (55.68) | < .0001 |
| Dyslipidemia, n (%) | 851,217 (37.6) | 851,178 (37.6) | 39 (24.22) | 0.0005 | 850,908 (37.6) | 309 (34.76) | 0.0801 | 849,590 (37.59) | 1627 (41.69) | < .0001 | 850,912 (37.6) | 305 (37.98) | 0.823 | 842,921 (37.56) | 8296 (42.7) | < .0001 |
| Use of insulin, n (%) | 167,639 (14.1) | 167,616 (14.09) | 23 (28.75) | 0.0002 | 167,536 (14.09) | 103 (20) | 0.0001 | 167,021 (14.08) | 618 (22.26) | < .0001 | 167,573 (14.1) | 66 (15.64) | 0.362 | 165,575 (14.06) | 2064 (17.77) | < .0001 |
| Number of oral hypoglycemic agents used ≥ 3, n (%) | 299,387 (13.22) | 299,371 (13.22) | 16 (9.94) | 0.2183 | 299,278 (13.23) | 109 (12.26) | 0.3962 | 298,801 (13.22) | 586 (15.01) | 0.001 | 299,295 (13.23) | 92 (11.46) | 0.1392 | 296,406 (13.21) | 2981 (15.34) | < .0001 |
| Duration of type 2 diabetes ≥ 5 years, n (%) | 619,733 (27.38) | 619,701 (27.38) | 32 (19.88) | 0.0328 | 619,482 (27.37) | 251 (28.23) | 0.5657 | 618,290 (27.36) | 1443 (36.97) | < .0001 | 619,533 (27.38) | 200 (24.91) | 0.1166 | 613,809 (27.35) | 5924 (30.49) | < .0001 |
| Depression, n (%) | 110,525 (4.88) | 110,510 (4.88) | 15 (9.32) | 0.009 | 110,458 (4.88) | 67 (7.54) | 0.0002 | 110,089 (4.87) | 436 (11.17) | < .0001 | 110,463 (4.88) | 62 (7.72) | 0.0002 | 109,197 (4.87) | 1328 (6.84) | < .0001 |
| Fasting glucose, mg/dL, Mean ± SD | 138.46 ± 48.16 | 138.46 ± 48.16 | 128.45 ± 39.55 | 0.0083 | 138.47 ± 48.16 | 128.42 ± 41 | < .0001 | 138.5 ± 48.16 | 118.96 ± 42.36 | < .0001 | 138.46 ± 48.16 | 131.98 ± 51.63 | 0.0001 | 138.48 ± 48.16 | 136.25 ± 47.46 | < .0001 |
| Systolic BP, mmHg, Mean ± SD | 128.73 ± 15.79 | 128.73 ± 15.79 | 123.47 ± 14.88 | < .0001 | 128.73 ± 15.79 | 125.79 ± 14.97 | < .0001 | 128.73 ± 15.79 | 128.94 ± 16.52 | 0.3558 | 128.73 ± 15.79 | 127.79 ± 15.3 | 0.0901 | 128.73 ± 15.79 | 128.55 ± 15.49 | 0.1062 |
| Diastolic BP, mmHg, Mean ± SD | 79.17 ± 10.27 | 79.17 ± 10.27 | 76.44 ± 9.14 | 0.0007 | 79.18 ± 10.27 | 77.3 ± 9.62 | < .0001 | 79.18 ± 10.27 | 78.01 ± 10.1 | < .0001 | 79.17 ± 10.27 | 79.23 ± 10.48 | 0.8801 | 79.18 ± 10.28 | 78.95 ± 10.12 | 0.0025 |
| Total cholesterol, mg/dL, Mean ± SD | 198.54 ± 46.39 | 198.54 ± 46.39 | 179.81 ± 42.65 | < .0001 | 198.55 ± 46.39 | 189.24 ± 38.26 | < .0001 | 198.55 ± 46.4 | 192.25 ± 40.64 | 0.0029 | 198.54 ± 46.39 | 191.87 ± 39.35 | < .0001 | 198.54 ± 46.41 | 198.33 ± 44.31 | 0.5293 |
| HDL cholesterol, mg/dL, Mean ± SD | 51.44 ± 13.49 | 51.44 ± 13.49 | 49.71 ± 14.41 | 0.1028 | 51.44 ± 13.49 | 51.17 ± 13.59 | 0.5457 | 51.43 ± 13.48 | 55.64 ± 14.5 | < .0001 | 51.44 ± 13.49 | 51.15 ± 13.68 | 0.537 | 51.44 ± 13.48 | 51.35 ± 13.58 | 0.3328 |
| LDL cholesterol, mg/dL, Mean ± SD | 113.81 ± 88.05 | 113.81 ± 88.05 | 98.3 ± 38.12 | 0.0254 | 113.81 ± 88.06 | 108.85 ± 34.34 | 0.0929 | 113.82 ± 88.1 | 109.38 ± 44 | 0.1495 | 113.81 ± 88.06 | 108.53 ± 34.9 | 0.0892 | 113.82 ± 88.3 | 113.03 ± 50.07 | 0.2148 |
| Triglyceride, mg/dL, Median (IQR) | 145.99 (145.88–146.1) | 145.99 (145.88–146.1) | 135.97 (124.79–148.16) | 0.1164 | 146 (145.89–146.11) | 124.6 (120.11–129.27) | < .0001 | 146.04 (145.93–146.14) | 121.92 (120.08–123.8) | < .0001 | 145.99 (145.88–146.1) | 137.81 (132.83–142.97) | 0.0044 | 145.98 (145.87–146.09) | 147.66 (146.51–148.82) | 0.0054 |
CD crohn's disease, UC ulcerative colitis, RA rheumatoid arthritis, AS ankylosing spondylitis, PsO psoriasis, BMI body mass index, BP blood pressure, HDL high density lipoprotein, LDL low density lipoprotein, SD standard deviation, IQR interquartile range.
Ethical approval and consent to participate
This study was approved by the Institutional Review Board (IRB) of Gangnam Severance Hospital (IRB No: 3-2020-0269). Owing to the retrospective nature of this study, the requirement for informed consent was waived.
Results
Baseline characteristics
The baseline characteristics of the 2,263,853 patients with type 2 diabetes, and the comparisons of their characteristics according to each IMID are summarized in Table 1. Of the 2,263,853 patients with type 2 diabetes, 161 (0.007%), 889 (0.039%), 3903 (0.159%), 803 (0.035%), and 19,428 (0.858%) patients had CD, UC, RA, AS, and PsO, respectively. The mean age of the study population was 55.65 ± 12.31 years, and 1,388,001 (61.31%) patients were male.
The proportion of males as well as the mean age differed between groups of patients with each IMID. In comparisons with the respective controls, in those with CD and those with AS, males and younger age were commoner; in those with UC and those with PsO, males and older age were commoner; and in those with RA, females and older age were commoner. According to each IMID, the proportions of current smokers, heavy alcohol drinkers, and those who perform regular physical activity varied. In comparisons with their respective controls, those with CD included a lower proportion of heavy alcohol drinkers; those with UC included a lower proportion of current smokers and heavy alcohol drinkers; those with RA included a lower proportion of current smokers, heavy alcohol drinkers, and those who perform regular physical activity; those with AS included a higher proportion of current smokers and lower proportion of patients who perform regular physical activity; and those with PsO included a higher proportion of current smokers and those who perform regular physical activity. The presence of other cardiovascular risk factors varied between groups of patients with each IMID as well. Hypertension and dyslipidemia were less common in patients with CD; hypertension was less common in patients with UC; and hypertension and dyslipidemia were commoner in patients with RA and those with PsO, in comparisons with their respective controls.
Incidence of cardiovascular mortality according to IMIDs
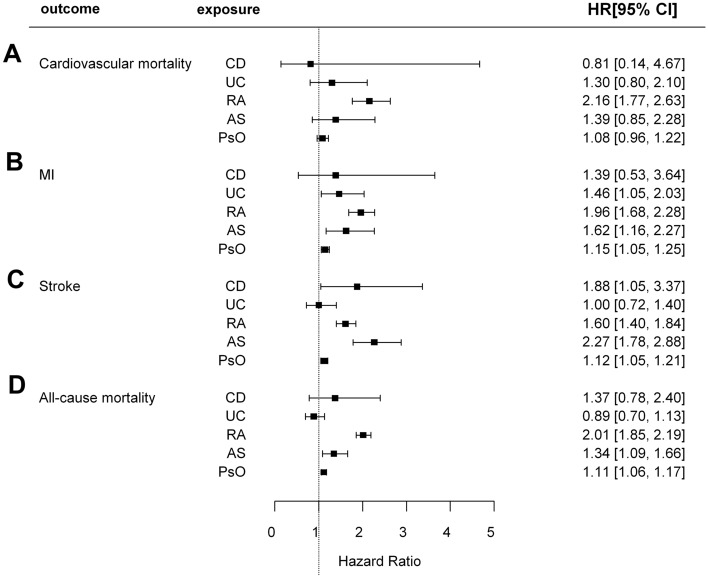
The incidences of cardiovascular mortality in patients with type 2 diabetes with CD, UC, RA, AS, and PsO were 1.67, 2.28, 8.56, 2.04, and 2.40 per 1000 person-years, respectively. The cumulative incidence of cardiovascular mortality according to the presence of each IMID is depicted in Fig. 2A. Patients with RA (p < 0.001) and PsO (p < 0.001) had a significantly higher cumulative incidence of cardiovascular mortality than those without RA and PsO, respectively. Compared with their respective controls, patients with RA (unadjusted HR: 2.855 [95% CI 2.421, 3.367]) and PsO (unadjusted HR: 1.273 [95% CI 1.144, 1.418]) had a significantly higher risk of cardiovascular mortality in the univariable model (model 1). The higher risk of cardiovascular mortality remained statistically significant only in patients with RA in the multivariable models (model 2–6) that were adjusted for potential confounders (model 6, adjusted HR: 2.278 [95% CI 1.931, 2.689]). Similarly, IPTW-adjusted analysis revealed higher risk of cardiovascular mortality in patients with RA (IPTW-adjusted HR: 2.156 [95% CI 1.769, 2.627]). There was no difference in the cardiovascular mortality between patients with type 2 diabetes based on the presence of CD, UC, and AS, respectively (Table 2 and Fig. 3).
Figure 2.
Comparison of cumulative incidence of (A) cardiovascular mortality, (B) MI, (C), stroke, and (D) all-cause mortality according to the presence of each IMID. CD, crohn’s disease; UC, ulcerative colitis; RA, rheumatoid arthritis; AS, ankylosing spondylitis; PsO, psoriasis; MI, myocardial infarction; IMIDs, immune-mediated inflammatory disease.
Table 2.
Clinical outcomes according to the presence of immune-mediated inflammatory diseases.
| Clinical outcomes | Disease group | Control group | HR (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of events/N | Incidence rate (/1000 person-years) | No. of events/N | Incidence rate (/1000 person-years) | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | IPTW-adjusted | |
| Cardiovascular mortality | |||||||||||
| CD | 2/161 | 1.67478 | 31,394/2,263,692 | 1.89188 | 0.886 (0.223,3.521) | 1.162 (0.290,4.645) | 1.194 (0.299,4.775) | 1.096 (0.274,4.384) | 1.543 (0.386,6.169) | 1.085 (0.271,4.337) | 0.812 (0.141,4.674) |
| UC | 15/889 | 2.28232 | 31,381/2,262,964 | 1.89171 | 1.202 (0.724,1.993) | 1.054 (0.636,1.749) | 1.084 (0.653,1.798) | 1.105 (0.666,1.834) | 0.933 (0.502,1.734) | 1.104 (0.665,1.831) | 1.298 (0.803,2.100) |
| RA | 142/3903 | 8.55730 | 31,254/2,259,950 | 1.88634 | 2.855 (2.421,3.367) | 2.471 (2.095,2.914) | 2.452 (2.079,2.892) | 2.270 (1.925,2.678) | 2.263 (1.884,2.718) | 2.278 (1.931,2.689) | 2.156 (1.769,2.627) |
| AS | 12/803 | 2.03583 | 31,384/2,263,050 | 1.89181 | 1.077 (0.612,1.896) | 1.663 (0.945,2.929) | 1.623 (0.922,2.858) | 1.592 (0.904,2.804) | 1.195 (0.537,2.660) | 1.632 (0.926,2.873) | 1.390 (0.847,2.282) |
| PsO | 336/19,428 | 2.39507 | 31,060/2,244,425 | 1.88757 | 1.273 (1.144,1.418) | 1.093 (0.981,1.217) | 1.079 (0.969,1.202) | 1.077 (0.967,1.199) | 1.029 (0.905,1.169) | 1.051 (0.944,1.170) | 1.081 (0.960,1.218) |
| MI | |||||||||||
| CD | 6/161 | 5.07558 | 59,656/2,263,692 | 3.63053 | 1.391 (0.626,3.095) | 1.633 (0.735,3.629) | 1.635 (0.735,3.634) | 1.654 (0.743,3.681) | 1.599 (0.600,4.261) | 1.608 (0.722,3.579) | 1.390 (0.530,3.643) |
| UC | 33/889 | 5.07397 | 59,629/2,262,964 | 3.63006 | 1.396 (0.993,1.963) | 1.285 (0.913,1.807) | 1.302 (0.926,1.831) | 1.327 (0.943,1.867) | 1.149 (0.749,1.762) | 1.332 (0.946,1.873) | 1.462 (1.051,2.032) |
| RA | 237/3903 | 9.03496 | 59,425/2,259,950 | 3.62199 | 2.532 (2.228,2.876) | 2.224 (1.957,2.527) | 2.165 (1.906,2.460) | 2.152 (1.894,2.445) | 1.940 (1.675,2.247) | 2.154 (1.895,2.447) | 1.958 (1.683,2.278) |
| AS | 25/803 | 4.29056 | 59,637/2,263,050 | 3.63040 | 1.197 (0.811,1.767) | 1.477 (0.998,2.186) | 1.419 (0.959,2.101) | 1.400 (0.946,2.072) | 1.748 (1.140,2.681) | 1.402 (0.947,2.075) | 1.624 (1.164,2.266) |
| PsO | 648/19,428 | 4.67600 | 59,014/2,244,425 | 3.62174 | 1.296 (1.200,1.400) | 1.181 (1.093,1.276) | 1.158 (1.071,1.251) | 1.143 (1.058,1.235) | 1.075 (0.979,1.180) | 1.115 (1.032,1.205) | 1.146 (1.055,1.246) |
| Stroke | |||||||||||
| CD | 10/161 | 8.59329 | 84,853/2,263,692 | 5.19290 | 1.670 (0.901,3.094) | 2.046 (1.101,3.802) | 2.140 (1.151,3.977) | 2.105 (1.133,3.912) | 2.837 (1.482,5.431) | 1.814 (1.074,3.063) | 1.877 (1.046,3.367) |
| UC | 33/889 | 5.07569 | 84,830/2,262,964 | 5.19319 | 0.977 (0.694,1.374) | 0.886 (0.630,1.246) | 0.928 (0.660,1.306) | 0.949 (0.674,1.335) | 0.914 (0.612,1.363) | 1.085 (0.844,1.395) | 1.000 (0.717,1.395) |
| RA | 224/3903 | 8.55730 | 84,639/2,259,950 | 5.18775 | 1.657 (1.453,1.889) | 1.405 (1.232,1.602) | 1.406 (1.233,1.603) | 1.382 (1.212,1.576) | 1.289 (1.111,1.496) | 1.702 (1.547,1.871) | 1.605 (1.396,1.845) |
| AS | 32/803 | 5.51876 | 84,831/2,263,050 | 5.19303 | 1.065 (0.753,1.506) | 1.448 (1.024,2.047) | 1.433 (1.013,2.027) | 1.412 (0.998,1.996) | 1.183 (0.754,1.854) | 1.420 (1.087,1.854) | 2.266 (1.782,2.882) |
| PsO | 913/19,428 | 6.63109 | 83,950/2,244,425 | 5.18093 | 1.282 (1.201,1.368) | 1.146 (1.074,1.223) | 1.139 (1.068,1.216) | 1.135 (1.064,1.212) | 1.110 (1.028,1.199) | 1.107 (1.052,1.166) | 1.123 (1.046,1.205) |
| All-cause mortality | |||||||||||
| CD | 16/161 | 13.3982 | 181,262/2,263,692 | 10.9233 | 1.246 (0.767,2.024) | 1.572 (0.963,2.566) | 1.623 (0.994,2.650) | 1.465 (0.897,2.391) | 1.436 (0.795,2.593) | 1.428 (0.875,2.330) | 1.372 (0.784,2.403) |
| UC | 73/889 | 11.1073 | 181,205/2,262,964 | 10.9234 | 1.000 (0.794,1.260) | 0.868 (0.690,1.092) | 0.898 (0.714,1.129) | 0.893 (0.710,1.124) | 0.849 (0.654,1.104) | 0.857 (0.682,1.079) | 0.887 (0.696,1.130) |
| RA | 750/3903 | 28.0406 | 180,528/2,259,950 | 10.8958 | 2.600 (2.421,2.794) | 2.439 (2.271,2.621) | 2.424 (2.256,2.604) | 2.207 (2.054,2.371) | 2.021 (1.863,2.193) | 2.130 (1.982,2.289) | 2.013 (1.849,2.192) |
| AS | 62/803 | 10.5184 | 181,216/2,263,050 | 10.9236 | 0.963 (0.751,1.235) | 1.355 (1.057,1.739) | 1.331 (1.038,1.707) | 1.311 (1.022,1.682) | 1.258 (0.923,1.715) | 1.324 (1.032,1.699) | 1.344 (1.089,1.658) |
| PsO | 2025/19,428 | 14.4346 | 179,253/2,244,425 | 10.8935 | 1.329 (1.272,1.389) | 1.117 (1.069,1.167) | 1.105 (1.057,1.154) | 1.117 (1.069,1.167) | 1.056 (1.003,1.112) | 1.080 (1.034,1.129) | 1.115 (1.062,1.171) |
HR hazard ratio, CI confidence interval, CD Crohn's disease, UC ulcerative colitis, RA rheumatoid arthritis, AS ankylosing spondylitis, PsO psoriasis, MI myocardial infarction, IPTW inverse probability of treatment weighting.
Model 1 adjusted for none of the covariates (univariable analysis).
Model 2 adjusted for age and sex.
Model 3 adjusted for age, sex, smoking, alcohol consumption, and regular physical activity.
Model 4 adjusted for age, sex, smoking, alcohol consumption, regular physical activity, hypertension, dyslipidemia, and BMI.
Model 5 adjusted for age, sex, smoking, alcohol consumption, regular physical activity, hypertension, dyslipidemia, BMI, use of insulin, number of oral hypoglycemic agent ≥ 3, duration of type 2 diabetes ≥ 5 years, and depression.
Model 6 adjusted for age, sex, smoking, alcohol consumption, regular physical activity, hypertension, dyslipidemia, BMI, use of insulin, number of oral hypoglycemic agent ≥ 3, duration of type 2 diabetes ≥ 5 years, depression, waist circumference, fasting glucose, systolic BP, diastolic BP, total cholesterol, LDL-cholesterol, and triglyceride.
Figure 3.
A forest plot showing IPTW-adjusted HRs and 95% CIs for (A) cardiovascular mortality, (B) MI, (C), stroke, and (D) all-cause mortality. IPTW, inverse probability of treatment weighting; HR, hazard ratio; CI, confidence interval; CD, Crohn’s disease; UC, ulcerative colitis; RA, rheumatoid arthritis; AS, ankylosing spondylitis; PsO, psoriasis; MI, myocardial infarction.
Incidence of MI and stroke, and all-cause mortality rate according to IMIDs
The incidences of MI in patients with type 2 diabetes with CD, UC, RA, AS, and PsO were 5.08, 5.07, 9.03, 4.29, and 4.68 per 1000 person-years, respectively. The cumulative incidence of MI according to each IMID is depicted in Fig. 2B. Patients with RA (p < 0.001) and PsO (p < 0.001) had a significantly higher cumulative incidence of MI than those without RA and PsO, respectively. In the univariable model (model 1), patients with RA (unadjusted HR: 2.532 [95% CI 2.228, 2.876]) and those with PsO (unadjusted HR: 1.296 [95% CI 1.200, 1.400]) had significantly higher risks of MI. After adjusting for the potential confounders, patients with RA continued to demonstrate significantly higher risk of MI (model 6, adjusted HR: 2.154 [95% CI 1.895, 2.447]), as well as the patients with PsO (model 6, adjusted HR: 1.115 [95% CI 1.032, 1.205]). These associations were also observed in the IPTW-adjusted analysis: higher risk of MI in patients with RA (IPTW-adjusted HR: 1.958 [95% CI 1.683, 2.278]), and those with PsO (IPTW-adjusted HR: 1.146 [95% CI 1.053, 1.246]). Moreover, patients with UC (IPTW-adjusted HR: 1.462 [95% CI 1.051, 2.032]) and those with AS (IPTW-adjusted HR: 1.624 [95% CI 1.164, 2.266]) were newly identified to have a significantly higher risk of MI in the IPTW-adjusted analysis. There were no differences in the risk of MI in patients with type 2 diabetes with CD (Table 2 and Fig. 3).
The incidences of stroke in patients with type 2 diabetes with CD, UC, RA, AS, and PsO were 8.59, 5.08, 8.56, 5.52, and 6.63 per 1000 person-years, respectively. The cumulative incidence of stroke according to each IMID is depicted in Fig. 2C. Patients with RA (p < 0.001) and PsO (p < 0.001) had a significantly higher cumulative incidence of stroke than those without RA and PsO, respectively. Patients with RA (unadjusted HR: 1.657 [95% CI 1.453, 1.889]) and PsO (unadjusted HR: 1.282 [95% CI 1.201, 1.368]) had a significantly higher risk of stroke in the univariable model (model 1) compared with their respective controls. The higher risk of stroke remained statistically significant in the multivariable models in patients with RA (model 6, adjusted HR: 1.702 [95% CI 1.547, 1.871]) and those with PsO (model 6, adjusted HR: 1.107 [95% CI 1.052, 1.166]). Patients with type 2 diabetes with CD (adjusted HR: 1.814 [95% CI 1.074, 3.063]) and those with AS (adjusted HR: 1.420 [95% CI 1.087, 1.854]) were newly identified to have a significantly higher risk of stroke in model 6. Similar associations were also observed in the IPTW-adjusted analysis: higher risk of stroke in patients with CD (IPTW-adjusted HR: 1.877 [95% CI 1.046, 3.367]), those with RA (IPTW-adjusted HR: 1.605 [95% CI 1.396, 1.845]), those with AS (IPTW-adjusted HR: 2.266 [95% CI 1.782, 2.882]), and those with PsO (IPTW-adjusted HR: 1.123 [95% CI 1.046, 1.205]). There was no difference in the risk of stroke in patients with type 2 diabetes with UC (Table 2 and Fig. 3).
The rates of all-cause mortality in patients with type 2 diabetes with CD, UC, RA, AS, and PsO were 13.40, 11.11, 28.04, 10.52, and 14.43 per 1000 person-years, respectively. The cumulative incidence of all-cause mortality according to each IMID is depicted in Fig. 2D. Patients with RA (p < 0.001) and PsO (p < 0.001) had a significantly higher cumulative incidence of all-cause mortality than those without RA and PsO, respectively. In the univariable model (model 1), patients with RA (unadjusted HR: 2.600 [95% CI 2.421, 2.794]) and PsO (unadjusted HR: 1.329 [95% CI 1.272, 1.389]) had a significantly higher risk of all-cause mortality—both these risk remained statistically significant in all the multivariable models (model 6, RA, adjusted HR: 2.130 [95% CI 1.982, 2.289]; and model 6, PsO, adjusted HR: 1.080 [95% CI 1.034, 1.129]). Patients with type 2 diabetes with AS (adjusted HR: 1.324 [95% CI 1.032, 1.699]) were newly identified to have a significantly higher risk of all-cause mortality in model 6. Similar associations were also observed in the IPTW-adjusted analysis: higher risk of all-cause mortality in patients with RA (IPTW-adjusted HR: 2.013 [95% CI 1.849, 2.192]), those with AS (IPTW-adjusted HR: 1.344 [95% CI 1.089, 1.658]), and those with PsO (IPTW-adjusted HR: 1.115 [95% CI 1.062, 1.171]). There were no differences in the rates of all-cause mortality between patients with type 2 diabetes with CD, and UC (Table 2 and Fig. 3).
Discussion
In this population-based cohort study, we found significant associations between the presence of IMIDs and the risks of cardiovascular morbidity and mortality in patients with type 2 diabetes. Notably, the effects on cardiovascular morbidity and mortality varied between IMIDs. CD was associated with a higher risk of stroke; UC was associated with a higher risk of MI; RA was associated with a higher risk of cardiovascular mortality, MI, stroke, and all-cause mortality; AS was associated with a higher risk of MI, stroke, and all-cause mortality; and PsO was associated with a higher risk of MI, stroke, and all-cause mortality. These findings were observed in multivariable models adjusted for potential confounders, such as the traditional cardiovascular risk factors (age, sex, hypertension, dyslipidemia, smoking, and obesity)25, as well as in the IPTW-adjusted analyses, thus suggesting that IMIDs should be considered independent risk factors of cardiovascular disease in patients with type 2 diabetes. To our knowledge, this is the first study to comprehensively evaluate the effects of IMIDs on cardiovascular morbidity and mortality in patients with type 2 diabetes. The association between each and every IMID with risk of cardiovascular morbidity and mortality observed in our study reflects that chronic inflammation is an important cardiovascular risk factor. Considering that cardiovascular diseases cause substantial economic burden and adversely affect the quality of life in patients with type 2 diabetes, patients with concomitant IMIDs definitely deserve more stringent monitoring for cardiovascular morbidity and mortality.
Among the IMIDs, RA was the most potent risk factor of cardiovascular mortality, MI, and all-cause mortality with approximately a two-fold increase in the risk, while AS was the most potent risk factor of stroke with approximately a two-fold increase in the risk. The effect sizes of these diseases on the cardiovascular outcomes were larger than that observed in the general population15,26–28. Previous studies have reported increased risk of MI by approximately 68% and that of cerebrovascular accidents by approximately 41% in patients with RA when compared with the general population26–28. In addition, a meta-analysis reported that AS was associated with a 50% increased risk of stroke15. Given that type 2 diabetes itself is associated with an increased risk of cardiovascular morbidity and mortality2–5, the effect sizes of RA and AS on the cardiovascular outcomes observed in this study are considerable. Hence, patients with type 2 diabetes who also have RA or AS could benefit from a more vigilant surveillance for cardiovascular diseases and a tighter control of modifiable cardiovascular risk factors.
In regard to IBD, CD, but not UC, demonstrated a significant association with stroke. Similarly, a recent study that assessed the risk of stroke in patients with IBD reported a higher risk of stroke in CD (HR: 1.50 [95% CI 1.10, 2.06]) but not in UC (HR: 1.17 [95% CI 0.90, 1.52]) when compared with those without IBD29. Additionally, a meta-analysis of articles published before July 2020 demonstrated that CD was associated with a 25% increased risk of stroke30. Inflammation is a major contributor to accelerated atherosclerosis, which consequently results in cardiovascular morbidities31,32. Considering that UC is less aggressive and has less frequent extra-intestinal manifestations than CD33, the lower inflammatory burden of UC than that in CD could be a possible explanation for the lack of association between UC and stroke. Regarding other outcomes, UC was associated with a higher risk of MI. This is similar to the findings of a previous study in the general population that demonstrated a higher risk of MI in patients with IBD (OR: 1.25 [95% CI 1.24, 1.27])13. However, in our study, CD was not associated with a higher risk of MI. It should be noted that in our study, the HRs of CD had wide 95% CIs, probably due to a small number of patients (n = 161). The effect size of CD on MI (IPTW-adjusted: 1.390) was numerically comparable to that of UC on MI (IPTW-adjusted HR: 1.462), and even higher than that reported in the previous study (OR 1.25)13. Therefore, although the statistical significance was not reached in the association between CD and MI, patients with CD might still benefit clinically from stringent monitoring for MI.
Regarding AS, a meta-analysis reported a higher risk of MI (OR 1.60 [95% CI 1.32, 1.93]) and stroke (OR 1.50 [95% CI 1.39, 1.62]) in patients with AS in the general population15. Another study demonstrated a significantly higher risk of cardiovascular mortality (HR 1.36 [95% CI 1.13, 1.65]) in patients with AS34. We also observed a significantly higher risk of MI (IPTW-adjusted HR: 1.624 [95% CI 1.164, 2.266]) and stroke (IPTW-adjusted HR 2.266 [95% CI 1.782, 2.882]) in patients with type 2 diabetes with a larger effect size than that observed in the general population15. Moreover, associations of AS with all cause-mortality was also observed in this study. This might be explained by the relatively older age of patients with type 2 diabetes and AS. The mean age of the patients with type 2 diabetes and AS in our study was 50.05 years, whereas it was 45.55 years in the previous study34. The older age of patients with AS in our cohort may have accentuated the effect of AS on MI, stroke, and all-cause mortality.
PsO has been reported to increase the risk of MI (OR 1.25)16. In our study, PsO was associated with only a modestly higher risk of MI (IPTW-adjusted HR 1.146 [95% CI 1.055, 1.246]). Furthermore, PsO was also associated with a modestly higher risk of stroke and all-cause mortality (stroke, IPTW-adjusted HR: 1.123 [95% CI 1.046, 1.205]; all-cause mortality, IPTW-adjusted HR: 1.115 [95% CI 1.062, 1.171]). A population-based study that investigated the epidemiology of PsO in Korea reported that mild PsO is 7–9 times commoner than severe PsO23. It is possible that the low proportion of patients with severe PsO in the Korean population may have led to the smaller effect size. Therefore, although the effect size was relatively small compared with those of other IMIDs, PsO deserves close attention in patients with type 2 diabetes.
There are some limitations to this study. First, this was a retrospective observational study. Although we adjusted for a number of potential confounders in the multivariable models, the possibility of confounding by unmeasured covariates exists. For instance, data on the use of medications for IMIDs and the disease activities of these IMIDs, which are potential confounders for cardiovascular outcomes25, were not available. In addition, hemoglobin A1c levels were also not available. Second, as this was a Korean population-based study, the results may not be generalizable to other populations. Nonetheless, this study has a strength in that a large cohort of patients with type 2 diabetes extracted from the nationwide population database was used. This enabled us to evaluate the effects of IMIDs, which are relatively rare diseases, on the cardiovascular outcomes in patients with type 2 diabetes.
Conclusions
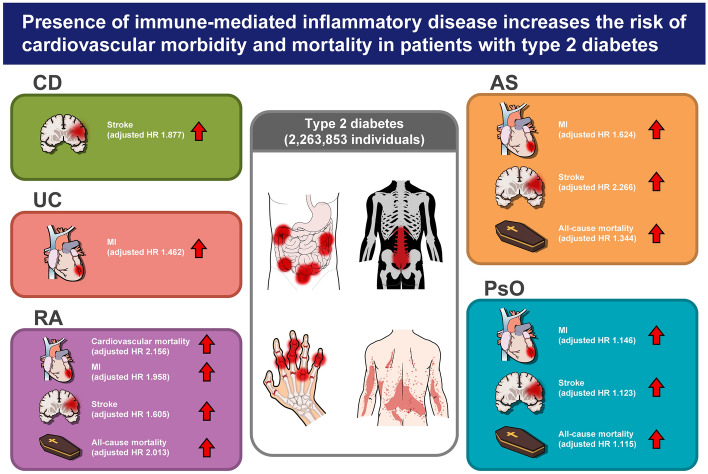
In conclusion, the presence of IMIDs in patients with type 2 diabetes was associated with a higher risk of cardiovascular morbidity and mortality (Fig. 4). Particularly CD was associated with a higher risk of stroke; UC was associated with a higher risk of MI; RA was associated with a higher risk of cardiovascular mortality, MI, stroke, and all-cause mortality; AS was associated with a higher risk of MI, stroke, and all-cause mortality; and PsO was associated with a higher risk of MI, stroke, and all-cause mortality. Therefore, patients with type 2 diabetes with concomitant IMIDs require meticulous surveillance for cardiovascular morbidity and mortality.
Figure 4.
Summary of the present nationwide population-based study. CD, crohn’s disease; UC, ulcerative colitis; RA, rheumatoid arthritis; AS, ankylosing spondylitis; PsO, psoriasis; HR, hazard ratio.
Acknowledgements
We would like to thank Seung Woo Lee for the statistical support, and MID (Medical Illustration & Design), a part of the Medical Research Support Services of Yonsei University College of Medicine, for providing excellent support with medical illustration.
Abbreviations
- IMIDs
Immune-mediated inflammatory diseases
- CD
Crohn’s disease
- UC
Ulcerative colitis
- RA
Rheumatoid arthritis
- AS
Ankylosing spondylitis
- PsO
Psoriasis
- IBD
Inflammatory bowel disease
- MI
Myocardial infarction
- OR
Odds ratio
- NHIS
National Health Insurance Service
- RID
Rare intractable disease
- IRB
Institutional review board
- HR
Hazard ratio
- CI
Confidence interval
Author contributions
O.C.K., K.H., and J.C. contributed to the study concept and design; acquisition, analysis, and interpretation of the data; and drafting and editing the manuscript. R.K., S.W.H., J.-H.K., Y.H.Y., H.P., and M.-C.P. contributed to the interpretation of the data and critical review of the manuscript. K.H., and J.C. are the guarantor of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of data analysis.
Funding
This study was supported by a new faculty research seed money grant of Yonsei University College of Medicine for 2021 (2021-32-0053).
Data availability
All data generated or analyzed during this study are included in this article.
Competing interests
Dr. Chun received a grant given by Eisai Co. All other authors disclose that they have no financial, professional, or personal conflicts related to this publication.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A list of authors and their affiliations appears at the end of the paper.
Contributor Information
Kyungdo Han, Email: hkd917@naver.com.
Jaeyoung Chun, Email: j40479@gmail.com.
Gastroenterology, Neurology and Rheumatology National Data Science Research (GUARANTEE) Group:
Oh Chan Kwon, Kyungdo Han, Jaeyoung Chun, Ryul Kim, Seung Wook Hong, Jie-Hyun Kim, Young Hoon Youn, Hyojin Park, and Min-Chan Park
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diab. Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Sarwar N, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/s0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: Epidemiology, pathophysiology, and management. JAMA. 2002;287:2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 4.Low-Wang CC, Hess CN, Hiatt WR, Goldfine AB. Clinical update: Cardiovascular disease in diabetes mellitus: Atherosclerotic cardiovascular disease and heart failure in type 2 diabetes mellitus: Mechanisms, management, and clinical considerations. Circulation. 2016;133:2459–2502. doi: 10.1161/circulationaha.116.022194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tancredi M, et al. Excess mortality among persons with type 2 diabetes. N. Engl. J. Med. 2015;373:1720–1732. doi: 10.1056/NEJMoa1504347. [DOI] [PubMed] [Google Scholar]
- 6.Bertoluci MC, Rocha VZ. Cardiovascular risk assessment in patients with diabetes. Diabetol. Metab. Syndr. 2017;9:25. doi: 10.1186/s13098-017-0225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schett G, McInnes IB, Neurath MF. Reframing immune-mediated inflammatory diseases through signature cytokine hubs. N. Engl. J. Med. 2021;385:628–639. doi: 10.1056/NEJMra1909094. [DOI] [PubMed] [Google Scholar]
- 8.Baumgart DC, Sandborn WJ. Crohn's disease. Lancet. 2012;380:1590–1605. doi: 10.1016/s0140-6736(12)60026-9. [DOI] [PubMed] [Google Scholar]
- 9.Danese S, Fiocchi C. Ulcerative colitis. N. Engl. J. Med. 2011;365:1713–1725. doi: 10.1056/NEJMra1102942. [DOI] [PubMed] [Google Scholar]
- 10.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 11.Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N. Engl. J. Med. 2017;376:957–970. doi: 10.1056/NEJMra1505557. [DOI] [PubMed] [Google Scholar]
- 12.Taurog JD, Chhabra A, Colbert RA. Ankylosing spondylitis and axial spondyloarthritis. N. Engl. J. Med. 2016;374:2563–2574. doi: 10.1056/NEJMra1406182. [DOI] [PubMed] [Google Scholar]
- 13.Panhwar MS, et al. Risk of myocardial infarction in inflammatory bowel disease: A population-based national study. Inflamm. Bowel Dis. 2019;25:1080–1087. doi: 10.1093/ibd/izy354. [DOI] [PubMed] [Google Scholar]
- 14.Avina-Zubieta JA, Thomas J, Sadatsafavi M, Lehman AJ, Lacaille D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: A meta-analysis of observational studies. Ann. Rheum. Dis. 2012;71:1524–1529. doi: 10.1136/annrheumdis-2011-200726. [DOI] [PubMed] [Google Scholar]
- 15.Mathieu S, Pereira B, Soubrier M. Cardiovascular events in ankylosing spondylitis: An updated meta-analysis. Semin. Arthritis Rheum. 2015;44:551–555. doi: 10.1016/j.semarthrit.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Horreau C, et al. Cardiovascular morbidity and mortality in psoriasis and psoriatic arthritis: A systematic literature review. J. Eur. Acad. Dermatol. Venereol. 2013;27(Suppl 3):12–29. doi: 10.1111/jdv.12163. [DOI] [PubMed] [Google Scholar]
- 17.Kim JA, Yoon S, Kim LY, Kim DS. Towards actualizing the value potential of Korea health insurance review and assessment (HIRA) data as a resource for health research: Strengths, limitations, applications, and strategies for optimal use of HIRA data. J. Korean Med. Sci. 2017;32:718–728. doi: 10.3346/jkms.2017.32.5.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim WH, et al. Proteinuria detected by urine dipstick test as a risk factor for atrial fibrillation: A nationwide population-based study. Sci. Rep. 2017;7:6324. doi: 10.1038/s41598-017-06579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nam GE, et al. Body weight variability and the risk of cardiovascular outcomes and mortality in patients with type 2 diabetes: A nationwide cohort study. Diab. Care. 2020;43:2234–2241. doi: 10.2337/dc19-2552. [DOI] [PubMed] [Google Scholar]
- 20.Kang EA, et al. Periodontitis combined with smoking increases risk of the ulcerative colitis: A national cohort study. World J. Gastroenterol. 2020;26:5661–5672. doi: 10.3748/wjg.v26.i37.5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J, et al. Increased risk of idiopathic pulmonary fibrosis in inflammatory bowel disease: A nationwide study. J. Gastroenterol. Hepatol. 2020;35:249–255. doi: 10.1111/jgh.14838. [DOI] [PubMed] [Google Scholar]
- 22.Lee JS, et al. Reasons for the high cesarean delivery rate among women with ankylosing spondylitis: Using the Korean national health insurance database. J. Rheumatol. 2020;47:668–673. doi: 10.3899/jrheum.190754. [DOI] [PubMed] [Google Scholar]
- 23.Han JH, et al. Epidemiology and medication trends in patients with psoriasis: A nationwide population-based cohort study from Korea. Acta Derm. Venereol. 2018;98:396–400. doi: 10.2340/00015555-2877. [DOI] [PubMed] [Google Scholar]
- 24.Hong S, et al. Association between obesity and cardiovascular disease in elderly patients with diabetes: A retrospective cohort study. J. Clin. Endocrinol. Metab. 2021 doi: 10.1210/clinem/dgab714. [DOI] [PubMed] [Google Scholar]
- 25.Nurmohamed MT, Heslinga M, Kitas GD. Cardiovascular comorbidity in rheumatic diseases. Nat. Rev. Rheumatol. 2015;11:693–704. doi: 10.1038/nrrheum.2015.112. [DOI] [PubMed] [Google Scholar]
- 26.Solomon DH, et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003;107:1303–1307. doi: 10.1161/01.cir.0000054612.26458.b2. [DOI] [PubMed] [Google Scholar]
- 27.Turesson C, Jarenros A, Jacobsson L. Increased incidence of cardiovascular disease in patients with rheumatoid arthritis: Results from a community based study. Ann. Rheum Dis. 2004;63:952–955. doi: 10.1136/ard.2003.018101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lévy L, Fautrel B, Barnetche T, Schaeverbeke T. Incidence and risk of fatal myocardial infarction and stroke events in rheumatoid arthritis patients: A systematic review of the literature. Clin. Exp. Rheumatol. 2008;26:673–679. [PubMed] [Google Scholar]
- 29.Tanislav C, Trommer K, Labenz C, Kostev K. Inflammatory bowel disease as a precondition for stroke or TIA: A Matter of crohn's disease rather than ulcerative colitis. J. Stroke Cerebrovasc. Dis. 2021;30:105787. doi: 10.1016/j.jstrokecerebrovasdis.2021.105787. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y, Wang X. Increased risk of stroke among patients with inflammatory bowel disease: A PRISMA-compliant meta-analysis. Brain Behav. 2021;11:e02159. doi: 10.1002/brb3.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weissberg PL, Bennett MR. Atherosclerosis: An inflammatory disease. N. Engl. J. Med. 1999;340:1928–1929. doi: 10.1056/NEJM199906173402418. [DOI] [PubMed] [Google Scholar]
- 32.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 33.Herzog D, et al. Age at disease onset of inflammatory bowel disease is associated with later extraintestinal manifestations and complications. Eur. J. Gastroenterol. Hepatol. 2018;30:598–607. doi: 10.1097/meg.0000000000001072. [DOI] [PubMed] [Google Scholar]
- 34.Haroon NN, Paterson JM, Li P, Inman RD, Haroon N. Patients with ankylosing spondylitis have increased cardiovascular and cerebrovascular mortality: A population-based study. Ann. Intern. Med. 2015;163:409–416. doi: 10.7326/m14-2470. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article.