Abstract
Advances in medical technology do not follow a smooth process and are highly variable. Implementation can occasionally be rapid, but often faces varying degrees of resistance resulting at the very least in delayed implementation. Using qualitative comparative analysis, we have evaluated numerous technological advances from the perspective of how they were introduced, implemented, and opposed. Resistance varies from benign — often happening because of inertia or lack of resources to more active forms, including outright opposition using both appropriate and inappropriate methods to resist/delay changes in care. Today, even public health has become politicized, having nothing to do with the underlying science, but having catastrophic results. Two other corroding influences are marketing pressure from the private sector and vested interests in favor of one outcome or another. This also applies to governmental agencies. There are a number of ways in which papers have been buried including putting the thumb on the scale where reviewers can sabotage new ideas. Unless we learn to harness new technologies earlier in their life course and understand how to maneuver around the pillars of obstruction to their implementation, we will not be able to provide medical care at the forefront of technological capabilities.
Keywords: Technology advancement, Resistance to change, Peer review, Conflicts of interest, Editorial process
Introduction
This paper derives from the keynote address at the Central Association of Obstetricians and Gynecologists (CAOG) in 2021 delivered by one of us (MIE). It is a hybrid between a review of the process of implementation of new technologies, our personal experiences with resistance to new ideas, and a perspective commentary on such. The focus is on how implementation of new technologies happens in actual practice vs the theoretical constructs of how evidence-based evaluations should lead to progress. As such, it is intended to highlight some important issues on the subject that would not be clear if the manuscript were constrained by the standard structure for primary scientific or even generic review articles. Some critics have stated that the topic of resistance to change does not belong in a “academic” journal. We respectfully disagree and believe that the traditional academic and even basic science audience represents precisely the people who most need to understand the stated and unstated processes that determine whether a new medical technology becomes part of mainstream care in months, decades, or not at all.
For the past couple of decades, an increasing proportion of high-tech science, often emanating from nonmedical precursors, has worked its way into clinical practice. An ever-increasing amount of research now has industrial and entrepreneurial funding rather than traditional university and grant sources that were predominant for several previous decades [1–3]. We have written previously on how the change in funding from academic to business has also changed the ethical foundations of such research [4]. The net effect has diminished some of the typical restraints paramount in traditional academic publications and have been sometimes corroded by a blatant sales pitch mentality. As a consequence, the financial stakes of research have increased dramatically, and incentives to influence the process have likewise risen.
When I (MIE) was President of the CAOG in 2007, my keynote speaker was Dr. Ruth Hanft who was Deputy Assistant Secretary and Director of the office of Health Research, Statistics, and Technology in the Department of Health and Human Services in the Carter Administration. Her talk focused on the political realities of emerging advances and technology in medicine including, figuratively, the bullet trains to get there faster and the landmines on the tracks trying to stop progress. Her thesis and warning to us was that no matter how honorable and well-intentioned medical innovation might be, to the governmental establishment, bureaucrats, and some powerful constituencies, we are just another special interest group seeking favor — no different than the airlines, broccoli growers, or coal miners. To change anything, there has to be both good reason and widespread support from many key players — some of whom are purposely not visible.
My presidential talk in 2007, entitled “Overcoming Militant Mediocrity,” traced the evolution from individual physician autonomy and internalized responsibility to what I termed “amorphous accountability” using the analogy of being taught as a resident in the early 1980s to be Don Quixote — knocking down windmills to protect our patients, gradually evolving over the past 25 years into being the 3rd violin on the left — do not squeak [4]. In military terms, being a department chairman used to be like being a colonel; now you are a sergeant. You do not make policy; you just enforce it. Our thesis today blends Ruth’s philosophy, and what we believe she would say today were it possible for her to give it, combined with ours. However, it is now being played on a much rougher playing field where Robert’s Rules of Order are considered a useless anachronism.
The evolution and acceptance of new technology in medical practice have never been noted for smooth transitions. Rather, there are major leaps followed by periods of “dotting the i’s and crossing the t’s” at which time, the establishment, often begrudgingly, eventually adopts the new approach [5, 6]. Progress follows the model — not of a Pitocin induction with gradual linear progress, but more like a prostaglandin, with seemingly, nothing, nothing, and nothing happening — and then boom — it is done. However, change does not happen for the majority of providers, until they become uncomfortable with the current situation, and only then do they become motivated to change. Commonly, particularly in obstetrics, fear of litigation for not doing so is a primary motivating factor [5–7]. In this paper, we explore three different ways of thinking about progress or lack thereof: (1) using the analogy of strategies and player’s roles on a football team, (2) as a comparative analysis problem and exercise, and (3) as a general technology change issue.
Change as the Balance of Offense and Defense on a Football Team
Metaphorically, we think of overall progress as involving components of a football team. On all teams there is an offense comprising play makers (quarterbacks, running backs, and receivers) and infrastructure (offensive line).In science, we have basic and clinical innovators, translational academics who develop a basic or public health concept and show how it can be introduced. Then, there are implementors who deploy the concept into actual use [5, 6]. The infrastructure plows the road. In the technology assessment field, innovation is commonly described as having two phases — development in which there is a new idea, people work on it, test it, publish, patent, and tinker with it to tertiary or “quaternary” introduction. Then, there is diffusion, when the concept spreads out to the community and eventually becomes widespread and routine (Table 1).
Table 1.
Technology creation and assessment
| Development | Diffusion |
|---|---|
|
Innovators: Basic, clinical, translational Commonly but not always |
•Implementors |
| Academics | -Spread out into the community |
| Conceptualize | -Utilization grows rapidly |
| Study | -Complications skyrocket |
| Tinker | -Eventually calms down with experience |
| Publish | •Infrastructure |
| Patent? | -Insurance coverage begins |
| Infrastructure | -Goes from tertiary to routine |
| Plow the road for progress |
However, there is also a “defense” that may do whatever it can to protect the status quo and can push back with a trench warfare mentality against any changes. Here again, the infrastructure is analogous to the defensive tackles who stand in the way of progress and the playmakers (edge rushers who throttle a quarterback before he has the time to develop plays, defensive linemen and linebackers who stifle running backs before they can muscle forward, and shut down corners who try to prevent the innovative long ball). All are attempting to wreak havoc and blow up the enterprise. Most analyses have focused on the “offense.” Here we focus on the “defense.”
There are several types of defense or resistance employed depending upon the situation. When there are no obvious vested interests in place, the primary explanation of “defense” may simply be inertia, lack of will, or resources (including societal will and finances) to make something good happen. At the other extreme, when vested interests are entrenched and dominant, there can be fierce efforts at multiple levels, including a distortion of the peer review process to inhibit/prevent the concept getting well known.
The football metaphor identifies some of the players and seems to sensitize us to the basic idea that progress is a competition. The nexus of medical technology progress and resistance, however, is a complicated one. There are, for example, enormously important situations that fall outside of the central focus of the argument we wish to make. Egregious breakdowns in the normative protocols that govern the conduct of medical experiments, such as Tuskegee, are fundamentally dangerous. In the Tuskegee study, there was intentional malfeasance to patients — failing to alter the nature of treatment for syphilis after the discovery, vetting, and dissemination of penicillin [8]. This example serves as a compelling reminder of the obstetric mantra that Primum non Nocere (“first, do no harm”) must always be part of the calculation of change [4]. Certainly, Tuskegee speaks to a culture in which those who were not only primarily patients but also study participants, who had a good chance of treatment success, could nevertheless still be treated as being expendable if done in the name of fostering the advancement of medical science. The football analogy would be players in a game being expected to play while significantly hurt, play dirty in order to win the game, steal the signals from the other team, or bribe/blackmail the referee.
The football analogy, however, does not get us very deep into an analysis that can help us tease out important factors or move us beyond the shock that the construct of blatant malfeasance as in the Tuskegee experiment causes, nor does it help us understand more common situations in which there are cultural, reputational, political, or financial interests tied to existing and new technologies that predominate. Yet, these are indications that under certain conditions, the normal conduct of science can be undermined and corrupted.
Configurations of Factors Having Different Outcomes
We have identified several patterns, mostly here related to pregnancy management, with respect to both the speed and variability of adoption of newer technologies. Each pattern consists of a combination of characteristics: (1) the setting in which the technology has been introduced such as the level of organization and funding of the medical infrastructure or the degree of politicization surrounding medical innovations, (2) the relative advantages of the new technology (including the extent of the comfort with existing technologies) a (3) the level and extent of financial and reputational investment in existing technologies, and so on. Very similar developments may play out very differently depending on other, not always visible, factors that need to be considered. Our goal here is to find some order among these seemingly disparate examples of resistance and investigate the importance of a few factors that a case-by-case analysis suggests are relevant.
QCA Methods
To explore combinations of conditions or configurations of dimensions that may be associated with rapid adoption of new technologies, as opposed to mixed adoption, we use the general framework provided by qualitative comparative analysis (QCA). Developed by Charles Ragin (1987), QCA is particularly useful when phenomena are difficult to study, especially when context shapes the results of causal mechanisms, and samples are small [9]. QCA has been widely used in the social science, organizational, and medical literatures for decades [10, 11]. Its use has exploded since 2007 [12]. There have been numerous analyses of innovation more generally in different societies and contexts that have used such an approach [10, 12–17].
QCA is more deterministic than probabilistic. It fundamentally embraces conditionality so that the researcher is always sensitized to the combinations of conditions that might be influential rather than the possible influence of single factors that may have an influence “with other factors held constant.” QCA’s most interesting feature is that it permits active consideration of alternative combinations of conditions that might lead to a particular outcome (equifinality) — quite similar to a clinician’s consideration of alternate combinations of symptoms that might indicate the presence of a particular disease.
Table 2 summarizes the cases selected for analysis and the coding of causal factors deemed germane to the analysis, together with a code for the level of acceptance associated with each combination of causal factors [9]. Each row in the table contains the codes for the pertinent causal factors and the level of acceptance (the dependent variable, in red). We dichotomize these decisions for the sake of simplicity. Furthermore, a constant challenge for comparative research is limiting the number of factors considered so as to keep the number of discrete configurations or combinations of factors (which doubles with each additional causal factor) from becoming prohibitively high. Hence, we have limited the number of causal factors to five.
Table 2.
Table for configurational analysis (uppercase letters signify a “high” coding on a factor; lowercase letters signify a “low” coding on a factor)
| Cases | Compelling need (size of population at risk, serious medical problem, and inutility of existing technologies) [N,n] | Existing medical infrastructure development, funding, and organization [I,i] | Degree of political polarization as a source of pushback [P,p] | Strong marketing for one technology over another, including exaggerated claims of performance and risks [M,m] | Degree of vested reputational and financial investments in existing technology [V,v] | Level of acceptance [A,a] |
|---|---|---|---|---|---|---|
| Use of antibiotics in wartime to treat combat injuries | N | I | p | m | v | A |
| COVID-19 vaccine development and use to fight pandemic | N | I | P | m | v | a (bi-modal) |
| Rhogam (in the developed world) to combat Rh disease | N | I | p | m | v | A |
| Rhogam (in the developing world) to prevent Rh disease | N | I | p | m | v | a (slow) |
| Antenatal steroid therapy to prevent/reduce neonatal respiratory distress | N | I | p | m | v | a (delayed) |
| High MSAFP to screen for NTDs | N | I | p | m | v | A |
| Low MSAFP, multiple markers, and NT in Europe to screen for DS and other genetic abnormalities | N | I | p | m | v | A (rapid) |
| Low MSAFP, multiple markers, and NT in USA to screen for DS and other genetic abnormalities | N | I | p | m | v | A low (delayed) |
| NIPT for trisomy 21 and some other genetic abnormalities | N | I | p | M | v | A (rapid) |
| Microarray technology as a diagnostic test for abnormal copy number variants | N | I | p | M | v | a (bimodal) |
| Traditional electronic fetal monitoring to improve identification of risk for stillbirths and CP | N | I | p | m | v | A |
| New approaches to EFM | N | I | p | M | V | Too early to evaluate |
The causal factors we have chosen are defied here. (1) Compelling need is derived from three sources: the viability of existing options for treatment, the size of the at-risk population, and the seriousness of the condition. It is scored high (N) when the existing treatment options are limited, the condition is serious, the size of the at-risk population is large, and it is scored low (n) if otherwise. We will show that on occasion, there is a difference between the objective and perceived need. (2) Medical infrastructure is scored high (I) when the medical infrastructure of a nation is well-developed and funded, and low (i) otherwise. (3) Politicization is scored high (P) when the introduction of the new technology is highly politicized, and low (p) otherwise. Politicization could favor either existing technologies or new technologies. (4) Marketing is scored high (M) when the marketing efforts behind the distribution of the technology are both intense and characterized by exaggerated claims, and low (m) otherwise. 5. Vested interests are scored high (V) when there are both reputational and financial commitments defending the existing technology at the individual and/or organizational level, and low (v) otherwise. Finally, the outcome of interest is the nature of the Acceptance of the new technology. It is scored as high (A) when there is wide acceptance of the new technology with only modest pushback, and low (a) otherwise. If all five of the codes were positive, the configuration would be “NIPMV,” and if all were negative, it would be “nipmv.”
We analyzed thirteen diverse cases, and we are interested in understanding the sources of both high and low acceptances, whether the latter be ones that never gets off the ground, had delayed acceptance, or had uneven (often bimodal) acceptance. With 13 cases and 5 factors, we cannot make more than preliminary claims regarding causation, but we can highlight configurations of causes that are important to consider. Though we believe that the development of a tentative model is useful, we can reliably evaluate neither the consistency of configurations (the percentage of time that a particular configuration is associated with low acceptance) nor their coverage (the percentage of all cases of low acceptance that is associated with a particular configuration) because of the small number of cases (13) relative to the number of factors that we believe must be considered (5) and the modest number of cases associated with some of the configurations.
QCA Comparative Analysis Results
Compelling need and medical infrastructure have disproportionately high codes. All 13 cases studied have high compelling need — reflecting the fact that cases were chosen for inclusion in terms of their objective status as breakthrough technologies where the need was high. Adequate medical infrastructure was coded high in 12 of 13 cases. Both are clearly likely to be present when acceptance of medical technologies is high.
It might be of academic interest to examine cases such as the century’s long history of arguably nutritional supplements to assess the impact of variations in need, and where the claims of efficacy are often exaggerated in the spirit of snake oil sales. However, we have limited our sample of cases to what would generally be considered by knowledgeable people to have been real breakthroughs. Two points are in need of mention here. First, as a field matures, “next best alternatives” (i.e., existing technologies) will organically have become more widely adopted and then defended. Second, perception and reality do not always align for these cases — an application of the Thomas Theorem (1928): “If men define situations as real, they are real in their consequences” [18]. We have chosen to define need in terms of the longer-term, more objective underlying reality rather than various perceptions of it — though it is undeniable that perceptions alter how people react to a situation.
COVID, frankly, has dramatically reinforced our sense of the importance of doing so [19–23]. Some people have been persuaded that the virus was no worse than the flu, that it would disappear “in no time,” that vaccinations have side effects which are more dangerous than the disease, and that the vaccines that were developed are a plot to control humanity in some form. Such perceptions clearly alter the chances that “believers” would get vaccinated, wear masks, or avoid large crowds. However, that does not alter the fundamental truth that the virus in its several forms is inherently dangerous, that a lot of people were at risk, and that the existing treatments at the beginning of the pandemic were very limited in efficacy.
Similarly, physicians may be “comfortable enough” with existing treatments, may tend to disparage technologies that are not developed “in house,” and/or they may significantly underestimate the potential legal consequences of not migrating to the newer technology [4]. The factor of medical infrastructure, on the other hand, is little more than common sense: If the logistics for safely and reliably providing access do not exist, how could acceptance be accomplished? These two factors — in spite of being almost constant — do help us understand the importance of different configurations in causing lack of acceptance.
Leaving aside for the moment the single case in which there was inadequate infrastructure (Rhogam in developing countries), which resulted in lack of acceptance and implementation, let us interrogate the remaining 12 cases, of which 7 had high acceptance and 5 of which did not. QCA permits us to rigorously examine the alternative configurations of causal factors that are associated acceptance or lack thereof. Of the 7 high-acceptance cases, 6 had an NIpmv configuration of the category scores. Specifically, there was minimal or nonexistent politicization, low market intrusion, and minimal reputational and/or financial stakes in the existing technology, but there was high compelling need and adequate infrastructure. In our data, 85.7% of the time that the NIpmv configuration occurs, it is associated with high acceptance. In the language of QCA, this is a highly consistent pattern, albeit having only a modest number of cases.
The only alternative path to acceptance (NIpMv) involved noninvasive prenatal testing (NIPT), for which marketing intrusion has been pronounced (and in some cases, financial considerations appear to have been pronounced as well). NIPT is interesting because its level of acceptance was far greater than might be expected based upon other new screening methods, owing in part (we will argue) to its ability to provide early identification of fetal sex. The collateral damage was its capacity to undermine the integrity of the normal process of the conduct of science and the review process. This configuration, however, was associated with high acceptance only 14.5% of the time. As we will see, it is much more revealing when it comes to nonacceptance. In equation form (as is used routinely in QCA analysis), then, we can summarize the conditions of acceptance as follows:
| 1 |
This focuses attention on the fact that acceptance is achieved when high compelling need and high infrastructure are combined with low politicization, low market intrusion, and low prior existing vested interests.
For the 5 cases that did not gain widespread acceptance in a timely fashion, we can then ask the important question: In spite of a high compelling need and adequate medical infrastructure, how is it that wide and rapid acceptance was not forthcoming? In the 4 of the 5 cases in which acceptance was problematic in spite of having high compelling need and adequate infrastructure, politicization, marketing intrusion, or vested interests were present. The “ + ” sign (below) between the terms should be read as “or,” which introduces the real strength of QCA because it shows alternative ways that the same result can be achieved, with all combinations of conditions considered.
As before, these results may be summarized in equation form:
| 2 |
There was one instance of the NIPmv configuration, with heavy politicization, and it was associated with low acceptance (COVID). This means that in 100% of the time that such a configuration occurred, it was associated with low acceptance. But of course, it is only one case. The same is true for the configuration NIpmV, in which vested interests have distorted the process: There was only one case, and it was associated with low acceptance. There were three cases in which market interests were prominent, yielding the configuration, NIpMv. In two of these cases, 17-OHPC and microarray, the configuration was associated with low acceptance, and in one case, it was associated with high acceptance (NIPT). Typically, one uses the percent of time that a configuration is associated with an outcome (here, 67%), to decide whether a configuration is reliably associated with an outcome. A figure of 67% is a bit light for concluding even tentatively that market intrusion is associated with low acceptance.
What we can argue, with some confidence, is that market intrusion is associated with the risk of distortions in the normal process of vetting and adopting a new technology. In the case of NIPT, for example, there are clear signs of exaggerated claims both with respect to the value of NIPT and the procedural risks associated with obtaining specimens for microarrays. Hence, we are comfortable arguing that each of these factors (market intrusion [M], politicization [P], and vested interests [V]) has the potential to undermine the normal conduct of science and review.
Politicization, as we have seen from the COVID-19 fiasco, has been the most dangerous [19–23]. However, the over-hyping of the effectiveness of certain (bogus) tests, procedures, and treatments by the private sector can distort the normal operation in different ways. There were two cases of this: The case of microarray technology, which suffered from market intrusion at the same time that NIPT was profiting from it: Microarray technologies were being underutilized while NIPT was being overutilized [24–26]. Competition among various vested interests (companies and sometimes universities) often manifests in many forms, including conferences for marketing, promotions, and sometimes patent litigation.
Many individuals have had sources of income and reputation become tied to particular technologies. This is an entrenched form of vested interests. Suffice it to say at this point that there is a level of cultural inertia and individual comfortableness with existing technological approaches that must be overcome.
QCA Individual Cases and Discussion
To fully appreciate the results, we have tentatively shown in our comparative analysis of the acceptance of innovations the cases themselves — and some of the comparisons between them need greater discussion. An important consideration in the development and implementation of a new technology is the sense that there is a compelling need for it (Table 2). There are three parts to this assessment: The first is the size of the population at risk; the second is the seriousness of the condition; and the third is the relative inutility of existing technologies. When these are all combined, the extent to which a new technology fills a compelling need can be the cornerstone of a configuration that yields rapid acceptance, as we have seen from the QCA analysis.
However, this can break down in a couple of different ways, as perception and reality become discordant. First might be a situation in which the primary stumbling block to an orderly process of introduction of new technologies seems to be nothing more than what might be called cultural inertia. Such is characterized by a general lack of understanding of the advantages of newer approaches combined perhaps with an underestimation of the legal risks associated with not adopting newer approaches.
Rhogam
Given a sense of the compelling nature of a new technology, one straightforward source of divergent outcomes is the level of development and funding of the medical infrastructure. Sometimes the failure of a vaccination campaign or the adoption of new technologies may be attributable to nothing more than the fact that the basic medical infrastructure cannot support the measures needed to effectively negotiate the route towards introduction [27, 28]. Usually, the situation is more complicated. The capacity of the medical infrastructure is only part of a more complicated configuration of causes that together may be sufficient to sabotage successful implementation.
Before the introduction of Rhogam, since there was no adequate treatment of serious erythroblastosis fetalis (or RH disease), tens of thousands of newborns with such hemolytic disease died each year, and RH disease also caused brain damage in thousands more [29–32]. It was a huge problem demanding a solution (high compelling need). If compelling need was the only important factor, then the results would have been the same worldwide, but they were not. In the developed world, in spite of some resistance from the blood bank community (that was countered by two champions for Rhogam), adoption was reasonably rapid and very effective. Yet in the developing world, in spite of continuing NGO efforts to counter the problem, hundreds of thousands of infants have continued to die from Rh disease: The lack of a well-funded infrastructure has prevented the adoption of Rhogam in any meaningful way [32]. Hence, the adequacy of the medical infrastructure can create a divergence in the outcome of a new technological breakthrough that has no existing competition: When it is adequate, adoption can occur; when it is not adequate, in spite of the fact that the compelling need is high in both situations, adoption fails or is long-delayed and uneven.
Penicillin
The consideration of medical infrastructure segues into a discussion of a second configuration with a potentially divergent pattern. Alexander Fleming discovered penicillin in 1928, but it remained in limited quantity and was very expensive until several US drug companies were recruited to mass produce penicillin for the allied forces in WWII [33, 34]. The straightforward capacity to mass produce a drug that was going to primarily benefit soldiers fighting in a war also led to its wide civilian adoption in the US and acceptance by an overwhelming proportion of Americans (beyond the military). However, it is more plausible to believe that rapid and general acceptance was facilitated by the fact that the US population was galvanized by the attack on Pearl Harbor and united behind the war effort in a way that we have rarely seen since [35].
Thinking of this configurationally, three factors seem important: (1) A compelling need for such an antibiotic existed (huge numbers of soldiers were at risk and there were limited treatment options), (2) a capacity to mass produce and distribute the drug existed, and (3) importantly, a united population behind almost anything that facilitated the war effort was present. Under these conditions, wide acceptance was achieved both during and continuing after the war.
Antenatal Steroids
In the 1980s, antenatal steroid therapy to accelerate pulmonary maturation for premature human babies was developed, published, and introduced into general practice [36, 37]. The need was objectively compelling, and the manufacturing capability certainly existed. Furthermore, there was little politicization and only minimal intrusiveness from the market. However, the uptake was very slow at the beginning, having no reasonable scientific basis, particularly in the USA, where it was readily available. Mont Liggins, the New Zealand Professor who did the pioneering work on sheep, when asked why it took so long to be generally accepted, responded that many think that no good can come from the Colonies [38].
After multiple traditional educational efforts failed, the National Institute for Child Health and Human Development (NICHD) put on a consensus conference in 1994, producing a conference proceeding’s book, national news stories, and publications [39, 40]. The intent was to create a clear paper trail that would get the attention of practicing physicians (i.e., hit them over the head) to encourage them but also clearly warning that failure to follow the guidelines would be considered indefensible in court.
It took the stick to achieve the change that no carrot could. This strikes us as an example of cultural inertia rather than vested interests motivating resistance, yet the impact is similar: Physicians and institutions were just used to addressing problems of pulmonary maturation in “standard,” although inadequate, ways. The perception of the compellingness of the breakthrough, in other words, was out of line with the actual advantages of adopting the newer technology.
MSAFP Evolution
Amniotic fluid alpha fetoprotein to diagnose neural tube defects was an important advance first published in 1972 [41]. In some areas of the world, NTD incidence was almost 1%, and ultrasound was not yet in practice. However, since 95% of NTDs did not occur to known at risk families and universal amniocentesis was not feasible, maternal serum screening was initiated in 1973 as a screening test to determine who had high enough risk to warrant an amniocentesis — whose procedural risk was then commonly quoted as 2% [42].
The uptake of maternal serum alpha fetoprotein (MSAFP) was characterized by cultural inertia and some push back by those opposed because of an abortion option it might create. The acceptance of screening for NTDs and aneuploidy was much slower in the USA than in much of the developed world [43]. The delayed acceptance of MSAFP screening in the 1970s and 1980s was potentiated by academically powerful pundits who adopted a position that American obstetricians could not be trusted to use a screening test without having numerous patients prematurely terminating their pregnancies because of an abnormal screening test and not waiting for an actual diagnosis [43].
This assessment ignored decades of successful experience with Pap smears as a screening test for cervical cancer without patients having unnecessary or inappropriate hysterectomies before their actual diagnosis. Combined reticence from both ACOG and the American Academy of Pediatricians delayed the acceptance of MSAFP as it was practiced in the UK for several years. Actual successful data eventually moderated to a degree the perceptions of US physicians and institutions. In 1986, nearly a decade after the UK had routinely adopted MSAFP for NTDs, the American College of Obstetricians and Gynecologists (ACOG), in an attempt to protect its membership, sent a note within its routine monthly mailing to the membership warning that while ACOG did not see MSAFP screening as being required, failure to do so could expose obstetricians to litigation in the event of an affected baby. The mailing actually had exactly the opposite of the intended effect. For all practical purposes, it made offering MSAFP a required standard of care [44]. In a Machiavellian construct, the proper result was achieved by a method its authors never would have intended, i.e., increased medicolegal exposure [4].
With the introduction of low MSAFP for Down syndrome (DS) screening — also in the mid 1980s, the market, based upon patient interest, vastly increased [44]. However, the need for standardization of methodologies and programmatic methods was often ignored with serious consequences for statistical performance [44–47]. For example, in 1994 by when multiple markers had already replaced single MSAFP for DS screening, one major lab noted that 20% of its requisitions were still for single MSAFP (unpublished).
Resistance to adoption of MSAFP and later multiple markers for Down syndrome spans the spectrum from benign inertia to elements of opposition because of the abortion issue. Over the past 3 decades, acceptance of screening (by whatever methodology) has remained about 67%. However, it is not 67% of everyone’s practice. Some physicians have nearly 100% of their patients having screening or diagnosis [47]. Other practices have near 0% uptake suggesting that religious and political considerations of both physician and patients are a major driving force in decisions made.
Also, in the early 1990s, several papers — most prominently coming from the Fetal Medicine Foundation in London — demonstrated that an enlarged nuchal translucency (NT) measurement combined with free β hCG and PAPP-A, at about 12 weeks, was an improved marker for DS and many other conditions [48, 49]. NT measurement, however, requires a precise methodology that can achieve similar coefficients of variance for ultrasound measurements comparable to those expected for lab tests [50–52]. Even reasonably trained physicians or sonographers could not just begin to do NT scans and have accurate, reproducible readings. To avoid the well-known increase in mistakes occurring as a technology diffuses into practice, the FMF established standards and set up numerous training programs worldwide [50–52].
We ran the FMF’s US program. Multiple papers documented the teaching process, examinations of data to prove quality improvements over time leading to certification, and increasing statistical performance metrics of screening [53–55]. Later, the SMFM established its own program. Once again, the UK and Europe were faster to adopt the program with the required training and certification.
Many American physicians and organizations simply refused to accept that any specific training was necessary [45, 53–55]. Both ACOG and the Society for Maternal–Fetal Medicine (SMFM) were largely silent for several years. Screening utilization increased, but American performance was suboptimal showing poor quality control. We submitted a paper on the problems of NT screening in the USA to Obstetrics & Gynecology (the Green Journal) — the official journal of ACOG. The paper was rejected on the basis of what we considered to be bizarre reviewer criticisms [53]. A few weeks later, I happened to see Jim Scott, then the editor, told him the story, and that in 30 years of publishing and reviewing extensively for the Green journal, I had never before asked for a paper to be reconsidered. He told me to submit a revised version directly to him and respond to the reviewer’s criticisms. Looking at the actual comments from the original submission is very revealing.
Criticism: This is a small study.
Answer: No, this is actually the biggest such study ever done in the United States.
Criticism: The performance differences between the UK and US were not significant.
Answer: they were significant to p < 0.001.
Criticism: any differences did not have any clinical implication.
Answer: US OB GYNS missed at least 100 Down syndrome cases a year that would have been found with UK level performance [56].
The paper went from being rejected to being the lead article with a published editorial pleading for improved American performance [53].
COVID-19
Contrasting the development of penicillin with the development and distribution of COVID-19 vaccines, again, there was a compelling epidemiological need and a capacity for mass-producing different versions of the vaccine. What makes this configuration different from that surrounding the acceptance of penicillin is the extraordinary levels of politicization and polarization of the disease and its treatment from conservative politicians and the media [57]. The result of this polarization was a bifurcated level of acceptance, documented in state-by-state and county-by-county analyses over time, with more liberal and urban areas having much higher vaccination rates, and as a direct result, they had lower rates of onset, hospitalization, and mortality as compared to more conservative and more rural areas of the country.
Politicization not only undermined the acceptance of vaccines by the public, but it also weakened the stature of the Centers for Disease Control both nationally and internationally. Its independence was undermined impairing its credibility to provide coherent and consistent guidance regarding how to cope with the outbreak, weakening the credibility of its reports, and corrupting the normal scientific processes characterizing the institution [58]. The critical, differentiating condition in these configurations was the level of politicization and polarization — minimal in the case of penicillin and omnipresent in the case of COVID-19. Politicization cannot be thought of as being orthogonal to the other elements in the configuration. Not only was the credibility of vaccination efforts undermined, but bogus alternative treatments were touted. As a result, the normal practice of science was belittled, and myths about COVID-19 required constant debunking [59, 60].
Fetal Therapy and Fetal Reduction
A somewhat simpler example is represented by the initiation and development of fetal therapy and fetal reduction — simpler because there was an empty playing field until the first efforts of both were developed. The analysis of several technologies depends upon extensively when in the course of their development one is asking the question. For fetal therapy and fetal reduction, there was a clear, compelling need for them and the basis for early work. For fetal therapy, pioneers such as Michael Harrison and his group at University of California, San Francisco, spent nearly a decade with animal work to get to the point of the first attempts at open fetal surgery for diaphragmatic hernia [61]. Then, there were several years of initial clinical attempts with very limited support from obstetricians proximate to UCSF. In fact, four of the first 8 cases it took to achieve the first success came from MIE then in Detroit — nearly 2000 miles away [62]. Thus, there was compelling need, an infrastructure at least in limited centers to do the work, no real political resistance, no marketplace competition, and no prior existing vested interests. Organizations such as the International Fetal Medicine and Surgery Society were created and continues to exist as a worldwide collaboration of investigators freely sharing their expertise to move the field forward [63].
As fetal therapy developed, the number of centers multiplied to the point currently, where there is actually significant competition for patients. One of the issues as technology moves from development to diffusion is the many times observed history that the people who are the “second generation” to get into the field frankly are beaten to it by people who should not be second, because they do not have the experience and training [5, 6]. Rather, they see the marketing opportunity and seize upon it. Such leads to the general observation that as the transition occurs, the cases increase, and the complications skyrocket. Eventually, it sorts out.
For fetal reduction, the need was clear, and there were people with the expertise to perform it [64]. There was no real ability to have an animal model, and it was introduced by a tiny group including us that began it as an innovative, compassionate care procedure [65, 66]. The experiences lead to a number of individual and multicenter collaborative publications [67]. Now, almost 40 years later, a larger number of groups perform it, although there is wide variability of the services and expertise provided. The resistance mostly comes from those with moral objections to it, but at least historically, it has not been at a scale that has impeded either operation or progress.
17-OHPC
Differentiating conditions in developed societies may take forms other than politicization and polarization. A continuing challenge for health systems in such societies is balancing patient safety and efficacy against patient access, a problem that may be exacerbated as the private sector ineluctably becomes involved. Two loosely related examples of this involvement in the USA illustrate some of the complexities generated as the market insinuates itself into the development, distribution, and cost of new technologies: the prevention of preterm birth (PTB) and the screening for trisomy 21 and other genetic disorders.
Prevention of PTB is a paramount concern in modern obstetrics [68]. Multiple studies have shown variable to even diametrically opposing results for preventing PTB using various forms of progesterone, cerclages, and pessaries [68–70]. Thus, PTB clearly presents a compelling need, regardless of some variability as to the demarcation of weeks of gestation used in the definition of PTB. Vaginal suppository progesterone and some compounded versions of intra-muscular injection of 17α-hydroxyprogesterone caproate (17-OHPC) were used off-label and made inexpensively for years, the latter with recalls for poor quality [71].
A large multicenter international study under the direction of the Pregnancy Research Branch of NIH based at Wayne State University demonstrated significant efficacy of such suppositories [69–75]. Despite that, the US Preventative Services Task Force (USPTF) refused to endorse their use after performing a very unconventional and convoluted statistical analysis in which the USPTF ignored significant components of the study data and focused on only a subset (following the findings of one US-based center rather than the findings of three European centers) [69–75]. Concurrently, a company produced Makena and obtained FDA approval for weekly injections of 17α-hydroxyprogesterone caproate under an Accelerated Approval Program — set up to permit rapid development of drugs that addressed serious medical problems. The program was designed for situations for which there were only poor treatment options.
There were objections to the approval on the basis of somewhat flawed clinical trial (the placebo group had a higher rate of prior preterm births, a key risk factor for future potential future PTBs). Makena was then marketed with a price tag of over $1500 per week, generated revenue of over a billion dollars from 2015 to 2018, increasing the price of treatments by nearly 100-fold. Care could cost upwards of $30,000 per pregnancy vs. only several hundred for the suppositories. In response, the FDA formed an advisory group to review the evidence regarding Makena and its efficacy. First, the FDA in a very unusual posture allowed the home-brew compound pharmacy products to remain on the market. Then, the FDA revoked its authorization of Makena [74, 75]. This has been challenged.
Assessment of PTB treatments requires multiple levels of queries. First, the idea that a serious problem like PTB, with risks in part determined by a multitude of social determinants of health, could be treated as a single problem amenable to any single therapy is certainly not a given [69–75]. Second, the variability in quality of compounded treatments (which are not as tightly controlled as pharmaceutical company products but in which physicians had considerable confidence) needed to be understood. Third, a major multicenter study was methodologically flawed in that it had a higher level of risk factors in the placebo group than in the treatment group. Furthermore, the study ran almost 5 years longer than the average for such studies. The FDA emphasized the need for standardization, cited the recall problems of compounded 17-OHPC, and downplayed the utility of vaginal suppositories [76–78]. These approaches conflicted with the confidence that many physicians had developed in these treatments.
In short, the situation regarding the acceptance of Makena versus the alternative, cheaper, existing treatments for PTB as a compelling problem was murky and played out over time. Furthermore, the operation of the market and the operation of politicization are similar in that they both influence the other elements of their configurations. There was a compelling need for new approaches and the capacity to manufacture and deliver them. Nevertheless, the not-too-invisible hand of the market seems to have shaped processes at several points, perhaps untraceable, putting well-meaning efforts of the FDA to use the tools provided by the Accelerated Approval Program in the position of having to reverse course once the unintended consequence of a 100-fold price increase became apparent.
Configurationally, this leaves us with a compelling need, somewhat diminished treatment alternatives, a high capacity for producing FDA-approved 17-OHPC, and an ability to alter financial metrics by dramatically raising prices. This configuration resulted in a mixed scenario in which there was initial acceptance of Makena’s 17-OHPC until the FDA intervened by sanctioning the product. Rather than being complicated by mixed acceptance at the same point in time, as in the case with politicization, this divergence took place over time. The operation of the market, in short, can generate outcomes that can take years to bring back under control.
NIPT and Microarray Technologies
NIPT and microarray technologies were developed contemporaneously and offer some further considerations with respect to the operation of the market. Despite often misleading marketing, NIPT is a screening test from a maternal blood draw which has no real procedural risk attached; conversely, a microarray is a diagnostic test using samples from CVS or amniocentesis and carries a small, but nonzero, procedural risk. Microarrays were developed using traditional research approaches with NIH grant funded studies, multiple national conference presentations, and several refereed papers culminating in an NICHD funded multicenter study published in the New England Journal in 2012 [79–81]. Only then was it introduced into tertiary practice (Table 3).
Table 3.
Academia vs industry
| Microarray | NIPT |
|---|---|
| •Grants | •Industry money |
| •Multiple publications | •Engineers not physicians |
|
•NICHD multicenter trial - Published NEJM 2012 |
•Data fraud scandal |
| •Utilization increased slowly over last decade — primarily from tertiary centers | •One publication then heavily marketed |
| •Can find much more than NIPT | •Heavy sales push |
| •Utilization skyrockets primarily because of fetal sex identification | |
| •Procedures are suddenly dangerous | |
| •Finds much less than microarray |
In 2002, after fetal cells in the maternal blood were shown in an NICHD-funded multicenter trial — not to be ready for prime time [82], attention turned to cell-free fetal DNA commonly known as noninvasive prenatal testing (NIPT) [83, 84]. NIPT was primarily developed by industry, pioneered by engineers, not doctors, survived a serious data fraud scandal and hit the US market in 2012 (as a laboratory-developed test or “home brew”) after only a single refereed research paper was finally published [85] (Table 3).
These profiles generated very different acceptance rates. Heavy marketing generated acceptance for NIPT in the developed world, likely fueled because it provided early identification of fetal gender. Furthermore, laboratory sales staff also commonly convinced primary OB GYNS that NIPT could do “everything” that an amnio could and without the risk of procedures which suddenly had become “dangerous” [86, 87]. NIPT was initially approved for high-risk patients, but utilization rapidly crossed into the low-risk population attributable to fetal sex identification and the tremendous financial opportunity for the labs by logarithmically expanding the target market [25, 26]. NIPT seemed perfect for families who, as one commentor put it, “Don’t want no risk and don’t want no problems” [88].
In spite of the fact that the moral and practical concerns of parents may differ substantially, relatively high acceptance rates for NIPT also exist in low- and middle-income countries (close to 100 countries altogether at this point). Furthermore, there is growing concern among some circles that the “selling” of tests is undermining the traditional interests of doctors in their patients [4]. As a direct consequence of NIPT, utilization of CVS and amniocentesis has plummeted despite the fact that their specimens analyzed with microarray can often find nearly 10 × more problems than NIPT [24–26]. Such has resulted in what we have been called an “epidemic of abnormalities” missed because of NIPT. The medical care of missed cases’ costs in the USA is also about 3 times more than DS [24–26].
Electronic Fetal Monitoring
In the late 1960s and early 1970s, electronic fetal monitoring (EFM) was developed at a number of centers — perhaps most notably, the work of Dr. Edward Hon — first at Yale and then University of Southern California. It was intended to attack the problem of stillbirths of patients in labor, and early work showed it to be very successful at that [89, 90]. There was a compelling need, the infrastructure in the USA and much of the developed world to add the machinery to the armamentarium of labor and delivery, no prior existing technologies that worked across the board, and no political resistance to it. There was rapid acceptance and implementation long before extensive studies had been done. In many ways, it was analogous to the almost immediate discontinuation of diethylstilbestrol after Herbst et al. showed its association with clear cell adenocarcinoma in teenage girls who had been exposed in utero [91]. Eventually, its role morphed from one focused on the prevention of death to a more nuanced evaluation of fetal status and the prevention of morbidity. As the goal posts moved away from the very clear demarcation of dead/alive to fetal well-being, the ability of the technique to predict and prevent such became more problematic.
Earlier we discussed cultural inertia, operationalized as a certain level of comfort with existing technologies and a lack of appreciation of the possible legal consequences deriving from not using a newer technology, as a possible factor in the weakening of compelling need. While a case could be made that this is a mild expression of vested interests, we believe that vested interests should only be used when individuals have a reputational and/or financial stake in a particular technology. Consideration of neural tube defects beginning in the 1970s and Down syndrome in the 1980s as described above as resistance was more about the control of medical public policy by national organizations rather than the financial goals of proponents trying to develop such [43].
Some of the elements we have talked about before are represented here, such as a difference in adoption trajectories (even in the developed world) between the general area in which a breakthrough is made and more distant areas. However, the distortions generated by politicization and market pressure do not seem present here, at least directly.
There are four morals of this story. First, practitioners and institutions can become attached to particular ways of doing things — even well beyond their “time,” and they will act in ways to protect what is seen as their culture of care. A second source of attachment is their position in the “pecking order,” and these reputational factors can be enormously influential. Third, reputational factors become even more prominent when they are combined with perks, stipends, and/or profits from investments in these technologies. Fourth, vested interests can insinuate themselves into the normal review process and suppress the appearance and acceptance of newer approaches unless exceptional circumstances intervene. No system can depend on “exceptional circumstances” to realign the integrity of the process.
Vested interests can be a huge obstacle to change. However, the issue can work both ways because there is also a need to formally question papers that got published that should not have been. The number of papers that medical/scientific journals have retracted because of varying forms of malfeasance has increased logarithmically in the last 25 years [92–97]. The most notable example was the Lancet with Andrew Wakefield over the claimed association of autism from vaccines [97]. The retraction came only after a large effort from many quarters concerning the validity of his data and discovery of the author’s significant, undisclosed financial conflicts.
The acceptance of new technologies, therapeutics, and procedures into medical practice requires publications in the medical literature so that they can be peer reviewed, challenged, modified, improved, and finally considered suitable for use [97–99]. Only then can they get in line for insurance coverage required to potentiate widespread use. Without publications, concepts are treated, at best for a short period of time as investigational and preliminary, but depending upon the circumstances and who is proposing it, are often derided from the beginning as “junk science” [99].
Such a multistep vetting process has been applied to virtually every new concept — except NIPT 10 years ago and electronic fetal monitoring (EFM) 50 years ago. Both of them were rapidly implemented into widespread clinical care long before there were rigorous studies to verify their assertions. Once methods are established in practice, counterarguments have an uphill fight in trying to challenge them (Table 4).
Table 4.
Barriers to acceptance of technologies
| Offense | Defense |
|---|---|
|
•Studies •Publications •Modifications •Brought into tertiary practice •Diffuse to more general use •Fight for insurance coverage |
•Demanding multiple vetting steps before acceptance •Trashing selected new ideas as: -Junk science -Investigational -Preliminary •Major exceptions: -NIPT -Fetal monitoring |
The medical journals — particularly the ones with good reputations — are both the accelerators of concept acceptance, but they are also major gate keepers [97–100]. They keep out what they consider bad science, — and this is absolutely essential. However, they sometimes put their thumb on the scale favoring one side or the other in a controversy [97–100]. They sometimes downplay demands for rigor admitting privately that it was to supposedly minimize medicolegal exposures. Fights often concern concepts whose primary problem is that they can threaten the established groupthink or the privileged position of certain individuals, societies, or companies. Inertia, in short, can evolve into vested interests as reputations and income flows become attached to the relative dominance of particular technologies.
The more disruptive the technology, the more intense can be the pushback from those who control the current construct. There are some journals, notably the Lancet, which have as a primary mission to be the first to publish major new concepts. They tacitly acknowledge being willing to take some calculated risks of having to retract and admit mistakes in overreaching. We believe there is a place for such a journal philosophy as long as it is acknowledged. In contrast, the major establishment obstetrical journals have historically been extremely reticent to publish very disruptive studies that challenge the accepted order.
The tools through which journals exercise their authority is through the use of supposedly neutral referees and the discretion of the editors towards giving the benefit of the doubt to the offense or the defense [97, 98]. Of course, the majority of methods to achieve such never see the light of day, thus allowing for sometimes egregious commentaries to go unchallenged. As an example, over the past several years, our own work has focused on developing a new approach to EFM [101–113]. It has attacked the very poor performance metrics for EFM that even its own key opinion leaders admit and essentially proposed that various kinds of risk factors and uterine contraction frequency be used to contextualize EFM data and expand the clinical utility of EFM-based predictive models [114–116]. Conceptually, this should have been seen as a relatively minor change but with important impact, i.e., recognizing that the effectiveness of a technology might be conditioned by other factors [117–120]. Nevertheless, the resistance to even considering a new approach has been considerable, sometimes publicly including personal invectives even on paper. One EFM key opinion leader wrote in a published review paper: “another group has proposed a risk-scoring system, dubbed the Fetal Reserve index….” [121] (The bold is ours not the author’s.) In our opinion, the end effect is to try to hide new, promising approaches from the public using the privilege and hegemony of the review system. This is exemplified by some reviewer’s comments to these papers. For example (paraphrasing):
Criticism: Even a highly significant case/control series (on this subject) cannot be published. The authors must first do a very large randomized clinical trial (costing millions of dollars) proving it works. However, since the size of such a trial required would be virtually impossible to accomplish, the authors should just give up.
Answer: Not all science is best evaluated or requires a randomized trial design, as they typically are high on internal validity but low on external validity. Bluntly, there has never been a randomized trial showing that if you jump out of an airplane, having a parachute is good for you [122]. There are a lot of anecdotal data, you can call that a case control series, and if there were a randomized trial, would you volunteer for it? There are many legitimate study designs. For rare occurrence conditions, case control and cohort designs are very common and completely accepted. Actually, this is how most molecular markers for genetic disorders and cancer have been introduced [79–81].
Criticism: Everyone knows all cerebral palsy (CP) is genetic, so go study the genetics for 10 years first.
Answer: No, not all CP is genetic, and don’t expect the people in the United States, let alone Somalia, to get such testing anytime soon.
Criticism: in studies in which we used fetal scalp sample data from the 1st stage, the reviewer trashed any use of scalp samples as completely bogus and unsuitable for publication. They cited in 2020 a 1974 study in the South African Medical Journal [123]. Never mind that paper was largely discounted at the time, and fetal scalp sampling continued to be routine well into the 1980s — years after that paper. That paper argued that scalp sample pH taken from areas of caput when the fetus was crowning was different than areas without caput and as such scalp samples were useless.
Answer: Such differences at the very last minute of labor have absolutely no relevance to samples taken at 4cm, when for 99% of cases, there is no caput yet.
The unifying theme to all these criticisms is that they lead to the primary rejection of the papers with no opportunity to respond to even absurd criticisms. All these papers were eventually published by other reputable journals [101–113]. If they are looking for it, editors and experienced reviewers can usually distinguish between a legitimate review and “hit jobs.” The latter often have a litany of criticisms about failure to discuss this or that which were clearly already in the paper. We have also seen passive aggressive maneuvers such as having submissions buried for months at journals before a bogus review was belatedly issued that rejected the paper.
Other mechanisms include having excellent papers be declined immediately without any outside review that would leave a paper trail, choosing reviewers who conveniently do not understand or misconstrue the new technology or decide no paper can be published until every last-minute detail of a new approach has been completely vetted. When disruptive methods do get published, but in less prominent journals, then attacks on the new technology say: This cannot be important because it has not been published in the very top journals (circular logic). As Upton Sinclair noted: “Never expect a man to understand something, if his livelihood depends upon not understanding it” [124]. Dr. Roberto Romero, a longtime friend and colleague, often laments in his lectures the sayings of William James, a nineteenth century physician and philosopher: “First, they say it’s not true. Then they admit it’s true, but it’s not important. Finally, they say it’s true and important, but it’s not new” [125].
All of this can produce a paralyzing “don’t rock the boat mentality.” To quote President Andrew Shepherd from the movie The American President: “I was so busy keeping my job, I forgot to do my job” [126]. We have noted that some journals may develop a strategy of being the place to publish disruptive ideas. We would argue that a different mechanism might be useful, one in which the capacity of authors to effectively respond to criticisms is strengthened. There needs to be an automatic rebuttal right against alleged “hit jobs” by journals, editors, and reviewers. If authors do not get an opportunity to respond to the criticisms, even outlandish reviewer claims (made for whatever reason) can mostly go unchecked, as most editors do not go back and review a paper in depth before rendering a judgment. Some social science journals have from time to time permitted simultaneous publication of articles, critiques, and responses when an issue holds promise of being enlightening both for the readership and for the defenders of each position. Put simply, published critiques tend to be more well-reasoned and fact-based.
Beyond journal reviews, the scientific method that has been long accepted is one of looking at a status quo, formulating a hypothesis to better explain or treat it, reporting the results, and if successful, having it be accepted to become standard. There are incentives for both the offense and the defense. Eventually, the process starts all over again for the next generation of development. Politicization, market influences, and vested interests can distort the normal operation of scientific review — and even override it, as can be seen in the case of NIPT. And there are, of course, extraordinary examples of fraud and greed with respect to new procedures and drug prices that do not even need to be mentioned to be understood.
Resistance to change is often substantively and morally legitimate and may involve analysis of the trade-offs of new capabilities at the expense of losing others. For example, NIPT does allow more patients to have access to a genetic evaluation, but it actually provides considerably less information for the individual patient than diagnostic procedures with modern laboratory analyses [24–26, 85, 86]. Reasonable people can have different opinions as to where they fall on that issue, and there is an extensive literature espousing viewpoints all along the spectrum. The inputs into the “tradeoff,” in such a debate however, often go far beyond typical scientific metrics such as comparing increased sensitivity for increased costs. Religious, academic, political, ideological, and economic factors are often unstated, but may actually form the basis — consciously or unconsciously — for decisions [125]. Indeed, as we have seen in the multidimensional examples we have discussed, market pressures, politicization, and vested interests may be vitally important in determining at least the short-term acceptance of newer technologies.
Borrowing the Logic of Multidisciplinary Marketing Studies
Looking at all this through a different lens might be helpful. Marketing studies, which have been built on decades of legitimate and sometimes academic research, routinely describe the population as “early, middle, and late adopters” of something new. This is usually graphed as a typical bell curve. The same concepts apply to medical technologies. Here, building upon Geoffrey Moore (a marketing expert), 5 groups have been identified (Fig. 1) [7].
Innovators pursue new technology aggressively — often seeking them out before formal introduction. Traditionally in medicine, such has been done mostly in academic settings under IRB supervision. Lately, new genetic technologies such as NIPT were developed primarily in industry by engineers and Ph.D.’s and rapidly introduced — often as laboratory-developed tests (previously called “home brews”) circumnavigating many FDA regulations.
Early adopters buy into the new approach early in the life cycle, but they are not technology buffs. Rather, they find it easy to imagine, understand, and appreciate the benefits of the new technology. Robotic surgery is a good example.
Early majority share some of the early adopter’s ability to relate to the new technology, but they are driven by a strong primacy of practicality in their marketplace. They have a “wait and see” approach before taking the plunge. Uptake of NT screening in the USA was mostly divided into the early and late majority groups.
Late majority are fairly similar to the early majority, but they do not implement until something has become a clearly established standard and, even then, need “hand holding” and support. Steroids for prematurity are a classic example.
Laggards simply do not want anything to do with the new technology. They will only change when absolutely forced to do so. People, still using single MSAFP years after multiple markers became standard, would fall in this category. From a “marketing” perspective, the general consensus is not to waste your time trying to convince them. The return is not worth the effort. In medical practice, however, we do not have that luxury.
Fig. 1.
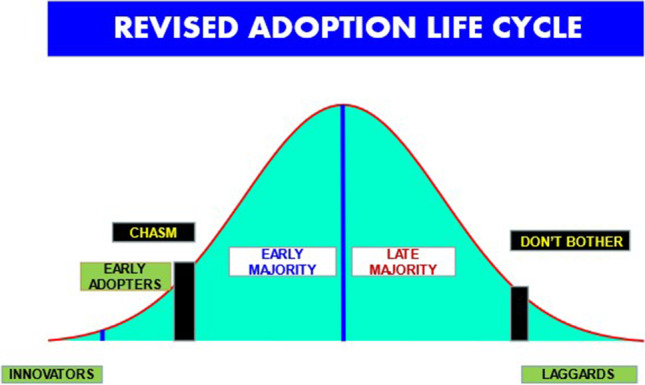
Progression across the groups is neither smooth nor linear
Progression across the groups is not smooth. It merely papers over the reality. In fact, there really is a wide chasm between the combination of the innovators plus early adopter groups as distinct from the remaining categories. There are substantial differences in mentality across the divide. Breaching the chasm — in effect landing on Omaha Beach — to get the early majority group to accept a new technology requires a complete reframing of the enterprise. A practical hook is needed to produce a substantive, conceptual pivot presented to the community from a “product/technology centric focus” to a “market centric” emphasis — without the extreme market pressure that we have seen can distort the adoption process. Each step requires a different promotional “pitch” focusing on the issues that are most important to the current focus group which may have been completely irrelevant to the previous one. Sustainable success only comes by co-opting the majority.
Twenty-five years ago, I was asked to lecture on technology assessment to the ACOG leadership at the Annual Clinical Meeting. My argument then, and ours now, is that ACOG worries much too much about the borderline between lousy practice and malpractice (i.e., the border between the late majority and the laggards) — and not enough about the transition from early adopters to early majority [6, 7].
The reality is that for the successful introduction of any new technology, it must overcome a number of obstacles. Strategies will obviously vary by situation, but the principles are essentially the same. To quote Moore: “Almost all successful crossings happen in business markets where the economic and technical resources can absorb the challenges of an immature product and service offerings” [7].
Even before COVID, the world had become essentially borderless with a well-developed connectivity of academicians. More recently, however, multinational billion-dollar companies have become dominant, and the stakes for “holding the high ground” have increased dramatically. When people’s academic reputations, incomes, and position on the “pecking order” become threatened, the guard rails can come off. Everything can become vicious. However, this is not new. Even the Nobel laureate Max Planck said over a 100 years ago: “A new scientific truth does not triumph by convincing its opponents and making them see the light, but rather because its opponents eventually die, and a new generation grows up that is familiar with it” [127].
The standards of business conflict have to a considerable extent replaced the Hippocratic ethical foundation of medicine. Primum non nocere is now often loosely translated as “no harm in just once” [4]. In the early 1990s, I took, a “mini-MBA” course sponsored by ACOG, and Wyeth run by Harvard business school faculty which was designed for OB GYN chairs and emerging national leaders. They used a case study in which we were given the ground rules of the game, but the upshot was that the only way to win was to cheat — and that was the lesson the businesspeople taught us we were supposed to learn.
How Do We Fix This?
The examples cited here represent a microcosm of the interplay between the new and the old realities. The players and coaches for both offense and defense have gotten better, more sophisticated, and much more aggressive. As compared to most industries, medicine has been on the very slow end to adopt new technologies [4]. Furthermore, obstetrician and gynecologists tend to be at the slower, “by the book,” end of the medicine spectrum, and on the whole, are much less entrepreneurial and open to changing paradigms than some other specialties. This is in considerable part out of fear of liability exposure for deviating off the “yellow brick road.” Something “new” is usually initially internalized primarily as an exposure risk. Only when failure to implement something emerges to carry more of an exposure than not does the switch flips.
Unfortunately, the academic establishment has become complicit, in part, because the criteria to be appointed to leadership are now dramatically different than when I began my career [1]. The vast majority of doctors under age 50 do not even know that it was ever different. Department chairs are now primarily middle-management business administrators rather than academic scholars and role models. The shift of culture is that doctors now mostly see themselves as having a job rather than a career. Having corporate executives with MBAs rather than clinicians controlling medical care has altered much of the incentive structure of even academic medicine. Faculties are still routinely told that they are judged by their overall performance in education, research, and patient care. However, when they find out they are actually only rewarded based on their practice receipts, most then give short shrift to the other two and get little pushback from administration. Some departments forbid faculty to do any clinical research without dedicated funding to compensate for their lost time (from patient care). Doing research, even on your own time, is now often considered unacceptable (private conversation). Hence, developing ways of analyzing and studying new ideas at the national and local levels need to be found.
The cadre of investigators at both the basic and translational levels has shrunk considerably, dedicated research time is a rare luxury, and the direct and indirect fundings to support such endeavors have largely diminished [127]. Multicenter research collaboratives such as the Maternal–Fetal Medicine (MFM) Network and Gynecologic Oncology Group (GOG) have in many fields had to replace individual centers of excellence to obtain funding and generate enough data to produce anything meaningful. The result is a homogenization of thought, diminishment of creativity, and a hegemony of mediocrity of lowest common denominator groupthink that makes the critical breakthrough by an iconoclastic investigator that much more difficult to occur.
The hit is particularly prominent in the development phase of technology. We have to engender a culture of encouraging innovation and individualization of approaches, while supporting baseline quality of care standardization for routine situations. As a resident, I vividly remember a faculty member who could recite 98 reasons not to try something new but could never visualize the 2 compelling reasons to actually do so. Conceptually, an expert pontificates on the old ideas. An entrepreneur creates new ones.
At the same time, outside control of medicine has led to the denigration of the concept of actually requiring scientific rigor in our decision making and policy prescriptions. Examples include the tragedy of partisan politics in the resistance against COVID-19 prevention and bogus treatments, e.g., Ivermectin [128]. We have only ourselves to blame for lowering the bar for gibberish to be given credibility, but unfortunately, it is now a significant part of the overall resistance to change that hampers advancement in all endeavors. Until we understand a problem and the motives, we have little ability to fix it. As Winston Churchill said: “you can always count on the Americans to get it right, but only after they have tried everything else first” [129].
We need a new model for balancing the potential of cutting-edge science, the ability to get around or through the barriers of intransigence, yet have respect for the responsibility for excellence and integrity required to ensure that a new generation of vested interests just does not replace the old ones. We also need to understand the balance in the new world between academically funded and industry-funded research, yet we must realize that many of the paradigms of clinical care as well as research need to be rethought in light of new budget realities that will require us to achieve far more using less resources [130–132]. The logarithmic increase in our electronic ability to communicate globally [127] has dented but not eliminated the stranglehold of the Brahmins of the last generation. New platforms for information do allow communicating outside of the hierarchical establishment creating the potential for instantaneous awareness among vast numbers of physicians and policy makers, but they are still considered second tier and denigrated [133, 134]. Historically, physicians, MASH not-withstanding, have had higher than average respect for societal authority, so mobilizing large-scale change will be difficult. However, as a younger generation of physicians, having grown up in the computer age, moves into positions of substantive leadership, multiple collateral circulations around roadblocks should be possible [134]. We need them now. Traditional journals offer a time-tested way of regulating such efforts by exposing them to peer review. These need to be expanded so that the potential for theoretical and empirically based exchanges may take place — emulating and expanding the common practice of publishing commentaries and responses. These efforts could be supplemented by the emerging presence of electronic vehicles for distributing research and permitting closer examination and exchange of ideas in real time.
A fuller understanding of the obstacles to the introduction of new technologies — over and above — the actual scientific base of proving it actually works — is required to advance care and quality of life. With a few notable exceptions such as the COVID vaccine — as a worldwide crisis, medicine has been a late majority to a laggard player. Much of the resistance has been the dominance of what should be peripheral issues, but the Wizard of Oz was behind the curtain pulling the strings. Patients are commonly told that we cannot treat something until we have diagnosed it. Until we come to grips with the entirety of the breadth and depth of both legitimate and corrupt resistance to change, we will not be able to escape the morass.
Data Availability
All previously published.
Code Availability
N/A.
Declarations
Ethics/Institutional Review Board
This manuscript does not present any original patient study data or reveal any patient data, so no IRB approval was needed.
Consent to Participate
N/A
Consent for Publication
N/A
Conflict of Interest
Dr. Evans and Britt have publications on some of the topics detailed. Dr. Evans has patents on the Fetal Reserve Index (which is not mentioned in the manuscript), but the topic of electronic fetal monitoring is.
Footnotes
Keynote address at the 88th annual meeting of the Central Association of Obstetricians and Gynecologists, Napa California October 6–9, 2021
References
- 1.McGeary Michael, Hanna Kathi E., eds. (2004). Sources of funding for biomedical research. Strategies to Leverage Research Funding. pp. 37–54. ISBN 978–0–309–09277–7.
- 2.Mervis, Jeffrey (2017). Data check: U.S. Government share of basic research funding falls below 50%. Science. 10.1126/science.aal0890.
- 3.Slaughter S, Rhoades G. The emergence of a competitiveness research and development policy coalition and the commercialization of academic science and technology. Sci Technol Human Values. 2016;21(3):303–339. doi: 10.1177/016224399602100303.JSTOR689710. [DOI] [Google Scholar]
- 4.Evans MI. Overcoming militant mediocrity. Am J Obstet Gynecol. 2008;198:656–661. doi: 10.1016/j.ajog.2007.11.056. [DOI] [PubMed] [Google Scholar]
- 5.Cohen A, Hanft R. Policy directions for effctive evaluation and management. In: Hanft ABCaRS, editor. Technology in American health care. Ann Arbor, MI: University of Michigan Press; 1994. p. 480.
- 6.Evans M, Hanft R. The introduction of new technologies. ACOG Clinical Seminars. Washington: ACOG; 1997. p. 3.
- 7.Moore GA: Crossing the chasm: marketing and selling discruptive products to mainstream customers (3rd ed) Harper Business New York 2014.
- 8.The Tuskegee timeline. Centers for Disease Control and Prevention https://www.cdc.gov/tuskegee/timeline.htm
- 9.Ragin, C. (1987). The comparative method. University of California Press.
- 10.Fernández-Esquinas M, Sánchez-Rodríguez MI, Pedraza-Rodríguez JA, et al. The use of QCA in science, technology and innovation studies: a review of the literature and an empirical application to knowledge transfer. Scientometrics. 2021;126:6349–6382. doi: 10.1007/s11192-021-04012-y. [DOI] [Google Scholar]
- 11.Britt DW. Beyond elaborating the obvious: context-dependent parent-involvement scenarios in a preschool program. Appl Behav Sci Rev 6,179–197.
- 12.Roig-Tierno N, Gonzalez-Cruz TF, Llopis-Martinez J. An overview of qualitative comparative analysis: a bibliometric analysis. J Innov Knowl. 2017;2:15–23. doi: 10.1016/j.jik.2016.12.002. [DOI] [Google Scholar]
- 13.Álvarez-Coque JMG, Mas-Verdú F, Roig-Tierno N. Technological innovation versus non-technological innovation: different conditions in different regional contexts? Qual Quant. 2017;51(5):1955–1967. doi: 10.1007/s11135-016-0394-2. [DOI] [Google Scholar]
- 14.Arundel A. Rethinking the effect of risk aversion on the benefits of service innovations in public administration agencies. Res Policy. 2017;46(5):900–910. doi: 10.1016/j.respol.2017.03.009. [DOI] [Google Scholar]
- 15.Ganter A, Hecker A. Configurational paths to organizational innovation: qualitative comparative analyses of antecedents and contingencies. J Bus Res. 2014;67(6):1285–1292. doi: 10.1016/j.jbusres.2013.03.004. [DOI] [Google Scholar]
- 16.Leal-Rodríguez AL, Ariza-Montes JA, Roldán JL, Leal-Millán AG. Absorptive capacity, innovation and cultural barriers: a conditional mediation model. J Bus Res. 2014;67:763–768. doi: 10.1016/j.jbusres.2013.11.041. [DOI] [Google Scholar]
- 17.Rui Li, QT and Wang Y, A qualitative comparative analysis (QCA) of innovation network attributes, 2015 International Conference on Logistics, Informatics and Service Sciences (LISS), 2015, pp. 1–6, 10.1109/LISS.2015.7369615.
- 18.Thomas WI, Thomas DS. The child in America: behavior problems and programs. New York: Knopf; 1928. pp. 571–572. [Google Scholar]
- 19.Mylan S, Hardman C. COVID-19, cults, and the ani-vax movement. Lancet. 2021;397:1181. doi: 10.1016/S0140-6736(21)00443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burgess RA, Osborne RH, Yongabi KA, Greenhalgh T, Gurdasani D, Kang G, et al. The COVID-19 vaccine rush: participatory community engagement matters more than ever. Lancet. 2021;397:8–10. doi: 10.1016/S0140-6736(20)32642-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothwell J, Makrisis C: Politics is wrecking America’s pandemic response. https://www.brookings.edu/blog/up-front/2020/09/17/politics-is-wrecking-americas-pandemic-response/ Accessed 10/5/21
- 22.Kates J, Tolbert J, Orgera K (2021) The red/blue divide in COVID-19 vaccination rates. Kaiser Family Foundation (https://www.kff.org/policy-watch/the-red-blue-divide-in-covid-19-vaccination-rates/, downloaded from the KFF website 10/20/21)
- 23.Dr. Fauci says the Covid outbreak isn’t a political issues: “you can’t run away from the data.” https://www.cnbc.com/2020/11/17/dr-fauci-says-the-covid-outbreak-isnt-a-political-issue-you-cant-run-away-from-the-data.html
- 24.Evans MI, Andriole S, Curtis J, Evans SM, Kessler AA, Rubenstein AF. The epidemic of abnormal copy number variants missed because of reliance upon reliance upon noninvasive prenatal screening. Prenat Diagn. 2018;38:730–734. doi: 10.1002/pd.5275. [DOI] [PubMed] [Google Scholar]
- 25.Evans MI, Wapner RJ, Berkowitz RL. Non invasive prenatal screening or advanced diagnostic testing: caveat emptor. Am J Obstet Gynecol. 2016;215:298–305. doi: 10.1016/j.ajog.2016.04.029. [DOI] [PubMed] [Google Scholar]
- 26.Evans MI, Evans SM, Bennett TA, Wapner RJ. The price of abandoning diagnostic testing for cell free fetal DNA Screening. Prenat Diagn. 2018;38:243–245. doi: 10.1002/pd5226. [DOI] [PubMed] [Google Scholar]
- 27.Glatman-Freedman A, Cohen M-L, Nichols KA, Porges RF, Saludes IR, et al. (2010) Factors affecting the introduction of new vaccines to poor nations: a comparative study of the haemophilus influenzae type B and hepatitis B vaccines. PLoS ONE 5(11): e13802. 10.1371/journal.pone.0013802 (available online: http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0013802) [DOI] [PMC free article] [PubMed]
- 28.Maternal Mortality statistics: https://ourworldindata.org/maternal-mortality accessed 10/5/21
- 29.Freda VJ, Gorman JG, Pollack W, Bowe E. Prevention of Rh hemolytic disease – 10 years’ clinical experience with Rh immune globulin N Engl. J Med. 1975;292:1014–1016. doi: 10.1056/NEJM197505082921906. [DOI] [PubMed] [Google Scholar]
- 30.Zipursky A, Paul VK. The global burden of Rh disease. Arch Dis Child Fetal Neonatal Ed. 2011;196:F84–F85. doi: 10.1136/adc.2009.181172. [DOI] [PubMed] [Google Scholar]
- 31.Eastman NJ, Hellman LM. Williams Obstetrics. 13. New York: Appelton-Century Crofts; 1966. pp. 1010–1030. [Google Scholar]
- 32.Neighbor J. (2018) RhoGAM at 50: a Columbia drug still saving lives of newborns. Downloaded from the Columbia University Irving Medical Center website on 10/19/21 (https://www.cuimc.columbia.edu/news/rhogam-50-columbia-drug-still-saving-lives-newborns)
- 33.Bernard D: How a miracle drug changed the fight against infection during World War II. Wash Post July 11, 2020. https://www.washingtonpost.com/history/2020/07/11/penicillin-coronavirus-florey-wwii-infection/
- 34.The National WWII Museum. “Thanks to penicillin…he will come home!” The challenge of mass production. Downloaded from www.nationalww2museum.org , May, 2022).
- 35.Shirley C, Mauer S (2016). The attack on Pearl Harbor united Americans like no other event in our history: 75 years ago was he last time the nation truly came together. Washington Post: Post everything 12/07/2016 (downloaded May, 2022).
- 36.Crowly PA. Antenatal corticosteroid therapy: a meta-analysis of the randomized trials 1972–1994. Am J Obstet Gynecol. 1995;173:322–355. doi: 10.1016/0002-9378(95)90222-8. [DOI] [PubMed] [Google Scholar]
- 37.Roberts D, Brown J, Medley N, Dalziel SR: Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev 2017;CDC004454 10.1002/14651858.CD004454.pub3 [DOI] [PMC free article] [PubMed]
- 38.Norwitz ER, Greenberg JA. Beyond antenatal corticosteroids: what did Mont Liggins teach us? Rev Obstet Gynecol. 2010;3(3):79–80. [PMC free article] [PubMed] [Google Scholar]
- 39.Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Statement. 1994 Feb 28-Mar 2;12(2):1–24. PMID: 7728157. [PubMed]
- 40.Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Development panel on the effect of corticosteroids for fetal maturation on perinatal outcomes. JAMA 1995;273:413–418 [DOI] [PubMed]
- 41.Brock DJH, Sutcliffe. Alpha-fetoprotein in the antenatal diagnosis of anencephaly and spina bifida. Lancet 1972;2:197–199 [DOI] [PubMed]
- 42.Brock DJH, Bolton AE, Monaghan JM. Prenatal diagnosis of anencephaly through maternal serum alpha-fetoprotein measurements. Lancet ii:923–924, 1973. [DOI] [PubMed]
- 43.Gastel B, Haddow JE, Fletcher JC, Neale A: Maternal serum alpha-fetoprotein: issues in the prenatal screening an diagnosis of neural tube defects. NCHCT Conference Proceedings. US Gov’t Printing Office. Washington DC 1981.
- 44.Cuckle HS. Maternal serum screening policy for Down’s syndrome. The Lancet. 1992;340:799. doi: 10.1016/0140-6736(92)92349-K. [DOI] [PubMed] [Google Scholar]
- 45.Evans MI, O’Brien JE, Dvorin E, et al. Standardization of methods reduces variability: explanation for the historical discrepancies in biochemical screening. Genet Test. 2003;7:81–83. doi: 10.1089/109065703321561001. [DOI] [PubMed] [Google Scholar]
- 46.Brooks K, Chik L, O’Brien JE, Critchfield G, Ayoub M, Johnson MP, Evans MI. Variability of adjustments to indices in determining risk in biochemical screening. Fetal Diagn Ther. 1999;14(1):41–46. doi: 10.1159/000020887. [DOI] [PubMed] [Google Scholar]
- 47.Evans MI, O'Brien JE, Dvorin E, Johnson MP. Biochemical screening. Curr Opin Obstet Gynecol. 1994;6:453–458. doi: 10.1097/00001703-199410000-00011. [DOI] [PubMed] [Google Scholar]
- 48.Nicolaides KH: The 11-14 week scan. Fetal Medicine Foundation, London 2004.
- 49.Nicolaides KH. Screening for fetal aneuploidies at 11–13 weeks. Prenat Diagn. 2011;31:7–15. doi: 10.1002/pd.2637. [DOI] [PubMed] [Google Scholar]
- 50.Cuckle H, Platt LD, Thornburg LL, Bromley B, Fuchs K, Abuhamad A, et al. Nuchal translucency quality review (NTQR) program: first one and half million results. Ultrasound Obstet Gynecol. 2015;45:199–204. doi: 10.1002/uog.13390. [DOI] [PubMed] [Google Scholar]
- 51.Nicolaides KH, Bindra R, Heath V, Cicero S. One-stop clinic for assessment of risk of chromosomal defects at 12 weeks of gestation. J Matern Fetal Neonatal Med. 2002;12(1):9–18. doi: 10.1080/jmf.12.1.9.18. [DOI] [PubMed] [Google Scholar]
- 52.Evans MI, Van Decruyes H, Nicolaides KH. Nuchal translucency (NT) measurements for 1st trimester screening: the “price” of inaccuracy. Fetal Diagn Ther. 2007;22:401–404. doi: 10.1159/000106342. [DOI] [PubMed] [Google Scholar]
- 53.Evans MI, Krantz DA, Hallahan TW, Sherwin JE. Undermeasurement of nuchal translucencies: implications for screening. Obstet Gynecol. 2010;116:815–818. doi: 10.1097/AOG.0b013e3181f23ae3. [DOI] [PubMed] [Google Scholar]
- 54.Evans MI, Krantz D, Hallahan T, Sherwin J. Impact of NT credentialing by FMF, NTQR, or both upon screening distribution and performance. Ultrasound Obstet Gynecol. 2012;39:181–184. doi: 10.1002/uog.9023. [DOI] [PubMed] [Google Scholar]
- 55.Evans MI, Krantz DA, Hallahan T, Sherwin J, Britt DW: Quality of nuchal translucency measurements correlates with broader aspects of program rigor and culture of excellence. Fetal Diagn Ther 2013;33:230–234 10.1159/000346418 [DOI] [PubMed]
- 56.Evans MI, Brabbing-Goldstein D, Evans SM, Yaron Y: Prenatal diagnosis in the molecular age – indications, procedures, and laboratory techniques in Boardman JP, Groves A, Ramasethu J eds: Avery & MacDonald’s neonatology: pathophysiology and management of the newborn (8th ed) Wolters Kluwers/Lippincott Williams and Wilkins Publishing Co. Philadelphia, PA 2021 pp 109–136.
- 57.Stroebe W, vanDellen MR, Abakoumkin G, et al. Politicization of COVID-19 health-protective behaviors in the United States: longitudinal and cross-national evidence. PLoS ONE. 2021;16(10):e0256740. doi: 10.1371/journal.pone.0256740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hart PS, Chinn S, Soroka S. Politicization and polarization in COVID-19 news coverage. Sci Commun. 2020;42(5):679–697. doi: 10.1177/1075547020950735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Piller C (2020) Undermining CDC. Science October 14. Available on line at: https://www.science.org/content/article/inside-story-how-trumps-covid-19-coordinator-undermined-cdc
- 60.Mayo Clinic Staff (2022) Debunking COVID-19 myths (https://www.mayoclinic.org/diseases-conditions/coronavirus/in-depth/coronavirus-myths/art-20485720).
- 61.Harrison MR, Evans MI, Adzick NS, Holzgreve W (eds). The unborn patient: the art and science of fetal therapy, 3rd Ed. W.B. Saunders Publishing Company, Philadelphia, 2001
- 62.Harrison MR, Longaker MT, Adzick NS, Goldberg JD, Rosen MA, Filly RA, Evans MI, Golbus MS. Successful repair in utero of a fetal diaphragmatic hernia after removal of herniated viscera from the left thorax. NEJM. 1990;322:1582–1584. doi: 10.1056/NEJM199005313222207. [DOI] [PubMed] [Google Scholar]
- 63.Moon-Grady AJ, Baschat A, Cass D, Choolani M, Copel J, Crombelholme TM, Deprest J, Emery SP, Evans MI, Luks F, Norton M, Ryan G, Tsao K, Welch R, Harrison MR. Fetal Treatment 2017: the evolution of fetal therapy centers – a joint opinion from the international fetal medicine and Surgical Society (IFMSS) and the North American Fetal Therapy Network (NAFNet) Fetal Diagn Ther. 2017;42:241–248. doi: 10.1159/000475929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Evans MI, Andriole SA, Britt DW. Fetal reduction – 25 years’ experience. Fetal Diagn Ther. 2014;35:69–82. doi: 10.1159/000357974. [DOI] [PubMed] [Google Scholar]
- 65.Evans MI, Fletcher JC, Zador IE, Newton BW, Struyk CK, Quigg MH. Selective first trimester termination in octuplet and quadruplet pregnancies: clinical and ethical issues. Obstet Gynecol. 1988;71:289–296. [PubMed] [Google Scholar]
- 66.Berkowitz RL, Lynch L, Chitkara U, et al. Selective reduction of multiple pregnancies in the first trimester. N Engl J Med. 1988;318:1043. doi: 10.1056/NEJM198804213181607. [DOI] [PubMed] [Google Scholar]
- 67.Evans MI, Dommergues M, Wapner RJ, Lynch L, Dumez Y, Goldberg JD, Zador IE, Nicolaides KH, Johnson MP, Golbus MS, Boulot P, Berkowitz RL. Efficacy of transabdominal multifetal pregnancy reduction: collaborative experience among the world’s largest centers. Obstet Gynecol. 1993;46:537–541. [PubMed] [Google Scholar]
- 68.Conde-Agudelo A, Romero R, Da Fonseca E, O’Brien JM, Cetingoz E, et al: Vaginal progesterone is as effective as cervical cerclage to prevent preterm birth in women with a singleton gestation, previous spontaneous preterm birth, and a short cervix. Updated indirect comparison meta-analysis. Am J Obstet Gynecol 2018;219:10–25 [DOI] [PMC free article] [PubMed]
- 69.Romero R, Conde-Agudelo A, Da Fonseca E, O’Brien JM, Cetingoz E, Creasy GW, et al. Vaginal progesterone for preventing preterm birth and adverse perinatal outcomes. Am J Obstet Gynecol. 2018;218:161–180. doi: 10.1016/j.ajog.2017.11.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Romero R, Conde-Agudelo A, El-Refail W, Rode L, Brizot ML, Cetingoz E, et al. Vaginal progesterone decreased preterm birth and neonatal morbidity and mortality in women with a twin gestation and a short cervix: an updated meta-analysis of individual patient data. Ultrasound Obstet Gynecol. 2017;49:303–314. doi: 10.1002/uog.17397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khalifeh SG, Elimian A, Bahrami E, Chaman-Ara K, Bahrami MA, Berghella V. Vaginal progesterone vs intramuscular 17 alpha-hydroxyprogesterone caproate for prevention of recurrent spontaneous preterm birth in singleton gestations: systematic review and meta-analysis of randomized controlled trials. Ultrasound Obstet Gynecol. 2017;49:315–321. doi: 10.1002/uog.17245. [DOI] [PubMed] [Google Scholar]
- 72.Godlewski BJ, Sobolik LI, King VJ, Harrod CS. Accelerated approval of 17 α-hydroxyprogesterone caproate: a cautionary tale. Obstet Gynecol. 2020;135:1207–13. [DOI] [PubMed]
- 73.Gandell DL, Randel MD, Gudeman JL. FDA approved vs pharmacy compounded 17 OHPC-current issues for obstetricians to consider in reducing preterm birth. Curr Med Res Opin. 2020;36:1393–1401. doi: 10.1080/03007995.2020.1783220. [DOI] [PubMed] [Google Scholar]
- 74.Chang HH, Larson J, Blencowe H, Spong CY, Howson CP, Cairns-Smith S, et al: Preventing preterm births: analysis of trends and potential reductions with interventions in 39 countries with very high human development index. Lancet 2012; 381:223–234 10.1016/S0140-6736(12)61856-X [DOI] [PMC free article] [PubMed]
- 75.Khandelwal M. Vaginal progesterone in risk reduction of preterm birth in women with short cervix in the midtrimster of pregnancy. Int J Wom Health. 2012;4:481–490. doi: 10.2147/IJWH.S28944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Godlewski BJ, Sobolik LI, King VJ, Harrod CS. Accelerated approval of 17 alpha hydroxyprogesterone caproate: a cautionary table. Obstet Gynecol. 2020;135:1207–1213. doi: 10.1097/AOG.0000000000003787. [DOI] [PubMed] [Google Scholar]
- 77.Gandell DL, Randel MD, Gudeman JL. FDA approved vs pharmacy compounded 17 OHPC-current issues for obstetricians to consider in reducing preterm birth. Curr Med Res Opin. 2020;36:1393–1401. doi: 10.1080/03007995.2020.1783220. [DOI] [PubMed] [Google Scholar]
- 78.Shaffer LM, Dabell MP, Fisher AJ, Coppinger J, Bandholz AM, Ellison JW, et al. Experience with microarray-based comparative genomic hybridization for prenatal diagnosis in over 5000 pregnancies. Prenat Diagn. 2012;32:976–985. doi: 10.1002/pd.3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shaffer LG, Rosenfeld JA, Dabell MP, Coppinger J, Bandholz AM, Ellison JW, et al. Detection rates of clinically significant genomic alterations by microarray analysis for specific anomalies detected by ultrasound. Prenat Diagn. 2015;32:986–995. doi: 10.1002/pd.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wapner RJ, Martin CL, Levy B, et al. Chromosomal microarray versus karyotyping for prenatal diagnosis. N Engl J Med. 2012;367:2175–2184. doi: 10.1056/NEJMoa1203382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bianchi DW, Simpson JL, Jackson LG, Elias S, Holzgreve W, Evans MI, Dukes KA, Sullivan LM, Klinger KW, Bischoff FZ, Hahn S, Johnson KL, Lewis D, Wapner RJ, de la Cruz F. Fetal gender and aneuploidy detection using fetal cells in maternal blood: analysis of NIFTY I data. Prenat Diagn. 2002;22:609–615. doi: 10.1002/pd.347. [DOI] [PubMed] [Google Scholar]
- 82.Lo YM, Corbetta N, Chamberlain PF, Rai V, Sargent IL, Redman CS, Wainscoat JS. Presence of fetal DNA in maternal plasma and serum. Lancet. 1997;350:485–487. doi: 10.1016/S0140-6736(97)02174-0. [DOI] [PubMed] [Google Scholar]
- 83.Lo YMD. Non-invasive prenatal testing using massively parallel sequencing of maternal plasma DNA: from molecular sequencing of maternal plasma DNA: from molecular karyotyping to fetal whole-genome sequencing. Reprod Biomed online. 2013;27:593–598. doi: 10.1016/j.rbmo.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 84.Ehrich M, Nelson MR, Stanssens P, Zabeau M, Liloglou T, Xinarianos G, et al. Noninvasive detection of fetal trisomy 21 by sequencing of DNA in maternal blood. Am J Obstet Gynecol. 2011;204:205.e201–205.e211. doi: 10.1016/j.ajog.2010.12.060. [DOI] [PubMed] [Google Scholar]
- 85.Dar P, Norton ME: Primary cell free DNA screening or contingent screening for common trisomies. Am J Obstet Gynecol 2022 (in press) [DOI] [PubMed]
- 86.Dar P, Jacobson B, Clifton R, Egbert M, Malone F, Wapner RJ, et al: Cell-free DNA screening for prenatal detection of 22q11.2 deletion syndrome. Am J Obstet Gynecol 2022; in press. [DOI] [PubMed]
- 87.Allyse M, Sayres LC, Goodspeed T, Michie M, Cho MK. “Don’t want no risk and don’t want no problems”: public understandings of the risks and benefits of noninvasive prenatal testing in the United States. AJOB Empirical Bioethics. 2015;6(1):5–20. doi: 10.1080/23294515.2014.994722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hon EH. The fetal heart rate patterns preceding death in utero. Am J Obstet Gynecol. 1959;78:47–56. doi: 10.1016/0002-9378(59)90639-8. [DOI] [PubMed] [Google Scholar]
- 89.Paul RH, Hon EH: Clinical fetal momitoring. V. Effect on perinatal outcome. Am J Obstet Gynecol 1974;118:529–533. [DOI] [PubMed]
- 90.Herbst AL, Ulfelder H, Poskanzer DC. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. N Engl J Med. 1971;284(15):878–81. [DOI] [PubMed]
- 91.Grimes DA, Peipert JF. Electronic fetal monitoring as a public health screening program: the arithmetic of failure. Obstet Gynecol. 2010;116(6):1397–1400. doi: 10.1097/AOG.0b013e3181fae39f. [DOI] [PubMed] [Google Scholar]
- 92.Godlee F, Marcovitch H. Wakefield’s article linking MMR vaccine and autism was fraudulent. Brit Med J. 2011;342:c7452. doi: 10.1136/bmj.c7452. [DOI] [PubMed] [Google Scholar]
- 93.Brainard J. Rethinking retractions. Science. 2018;362:390–393. doi: 10.1126/science.362.6413.390. [DOI] [PubMed] [Google Scholar]
- 94.Berg J. Imagine a world without facts. Science. 2018;362:379. doi: 10.1126/science.aav7494. [DOI] [PubMed] [Google Scholar]
- 95.Chauca FB. Good science, bad science and junk science. Am J Orthodontics & Dentofacial Orthopedics. 2017;151:637–638. doi: 10.1016/j.ajodo.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 96.Wakefield AJ, Murch SH, Anthony A, Linnell J, Casson DM, Malik M, et al. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in Children. Lancet. 1998;351:637–641. doi: 10.1016/S0140-6736(97)11096-0. [DOI] [PubMed] [Google Scholar]
- 97.Drazen JM. Fifteen years. NEJM. 2015;373:1774–1775. doi: 10.1056/NEJMe1512739. [DOI] [PubMed] [Google Scholar]
- 98.Chusid MJ, Casper JT, Camitta BM: Editors have ethical responsibilities, too. NEJM 1984;3`11:990–991 [DOI] [PubMed]
- 99.Fisher PG. Disproving junk science. J Pediatr. 2019;209:1. doi: 10.1016/j.jpeds.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 100.Eden RD, Evans MI, Evans SM, Schifrin BS. The “fetal reserve index”: re-engineering the interpretation and responses to fetal heart rate patterns. Fetal Diagn Ther. 2018;43:90–104. doi: 10.1159/000475927. [DOI] [PubMed] [Google Scholar]
- 101.Eden RD, Evans MI, Evans SM, Schifrin BS. Reengineering electronic fetal monitoring interpretation: using the fetal reserve index to anticipate the need for emergent operative delivery. Reprod Sci. 2018;25:487–497. doi: 10.1177/1933719117737849. [DOI] [PubMed] [Google Scholar]
- 102.Britt DW, Evans MI, Schifrin BS, Eden RD. Refining the prediction and prevention of emergency operative deliveries with the fetal reserve index. Fetal Diagn Ther. 2018;21:1–7. doi: 10.1159/000494617. [DOI] [PubMed] [Google Scholar]
- 103.Eden RD, Evans MI, Britt DW, Evans SM, Schifrin BS. Safely lowering the emergency cesarean and operative vaginal delivery rates using the “fetal reserve index” using the fetal reduction index. J Matern Fet Neonal Med. 2019;32:2561–2569. doi: 10.1080/14767058.2018.1519799. [DOI] [PubMed] [Google Scholar]
- 104.Evans MI, Eden RD, Britt DW, Evans SM, Schifrin BS. Reconceptualizing fetal monitoring. Eur J Gynecol Obstet. 2019;1:10–17. [Google Scholar]
- 105.Evans MI, Britt DW, Eden RD, Gallagher P, Evans SM, Schifrin BS. The fetal reserve index significantly outperforms ACOG Category system in predicting cord blood base excess and pH: a methodological failure of the category system. Reprod Sci. 2019;26:858–863. doi: 10.1177/1933719119833796. [DOI] [PubMed] [Google Scholar]
- 106.Evans MI, Eden RD, Britt DW, Evans SM, Schifrin BS. Re-engineering the interpretation of electronic fetal monitoring to identify reversible risk for cerebral palsy: a case control series. J Matern Fetal Neonatal Med. 2018:1–9. [DOI] [PubMed]
- 107.Eden RD, Evans MI, Britt DW, Evans SM, Gallagher P, Schifrin BS. Combined prenatal and postnatal prediction of early neonatal compromise. J Matern Fet Neo Med. 2021 doi: 10.1080/14767058.2019.1676714. [DOI] [PubMed] [Google Scholar]
- 108.Evans MI, Britt DW, Evans SM. Midforceps didn’t cause “compromised babies” – “compromise” caused forceps: an approach towards safely lowering the cesarean delivery rate. J Matern Fet Neo Med (in press) 2021;10(1080/14767058):1876657. doi: 10.1080/14767058.2021.1876657. [DOI] [PubMed] [Google Scholar]
- 109.Evans MI, Britt DW, Eden RD, Evans SM, Schifrin BS: Earlier and improved screening for impending fetal compromise. J Matern Fet Neo Med (in press) 10.1080/14767058.2020.1811670 [DOI] [PubMed]
- 110.Evans MI, Britt DW, Worth J, Mussalli G, Evans SM, Devoe LD: Uterine contraction frequency in the last hour of labor: how many contractions are too many? J Matern Fet Neo Med (in press) [DOI] [PubMed]
- 111.Evans MI, Britt DW, Evans SM, Devoe LD: Changing perspectives of electronic fetal monitoring. Reprod Sci (in press) 10.1007/s43032-021-00749-2 [DOI] [PMC free article] [PubMed]
- 112.Evans MI, Britt DW, Evans SM, Devoe LD: Evolving frameworks for the foundation and practice of electronic fetal monitoring. Mat Fet Med 2022 (in press)
- 113.Clark SL, Nageotte MP, Garite TJ, Freeman RK, Miller DA, Simpson KR, et al. Intrapartum management of category II fetal heart rate tracings: towards standardization of care. Am J Obstet Gyn. 2013;209:89–97. doi: 10.1016/j.ajog.2013.04.030. [DOI] [PubMed] [Google Scholar]
- 114.Clark SL, Hamilton E, Garite TJ, Timmins A, Warrick PA, Smith S. The limits of electronic fetal heart rate monitoring in the prevention of neonatal metabolic acidemia. Am J Obstet Gynecol. 2017;216(163):e1–6. doi: 10.1016/j.ajog.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 115.Sartwelle TP, Johnston JC. Continuous electronic fetal monitoring during labor: a critique and a reply to contemporary proponents. Surg J. 2018;4:e23–e28. doi: 10.1055/s-0038-1632404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nelson KB, Ellenberg JH: Antecedents of cerebral palsy. I. Univariate analysis of risks: Am J Dis Child 1985;60:1031–1038. [DOI] [PubMed]
- 117.Nelson KB, Ellenberg JH. Antecedents of cerebral palsy. NEJM. 1986;315:81–86. doi: 10.1056/NEJM198607103150202. [DOI] [PubMed] [Google Scholar]
- 118.Ellenberg JH, Nelson KB. The association of cerebral palsy with birth asphyxia: a definitional quagmire. Dev Med Child Neurol. 2013;55:210–216. doi: 10.1111/dmcn.12016. [DOI] [PubMed] [Google Scholar]
- 119.Johnson GJ, Salmanian B, Dennings SG, Belfort MA, Sundgren NC, Clark SL. Relationship between umbilical cord gas values and neonatal outcomes. Obstet Gynecol. 2021;131:366–373. doi: 10.1097/AOG.0000000000004515. [DOI] [PubMed] [Google Scholar]
- 120.Miller DA: Intrapartum fetal heart rate assessment – up to date 2019 https://www.uptodate.com/contents/table-of-contents/obstetrics-gynecology-and-womens-health
- 121.Smith GGS, Pell JP. Parachute use to prevent death and major trauma related to gravitational challenge: systematic review of randomized controlled trials. Brit Med J. 2009;327:1459–1461. doi: 10.1136/bmj.327.7429.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Odendaal H. Factors influencing the pH value of foetal scalp blood with special reference to caput succedaneum. S Afr Med J. 1974;48:59–62. [PubMed] [Google Scholar]
- 123.Upton Sinclair biography. Wikipedia https://en.wikipedia.org/wiki/Upton_Sinclair accessed Oct 4 2021.
- 124.William James Biography. Stanford encyclopedia of philosophy. https://plato.stanford.edu/entries/james/ accessed Oct 4, 2021.
- 125.American President Movie 1995. Wikipedia https://en.wikipedia.org/wiki/The_American_President
- 126.Max Planck Biography https://en.wikipedia.org/wiki/Max_Planck Accessed Oct 4, 2021.
- 127.Packalen M, Bhattacharya J. NIH funding and the pursuit of edge science. Proc Natl Acad Sci. 2020;117:12011–12016. doi: 10.1073/pnas.1910160117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Why you should not use Ivermectin to treat or prevent COVID-19. US Food and Drug Admin Sept 9, 2021 https://www.fda.gov/consumers/consumer-updates/why-you-should-not-use-ivermectin-treat-or-prevent-covid-19
- 129.Winston Churchill quotes https://www.brainyquote.com/authors/winston-churchill-quotes Accessed Oct 4, 2021.
- 130.Evans MI, Vermeesch JR. Current controversies in prenatal diagnosis 3: industry drives innovation in research and clinical application of genetic prenatal diagnosis and screening. Prenat Diagn. 2016;36(13):1172–1177. doi: 10.1002/pd.4967. [DOI] [PubMed] [Google Scholar]
- 131.Britt DW, Eden RD, Evans MI. Matching risk and resources in high-risk pregnancies. J Matern Fetal Neonatal Med. 2006;19(10):645–650. doi: 10.1080/14767050600850449. [DOI] [PubMed] [Google Scholar]
- 132.Nicolaides KH. Turning the pyramid of prenatal care. Fetal Diagn Ther. 2011;29(3):183–196. doi: 10.1159/000324320. [DOI] [PubMed] [Google Scholar]
- 133.Britt DW. A conceptual introduction to modeling: qualitative and quantitative perspectives. Mahwah, NJ: Lawrence Erlbaum Associates; 1997. [Google Scholar]
- 134.McAffe A, Brynjolfsson E; Harnessing our digital future. 2017 Norton & Company N & Company NY
- 135.Godlee F. The fraud behind the MMR scare. Brit Med J. 2011;342:d22. doi: 10.1136/bmj.d22. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All previously published.
N/A.


