Abstract
The South Asian population is rapidly ageing and sarcopenia is likely to become a huge burden in this region if proper action is not taken in time. Several sarcopenia guidelines are available, from the western world and from East Asia. However, these guidelines are not fully relevant for the South Asian healthcare ecosystem. South Asia is ethnically, culturally, and phenotypically unique. Additionally, the region is seeing an increase in non-communicable lifestyle disease and obesity. Both these conditions can lead to sarcopenia. However, secondary sarcopenia and sarcopenic obesity are either not dealt with in detail or are missing in other guidelines. Hence, we present a consensus on the screening, diagnosis and management of sarcopenia, which addresses the gaps in the current guidelines. This South Asian consensus gives equal importance to muscle function, muscle strength, and muscle mass; provides cost-effective clinical and easy to implement solutions; highlights secondary sarcopenia and sarcopenic obesity; lists commonly used biomarkers; reminds us that osteo-arthro-muscular triad should be seen as a single entity to address sarcopenia; stresses on prevention over treatment; and prioritizes non-pharmacological over pharmacological management. As literature is scarce from this region, the authors call for more South Asian research guided interventions.
Keywords: Sarcopenia, Sarcopenic obesity, Guidelines, Secondary sarcopenia, South Asian, Body composition
1. Epidemiology
Sarcopenia is a global problem. A large multi-center study from 9 countries (Finland, Poland, Spain, China, Ghana, India, Mexico, Russia, and South Africa) across 3 continents reported overall sarcopenia prevalence of 15.2% [1]. A systematic review and meta-analysis showed that sarcopenia prevalence varies with patient population and reported sarcopenia prevalence of 11% and 9% in community-dwelling men and women, respectively; the corresponding prevalence in nursing-homes was 51% and 31%, respectively, and in hospitalized men and women was 23% and 24%. Respectively [2].
The prevalence of sarcopenia in Asia has mostly been reported from community dwelling older men and women of East Asia; 40.3% and 41.3%, respectively (2020, Korea) [3], 11.5% and 16.7%, respectively (2021, Japan residents participating in health check-ups) [4]; and 9.8% and 10.1%, respectively (2019, Japan, pooled prevalence from systemic review and meta-analysis) [5].
Literature on sarcopenia prevalence from South Asia is largely lacking. Medline and Google Scholar search reveals that sarcopenia prevalence has not been reported from Afghanistan, Pakistan, Nepal, Bhutan, Myanmar, Maldives, and Mauritius. A global study reported sarcopenia prevalence of 17.5% in Indian elderly (≥ 65 years) [1]. Indian studies have reported a prevalence of 14.2% (≥ 60 years) from South India [6] and 3.2% in community dwelling North Indians (≥ 20 years; younger population in SO-CUBES study) [7]. According to the SARIR Study from Iran the prevalence of presarcopenia (52.7% vs 25.3%), sarcopenia (20.7% vs 15.3%), and severe sarcopenia (6% vs 5.3%), were higher in men than women (> 55 years) [8]. The prevalence of sarcopenia was found to be 41.8% in the elderly (> 60 years) Surabaya Community of Indonesia [9].
Secondary sarcopenia prevalence has also been captured from South Asia, albeit the literature is scarce. A study from Sri Lanka in middle-aged (20–40 years) women reported pre-sarcopenia, sarcopenia, and severe-sarcopenia prevalence of 3.0%, 2.2%, 0.7% respectively [10]. The corresponding values for premenopausal women were 1.0%, 1.0%, 0.0%; and for postmenopausal women (51–60 years) were 4.2%, 3.0%, 1.2%, respectively [10]. The study showed that though prevalence of sarcopenia of any severity (including severe sarcopenia) was highest in post-menopausal women, presarcopenia and sarcopenia was also seen in younger age women.
Sarcopenia is more prevalent in patients with comorbidities and thus secondary sarcopenia needs to be tackled early. A study from Bangladesh evaluating sarcopenia in chronic obstructive disease (COPD) patients reported a prevalence of 26% [11]. Another study from Thailand reported a prevalence of 24% in patients with COPD [12]. Similarly, high sarcopenia prevalence (63.5%) has been reported in patients with hip fractures [13]. Another study from Iran reported a sarcopenia prevalence of 11.5% in peritoneal dialysis (PD) patients [14].
2. Sarcopenia as a syndrome
The group identifies sarcopenia as a multifactorial multi-causative syndrome. Primary and secondary sarcopenia can be distinguished by their etiology, but overlap each other in terms of clinical presentation and management strategies.
2.1. Primary sarcopenia
The loss of muscle mass and/or function and strength seen in elderly and geriatric is considered as primary sarcopenia [[15], [16], [17], [18]] It is a complex geriatric syndrome of multifactorial pathogenesis (Fig. 1) [19]. Since the majority of elderly lead a sedentary lifestyle, they are at increased risk of developing sarcopenia and mobility disorders [[20], [21], [22]].
Fig. 1.
2.2. Secondary sarcopenia
Patients in any age group, including younger patients may present with muscle wasting, loss of strength, and/or function due to an underlying cause. This is called secondary sarcopenia [15,18,19]. The various causes of secondary sarcopenia can be broadly classified as medical, endocrine, and metabolic (Table 1). The secondary causes of sarcopenia can be screened for using disease specific clinical, biochemical and imaging parameters (Table 2).
Table 1.
Causes and pathophysiology of secondary sarcopenia.
Common themes leading to secondary sarcopenia in patients with comorbidities:
| ||||
|---|---|---|---|---|
| Medical | ||||
| Condition | Pathophysiology in sarcopenia | Condition | Pathophysiology in sarcopenia | |
| CKD, ESRD, CRF [150] | Balance between skeletal muscle regeneration and catabolism is altered because of uremia | Dementia |
|
|
| CHF [160] |
|
Polypharmacy [18] | Mainly anorexia induced by effects of multiple drugs | |
| Cirrhosis [161] |
|
Psychological/psychiatric: Depression, anorexia nervosa |
|
|
| COPD [162] |
|
Dysphagia [18] | Poor nutritional intake | |
| Stroke [163] |
|
Malabsorption syndromes | Deficiency of proteins and important nutrients required for muscle growth and metabolism | |
| Malignancy [164,165] |
|
HIV and AIDS [166] |
|
|
| Trauma, burns [167,168] |
|
Sepsis [169] |
|
|
| Arthritis (Osteoarthritis and rheumatoid arthritis) [23,170] |
|
COVID-19 [158] |
|
|
| Neurological disorders [40] | Primary muscular disease | Chronic inflammatory demyelinating polyneuropathy, Motor neuron disease, Myasthenia Gravis |
|
|
| Endocrine | Malnutrition | |||
| Condition | Pathophysiology in sarcopenia | Condition | Pathophysiology in sarcopenia | |
| Hypothyroidism and hyperthyroidism [171,172,172] |
|
Protein energy malnutrition/isolated protein undernutrition |
|
|
| Diabetes [173,174] |
|
Overnutrition 175 |
|
|
| ||||
| ||||
| ||||
| ||||
| Osteoporosis [120,121,176] |
|
|||
| Hypogonadism and menopause [[177], [178], [179]] |
|
|||
| ||||
| ||||
| ||||
| ||||
| ||||
| Obesity [175] |
|
|||
| ||||
| ||||
| ||||
| Others | Metabolic | |||
| Condition | Pathophysiology in sarcopenia | Condition | Pathophysiology in sarcopenia | |
| Ethnic variations | Due to genetic predisposition | Fatty liver/NAFLD [180] |
|
|
| Muscle wasting as side effect of concomitant medications [166,181]: statins, sulfonylureas, glinides, antiretroviral therapy | ||||
AGES, advanced glycated end products; AIDS, acquired immunodeficiency syndrome; CHF, congestive heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus 2019; CRF, chronic renal failure; CRP, C-reactive protein; DHEA, dehydroepiandrosterone; ESRD, end stage renal disease; FSH, follicular stimulating hormone; FT3, free tri-iodothyronine; HIV, human immunodeficiency virus; IGF-1, insulin-like growth factor I; NAFLD, nonalcoholic fatty liver disease; TNF-α, tumor necrosis factor-alpha.
Table 2.
South Asian definition of sarcopenia and diagnostic tools for sarcopenia.
| Sarcopenia: 2 of 3 rule: any two of the following should be present: muscle strength, muscle function and muscle mass | |||||
|---|---|---|---|---|---|
| Clinical (primary modality of diagnosis) | |||||
| Muscle strength | Muscle Function | Muscle Mass | |||
| Hand grip [19] Lower limb muscle strength [82] |
Walking speed Sit-to stand [87] Chair stand test [88] SPPB [19] |
Anthropometry CC/MAC/Thigh circumference BMI: weight and height Waist circumference |
|||
| Imaging [18,19] | |||||
| |||||
| |||||
| |||||
| Biochemistry (proposed biomarkers) | |||||
| Common laboratory tests [19,[182], [183], [184], [185]] Can be done at community/PHC level |
Biomarker/s | Level in sarcopenia | What do biomarker levels in sarcopenia indicate? | ||
| Albumin [186]; total protein [186], hemoglobin, vitamin D [187]; uric acid; magnesium, calcium [186], uric acid [186], triglycerides [186] | ▼ | Inadequate intake/underproduction | |||
| Testosterone [188] | ▼ | Decreased muscle growth as it is muscle growth promoter | |||
| Estrogen [189] | ▼ | Loss of beneficial effect of estrogen on skeletal muscle proliferation; increased inflammatory stress damage | |||
| Creatine [190] | ▼ | Reduced muscle turnover | |||
| CPK | ▲ | Muscle damage and inflammation | |||
| CRP, ESR [186,191] | ▲ | Muscle inflammation | |||
| Specialized laboratory tests [19,94,185] Not routinely done; many are of academic interest and yet to be clinically relevant |
Leptin [192] | ▲ | Impaired physical function | ||
| Also increased in obesity induced sarcopenia (sarcopenic obesity) | |||||
| Follistatin [193]; brain-derived neurotrophic factor; bone morphogenetic proteins; IGF-1 [194]; growth hormone [194]; | ▼ | Decreased muscle growth as individually each is a muscle growth promoter | |||
| DHEAS [195,196] | |||||
| Serum cortisol/DHEAS ratio [196] | ▲ | Decreased muscle mass and strength, especially in diabetes mellitus patient | |||
| Oxidized low-density lipoprotein | ▲ | The decrease in pro-oxidants levels reduce muscle protective action | |||
| Selenium | ▼ | Inadequate intake | |||
| vitamin C and vitamin E | ▼ | The decrease in antioxidants levels reduce muscle protective action | |||
| Advanced glycosylation end-products (AGEs) [197] | ▲ | Altered muscle hemostasis (promoting muscle wasting), increased inflammation | |||
| Also increased in sarcopenia secondary to diabetes, cancer, inflammatory skeletal muscle diseases and myopathies | |||||
| Protein carbonyls [198] | ▲ | Oxidative damage to muscle proteins; reduced muscle strength | |||
| Adiponectin [186,199] | ▲ | Increase muscle inflammation and muscle metabolism | |||
| Myostatin [200,201]; Growth differentiation factor-15; Activins A and B; | ▲ | Decreased muscle growth as individually each is a muscle growth suppressor | |||
| Tumor growth factor β | |||||
| N-terminal type III procollagene | ▼ | Decreased muscle remodeling | |||
| Interleukin 6 [191,202], | ▲ | Muscle inflammation | |||
| GM-CSF, interferon γ, | |||||
| P-selectin, | |||||
| Tumor necrosis factor α [202]; Interleukin 1 and 8; | ▼ | Muscle inflammation | |||
| Butyryl-cholinesterase, myeloperoxidase, MCP-1, macrophage inflammatory protein 1-α, PDGF BB | |||||
| 3-methylhistidine [203] | ▲ | Proteolysis of myofibrils | |||
| Skeletal muscle-specific troponin T | ▲ | Contractile insufficiency | |||
| CAF [204] | ▲ | Impairment or degeneration of neuromuscular junctions | |||
| Complement protein C1q | ▲ | Physical inactivity | |||
| Cystatin C [205,206] | ▲ | Decreased muscle mass | |||
| Secreted protein acidic and rich in cysteine (SPARC) [207] | ▲ | Reduced myogenesis | |||
| Osteonectin, P3NP [208], fatty acid-binding protein-3, irisin [209], CAF, and macrophage migration inhibitory factor [207] | ▲ | These markers are increased in sarcopenia secondary to COPD or CHF | |||
| Various MOAs: | |||||
| Increased muscle inflammation, | |||||
| Reduced HGS | |||||
| Reduced appendicular lean mass/height [2] | |||||
| Pre-Albumin | ▼ | Nutrition | |||
BIA, Bio-electrical impedance analysis; CAF, c-terminal agrin fragment-22; CC, calf circumference; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CPK, creatinine phosphokinase; CRP, C-reactive protein; CT, computed tomography, DHEAS, dehydroepiandrosterone sulfate; DXA, Dual-energy X-ray absorptiometry; ESR, erythrocyte sedimentation rate; GM-CSF, granulocyte-monocyte colony-stimulating factor; HGS, hand grip strength; IGF-1, insulin like growth factor-1; MCP-1, monocyte chemoattractant protein 1; MOA, mechanism of action; MRI, magnetic resonance imaging; P3NP, procollagen type-III amino-terminal pro-peptide; PHC, primary health care; PDGF, platelet-derived growth factor BB; SPPB, Short Physical Performance Battery; USG-M, skeletal muscle ultrasound; ▼, decrease; ▲, increase.
Secondary sarcopenia is thought to result from a complex interplay of chronic and systemic inflammation, immobilization, and undernutrition (reduced or inappropriate intake) that result in increased protein breakdown (catabolism) and reduced protein synthesis (anabolism) in the muscles [18].
The secondary causes of sarcopenia result from comorbidities that affect the patient's osteo-arthro-muscular triad or the bio-psycho-social triad in some way, leading to poor quality of life (QoL). There is abundant literature to support the osteo-arthro-muscular triad [[23], [24], [25], [26], [27], [28]] or the bio-psycho-social triad [[29], [30], [31], [32]] comorbidities as a cause of secondary sarcopenia. Corresponding literature from South Asia includes an article from Pakistan, that mentions that major depression is associated with low QoL and leads to sarcopenia with loss of muscle mass and function [33]. Major depression starts a vicious cycle of low energy, low interest in surroundings, low physical activity, poor nutrition intake, poor mental health, and sarcopenia [33].
An Indian study screened the elderly patients (≥ 65 years; n = 100) presenting to the outpatient department of Geriatric Medicine in a tertiary care hospital for sarcopenia [34]. The study also used known standardized screening questionnaires and tools to assess various geriatric syndromes, cognition and QoL. The study found that osteoporosis, hypothyroidism, and neurological disease were the only comorbidities that correlated positively with sarcopenia [34]. Of these, only osteoporosis was significantly associated with sarcopenia. (P < 0.01). On the other hand, nutrition, cognition, and QoL had a negative correlation with sarcopenia [34]. In another study on the same patient group, one-repetition-maximum (1-RM) knee extension (1-RMKE) RM knee extension was evaluated to assess lower extremity muscle strength (LEMS) [35]. Low 1-RMEK was found to be significantly associated with malnutrition (P = 0.001), dementia (P = 0.016), and depression (P = 0.047) [35].
Similarly, literature on various other causes of secondary sarcopenia is slowly emerging from South Asia.
A recent study from Bangladesh evaluating prevalence and factors associated with sarcopenia in patients with COPD noted that one third of study patients had sarcopenia. Independent factors associated with sarcopenia were age (> 70 years), body mass index (BMI), COPD severity, and dyspnea severity on Modified Medical Research Council (MMRC scale) [11].
Another recent study from Sri Lanka looked at factors associated with sarcopenia in randomly selected 184 premenopausal and 166 postmenopausal women [36]. Appendicular skeletal mass index (ASMI) was significantly associated with BMI, total-body-fat-mass (TBFM), weight and HGS in premenopausal women (adjusted R2 = 0.85) and with BMI, weight, TBFM, hip-circumference and fasting insulin in postmenopausal women (adjusted R2 = 0.80). Of all the factors, BMI had the strongest association with ASMI in both premenopausal (r = 0.87, R2 = 0.76) and premenopausal (r = 0.87, R2 = 0.76) women [36].
An Indian study screened the elderly patients (≥ 65 years; n = 100) presenting to the outpatient department of Geriatric Medicine in a tertiary care hospital for sarcopenia [23]. The study also used known standardized screening questionnaires and tools to assess various geriatric syndromes, cognition and QoL. The study found that osteoporosis, hypothyroidism and neurological disease were the only comorbidities that correlated positively with sarcopenia [23]. Of these, only osteoporosis was significantly associated with sarcopenia. (P < 0.01). On the other hand, nutrition, cognition and QoL had a negative correlation with sarcopenia [23]. In another study on the same patient group, one-repetition-maximum (1-RM) knee extension (1-RMKE) 1-RM knee extension was evaluated to assess lower extremity muscle strength (LEMS). Low 1-RMEK was found to be significantly associated with malnutrition (P = 0.001), dementia (P = 0.016), and depression (P = 0.047)[24].
Similarly, literature on various other causes of secondary sarcopenia is slowly emerging from South Asia.
A recent study from Bangladesh evaluating prevalence and factors associated with sarcopenia in patients with COPD noted that one third of study patients had sarcopenia. Independent factors associated with sarcopenia were age (> 70 years), body mass index (BMI), COPD severity, and dyspnea severity on Modified Medical Research Council (MMRC scale) [11].
Another recent study from Sri Lanka looked at factors associated with sarcopenia in randomly selected 184 premenopausal and 166 postmenopausal women [36]. Appendicular skeletal mass index (ASMI) was significantly associated with BMI, total-body-fat-mass (TBFM), weight and HGS in premenopausal women (adjusted R2 = 0.85) and with BMI, weight, TBFM, hip-circumference and fasting insulin in postmenopausal women (adjusted R2 = 0.80). Of all the factors, BMI had the strongest association with ASMI in both premenopausal (r = 0.87, R2 = 0.76) and premenopausal (r = 0.87, R2 = 0.76) women [36].
2.3. Sarcopenic-obesity
A unique clinical presentation, sarcopenic obesity is defined as the co-existence of sarcopenia and obesity [[37], [38], [39]]. The condition can be defined based on values of ASMI/height squared, ASMI/bodyweight, BMI, body fat percentage, and/or waist circumference [39].
An article from Pakistan mentions that loss of muscle mass with an increase in adipose tissue (sarcopenic-obesity) causes metabolic imbalances associated with increase in fat, cortisol levels and pro-inflammatory cytokines, and decrease in protein and bone mass [33]. These metabolic imbalances increase the risk of cardiovascular and cerebrovascular events [33].
The SO-CUBES study from India used different methods to assess sarcopenic obesity in community dwelling elderly (n = 804; ≥65 years). The prevalence of sarcopenic obesity was 5.4%, 5.4%, and 6.3% based on BMI, waist circumference, and fat mass percent, respectively [7].
Another study from India compared the prevalence of sarcopenic obesity in 30 patients with motor neuron disease (MND) and amyotrophic lateral sclerosis (MND-ALS) and 33 controls (mean age 59.2 vs 61.2 years). The prevalence of sarcopenic obesity, defined in the study as fat mass percentage > 27% + appendicular lean mass (ALM)/BMI < 0.786 kg/kg/m2, was higher in MND-ALS group compared to controls (44.5% vs 16.7%; P = 0.03) [40].
3. Consensus process
The South Asian Working Action Group on Sarcopenia (SWAG-SARCO) consists of experts from the field of geriatrics, endocrinology, and general medicine from the South Asian and neighboring countries (Bangladesh, Bhutan, India, Indonesia, Iran, Maldives, Mauritius, Myanmar, Nepal, Pakistan, and Sri Lanka). The experts came together through several virtual meetings in the months of June and July 2021, to formulate a consensus on definition, prevention, diagnosis, and management of sarcopenia. Prior to the virtual meets, the experts collated data from their individual countries, studied international and Asian guidelines in detail and scoured through latest available literature in English language on sarcopenia retrieved from Medline and Google Scholar. The members of the group presented their findings during the virtual meet, and discussed the available evidence and research gaps. Through general consensus, they formulated the definition of sarcopenia and recommended practical ways to prevent, diagnose, and manage sarcopenia.
The experts acknowledge that definitive answers do not exist for many research questions, and have therefore, attempted to create a framework that will improve clinical practice, focus on sarcopenia, and case finding, and help in management and monitoring of sarcopenia in cost restrained settings. The consensus is based on clinical parameters, followed by biochemical markers and imaging to be used only in select cases. The recommendations have been made keeping the unique needs of the South Asian population; however, it is felt that these recommendations will hold universal application and acceptance. The novel approach should also help enhance research and promote advocacy for sarcopenia care.
3.1. Existing definitions of sarcopenia: applicability to South Asia
Sarcopenia has been a topic of interest for years. In 2010, the European Working Group on Sarcopenia in Older People (EWGSOP) [41] formulated their guidelines for sarcopenia followed by the International Working Group on Sarcopenia (IWGS) [42]in 2011, Asian Working Group for Sarcopenia (AWGS) [43], and the Foundation for the National Institutes of Health (FNIH) 44in 2014. The recent guidelines include the Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition) 45in 2016, Clinical Practice Guideline for Sarcopenia from Japan [39], the Revised European Working Group on Sarcopenia in Older People (EWGSOP2) 46and International Clinical Practice Guidelines for Sarcopenia (ICFSR) 47in 2018, followed by Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment [48]. In 2020, the Position Statements of the Sarcopenia Definition and Outcomes Consortium (SDOC) were published [49].
As science progresses, so does the knowledge of sarcopenia, and this lead to the revision of some of the guidelines like the EWGSOP and AWGS. However, research, clinical evidence, and scientific information on sarcopenia are still evolving. There are regional and ethnic differences between the western world and Asia and even within the Asian community, and healthcare systems also vary. Hence, our group studied the various guidelines (Table 3) to understand how they define and diagnose sarcopenia. This helped the experts understand the limitations in the existing guidelines that would prevent their applicability to South Asia. This also helped identify strengths of the guidelines in guiding our decisions to formulate a definition for the South Asian community and chalk out an effective, economic, clinical and community level screening, diagnostic and management pathway for sarcopenia.
Table 3.
Comparing sarcopenia definition and diagnosis across guidelines.
| Guideline | Definition | Strengths (Aligned with South Asian perspective) | Limitations (Not aligned with South Asian perspective) |
|---|---|---|---|
| International Guidelines | |||
| EWGSOP [41] | “The EWGSOP recommends using the presence of both low muscle mass and low muscle function (strength or performance) for the diagnosis of “with diagnosis based on documentation of low muscle mass with either low muscle strength or low physical performance |
|
|
| EWGSOP2 [46] | “In its 2018 definition, EWGSOP2 uses low muscle strength as the primary parameter of sarcopenia; muscle strength is presently the most reliable measure of muscle function. Specifically, sarcopenia is probable when low muscle strength is detected. A sarcopenia diagnosis is confirmed by the presence of low muscle quantity or quality. When low muscle strength, low muscle quantity/quality and low physical performance are all detected, sarcopenia is considered severe.” |
|
|
| ICFSR [47] | Evidence based guideline for screening, diagnosis and management; no definition proposed General definition of sarcopenia is accepted: “Sarcopenia is defined as an age-associated loss of skeletal muscle function and muscle mass, and is common in older adults” |
|
|
| FNIH [44] | No definition proposed |
|
|
| International Working Group on Sarcopenia (IWGS) [42] | “Sarcopenia is the age-associated loss of skeletal muscle mass and function. Sarcopenia is a complex syndrome that is associated with muscle mass loss alone or in conjunction with increased fat mass. The causes of sarcopenia are multifactorial and can include disuse, changing endocrine function, chronic diseases, inflammation, insulin resistance, and nutritional deficiencies. While cachexia may be a component of sarcopenia, the two conditions are not the same.” |
|
|
| Position Statements of the Sarcopenia Definition and Outcomes Consortium (SDOC) [49] | “The Panel agreed that both weakness defined by low grip strength and slowness defined by low usual gait speed should be included in the definition of sarcopenia.” | “Identified grip strength – either absolute or scaled to measures of body size – as an important discriminator of slowness” Does not recommend the use lean mass measured by DXA in the definition of sarcopenia |
No recommendations for secondary sarcopenia |
| Asian Guidelines | |||
| AWGS 2014 [43] | Both define sarcopenia as “age-related loss of muscle mass, plus low muscle strength, and/or low physical performance” without reference to comorbidity |
|
|
| AWGS 2019 Consensus Update on Sarcopenia Diagnosis and Treatment [48] |
|
|
|
| JSH [45] | No definition proposed |
|
|
| Clinical Practice Guideline for Sarcopenia from Japan [39] | “Sarcopenia is generally defined as a decrease in skeletal muscle mass and muscle strength or physical function, such as gait speed, observed in elderly individuals.” |
|
|
| South Asian Consensus | Strengths (Can be applied globally) | Limitations (for global use) | |
| The SWAG-SARCO 2021 | The group defines sarcopenia as a condition in which any two of the following parameters are suboptimal, as assessed by clinical, biochemical and/or imaging modalities: muscle function, muscle strength and muscle mass |
|
|
AWGS, Asian Working Group for Sarcopenia; EWGSOP, European Working Group on Sarcopenia in Older People; EWGSOP2, Revised European Working Group on Sarcopenia in Older People; FNIH, Foundation for the National Institutes of Health; ICFSR. International Clinical Practice Guidelines for Sarcopenia; IWGS, International Working Group on Sarcopenia; JSH, Japanese Society of Hematology; PEM, protein energy malnutrition; SWAG-SARCO, South Asian Working Action Group on Sarcopenia.
3.2. Need for South Asian definition
Asian community is culturally and ethnically different from the western world [48]. Other major anthropometric and phenotype differences set the Asian community apart from the western world [50]. Asians have relatively smaller body size and higher adiposity than the western population and lead a less mechanized and more physically active lifestyle, in general [48,51]. Also, muscle strength and mass is lower in South Asians than Caucasians [52]. The SDOC also supports the notion that the definition of sarcopenia should be scaled according to body size [49]. Hence, international guidelines can be adopted for the Asian and South Asian community only with caveats and concerns.
In this context the South Asian community is ethnically and phenotypically different from Eastern and South Eastern Asia (which include countries like China, Hong Kong, Japan, Singapore, South Korea, Taiwan, and Thailand) [48,53,54]. These ethnic differences in body composition have clinical significance [[55], [56], [57]]. A known phenotype of South Asian obesity is the ‘thin-fat obesity’ or ‘sarcopenic obesity’, also known as normal weight obesity that has similar cardio-metabolic risk as obesity [37,38,58,59]. This phenotype typical of South Asian community is not seen in Eastern and South Eastern Asian communities. Hence, AWGS (2014 and 2019) guidelines are largely not applicable in South Asia. Using AWGS cut-offs can produce results that are not typical of the South Asian community. This was reflected by a study conducted on elderly outpatients (≥ 65 years; n = 100) presenting to the Geriatric Medicine department in a tertiary care hospital from India [34]. The study used AWGS 2014 cutoffs for hand grip strength (HGS), gait speed, and ASMI to diagnose sarcopenia. The prevalence of sarcopenia using AWGS 2014 cutoffs was 53%, which was much higher [34] than the prevalence reported from various regions of India (17.5% [1], 14.2% 6) and Asia (Singapore 27% [60], Malaysia 28.5% [61]). One of the reasons for this high prevalence in the study population was because the AWGS low muscle mass cutoff is much higher than the cutoff which can be used for Indian older adults. Thus, many older Indian adults with muscle mass levels normal for South Asian population were flagged as sarcopenic by the study. Similarly, a Malaysian study using the EWGSOP muscle-mass cutoff for older people reported sarcopenia in 51% of the population [62]. This is because the western cutoffs for sarcopenia are higher than that of AWGS as their body mass is more than that of Asians. Hence, older Malaysian adults without sarcopenia were flagged as sarcopenic.
Unlike South East Asia which has a high proportion of ageing population for years, the South Asian population, which was earlier comparatively young, is now increasingly ageing [63,64]. A recent publication from India noted that population is ageing globally in the 21st century with India having the second highest elderly population in the world [65]. The ageing population is other regions of South Asia is also rising with rise in life expectancy at birth [66], changing lifestyle, and improvement in medical facilities and sanitation [65,[67], [68], [69]]. The proportion of population aged ≥ 60 years in South Asia is expected to increase to 10% by 2025 and 19% by 2050 [64]. This means that nearly one in five South Asians will be an older person in 2050 [63]. Therefore, there is a felt need to formulate a way to screen, diagnose, and manage age related sarcopenia in South Asia.
South Asian countries are mostly low middle income countries and face resource and cost challenges [70]. The guidance should be appropriate for the local healthcare delivery system, which places greater reliance on clinical medicine than investigations. Therefore, as shown in Table 3, most definitions of sarcopenia are based on diagnosis through DXA and BIA, which are costly tests and not available at all centers in South Asia. On the other hand, this region is developing fast, and there is dual burden of over and undernutrition. Overnutrition results in metabolic challenges as discussed earlier and sarcopenic obesity [37,38]. South Asians are increasingly leading a sedentary life style, and the region is seeing an increase in lifestyle and economic growth related non-communicable diseases in middle-aged and elderly which likely to increase secondary sarcopenia burden in this region [67,68,[70], [71], [72]].
Hence, there is a need to look at secondary causes of sarcopenia in the South Asian population. The EWGSOP2 also acknowledges that sarcopenia is increasingly being recognized earlier in adult life cycle, and apart from age there are many other contributing factors that define sarcopenia phenotype [46].
Literature from South Asia was scarce until 2014 [43]. However, new data and publications are slowly emerging from this region [6,11,33,34,36,52,57,73]. This has been helpful in understanding sarcopenia from South Asian perspective, and in formulating a consensus on screening, diagnosing, and managing sarcopenia in this patient population.
4. South Asian definition and diagnosis of sarcopenia
4.1. Definition
The SWAG-SARCO 2021 defines sarcopenia as a syndrome in which any 2of the following parameters, as assessed by clinical, biochemical and/or imaging modalities, are suboptimal: muscle function, muscle strength and muscle mass (Table 2).
4.2. Rationale behind South Asian definition
4.2.1. Diagnosis of sarcopenia
Muscle mass, function and strength have also been used by various guidelines in varying proportion for defining sarcopenia. The EWGSOP2 gave importance to low muscle strength over low muscle mass [46]. On the other hand, AWGS 2019 and EWGSOP gave priority to muscle mass over muscle strength [41,48]. SDOC gives importance to muscle strength and function, adjusted to body size but does not recommend the inclusion of dual-energy X-ray absorptiometry (DXA) measured lean muscle mass in the definition of sarcopenia [49]. However, the SWAG-SARCO 2021 group gives equal importance to muscle strength, muscle function and mass while diagnosing sarcopenia. All 3 muscle components have been linked to adverse outcomes, either independently or in various combinations [49,74]. Therefore, the South Asian guidelines are marked of greater equipoise. However, the group acknowledges that all 3 are unlikely to be present in a patient and therefore the diagnosis of sarcopenia may be missed in many patients. Hence, the SWAG-SARCO 2021 defines sarcopenia as the loss of any 2 of the muscle components (mass, function, or strength), without giving importance to any 1 component over the other.
The definition proposed by SWAG-SARCO 2021 is based on an approach similar to that followed by experts working in other disease areas, such as psychiatry [75], rheumatology [76], polycystic ovary syndrome (PCOS) [77], and metabolic syndrome [78]. The EWGSOP also uses the two-by-three rule to diagnose sarcopenia but makes documentation of low muscle mass mandatory for diagnosis along with either low muscle strength or low physical performance [41]. The rationale for using this approach is based on the acceptance that all disease criteria will not be present in 1patient. Therefore, using the approach to diagnose a condition based on presence of majority of criteria decreases the chance of missing a diagnosis and thereby increases the sensitivity of diagnosing sarcopenia by using this definition. Overall a syndromic approach for diagnosis of sarcopenia is proposed by the SWAG-SARCO 2021.
Additionally, while defining sarcopenia, the AWGS 2019 did not consider muscle wasting caused predominantly due to a coexisting condition [48]. However, though skeletal muscle loss due to cachexia (caused by underlying illness), inactivity (decreased muscle synthesis), and sarcopenia (age-related) have been considered as separate conditions [79], secondary sarcopenia inclusive of cachexia and inactivity is a well-recognized entity [11,18,19,34,36,80], and also accepted by EWGSOP2 2019 [46].
Hence, the SWAG-SARCO 2021 felt the need to consider age related and other causes of sarcopenia (including concomitant medications), since this allowed diagnosis of any condition that could result in a sarcopenia-like state. Alternatively, this definition may allow physicians to diagnose an underlying condition in a patient presenting with sarcopenia-like features. Thus, the proposed definition allows a wide spectrum of disease to be identified; and identification is the first step to address and optimize. Moreover, many patients in the geriatric population have underlying comorbidities that could cause sarcopenia, thereby making it difficult to segregate primary from secondary sarcopenia.
The SWAG-SARCO 2021 definition of sarcopenia therefore reflects the multifactorial etiology, multifaceted clinical presentation, and multi-dimensional impact of the sarcopenia as a syndrome. It accepts the limitation that all the conditions/symptoms/signs are unlikely to be present in 1patient. Therefore, the definition aims to identify all possible cases of sarcopenia and allows them to be assessed further. It promotes clinical medicine, while fully respecting advances in imaging and biochemistry. The definition will maintain its relevance in the face of developments in diagnostic science in the years to come.
5. Clinical tools to assess muscle strength, function and mass
5.1. Muscle strength
Loss of muscle strength is also known as dynapenia [81]. Deterioration in muscle mass is slow and difficult to measure compared to muscle strength and function. Hand grip strength (HGS) is the most easy way to clinically asses muscle strength of upper limb using hand-held dynometer (HHD) [19]. Similarly LEMS can be measured using isokinetic dynamometers or HHDs [82]. Though isokinetic dynamometers are more precise than HHDs, they are bulky and costly instruments that find little use in community settings. Hence HHDs are usually used to assess LEMS [82]. Muscle strength can also be measured using manual muscle testing (MMT), but this brings a lot of subjectivity to assessment and therefore, it's use is restricted only for settings where HHDs are not available [82]. HGS and LEMS are measured for both the upper and lower limbs, respectively [83]. Three readings are usually taken on each limb after giving some in-between resting interval; then the highest reading is recorded as HGS or LEMS [[83], [84], [85]]. However, some clinicians prefer to use the mean of the 3 readings [85]. The HGS and LEMS values vary with the type of dynometer used, the encouragement given during the test, and whether highest or mean of the readings is taken [85]. Hence, experts recommend that the same methodology and dynometer (type of dynometer if not possible to use the same machine) should be used for the patient all throughout the management of sarcopenia.
In settings where a dynamometer is not available, HGS is measured by asking the patient to lift 1 kg weight by lifting the arm above the shoulder for at least 30 s, all the time keeping the elbow fully extended [86]. HGS is considered normal if the patient is able to hold the weight for at least 30 s in that position. LEMS can be measured by using 1-RMKE method. This was evaluated in a study conducted on 100 older adults (≥ 65 years) attending outpatient clinics in India [35]. The test was done by asking the patient to sit comfortably on a standard quadriceps chair, back straight against the backrest, and legs hanging freely from the chair. Complete extension of the knee joint was measured by asking the patient to lift increasing weight with the leg against gravity. The average 1-RMKE of both legs is taken as the 1-RMKE value for the patient [35]. The study also looked at the association of 1-RMKE values with HGS and geriatric syndromes. In this study, 1-RMKE had a median value of 2.29 (0.5–10.0) and HGS of 17.5 (0–78). The correlation between 1-RMKE and HGS was moderately significant (r = 0.491, P < 0.001). Low 1-RMKE was significantly associated with female sex and sarcopenia (P < 0.001 for both) [35]. The EWGSOP [41] guidelines do note knee flexion extension as a reliable method to measure LEMS.
5.2. Muscle function
A 4-m gait speed test is a reliable way to assess muscle function and performance [83]. Time taken by the patient to walk for a distance of 4 m (on a flat surface) is noted as the gait speed. A normal person usually takes less than 5 s to cover the distance; thereby a gait speed of < 0.8 m/s is considered normal [83]. Gait speed is considered low if the patient takes longer than 5 s to walk this distance. This method also tests the patient's balance [83].
Muscle function can also be assessed using sit-to-stand (STS) tests, which are mainly of 2 types, 5 times STS (5TSTS), and 30 s Chair Stand Test (30CST) [87]. The 2STS types correlate strongly with gait speed (P < 0.01 for both), endurance (P < 0.01 for both) and dynamic balance (P < 0.01 for both) [87].
The chair stand test is a reliable easy to perform test at community level as its results correlate directly with sarcopenia. Each 1 s increment in performing the test increases the probability of sarcopenia by 8% [88].
Muscle function can also be assessed using a more comprehensive test such as the Short Physical Performance Battery (SPPB) [19]. SPPB tests the patient's ability to stand with the feet together in 3 positions: side-by-side, semi-tandem, and tandem positions. It also assesses time taken to walk 8 feet and average time taken to rise from a chair and return to seated position (over 5 efforts) [19].
The timed up-and-go test (TUGT) is another way to evaluate muscle function and performance. The patient gets up from chair, walks for 3 m, turns back, walks again, and sits down [83,89]. A normal individual usually performs TUGT in less than 10.2 s to complete the test [89]. The western population clocks higher TUGT time of 12.3 s [89]. Other tests that have been used to evaluate muscle function and performance include 6 min-walk time, 400-m walk time, and climbing up the stairs [83].
5.3. Muscle mass
Though DXA is a reliable test for measuring skeletal muscle mass (SMM), it is an expensive test that is not available across centers in South Asia, nor is it affordable by all. The SDOC also noted that when DXA was used to measure lean mass in community-dwelling older adults, the measured mass (with or without adjustment for body size) did not correlate with adverse outcomes such as sarcopenia [49].
Therefore, clinical and economic tools such as mid-arm circumference (MAC) or mid-upper arm circumference (MUAC), and calf circumference (CC) can be used instead as they also correlate very well with SMM [83]. Studies from Bangladesh and Nepal, respectively, show that MUAC is also useful in measuring chronic energy deficiency (CED) [90]and underweight [91]. Hence, MUAC may also be useful to assess conditions such as CED and underweight, which can lead to sarcopenia.
A non-elastic tape can be used to measure the maximum circumference (girth). This method is moderate to highly sensitivity and specific in predicting sarcopenia [48]. MAC is measured with patient flexing biceps with arm raised at shoulder level and elbow bent at 90° angle [83]. CC is measured with the patient standing with feet shoulder-wide apart [83].
When CC, MAC is used for measuring muscle mass, it is important to rule out fat mass present in the subcutaneous tissue to obtain the actual muscle mass. This is not routinely practiced in clinics and the measured CC or MAC is taken as the muscle mass. This is likely to give proportionately high muscle mass, especially in patients with sarcopenic obesity. Hence, it is suggested that fat mass should be excluded by measuring the skin fold thickness to obtain the actual mid-arm muscular circumference (MAMC). One way of estimating MAMC is by using triceps skinfold (TSF) thickness [92]. TSF is measured on the posterior surface of the arm, midway between the olecranon and the acromion process using a skinfold caliper. Then MAMC is calculated using the equation: MAMC (cm) = MUAC (cm) – [TSF (mm) x 0.314 92. This method of calculating the MAMC may be tried at community levels to assess for feasibility and to create normative data.
6. Proposed biochemical biomarkers for sarcopenia
Most of the pathophysiological pathways of sarcopenia have their own biomarkers [19,93,94] as shown in Table 2. Some biochemical markers are routinely done at community level as shown in Table 2. Other biomarkers, as discussed in Table 2 are expensive, not widely done at all centers, and may be done either for research purposes or as per the discretion of the treating physician …
Though the currently available biomarkers have been found to be raised in patient diagnosed with sarcopenia through SARC-F and SPPB, they are neither specific nor sensitive enough as stand-alone diagnostic criteria for sarcopenia [19,93,94]. Also, a single biomarker is less predictive of sarcopenia than a battery of biomarkers [93,94].
Therefore, experts place biochemical measures as second in importance after clinical measures. In patients where biochemical assessment is thought to add value to treatment (eg, secondary sarcopenia), the experts stress on using serial biochemical assessment, as a rising/falling trend is of more importance than a single value. The experts also suggest that normal local laboratory ranges for each biomarker may be used to guide decision making. However, the experts also acknowledge that future work is required to ascertain each biomarker's predictive accuracy.
7. Imaging as a tool to diagnose sarcopenia
Various imaging modalities can be used to screen for or diagnose sarcopenia. Computed tomography (CT), DXA, and bio-electrical impedance analysis (BIA) have been effectively used to define sarcopenia [95]. Reduced or low lean muscle mass detected through all the 3 modalities has been found to be associated with increased mortality [95].
CT and magnetic resonance imaging (MRI) can be used as reliable imaging techniques as they provide exact measurement of muscle mass, and information on muscle density and fatty infiltration [96,97]. CT and MRI also help to accurately differentiate between lean body tissue, and fat [82]. However, their cost, possibility of radiation hazard and the fact that they are not easily portable or available across centers, limits their use.
Though skeletal muscle ultrasound (USG-M) is a relatively cheaper imaging technique than CT/MRI, yet USG-M is not as sensitive in detecting sarcopenia due to many technical (type of probe, its pressure, position and inclination; site of muscle) and patient (supine/prone; sitting/standing; cognitive impairment) related factors [19]. On the other hand, 2 studies, one from Japan [98] and another from Turkey [99], reported that gastrocnemius thickness measured by USG was more likely to predict low HGS than skeletal muscle index [SMI] or appendicular skeletal muscle index [ASMI]) measured by BIA [98,99]. Therefore, given its low cost and easy availability at most centers in South Asia, USG may be used as an imaging tool to assess muscle mass. Also, USG M by the same observer can be used for sequential monitoring of muscle mass [100].
DXA and BIA are reliable tests. However, they are costly and available only in select centers in South Asia, and hence cannot be used routinely. Despite the high cost, DXA is a useful tool that can help assess multiple parameters such as fat mass, lean soft tissue mass, and bone mineral content [101,102]. The assessment can be localized or involve the whole body, and helps to differentiate between sarcopenia, obesity, sarcopenic-obesity, and osteoporosis. Appendicular lean mass (ALM) adjusted for BMI or height can help differentiate between physiological (primary) and pathological (secondary) sarcopenia [101,102].
BIA helps assess and normalize total skeletal muscle mass (SMI or ASMI) with respect to height [103]. Single frequency BIA (SF-BIA) cannot penetrate tissue completely; and therefore cannot be used to measure the entire muscle volume [104]. Conversely, multiple frequency BIA (MF-BIA) can accurately measure body composition (total skeletal mass and body fat mass) as it uses several current frequencies [104]. MF-BIA is a better method to assess ASM in sarcopenia than SF-BIA [105].
BIA is found to be as good as axial CT scan, calf circumference, or grip strength for screening sarcopenia, especially in patients with malignancy [106]. Though BIA is less expensive than DXA, its reliability is compromised at BMI of ≥ 35 kg/m282, and hence may not be very useful in sarcopenic obesity. Nevertheless, given the portability of BIA as compared to DXA, BIA can be used for mass screening in the community.
Imaging has also been used to study or define sarcopenia in India. A study from South India looked at prevalence of sarcopenia in patients undergoing abdominal CT scan for any reason [73]. In this study, Sreepriya et al [107] considered lumbar skeletal muscle index ≤ 38.5 cm2/m2 in females and ≤ 52.4 cm2/m2 in males as radiologically significant sarcopenia on CT. Similarly, another single center study from India used CT scan to measure psoas muscle mass and density, and defined sarcopenia at 5th percentile and provided separate cutoff points for males and females. The 5th percentile values for psoas mass and density for men were 220.7 mm2/m2 and 0.56 Hounsfield units (HU)/kg, respectively; the corresponding values for females were 149.5 mm2/m2 and 0.53 HU/kg, respectively. However, these are small studies and their results cannot be extrapolated to community and clinical settings. The experts recommend that imaging should be used only in select cases as per discretion of treating physicians. Imaging should ideally be used where clinical and biochemical measures are not able to guide diagnostic or treatment decisions. Choice of imaging depends on availability, accessibility and affordability.
8. Using questionnaires to screen for and diagnose sarcopenia
In addition to clinical assessment, sarcopenia can also be defined by simple functional questions [94]. The SARC-F questionnaire helps screen 5 sarcopenia-related components, Strength, Assistance with walking, Rise from a chair, Climb stairs and Falls (Table 4) [108]. On a score scale of 0–10, a score ≥ 4 is predictive of sarcopenia [108]. The ICFSR [47], EWGSOP2 [46] and AWGS 2019 Consensus Update on Sarcopenia Diagnosis and Treatment [48] recommend the use of SARC-F to screen for sarcopenia. A study found SARC-F score ≥ 4 as a useful tool in screening sarcopenia in patients with hip fractures with reliable sensitivity (95.35%), specificity (56.94%), positive predictive value (PPV; 56.94%), and negative predictive value (NPV; 71.3%) [13].
Table 4.
SARC-F and SARC-CalF questionnaires for sarcopenia [111].
| Components | Questions | SARC-F score | SARC-CalF score |
|---|---|---|---|
| Strength | How much difficulty do you have in lifting and carrying 10 pounds (approximately 4.5 kg)? | None = 0 | None = 0 |
| Some = 1 | Some = 1 | ||
| A lot or unable = 2 | A lot or unable = 2 | ||
| Assistance in walking | How much difficulty do you have walking across a room? | None = 0 | None = 0 |
| Some = 1 | Some = 1 | ||
| A lot, use aids, or unable = 2 | A lot, use aids, or unable = 2 | ||
| Rise from a chair | How much difficulty do you have transferring from a chair or bed? | None = 0 | None = 0 |
| Some = 1 | Some = 1 | ||
| A lot or unable without help = 2 | A lot or unable without help = 2 | ||
| Climb stairs | How much difficulty do you have climbing a flight of 10 stairs? | None = 0 | None = 0 |
| Some = 1 | Some = 1 | ||
| A lot or unable = 2 | A lot or unable = 2 | ||
| Falls | How many times have you fallen in the past year? | None = 0 | None = 0 |
| 1‒3 falls = 1 | 1‒3 falls = 1 | ||
| 4 or more falls = 2 | 4 or more falls = 2 | ||
| Calf circumference | Females: | ||
| >33 cm = 0 | |||
| ≤33 cm = 10 | |||
| Males: | |||
| >34 cm = 0 | |||
| ≤34 cm = 10 |
The AWGS 2019, in addition, also recommends the use of SARC-CalF questionnaire for screening, which combines calf circumference with SARC-F [48,109]. A score of ≥11 is predictive of sarcopenia [110,111]. A study from Indonesia found that combining calf circumference and thigh circumference with the SARC-F questionnaire had a sensitivity, specificity, PPV, and NPV of 15.79%; 99.01%; 75.00%; and 86.21%, respectively, for predicting sarcopenia in 120 elderly outpatients [112].
Of the 2, SARC-CalF is found to be more sensitive and accurate than SARC-F in screening and diagnosing sarcopenia in community dwelling elders and in clinical practice as well [110,111]. In patients with clinical suspicion of sarcopenia, the experts recommend that SARC-F (score ≥ 4) or SACF-CalF (score ≥ 11) can be used to screen for sarcopenia.
9. Literature to support diagnostic cut offs in South Asia
The Sarcopenia-Chandigarh Urban Bone Epidemiological Study (Sarco-CUBES) 52study from India looked for diagnostic cut-offs and biochemical predictors of sarcopenia in healthy adults. Muscle mass, strength, and physical performance were expressed as ASMI, grip strength (dominant hand), and gait speed, respectively. Grip strength and ASMI < 2SD of young reference population (20–39 years) was taken as a cutoff for muscle strength and mass, respectively. A gait speed ≤ 0.8 m/s was considered as poor physical performance [52]. A grip strength < 27.5 kg in males and <18.0 kg in females was defined as low muscle strength, and an ASMI < 6.11 kg/m2 in males and < 4.61 kg/m2 in females was defined as low muscle mass.
In their study on elderly in South India, Shaikh et al [6] used gait speed to screen for sarcopenia. A gait speed of < 0.8 m/s (not validated in India but used based on available literature) was considered as sarcopenia [6]. Elderly with normal gait speed but suspected to have sarcopenia were further investigated with HGS with HHD. A grip strength of < 30 kg in men and < 20 kg in women was considered as sarcopenia. The ASMI of these patients was calculated using Lee's equation [113] for Asians. ASMI of < 7.0 kg/m2 in men and < 5.7 kg/m2 in women was considered low [6].
A cross-sectional study from Sri Lanka in middle-aged (20–40 years) women (n = 117) determined the following cutoff values for the 3main measures of sarcopenia; 5.03 kg/m2 for ASMI) derived from Appendicular Skeletal Muscle Mass (ASMM, kg) measured by DXA and adjusted for height (ASMM/height [2]), 9.66 kg for hand grip strength (HGS) and 0.96 m/s for gait speed [10].
In a study from India, researchers used BIA to derive a novel predictive equation for fat free mass (FFM) based on birth weight: “FFM = 32.637 + (−0.222 X age) + (−32.51 X waist-to-hip ratio) + (0.33 X body mass index) + (1.58 × 1 or 2 (1 = normal birth weight, 2 = low birth weight) + (0.510 X waist circumference)” [114]. This equation needs to be validated in larger populations.
A study from Indonesia showed good diagnostic accuracy of combining muscle mass using calf circumference (< 34 cm in men and < 29 cm in women) and thigh circumference (< 49 cm in men and < 44 cm in women) with muscle function assessed through the SARC-F questionnaire (score of ≥ 4) in predicting sarcopenia in 120 elderly outpatients [112].
Literature to derive diagnostic cutoff for sarcopenia is largely lacking from South Asia. Diagnostic cutoffs used in western guidelines or in AWGS 2019 have not been validated in South Asia. This is definitely an area for further research in coming years. Also, a couple of studies from this region have reported different cutoffs for the same measure-thereby raising a significant unmet need to standardize the cutoffs for South Asia. The SWAG-SARCO group thus provided the cutoff recommendations in Table 5 based on the literature available from this region. If no diagnostic cutoffs were available for a measure, then the experts recommend using the global/western cutoffs until these cutoffs are validated in the South Asian population. If different cutoffs were reported for a measure from South Asia, the experts recommend that physicians can use either until a particular value is validated/standardized for this region.
Table 5.
Diagnostic cutoffs of various tests for sarcopenia in Asian, international and South Asian guidelines.
| Parameter | International |
Asian |
South Asian |
||||||
|---|---|---|---|---|---|---|---|---|---|
| EWGSOP [41] | EWGSOP2 [46] | IWGS [42] | FNIH [44] | ICFSR [47] | AWGS 2014 consensus [43] | AWGS 2019 Consensus Update on Sarcopenia Diagnosis and Treatment [48] | JSH [45] (cut-off for sarcopenia in liver disease) | SWAG-SARCO 2021 | |
| Diagnostic tools/Screening | Muscle mass using DXA, BIA Muscle strength by grip strength Muscle function/performance by gait speed, SPPB |
Muscle mass using DXA, BIA, Muscle strength by grip strength Muscle function/performance by gait speed, SPPB, or TUG |
DXA, gait speed | DXA, gait speed and grip strength | Screen using gait speed, or with the SARC-F Diagnosis using DXA, walking speed, grip strength |
Screening with hand grip and gait speed; history taking; height-adjusted skeletal muscle mass by DXA | Screening with calf circumference or SARC-F or SARC-CalF questionnaires | Grip strength, gait speed, DXA, BIA | Screening with clinical suspicion + calf circumference or SARC-F or SARC-CalF questionnaires |
| Hand grip strength | Male: <30 kg | Male: <27 kg | Male: <26 kg | Cutoff to match population characteristics | Male: <26 kg | Male: <28 kg | Male: <26 kg | Male: | |
| Female: <20 kg | Female: <16 kg | Female: <16 kg | Female: <16 kg | Female<18 kg | Female: <16 kg | <27.5 kg Female: < 18 kg52 | |||
| LEMS: one-repetition-maximum (1-RM) knee extension | Not covered | Not covered | Male: <18 kg | Not covered | Median values Indian study:2.29 (0.5–10.0)35 | ||||
| Knee flexion/extension cited as literature | Female:<16 kg (not recommended but cited as literature) | Can use AWGS 2014 [43] values until further research from South Asia | |||||||
| Gait speed (6-m walk test) | <1 m/s SPPB 0–6 Low performance SPPB 7–9 Intermediate performance SPPB 10–12 High Performance |
≤0.8 m/s 400 m walk test Non-completion or ≥6 min for completion |
<1.0 m/s (4- minute test) | ≤0.8 m/s | Cut off to match population characteristics | <0.8 m/s | <1.0 m/s | ≤0.8 m/s | India: ≤0.8 m/s52 Sri Lanka: ≤0.96 m/s10 ≤0.8 m/s is validated in other guidelines as well |
| Short Physical Performance Battery score (including chair stand test)/TUGT | ≤8 | >15 s for 5 rises (chair stand test) TUG: ≥20 s |
Not covered | Considered sarcopenic if unable to rise from chair unassisted DXA not required for diagnosis |
≤9, or 5-time chair stand test ≥12 s | Not covered | TUGT: <10.2 s [89] Can use AWGS 2014 [43] values until further research from South Asia |
||
| Calf circumference | Not recommended as vulnerable to errors | Recommended only if no other method available. No cutoffs | Cut off to match population characteristics | Not covered | Men:<34 cm Women: <33 cm | No covered | Men:<34 cm Women: <33 cm Validated for South East Asians |
||
| SARC-F | Not covered | Use SARC-F (≥4) or clinical suspicion for screening. | Cutoff to match population characteristics | Not covered | ≥4 | Not covered | ≥4 Internationally validated score |
||
| SARC-CalF | Not covered | Not covered | No covered | Not covered | ≥11 | No covered | ≥11 Validated in South East Asians |
||
| ALM/height [2] | Male: ≤7.23 kg/m2 | Male: ≤7 kg/m2 | Male: ≤7.23 kg/m2 | Not covered | Male: ≤7.0 kg/m2 | Not covered | India: ASMI (Lee's equation for Asians) Male: <7.0 kg/m2 Female: <5.7 kg/m26 Validated in South East Asians |
||
| Female: ≤5.67 kg/m2 | Female: ≤5.5 kg/m2 | Female: ≤5.67 kg/m2 | Female: ≤5.7 kg/m2 | ||||||
| Height-adjusted muscle mass: DXA | Male: ≤7.23 kg/m2 | Male: ≤7.23 kg/m2 | Male: ≤7.23 kg/m2 | Cut off to match population characteristics | Male: <7.0 kg/m2 | Male: <7.0 kg/m2 | Male:<7.0 kg/m2 | Sri Lanka: 5.03 kg/m210 | |
| Female: ≤5.67 kg/m2 | Female: ≤5.67 kg/m2 | Female: ≤5.67 kg/m2 | Female: <5.4 kg/m2 | Female: <5.4 kg/m2 | Female:<5.4 kg/m2 | Can use AWGS values until further research from South Asia | |||
| Bio-electrical impedance analysis | SM/height [2] Male: 8.87 kg/m2 Female: 6.42 kg/m2 absolute muscle mass/height [2] Male: Severe sarcopenia ≤8.50 kg/m2 Moderate sarcopenia 8.51–10.75 kg/m2 Normal muscle ≥10.76 kg/m2 Female: Severe sarcopenia ≤5.75 kg/m2 Moderate sarcopenia 5.76–6.75 kg/m2 Normal muscle ≥6.76 kg/m2 |
Cutoff to match population characteristics | Male: <7.0 kg/m2 Female: <5.7 kg/m2 |
Male: <7.0 kg/m2 Female: <5.7 kg/m2 |
Male:<7.0 kg/m2 Female:<5.7 kg/m2 |
India: FFM = 32.637 + (−0.222 X age) + (−32.51 X waist-to-hip ratio) + (0.33 X body mass index) + (1.58 × 1 or 2 (1 = normal birth weight, 2 = low birth weight) + (0.510 X waist circumference) [114] Can use AWGS values until further research from South Asia |
|||
| Ultrasound | No values have been validated or reported in any guidelines as cut-offs for screening sarcopenia. | ||||||||
ALMBMI, ratio of appendicular lean mass over body mass index; ALM/ht [2], ratio of appendicular lean mass over height squared; AWGS, Asian Working Group for Sarcopenia; EWGSOP, European Working Group on Sarcopenia in Older People; EWGSOP2, Revised European Working Group on Sarcopenia in Older People; FFM, fat free mass; FNIH, Foundation for the National Institutes of Health; ICFSR. International Clinical Practice Guidelines for Sarcopenia; IWGS, International Working Group on Sarcopenia; LEMS, lower extremity muscle strength; SWAG-SARCO, South Asian Working Action Group on Sarcopenia; SM, skeletal muscle mass; TUG, Timed -up-and-go test.
Note: No diagnostic cut-offs were provided by the Clinical Practice Guideline for Sarcopenia from Japan [39].
10. Algorithm for screening and diagnosing sarcopenia
SWAG-SARCO proposes the 5-S pathway for screening and diagnosing sarcopenia: Suspect, Screening, Secondary sarcopenia (including concomitant therapy), Severity (muscle strength, function, and/or mass), and Shared decision making (Box 1, Box 2). These recommendations follows the same idea/pattern followed by the EWGSOP2 guidelines, that recommend the F-A-C-S pathway for screening and diagnosing sarcopenia [46]. The pathway includes 4 steps, Finding case, Assessing, Confirming, and Severity. A recent cost-effective analysis comparing various sarcopenia screening methods, sarcopenia scoring assessment models (SarSA-Mod), EWGSOP method, Mini sarcopenia risk assessment (MSRA), and SARC-F found that the EWGSOP method was the most economical [115].
Box 1. (Highlights of SWAG-SARCO).
-
•
First expert opinion on sarcopenia from South Asia
-
•
Formulated after comparing and contrasting with other guidelines and identifying gaps
-
•
Provides practical definition and solutions; focus on cost effective strategies
-
•
Advocates algorithmic approach. Adapts the 5-S pathway: Suspect, Screening, Secondary sarcopenia (including concomitant therapy), Severity (muscle strength, function, and/or mass), and Shared decision making
-
•
Recognizes muscle strength and function over muscle mass
-
•
Gives importance to secondary sarcopenia and sarcopenic obesity
-
•
Tries to suggest biomarkers for diagnosing sarcopenia
-
•
Gives importance to osteo-arthro-muscular triad and biopsychosocial environmental triad
-
•
Provides practical and cost effective management strategies
-
•
Discusses advantages and disadvantages of both non-pharmacological and pharmacological approaches
Alt-text: Box 1
Box 2. 5-S Pathway.
Suspect sarcopenia in all patients in geriatric age group.
Screening: SARC-F questionnaire, grip strength and/or a chair stand test.
Secondary sarcopenia (including concomitant therapy): Assess all possibilities.
Severity (muscle strength, function, and/or mass): gait speed, SPPB, TUGT and 400-m walk tests; biomarkers and imaging decision case by case.
Shared decision making for optimal management.
Alt-text: Box 2
The EWGSOP2 F-A-C-S pathway recommends case finding or screening through SARC-F questionnaire or clinical suspicion; followed by assessment using grip strength or a chair stand test (other measures of strength such as knee flexion/extension can be used in special cases); thereafter confirm by DXA in clinical practice (research studies: use BIA, CT or MRI); lastly severity can be assessed by performance measures such as gait speed, SPPB, TUG, and 400-m walk tests [46].
Similarly, the SWAG-SARCO's 5-S pathway recommends that sarcopenia should be suspected in all patients in the geriatric age group, especially those having geriatric syndromes. SARC-F questionnaire, grip strength and/or a chair stand test can be used for screening. Comorbidities and concomitant medications (Table 1) likely to be causing or worsening sarcopenia should be assessed. Severity should be assessed through gait speed, SPPB, TUGT, and 400-m walk tests. If laboratory studies are being done routinely for comorbidities, they may be used to strengthen the diagnosis; however, biomarker analysis may not have a screening and diagnostic implication. Imaging such as CT, MRI, USG, DXA or BIA may be done as per the need (to diagnose and assess severity), availability, and affordability. The experts believe that the 5-S pathway will be most clinically and cost effective pathway for screening and diagnosing sarcopenia. The various cutoffs recommended by SWAG-SARCO for each of the mentioned tests is mentioned in Table 5.
The experts stressed that the entire 5-S pathway does not need to be followed for all patients. Any patient suspected, screened, and assessed for secondary sarcopenia should be managed through non-pharmacological means without undergoing severity assessment. Non-pharmacological treatment should be started through shared decision making between the physician, dietician/nutritionist, physiotherapist and psychologist if required. Severity assessment should ideally be performed in patients who do not respond to non-pharmacological treatment, who need upfront pharmacological treatment or who in the physician's judgment need severity triage for optimal management.
The experts gave importance to clinical measures over biochemical and imaging. They concurred that no single measure alone can diagnose sarcopenia. The experts also agreed that a patient should be screened and followed-up using the same tool. They noted that different tools have different sensitivity in detecting sarcopenia. For example, the prevalence of sarcopenia in a given population can vary if weight-adjusted SMI (wSMI) is used instead of height-adjusted SMI (hSMI) [116]. The prevalence is lower when hSMI is used instead of wSMI and this difference is more marked in women [117]. A study from Asia evaluating the most effective screening method in the Asian population concluded that wSMI maybe preferred to assess sarcopenia prevalence among women, and hSMI for men [116]. HGS is a more sensitive method for sarcopenia prevalence compared to ASMI when the study population has both males and females [116].
The experts noted that all measures of sarcopenia are inter-related and dependent on many patient-related factors. A recent Sri Lankan paper reported that HGS showed significant associations with ASMM, total-body-bone-mineral-content, pattern of physical activities (PA score), age and weight (adjusted R2 = 0.33) in premenopausal women, and with ASMM and height (adjusted R2 = 0.23) in postmenopausal women [36]. Of all the factors identified, ASMM had the strongest correlation with HGS in both premenopausal (r = 0.44, R2 = 0.20) and postmenopausal women (r = 0.44, R2 = 0.20). On the other hand, HGS was significantly associated with height, BMI and energy consumption (adjusted R2 = 0.13) in premenopausal and with carbohydrate consumption and total-body-bone-mineral-density (adjusted R2 = 0.09) in postmenopausal women [36,118]. Of all the factors associated with HGS, height had the strongest association with HGS in postmenopausal women (r = 0.28, R2 = 0.08) and with carbohydrate consumption in premenopausal women (r = 0.24, R2 = 0.06) [36]. Another study from China reported that HGS was significantly associated with physical disability and not visual disability (adjusted prevalence ratios [aPR]: 1.69; 95% CI, 1.88–2.42); depressive symptoms (aPR, 1.31; 95% CI, 1.04–1.62) and Chinese version of Physical Activity Scale for the Elderly (PASE) score (aPR, 0.99; 95% CI, 0.99–1.00) [119].
11. Sarcopenia through a prevention prism
Though sarcopenia is a muscle-related syndrome, muscle health cannot be considered in isolation as muscles are an integral part of the osteo-arthro-muscular (OAM) triad [120,121]. Secretary factors from muscles regulate bone development or remodeling and those from bone regulate muscle growth and metabolism [120]. Hence, optimally functioning OAM triad is required to prevent sarcopenia. The group proposes broadening the OAM triad concept to include neuropathy (MOAN), as this will provide comprehensive coverage of the various anatomical and functional factors which are associated with sarcopenia.
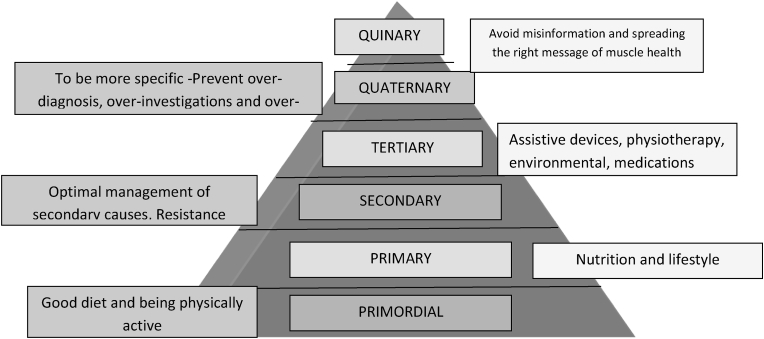
The prevention prism of sarcopenia has 6 levels: primordial prevention, primary prevention, secondary prevention, tertiary prevention, quaternary prevention and quinary prevention (Fig. 2).
Fig. 2.
Sarcopenia prevention prism.
Primordial prevention is aimed at preventing the development of risk factors for sarcopenia. This includes maintaining dietary diversity and protein intake as per recommended dietary allowance (RDA) (1–1.2 g/kg) [70,122]. It also includes encouraging South Asian physical activity practices 123such as using oriental style of toilets (squatting), sitting on the floor cross-egged for doing day to day activities, and non-mechanized ways of carrying out activities of daily living such as washing clothes with hands, using bucket and mug to bathe, squatting to mop floors, and grinding using stone grinder.
Primary prevention comes into play when risk factors for sarcopenia are present. Nutritional lifestyle adjustments include eating a balanced diet and taking adequate protein. Physical lifestyle adjustments include using stairs instead of lifts, avoiding uninterrupted sitting for long periods, taking short walk breaks in between desk work, etc. Appropriate diagnosis and timely management of probable causes of sarcopenia (Table 1) is also a part of primary prevention.
Secondary prevention of complications includes exercises and focuses on diet management for comorbid conditions. The exercises are aimed at strengthening the muscles, improving balance and gait, and preventing falls. Light strength training, flexibility and balance exercises and 30-min brisk walk as per patient's capacity are prescribed. Details of exercises are covered under the management section.
Tertiary prevention includes providing assistive devices and home-care equipment that are primarily aimed at preventing falls and easing the patient's movements during ADLs. Details are covered under the management section.
Quaternary prevention works to prevent over-diagnosis, over-investigations and over-treatment. Laboratory and imaging tests should only be prescribed for patients with high clinical suspicion of sarcopenia, if they are required for patient management. Pharmacotherapy should be avoided unless deemed absolutely necessary for patient management. The SWAG-SARCO group highlights the need for focusing on clinical modalities of diagnosis.
Quinary prevention, though discussed last, should be the backbone of prevention, as this is aimed at spreading the right message. Though spreading the right message is important, denouncing false information is equally important. All healthcare professionals should counsel their patients and peers about muscle health. All modalities of prevention and management should be discussed at each visit. Patients should be taught to recognize signs of sarcopenia and act upon them. Basics of care should be reinforced at each visit. Healthcare professionals also share a responsibility to advocate for persons living with sarcopenia and their treatment.
12. Management of sarcopenia
The main aim of treatment is to prevent deterioration in muscle performance-function. Secondarily, early treatment may be able to reduce or prevent falls and disability, thereby improving activities of daily living (ADL). Another goal is to reduce/prevent hospitalization, and reduce mortality in the affected patients. In their article, Afzal et al [33] note that QoL of patients impacted by sarcopenia can be improved significantly through the right treatment approach. An optimally functioning OAM triad is required to lead a good QoL and perform ADL safely. Hence, treatment is also aimed at optimizing the OAM triad.
Since sarcopenia is a multifactorial syndrome, no single treatment approach alone is sufficient to bring the desired improvement in muscle strength, function and muscle mass [83,124]. Therefore, treatment approach needs to be optimized and individualized 125 to AMEND muscle health through the right combination of Activity and exercise optimization; Medical and metabolic care; Environmental optimization, Nutritional optimization, and Drug therapy (Table 6).
Table 6.
Advantages and disadvantages of different management strategies for sarcopenia.
| Treatment strategy | Intervention | Advantages | Disadvantages |
|---|---|---|---|
| Nutritional supplementation or medical nutrition therapy (MNT) [48,70] | Protein supplements including whey protein [145,210] |
|
Does not improve the muscle strength and physical performance |
| |||
| Essential amino acid (EAA) supplementation [211] | Improves muscle mass and basal muscle protein synthesis | No improvement in muscle strength and physical performance | |
| β-hydroxy β-methylbutyric acid (HMB) supplementation [[212], [213], [214]] | No consistent results across studies regarding muscle mass, strength and physical performance. | ||
| Fatty acid supplementation (omega-3 fatty acids) [215,216] |
|
Need further investigation on the dosage and frequency use | |
| |||
| Creatine [217,218] |
|
|
|
| Exercise and physical activity [133,139,140,147] | Resistance training (weightlifting, pulling against resistance bands, or moving body parts against gravity) | Increased muscle mass and strength, skeletal muscle protein synthesis and muscle fiber size and improvement in physical performance |
|
| Aerobic exercise (jogging, cycling, brisk walking, dancing, climbing stairs, and treadmill) | Increase mitochondrial volume and activity | ||
| Balance (standing on heels or toes, tandem walking, walking on different types of surfaces) and flexibility (stretches, Tai Chi, yoga) | Stabilizes osteo-arthro-muscular triad | ||
| Environmental optimization [138,152] | Physical- Ramps, grab rails, types of toilets, other assistive devices | Aids in easing activities of daily living and prevents falls | May not be possible to use these across region, especially in rural areas |
| Psychological-support |
|
|
|
| |||
| |||
| Social or peer group support | Has not been explored in sarcopenia | ||
| Prevention of sarcopenia | Walking to work, climbing stairs, use of less technology | ||
| Medical optimization of comorbidities | Optimal medical management of causes of secondary sarcopenia through pharmacological and non-pharmacological methods (MNT, psychotherapy, exercise, etc.) | ||
| Pharmacotherapy | Vitamin D [25,83] | Increase muscle strength | No consistent results across studies |
| Testosterone [219] |
|
|
|
| Calcium supplementation [220,221] |
|
|
|
| Myostatin inhibitors [219] | Lean muscle mass is enhanced | Inconclusive evidence that enhanced muscle mass correlates with improved muscular strength and physical performance | |
| Growth hormone [19,222] |
|
|
|
| Alendronate [223,224] | Improves lumbar bone mineral density, muscle mass and handgrip strength | Not used in routine clinical practice Results mainly from clinical trials on osteoporosis patients |
|
| Hormone replacement therapy [225]. |
|
Effect not seen in women >65 years and in obese women | |
| Anabolic steroids [226,227] |
|
|
|
| ACE inhibitors [181,228] | Some evidence for increased exercise capacity |
|
|
12.1. Nutritional optimization
SMM loss in sarcopenia is often associated with protein, energy, or micronutrient deficiency [83]. Both, over and undernutrition coexist in South Asia [70,126]. This malnutrition is more prevalent in older adults at the community level [65,127].
Thus, nutrition plays a major role in sarcopenia management. Nutrition support is provided in form of medical nutrition therapy (MNT) by a registered healthcare professional and includes diet counseling and/or nutrient supplementation.
South Asian diet is usually calorie predominant with high carbohydrates, and does not meet the RDA for protein in adults [70,128]. This increases the risk of sarcopenic obesity as body composition changes are directly linked to nutrition [129]. On the other hand, protein energy malnutrition (PEM) is also seen in South Asian adults [126]. Low dietary protein and PEM have been linked to sarcopenia as protein is integral to muscle mass and function [[129], [130], [131], [132]]. Hence, MNT should be designed to increase the patient's current protein intake by at least 10% to a maximum of 1 g/kg body weight/day [70]. Patients having sarcopenic obesity will need a calorie restricted diet to lose fat [133]. Refined sugar, non-nutritive artificial sweeteners, and high fat diet (especially saturated fats) should be restricted in patients with sarcopenic obesity [70]. On the other hand, patients having PEM should be prescribed energy dense and protein rich food.
MNT should cater to individual needs and respect local taboos and traditions [70]. Diet should be individualized based on whether sarcopenia is primary, or secondary to comorbidities, or part of sarcopenic obesity. For example, elderly with sarcopenia often have vitamin D deficiency. Increasing vitamin D through supplements and pills and increasing their exposure to sun often helps in improving sarcopenia [134].
All dietary advice should also be customized based on affordability, availability, and patient's comorbidities (for example, protein needs to be strictly monitored in a patient with CKD) [70]. The affordability of nutrients, especially animal sourced protein (fish, chicken, eggs, and milk) in the South Asian community is largely poor [135]. However, there are some affordable options for vitamin A, calcium, iron, folate (green leafy vegetables, legumes, millets etc.), which can be incorporated into the local cuisine [135,136]. Millets are also affordable sources of protein and hence should be substituted for preparations made from wheat (chapatti, porridge, daliya, etc) and rice (khichadi, pulav, poha, dosa, idli, etc), and animal source of proteins [128,136,137]. Millets can be combined with legumes, pulses, animal proteins if affordable and locally available vegetables to improve the protein intake and reduce the carbohydrate intake [128,136,137]. MNT based on affordability will ensure that locally available and affordable diet options are provided to supplement protein and other deficiencies contributing to sarcopenia. Patients with sarcopenia who have poor access to cooking facilities, mobility restrictions, and/or chewing or swallowing difficulties may be prescribed locally available protein rich formula MNT, which could contain branched-chain amino acids and whey protein [48,70]. Mobile applications are now available to help select and order nutritious food, or learn easy recipes or set reminders to eat [138]. Patients may need nutritional rehabilitation and may need to be provided with adapted cutlery, plate, glasses and cups that are easy to hold and prevent spills [138].
12.2. Exercise and physical activity
Exercise and physical activity are different forms of physical activity. Exercise is a systematically designed structured set of movements with a specific goal in mind. Physical activity is any unstructured movement that increases energy expenditure [139]. Exercises are done over and above the physical activity required to perform ADLs [140]. Afzal et al. [3] from Pakistan advocate that aerobic exercises and energy expenditure through physical activity improves the overall physical and mental health, improves immunity, reduces osteoporosis and risk of fracture and falls; and there is an added benefit of an obvious decline in cardiovascular, respiratory, and metabolic cause related mortality [33].
A systematic review and meta-analysis showed that exercise significantly improves muscle strength (grip strength and timed 5 chair stands, P < 0.0001 for both) and physical performance (gait speed and TUGT, P < 0.00001 for both) but had no effect on SMM (P = 0.15 [141].
A combination of different types of exercises (resistance, aerobic, balance and flexibility) can be done to manage sarcopenia as they have different effects on muscle strength, function and muscle mass [133,139]. Additionally, a combination of different exercises can combat muscle fat infiltration and help in sarcopenic obesity [142]. Resistance exercises include weight lifting, pulling against resistance bands, or moving body parts against gravity [83]. Resistance training probably provides the best support in improving muscle mass, function and performance [[143], [144], [145], [146]]. It has been found to be effective in both primary and secondary sarcopenia [147] and helpful in improving gait and balance [147]. Aerobic and endurance exercises include jogging, cycling, brisk walking, dancing, climbing stairs, and treadmill [83,140,147]. Aerobic exercises help in muscle protein synthesis, decrease muscle catabolism, and bring about many positive cellular changes and thus help in increasing muscle mass, muscle strength, and aerobic capacity [133]. However, many patients with sarcopenia may not be able to perform aerobic exercises. Low impact exercises should be preferred over high impact ones, especially in persons with osteoporosis.
Flexibility exercises take care of the OAM triad by improving mobility of joints across all possible range of movements [140]. Stretches can be done as part of pre-exercise warm up or post-exercise cool down or can be done in form of Tai Chi and Yoga [140]. Balance exercises stabilize the OAM triad and are especially helpful in preventing falls [140]. Balance exercises can be done in form of standing on heels or toes, tandem walking, walking on different types of surfaces, etc [140]. Flexibility and balance exercises help in preventing falls as well [125].
Other than the usual exercises prescribed for sarcopenia, exercises specific for the secondary causes of sarcopenia (cardiac rehabilitation or weight loss) are also necessary [148]. Also, patients with sarcopenia secondary to comorbidities such as diabetes and CKD have a reduced functional performance that would need to be addressed during rehabilitation [[149], [150], [151]].
Exercises should be done under the guidance of a trained healthcare professional [70]. Patients should be encouraged to do the prescribed exercises regularly (at least 3 times a week) based on their exercise capacity [147]. Adequate warm up before resistance and aerobic exercises and adequate cool down post exercise should be ensured [147]. Balance, flexibility and strength training should ideally precede aerobic exercises to ensure stability and prevent falls [140]. Prescribed exercises should be gradually stepped up in intensity based on patient's tolerability and adequate protein intake should be ensured to build muscle mass during strength training [70,140,147].
12.3. Environmental optimization
The environment around a patient with sarcopenia has 3main components, biological or physical, psychological, and social. All the 3components are optimized to rehabilitate the patient into a more safely negotiable environment. Though, the environmental optimization is being considered under 3components, clinicians must remember that all 3components are inter-related and inter-dependent and therefore all 3 need to be optimized for the best result. Additionally, environmental optimization needs to be planned based on patient's physical and psychosocial surroundings.
12.3.1. Psychosocial optimization
Psychological component is very important in sarcopenia since the treatment is predominantly non-pharmacological. The first step is acceptance by the patient and the caregiver that patient has sarcopenia and needs rehabilitation. The next step is proper understanding of the rehabilitation program. Next in importance is adherence to the rehabilitation program. Self-motivation is very important to adhere to physical activity and exercise programs (concluded on basis of a systematic review of 66 studies [152]. Many patients may need psychological support and consultation with a trained clinical psychologist. This aspect is often ignored in routine geriatric care in South Asia, partially due to cultural cohesiveness of the South Asian family system [123,153,154]. However, with cultural shift towards nuclear families and adaptation of western culture, the geriatric age group now increasingly needs psychological support from a trained psychologist to keep them motivated [154]. Other reasons for poor psychological support in this age population is poor infrastructure, resource crunch, and poor recognition of mental health as an important aspect of care [123].
Social or peer support programs that have been found to be effective for treating conditions such as alcoholism [155] in South Asia have not been tried for sarcopenia. In fact, it is noted that social or peer support programs for sarcopenia are lacking the world over. Muscle health can be integrated in pre-existing elderly and menopause care programs.
12.3.2. Optimization of the physical environment
This is the most tangible optimization as the change is very evident. The physical environment around the patient can be improved through assistive technologies that enable the patient to independently and safely carry out ADLs and reduce functional decline [138].
Patient's home environment can made safe by adding cameras, in-home and wearable sensors, and connected devices that automate home environment [138]. ADLS can be made easy and safe through use of walking aids (eg, walking sticks and frames) or external orthoses, grab rails, wearable sensors, using western toilets and toilet chairs [138]. The rooms should be well lighted and have minimal furniture [138].
12.4. Medical optimization of secondary causes
All medical, metabolic, endocrine, and psychological issues that could be the cause of secondary sarcopenia should be addressed through pharmacological and non-pharmacological means (MNT, psychotherapy, exercise, etc).
12.4.1. Pharmacological
Most commonly used pharmacotherapeutic agents in the treatment of sarcopenia include vitamin D, calcium supplementation, testosterone, and hormone replacement therapy (menopausal women). However, none of the agents have been clinically proven to be the sole treatment option for sarcopenia. Hence, the experts stressed that pharmacological treatment should be the last option for managing sarcopenia. Additionally, it would increase the pill medication burden in a patient population already burdened with multiple pharmacotherapies because of their comorbidities. The experts also urge that all benefits and limitations of pharmacotherapy should be seriously weighed before starting the treatment. Table 6 provides the benefits and limitations of most commonly used pharmacotherapeutic agents. The experts acknowledge that further research and real world evidence is required to understand the role of pharmacotherapy in sarcopenia.
However, medications required to manage comorbidities can form an integral part of sarcopenia management. All efforts should be made to optimize the management of comorbidities likely to the secondary cause of sarcopenia (Table 1). Drugs likely to cause muscle wasting should be replaced with suitable alternatives.
13. COVID-19 and sarcopenia in South Asia: special considerations
Novel coronavirus 2019 (COVID-19) pandemic has worsened the biospychosocial sarcopenia environment in many ways. COVID-19 pandemic has exacerbated the malnutrition problem in South Asia [156]. Patients with COVID-19 have altered normal body functioning [157]. There is a notable increase in fat accumulation, sarcopenia and sarcopenic obesity [157]. Patients remain indoors for prolonged period of time due to imposed lockdowns and this decreases physical activity and increases unhealthy eating.
COVID-19 pandemic brought with it an increase in anxiety, loneliness, and depression for all age groups because of social isolation, unemployment, and continuous broadcast of depressive news [158]. This was not well recognized initially, and needs to be dealt with immediately as it likely to de-motivate those who already have sarcopenia. Also, the poor mental health causes reduction in physical activity which can lead to sarcopenia in this vulnerable age group [158]. However, South Asia is not ready to deal with additional mental health crunch due to poor resources and infrastructure, lack of trained professionals, patients paying out of pocket for their treatment (least expenditure on mental health), and lack of insurance and government policies covering mental health [158].
Hence, there is a felt need to educate clinicians, COVID-positive patients, and public in general about the risk of sarcopenia and sarcopenic obesity due to the abnormal virtual bubble created by the pandemic. Clinicians should provide or facilitate individualized MNT, exercise and counseling for all patients, and especially for COVID-positive cases due to change in body functioning [157,159]. A more aggressive approach than is usually adopted is required to combat sarcopenia during this pandemic [159].
14. Research gaps
The SWAG-SARCO 2021 group noted several gaps in the management of sarcopenia, not just in South Asia but world over:
-
•
Diagnostic cut-offs for South Asia have not been standardized based on longitudinal data
-
•
Primary sarcopenia is a well-recognized entity but not secondary sarcopenia. Sarcopenic obesity has not been studied in detail as a separate entity.
-
•
Focus is more on treatment than prevention; more on management than detection; more on drug therapy than non-pharmacological
-
•
Since secondary sarcopenia is not well recognized, aggressive action plans to prevent sarcopenia in younger population have not been made.
-
•
There is no clarity on the role of biomarkers in diagnosing, monitoring and managing sarcopenia.
-
•
Lack of head-to-head clinical trials comparing various screening and diagnostic modalities makes it difficult to set gold standards.
-
•
Lack of head-to-head clinical trials comparing various treatment strategies, either alone or in combination, makes it difficult to reach a consensus on best treatment strategies.
-
•
Psychosocial support strategies are largely lacking in South Asia.
-
•
Sarcopenia prevalence, burden, impact and management during COVID-19 pandemic have not been studied in detail. Hence, long-term impact is not known.
-
•
Support groups in the form of community health centers, integration of sarcopenia care in existing programs is lacking
15. Limitations of the consensus
During this whole process of consensus building, we realized we do not have high quality literary evidence from South Asia. Also, tools used for diagnosis and management of sarcopenia differed within the available resources. The South Asia region has many resource challenged settings. Hence, this consensus was developed using important points and tools from available international and Asian guidelines which could be extrapolated to the South Asian phenotype. The clinical experience and expertise of the experts who deliberated on all the available evidence and limitations added strength to the recommendations. Additionally, though the literature from South Asia mainly included small studies and lacked randomized trials, we have included this literature in the consensus as this was the only source of published evidence from this region. Other than this, we acknowledge that though we recommend the use of biomarkers for monitoring select patients, as per the discretion of the treating physician, there is no evidence on their sensitivity, specificity, and accuracy in predicting or diagnosing sarcopenia. By publishing this consensus, we are trying to bridge this gap, create awareness, encourage more publications, and hope to be able to revise the consensus recommendations in future based on strong evidence from this region.
16. Way forward
The SWAG-SARCO 2021 group has done its best to provide clinical and practical solutions for defining, screening, diagnosing, and treating sarcopenia. Attention has been given to prevention, secondary sarcopenia and sarcopenic phenotype in South Asia.
However, the experts recognize that tremendous efforts are required to bridge research gaps in South Asia. As this population is ageing at a fast pace, these gaps must be addressed as soon as possible. Well planned and sustained efforts are required to promote sarcopenia awareness in clinicians and patients alike. Preventive strategies should be stressed upon during all patient communications.
‘Well-elderly clinics’, dedicated sarcopenia clinics, sarcopenia support groups need to be established across the length and breadth of the region. Well-designed head to head comparative trials should be planned to understand best diagnostic and treatment strategies. Newer therapies and regenerative strategies should be given a boost in research areas. The SWAG-SARCO group advocates individualization of sarcopenia management achieved through shared decision making that incorporates the environmental biopsychosocial and OAM triads for holistic prevention and management strategies.
The group recognizes that sarcopenia knowledge in South Asia is evolving with time and believes this paper adds to the knowledge, provides practical solutions and a way forward.
CRediT author statement
Minakshi Dhar: Conceptualization, Formal analysis, Writing – original draft. Nitin Kapoor: Conceptualization, Formal analysis, Writing – original draft. Ketut Suastika: Conceptualization, Formal analysis, Writing – original draft. Mohammad E. Khamseh: Conceptualization, Formal analysis, Writing – original draft. Shahjada Selim: Conceptualization, Formal analysis, Writing – original draft. Vijay Kumar: Conceptualization, Formal analysis, Writing – original draft. Syed Abbas Raza: Conceptualization, Formal analysis, Writing – original draft. Umal Azmat: Conceptualization, Formal analysis, Writing – original draft. Monika Pathania: Methodology, Validation, Formal analysis, Data curation. Yovan Parikshat Rai Mahadeb: Methodology, Validation, Formal analysis, Data curation. Sunny Singhal: Methodology, Validation, Formal analysis, Data curation. Mohammad Wali Naseri: Methodology, Validation, Formal analysis, Data curation. IGP Suka Aryana: Methodology, Validation, Formal analysis, Data curation. Subarna Dhoj Thapa: Methodology, Validation, Formal analysis, Data curation. Jubbin Jacob: Methodology, Validation, Formal analysis, Data curation. Noel Somasundaram: Methodology, Validation, Formal analysis, Data curation. Ali Latheef: Resources. Guru Prasad Dhakal: Resources. Sanjay Kalra: Supervision, Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing
Conflicts of interest
The authors declare no competing interests.
Acknowledgments
The authors thank Dr. Kokil Mathur and Dr. Punit Srivastava of Mediception Science Pvt. Ltd. (www.mediception.com) for providing medical writing support during the preparation of this manuscript. ORCID Minakshi Dhar: 0000-0001-6336-5179. Nitin Kapoor: 0000-0002-9520-2072. Ketut Suastika: 0000-0001-7895-382X. Mohammad E. Khamseh: 0000-0003-4313-8440. Shahjada Selim: 0000-0001-7749-3542. Vijay Kumar: 0000-0002-8445-299X. Syed Abbas Raza: 0000-0003-4270-7939. Umal Azmat: 0000-0001-8545-7111. Monika Pathania: 0000-0003-3609-1162. Yovan Parikshat Rai Mahadeb: 0000-0001-6519-8407. Sunny Singhal: 0000-0002-9977-4182. Mohammad Wali Naseri: 0000-0002-2281-5361. IGP Suka Aryana: 0000-0001-8582-2254. Subarna Dhoj Thapa: 0000-0003-3568-1777. Jubbin Jacob: 0000-0003-1755-5523. Noel Somasundaram:0000-0002-6241-7501. Ali Latheef: 0000-0003-1358-3105. Guru Prasad Dhakal: 0000-0003-3207-0743Sanjay Kalra: 0000-0003-1308-121X.
Footnotes
Peer review under responsibility of The Korean Society of Osteoporosis.
References
- 1.Tyrovolas S., Koyanagi A., Olaya B., Ayuso-Mateos J.L., Miret M., Chatterji S., et al. Factors associated with skeletal muscle mass, sarcopenia, and sarcopenic obesity in older adults: a multi-continent study. J Cachexia Sarcopenia Muscle. 2016;7:312–321. doi: 10.1002/jcsm.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papadopoulou S.K., Tsintavis P., Potsaki P., Papandreou D. Differences in the prevalence of sarcopenia in community-dwelling, nursing home and hospitalized individuals. A systematic review and meta-analysis. J Nutr Health Aging. 2020;24:83–90. doi: 10.1007/s12603-019-1267-x. [DOI] [PubMed] [Google Scholar]
- 3.Chang H.-K., Lee J.-Y., Gil C.-R., Kim M.-K. Prevalence of sarcopenia in community-dwelling older adults according to simplified algorithms for sarcopenia consensus based on Asian Working Group for Sarcopenia. Clin Interv Aging. 2020;15:2291–2299. doi: 10.2147/CIA.S281131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kitamura A., Seino S., Abe T., Nofuji Y., Yokoyama Y., Amano H., et al. Sarcopenia: prevalence, associated factors, and the risk of mortality and disability in Japanese older adults. J Cachexia Sarcopenia Muscle. 2021;12:30–38. doi: 10.1002/jcsm.12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makizako H., Nakai Y., Tomioka K., Taniguchi Y. Prevalence of sarcopenia defined using the Asia Working Group for Sarcopenia criteria in Japanese community-dwelling older adults: a systematic review and meta-analysis. Physical Therapy Research. 2019;22:53–57. doi: 10.1298/ptr.R0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaikh N., Harshitha R., Bhargava M. Prevalence of sarcopenia in an elderly population in rural South India: a cross-sectional study. F1000Res. 2020;9:175. [Google Scholar]
- 7.Pal R., Bhadada S.K., Aggarwal A., Singh T. The prevalence of sarcopenic obesity in community-dwelling healthy Indian adults - the Sarcopenic Obesity-Chandigarh Urban Bone Epidemiological Study (SO-CUBES) Osteoporos Sarcopenia. 2021;7:24–29. doi: 10.1016/j.afos.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hashemi R., Shafiee G., Motlagh A.D., Pasalar P., Esmailzadeh A., Siassi F., et al. Sarcopenia and its associated factors in Iranian older individuals: results of SARIR study. Arch Gerontol Geriatr. 2016;66:18–22. doi: 10.1016/j.archger.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 9.Widajanti N., Ichwani J., Dharmanta R.S., Firdausi H., Haryono Y., Yulianti E., et al. Sarcopenia and frailty profile in the elderly community of Surabaya: a descriptive study. Acta Med Indones. 2020;52:5–13. [PubMed] [Google Scholar]
- 10.Rathnayake N., Alwis G., Lenora J., Lekamwasam S. Cutoff values for the determination of sarcopenia and the prevalence of the condition in middle-aged women: a study from Sri Lanka. Ceylon Med J. 2019;64:9–16. doi: 10.4038/cmj.v64i1.8834. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed S. Study of factors associated with sarcopenia in COPD patients. Med Today. 2021;33:50–53. [Google Scholar]
- 12.Limpawattana P., Inthasuwan P., Putraveephong S., Boonsawat W., Theerakulpisut D., Sawanyawisuth K. Sarcopenia in chronic obstructive pulmonary disease: a study of prevalence and associated factors in the Southeast Asian population. Chron Respir Dis. 2018;15:250–257. doi: 10.1177/1479972317743759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ha Y.-C., Won C.W., Kim M., Chun K.-J., Yoo J.-I. SARC-F as a useful tool for screening sarcopenia in elderly patients with hip fractures. J Nutr Health Aging. 2020;24:78–82. doi: 10.1007/s12603-019-1307-6. [DOI] [PubMed] [Google Scholar]
- 14.As’habi A., Najafi I., Tabibi H., Hedayati M. Prevalence of sarcopenia and dynapenia and their determinants in Iranian peritoneal dialysis patients. Iran J Kidney Dis. 2018;12:53–60. [PubMed] [Google Scholar]
- 15.Kalyani R.R., Corriere M., Ferrucci L. Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014;2:819–829. doi: 10.1016/S2213-8587(14)70034-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larsson L., Degens H., Li M., Salviati L., Lee Y il, Thompson W., et al. Sarcopenia: aging-related loss of muscle mass and function. Physiol Rev. 2019;99:427–511. doi: 10.1152/physrev.00061.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakuma K., Yamaguchi A. Sarcopenia and age-related endocrine function. International Journal of Endocrinology. 2012;2012 doi: 10.1155/2012/127362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saggini R., Carmignano S.M., Cosenza L., Palermo T., Bellomo R.G. IntechOpen; 2017. Sarcopenia in chronic illness and rehabilitative approaches. [DOI] [Google Scholar]
- 19.Liguori I., Russo G., Aran L., Bulli G., Curcio F., Della-Morte D., et al. Sarcopenia: assessment of disease burden and strategies to improve outcomes. Clin Interv Aging. 2018;13:913–927. doi: 10.2147/CIA.S149232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan A., Toor R., Amjad Q. Assessment and management of geriatric care in Pakistan. J Gerontol Geriatr Res. 2018;7 doi: 10.4172/2167-7182.1000488. [DOI] [Google Scholar]
- 21.Chang S.-F. [Sarcopenia in the elderly: diagnosis and treatment] Hu Li Za Zhi. 2014;61:101–105. doi: 10.6224/JN.61.2.101. [DOI] [PubMed] [Google Scholar]
- 22.Rezende LFM de, Rey-López J.P., Matsudo V.K.R., Luiz O., do C. Sedentary behavior and health outcomes among older adults: a systematic review. BMC Publ Health. 2014;14:333. doi: 10.1186/1471-2458-14-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doğan S.C., Hizmetli S., Hayta E., Kaptanoğlu E., Erselcan T., Güler E. Sarcopenia in women with rheumatoid arthritis. Eur J Rheumatol. 2015;2:57–61. doi: 10.5152/eurjrheum.2015.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vlietstra L., Stebbings S., Meredith-Jones K., Abbott J.H., Treharne G.J., Waters D.L. Sarcopenia in osteoarthritis and rheumatoid arthritis: the association with self-reported fatigue, physical function and obesity. PLoS One. 2019;14 doi: 10.1371/journal.pone.0217462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minamino H., Katsushima M., Torii M., Yamamoto W., Fujita Y., Ikeda K., et al. Serum vitamin D status inversely associates with a prevalence of severe sarcopenia among female patients with rheumatoid arthritis. Sci Rep. 2021;11:20485. doi: 10.1038/s41598-021-99894-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmio J., Udd B. Borderlines between sarcopenia and mild late-onset muscle disease. Front Aging Neurosci. 2014;6:267. doi: 10.3389/fnagi.2014.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Confortin S.C., Ono L.M., Marques L.P., Ceolin G., d'Orsi E., Barbosa A.R. Osteopenia/osteoporosis and its association with sarcopenia: EpiFloripa aging study 2013/2014. PJP. 2020;38:15–22. [Google Scholar]
- 28.Clynes M.A., Gregson C.L., Bruyère O., Cooper C., Dennison E.M. Osteosarcopenia: where osteoporosis and sarcopenia collide. Rheumatology. 2021;60:529–537. doi: 10.1093/rheumatology/keaa755. [DOI] [PubMed] [Google Scholar]
- 29.Chang K.-V., Hsu T.-H., Wu W.-T., Huang K.-C., Han D.-S. Is sarcopenia associated with depression? A systematic review and meta-analysis of observational studies. Age Ageing. 2017;46:738–746. doi: 10.1093/ageing/afx094. [DOI] [PubMed] [Google Scholar]
- 30.Yuenyongchaiwat K., Boonsinsukh R. Sarcopenia and its relationships with depression, cognition, and physical activity in Thai community-dwelling older adults. Current Gerontology and Geriatrics Research. 2020;2020 doi: 10.1155/2020/8041489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dorosty A., Arero G., Chamar M., Tavakoli S. Prevalence of sarcopenia and its association with socioeconomic status among the elderly in Tehran. Ethiop J Health Sci. 2016;26:389–396. doi: 10.4314/ejhs.v26i4.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swan L., Warters A., O'Sullivan M. Socioeconomic inequality and risk of sarcopenia in community-dwelling older adults. Clni Interv Aging. 2021;16:1119–1129. doi: 10.2147/CIA.S310774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Afzal A., Farooque U., Tauseef A., Poudel S. Quality of life can be improved dramatically in people suffering from depression and its associated sarcopenia. Archives of Clinical and Biomedical Research. 2020;4:465–467. [Google Scholar]
- 34.Singhal S., Dewangan G.C., Bansal R., Upadhyay A.D., Dwivedi S.N., Das C.J., et al. Sarcopenia and its association with geriatric syndromes and quality of life in older Indian outpatients - a cross-sectional pilot observational study. J Indian Acad Geosci. 2019;15:66. [Google Scholar]
- 35.Singhal S., Bansal R., Dewangan G.C., Upadhyay A.D., Dwivedi S.N., Chatterjee P., et al. Low one-repetition-maximum knee extension is significantly associated with poor grip strength, female sex, and various aging-related syndromes. Aging Med (Milton) 2020;3:125–131. doi: 10.1002/agm2.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rathnayake N., Alwis G., Lenora J., Lekamwasam S. Factors associated with measures of sarcopenia in pre and postmenopausal women. BMC Wom Health. 2021;21:5. doi: 10.1186/s12905-020-01153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kapoor N. In: Endotext. MDText.com. Feingold K.R., Anawalt B., Boyce A., Chrousos G., de Herder W.W., Dhatariya K., et al., editors. , Inc.; South Dartmouth (MA: 2000. Thin fat obesity: the Tropical phenotype of obesity.http://www.ncbi.nlm.nih.gov/books/NBK568563/ (accessed 7 Jun2021) [Google Scholar]
- 38.Kapoor N., Lotfaliany M., Sathish T., Thankappan K.R., Thomas N., Furler J., et al. Prevalence of normal weight obesity and its associated cardio-metabolic risk factors – results from the baseline data of the Kerala Diabetes Prevention Program (KDPP) PLoS One. 2020;15 doi: 10.1371/journal.pone.0237974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akishita M., Kozaki K., Iijima K., Tanaka T., Shibasaki K., Ogawa S., et al. Chapter 1 Definitions and diagnosis of sarcopenia. Geriatr Gerontol Int. 2018;18:7–12. doi: 10.1111/ggi.13311. [DOI] [PubMed] [Google Scholar]
- 40.Sooragonda B.G., Agarwal S., Benjamin R.N., Prabhakar A.T., Sivadasan A., Kapoor N., et al. Bone mineral density and body composition in males with motor neuron disease: a study from Teaching hospital in southern part of India. Ann Indian Acad Neurol. 2021;24:211–216. doi: 10.4103/aian.AIAN_293_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cruz-Jentoft A.J., Baeyens J.P., Bauer J.M., Boirie Y., Cederholm T., Landi F., et al. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fielding R.A., Vellas B., Evans W.J., Bhasin S., Morley J.E., Newman A.B., et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen L.-K., Liu L.-K., Woo J., Assantachai P., Auyeung T.-W., Bahyah K.S., et al. Sarcopenia in Asia: consensus report of the asian working group for sarcopenia. J Am Med Dir Assoc. 2014;15:95–101. doi: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 44.Studenski S.A., Peters K.W., Alley D.E., Cawthon P.M., McLean R.R., Harris T.B., et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69:547–558. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nishikawa H., Shiraki M., Hiramatsu A., Moriya K., Hino K., Nishiguchi S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol Res. 2016;46:951–963. doi: 10.1111/hepr.12774. [DOI] [PubMed] [Google Scholar]
- 46.Cruz-Jentoft A.J., Bahat G., Bauer J., Boirie Y., Bruyère O., Cederholm T., et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dent E., Morley J.E., Cruz-Jentoft A.J., Arai H., Kritchevsky S.B., Guralnik J., et al. International clinical practice guidelines for sarcopenia (ICFSR): screening, diagnosis and management. J Nutr Health Aging. 2018;22:1148–1161. doi: 10.1007/s12603-018-1139-9. [DOI] [PubMed] [Google Scholar]
- 48.Chen L.-K., Woo J., Assantachai P., Auyeung T.-W., Chou M.-Y., Iijima K., et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21:300–307.. doi: 10.1016/j.jamda.2019.12.012. e2. [DOI] [PubMed] [Google Scholar]
- 49.Bhasin S., Travison T.G., Manini T.M., Patel S., Pencina K.M., Fielding R.A., et al. Sarcopenia definition: the position statements of the sarcopenia definition and outcomes Consortium. J Am Geriatr Soc. 2020;68:1410–1418. doi: 10.1111/jgs.16372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kapoor N., Lotfaliany M., Sathish T., Thankappan K.R., Thomas N., Furler J., et al. Obesity indicators that best predict type 2 diabetes in an Indian population: insights from the Kerala Diabetes Prevention Program. J Nutr Sci. 2020;9 doi: 10.1017/jns.2020.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pomeroy E., Mushrif-Tripathy V., Cole T.J., Wells J.C.K., Stock J.T. Ancient origins of low lean mass among South Asians and implications for modern type 2 diabetes susceptibility. Sci Rep. 2019;9:10515. doi: 10.1038/s41598-019-46960-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pal R., Aggarwal A., Singh T., Sharma S., Khandelwal N., Garg A., et al. Diagnostic cut-offs, prevalence, and biochemical predictors of sarcopenia in healthy Indian adults: the Sarcopenia-Chandigarh Urban Bone Epidemiological Study (Sarco-CUBES) Eur Geriatr Med. 2020;11:725–736. doi: 10.1007/s41999-020-00332-z. [DOI] [PubMed] [Google Scholar]
- 53.Tätte K., Pagani L., Pathak A.K., Kõks S., Ho Duy B., Ho X.D., et al. The genetic legacy of continental scale admixture in Indian Austroasiatic speakers. Sci Rep. 2019;9:3818. doi: 10.1038/s41598-019-40399-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yeung W.-J.J., Desai S., Jones G.W. Families in southeast and South Asia. Annu Rev Sociol. 2018;44:469–495. [Google Scholar]
- 55.Kapoor N., Furler J., Paul T.V., Thomas N., Oldenburg B. The BMI-adiposity conundrum in South Asian populations: need for further research. J Biosoc Sci. 2019;51:619–621. doi: 10.1017/S0021932019000166. [DOI] [PubMed] [Google Scholar]
- 56.Oommen A.M., Kapoor N., Thomas N., George K. Prevalence and clinical characteristics of individuals with newly detected lean diabetes in Tamil Nadu, South India: a community-based cross-sectional study. Int J Diabetes Dev Ctries. 2019;39:680–684. [Google Scholar]
- 57.Palanisami S., Kulkarni V. Association between muscle mass and body mass index in elderly diabetic patients attending tertiary care center in Bangalore, India. Int J Med Stud. 2016;4:96–99. [Google Scholar]
- 58.Kapoor N., Lotfaliany M., Sathish T., Thankappan K.R., Tapp R.J., Thomas N., et al. Effect of a peer-led lifestyle intervention on individuals with normal weight obesity: insights from the Kerala diabetes prevention program. Clin Therapeut. 2020;42:1618-24. doi: 10.1016/j.clinthera.2020.06.007. [DOI] [PubMed] [Google Scholar]
- 59.Kapoor N., Furler J., Paul T.V., Thomas N., Oldenburg B. Normal weight obesity: an underrecognized problem in individuals of South asian descent. Clin Therapeut. 2019;41:1638–1642. doi: 10.1016/j.clinthera.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 60.Tan V.M.H., Pang B.W.J., Lau L.K., Jabbar K.A., Seah W.T., Chen K.K., et al. Malnutrition and sarcopenia in community-dwelling adults in Singapore: Yishun health study. J Nutr Health Aging. 2021;25:374–381. doi: 10.1007/s12603-020-1542-x. [DOI] [PubMed] [Google Scholar]
- 61.Sazlina S.-G., Lee P.Y., Chan Y.M., Hamid M.S.A., Tan N.C. The prevalence and factors associated with sarcopenia among community living elderly with type 2 diabetes mellitus in primary care clinics in Malaysia. PLoS One. 2020;15 doi: 10.1371/journal.pone.0233299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yap S.F., Boo N.Y., Pramod D.S., Thaw Z., Liew S.F., Woo L.F., et al. Risk factors associated with sarcopenia among independently mobile, institutionalised older people in the Klang valley of Malaysia: a cross-sectional study. Malays J Med Sci. 2020;27:120–128. doi: 10.21315/mjms2020.27.2.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mujahid G., Siddhisena K.A.P. Papers in population ageing: demographic prognosis for South Asia: a future of rapid ageing. UNFPA, United Nation Population Fund. 2009 https://ifa.ngo/wp-content/uploads/2012/12/059_South-Asia-Ageing.pdf [Google Scholar]
- 64.WHO . World Health Organization; 2021. Ageing and health in the South-East Asia region.https://www.who.int/westernpacific/health-topics/ageing (accessed 15 Jun2021) [Google Scholar]
- 65.Govind R., Rajeev J., Bhatt A.N. Malnutrition among community dwelling older adults in a rural block area of South India. J Fam Med Prim Care. 2020;9:5982. doi: 10.4103/jfmpc.jfmpc_1248_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Statista Research Department . Statista; 2021. South Asia: life expectancy by country 2020-2025.https://www.statista.com/statistics/591405/life-expectancy-at-birth-in-south-asia/ (accessed 15 Jun2021) [Google Scholar]
- 67.Sabzwari S.R., Azhar G. Humanitarian Library; 2014. Ageing in Pakistan—a new challenge.https://www.humanitarianlibrary.org/resource/ageing-pakistan%E2%80%94-new-challenge-0 (accessed 24 Jun2021) [Google Scholar]
- 68.Acharya S., Ghimire S., Jeffers E.M., Shrestha N. Health care utilization and health care expenditure of Nepali older adults. Front Public Health. 2019;7 doi: 10.3389/fpubh.2019.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ahmad A., Ashraf S. Geriatrics and gerontology: a neglected research area in SAARC countries. Age Ageing. 2015;44 doi: 10.1093/ageing/el_746. [DOI] [Google Scholar]
- 70.Kapoor N., Sahay R., Kalra S., Bajaj S., Dasgupta A., Shrestha D., et al. Consensus on medical nutrition therapy for diabesity (CoMeND) in adults: a South Asian perspective. Diabetes Metab Syndr Obes. 2021;14:1703–1728. doi: 10.2147/DMSO.S278928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tyagi P. Double Burden of Disease: double burden of communicable and non-communicable disease in old age in South Asia. Gravis. 2014 [Google Scholar]
- 72.Maharjan S., Timalsina R. Sedentary behavior and prevalence of obesity among nepalese middle aged adults. MOJ Gerontology & Geriatrics. 2017;1:105-9. [Google Scholar]
- 73.Sreepriya P.R., Pillai S.S., Nair A.N.K.K., Rahul A., Pillai S., Nair A.T.S. Prevalence and associated factors of sarcopenia among patients underwent abdominal CT scan in Tertiary Care Hospital of South India. J Frailty Sarcopenia Falls. 2020;5:79–85. doi: 10.22540/JFSF-05-079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Koon-Yee Lee G., Chun-Ming Au P., Hoi-Yee Li G., Chan M., Li H.-L., Man-Yung Cheung B., et al. Sarcopenia and mortality in different clinical conditions: a meta-analysis. Osteoporos Sarcopenia. 2021;7:S19–S27. doi: 10.1016/j.afos.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lara D.R., Bisol L.W., Ottoni G.L., de Carvalho H.W., Banerjee D., Golshan S., et al. Validation of the ‘rule of three’, the ‘red sign’ and temperament as behavioral markers of bipolar spectrum disorders in a large sample. J Affect Disord. 2015;183:195–204. doi: 10.1016/j.jad.2015.04.046. [DOI] [PubMed] [Google Scholar]
- 76.Haugeberg G., Orstavik R., Uhlig T., Falch J., Halse J., Kvien T. Clinical decision rules in rheumatoid arthritis: do they identify patients at high risk for osteoporosis? Testing clinical criteria in a population based cohort of patients with rheumatoid arthritis recruited from the Oslo Rheumatoid Arthritis Register. Ann Rheum Dis. 2002;61:1085–1089. doi: 10.1136/ard.61.12.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Williams T., Mortada R., Porter S. Diagnosis and treatment of polycystic ovary syndrome. AFP. 2016;94:106–113. [PubMed] [Google Scholar]
- 78.Huang P.L. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2:231–237. doi: 10.1242/dmm.001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Evans W.J. Skeletal muscle loss: cachexia, sarcopenia, and inactivity. Am J Clin Nutr. 2010;91:1123S–1127S. doi: 10.3945/ajcn.2010.28608A. [DOI] [PubMed] [Google Scholar]
- 80.Santilli V., Bernetti A., Mangone M., Paoloni M. Clinical definition of sarcopenia. Clin Cases Miner Bone Metab. 2014;11:177–180. [PMC free article] [PubMed] [Google Scholar]
- 81.Clark B.C., Manini T.M. Sarcopenia dynapenia. J Gerontol A Biol Sci Med Sci. 2008;63:829–834. doi: 10.1093/gerona/63.8.829. [DOI] [PubMed] [Google Scholar]
- 82.Nomura T., Kawae T., Kataoka H., Ikeda Y. Assessment of lower extremity muscle mass, muscle strength, and exercise therapy in elderly patients with diabetes mellitus. Environ Health Prev Med. 2018;23 doi: 10.1186/s12199-018-0710-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gupta O.P. Sarcopenia: a review. J Mahatma Gandhi Inst Med Sci. 2020;25:62–65. [Google Scholar]
- 84.Hogrel J.-Y. Grip strength measured by high precision dynamometry in healthy subjects from 5 to 80 years. BMC Muscoskel Disord. 2015;16:139. doi: 10.1186/s12891-015-0612-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Roberts H.C., Denison H.J., Martin H.J., Patel H.P., Syddall H., Cooper C., et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40:423–429. doi: 10.1093/ageing/afr051. [DOI] [PubMed] [Google Scholar]
- 86.Chatterjee P., Kandel R., Desai G.R., Chellaiyan V., Biswas A., Ab D. Development of simple diagnostic criteria for frailty syndrome in Indian elderly population. Int J Med Pharmaceut Sci. 2014;4:21–30. [Google Scholar]
- 87.Yee X.S., Ng Y.S., Allen J.C., Latib A., Tay E.L., Abu Bakar H.M., et al. Performance on sit-to-stand tests in relation to measures of functional fitness and sarcopenia diagnosis in community-dwelling older adults. European Review of Aging and Physical Activity. 2021;18:1. doi: 10.1186/s11556-020-00255-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pinheiro P.A., Carneiro J.a.O., Coqueiro R.S., Pereira R., Fernandes M.H. ‘Chair stand test’ as simple tool for sarcopenia screening in elderly women. J Nutr Health Aging. 2016;20:56–59. doi: 10.1007/s12603-016-0676-3. [DOI] [PubMed] [Google Scholar]
- 89.Choo P.L., Tou N.X., Jun Pang B.W., Lau L.K., Jabbar K.A., Seah W.T., et al. Timed up and go (TUG) reference values and predictive cutoffs for fall risk and disability in Singaporean community-dwelling adults: Yishun cross-sectional study and Singapore longitudinal aging study. J Am Med Dir Assoc. 2021 doi: 10.1016/j.jamda.2021.03.002. S1525-8610(21)00298-X. [DOI] [PubMed] [Google Scholar]
- 90.Chakraborty R., Bose K., Bisai S. Mid-upper arm circumference as a measure of nutritional status among adult Bengalee male slum dwellers of Kolkata, India: relationship with self reported morbidity. Anthropol Anzeiger. 2009;67:129–137. doi: 10.1127/0003-5548/2009/0017. [DOI] [PubMed] [Google Scholar]
- 91.Thorup L., Hamann S.A., Kallestrup P., Hjortdal V.E., Tripathee A., Neupane D., et al. Mid-upper arm circumference as an indicator of underweight in adults: a cross-sectional study from Nepal. BMC Publ Health. 2020;20:1187. doi: 10.1186/s12889-020-09294-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nunes G., Santos C.A., Barosa R., Fonseca C., Barata A.T., Fonseca J. Outcome and nutritional assessment OF chronic liver disease patients using anthropometry and subjective global assessment. Arq Gastroenterol. 2017;54:225–231. doi: 10.1590/S0004-2803.201700000-28. [DOI] [PubMed] [Google Scholar]
- 93.Calvani R., Picca A., Marini F., Biancolillo A., Cesari M., Pesce V., et al. The “BIOmarkers associated with Sarcopenia and PHysical frailty in EldeRly pErsons” (BIOSPHERE) study: rationale, design and methods. Eur J Intern Med. 2018;56:19–25. doi: 10.1016/j.ejim.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Qaisar R., Karim A., Muhammad T., Shah I., Khan J. Prediction of sarcopenia using a battery of circulating biomarkers. Sci Rep. 2021;11:8632. doi: 10.1038/s41598-021-87974-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li G.H.-Y., Lee G.K.-Y., Au P.C.-M., Chan M., Li H.-L., Cheung B.M.-Y., et al. The effect of different measurement modalities in the association of lean mass with mortality: a systematic review and meta-analysis. Osteoporos Sarcopenia. 2021;7:S13–S18. doi: 10.1016/j.afos.2021.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Goodpaster B.H., Thaete F.L., Kelley D.E. Composition of skeletal muscle evaluated with computed tomography. Ann N Y Acad Sci. 2000;904:18–24. doi: 10.1111/j.1749-6632.2000.tb06416.x. [DOI] [PubMed] [Google Scholar]
- 97.Engelke K., Museyko O., Wang L., Laredo J.-D. Quantitative analysis of skeletal muscle by computed tomography imaging—state of the art. J Orthop Translat. 2018;15:91–103. doi: 10.1016/j.jot.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yuguchi S., Asahi R., Kamo T., Azami M., Ogihara H. Gastrocnemius thickness by ultrasonography indicates the low skeletal muscle mass in Japanese elderly people. Arch Gerontol Geriatr. 2020;90 doi: 10.1016/j.archger.2020.104093. [DOI] [PubMed] [Google Scholar]
- 99.Sengul Aycicek G., Ozsurekci C., Caliskan H., Kizilarslanoglu M.C., Tuna Dogrul R., Balci C., et al. Ultrasonography versus bioelectrical impedance analysis: which predicts muscle strength better? Acta Clin Belg. 2021;76:204–208. doi: 10.1080/17843286.2019.1704989. [DOI] [PubMed] [Google Scholar]
- 100.Nakanishi N., Tsutsumi R., Okayama Y., Takashima T., Ueno Y., Itagaki T., et al. Monitoring of muscle mass in critically ill patients: comparison of ultrasound and two bioelectrical impedance analysis devices. Journal of Intensive Care. 2019;7:61. doi: 10.1186/s40560-019-0416-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Scafoglieri A., Clarys J.P. Dual energy X-ray absorptiometry: gold standard for muscle mass? Journal of Cachexia, Sarcopenia and Muscle. 2018;9:786–787. doi: 10.1002/jcsm.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Guglielmi G., Ponti F., Agostini M., Amadori M., Battista G., Bazzocchi A. The role of DXA in sarcopenia. Aging Clin Exp Res. 2016;28:1047–1060. doi: 10.1007/s40520-016-0589-3. [DOI] [PubMed] [Google Scholar]
- 103.Gonzalez M.C., Heymsfield S.B. Bioelectrical impedance analysis for diagnosing sarcopenia and cachexia: what are we really estimating? J Cachexia Sarcopenia Muscle. 2017;8:187–189. doi: 10.1002/jcsm.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fujii K., Ishizaki A., Ogawa A., Asami T., Kwon H., Tanaka A., et al. Validity of using multi-frequency bioelectrical impedance analysis to measure skeletal muscle mass in preschool children. J Phys Ther Sci. 2017;29:863–868. doi: 10.1589/jpts.29.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yamada Y., Watanabe Y., Ikenaga M., Yokoyama K., Yoshida T., Morimoto T., et al. Comparison of single- or multifrequency bioelectrical impedance analysis and spectroscopy for assessment of appendicular skeletal muscle in the elderly. J Appl Physiol. 2013;115:812–818. doi: 10.1152/japplphysiol.00010.2013. 1985. [DOI] [PubMed] [Google Scholar]
- 106.Aleixo G.F.P., Shachar S.S., Nyrop K.A., Muss H.B., Battaglini C.L., Williams G.R. Bioelectrical impedance analysis for the assessment of sarcopenia in patients with cancer: a systematic review. Oncol. 2020;25:170–182. doi: 10.1634/theoncologist.2019-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kapoor D., Piplani T., Singh A., Perwaiz A., Chaudhary A. Defining sarcopenia in the Indian population—a step forward. Indian J Surg. 2021;83:476–482. [Google Scholar]
- 108.Malmstrom T.K., Morley J.E. SARC-F: a simple questionnaire to rapidly diagnose sarcopenia. J Am Med Dir Assoc. 2013;14:531–532. doi: 10.1016/j.jamda.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 109.Lim W.S., Chew J., Lim J.P., Tay L., Hafizah N., Ding Y.Y. Case for validated instead of standard cut-offs for SARC-CalF. J Nutr Health Aging. 2019;23:393–395. doi: 10.1007/s12603-019-1177-y. [DOI] [PubMed] [Google Scholar]
- 110.Barbosa-Silva T.G., Menezes A.M.B., Bielemann R.M., Malmstrom T.K., Gonzalez M.C. Enhancing SARC-F: improving sarcopenia screening in the clinical practice. J Am Med Dir Assoc. 2016;17:1136–1141. doi: 10.1016/j.jamda.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 111.Yang M., Hu X., Xie L., Zhang L., Zhou J., Lin J., et al. Screening sarcopenia in community-dwelling older adults: SARC-F vs SARC-F combined with calf circumference (SARC-CalF) J Am Med Dir Assoc. 2018;19:277.e1–277.e8. doi: 10.1016/j.jamda.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 112.Mienche M., Setiati S., Setyohadi B., Kurniawan J., Laksmi P.W., Ariane A., et al. Diagnostic performance of calf circumference, thigh circumference, and SARC-F questionnaire to identify sarcopenia in elderly compared to asian working group for sarcopenia's diagnostic standard. Acta Med Indones. 2019;51:117–127. [PubMed] [Google Scholar]
- 113.Lee R.C., Wang Z., Heo M., Ross R., Janssen I., Heymsfield S.B. Total-body skeletal muscle mass: development and cross-validation of anthropometric prediction models. Am J Clin Nutr. 2000;72:796–803. doi: 10.1093/ajcn/72.3.796. [DOI] [PubMed] [Google Scholar]
- 114.Dasgupta R., Anoop S., Samuel P., Kurian M.E., Inbakumari M., Finney G., et al. Bioimpedance analysis with a novel predictive equation - a reliable technique to estimate fat free mass in birth weight based cohorts of Asian Indian males. Diabetes Metabol Syndr. 2019;13:738–742. doi: 10.1016/j.dsx.2018.11.070. [DOI] [PubMed] [Google Scholar]
- 115.Darvishi A., Hemami M.R., Shafiee G., Daroudi R., Mohseni M., Shekarabi F.H., et al. Sarcopenia screening strategies in older people: a cost effectiveness analysis in Iran. BMC Publ Health. 2021;21:926. doi: 10.1186/s12889-021-10511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lin Y.-C., Lu Y.-C., Chen F.-P., Lin Y.C., Cheung Y.-C., Chan W.P. Selecting appropriate sarcopenia screening methods for asian populations. J Clin Med. 2020;9:2333. doi: 10.3390/jcm9082333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Meng N.-H., Li C.-I., Liu C.-S., Lin C.-H., Lin W.-Y., Chang C.-K., et al. Comparison of height- and weight-adjusted sarcopenia in a Taiwanese metropolitan older population. Geriatr Gerontol Int. 2015;15:45–53. doi: 10.1111/ggi.12227. [DOI] [PubMed] [Google Scholar]
- 118.Rathnayake N., Lekamwasam S., Alwis G., Lenora J. 108 factors associated with hand grip strength of a group of pre and postmenopausal women from Sri Lanka. Age Ageing. 2019;48 iv18–iv27. [Google Scholar]
- 119.Fang Q., Zhu G., Huang J., Pan S., Fang M., Li Q., et al. Current status of sarcopenia in the disabled elderly of Chinese communities in shanghai: based on the updated EWGSOP consensus for sarcopenia. Front Med. 2020;7 doi: 10.3389/fmed.2020.552415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lo J.H., U K.P., Yiu T., Ong M.T., Lee W.Y. Sarcopenia: current treatments and new regenerative therapeutic approaches. Journal of Orthopaedic Translation. 2020;23:38–52. doi: 10.1016/j.jot.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Avin K.G., Bloomfield S.A., Gross T.S., Warden S.J. Biomechanical aspects of the muscle-bone interaction. Curr Osteoporos Rep. 2015;13:1–8. doi: 10.1007/s11914-014-0244-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kuzuya M., Sugimoto K., Suzuki T., Watanabe Y., Kamibayashi K., Kurihara T., et al. Chapter 3 prevention of sarcopenia. Geriatr Gerontol Int. 2018;18:23–27. doi: 10.1111/ggi.13321. [DOI] [PubMed] [Google Scholar]
- 123.Kadariya S., Gautam R., Aro A.R. Physical activity, mental health, and wellbeing among older adults in South and southeast Asia: a scoping review. BioMed Res Int. 2019;2019 doi: 10.1155/2019/6752182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Arai H., Wakabayashi H., Yoshimura Y., Yamada M., Kim H., Harada A. Chapter 4 treatment of sarcopenia. Geriatr Gerontol Int. 2018;18:28–44. doi: 10.1111/ggi.13322. [DOI] [PubMed] [Google Scholar]
- 125.Agostini F., Bernetti A., Di Giacomo G., Viva M.G., Paoloni M., Mangone M., et al. Rehabilitative good practices in the treatment of sarcopenia: a narrative review. Am J Phys Med Rehabil. 2021;100:280–287. doi: 10.1097/PHM.0000000000001572. [DOI] [PubMed] [Google Scholar]
- 126.Akhtar S. Malnutrition in South asia—a critical reappraisal. Crit Rev Food Sci Nutr. 2016;56:2320–2330. doi: 10.1080/10408398.2013.832143. [DOI] [PubMed] [Google Scholar]
- 127.Damayanthi H.D.W.T., Moy F.M., Abdullah K.L., Dharmaratne S.D. Prevalence of malnutrition and associated factors among community-dwelling older persons in Sri Lanka: a cross-sectional study. BMC Geriatr. 2018;18:199. doi: 10.1186/s12877-018-0892-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Salis S., Joseph M., Agarwala A., Sharma R., Kapoor N., Irani A.J. Medical nutrition therapy of pediatric type 1 diabetes mellitus in India: unique aspects and challenges. Pediatr Diabetes. 2021;22:93–100. doi: 10.1111/pedi.13080. [DOI] [PubMed] [Google Scholar]
- 129.Oh C., Jho S., No J.-K., Kim H.-S. Body composition changes were related to nutrient intakes in elderly men but elderly women had a higher prevalence of sarcopenic obesity in a population of Korean adults. Nutr Res. 2015;35:1–6. doi: 10.1016/j.nutres.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 130.Vandewoude M.F.J., Alish C.J., Sauer A.C., Hegazi R.A. Malnutrition-sarcopenia syndrome: is this the future of nutrition screening and assessment for older adults? Journal of Aging Research. 2012;2012 doi: 10.1155/2012/651570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sieber C.C. Malnutrition and sarcopenia. Aging Clin Exp Res. 2019;31:793–798. doi: 10.1007/s40520-019-01170-1. [DOI] [PubMed] [Google Scholar]
- 132.Dorhout B.G., Overdevest E., Tieland M., Nicolaou M., Weijs P.J.M., Snijder M.B., et al. Sarcopenia and its relation to protein intake across older ethnic populations in The Netherlands: the HELIUS study. Ethn Health. 2020:1–16. doi: 10.1080/13557858.2020.1814207. [DOI] [PubMed] [Google Scholar]
- 133.Yoo S.-Z., No M.-H., Heo J.-W., Park D.-H., Kang J.-H., Kim S.H., et al. Role of exercise in age-related sarcopenia. J Exerc Rehabil. 2018;14:551–558. doi: 10.12965/jer.1836268.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Siddiqee M.H., Bhattacharjee B., Siddiqi U.R., MeshbahurRahman M. High prevalence of vitamin D deficiency among the South Asian adults: a systematic review and meta-analysis. BMC Publ Health. 2021;21:1823. doi: 10.1186/s12889-021-11888-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ryckman T., Beal T., Nordhagen S., Murira Z., Torlesse H. Affordability of nutritious foods for complementary feeding in South Asia. Nutr Rev. 2021;79:52–68. doi: 10.1093/nutrit/nuaa139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Anitha S., Govindaraj M., Kane-Potaka J. Balanced amino acid and higher micronutrients in millets complements legumes for improved human dietary nutrition. Cereal Chem. 2020;97:74–84. [Google Scholar]
- 137.Mal B., Padulosi S., Ravi S.B. Bioversity international: Maccarese, Rome, Italy and the M.S. Swaminathan. Research Foundation; Chennai, India: 2010. Minor millets in South Asia: learnings from IFAD-NUS project in India and Nepal. [Google Scholar]
- 138.Scott R.A., Callisaya M.L., Duque G., Ebeling P.R., Scott D. Assistive technologies to overcome sarcopenia in ageing. Maturitas. 2018;112:78–84. doi: 10.1016/j.maturitas.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 139.Angulo J., El Assar M., Álvarez-Bustos A., Rodríguez-Mañas L. Physical activity and exercise: strategies to manage frailty. Redox Biol. 2020;35 doi: 10.1016/j.redox.2020.101513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Montero-Fernández N., Serra-Rexach J.A. Role of exercise on sarcopenia in the elderly. Eur J Phys Rehabil Med. 2013;49:131–143. [PubMed] [Google Scholar]
- 141.Bao W., Sun Y., Zhang T., Zou L., Wu X., Wang D., et al. Exercise programs for muscle mass, muscle strength and physical performance in older adults with sarcopenia: a systematic review and meta-analysis. Aging Dis. 2020;11:863–873. doi: 10.14336/AD.2019.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Goodpaster B.H., Chomentowski P., Ward B.K., Rossi A., Glynn N.W., Delmonico M.J., et al. Effects of physical activity on strength and skeletal muscle fat infiltration in older adults: a randomized controlled trial. J Appl Physiol. 2008;105:1498–1503. doi: 10.1152/japplphysiol.90425.2008. 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Devries M.C., Phillips S.M. Creatine supplementation during resistance training in older adults-a meta-analysis. Med Sci Sports Exerc. 2014;46:1194–1203. doi: 10.1249/MSS.0000000000000220. [DOI] [PubMed] [Google Scholar]
- 144.Gryson C., Ratel S., Rance M., Penando S., Bonhomme C., Le Ruyet P., et al. Four-month course of soluble milk proteins interacts with exercise to improve muscle strength and delay fatigue in elderly participants. J Am Med Dir Assoc. 2014;15:958. doi: 10.1016/j.jamda.2014.09.011. e1–9. [DOI] [PubMed] [Google Scholar]
- 145.Finger D., Goltz F.R., Umpierre D., Meyer E., Rosa L.H.T., Schneider C.D. Effects of protein supplementation in older adults undergoing resistance training: a systematic review and meta-analysis. Sports Med. 2015;45:245–255. doi: 10.1007/s40279-014-0269-4. [DOI] [PubMed] [Google Scholar]
- 146.de Mello R.G.B., Dalla Corte R.R., Gioscia J., Moriguchi E.H. Effects of physical exercise programs on sarcopenia management, dynapenia, and physical performance in the elderly: a systematic review of randomized clinical trials. J Aging Res. 2019;2019 doi: 10.1155/2019/1959486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Landi F., Marzetti E., Martone A.M., Bernabei R., Onder G. Exercise as a remedy for sarcopenia. Curr Opin Clin Nutr Metab Care. 2014;17:25–31. doi: 10.1097/MCO.0000000000000018. [DOI] [PubMed] [Google Scholar]
- 148.Harada H., Kai H., Niiyama H., Nishiyama Y., Katoh A., Yoshida N., et al. Effectiveness of cardiac rehabilitation for prevention and treatment of sarcopenia in patients with cardiovascular disease - a retrospective cross-sectional analysis. J Nutr Health Aging. 2017;21:449–456. doi: 10.1007/s12603-016-0743-9. [DOI] [PubMed] [Google Scholar]
- 149.Churilov I., Churilov L., Brock K., Murphy D., MacIsaac R.J., Ekinci E.I. Sarcopenia is associated with reduced function on admission to rehabilitation in patients with diabetes. J Clin Endocrinol Metab. 2021;106:e687–e695. doi: 10.1210/clinem/dgaa878. [DOI] [PubMed] [Google Scholar]
- 150.Moorthi R.N., Avin K.G. Clinical relevance of sarcopenia in chronic kidney disease. Curr Opin Nephrol Hypertens. 2017;26:219–228. doi: 10.1097/MNH.0000000000000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hirai K., Ookawara S., Morishita Y. Sarcopenia and physical inactivity in patients with chronic kidney disease. Nephro-Urol Mon. 2016;8 doi: 10.5812/numonthly.37443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Teixeira P.J., Carraça E.V., Markland D., Silva M.N., Ryan R.M. Exercise, physical activity, and self-determination theory: a systematic review. Int J Behav Nutr Phys Activ. 2012;9:78. doi: 10.1186/1479-5868-9-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Katiyar R., Ahmad S., Beg M., Baqar T. Geriatric mental health challenges in India- A review. J Evol Med Dent Sci. 2020;9:1787–1794. [Google Scholar]
- 154.Brosius C., Mandoki R. Heidelberg University Publishing (heiUP); 2020. Caring for old age : perspectives from South Asia.https://library.oapen.org/handle/20.500.12657/42464 (accessed 12 Jul2021) [Google Scholar]
- 155.South Asian Alcoholics Anonymous . SAMHIN; 2021. South Asian alcoholics anonymous (AA) meetings.https://samhin.org/alcoholics-anonymous/ (accessed 12 Jul2021) [Google Scholar]
- 156.Sharma D. South Asia@LSE; 2020. COVID-19 is exacerbating South Asia's impending food and malnutrition crisis.https://blogs.lse.ac.uk/southasia/2020/06/23/covid-19-is-exacerbating-south-asias-impending-food-and-malnutrition-crisis/ (accessed 14 Jun2021) [Google Scholar]
- 157.Cava E., Carbone S. Coronavirus disease 2019 pandemic and alterations of body composition. Curr Opin Clin Nutr Metab Care. 2021;24:229–235. doi: 10.1097/MCO.0000000000000740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mia M.A., Griffiths M.D. Can South Asian countries cope with the mental health crisis associated with COVID-19? Int J Ment Health Addiction. 2021 doi: 10.1007/s11469-021-00491-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wang P., Li Y., Wang Q. Sarcopenia: an underlying treatment target during the COVID-19 pandemic. Nutrition. 2021;84 doi: 10.1016/j.nut.2020.111104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Curcio F., Testa G., Liguori I., Papillo M., Flocco V., Panicara V., et al. Sarcopenia and heart failure. Nutrients. 2020;12:211. doi: 10.3390/nu12010211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bojko M. Causes of sarcopenia in liver cirrhosis. Clinical Liver Disease. 2019;14:167–170. doi: 10.1002/cld.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kim S.H., Shin M.J., Shin Y.B., Kim K.U. Sarcopenia associated with chronic obstructive pulmonary disease. J Bone Metab. 2019;26:65–74. doi: 10.11005/jbm.2019.26.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Scherbakov N., von Haehling S., Anker S.D., Dirnagl U., Doehner W. Stroke induced Sarcopenia: muscle wasting and disability after stroke. Int J Cardiol. 2013;170:89–94. doi: 10.1016/j.ijcard.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 164.Davis M.P., Panikkar R. Sarcopenia associated with chemotherapy and targeted agents for cancer therapy. Ann Palliat Med. 2019;8 doi: 10.21037/apm.2018.08.02. 8601–8101. [DOI] [PubMed] [Google Scholar]
- 165.Williams GrantR., Shachar S.S. What is the relationship between sarcopenia and cancer? ASCO Daily News. 2019 https://dailynews.ascopubs.org/do/10.1200/ADN.19.190231/full (accessed 12 Jul2021) [Google Scholar]
- 166.Oliveira V.H.F., Borsari A.L., Webel A.R., Erlandson K.M., Deminice R. Sarcopenia in people living with the Human Immunodeficiency Virus: a systematic review and meta-analysis. Eur J Clin Nutr. 2020;74:1009–1021. doi: 10.1038/s41430-020-0637-0. [DOI] [PubMed] [Google Scholar]
- 167.Klein G.L. Disruption of bone and skeletal muscle in severe burns. Bone Res. 2015;3:1–5. doi: 10.1038/boneres.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Polychronopoulou E., Herndon D.N., Porter C. The long-term impact of severe Burn Trauma on musculoskeletal health. J Burn Care Res. 2018;39:869–880. doi: 10.1093/jbcr/iry035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Schefold J.C., Bierbrauer J., Weber-Carstens S. Intensive care unit—acquired weakness (ICUAW) and muscle wasting in critically ill patients with severe sepsis and septic shock. J Cachexia Sarcopenia Muscle. 2010;1:147–157. doi: 10.1007/s13539-010-0010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pickering M.-E., Chapurlat R. Where two common conditions of aging meet: osteoarthritis and sarcopenia. Calcif Tissue Int. 2020;107:203–211. doi: 10.1007/s00223-020-00703-5. [DOI] [PubMed] [Google Scholar]
- 171.Bloise F.F., Oliveira T.S., Cordeiro A., Ortiga-Carvalho T.M. Thyroid hormones play role in sarcopenia and myopathies. Front Physiol. 2018;9:560. doi: 10.3389/fphys.2018.00560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Szlejf C., Suemoto C.K., Janovsky C.C.P.S., Barreto S.M., Diniz M. de FHS., Lotufo P.A., et al. Thyroid function and sarcopenia: results from the ELSA-Brasil study. J Am Geriatr Soc. 2020;68:1545–1553. doi: 10.1111/jgs.16416. [DOI] [PubMed] [Google Scholar]
- 173.Mesinovic J., Zengin A., De Courten B., Ebeling P.R., Scott D. Sarcopenia and type 2 diabetes mellitus: a bidirectional relationship. Diabetes Metab Syndr Obes. 2019;12:1057–1072. doi: 10.2147/DMSO.S186600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Khamseh M.E., Malek M., Aghili R., Emami Z. Sarcopenia and diabetes: pathogenesis and consequences. Diabetes & Vascular Disease. 2011;11:230–234. [Google Scholar]
- 175.Stenholm S., Harris T.B., Rantanen T., Visser M., Kritchevsky S.B., Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care. 2008;11:693–700. doi: 10.1097/MCO.0b013e328312c37d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kaji H. Interaction between muscle and bone. J Bone Metab. 2014;21:29–40. doi: 10.11005/jbm.2014.21.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Shin M.J., Jeon Y.K., Kim I.J. Testosterone and sarcopenia. World J Mens Health. 2018;36:192–198. doi: 10.5534/wjmh.180001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Messier V., Rabasa-Lhoret R., Barbat-Artigas S., Elisha B., Karelis A.D., Aubertin-Leheudre M. Menopause and sarcopenia: a potential role for sex hormones. Maturitas. 2011;68:331–336. doi: 10.1016/j.maturitas.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 179.Chidi-Ogbolu N., Baar K. Effect of estrogen on musculoskeletal performance and injury risk. Front Physiol. 2019 doi: 10.3389/fphys.2018.01834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mikolasevic I., Pavic T., Kanizaj T.F., Bender D.V., Domislovic V., Krznaric Z. Nonalcoholic fatty liver disease and sarcopenia: where do we stand? Canadian Journal of Gastroenterology and Hepatology. 2020;2020 doi: 10.1155/2020/8859719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Campins L., Camps M., Riera A., Pleguezuelos E., Yebenes J.C., Serra-Prat M. Oral drugs related with muscle wasting and sarcopenia. A review. PHA. 2017;99:1–8. doi: 10.1159/000448247. [DOI] [PubMed] [Google Scholar]
- 182.Baird M.F., Graham S.M., Baker J.S., Bickerstaff G.F. Creatine-kinase- and exercise-related muscle damage implications for muscle performance and recovery. Journal of Nutrition and Metabolism. 2012;2012 doi: 10.1155/2012/960363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kurita N., Kamitani T., Wada O., Shintani A., Mizuno K. Disentangling associations between serum muscle biomarkers and sarcopenia in the presence of pain and inflammation among patients with osteoarthritis: the SPSS-OK study. J Clin Rheumatol. 2021;27:56–63. doi: 10.1097/RHU.0000000000001156. [DOI] [PubMed] [Google Scholar]
- 184.Geraci A., Calvani R., Ferri E., Marzetti E., Arosio B., Cesari M. Sarcopenia and menopause: the role of estradiol. Front Endocrinol. 2021;12 doi: 10.3389/fendo.2021.682012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Marzetti E., Picca A., Marini F., Biancolillo A., Coelho-Junior H.J., Gervasoni J., et al. Inflammatory signatures in older persons with physical frailty and sarcopenia: the frailty ‘cytokinome’ at its core. Exp Gerontol. 2019;122:129–138. doi: 10.1016/j.exger.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 186.Can B., Kara O., Kizilarslanoglu M.C., Arik G., Aycicek G.S., Sumer F., et al. Serum markers of inflammation and oxidative stress in sarcopenia. Aging Clin Exp Res. 2017;29:745–752. doi: 10.1007/s40520-016-0626-2. [DOI] [PubMed] [Google Scholar]
- 187.Arik G., Ulger Z. Vitamin D in sarcopenia: understanding its role in pathogenesis, prevention and treatment. European Geriatric Medicine. 2016;7:207–213. [Google Scholar]
- 188.Yuki A., Otsuka R., Kozakai R., Kitamura I., Okura T., Ando F., et al. Relationship between low free testosterone levels and loss of muscle mass. Sci Rep. 2013;3:1818. doi: 10.1038/srep01818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Geraci A., Calvani R., Ferri E., Marzetti E., Arosio B., Cesari M. Sarcopenia and menopause: the role of estradiol. Front Endocrinol. 2021;12 doi: 10.3389/fendo.2021.682012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Evans W.J., Hellerstein M., Orwoll E., Cummings S., Cawthon P.M. D 3-Creatine dilution and the importance of accuracy in the assessment of skeletal muscle mass. Journal of Cachexia, Sarcopenia and Muscle. 2019;10:14–21. doi: 10.1002/jcsm.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Aryana S., Lestari A.W., Putrawan I.B., Purnami N.K.R., Astika I.N., Kuswardhani R.A.T. The the relationship between IL-6 and CRP with sarcopenia in indigenous elderly population at pedawa Village, Buleleng, Bali, Indonesia. Health Science Journal of Indonesia. 2018;9:37–44. [Google Scholar]
- 192.Lana A., Struijk E., Guallar-Castillón P., Martín-Moreno J.M., Rodríguez Artalejo F., Lopez-Garcia E. Leptin concentration and risk of impaired physical function in older adults: the Seniors-ENRICA cohort. Age Ageing. 2016;45:819–826. doi: 10.1093/ageing/afw142. [DOI] [PubMed] [Google Scholar]
- 193.Mafi F., Biglari S., Ghardashi Afousi A., Gaeini A.A. Improvement in skeletal muscle strength and plasma levels of follistatin and myostatin induced by an 8-week resistance training and epicatechin supplementation in sarcopenic older adults. J Aging Phys Activ. 2019;27:384–391. doi: 10.1123/japa.2017-0389. [DOI] [PubMed] [Google Scholar]
- 194.Bian A., Ma Y., Zhou X., Guo Y., Wang W., Zhang Y., et al. Association between sarcopenia and levels of growth hormone and insulin-like growth factor-1 in the elderly. BMC Muscoskel Disord. 2020;21:214. doi: 10.1186/s12891-020-03236-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Valenti G., Denti L., Maggio M., Ceda G., Volpato S., Bandinelli S., et al. Effect of DHEAS on skeletal muscle over the life span: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59:466–472. doi: 10.1093/gerona/59.5.m466. [DOI] [PubMed] [Google Scholar]
- 196.Yanagita I., Fujihara Y., Kitajima Y., Tajima M., Honda M., Kawajiri T., et al. A high serum cortisol/DHEA-S ratio is a risk factor for sarcopenia in elderly diabetic patients. J Endocr Soc. 2019;3:801–813. doi: 10.1210/js.2018-00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Riuzzi F., Sorci G., Sagheddu R., Chiappalupi S., Salvadori L., Donato R. RAGE in the pathophysiology of skeletal muscle: RAGE in skeletal muscle. Journal of Cachexia, Sarcopenia and Muscle. 2018;9:1213–1234. doi: 10.1002/jcsm.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Howard C., Ferrucci L., Sun K., Fried L.P., Walston J., Varadhan R., et al. Oxidative protein damage is associated with poor grip strength among older women living in the community. J Appl Physiol. 2007;103:17–20. doi: 10.1152/japplphysiol.00133.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Komici K., Dello Iacono A., De Luca A., Perrotta F., Bencivenga L., Rengo G., et al. Adiponectin and sarcopenia: a systematic review with meta-analysis. Front Endocrinol. 2021:12. doi: 10.3389/fendo.2021.576619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Baczek J., Silkiewicz M., Wojszel Z.B. Myostatin as a biomarker of muscle wasting and other pathologies-state of the art and knowledge gaps. Nutrients. 2020;12:2401. doi: 10.3390/nu12082401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Aryana S., Astika I., Kuswardhani T., Putrawan I., Purnami N., Putra W., et al. High myostatin serum related with high prevalence of sarcopenia among elderly population in pedawa Village, Bali, Indonesia. The Indonesian Biomedical Journal. 2019;11:293–298. [Google Scholar]
- 202.Bian A.-L., Hu H.-Y., Rong Y.-D., Wang J., Wang J.-X., Zhou X.-Z. A study on relationship between elderly sarcopenia and inflammatory factors IL-6 and TNF-α. Eur J Med Res. 2017;22:25. doi: 10.1186/s40001-017-0266-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kochlik B., Stuetz W., Pérès K., Féart C., Tegner J., Rodriguez-Mañas L., et al. Associations of plasma 3-methylhistidine with frailty status in French cohorts of the FRAILOMIC initiative. J Clin Med. 2019;8:1010. doi: 10.3390/jcm8071010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Drey M., Sieber C.C., Bauer J.M., Uter W., Dahinden P., Fariello R.G., et al. C-terminal Agrin Fragment as a potential marker for sarcopenia caused by degeneration of the neuromuscular junction. Exp Gerontol. 2013;48:76–80. doi: 10.1016/j.exger.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 205.Singhal S., Singh S., Upadhyay A.D., Dwivedi S.N., Das C.J., Mohta S., et al. Serum creatinine and cystatin C-based index can be a screening biomarker for sarcopenia in older population. Eur Geriatr Med. 2019;10:625–630. doi: 10.1007/s41999-019-00197-x. [DOI] [PubMed] [Google Scholar]
- 206.Ivey-Miranda J., Stewart B., Gomez N., Barnett J., Thomas A., Wycallis E., et al. Sarcopenia strongly affects serum levels of cystatin C in patients with heart failure. J Card Fail. 2019;25:S20–S21. [Google Scholar]
- 207.Kwak J.Y., Hwang H., Kim S.-K., Choi J.Y., Lee S.-M., Bang H., et al. Prediction of sarcopenia using a combination of multiple serum biomarkers. Sci Rep. 2018;8:8574. doi: 10.1038/s41598-018-26617-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Bhasin S., He E.J., Kawakubo M., Schroeder E.T., Yarasheski K., Opiteck G.J., et al. N-terminal propeptide of type III procollagen as a biomarker of anabolic response to recombinant human GH and testosterone. J Clin Endocrinol Metab. 2009;94:4224–4233. doi: 10.1210/jc.2009-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Chang J.S., Kim T.H., Nguyen T.T., Park K.-S., Kim N., Kong I.D. Circulating irisin levels as a predictive biomarker for sarcopenia: a cross-sectional community-based study: irisin as a biomarker for sarcopenia. Geriatr Gerontol Int. 2017;17:2266–2273. doi: 10.1111/ggi.13030. [DOI] [PubMed] [Google Scholar]
- 210.Bunout D., Barrera G., de la Maza P., Avendaño M., Gattas V., Petermann M., et al. The impact of nutritional supplementation and resistance training on the health functioning of free-living Chilean elders: results of 18 Months of follow-up. J Nutr. 2001;131:2441S–2446S. doi: 10.1093/jn/131.9.2441S. [DOI] [PubMed] [Google Scholar]
- 211.Dillon E.L., Sheffield-Moore M., Paddon-Jones D., Gilkison C., Sanford A.P., Casperson S.L., et al. Amino acid supplementation increases lean body mass, basal muscle protein synthesis, and insulin-like growth factor-I expression in older women. J Clin Endocrinol Metab. 2009;94:1630–1637. doi: 10.1210/jc.2008-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Stout J.R., Smith-Ryan A.E., Fukuda D.H., Kendall K.L., Moon J.R., Hoffman J.R., et al. Effect of calcium β-hydroxy-β-methylbutyrate (CaHMB) with and without resistance training in men and women 65+yrs: a randomized, double-blind pilot trial. Exp Gerontol. 2013;48:1303–1310. doi: 10.1016/j.exger.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 213.Deutz N.E.P., Pereira S.L., Hays N.P., Oliver J.S., Edens N.K., Evans C.M., et al. Effect of β-hydroxy-β-methylbutyrate (HMB) on lean body mass during 10 days of bed rest in older adults. Clin Nutr. 2013;32:704–712. doi: 10.1016/j.clnu.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 214.Vukovich M.D., Stubbs N.B., Bohlken R.M. Body composition in 70-Year-old adults responds to dietary β-Hydroxy-β-Methylbutyrate similarly to that of young adults. J Nutr. 2001;131:2049–2052. doi: 10.1093/jn/131.7.2049. [DOI] [PubMed] [Google Scholar]
- 215.Smith G.I., Julliand S., Reeds D.N., Sinacore D.R., Klein S., Mittendorfer B. Fish oil–derived n−3 PUFA therapy increases muscle mass and function in healthy older adults 1. Am J Clin Nutr. 2015;102:115–122. doi: 10.3945/ajcn.114.105833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Dupont J., Dedeyne L., Dalle S., Koppo K., Gielen E. The role of omega-3 in the prevention and treatment of sarcopenia. Aging Clin Exp Res. 2019;31:825–836. doi: 10.1007/s40520-019-01146-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Candow D.G., Forbes S.C., Chilibeck P.D., Cornish S.M., Antonio J., Kreider R.B. Variables influencing the effectiveness of creatine supplementation as a therapeutic intervention for sarcopenia. Front Nutr. 2019;6 doi: 10.3389/fnut.2019.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Dolan E., Artioli G.G., Pereira R.M.R., Gualano B. Muscular atrophy and sarcopenia in the elderly: is there a role for creatine supplementation? Biomolecules. 2019;9:642. doi: 10.3390/biom9110642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Hardee J.P., Lynch G.S. Current pharmacotherapies for sarcopenia. Expet Opin Pharmacother. 2019;20:1645–1657. doi: 10.1080/14656566.2019.1622093. [DOI] [PubMed] [Google Scholar]
- 220.Agrawal A., Suryakumar G., Rathor R. Role of defective Ca2+ signaling in skeletal muscle weakness: pharmacological implications. J Cell Commun Signal. 2018;12:645–659. doi: 10.1007/s12079-018-0477-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Sadiq N.M., Naganathan S., Badireddy M. StatPearls. StatPearls publishing: Treasure Island (FL) 2021. Hypercalcemia.http://www.ncbi.nlm.nih.gov/books/NBK430714/ (accessed 28 Aug2021) [Google Scholar]
- 222.Brioche T., Kireev R.A., Cuesta S., Gratas-Delamarche A., Tresguerres J.A., Gomez-Cabrera M.C., et al. Growth hormone replacement therapy prevents sarcopenia by a dual mechanism: improvement of protein balance and of antioxidant defenses. J Gerontol A Biol Sci Med Sci. 2014;69:1186–1198. doi: 10.1093/gerona/glt187. [DOI] [PubMed] [Google Scholar]
- 223.Chiu H.-C., Chiu C.-Y., Yang R.-S., Chan D.-C., Liu S.-H., Chiang C.-K. Preventing muscle wasting by osteoporosis drug alendronate in vitro and in myopathy models via sirtuin-3 down-regulation. Journal of Cachexia, Sarcopenia and Muscle. 2018;9:585–602. doi: 10.1002/jcsm.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Harada A., Ito S., Matsui Y., Sakai Y., Takemura M., Tokuda H., et al. Effect of alendronate on muscle mass: investigation in patients with osteoporosis. Osteoporosis and Sarcopenia. 2015;1:53–58. [Google Scholar]
- 225.Kim S.-W., Kim R. The association between hormone therapy and sarcopenia in postmenopausal women: the Korea National Health and Nutrition Examination Survey, 2008-2011. Menopause. 2020;27:506–511. doi: 10.1097/GME.0000000000001509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Supasyndh O., Satirapoj B., Aramwit P., Viroonudomphol D., Chaiprasert A., Thanachatwej V., et al. Effect of oral anabolic steroid on muscle strength and muscle growth in hemodialysis patients. Clin J Am Soc Nephrol. 2013;8:271–279. doi: 10.2215/CJN.00380112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Mandel S. Steroid myopathy. PGM (Postgrad Med) 1982;72:207–215. doi: 10.1080/00325481.1982.11716260. [DOI] [PubMed] [Google Scholar]
- 228.Sumukadas D., Struthers A.D., McMurdo M.E.T. Sarcopenia--a potential target for Angiotensin-converting enzyme inhibition? Gerontology. 2006;52:237–242. doi: 10.1159/000093656. [DOI] [PubMed] [Google Scholar]
- 229.Ali S., Garcia J.M. Sarcopenia, cachexia and aging: diagnosis, mechanisms and therapeutic options - a mini-review. GER. 2014;60:294–305. doi: 10.1159/000356760. [DOI] [PMC free article] [PubMed] [Google Scholar]