Abstract
Objectives
Patients with chronic kidney disease (CKD) are known to develop sarcopenia, an aging-related disorder, with low muscle mass, strength and physical performance. Ultrasound-derived thigh muscle and rectus femoris thickness (TMT and RFT) can be measured easily in clinical practice, but need validation for use in predialysis CKD (stages III through V) for muscle mass estimation. The study aims to compare ultrasound-derived TMT and RFT with bioelectrical impedance analysis (BIA)-derived muscle mass estimation in the diagnosis of sarcopenia in predialysis CKD.
Methods
Patients with stable CKD stage III, IV, V and not yet on dialysis were recruited, and underwent anthropometric assessment, BIA and ultrasound examination of midthigh region. Appendicular skeletal muscle index (ASMI)/height2 derived from BIA was taken as a standard for the diagnosis of low muscle mass. Gait speed and handgrip were also measured. The Asian Working Group criteria were applied. Cutoff values for low muscle mass by TMT and RFT were obtained using receiver operator curve (ROC) analysis.
Results
Of the total of 117 enrolled study participants, 52 (45%) had low muscle mass, 34 (29%) had sarcopenia, of whom 79% were male, majority (38%) were CKD stage IV and had a mean age of 58 years. Using ROC analysis, TMT cutoffs of 19 mm in males and 17 mm in females were computed. Comparison of TMT cutoffs and ASMI/h2 showed good agreement between the 2 methods using Bland-Altman plots.
Conclusions
Ultrasound-derived TMT and RFT can be used for muscle mass estimation in the diagnosis of sarcopenia.
Keywords: Sarcopenia, Chronic kidney disease, Ultrasound, Bioelectrical impedance
1. Introduction
Sarcopenia has been recognized as a prevalent condition in patients with chronic kidney disease (CKD), both prior to and after renal replacement therapies [1]. Following the initial definitions laid out a decade ago, many studies have focussed on the prevalence, determinants and outcomes of sarcopenia (as a concomitant condition to protein-energy wasting and cachexia) [[2], [3], [4]]. Patients with CKD have an accelerated loss of muscle mass, secondary to anorexia, inflammation, and increased protein catabolism, which then lead to reductions in muscle strength and physical performance, the other 2 essential prerequisites in the diagnosis of sarcopenia [5].
CKD-associated sarcopenia is a secondary form of sarcopenia, whose magnitude exceeds ageing-related loss of muscle mass, and involves increased muscle protein degradation, and atrophy of both type I and II fibres [6]. In the recent years, the focus of sarcopenia diagnosis has shifted from pure loss of muscle mass, to initial measurement of muscle strength and physical performance, prior to estimation of muscle mass in the current diagnostic algorithms by the leading societies, European Working Group on Sarcopenia in Older People (EGSWOP2) and the Asian Working Group for Sarcopenia [7,8]. This is justified by the stronger effect of muscle strength rather than mass, on hard clinical outcomes, such as mortality and hospitalization rates [9]. Hitherto, muscle mass estimation in the diagnosis of sarcopenia, has been done using anthropometric, bioelectrical impedance analysis (BIA) based, and imaging based [primarily computed tomography (CT) and magnetic resonance imaging (MRI)] methods [5]. The use of ultrasound (US) muscle thickness and cross-sectional area in the estimation of muscle mass, has recently been studied, though only sparse data exists for its use in patients with CKD [[10], [11], [12]]. Compared to CT and MRI, US has the advantages of lower cost and measurement time, as well as no radiation exposure. The use of thigh muscle and rectus femoris thickness over the cross-sectional area, and other muscle characteristics, is not well-validated in CKD populations, but was evaluated in the present study due to the ease of learning for nephrology trainees.
The present study aims to compare ultrasound-derived thigh and rectus muscle thickness and bioelectrical impedance-based appendicular skeletal muscle mass estimation, in the diagnosis of sarcopenia in patients with predialysis CKD (stages III through V), and to derive muscle thickness cutoffs for defining low muscle mass in a South Asian CKD population. CKD stages III through V were chosen during the study design, as sarcopenia and its diagnosis in patients with dialysis-requiring CKD is better studied than in predialysis stages, and is affected both by uremic milieu, and the catabolic effects of the hemodialysis session itself [5]. Also, early diagnosis of sarcopenia in a patient with predialysis CKD may present lost opportunities for management of the condition, especially so, in younger patients with CKD on protein-restricted diets. Earlier stages of CKD (I and II) constitute only a minor proportion of patients under nephrologists’ care, as the vast majority of CKD are diagnosed or referred to a nephrologist at more advanced stages. Based on a previous study done in an elderly non-CKD Asian population which showed a 4 mm difference in mean thigh muscle thickness between the sarcopenic and non-sarcopenic groups, an α cut-off of 0.05, β of 0.2, power of 80%, 112 patients were required to show differences in the means of sarcopenic versus non-sarcopenic adults with CKD [13].
2. Methods
The study was conducted in the outpatient clinic at the Department of Nephrology at a tertiary care teaching hospital in northern India, between September 2020 and February 2022. The study was approved by the institutional ethical committee, and was registered (CTRI/2019/10/021731). Patients were included in the study if they were diagnosed with chronic kidney disease stage III, IV, V but not yet on renal replacement therapy (according to KDIGO criteria for the diagnosis of CKD), had visited outpatient department at least twice in the year prior to enrolment, had no episode of acute kidney injury or intercurrent hospitalizations in the 6 months prior to enrolment, and were not on dialysis. The diagnosis of sarcopenia was established based on the Asian Working Group for Sarcopenia criteria. Low muscle strength was defined as a hand-grip strength of < 26 kg in males and < 18 kg in females, and low gait speed cut-off value was < 0.8 m/s. When a patient had low muscle strength and/or low gait speed, muscle mass (ASMI/h2) measured by BIA < 7.0 kg/m2 in males and < 5.7 kg/m2 in females, was defined as sarcopenia [8]. The study was performed in accordance with the guidelines laid out in the Declaration of Helsinki.
2.1. Clinical, anthropometric and laboratory assessments
Clinical details were collected using a questionnaire, and anthropometric measurements (height, weight, mid-upper arm circumference and skin fold thickness) were performed by a trained outpatient nurse. Biochemical parameters such as hemoglobin, serum creatinine and albumin were noted using the hospital information system. Muscle strength was estimated using an average of 3 measurements taken from a handheld digital handgrip dynamometer. Gait speed was estimated by walking the patient in a flat corridor.
2.2. Muscle mass estimation by bioelectrical impedance
The appendicular skeletal muscle mass was measured by positioning 6 electrodes using the InBody S310® (InBody, Seoul, Korea) bioelectrical impedance analyser. This machine measures the body composition according to the differences in conductivity of various compartments. The voltage is read between receiving electrodes and corresponds to the tissues’ opposition to the current flow. The appendicular skeletal muscle index ASMI/h2 was calculated as: ASMI = arm and leg skeletal muscle mass (kg)/height2(m2).
2.3. Thigh muscle thickness and rectus femoris thickness by ultrasound
The patient was seated on a chair in the ultrasound examination room, with the hip and knee joints placed at 90°. Using a tape measure, the midpoint of greater trochanter and patella were determined. At this point, the curvilinear transducer of the Sono Site M-TURBO ®ultrasound machine (Fujifilm Sonosite, Bothell, WA, USA) was placed perpendicular to the anterior thigh such that the distance from the femur is the shortest. Drawing an axial cross-section, the thigh muscle thickness (TMT) was measured as the distance between the anterior fascia of rectus femoris muscle and the posterior fascia of the vastus intermedius muscle. Similarly, rectus femoris thickness (RFT) was measured between the anterior and posterior fasciae of rectus femoris muscle. A single investigator (NR) performed ultrasound examination on all the study subjects.
2.4. Statistical analysis
Continuous variables were established as mean with standard deviation, and if nonparametric (using Kolmogorov-Smirnov test), then as medians with interquartile ranges. Categorical variables were expressed as percentages. Correlations were carried out using Pearson's correlation coefficient. Receiver-operator curve (ROC) analysis was used to determine cutoffs for reduced muscle mass when using ultrasound-derived TMT and RFT to diagnose sarcopenia. The area under the curve (AUC) was used to calculate sensitivity, specificity and the negative predictive value of the selected cutoffs for TMT and RFT. Bland-Altman plot analysis and Cohen's kappa were used to determine agreement between the 2 methods for defining low muscle mass.
3. Results
The study was conducted between January 2021 and February 2022 at a tertiary care teaching hospital in Northern India. A total of 117 patients with stable chronic kidney disease stages III to V (not on dialysis), were enrolled in the study. Fifty-two patients (45%) had low muscle mass and 34 (29%) were found to be sarcopenic according to the Asian Working Group definition. Table 1 shows that subjects with sarcopenia did not differ significantly from those without sarcopenia, in terms of age, sex, CKD stage, comorbidities, etc. Body weight and mid-upper arm circumference were expectedly lower in the group with sarcopenia.
Table 1.
Baseline clinical, anthropometric and biochemical parameters in study subjects with and without sarcopenia.
| Characteristics | With sarcopenia (n = 34), mean (SD) | Without sarcopenia (n = 83), mean (SD) | P-value |
|---|---|---|---|
| Age, yr | 55.7 (11.8) | 49.9 (15.3) | 0.096 |
| Sex | 27 males: 7 females | 61 males: 22 females | 0.184 |
| Duration of CKD, months | 18.8 (15.9) | 24.0 (20.4) | 0.101 |
| Serum creatinine, mg/dL) | 3.16 (1.42) | 3.43 (1.44) | 0.79 |
| Estimated GFR, (mL/min/1.73m2) | 26.56 (13.12) | 24.13 (11.86) | 0.322 |
| CKD stage 3a | 3 | 7 | 0.638 |
| 3b | 10 | 14 | |
| 4 | 13 | 45 | |
| 5 | 8 | 16 | |
| Underlying diabetes, % | 10 (29.4%) | 29 (35.3%) | 0.642 |
| Hypertension, % | 29 (85.2%) | 68 (82.9%) | 0.863 |
| Weight, kg | 55.1 (8.45) | 65.1 (13.3) | 0.017 |
| Height, m | 1.57 (0.08) | 1.59 (0.08) | 0.927 |
| Body mass index, kg/m2 | 22.5 (3.8) | 25.7 (5.19) | 0.268 |
| Mid upper arm circumference, cm | 24.2 (2.6) | 26.8 (4.3) | 0.032 |
| Skin fold thickness, mm | 17.4 (6.2) | 20.1 (9.2) | 0.113 |
| Hemoglobin, g/dl | 10.5 (1.92) | 10.7 (2.03) | 0.746 |
| Serum albumin, g/dl | 3.89 (0.57) | 3.96 (0.57) | 0.525 |
CKD, chronic kidney disease; GFR, glomerular filtration rate.
3.1. Muscle mass and strength parameters
Muscle mass measurements by bioimpedance and ultrasound are presented in Table 2. Majority of skeletal muscle mass indices, as well as ultrasound-derived thigh muscle thickness (TMT) and rectus femoris thickness (RFT) were expectedly lower in the group with sarcopenia. Fig. 1 and Fig. 2 show that ultrasound derived TMT and RFT correlate with ASM/h2 (r = 0.402, P < 0.001 for TMT, r = 0.438, P < 0.001 for RFT). Ultrasound-derived TMT and RFT correlated with average handgrip strength, walking speed, mid-upper arm circumference and serum albumin level.
Table 2.
Muscle mass, strength and gait in subjects with and without sarcopenia.
| Characteristics | Males (n = 88) |
Females (n = 29) |
||||
|---|---|---|---|---|---|---|
| With sarcopenia (n = 27) mean (SD) | Without sarcopenia (n = 61) mean (SD) | p-value | With sarcopenia (n = 7) mean (SD) | Without sarcopenia (n = 22) mean (SD) | P-value | |
| Total muscle mass, kg | 20.01 (2.58) | 24.42 (4.06) | 0.132 | 14.79 (1.35) | 18.01 (2.67) | 0.20 |
| SLM right upper limb, kg | 1.87 (0.35) | 2.35 (0.59) | 0.000 | 1.35 (0.17) | 1.61 (0.35) | 0.04 |
| SLM left upper limb, kg | 1.83 (0.32) | 2.28 (0.50) | 0.083 | 1.27 (0.18) | 1.65 (0.45) | 0.25 |
| SLM trunk, kg | 17.25 (2.17) | 19.91 (3.71) | 0.063 | 13.74 (1.12) | 15.63 (2.48) | 0.15 |
| SLM right lower limb, kg | 6.00 (0.92) | 7.54 (1.36) | 0.04 | 4.35 (0.54) | 5.39 (1.05) | 0.10 |
| SLM left lower limb, kg | 5.99 (0.99) | 7.43 (1.30) | 0.07 | 4.27 (0.51) | 5.29 (1.00) | 0.14 |
| Composite ASMI, kg | 15.71 (2.45) | 19.61 (3.44) | 0.000 | 11.25 (1.31) | 13.96 (2.52) | 0.05 |
| ASMI/height2, kg/m2 | 6.09 (0.63) | 7.43 (0.15) | 0.000 | 5.14 (0.53) | 6.19 (0.97) | 0.048 |
| Thigh muscle thickness, cm | 1.73 (0.35) | 2.14 (0.48) | 0.000 | 1.75 (0.65) | 2.01 (0.46) | 0.05 |
| Rectus femoris thickness, cm | 0.94 (0.26) | 1.14 (0.27) | 0.000 | 0.94 (0.26) | 1.14 (0.27) | 0.047 |
| Handgrip strength, kg | 22.0 (5.4) | 28.6 (7.8) | 0.018 | 14.5 (3.4) | 19.7 (2.8) | 0.07 |
| Gait speed, m/s | 0.76 (0.17) | 0.85 (0.12) | 0.000 | 0.75 (0.08) | 0.79 (0.15) | 0.16 |
SLM, skeletal lean mass; ASMI, appendicular skeletal muscle index.
Fig. 1.
Receiver-operator curves for ultrasound-derived thigh muscle thickness (a) for the diagnosis of sarcopenia in male (b) and female (c).
Fig. 2.
Receiver-operator curves for ultrasound-derived rectus femoris thickness (a) for the diagnosis of sarcopenia in male (b) and female (c).
3.2. Determination of ultrasound-derived TMT and RFT cutoffs
Table 3 shows the sensitivity, specificity and negative predictive values for ultrasound-derived TMT and RFT cutoffs in the diagnosis of sarcopenia. The area under the curve (AUC) in the receiver-operator curve (ROC) analysis was 0.75 in males and 0.59 in females using TMT, and the AUC was 0.75 in males and 0.63 in females while using RFT, confirming moderate reliability. Fig. 1a–c and 2a-c show the ROC curves displaying the AUC for TMT and RFT in males and females, respectively.
Table 3.
Sensitivity and specificity values for ultrasound-derived muscle thickness for diagnosis of sarcopenia.
| Method | Area under curve | Cutoffs | Sensitivity | Specificity | P-value |
|---|---|---|---|---|---|
| Thigh muscle thickness, cm | |||||
| Males | 0.748 | 1.9 | 70% | 70% | 0.000 |
| Females | 0.594 | 1.7 | 70% | 55% | 0.10 |
| Rectus femoris thickness, cm | |||||
| Males | 0.749 | 1.1 | 76% | 64% | 0.000 |
| Females | 0.628 | 1.0 | 70% | 55% | 0.09 |
3.3. Comparison of bioimpedance-based and ultrasound-derived measures in the diagnosis of sarcopenia
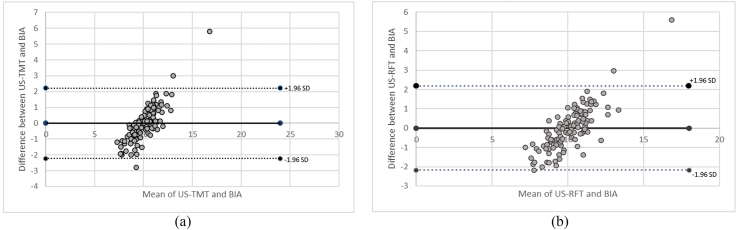
Cohen's kappa was run to determine the level of agreement between BIA-based ASMI/h2 and ultrasound-derived TMT cutoffs in the diagnosis of sarcopenia. There was moderate agreement between the 2 methods (κ = 0.59, 95% CI, 0.38-0.79, P = 0.001). The Bland-Altman plot analysis showed good agreement between the 2 methods while using TMT and RFT cutoffs derived in the study, as in Fig. 3a and b.
Fig. 3.
Bland-Altman plot of differences between low extremity muscle mass estimation (a: thigh muscle thickness; b: rectus femoris thickness) derived by ultrasound and bioelectrical impedance.
4. Discussion
The present study supports the use of skeletal muscle ultrasound as a tool for muscle mass assessment in patients with chronic kidney disease, as both ultrasound derived-TMT and RFT were found to be significantly correlated to the ASMI measured using bioelectrical impedance method.
While early definitions of sarcopenia identified it as an aging induced loss of muscle mass, with improved understanding over the years, the current operational definitions of sarcopenia require the presence of both low muscle mass as well as low strength. The Asian Working group diagnostic algorithm (which was utilized in the present study) involves evaluation of physical performance and muscle strength as a first step, and muscle mass estimation only when there is evidence of low muscle strength and/or physical performance, thus laying primary emphasis on functional disturbances, rather than anatomical ones. However, only few prior studies evaluated both muscle mass, strength and physical performance in stable CKD and reported much lower prevalence rates, from 10% to 24%, similar to the present study, thus supporting the hypothesis that not all muscle mass reduction equals sarcopenia, in patients with CKD [[14], [15], [16]].
Muscle mass reduction has a variable prevalence, ranging from 6% to 62% in patients with CKD, as evaluated by MUAC, CT, DXA or BIA, including a recent Indian study which found an unusually high prevalence of sarcopenia, up to 69% (using only muscle mass estimation, and not muscle strength; mean age up to 50.9 years) [[14], [15], [16], [17], [18]]. Up to 45% of the patients in the present study had low muscle mass by the BIA derived-ASMI/h2 criteria. In another multicenter study involving non-CKD subjects of various ethnicities, Indian participants were found to have significantly lower muscle mass than other ethnicities [19]. The role of ethnicity in predisposition to sarcopenia is presently unclear, when combined with the effects of metabolic acidosis, concomitant diabetes, hypothyroidism, protein-restricted diet, and fall in eGFR, all of which contribute to muscle wasting in CKD.
The majority of previous studies evaluating sarcopenia in predialysis CKD have focussed on elderly CKD patients [[12], [13], [14], [15]]. The mean age of enrolled participants in these studies, was consistently > 60 years. To our knowledge, only 2 studies examined sarcopenia in the setting of younger (< 60 years) predialysis CKD, a Brazilian study which compared MUAC, BIA and DXA-based muscle mass estimation with mortality (the mean age of participants in the study was 59.5 years), and the Indian study mentioned above, which had a mean age of 50.9 years, and in which BIA-based muscle wasting was seen in 69% of study subjects [14,18]. Our study population was also younger (mean age of 51.6 years), with 45% of the study subjects having low muscle mass based on the ASMI/h2 criteria and 29% being diagnosed with sarcopenia.
Sarcopenia is increasingly being recognized as a contributor to morbidity and mortality in patients with CKD, and on hemodialysis, but it is important to note that the diagnostic criteria for sarcopenia are not CKD-specific, and their performance in large CKD cohorts is not yet well studied. Diagnosing sarcopenia in patients with CKD can identify the subset of patients who can benefit the most from dietary and physical exercise related interventions. Therefore, it is important to reliably diagnose sarcopenia using commonly available equipment in nephrology practice. Ultrasound derived measures of muscle mass estimation have been hitherto used in a few studies, and demonstrated good correlation between the ultrasound and the bioelectrical impedance-based measures [[10], [11], [12]]. However, muscle mass cutoffs by both methods differ by sex and ethnicity, and may possibly be influenced by the underlying cause of sarcopenia. For example, a study done in an ageing Asian population with no comorbidities, highlighted TMT cutoffs of 36 mm in males and 32 mm in females, corresponding to the BIA-derived ASMI values of < 7.0 kg/m2 and < 5.7 kg/m2 in males and females respectively [13]. These were much higher than the TMT and RFT cutoffs derived in the present study done in prevalent patients with CKD stages III to V. Whether these differences are secondary to CKD per se, or South Asian ethnicity, or yet other factors, is unclear. With increasing focus on point-of-care ultrasound (POCUS) examination in nephrology, the use of easily reproducible measures of muscle mass such as TMT and RFT can be incorporated into diagnostic algorithms for sarcopenia in CKD clinics. Also, TMT and RFT are easier to measure than cross-sectional area (CSA), especially so for clinicians using bedside ultrasound.
The strengths of the study are the use of a homogenous, representative stable CKD study population, and diagnosing sarcopenia based on standardized measures of muscle mass, strength and physical performance, rather than relying only on muscle mass estimation alone. Also, all the ultrasound examinations were performed by a single operator, thus minimizing variability. To our knowledge, this is the first study to examine the prevalence of sarcopenia based on Asian Working Group criteria in a predialysis Indian CKD population. The study is limited by the lack of a validation cohort to compare ultrasound and bioimpedance based muscle mass estimation, using the cutoffs defined in this study. Inclusion of a control group of similar age group but with normal renal function, may have allowed for comparisons with the muscle mass and strength measured in patients with CKD. The limited number of patients per CKD stage does not allow for intra-stage comparisons. Further studies should look into dietary and exercise interventions in the progression of sarcopenia among patients with predialysis CKD.
5. Conclusions
The study found sarcopenia to be prevalent in up to 29% of participants. Ultrasound-derived thigh muscle and rectus femoris thickness correlated significantly with muscle mass estimation using bioelectrical impedance, and therefore, can be utilized in the diagnosis of sarcopenia in patients with predialysis CKD. The study proposes thigh muscle thickness cutoffs of 19 mm and 17 mm, for males and females respectively, for the diagnosis of low muscle mass in this South Asian ethnic population with CKD.
CRediT author statement
Namrata Rao S: Conceptualization, Methodology, Data curation, Formal analysis, Writing - Original Draft. Abhilash Chandra: Methodology, Validation, Formal analysis, Data Curation. Sai Saran: Data analysis, Resources, Writing - Review & Editing. Ayush Lohiya: Data analysis, revising, approval of final draft.
Conflicts of interest
The authors declare no competing interests.
Acknowledgments
ORCID
Namrata Rao S: 0000-0002-5733-4218. Abhilash Chandra: 0000-0002-9055-4351. Sai Saran: 0000-0002-6181-8661. Ayush Lohiya: 0000-0003-1429-7821.
Footnotes
Peer review under responsibility of The Korean Society of Osteoporosis.
References
- 1.Foley RN, Wang C, Ishani A, Collins AJ, Murray AM. Kidney function and sarcopenia in the United States general population: NHANES III. Am J Nephrol. 2007;27:279–286. doi: 10.1159/000101827. [DOI] [PubMed] [Google Scholar]
- 2.Stenvinkel P, Carrero J, von Walden F, Ikizler TA, Nader G. Muscle wasting in end-stage renal disease promulgates premature death: established emerging and potential novel treatment strategies. Nephrol Dial Transplant. 2016;31:1070–1077. doi: 10.1093/ndt/gfv122. [DOI] [PubMed] [Google Scholar]
- 3.Fouque D, Kalanter-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73:391–398. doi: 10.1038/sj.ki.5002585. [DOI] [PubMed] [Google Scholar]
- 4.Ortiz A, Sanchez-Nino MD. Sarcopenia in CKD: a roadmap from basic pathogenetic mechanisms to clinical trials. Clin Kidney J. 2019;12:110–112. doi: 10.1093/ckj/sfz001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sabatino A, Cuppari L, Stenvinkel P, Lindholm B, Avesani CM. Sarcopenia in chronic kidney disease: what have learned so far? J Nephrol. 2021;34:1347–1372. doi: 10.1007/s40620-020-00840-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molfino A, Chiappinni MG, Lvano A, Ammann T, Bollea MR, Alegiani F, et al. Effect of intensive nutritional counselling and support on clinical outcomes of hemodialysis patients. Nutrition. 2012;28:1012–1015. doi: 10.1016/j.nut.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Ceder-holm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21:300–307. doi: 10.1016/j.jamda.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Leong D, Teo K, Rangarajan S, Lopez-Jaramillo P, Avezum A, Orlandini A, et al. Prognostic value of grip strength: findings from the prospective urban rural epidemiology (PURE) study. Lancet. 2015;386:266–273. doi: 10.1016/S0140-6736(14)62000-6. [DOI] [PubMed] [Google Scholar]
- 10.Wilkinson TJ, Gore EF, Vadaszy N, Nixon DGD, Watson EL, Smith AC. Utility of ultrasound as a valid and accurate diagnostic tool for sarcopenia. J Ultrasound Med. 2021;40:457–467. doi: 10.1002/jum.15421. [DOI] [PubMed] [Google Scholar]
- 11.Wilkinson TJ, Gould DW, Nixon DG, Watson EL, Smith AC. Quality over quantity? Association of skeletal muscle myosteatosis and myofibrosis on physical function in chronic kidney disease. Nephrol Dial Transplant. 2019;10:748–755. doi: 10.1093/ndt/gfy139. [DOI] [PubMed] [Google Scholar]
- 12.de Souza V, Oliviera D, Cupolilo EN, Miranda CS, Colugnati FAB, et al. Rectus femoris muscle mass evaluation by ultrasound: facilitating sarcopenia diagnosis in pre-dialysis chronic kidney disease stages. Clinics. 2018;73:e391. doi: 10.6061/clinics/2018/e392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hida T, Ando K, Kobayashi K, Ito K, Tsushima M, Kobayakawa T, et al. Ultrasound measurement of thigh muscle thickness for assessment of sarcopenia. Nagoya J Med Sci. 2018;80:519–527. doi: 10.18999/nagjms.80.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pereira R, Cordeiro A, Avesani C, Carrero J, Lindholm B, Amadaro F, et al. Sarcopenia in chronic kidney disease on conservative therapy: prevalence and association with mortality. Nephrol Dial Transplant. 2015;30:1718–1725. doi: 10.1093/ndt/gfv133. [DOI] [PubMed] [Google Scholar]
- 15.Souza V, Oliviera D, Barbosa S, Correa J, Colugnati F, Mansur H, et al. Sarcopenia in patients with chronic kidney disease not yet on dialysis: analysis of the prevalence and associated factors. PLoS One. 2017;12(4) doi: 10.1371/journal.pone.0176230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vettoretti S, Caldiroli L, Armelloni S, Ferrari C, Cesari M, Messs P. Sarcopenia is associated with malnutrition but not with systemic inflammation in older persons with advanced CKD. Nutrients. 2019;11:1378. doi: 10.3390/nu11061378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanatani S, Izumiya Y, Onuoue Y, Tanaka T, Yamamoto M, Ishida T, et al. Non-invasive testing for sarcopenia predicts future cardiovascular events in patients with chronic kidney disease. Int J Cardiol. 2018;1:216–221. doi: 10.1016/j.ijcard.2018.03.064. [DOI] [PubMed] [Google Scholar]
- 18.Dubey AK, Sahoo J, Vairappan B, Parameswaran S, Priyamvada PS. Prevalence and determinants of sarcopenia in Indian patients with chronic kidney disease stage 3 & 4. Osteoporosis and Sarcopenia. 2021;7:153–158. doi: 10.1016/j.afos.2021.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tyrovolas S, Koyanagi B, Olaya JL, Ayuso-Mateos M, Miret S, Chaterji S, et al. Factors associated with skeletal muscle mass, sarcopenia, and sarcopenic obesity in older adults: a multi-continent study. J Cachexia Sarcopenia Muscle. 2016;7:312–321. doi: 10.1002/jcsm.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]