Abstract
Introduction
Coffee is one of the most frequently consumed beverages worldwide and has been found to have a wide assortment of health benefits. Although habitual coffee consumption is associated with a lower incidence of chronic kidney disease, an association between coffee and acute kidney injury (AKI) has not yet been revealed.
Methods
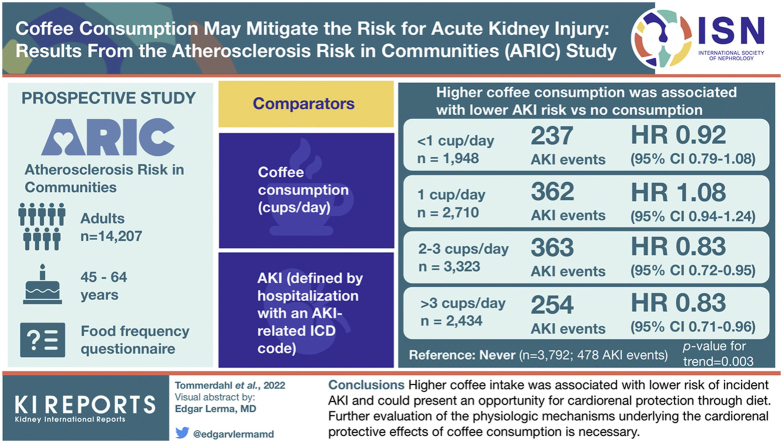
In the Atherosclerosis Risk in Communities (ARIC) Study, a prospective cohort study of 14,207 adults aged 45 to 64 years, coffee consumption (cups/d) was assessed at a single visit via food frequency questionnaires and compared with incident AKI defined by hospitalization with an AKI-related International Classification of Diseases code.
Results
In ARIC, there were 1694 cases of incident AKI in a median of 24 follow-up years. Higher coffee consumption was associated with lower AKI risk versus no consumption (hazard ratio [HR] <1 cup/d: 0.92 [95% CI: 0.79–1.08]; 1 cup/d: 1.08 [95% CI: 0.94–1.24]; 2 to 3 cups/d: 0.83 [95% CI: 0.72–0.95]; >3 cups/d: 0.83 [95% CI: 0.71–0.96]; reference: never, P = 0.003). Trends for AKI risk across coffee categories remained significant after multivariable adjustment for age, sex, race-center, education, total daily energy intake, physical activity, smoking, alcohol intake, diet quality (Dietary Approaches to Stop Hypertension [DASH] score), systolic blood pressure (BP), diabetes status, use of antihypertensive agents, estimated glomerular filtration rate (eGFR), and body mass index (BMI) (P = 0.02).
Conclusion
Higher coffee intake was associated with a lower risk of incident AKI and could present an opportunity for cardiorenal protection through diet. Further evaluation of the physiological mechanisms underlying the cardiorenal protective effects of coffee consumption is necessary.
Keywords: acute kidney injury, beverages, caffeine, coffee, incident AKI
Graphical abstract
Coffee, one of the most often consumed beverages worldwide, contains a wide variety of compounds, including caffeine, diterpenes, and chlorogenic acid, which fully develop after the bean roasting process and are reported to have an assortment of health benefits.1 Habitual coffee consumption is associated with the prevention of chronic and degenerative diseases, including type 2 diabetes, cardiovascular disease, and liver disease.1, 2, 3, 4 ARIC, a large population study of 14,207 participants aged 45 to 64 years, revealed that higher self-reported daily coffee consumption was associated with a lower risk of incident chronic kidney disease after adjustments were made for demographic, clinical, and dietary factors.5 Thus, habitual coffee consumption has a strong potential for reducing the risk of progressive kidney disease.
The most often studied compound in coffee is caffeine, a methylxanthine alkaloid and an adenosine receptor antagonist that significantly alters kidney function by rapid-acting mechanisms, including modification of the renin-angiotensin-aldosterone system, baseline renal plasma flow, hemodynamics, and natriuresis.6,7 Caffeine also inhibits sodium reabsorption in the proximal and distal tubules of the kidney, thus increasing solute and free water excretion,8 and has been postulated to fully inhibit the local tubule-glomerular feedback response to increase distal sodium delivery.9 Other less-studied bioactive compounds in coffee including chlorogenic acids, chlorogenic acid lactones, p-coumaric acid, nicotinic acid, theobromine, and trigonelline10,11 must also be considered as potential contributors to coffee’s cardiorenal protective effects, as many of these polyphenol compounds are potent plant-based antioxidants that have been found to improve generalized inflammation and oxidative stress,12 key factors in the development of AKI.13,14 For these reasons, coffee consumption may also mitigate the risk for developing AKI, yet it is unclear which individuals will experience the strongest cardiorenal protective effects.
We aimed to assess associations between coffee consumption and incident AKI in a large, diverse population of middle-aged adults and hypothesized that habitual coffee consumption would be associated with a lower risk of incident AKI because of the cardiorenal protective properties of both coffee and caffeine.
Methods
Participants
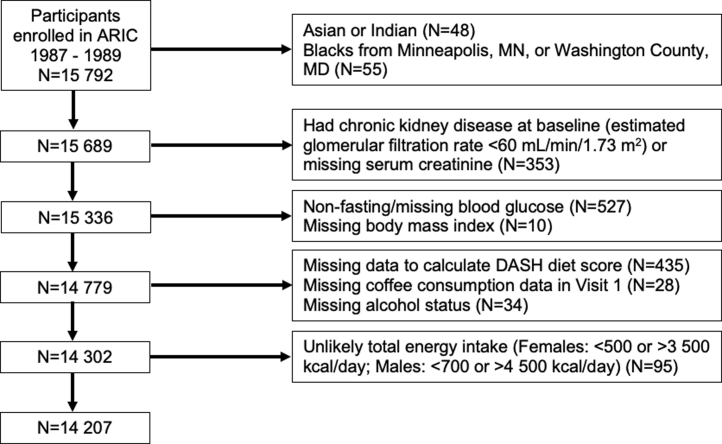
The ARIC study included a cohort of 15,792 adults aged 45 to 64 years from 4 different US communities recruited from 1987 to 1989. Participants completed 7 subsequent follow-up visits. The study protocol was approved at each study site, and all participants were provided written documentation of informed consent. After exclusions, a total of 14 207 study participants were included in our analysis (Figure 1).
Figure 1.
Flow diagram of study participants for the ARIC study. ARIC, Atherosclerosis Risk In Communities study; BMI, body mass index; CKD; chronic kidney disease; DASH diet score, Dietary Approaches to Stop Hypertension diet score; eGFR, estimated glomerular filtration rate; MD, Maryland; MN, Minnesota; serum Cr, serum creatinine.
Coffee Consumption Assessments
Coffee consumption was assessed by self-report using a semiquantitative food frequency questionnaire involving 66 questions administered by a trained interviewer at visit 1 (1987–1989). Participants reported their average frequency of consumption of an 8-oz cup of regular coffee over the preceding year with the following choices: almost never; 1 to 3 cups/mo; 1 cup/wk; 2 to 4 cups/wk; 5 to 6 cups/wk; 1 cup/d; 2 to 3 cups/d; 4 to 6 cups/d; and >6 cups/d. We collapsed the aforementioned 9-category variable into a 5-category variable with the following choices: never (including participants who indicated “almost never” coffee consumption); <1 cup/d (including participants who indicated “1–3 cup/mo,” “1 cup/wk,” “2–4 cups/wk,” and “5–6 cups/wk”); 1 cup/d; 2 to 3 cups/d; and >3 cups/d (including participants who indicated “4–6 cups/d” and “>6 cups/d”).
Outcomes and Covariates Assessed
Incident AKI was defined as a hospitalization including an International Classification of Diseases code indicating AKI during the follow-up period. The eGFR was calculated with the 2009 Chronic Kidney Disease Epidemiology Collaboration equation using serum creatinine measured by the modified kinetic Jaffé method. Diabetes was defined as baseline fasting blood glucose ≥126 mg/dl; random blood glucose ≥200 mg/dl; self-reported history of a physician diagnosis of diabetes; or use of any antihyperglycemic medication in the preceding 2 weeks. Hypertension was defined as follows: systolic BP (SBP) ≥140 mm Hg; diastolic BP (DBP) ≥90 mm Hg; or use of any antihypertensive medication in the preceding 2 weeks.
Adjustments were made for demographics, lifestyle, and clinical factors, including age, sex, race, smoking status, physical activity level (scored from 1 [low] to 5 [high] based on time and intensity dedicated to sports and nonsport leisure exercise), alcohol intake status (never, former, current moderate [<1 drink/d for women or <2 drinks/d for men], or current heavy [≥1 drink/d for women or ≥2 drinks/d for men]), education level, SBP, DBP, fasting blood glucose, eGFR, diabetes status, hypertension status, use of antihypertensive medication, and study center.15 Race and study center were combined as a single variable, as racial distributions across study centers were nonuniform. As a general measure of diet quality, a DASH diet score16 was calculated based on a summation of the following criteria, each scored from 1 (low) to 5 (high) and based on ranked distribution in quintiles: high intake of whole grains, fruits, vegetables, nuts and legumes, and low-fat dairy; and low intake of sodium, red and processed meat, and sweetened beverages. Total daily energy intake was reported in kcal/d.
Statistical Analysis
We report demographic and clinical risk factors per predetermined categories of self-reported habitual coffee consumption. Detailed statistical analyses procedures were previously reported.5 Cox proportional hazards regression models were used to estimate HRs and 95% CIs for incident AKI per coffee consumption categories. We present comparisons of individuals who report previously consuming coffee to those who report never having consumed coffee. We also evaluated the following models: model 1 was unadjusted (i.e., no covariates); model 2 was adjusted for demographic characteristics (age, sex, race-center), education as a proxy for socioeconomic status, dietary factors (total energy intake and DASH diet score as a measure of diet quality), and health-related behaviors (physical activity, smoking status, and alcohol intake status); and model 3 was adjusted for model 2 variables in addition to potential mediating factors that could contribute to the relationship between coffee and AKI (SBP, diabetes status, antihypertensive medication use, baseline eGFR, and BMI). We tested interaction terms between potential effect modifiers (i.e., sex, smoking, diabetes, race, physical activity, and DASH diet score) and coffee consumption on incident AKI using the likelihood ratio test. We assessed the proportionality assumption in cause-specific models based on Schoenfeld residuals. P values were calculated for trends across coffee consumption categories using an ordinal variable corresponding to the categories of coffee consumption. Statistical significance was set a priori at P < 0.05, and all P values were 2-tailed. Analyses were performed using Stata version 16.1 (StataCorp, College Station, TX).
Results
Baseline Characteristics
We provide data from 14,207 adult participants with the following self-endorsed coffee drinking habits: 27% never drank coffee; 14% drank <1 cup/d; 19% drank 1 cup/d; 23% drank 2 to 3 cups/d; and 17% drank >3 cups/d (Table 1 and Figure 1). Coffee consumption was highest in males, individuals of White race/ethnicity, current smokers, and people without a concurrent diabetes diagnosis. Individuals with lower BMI, SBP, and DBP, and fasting blood glucose and people with a higher total energy intake per day were also more likely to consume more coffee. eGFR was slightly lower for higher categories of coffee consumption (P < 0.001).
Table 1.
Baseline demographic characteristics of participants in the ARIC study according to categories of coffee consumption
| Baseline characteristics | Coffee consumption categories |
P value | ||||
|---|---|---|---|---|---|---|
| Never (n = 3792) | <1 cup/d (n = 1948) | 1 cup/d (n = 2710) | 2–3 cups/d (n = 3323) | >3 cups/d (n = 2434) | ||
| Age (yr) | 54 ± 6 | 54 ± 6 | 55 ± 6 | 54 ± 6 | 54 ± 6 | <0.001 |
| Sex, % female | 2382 (62.8) | 1045 (53.6) | 1558 (57.5) | 1739 (52.3) | 1178 (48.4) | <0.001 |
| Race, % black | 1313 (34.6) | 459 (23.6) | 1007 (37.2) | 581 (17.5) | 157 (6.5) | <0.001 |
| Education level (%) | ||||||
| Less than high school | 916 (24.2) | 394 (20.2) | 822 (30.3) | 654 (19.7) | 448 (18.4) | <0.001 |
| High school or equivalent | 1524 (40.2) | 816 (41.9) | 1006 (37.1) | 1430 (43.0) | 1089 (44.7) | |
| College or above | 1352 (35.7) | 738 (37.9) | 882 (32.6) | 1239 (37.3) | 897 (36.9) | |
| Center (%) | ||||||
| Forsyth County, NC | 854 (22.5) | 475 (24.4) | 755 (27.9) | 998 (30.0) | 596 (24.5) | <0.001 |
| Jackson, MS | 1144 (30.2) | 399 (20.5) | 912 (33.7) | 535 (16.1) | 132 (5.4) | |
| Minneapolis, MN | 814 (21.5) | 568 (29.2) | 396 (14.6) | 899 (27.1) | 1047 (43.0) | |
| Washington County, MD | 980 (25.8) | 506 (26.0) | 647 (23.9) | 891 (26.8) | 659 (27.1) | |
| Physical activity index scorea | 2.4 ± 0.8 | 2.5 ± 0.8 | 2.4 ± 0.8 | 2.5 ± 0.8 | 2.5 ± 0.8 | <0.001 |
| Smoking status (%) | ||||||
| Never smoker | 1990 (52.5) | 944 (48.5) | 1238 (45.7) | 1231 (37.0) | 553 (22.7) | <0.001 |
| Former smoker | 1073 (28.3) | 631 (32.4) | 856 (31.6) | 1199 (36.1) | 843 (34.6) | |
| Current smoker | 729 (19.2) | 373 (19.2) | 616 (22.7) | 893 (26.9) | 1038 (42.7) | |
| BMI (kg/m2) | 28 ± 6 | 28 ± 5 | 28 ± 6 | 27 ± 5 | 27 ± 5 | <0.001 |
| Total energy intake (kcal/d) | 1561 ± 594 | 1592 ± 581 | 1614 ± 595 | 1630 ± 586 | 1728 ± 619 | <0.001 |
| DASH diet score (8–40) | 25 ± 5 | 24 ± 5 | 24 ± 5 | 24 ± 5 | 24 ± 5 | <0.001 |
| SBP (mm Hg) | 123 ± 19 | 121 ± 18 | 123 ± 19 | 120 ± 18 | 117 ± 17 | <0.001 |
| DBP (mm Hg) | 75 ± 11 | 74 ± 11 | 75 ± 12 | 73 ± 11 | 71 ± 11 | <0.001 |
| MAP (mm Hg) | 91 ± 13 | 90 ± 12 | 91 ± 13 | 89 ± 12 | 87 ± 12 | <0.001 |
| Diabetes (%) | 465 (12.3) | 208 (10.7) | 337 (12.4) | 304 (9.2) | 143 (5.9) | <0.001 |
| Hypertension (%) | 1464 (38.6) | 673 (34.6) | 1100 (40.6) | 990 (29.8) | 549 (22.6) | <0.001 |
| Antihypertensive medication (%) | 1299 (34.3) | 608 (31.2) | 962 (35.6) | 851 (25.6) | 472 (19.4) | <0.001 |
| ACE inhibitors (%) | 130 (3.4) | 52 (2.7) | 95 (3.5) | 78 (2.4) | 55 (2.3) | 0.005 |
| Fasting blood glucose (mmol/l) | 6.0 ± 2.1 | 5.9 ± 1.7 | 6.1 ± 2.1 | 5.9 ± 1.8 | 5.7 ± 1.4 | <0.001 |
| Serum creatinine (μmol/l) | 95.3 ± 16.1 | 96.6 ± 16.1 | 96.3 ± 16.1 | 96.7 ± 15.7 | 96.0 ± 14.9 | 0.001 |
| Serum potassium (mmol/l) | 4.4 ± 0.5 | 4.4 ± 0.5 | 4.3 ± 0.5 | 4.5 ± 0.5 | 4.6 ± 0.5 | <0.001 |
| Serum magnesium (mmol/l) | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | <0.001 |
| eGFR (ml/min per 1.73 m2) | 104 ± 15 | 103 ± 15 | 104 ± 15 | 102 ± 13 | 102 ± 12 | <0.001 |
| Alcohol intake status (%)b | ||||||
| Never | 1309 (34.5) | 478 (24.5) | 809 (29.9) | 629 (18.9) | 286 (11.8) | <0.001 |
| Former | 742 (19.6) | 317 (16.3) | 525 (19.4) | 554 (16.7) | 471 (19.4) | |
| Current (moderate) | 256 (6.8) | 177 (9.1) | 222 (8.2) | 407 (12.3) | 345 (14.2) | |
| Current (heavy) | 1485 (39.2) | 976 (50.1) | 1154 (42.6) | 1733 (52.2) | 1332 (54.7) | |
ACE, angiotensin-converting enzyme; ANOVA, analysis of variance; DASH, Dietary Approaches to Stop Hypertension; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; MAP, mean arterial pressure; SBP, systolic blood pressure; UACR, urinary albumin-to-creatinine ratio.
Data for categorical variables are presented as n (%) and were analyzed by χ2 tests. Data for continuous variables are presented as mean ± SD and were analyzed by ANOVA test.
The physical activity index included considerations of both time and intensity of the exercise or sporting activity completed during leisure time, with scores ranging from 1 (lowest) to 5 (highest).
Moderate alcohol intake was defined as follows: <1 alcoholic beverage per day for women or <2 alcoholic beverages per day for men. Heavy alcohol intake was defined as ≥1 alcoholic beverage per day for women or ≥2 alcoholic beverages per day for men.
Association Between Coffee Consumption and AKI
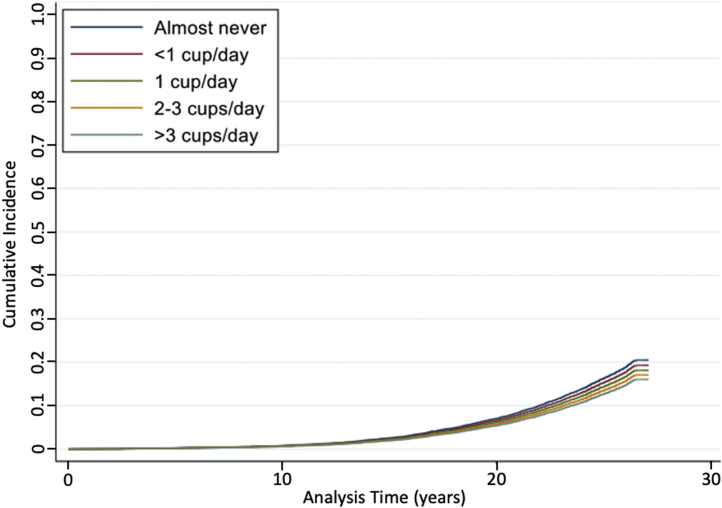
A total of 1694 AKI events were recorded for all study participants throughout a median of 24 years of follow-up. Any frequency of coffee consumption was associated with a lower risk of incident AKI compared with abstention from coffee consumption (Table 2). The cumulative proportions of participants experiencing AKI events at each of the self-reported coffee consumption categories over time are presented in Figure 2. After accounting for demographic characteristics (age, sex, race-center), socioeconomic status (education), lifestyle factors (physical activity, smoking, alcohol intake status), and other dietary factors (total energy intake, DASH diet score), there was a 15% lower risk of AKI for those who consumed any coffee versus never (model 2 HR: 0.85, 95% CI: 0.76–0.94, P = 0.002). The results were slightly attenuated but remained statistically significant after accounting for potential mediators of the association between coffee and AKI (i.e., SBP, diabetes status, use of antihypertensive medications, eGFR, and BMI) (model 3 HR: 0.89, 95% CI: 0.80–0.99, P = 0.03).
Table 2.
Risk for AKI in adult participants from the ARIC study for those consuming any amount of coffee versus never consuming coffee
| Outcome | Coffee consumption categories |
P value | |
|---|---|---|---|
| Never (n = 3792) | Any (n = 10,415) | ||
| AKI events (n) | 478 | 1 216 | |
| Incidence (per 1000 person-yr) | 6.0 (5.5–6.6) | 5.5 (5.2–5.8) | |
| Model 1 | 1 (reference) | 0.91 (0.82–1.01) | 0.07 |
| Model 2 | 1 (reference) | 0.85 (0.76–0.94) | 0.002 |
| Model 3 | 1 (reference) | 0.89 (0.80–0.99) | 0.03 |
AKI, acute kidney injury; ARIC, Atherosclerosis Risk In Communities; DASH, Dietary Approaches to Stop Hypertension.
Model 1: Unadjusted (no covariates).
Model 2: Variables include age, sex, race-center, education, total energy intake, physical activity, smoking, alcohol intake status, and DASH diet score.
Model 3: All model 2 variables plus systolic blood pressure, diabetes status, use of antihypertensive medications, estimated glomerular filtration rate, and body mass index.
Data presented as hazard ratio (95% CI) unless otherwise noted.
Figure 2.
Cumulative incidence of AKI by coffee consumption category in the ARIC study. AKI, acute kidney injury; ARIC, Atherosclerosis Risk In Communities study.
Analysis of more specific coffee consumption categories revealed statistically significant trend for lower AKI risk with a higher frequency of coffee consumption in all 3 models (model 1: P = 0.003, model 2: P < 0.001, model 3: P = 0.02) (Table 3). In model 2, which adjusted for demographic factors, education, dietary factors, and health behaviors, there was a 22% to 23% lower risk of AKI for those who consumed >2 cups/d of coffee relative to never consumers (HR for 2 to 3 cups/d: 0.77 [95% CI: 0.67–0.89] and >3 cups/d: 0.78 [95% CI: 0.67–0.89]; P for trend < 0.001). After accounting for all factors in model 2 in addition to potential mediating factors (SBP, diabetes status, use of hypertensive medications, eGFR, and BMI) in model 3, the results were attenuated but the trend remained statistically significant (HR for 2–3 cups/d 0.83 [95% CI: 0.72–0.95]; >3 cups/d: 0.88 [95% CI: 0.75–1.04]; P for trend = 0.02).
Table 3.
Risk for AKI in adult participants from the ARIC study according to average daily coffee consumption
| Outcome | Coffee consumption categories |
P value for trend | ||||
|---|---|---|---|---|---|---|
| Never (n = 3792) | <1 cup/d (n = 1948) | 1 cup/d (n = 2710) | 2–3 cups/d (n = 3323) | >3 cups/d (n = 2434) | ||
| AKI events (n) | 478 | 237 | 362 | 363 | 254 | |
| Incidence (per 1000 person-yr) | 6.0 (5.5–6.6) | 5.7 (5.0–6.4) | 6.4 (5.8–7.1) | 5.1 (4.6–5.6) | 5.0 (4.4–5.6) | |
| Model 1 | 1 (reference) | 0.92 (0.79–1.08) | 1.08 (0.94–1.24) | 0.83 (0.72–0.95) | 0.83 (0.71–0.96) | 0.003 |
| Model 2 | 1 (reference) | 0.91 (0.77–1.06) | 0.93 (0.81–1.06) | 0.77 (0.67–0.89) | 0.78 (0.67–0.89) | <0.001 |
| Model 3 | 1 (reference) | 0.91 (0.79–1.08) | 0.94 (0.82–1.07) | 0.83 (0.72–0.95) | 0.88 (0.75–1.04) | 0.02 |
AKI, acute kidney injury; ARIC, Atherosclerosis Risk In Communities; DASH, Dietary Approaches to Stop Hypertension.
Model 1: Unadjusted (no covariates).
Model 2: Variables included age, sex, race-center, education, total energy intake, physical activity, smoking, alcohol intake status, and DASH diet score.
Model 3: All Model 2 variables plus systolic blood pressure, diabetes status, use of antihypertensive medications, estimated glomerular filtration rate, and body mass index.
Data presented as hazard ratio (95% CI) unless otherwise noted.
Discussion
In ARIC, higher amounts of average daily coffee consumption were associated with a lower risk of incident AKI. Coffee consumption was highest in males, White participants, smokers, individuals without diabetes, and people with lean BMIes, normal BP, and higher total energy intake per day. When we adjusted for age, sex, race-center, education, total energy intake, physical activity, smoking, alcohol intake, diet, BP, diabetes status, use of antihypertensive medication, kidney function, and BMI, individuals who consumed any amount of daily coffee still had an 11% lower risk of developing AKI compared with individuals who had never consumed coffee. AKI risk reduction was dose-dependent, with the most substantial reductions observed in the group that consumed 2 to 3 cups/d of coffee. The underlying mechanism for this may be that either bioactive compounds and/or caffeine in coffee improve kidney oxygenation through attenuation of renal oxygen consumption while maintaining both GFR and renal plasma flow. However, mechanistic studies have yet to be completed to explore the effects of coffee consumption on intraglomerular hemodynamic function or kidney oxygen availability. Consequently, it is necessary to further investigate the possible kidney protective mechanisms of coffee consumption.
AKI represents a significant public health problem, with the incidence of 0.25% in the general population increasing to 18% in the subset of individuals who are hospitalized annually.17 AKI pathophysiology is multifaceted and often relates to a focal mismatch between impaired delivery of oxygen and nutrients to the nephrons and increased energy and oxygen demands of the kidney.18 Habitual coffee consumption has been strongly associated with nephroprotection against chronic kidney disease,5 yet the underlying physiological mechanisms for this effect remain unclear and have not previously been verified in the setting of AKI. Caffeine modifies kidney function through a variety of mechanisms, including natriuresis, modification of kidney hemodynamics, and the renin-angiotensin-aldosterone system,6,7 important factors in the development of AKI, particularly in high-risk populations with concurrent illness, injury, or comorbidities. Caffeine has also been postulated to inhibit expression of the sodium/potassium adenosine triphosphatase and isoform 3 of the sodium/proton exchanger. These proteins are integral to passive and active renal tubular sodium reabsorption and represent the largest source of oxygen consumption in the kidney, thereby possibly decreasing renal oxygen consumption without impairing renal plasma flow or GFR.19 In a large cohort of middle-aged adults, we observed that a lower AKI risk was associated with higher coffee consumption, providing further support for a beneficial effect of caffeine-containing coffee on kidney health, particularly in older individuals already at risk of age-related declines in baseline kidney function.
Other important nonrenal mechanisms have been hypothesized to explain the potential protective effects of coffee consumption, including reduced inflammation, enhanced endothelial function, and improved insulin sensitivity.20, 21, 22 Indeed, in preclinical studies, dose-dependent treatment with a 5-day course of chlorogenic acid–containing green coffee beans was reported to significantly lower serum urea, creatinine, and potassium, while increasing serum sodium, glutathione, superoxide dismutase, and catalase in rats with gentamicin-induced AKI.23 These green coffee beans may down-regulate the p53 transcription factor, thereby attenuating inflammation, oxidative stress, and apoptosis factors in the renal tubules.23 The environment in which the coffee exerts its effects on the kidneys must also be considered. In ARIC, we analyzed the incidence of AKI in the setting of concurrent hospitalization (i.e., AKI was defined by AKI-related hospitalizations), a particularly high-risk state. We postulate that coffee consumption may exhibit its most protective effects in the setting of a nephrogenic insult combining systemic inflammatory effects and hemodynamic dysregulation, resulting in a metabolic imbalance that generates significant oxidative stress within the kidney.24 In a comprehensive review of 26 human intervention studies evaluating the effects of coffee on oxidative stress, results for the effects of coffee on total plasma antioxidant capacity, antioxidant enzymes, protein damage, and lipid damage were largely mixed.25 However, coffee consistently increased systemic glutathione levels while reducing spontaneous DNA strand breaks, oxidized DNA bases, and endogenous and oxidatively induced DNA damage.26, 27, 28, 29, 30 The high variability in results to date may be explained by high levels of heterogeneity between coffee-related methods used in published clinical trials, including differences in the types of coffee studied, their caffeine and bioactive compound contents, duration of coffee exposure, and techniques for evaluating outcomes.25 Consequently, greater attention to confounding variables and standardization of coffee exposure is necessary to develop large-scale clinical intervention studies aiming to elucidate the effects of coffee on important outcomes including systemic inflammation and oxidative stress.
Strengths of ARIC include its large sample size from 4 diverse US communities, adjustment for a wide variety of possible covariates, and exclusion of individuals with baseline chronic kidney disease (i.e., baseline eGFR <60 ml/min per 1.73 m2) who may be at higher risk for developing AKI. Limitations of ARIC include the use of a food frequency questionnaire that relied on participant recollection, rather than direct measurement, to assess self-reported average daily coffee consumption. In addition, each coffee type consumed contains a variable amount of caffeine, and differences in brew-related factors such as brew time, roasting temperature, and grind size may have significant effects on the presence of bioactive compounds, such as chlorogenic acids and acid lactones, nicotinic acids, trigonelline, and theobromide,10,11 which may affect the risk for developing AKI. However, it is notable that participants were asked to quantify their consumption of regular, rather than decaffeinated coffee. Coffee additives such as milk, half-and-half, creamer, sugar, or sweeteners could also influence outcomes and warrant further investigation. In addition, consumption of other types of caffeinated beverages such as tea or soda should also be considered as a possible confounding factor. Further limitations of this study include its reliance on the inclusion of AKI on the problem list during inpatient hospitalization and the potential for confounding effects from differences in etiologies for participant hospitalization. We contend that although we obtained data for this study through rigorous standard processes and accounted for many potential confounding factors through modeling, the risk for residual confounding because of the use of imprecise measures remains.
In conclusion, in a large, prospective cohort study of 14,207 middle-aged adults, higher coffee consumption was associated with a lower risk of incident AKI. Our data support chronic coffee consumption as an opportunity for cardiorenal protection through diet, particularly for the prevention of AKI hospitalizations or procedures. Larger studies evaluating the effects of coffee consumption on kidney perfusion and oxygenation in individuals with impaired kidney function at high-risk for AKI, including the effects of coffee on anti-inflammatory and antioxidant outcomes, are necessary to fully explain its potential cardiorenal protective effects.
Disclosure
PB has reported acting as a consultant for AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Eli Lilly, LG Chem, Sanofi, Novo Nordisk, and Horizon Pharma and serving on the advisory boards of AstraZeneca, Bayer, Boehringer Ingelheim, Novo Nordisk, and XORTX. CRP has reported serving as a member of the advisory board of and owning equity in RenalytixAI and as a consultant for Genfit and Novartis.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions. The ARIC study has been funded in whole or in part with federal funds from the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (NIH), Department of Health and Human Services, under contract numbers (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700004I, HHSN268201700005I). KLT receives salary and research support from the NIH/NHLBI (K23 HL159292), Children’s Hospital Colorado Research Institute Research Scholar Award, University of Colorado Diabetes Research Center (P30 DK116073), Ludeman Family Center for Women’s Health Research at the University of Colorado, ISPAD-JDRF Research Fellowship, and the Department of Pediatrics, Section of Endocrinology at the University of Colorado School of Medicine. CMR was supported by grants from the NIH/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (K01 DK107782, R03 DK128386) and the NIH/NHLBI (R01 HL153178). ES was supported by the NIH/NHLBI (K24 HL152440). PB receives salary and research support from NIDDK (R01 DK129211, R21 DK129720, K23 DK116720, UC DK114886, and P30 DK116073), JDRF (2-SRA-2019-845-S-B, 3-SRA-2017-424-M-B, 3-SRA-2022-1097-M-B), Boettcher Foundation, American Heart Association (20IPA35260142), Ludeman Family Center for Women’s Health Research at the University of Colorado, the Department of Pediatrics, Section of Endocrinology and Barbara Davis Center for Diabetes at University of Colorado School of Medicine. CRP is supported by NIH/NHLBI (R01 HL085757) and NIH/NIDDK (UH3 DK114866, U01 DK106962, R01 DK093770). The funders had no role in the study design; collection, analysis, and interpretation of these data; writing the report; or the decision to submit the report. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the US government. Trial Registration:
ClinicalTrials.govNCT00005131 (ARIC).
Author Contributions
KLT analyzed and interpreted data and wrote the manuscript. EAH, ES, LMS, JC, and MEG contributed to the development of the research idea and study design. EAH analyzed and interpreted the data. PB and CMR analyzed and interpreted the data and contributed to the discussion. CRP designed the study, analyzed and interpreted the data, and contributed to the discussion. All c-oauthors reviewed and edited the manuscript. CRP and CMR are the guarantor of this work and have full access to the data sets and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
STROBE Statement.
Supplementary Material
STROBE Statement.
References
- 1.Ludwig I.A., Clifford M.N., Lean M.E., Ashihara H., Crozier A. Coffee: biochemistry and potential impact on health. Food Funct. 2014;5:1695–1717. doi: 10.1039/c4fo00042k. [DOI] [PubMed] [Google Scholar]
- 2.van Dieren S., Uiterwaal C.S., van der Schouw Y.T., et al. Coffee and tea consumption and risk of type 2 diabetes. Diabetologia. 2009;52:2561–2569. doi: 10.1007/s00125-009-1516-3. [DOI] [PubMed] [Google Scholar]
- 3.van Dam R.M. Coffee consumption and risk of type 2 diabetes, cardiovascular diseases, and cancer. Appl Physiol Nutr Metab. 2008;33:1269–1283. doi: 10.1139/H08-120. [DOI] [PubMed] [Google Scholar]
- 4.Hu E.A., Lazo M., Selvin E., et al. Coffee consumption and liver-related hospitalizations and deaths in the ARIC study. Eur J Clin Nutr. 2019;73:1133–1140. doi: 10.1038/s41430-018-0346-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu E.A., Selvin E., Grams M.E., Steffen L.M., Coresh J., Rebholz C.M. Coffee consumption and incident kidney disease: results from the atherosclerosis risk in communities (ARIC) study. Am J Kidney Dis. 2018;72:214–222. doi: 10.1053/j.ajkd.2018.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown N.J., Ryder D., Nadeau J. Caffeine attenuates the renal vascular response to angiotensin II infusion. Hypertension. 1993;22:847–852. doi: 10.1161/01.hyp.22.6.847. [DOI] [PubMed] [Google Scholar]
- 7.Passmore A.P., Kondowe G.B., Johnston G.D. Renal and cardiovascular effects of caffeine: a dose-response study. Clin Sci (Lond) 1987;72:749–756. doi: 10.1042/cs0720749. [DOI] [PubMed] [Google Scholar]
- 8.Shirley D.G., Walter S.J., Noormohamed F.H. Natriuretic effect of caffeine: assessment of segmental sodium reabsorption in humans. Clin Sci (Lond) 2002;103:461–466. doi: 10.1042/cs1030461. [DOI] [PubMed] [Google Scholar]
- 9.Marx B., Scuvée É., Scuvée-Moreau J., Seutin V., Jouret F. [Mechanisms of caffeine-induced diuresis] Med Sci (Paris) 2016;32:485–490. doi: 10.1051/medsci/20163205015. [DOI] [PubMed] [Google Scholar]
- 10.Rodrigues N.P., Bragagnolo N. Identification and quantification of bioactive compounds in coffee brews by HPLC-DAD-MSn. J Food Compos Anal. 2013;32:105–115. doi: 10.1016/j.jfca.2013.09.002. [DOI] [Google Scholar]
- 11.Fuller M., Rao N.Z. The effect of time, roasting temperature, and grind size on caffeine and chlorogenic acid concentrations in cold brew coffee. Sci Rep. 2017;7:17979. doi: 10.1038/s41598-017-18247-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iglesias-Aguirre C.E., Cortes-Martin A., Avila-Galvez M.A., et al. Main drivers of (poly) phenol effects on human health: metabolite production and/or gut microbiota-associated metabotypes? Food Funct. 2021;12:10324–10355. doi: 10.1039/d1fo02033a. [DOI] [PubMed] [Google Scholar]
- 13.Rabb H., Griffin M.D., McKay D.B., et al. Inflammation in AKI: current understanding, key questions, and knowledge gaps. J Am Soc Nephrol. 2016;27:371–379. doi: 10.1681/ASN.2015030261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sureshbabu A., Ryter S.W., Choi M.E. Oxidative stress and autophagy: crucial modulators of kidney injury. Redox Biol. 2015;4:208–214. doi: 10.1016/j.redox.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wright J.D., Folsom A.R., Coresh J., et al. The ARIC (Atherosclerosis Risk In Communities) Study: JACC Focus Seminar 3/8. J Am Coll Cardiol. 2021;77:2939–2959. doi: 10.1016/j.jacc.2021.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rebholz C.M., Crews D.C., Grams M.E., et al. DASH (Dietary Approaches to Stop Hypertension) diet and risk of subsequent kidney disease. Am J Kidney Dis. 2016;68:853–861. doi: 10.1053/j.ajkd.2016.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palevsky P.M., Liu K.D., Brophy P.D., et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis. 2013;61:649–672. doi: 10.1053/j.ajkd.2013.02.349. [DOI] [PubMed] [Google Scholar]
- 18.Tögel F., Westenfelder C. Recent advances in the understanding of acute kidney injury. F1000Prime Rep. 2014;6:83. doi: 10.12703/P6-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J., Ha J.H., Kim S., Oh Y., Kim S.W. Caffeine decreases the expression of Na+/K+-ATPase and the type 3 Na+/H+ exchanger in rat kidney. Clin Exp Pharmacol Physiol. 2002;29:559–563. doi: 10.1046/j.1440-1681.2002.03697.x. [DOI] [PubMed] [Google Scholar]
- 20.Loftfield E., Shiels M.S., Graubard B.I., et al. Associations of coffee drinking with systemic immune and inflammatory markers. Cancer Epidemiol Biomarkers Prev. 2015;24:1052–1060. doi: 10.1158/1055-9965.EPI-15-0038-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobs S., Kroger J., Floegel A., et al. Evaluation of various biomarkers as potential mediators of the association between coffee consumption and incident type 2 diabetes in the EPIC-Potsdam Study. Am J Clin Nutr. 2014;100:891–900. doi: 10.3945/ajcn.113.080317. [DOI] [PubMed] [Google Scholar]
- 22.Lopez-Garcia E., van Dam R.M., Qi L., Hu F.B. Coffee consumption and markers of inflammation and endothelial dysfunction in healthy and diabetic women. Am J Clin Nutr. 2006;84:888–893. doi: 10.1093/ajcn/84.4.888. [DOI] [PubMed] [Google Scholar]
- 23.Bhattacharyya S., Kumar R., Sengupta G., Hazra A.K., Sur T.K. Chlorogenic acid enriched green coffee ameliorated renal injury in rats. Mymensingh Med J. 2020;29:991–1000. [PubMed] [Google Scholar]
- 24.Pavlakou P., Liakopoulos V., Eleftheriadis T., Mitsis M., Dounousi E. Oxidative stress and acute kidney injury in critical illness: pathophysiologic mechanisms-biomarkers-interventions, and future perspectives. Oxid Med Cell Longev. 2017;2017:6193694. doi: 10.1155/2017/6193694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martini D., Del Bo' C., Tassotti M., et al. Coffee consumption and oxidative stress: a review of human intervention studies. Molecules. 2016;21:979. doi: 10.3390/molecules21080979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bakuradze T., Lang R., Hofmann T., et al. Consumption of a dark roast coffee decreases the level of spontaneous DNA strand breaks: a randomized controlled trial. Eur J Nutr. 2015;54:149–156. doi: 10.1007/s00394-014-0696-x. [DOI] [PubMed] [Google Scholar]
- 27.Bakuradze T., Lang R., Hofmann T., et al. Coffee consumption rapidly reduces background DNA strand breaks in healthy humans: results of a short-term repeated uptake intervention study. Mol Nutr Food Res. 2016;60:682–686. doi: 10.1002/mnfr.201500668. [DOI] [PubMed] [Google Scholar]
- 28.Mišík M., Hoelzl C., Wagner K.H., et al. Impact of paper filtered coffee on oxidative DNA-damage: results of a clinical trial. Mutat Res. 2010;692:42–48. doi: 10.1016/j.mrfmmm.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Hoelzl C., Knasmüller S., Wagner K.H., et al. Instant coffee with high chlorogenic acid levels protects humans against oxidative damage of macromolecules. Mol Nutr Food Res. 2010;54:1722–1733. doi: 10.1002/mnfr.201000048. [DOI] [PubMed] [Google Scholar]
- 30.Bichler J., Cavin C., Simic T., et al. Coffee consumption protects human lymphocytes against oxidative and 3-amino-1-methyl-5H-pyrido[4,3-b]indole acetate (Trp-P-2) induced DNA-damage: results of an experimental study with human volunteers. Food Chem Toxicol. 2007;45:1428–1436. doi: 10.1016/j.fct.2007.02.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.