Abstract
SGLT2 inhibitors have emerged as a key disease-modifying therapy to prevent the progression of chronic kidney disease (CKD). These agents prevent decline in kidney function through reduction in glomerular hypertension mediated through tubuloglomerular feedback independent of their effect on glycemic control. The proliferation of clinical trials on SGLT2 inhibitors has rapidly expanded the approved clinical indications for these agents beyond patients with diabetes mellitus (DM). We review the current indications for SGLT2 inhibitors in patients with and without diabetic kidney disease, including new evidence for use in patients with heart failure with or without reduced ejection fraction, stage 4 CKD, and chronic glomerulonephritis. The EMPA-KIDNEY trial was recently stopped early for efficacy suggesting that SGLT2 inhibitors may soon be indicated for patients with CKD without albuminuria. We review practical considerations for prescription of SGLT2 inhibitors, including the anticipated acute decline in estimated glomerular filtration rate (eGFR) on initiation, initiating the lowest dosage used in clinical trials, volume status considerations, and adverse event mitigation. Combination therapy in patients with DM may be considered with agents, including glucagon-like peptide-1 receptor agonists (GLP-1-RAs), novel mineralocorticoid receptor antagonists, and selective endothelin receptor antagonists to reduce residual albuminuria and cardiovascular risk.
Keywords: chronic kidney disease, diabetes, diabetic kidney disease, glomerulonephritis, heart failure, SGLT2 inhibitors
In 2008, the US Food and Drug Administration mandated that new glucose-lowering therapies undergo cardiovascular outcome trials (CVOTs).1 This led to the approval of SGLT2 inhibitors, of which 4—canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin—are available in North America, whereas sotagliflozin, a dual SGLT1 and SGLT2 inhibitor, is approved in Europe, and other specific agents are available in Japan.
SGLT2 inhibitors have revolutionized the treatment of patients with type 2 DM (T2DM) with established or at risk for atherosclerotic cardiovascular disease (ASCVD) and patients with diabetic kidney disease.2, 3, 4, 5 The beneficial effects from SGLT2 inhibitors are apparent shortly after drug initiation suggesting mechanisms independent of glycemic control.6 On the basis of emerging evidence, SGLT2 inhibitors are now transforming the management of heart failure and CKD in patients with and without T2DM.7, 8, 9 Despite these proven clinical benefits, the mechanisms of benefit from SGLT2 inhibitors have not been fully elucidated. Nephrologists now play a key role in prescribing SGLT2 inhibitors as nephroprotective agents in our effort to reduce the global burden of kidney disease. We will provide an overview of SGLT2 inhibitors, their current indications and practical considerations in prescribing these agents.
Systemic Effects and Mechanisms of Action
SGLT2 inhibitors have been found to reduce hemoglobin A1c (HbA1c) by 0.6% to 1% in patients with T2DM and preserved renal function.10,11 This effect is primarily mediated by glucosuria resulting from blockade of the SGLT2 channel predominantly localized to the S1 segment of the proximal convoluted tubule, which is responsible for >90% absorption of filtered glucose.12 The resulting glucosuria can exceed 100 g/d in individuals with T2DM and 50 to 60 g/d in nondiabetics. The glucose-lowering effect of SGLT2 inhibitors is attenuated in patients with eGFR <60 ml/min per 1.73 m2 and minimal when eGFR is <30 ml/min per 1.73 m2.13 Caloric loss from glucosuria typically results in 1 to 3 kg weight loss,14 most of which is fat,15,16 and greater weight loss is observed in patients with higher baseline HbA1c.17
Mechanisms of Action for End-Organ Protection
SGLT2 inhibitors are associated with a sustained modest reduction in systolic blood pressure (BP) of approximately 3 to 6 mm Hg and diastolic BP of approximately 1 to 2 mm Hg.18,19 BP lowering is mediated through natriuresis and associated plasma volume contraction,20 reduction in arterial stiffness,21 and improvement in endothelial function.22 Reduction in BP is generally observed irrespective of hypertension status23 and is also achieved in patients with lower eGFR level.24
In patients with diabetes, decreased sodium delivery to the macula densa results in increased proximal tubular sodium reabsorption and afferent arteriolar vasodilatation by tubuloglomerular feedback leading to glomerular hypertension and hyperfiltration.25 The primary mechanism by which SGLT2 inhibitors are thought to be nephroprotective is through increasing distal sodium delivery and inhibiting tubuloglomerular feedback resulting in afferent vasoconstriction and reduction in intraglomerular pressure.26,27 A marker of this reduction in intraglomerular pressure is the reduction in albuminuria, which is largely independent of concomitant changes in metabolic parameters or eGFR.28
Other putative mechanisms through which SGLT2 inhibitors may be beneficial include reduction in inflammatory mediators, including interleukin-6, nuclear factor-kB, and profibrotic factors, such as transforming growth factor-ß.24,29 In addition, by conserving energy required to reabsorb the filtered load of glucose and associated sodium, SGLT2 inhibition may attenuate renal hypoxia and is simultaneously associated with a rise in hematocrit level.24,30,31
SGLT2 Inhibitors and Potassium
Hyperkalemia is a frequent clinical challenge in the care of patients with CKD and may prohibit uptitration of renin-angiotensin-aldosterone system (RAAS) inhibitor blockade. SGLT2 inhibitors may enhance kaliuresis by increasing distal delivery of sodium and stimulating aldosterone.25 In CREDENCE, which included patients with T2DM and CKD on RAAS blockade, canagliflozin reduced the incidence of hyperkalemia (K ≥ 6.0) by 23% without causing hypokalemia (<3.5 mmol/l) and the need for new potassium binder usage in those treated with canagliflozin by 22%.32
Dual SGLT1 and SGLT2 Inhibitors
Sotagliflozin is the first dual SGLT1 and SGLT2 inhibitor and is approved in Europe for both type 1 DM and T2DM. It has been postulated that SGLT1 inhibition delays intestinal glucose absorption and reduces postprandial glucose levels.33, 34, 35 Furthermore, SGLT1 contributes to distal proximal tubular glucose reabsorption following SGLT2 inhibition when tubular glucose concentrations are increased, which may result in additional glucosuric effects in patients with more advanced CKD.36 In the SCORED trial, 10,584 patients with T2DM, eGFR 25 to 60 ml/min per 1.73 m2 with or without albuminuria were enrolled. However, this trial ended early at 16 months because of loss of funding. The primary end point (cardiovascular death, heart failure hospitalizations, and urgent heart failure visits) was reduced by 26% with sotagliflozin despite the relatively short trial duration (hazard ratio [HR] 0.74, 95% CI 0.63–0.88).37 In the SOLOIST-WHF trial, initiation of sotagliflozin before or shortly following discharge reduced cardiovascular hospitalization or death and urgent heart failure visits.35 SGLT1 inhibition may result in increased rates of diarrhea, and the additional benefit of SGLT1 blockade to SGLT2 inhibition is not yet fully understood, although sotagliflozin does reduce hyperglycemia even in patients with CKD stage 4.38
Current Indications for SGLT2 Inhibitors
Indications for SGLT2 inhibitors have expanded based on growing evidence from randomized controlled trials and fall broadly into the following 5 categories: glycemic control/metabolic risk, reduction in ASCVD, heart failure, diabetic kidney disease with albuminuria, nondiabetic CKD with albuminuria (Table 1).
Table 1.
Current indications for SGLT2 inhibitors
| Indication | Criteria | Kidney function |
|---|---|---|
| Congestive heart failure |
|
|
| Glycemic control or metabolic risk |
|
|
| ||
| ||
| Reduction in ASCVD |
|
|
| Diabetic kidney disease |
|
|
| Nondiabetic kidney disease |
|
|
ASCVD, atherosclerotic cardiovascular disease; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate (CKD-EPI); FSGS, focal segmental glomerulosclerosis; HbA1c, hemoglobin A1c; LDL, low-density lipoprotein; NT-proBNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association; UACR, urine microalbumin-to-creatinine ratio.
Atherosclerotic cardiovascular disease is defined as ischemic heart disease, ischemic cerebrovascular disease, or peripheral artery disease. High risk for atherosclerotic cardiovascular disease is defined as age ≥55 years in men and ≥60 years in women and one or more of the following risk factors: hypertension, dyslipidemia (LDL >130 mg/dl or use of lipid-lowering therapies), or tobacco use.
The EMPA-KIDNEY trial was stopped early for efficacy and included patients with diabetic kidney disease and nondiabetic kidney disease with eGFR 20 to 45 ml/min per 1.73 m2 regardless of UACR or eGFR 45 to 90 ml/min per 1.73 m2 with UACR ≥200 mg/g; however, results have not yet been presented or published.
Kidney Outcomes From CVOTs
CVOTs including EMPA-REG OUTCOME, CANVAS, DECLARE-TIMI-58, VERTIS CV, and SCORED revealed the benefit of SGLT2 inhibitors in improving cardiovascular outcomes in patients with T2DM with varying risks for ASCVD.2, 3, 4, 5,37 On the basis of the results of CVOTs, the Kidney Disease: Improving Global Outcomes 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease now supports SGLT2 inhibitors or metformin as first-line treatment for T2DM for glycemic control.39,40
Secondary analysis of renal outcomes from CVOTs was the first to suggest potential benefit in patients with kidney disease. In EMPA-REG OUTCOME which included 7020 patients with T2DM with established ASCVD and enrolled patients with eGFR ≥30 ml/min per 1.73 m2, the renal composite outcome of end-stage kidney disease (ESKD) and doubling of serum creatinine was lower with empagliflozin (HR 0.54, 95% CI 0.40–0.75), with a reduction in ESKD (HR 0.45, 95% CI 0.21–0.97) and doubling in serum creatinine (HR 0.56, 95% CI 0.39–0.79).4 As a consequence of the reduction in intraglomerular hypertension and other protective pathways discussed previously, albuminuria decreases by 30% to 50% regardless of baseline albuminuria within the span of weeks in response to SGLT2 inhibition. For example, in EMPA-REG OUTCOME, patients taking empagliflozin had a reduction in albuminuria of 7% in those with normoalbuminuria, 25% with microalbuminuria, and 32% with macroalbuminuria, which was sustained when measured at follow-up at 164 weeks.28 On stopping these agents, albuminuria increases within weeks suggesting a contribution from underlying hemodynamic mechanisms.
In the CANVAS Program, which enrolled patients with T2DM with high cardiovascular risk and eGFR >30 ml/min per 1.73 m2, the renal composite outcome was also lower with canagliflozin (HR 0.53, 95% CI 0.33–0.84).3 DECLARE-TIMI 58, which only included patients with T2DM with established or multiple risk factors for ASCVD and eGFR >60 ml/min per 1.73 m2, similarly favored SGLT2 inhibitor use, which reduced the composite renal outcome of sustained eGFR decline of ≥40%, ESKD, or renal death (HR 0.53, 95% CI 0.43–0.66).2 Despite the positive outcomes, CVOTs were not powered for kidney-related outcomes and patients with CKD comprised <30% of the study cohorts but informed subsequent dedicated trials for patients with kidney disease.41 In VERTIS CV, ertugliflozin was associated with preservation of eGFR decline by >0.75 ml/min per 1.73 m2 per year with greater benefit in reducing heart failure hospitalizations in those with more advanced CKD.5,42, 43, 44 In SCORED, which included patients with CKD with eGFR 25 to 60 ml/min per 1.73 m2, a secondary kidney end point was not significantly different between sotagliflozin and placebo (HR 0.71, 95% CI 0.46–1.08), although the trial was terminated early and likely not of a sufficient duration to detect these differences in composite end points.37
CKD Trials
CREDENCE and DAPA-CKD are seminal randomized controlled trials which specifically evaluated the effect of SGLT2 inhibitors on a primary kidney end point and ultimately provide the strongest evidence for use in patients with CKD (Table 2). CREDENCE included 4401 patients aged ≥30 years with T2DM with albuminuria (microalbumin-to-creatinine ratio [ACR] 300–5000 mg/g), eGFR 30 to 90 ml/min per 1.73 m2, HbA1c 6.5% to 12%, on maximal tolerated RAAS blockade. The trial was stopped early after a median follow-up of 2.62 years because of benefit found in the interim analysis. The primary composite of doubling of creatinine, ESKD, and death from renal or cardiovascular causes was reduced by 30% with canagliflozin. Benefit was consistent across renal end points with lower risk of doubling serum creatinine (HR 0.60, 95% CI 0.48–0.76) and ESKD (HR 0.68, 95% CI 0.54–0.86). Decline in eGFR was lower in the canagliflozin group (−3.19 ml/min per 1.73 m2 per year) in comparison to −4.71 ml/min per 1.73 m2 per year in the placebo group. This finding was observed despite only modest changes in blood glucose, weight, and BP. Extrapolating between-group differences in eGFR loss to the average 63-year-old patient from CREDENCE with an eGFR of 56 ml/min per 1.73 m2 would result in a delay in progression to ESKD by as much as 15 years.45
Table 2.
Characteristics of participants in SGLT2 inhibitor trials that specifically recruited patients with CKD
| Characteristics | CREDENCE46 | DAPA-CKD8 | SCORED37 | EMPA-KIDNEY58 |
|---|---|---|---|---|
| Number of participants | 4401 | 4304 | 10584 | 6609 |
| Mean age (yr) | 63 | 61.8 | 69 | 63.8 |
| Female (%) | 1494 (33.9) | 1425 (33.1) | 4754 (44.9) | 2192 (33) |
| UACR (mg/g) Median (IQR) |
927 (463–1833) | 949.3 | 74 (17–481) | 412 (94–1190) |
| eGFR (ml/min per 1.73 m2) mean (SD) |
56.2 (18.2) | 43.1 (12.4) | 44.5 | 37.5 (14.8) |
| eGFR categories (%) | ||||
| ≥45 ml/min per 1.73 m2 | 3035 (69) | 1782 (41.4) | 5116 (48.3) | 1424 (22) |
| ≥30–44 ml/min per 1.73 m2 | 1191 (27.1) | 1898 (44.1) | 4655 (43.9) | 2905 (44) |
| <30 ml/min per 1.73 m2 | 174 (3.9) | 624 (14.5) | 813 (7.8) | 2280 (34) |
| Prior DM (%) | 4401 (100) | 2888 (67.1) | 10,584 (100) | 3039 (46) |
| Baseline RAAS inhibitor (%) | 4395 (99) | 4224 (98.1) | 9365 (88.5) | 5613 (84.9) |
| Primary kidney disease (%) | ||||
| Diabetic kidney disease | 4401 (100) | 2510 (58.3) | 10,584 (100) | 2057 (31) |
| Ischemic/hypertensive nephropathy | 687 (16) | 1445 (22) | ||
| Glomerular disease | 695 (16.1) | 1669 (25) | ||
| IgA nephropathy | 270 (6.3) | 817 (12) | ||
| Focal segmental glomerulosclerosis | 115 (2.7) | 195 (3.0) | ||
| Membranous nephropathy | 43 (1.0) | 96 (1.0) | ||
| Minimal change disease | 11 (0.3) | 14 (<1) | ||
| Other glomerular disease | 256 (5.9) | 547 (8.0) | ||
| Unknown | 214 (5) | 630 (10) | ||
| Other | 198 (4.6) | 808 (12) | ||
CKD, chronic kidney disease; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate (CKD-EPI); IQR, interquartile range; RAAS, renin-angiotensin-aldosterone inhibitor; UACR, urine microalbumin-to-creatinine ratio.
DAPA-CKD enrolled 4304 adults with both diabetic and nondiabetic kidney diseases with eGFR 25 to 75 ml/min per 1.73 m2, ACR 200 to 5000 mg/g on maximal tolerated RAAS blockade and followed participants for a median of 2.4 years.8 Dapagliflozin reduced the primary composite outcome of sustained decline in the estimated GFR by >50%, ESKD, and renal or cardiovascular death by 39% with a number need to treat of 19. Importantly, the effects of dapagliflozin were similar in patients with T2DM (HR 0.64, 95% CI 0.52–0.79) or without T2DM (HR 0.50, 95% CI 0.35–0.72). All individual components of the renal end point had benefit with the risk of ESKD reduced by 36% and 50% eGFR decline reduced by 47%. The risk for hospitalization for heart failure or cardiovascular was reduced by 29% similar to previous CVOTs. The participants had a mean baseline eGFR of 43 ml/min per 1.73 m2, and the slope of eGFR decline from baseline to 30 months was −2.86 ml/min per 1.73 m2 for dapagliflozin and −3.79 ml/min per 1.73 m2 per year for placebo, resulting in a between-group difference of 0.93 ml/min per 1.73 m2 per year (95% CI 0.61–1.25). Both CREDENCE and DAPA-CKD represent a strong win for the field of nephrology, collectively revealing impressive benefit of SGLT2 inhibitors on hard renal end points in patients with CKD with albuminuria regardless of diabetes status.
From a safety perspective, similar to CVOTs, a higher incidence of genital mycotic infection (GMI) noted was also observed, although reassuringly, no increase in serious volume depletion, hypotension, or hypoglycemia was observed in CREDENCE or DAPA-CKD.8,46 In CREDENCE, the incidence of diabetic ketoacidosis (DKA) was higher with canagliflozin treatment, although absolute rates were low (2.2 vs. 0.2 per 1000 patient-years). In DAPA-CKD, no cases of DKA were reported with dapagliflozin treatment; however, all patients should receive counseling regarding “sick day” medication management upon SGLT2 inhibitor prescription.
SGLT2 Inhibitors in Heart Failure
Subsequent to the consistent benefits in heart failure hospitalization risk reported in CVOTs,2, 3, 4 in patients with established heart failure with reduced left ventricular ejection fraction (≤40%), a reduction in the composite of heart failure hospitalizations or cardiovascular death was observed in both DAPA-HF and EMPEROR-Reduced, independent of diabetes status.7,47 The DAPA-HF trial compared dapagliflozin with placebo in adults with heart failure with left ventricular ejection fraction ≤40%, elevated N-terminal pro-brain natriuretic peptide, and eGFR ≥30 ml/min per 1.73 m2 on a background standard of care. The primary composite outcome of cardiovascular death, heart failure hospitalization, or urgent heart failure visit was reduced by 26%. In EMPEROR-Reduced, which included adults with chronic heart failure left ventricular ejection fraction ≤40%, New York Heart Association II to IV, elevated N-terminal pro-brain natriuretic peptide, on appropriate heart failure therapy with eGFR >20 ml/min per 1.73 m2, dapagliflozin reduced the primary composite outcome by 25%. The composite kidney outcome (≥50% sustained decline in eGFR, ESKD, and renal death) was reduced with dapagliflozin (HR 0.71, 95% CI 0.44–1.16). Annual change in eGFR was −3.10 ml/min per 1.73 m2 in the placebo group versus −2.05 ml/min per 1.73 m2 per year in the dapagliflozin group (95% CI −2.36 to −1.75) with no difference by diabetes status.
Historically, therapies have been lacking in patients with heart failure with preserved ejection fraction (>40%). However, in the EMPEROR-Preserved trial, which enrolled patients with left ventricular ejection fraction >40% and included patients with eGFR >20 ml/min per 1.73 m2, empagliflozin reduced the combined risk of cardiovascular death or hospitalization for heart failure (HR 0.79, 95% CI 0.69–0.90) regardless of the presence or absence of diabetes.9 In EMPEROR-Preserved, 50% of participants had eGFR <60 ml/min per 1.73 m2 and had similar benefit for the primary outcome (HR 0.78, 95% CI 0.66–0.91). Similarly, consistent benefit on heart failure end points was observed in subgroup analysis of patients with eGFR <60 ml/min per 1.73 m2 in DAPA-HF and EMPEROR-Reduced.48 Together, these trials provide the evidence to safely support SGLT2 inhibitor use in all patients with heart failure irrespective of ejection fraction, down to an eGFR of 20 ml/min per 1.73 m2. The recently completed EMPULSE trial revealed clinical benefit with in-hospital empagliflozin treatment among patients admitted with acute heart failure regardless of left ventricular ejection fraction. In this trial, empagliflozin was well tolerated with renal failure occurring in 7.7% in those receiving empagliflozin in comparison to 12.1% with placebo.49
Real-World Effectiveness Studies
Real-world effectiveness studies have confirmed that the benefits of SGLT2 inhibitors extend to routine clinical practice. CVD-REAL 3, a multinational observational cohort study, assessed kidney outcomes in 35,561 patients initiating SGLT2 inhibitors propensity matched to other glucose-lowering agents and found that SGLT2 inhibitors reduced eGFR decline by 1.53 ml/min per 1.73 m2 per year (95% CI 1.34–1.72). Similar to randomized controlled trials, the composite outcome of 50% decline in eGFR or progression to ESKD was lower with SGLT2 inhibitors.50, 51, 52 From a safety perspective, similar to results of clinical trials,53 in real-world evidence studies, SGLT2 inhibitors reduce the risk of acute kidney injury compared with other glucose-lowering agents,54 potentially as a result of improved kidney oxygenation and kidney perfusion.55
Stage 4 CKD
The most robust evidence for use of SGLT2 inhibitors in stage 4 CKD is a prespecified analysis of DAPA-CKD in 624 of 4304 patients (14%) with baseline eGFR 25 to 30 ml/min per 1.73 m2. Consistent with results from the overall trial, a 27% reduction in the primary composite end point (50% sustained decline in eGFR, ESKD, or kidney/cardiovascular death) was observed. Dapagliflozin resulted in a 28% reduction in the risk for ESKD, with an eGFR slope decline of 2.15 ml/min per 1.73 m2 in the dapagliflozin group in comparison to 3.38 ml/min per 1.73 m2 in the placebo group with separation of the eGFR curves evident by 16 months. No difference in adverse events, including renal related or volume depletion, was noted. Furthermore, no significant heterogeneity by diabetes status or albuminuria was observed. Although evidence for kidney-related end points remains limited for patients with eGFR <25 ml/min per 1.73 m2, it should be emphasized that SGLT2 inhibitors may be continued until patients are on dialysis.
Patients With CKD Without Albuminuria
Meta-analysis of CVOTs has revealed that the benefits of SGLT2 inhibitors on delaying CKD progression are consistent regardless of baseline albuminuria.2, 3, 4,46,56 To definitively determine benefits in patients with low eGFR and low urine ACR (UACR), the EMPA-KIDNEY trial included adults with or without diabetes with eGFR 20 to 45 regardless of albuminuria or eGFR 45 to 90 ml/min per 1.73 m2 with UACR ≥ 200 mg/g on maximally tolerated RAAS blockade.57 This study enrolled 6609 patients with a mean eGFR of 37.5 ml/min per 1.73 m2. Notably, this cohort includes patients with glomerular disease (n = 1669) and hypertensive/renovascular disease (n = 1444).58 The primary outcome of this trial was a sustained ≥40% decline in eGFR, ESKD, or death from renal or cardiovascular causes. The EMPA-KIDNEY trial was stopped early in March 2022 for efficacy suggesting that CKD patients without albuminuria also benefit from SGLT2 inhibitors and will soon markedly expand the population eligible for therapy.59
SGLT2 Inhibitors in Glomerulonephritis
Although patients with glomerulonephritis most often require immunosuppressive therapy, those who develop CKD secondary to chronic damage or scarring may share a common final pathway mediated by hyperfiltration, which may be amenable to SGLT2 inhibition. In the TRANSLATE study, short-term treatment with dapagliflozin did not significantly alter renal hemodynamics or reduce proteinuria in 10 patients with focal segmental glomerulosclerosis (FSGS).60 Similarly, the DIAMOND trial first evaluated this hypothesis in patients without diabetes CKD with eGFR > 25 ml/min per 1.73 m2 and 500 to 3500 mg/d proteinuria, including patients with IgA nephropathy (n = 25) and FSGS (n = 11).61 Dapagliflozin was associated with an acute dip in eGFR on initiation suggestive of a beneficial hemodynamic effect, but it did not result in a significant reduction in proteinuria compared with placebo in a 6-week treatment period, and the 17% reduction in UACR also did not reach significance.61
DAPA-CKD was the largest trial studying use of SGLT2 inhibitors in patients with chronic glomerulonephritis (n = 695) to date, although patients with a history of immunosuppression in the prior 6 months were excluded.8,62 DAPA-CKD included 270 participants with IgA nephropathy, of whom 254 (94%) had pathologic confirmation by kidney biopsy. The mean eGFR of participants was 43.8 ml/min per 1.73 m2 with a median ACR 900 mg/g, who were followed for a median of 2.1 years. In a prespecified analysis of IgA nephropathy participants, the primary composite kidney outcome was lower for patients with dapagliflozin (HR 0.29, 95% CI 0.12–0.73) with a mean annual rate of eGFR decline of 3.5 ml/min per 1.73 m2 with dapagliflozin and 4.7 ml/min per 1.73 m2 with placebo. Furthermore, dapagliflozin resulted in a 26% reduction in albuminuria in comparison to placebo. Interestingly, the primary outcome event rate was more than double in the placebo group (24% at 32 months), compared with what would have been predicted for the average DAPA-CKD patient using the international IgA nephropathy risk prediction tool, suggesting a high-risk group of participants. Nevertheless, the overall findings were supportive of SGLT2 inhibitor use in IgA nephropathy.63,64
For FSGS, a prespecified analysis of DAPA-CKD included 115 individuals with FSGS, of which 105 (90%) were biopsy proven.65 The primary composite kidney outcome did not reach statistical significance (HR 0.62, 95% CI 0.17–2.17). However, participants treated with dapagliflozin had 26.1% reduction in albuminuria compared with 9.9% in placebo which persisted after a year. Furthermore, the annual mean rate of eGFR decline was lower in those receiving dapagliflozin (−1.9 ml/min per 1.73 m2, 95% CI −3.0 to −0.9) in comparison to placebo (−4.0 ml/min per 1.73 m2, 95% CI −4.9 to −3.0).
Reduction in albuminuria has been used as a useful surrogate marker in clinical trials for FSGS, and although the primary outcome in DAPA-CKD was not significant in participants with this condition, attenuation of eGFR decline with dapagliflozin supports long-term benefit in patients with FSGS. For example, by modifying the eGFR slope, a hypothetical DAPA-CKD patient with a mean baseline eGFR of 43 ml/min per 1.73 m2 would have an 8-year delay in reaching eGFR of 10 ml/min per 1.73 m2. It may also be relevant that FSGS is a heterogeneous disease entity, and the exclusion of recent immunosuppression suggests that most patients with FSGS in DAPA-CKD may have had secondary etiologies. In patients with both IgA nephropathy and FSGS, SGLT2 inhibitors were well tolerated with no cases of major hypoglycemia or DKA in those receiving dapagliflozin.
In a prespecified analysis of DAPA-CKD, patients with T2DM had a 35.1% reduction in UACR in comparison to 14.8% in nondiabetics suggesting attenuated reduction in intraglomerular hypertension in nondiabetics. However, dapagliflozin had similar effects on kidney outcomes regardless of DM status suggesting kidney protective effects of SGLT2 inhibitors in nondiabetic patients may be partially mediated by mechanisms beyond inhibiting tubuloglomerular feedback, such as reduction in tubular workload, increased autophagy, and anti-inflammatory or antifibrotic effects.66
In both DIAMOND and DAPA-CKD,8,61 patients were required to be on the maximal tolerated dose of RAAS blockade. Given the positive findings from DAPA-CKD in patients with chronic glomerulonephritis, in patients with IgA nephropathy and FSGS, SGLT2 inhibitors should be considered as a component of “conservative care” for those with proteinuria. However, it should be emphasized that despite the benefit of SGLT2 inhibitors in IgA nephropathy and FSGS, these therapies should not be used in lieu of immunosuppression when clinically indicated. Heightened vigilance for infectious complications should also be considered in those receiving immunosuppression given that those receiving immunosuppression were excluded from clinical trials. Patients with lupus nephritis and antineutrophil cytoplasmic antibody vasculitis have also not been studied to date because of the relapsing and remitting nature of the disease processes, but future use may consider whether there is role for SGLT2 inhibitors in those who have achieved remission and are considered to have stable or inactive disease.67
Adverse Effects and Mitigation
Although SGLT2 inhibitors are generally well tolerated by most patients, clinicians should take potential adverse effects into consideration when prescribing these agents. Patients should be counseled regarding potential adverse events, as the risk may be reduced when appropriate mitigation strategies are followed.
Infectious Complications
Owing to glucosuria, SGLT2 inhibitors are associated with a 2- to 4-fold increased risk for GMIs.68, 69, 70 Furthermore, the increased risk of GMIs versus placebo was similar across all SGLT2 inhibitors.71 A retrospective analysis found that GMIs were most common in the first months after initiating SGLT2 inhibitors and are more common in women and those with prior GMIs.72 Strategies to reduce this risk include counseling the patient to maintain genital hygiene, including keeping the genital region dry. Prior history of GMI is not a contraindication to treatment, and prophylactic topical treatment with antifungal agents can be considered in high-risk individuals. If a patient develops an uncomplicated fungal infection, this may be easily treated with a single dose of an oral agent, such as 150 mg of fluconazole, and discontinuation of the SGLT2 inhibitor is typically not required.73
Fournier’s gangrene is an extremely rare, life-threatening condition associated with necrotizing fasciitis of perineal soft tissue. In 2018, US Food and Drug Administration identified 55 unique cases of Fournier’s gangrene in patients receiving SGLT2 inhibitors,74 and a meta-analysis involving 84 trials and including >42,000 patients receiving SGLT2 inhibitors found no difference in the risk of Fournier’s gangrene with SGLT2 inhibitor use.75 Although earlier studies suggested that SGLT2 inhibitors were associated with possible increased risk for urinary tract infections,10,76,77 subsequent randomized controlled trials have revealed no association.2, 3, 4,46 However, in patients with a history of complicated or recurrent urinary tract infections including those with chronic indwelling Foley catheters, SGLT2 inhibitors should be used with caution.
Amputation
The CANVAS Program raised a concern regarding an association between canagliflozin and minor and major amputations.3 However, CREDENCE reported similar incidences of amputation in both the canagliflozin and placebo groups.46 A real-world meta-analysis comparing canagliflozin and other antihyperglycemic agents did not find any association with amputation, and the black box warning for amputation was removed in 2020.78 No subsequent trials have revealed any association between other SGLT2 inhibitors and amputation risk.79,80 In clinical practice, routine foot care is recommended in all patients with diabetes, and it is also important to identify patients who may have an indication for SGLT2 inhibition on the basis of peripheral vascular disease, to reduce ASCVD risk.
Fractures
The CANVAS Program reported a link between canagliflozin and increased risks of fractures.3 However, in other studies involving canagliflozin, including CREDENCE, no such safety signal was detected.46,81 Meta-analyses including canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin68,82,83 have found no significant association with fracture.84
Diabetic Ketoacidosis
SGLT2 inhibitors are rarely associated with euglycemic DKA resulting in a US Food and Drug Administration warning in 2015. The frequency of DKA reported in CVOTs in patients with T2DM receiving SGLT2 inhibitors was low,2, 3, 4, 5 but occurred in 4% to 6% of patients with type 1 DM.85,86 Reports analyzing the US Food and Drug Administration Adverse Events Reporting System found a 7-fold higher rate of DKA with SGLT2 inhibitors in patients with T2DM when compared with dipeptidyl peptidase-4 inhibitor therapy, of which 71% had euglycemia.87 The risk factors for SGLT2 inhibitor-associated ketoacidosis include >20% insulin dose reduction, lean body habitus, women, surgical stress, trauma, intercurrent illness, alcohol abuse, and patients with latent autoimmune diabetes of adulthood.88 All patients being initiated on SGLT2 inhibitor must be counseled regarding risk of DKA. It is recommended that SGLT2 inhibitors be held 2 to 3 days prior to scheduled surgery. Strategies to reduce the risk of DKA include avoiding >20% reduction in insulin dose, careful monitoring following insulin dose changes, and discontinuation of SGLT2 inhibitors during episodes of acute illness, vomiting, diarrhea, or inability to eat or drink. In high-risk circumstances, monitoring of urine ketones can be considered. Following acute illness, SGLT2 inhibitors may be typically resumed 24 to 48 hours following recovery.
Practical Considerations
Accepting the Acute “Dip” in eGFR
The major mechanism by which SGLT2 inhibitors are thought to delay CKD progression is through reduction in glomerular hyperfiltration and tubuloglomerular feedback. SGLT2 inhibitors are well recognized to result in an acute transient reduction in GFR through a reduction in glomerular hypertension analogous to the mechanism of RAAS blockade. The dip in eGFR following SGLT2 inhibitor frequently elicits concern among clinicians, which may lead to inappropriate discontinuation of an effective therapy. The urge to discontinue the SGLT2 inhibitor because of a rise in serum creatinine should be resisted in most patients and efforts should be made to maintain patients on SGLT2 inhibitors given their cardiorenal benefits. In fact, a larger magnitude of dip in eGFR correlates with greater long-term benefit and therefore should be viewed as evidence of a positive hemodynamic effect.89 Furthermore, concerns regarding the incidence of acute kidney injury with SGLT2 inhibitors have been allayed by meta-analyses from clinical trials and propensity-matched observational studies, which have found that SGLT2 inhibitors are associated with lower rates of acute kidney injury.54,56,90
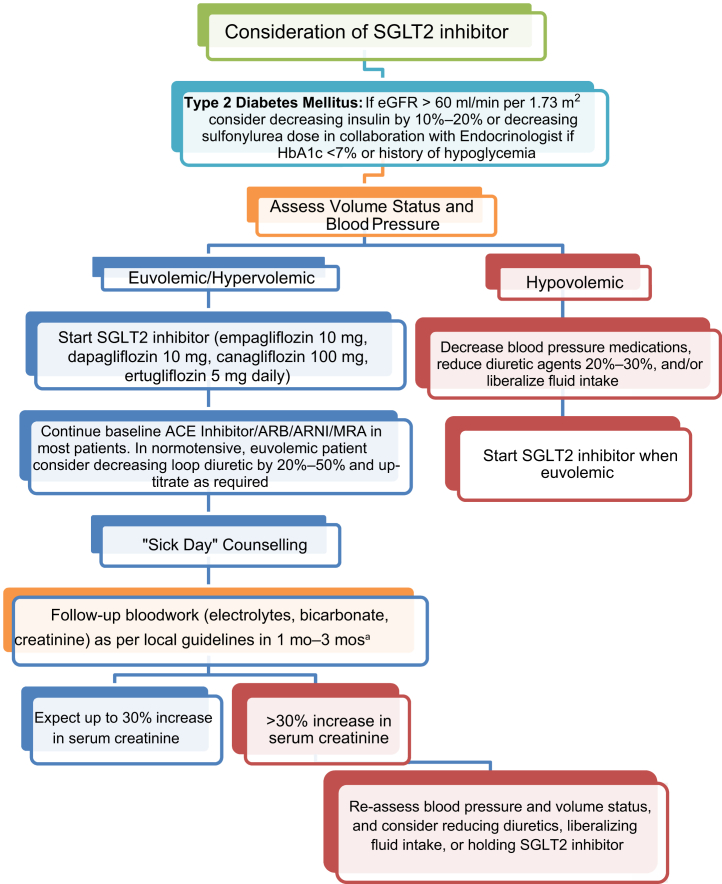
It remains uncertain whether it is necessary to monitor serum creatinine changes shortly after SGLT2 inhibitor initiation; however, it is reasonable to monitor kidney function 1 month after initiation in higher risk patients, including those with a history of prior acute kidney injury, advanced CKD, or in those in whom there is increased concern regarding volume depletion. Traditionally, an increase in serum creatinine level by up to 30% from baseline is considered acceptable. If the level rises beyond this threshold, the patient should undergo a careful reassessment of volume status and a decision made about whether to hold the SGLT2 inhibitor temporarily and then consider rechallenging the patient once appropriate (Figure 1).
Figure 1.
Proposed algorithm for SGLT2 inhibitor initiation. ∗Consider earlier bloodwork in higher risk: stage ≥3B CKD, prior episode(s) of acute kidney injury, or at risk for volume depletion. ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; ARNI, angiotensin receptor-neprilysin inhibitor; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; MRA, mineralocorticoid receptor antagonist.
Consideration of Diuretic Effect and Volume Status
SGLT2 inhibitors result in an osmotic diuresis that seems to be additive to loop diuretics. Favorable properties in comparison to loop diuretics include a reduction in serum uric acid and that hypokalemia or hypomagnesemia is uncommon.91,92 Although precise quantification of additional diuresis is challenging, in RECEDE-HF patients with T2DM and heart failure with reduced ejection fraction taking regular loop diuretic and empagliflozin 25 mg daily had a mean increase in 24-hour urine volume of 535 ml 3 days after SGLT2 inhibitor initiation and 545 ml by 6 weeks.93 Correspondingly, in patients on maintenance loop diuretics, reduction in diuretic dosage should be considered with SGLT2 inhibitor if they are not volume overloaded on clinical examination. This should also be considered in patients initiating medications with even modest diuretic effects, including mineralocorticoid receptor antagonists or angiotensin receptor-neprilysin inhibitors. Patients taking SGLT2 inhibitors may be at risk of volume depletion, during episodes of acute illness associated with nausea, vomiting, or diarrhea. Correspondingly, patients should be provided with sick day advice, whereby patients are advised to hold their SGLT2 inhibitor until resolution of symptoms. Some patients including those with heart failure may require liberalization of fluid intake if they are euvolemic when initiating SGLT2 inhibitors.
Specific Agents and Dose Considerations
In clinical trials, a dose–response relationship has not been observed for cardiorenal outcomes. Therefore, patients may be initiated on the lowest SGLT2 inhibitor dose available: canagliflozin 100 mg daily, dapagliflozin 10 mg daily, empagliflozin 10 mg daily, or ertugliflozin 5 mg daily.
Combination With GLP-1 Receptor Agonists
Although SGLT2 inhibitors offer a clear benefit in slowing progression of CKD, GLP-1-RAs also have cardiorenal benefit in patients with diabetic kidney disease. The GLP-1-RA agonists are optimal agents for patients with established ASCVD and act by increasing glucose-dependent insulin secretion, decreasing glucagon secretion, and delaying gastric emptying.94 GLP-1-RAs are an established disease-modifying treatment for T2DM with known beneficial effects on glycemic control, weight loss, BP control, and reduction in cardiovascular events. The proposed mechanisms of benefit on kidney disease progression include natriuresis through inhibition of proximal tubular NHE3-dependent sodium reabsorption and reduction in albuminuria by decreasing renal inflammation or antioxidative effects.95,96
On the basis of available data, SGLT2 inhibitors are clearly favored for preventing progression of CKD (with or without cardiovascular disease) and in patients with heart failure.39,40 In comparison, GLP-1-RAs may be preferred in patients with obesity/obesity-related complications or established ASCVD including stroke, particularly given that SGLT2 inhibitors have not been found to reduce the incidence of stroke.97
On the basis of complementary mechanisms of action and metabolic effects, the combination of SGLT2 inhibition plus GLP-1-RA therapy is an attractive option to enhance weight loss and reduce major adverse cardiovascular events in selected patients. Data on combination therapy have, however, been sparse. The DURATION-8 study evaluated the use of SGLT2 inhibition (dapagliflozin) and GLP-1-RA (once-weekly exenatide), which reduced HbA1c <0.4%, but found an additive BP reduction (4.2 mm Hg). An additive effect on weight loss was also observed in AWARD-10, which studied the GLP-1-RA dulaglutide versus placebo in patients already on SGLT2 inhibitors with 0.9 kg additional weight loss,98 although SUSTAIN-9 found that semaglutide superimposed on SGLT2 inhibition resulted in 3.8 kg of additional weight loss and a reduction of HbA1c of 1.42.99 In EXSCEL, a post hoc analysis which evaluated the combination of SGLT2 inhibitors and once-weekly exenatide, improvements in all-cause mortality and major adverse cardiovascular events were observed in addition to a nominal significant improvement in preventing decline in eGFR in comparison to patients not on SGLT2 inhibitors.100 Although SGLT2 inhibitors are preferred to delay progression of CKD, patients with residual albuminuria may benefit from the addition of a GLP-1-RA to reduce this risk further. Furthermore, GLP-1-RA may be useful in low eGFR settings, where SGLT2 inhibitor or RAAS blockade titration is not possible. In clinical practice, patients with T2DM may have indications for both agents, and sequential prescription can be considered. The ongoing FLOW trial (NCT03819153) will determine whether the GLP-1-RA semaglutide delays CKD progression in T2DM patients with eGFR 50 to 75 ml/min per 1.73 m2 and UACR 300 to 5000 mg/g or eGFR 25 to 50 ml/min per 1.73 m2 and ACR 100 to 5000 mg/g on a background of RAAS blockade.
Combination With Mineralocorticoid Receptor Antagonists and Endothelin Receptor Antagonists
The nonsteroidal, selective mineralocorticoid receptor antagonist finerenone exhibits beneficial effects on reducing fibrosis and inflammation and was found in the FIDELIO-DKD trial to reduce albuminuria, CKD progression, and cardiovascular events in T2DM patients with eGFR 25 to 60 ml/min per 1.73 m2 and UACR 30 to 300 mg/g or eGFR 25 to 75 ml/min per 1.73 m2 and UACR 300 to 5000 mg/g. The FIGARO-DKD trial further revealed similar benefit in a broader population including stages 2 to 4 CKD with moderately elevated albuminuria (30–300mg/g UACR) or stages 1 to 2 CKD with severely elevated albuminuria (300–5000 mg/g). A recent subgroup analysis of DAPA-CKD in 229 patients found similar safety and effectiveness in reducing kidney end points with combination therapy with SGLT2 inhibitors and mineralocorticoid receptor antagonists, although further studies on added benefit of combination therapy are needed.101
The SONAR trial evaluated the selective endothelin A receptor antagonist atrasentan in adults with T2DM with eGFR 25 to 75 ml/min per 1.73 m2 and UACR 300 to 5000 mg/g on maximally tolerated RAAS blockade carefully selected to have 30% reduction in UACR and no clinically significant fluid retention during an enrichment period. The composite kidney end point of sustained doubling in serum creatinine, ESKD, or kidney death was reduced by 35% with atrasentan treatment.102 However, in this study, only 1.4% of the cohort was on an SGLT2 inhibitor and the benefit conferred by combination therapy with SGLT2 inhibitors remains unclear.
Conclusion
SGLT2 inhibitors have emerged as a key therapy to prevent progression of CKD in patients with albuminuria with or without diabetes including patients with IgA nephropathy, FSGS, and heart failure. Although the indications for SGLT2 inhibitors have expanded rapidly, data remain scarce in transplant recipients or patients with ESKD and future studies should evaluate their safety and effectiveness in these populations.103, 104, 105 Nephrology has entered an exciting era in the development of novel therapeutics for our patients. Although SGLT2 inhibitors were found to have cardiorenal benefit, there remains a large unmet need to reduce remaining risk in patients with CKD. Novel mineralocorticoid receptor antagonists and selective endothelin A receptor antagonists have been found to be effective treatments for diabetic kidney disease, and future studies will be required to evaluate benefits with combination therapy with SGLT2 inhibitors to reduce residual albuminuria and further reduce cardiovascular risk.101,106
Disclosure
KY has received speaking honorarium from AstraZeneca. DZIC has received consulting fees or speaking honorarium or both from Janssen, Bayer, Boehringer Ingelheim, Eli Lilly, AstraZeneca, Merck & Co Inc., Prometic, and Sanofi and has received operating funds from Janssen, Boehringer Ingelheim, Eli Lilly, Sanofi, AstraZeneca, and Merck & Co Inc. All the other authors declared no competing interests.
Acknowledgments
IA is supported by King Abdulaziz University, Jeddah, Saudi Arabia. DZIC is supported by a Department of Medicine, University of Toronto Merit Award, and receives support from the Canadian Institutes of Health Research, Diabetes Canada, and the Heart & Stroke/Richard Lewar Centre of Excellence in Cardiovascular Research.
Author Contributions
KY, AD, IA, and DZIC all contributed to the writing of the manuscript, provided critical edits, and reviewed and approved the final manuscript.
References
- 1.US Department of Health and Human Services Food and Drug Administration Guidance for industry diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. US Department of Health and Human Services Food and Drug Administration. Published 2008. https://www.fda.gov/media/71297/download
- 2.Wiviott S.D., Raz I., Bonaca M.P., et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2018;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 3.Neal B., Perkovic V., Mahaffey K.W., et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 4.Zinman B., Wanner C., Lachin J.M., et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 5.Cannon C.P., Pratley R., Dagogo-Jack S., et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383:1425–1435. doi: 10.1056/NEJMoa2004967. [DOI] [PubMed] [Google Scholar]
- 6.Holman R.R., Paul S.K., Bethel M.A., et al. 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 7.McMurray J.J.V., Solomon S.D., Inzucchi S.E., et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 8.Heerspink H.J.L., Stefánsson B.V., Correa-Rotter R., et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 9.Anker S.D., Butler J., Filippatos G., et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–1461. doi: 10.1056/NEJMoa2107038. [DOI] [PubMed] [Google Scholar]
- 10.Vasilakou D., Karagiannis T., Athanasiadou E., et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2013;159:262–274. doi: 10.7326/0003-4819-159-4-201308200-00007. [DOI] [PubMed] [Google Scholar]
- 11.Fujita Y., Inagaki N. Renal sodium glucose cotransporter 2 inhibitors as a novel therapeutic approach to treatment of type 2 diabetes: clinical data and mechanism of action. J Diabetes Investig. 2014;5:265–275. doi: 10.1111/jdi.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vallon V., Platt K.A., Cunard R., et al. SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol. 2011;22:104–112. doi: 10.1681/ASN.2010030246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cherney D.Z.I., Cooper M.E., Tikkanen I., et al. Pooled analysis of phase III trials indicate contrasting influences of renal function on blood pressure, body weight, and HbA1c reductions with empagliflozin. Kidney Int. 2018;93:231–244. doi: 10.1016/j.kint.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 14.Lee P.C., Ganguly S., Goh S.Y. Weight loss associated with sodium-glucose cotransporter-2 inhibition: a review of evidence and underlying mechanisms. Obes Rev. 2018;19:1630–1641. doi: 10.1111/obr.12755. [DOI] [PubMed] [Google Scholar]
- 15.Bolinder J., Ljunggren O., Kullberg J., et al. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab. 2012;97:1020–1031. doi: 10.1210/jc.2011-2260. [DOI] [PubMed] [Google Scholar]
- 16.Schork A., Saynisch J., Vosseler A., et al. Effect of SGLT2 inhibitors on body composition, fluid status and renin-angiotensin-aldosterone system in type 2 diabetes: a prospective study using bioimpedance spectroscopy. Cardiovasc Diabetol. 2019;18:46. doi: 10.1186/s12933-019-0852-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurinami N., Sugiyama S., Nishimura H., et al. Clinical factors associated with initial decrease in body-fat percentage induced by add-on sodium-glucose co-transporter 2 inhibitors in patient with type 2 diabetes mellitus. Clin Drug Investig. 2018;38:19–27. doi: 10.1007/s40261-017-0580-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zaccardi F., Webb D.R., Htike Z.Z., et al. Efficacy and safety of sodium-glucose co-transporter-2 inhibitors in type 2 diabetes mellitus: systematic review and network meta-analysis. Diabetes Obes Metab. 2016;18:783–794. doi: 10.1111/dom.12670. [DOI] [PubMed] [Google Scholar]
- 19.Thomas M.C., Cherney D.Z.I. The actions of SGLT2 inhibitors on metabolism, renal function and blood pressure. Diabetologia. 2018;61:2098–2107. doi: 10.1007/s00125-018-4669-0. [DOI] [PubMed] [Google Scholar]
- 20.Lambers Heerspink H.J., de Zeeuw D., Wie L., et al. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15:853–862. doi: 10.1111/dom.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cherney D.Z., Perkins B.A., Soleymanlou N., et al. The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc Diabetol. 2014;13:28. doi: 10.1186/1475-2840-13-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sugiyama S., Jinnouchi H., Kurinami N., et al. The SGLT2 inhibitor dapagliflozin significantly improves the peripheral microvascular endothelial function in patients with uncontrolled type 2 diabetes mellitus. Intern Med. 2018;57:2147–2156. doi: 10.2169/internalmedicine.0701-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazidi M., Rezaie P., Gao H.K., Kengne A.P. Effect of sodium-glucose cotransport-2 inhibitors on blood pressure in people with type 2 diabetes mellitus: a systematic review and meta-analysis of 43 randomized control trials with 22 528 patients. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.004007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dekkers C.C.J., Petrykiv S., Laverman G.D., et al. Effects of the SGLT-2 inhibitor dapagliflozin on glomerular and tubular injury markers. Diabetes Obes Metab. 2018;20:1988–1993. doi: 10.1111/dom.13301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heerspink H.J., Perkins B.A., Fitchett D.H., et al. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134:752–772. doi: 10.1161/CIRCULATIONAHA.116.021887. [DOI] [PubMed] [Google Scholar]
- 26.Skrtic M., Yang G.K., Perkins B.A., et al. Characterisation of glomerular haemodynamic responses to SGLT2 inhibition in patients with type 1 diabetes and renal hyperfiltration. Diabetologia. 2014;57:2599–2602. doi: 10.1007/s00125-014-3396-4. [DOI] [PubMed] [Google Scholar]
- 27.Vallon V., Thomson S.C. Targeting renal glucose reabsorption to treat hyperglycaemia: the pleiotropic effects of SGLT2 inhibition. Diabetologia. 2017;60:215–225. doi: 10.1007/s00125-016-4157-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cherney D.Z.I., Zinman B., Inzucchi S.E., et al. Effects of empagliflozin on the urinary albumin-to-creatinine ratio in patients with type 2 diabetes and established cardiovascular disease: an exploratory analysis from the EMPA-REG OUTCOME randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017;5:610–621. doi: 10.1016/S2213-8587(17)30182-1. [DOI] [PubMed] [Google Scholar]
- 29.Panchapakesan U., Pegg K., Gross S., et al. Effects of SGLT2 inhibition in human kidney proximal tubular cells—renoprotection in diabetic nephropathy? PLoS One. 2013;8:e54442. doi: 10.1371/journal.pone.0054442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sano M., Takei M., Shiraishi Y., Suzuki Y. Increased hematocrit during sodium-glucose cotransporter 2 inhibitor therapy indicates recovery of tubulointerstitial function in diabetic kidneys. J Clin Med Res. 2016;8:844–847. doi: 10.14740/jocmr2760w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heerspink H.J.L., Kosiborod M., Inzucchi S.E., Cherney D.Z.I. Renoprotective effects of sodium-glucose cotransporter-2 inhibitors. Kidney Int. 2018;94:26–39. doi: 10.1016/j.kint.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 32.Neuen B.L., Oshima M., Perkovic V., et al. Effects of canagliflozin on serum potassium in people with diabetes and chronic kidney disease: the CREDENCE trial. Eur Heart J. 2021;42:4891–4901. doi: 10.1093/eurheartj/ehab497. [DOI] [PubMed] [Google Scholar]
- 33.Garg S.K., Henry R.R., Banks P., et al. Effects of sotagliflozin added to insulin in patients with type 1 diabetes. N Engl J Med. 2017;377:2337–2348. doi: 10.1056/NEJMoa1708337. [DOI] [PubMed] [Google Scholar]
- 34.Powell D.R., Zambrowicz B., Morrow L., et al. Sotagliflozin decreases postprandial glucose and insulin concentrations by delaying intestinal glucose absorption. J Clin Endocrinol Metab. 2020;105:e1235–e1249. doi: 10.1210/clinem/dgz258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhatt D.L., Szarek M., Steg P.G., et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2020;384:117–128. doi: 10.1056/NEJMoa2030183. [DOI] [PubMed] [Google Scholar]
- 36.Pitt B., Bhatt D.L. Does SGLT1 inhibition add benefit to SGLT2 inhibition in type 2 diabetes? Circulation. 2021;144:4–6. doi: 10.1161/CIRCULATIONAHA.121.054442. [DOI] [PubMed] [Google Scholar]
- 37.Bhatt D.L., Szarek M., Pitt B., et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med. 2020;384:129–139. doi: 10.1056/NEJMoa2030186. [DOI] [PubMed] [Google Scholar]
- 38.Cherney D.Z.I., Ferrannini E., Umpierrez G.E., et al. Efficacy and safety of sotagliflozin in patients with type 2 diabetes and severe renal impairment. Diabetes Obes Metab. 2021;23:2632–2642. doi: 10.1111/dom.14513. [DOI] [PubMed] [Google Scholar]
- 39.de Boer I.H., Caramori M.L., Chan J.C.N., et al. Executive summary of the 2020 KDIGO Diabetes Management in CKD Guideline: evidence-based advances in monitoring and treatment. Kidney Int. 2020;98:839–848. doi: 10.1016/j.kint.2020.06.024. [DOI] [PubMed] [Google Scholar]
- 40.American Diabetes Association Professional Practice Committee 10. Cardiovascular disease and risk management. Diabetes Care. 2021;44:S125–S150. doi: 10.2337/dc21-S010. [DOI] [PubMed] [Google Scholar]
- 41.Cherney D.Z.I., Odutayo A., Verma S. A big win for diabetic kidney disease: CREDENCE. Cell Metab. 2019;29:1024–1027. doi: 10.1016/j.cmet.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 42.Cherney D.Z.I., Cosentino F., Dagogo-Jack S., et al. Ertugliflozin and slope of chronic eGFR: prespecified analyses from the randomized VERTIS CV Trial. Clin J Am Soc Nephrol. 2021;16:1345–1354. doi: 10.2215/CJN.01130121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cherney D.Z.I., McGuire D.K., Charbonnel B., et al. Gradient of risk and associations with cardiovascular efficacy of ertugliflozin by measures of kidney function: observations from VERTIS CV. Circulation. 2021;143:602–605. doi: 10.1161/CIRCULATIONAHA.120.051901. [DOI] [PubMed] [Google Scholar]
- 44.Cherney D.Z.I., Dagogo-Jack S., McGuire D.K., et al. Kidney outcomes using a sustained ≥40% decline in eGFR: a meta-analysis of SGLT2 inhibitor trials. Clin Cardiol. 2021;44:1139–1143. doi: 10.1002/clc.23665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meraz-Muñoz A.Y., Weinstein J., Wald R. eGFR decline after SGLT2 inhibitor initiation: the tortoise and the hare reimagined. Kidney360. 2021;2:1042. doi: 10.34067/KID.0001172021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perkovic V., Jardine M.J., Neal B., et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 47.Packer M., Anker S.D., Butler J., et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–1424. doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 48.van der Aart-van der Beek A.B., de Boer R.A., Heerspink H.J.L. Kidney and heart failure outcomes associated with SGLT2 inhibitor use. Nat Rev Nephrol. 2022;18:294–306. doi: 10.1038/s41581-022-00535-6. [DOI] [PubMed] [Google Scholar]
- 49.Voors A.A., Angermann C.E., Teerlink J.R., et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med. 2022;28:568–574. doi: 10.1038/s41591-021-01659-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heerspink H.J.L., Karasik A., Thuresson M., et al. Kidney outcomes associated with use of SGLT2 inhibitors in real-world clinical practice (CVD-REAL 3): a multinational observational cohort study. Lancet Diabetes Endocrinol. 2020;8:27–35. doi: 10.1016/S2213-8587(19)30384-5. [DOI] [PubMed] [Google Scholar]
- 51.Pasternak B., Wintzell V., Melbye M., et al. Use of sodium-glucose co-transporter 2 inhibitors and risk of serious renal events: Scandinavian cohort study. BMJ. 2020;369:m1186. doi: 10.1136/bmj.m1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koh E.S., Han K., Nam Y.S., et al. Renal outcomes and all-cause death associated with sodium-glucose co-transporter-2 inhibitors versus other glucose-lowering drugs (CVD-REAL 3 Korea) Diabetes Obes Metab. 2021;23:455–466. doi: 10.1111/dom.14239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heerspink H.J.L., Cherney D., Postmus D., et al. A pre-specified analysis of the Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) randomized controlled trial on the incidence of abrupt declines in kidney function. Kidney Int. 2022;101:174–184. doi: 10.1016/j.kint.2021.09.005. [DOI] [PubMed] [Google Scholar]
- 54.Iskander C., Cherney D.Z., Clemens K.K., et al. Use of sodium-glucose cotransporter-2 inhibitors and risk of acute kidney injury in older adults with diabetes: a population-based cohort study. CMAJ. 2020;192:E351–E360. doi: 10.1503/cmaj.191283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Raalte D.H., Cherney D.Z.I. Sodium glucose cotransporter 2 inhibition and renal ischemia: implications for future clinical trials. Kidney Int. 2018;94:459–462. doi: 10.1016/j.kint.2018.05.026. [DOI] [PubMed] [Google Scholar]
- 56.Neuen B.L., Young T., Heerspink H.J.L., et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2019;7:845–854. doi: 10.1016/S2213-8587(19)30256-6. [DOI] [PubMed] [Google Scholar]
- 57.Herrington W.G., Preiss D., Haynes R., et al. The potential for improving cardio-renal outcomes by sodium-glucose co-transporter-2 inhibition in people with chronic kidney disease: a rationale for the EMPA-KIDNEY study. Clin Kidney J. 2018;11:749–761. doi: 10.1093/ckj/sfy090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Herrington W. Design, recruitment, and baseline characteristics of the EMPA-KIDNEY trial. Nephrol Dial Transplant. 2022:1–13. doi: 10.1093/ndt/gfac040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jardiance Phase III EMPA-KIDNEY trial will stop early due to clear positive efficacy in people with chronic kidney disease. Eli Lilly. https://investor.lilly.com/news-releases/news-release-details/jardiancer-phase-iii-empa-kidney-trial-will-stop-early-due-clear Published 2022.
- 60.Rajasekeran H., Reich H.N., Hladunewich M.A., et al. Dapagliflozin in focal segmental glomerulosclerosis: a combined human-rodent pilot study. Am J Physiol Ren Physiol. 2018;314:F412–F422. doi: 10.1152/ajprenal.00445.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cherney D.Z.I., Dekkers C.C.J., Barbour S.J., et al. Effects of the SGLT2 inhibitor dapagliflozin on proteinuria in non-diabetic patients with chronic kidney disease (DIAMOND): a randomised, double-blind, crossover trial. Lancet Diabetes Endocrinol. 2020;8:582–593. doi: 10.1016/S2213-8587(20)30162-5. [DOI] [PubMed] [Google Scholar]
- 62.Wheeler D.C., Stefansson B.V., Batiushin M., et al. The dapagliflozin and prevention of adverse outcomes in chronic kidney disease (DAPA-CKD) trial: baseline characteristics. Nephrol Dial Transplant. 2020;35:1700–1711. doi: 10.1093/ndt/gfaa234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wheeler D.C., Toto R.D., Stefánsson B.V., et al. A pre-specified analysis of the DAPA-CKD trial demonstrates the effects of dapagliflozin on major adverse kidney events in patients with IgA nephropathy. Kidney Int. 2021;100:215–224. doi: 10.1016/j.kint.2021.03.033. [DOI] [PubMed] [Google Scholar]
- 64.Barratt J., Floege J. SGLT-2 inhibition in IgA nephropathy: the new standard of care? Kidney Int. 2021;100:24–26. doi: 10.1016/j.kint.2021.04.002. [DOI] [PubMed] [Google Scholar]
- 65.Wheeler D.C., Jongs N., Stefansson B.V., et al. Safety and efficacy of dapagliflozin in patients with focal segmental glomerulosclerosis: a prespecified analysis of the DAPA-CKD trial. Nephrol Dial Transplant. 2022:1–10. doi: 10.1093/ndt/gfab335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jongs N., Greene T., Chertow G.M., et al. Effect of dapagliflozin on urinary albumin excretion in patients with chronic kidney disease with and without type 2 diabetes: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021;9:755–766. doi: 10.1016/S2213-8587(21)00243-6. [DOI] [PubMed] [Google Scholar]
- 67.Anders H.J., Peired A.J., Romagnani P. SGLT2 inhibition requires reconsideration of fundamental paradigms in chronic kidney disease, diabetic nephropathy, IgA nephropathy and podocytopathies with FSGS lesions. Nephrol Dial Transplant. 2020:1–7. doi: 10.1093/ndt/gfaa329. [DOI] [PubMed] [Google Scholar]
- 68.Toyama T., Neuen B.L., Jun M., et al. Effect of SGLT2 inhibitors on cardiovascular, renal and safety outcomes in patients with type 2 diabetes mellitus and chronic kidney disease: a systematic review and meta-analysis. Diabetes Obes Metab. 2019;21:1237–1250. doi: 10.1111/dom.13648. [DOI] [PubMed] [Google Scholar]
- 69.Nakamura A., Miyoshi H., Kameda H., et al. Impact of sodium-glucose cotransporter 2 inhibitors on renal function in participants with type 2 diabetes and chronic kidney disease with normoalbuminuria. Diabetol Metab Syndr. 2020;12:4. doi: 10.1186/s13098-020-0516-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lega I.C., Bronskill S.E., Campitelli M.A., et al. Sodium glucose cotransporter 2 inhibitors and risk of genital mycotic and urinary tract infection: a population-based study of older women and men with diabetes. Diabetes Obes Metab. 2019;21:2394–2404. doi: 10.1111/dom.13820. [DOI] [PubMed] [Google Scholar]
- 71.Puckrin R., Saltiel M.P., Reynier P., et al. SGLT-2 inhibitors and the risk of infections: a systematic review and meta-analysis of randomized controlled trials. Acta Diabetol. 2018;55:503–514. doi: 10.1007/s00592-018-1116-0. [DOI] [PubMed] [Google Scholar]
- 72.Thong K.Y., Yadagiri M., Barnes D.J., et al. Clinical risk factors predicting genital fungal infections with sodium-glucose cotransporter 2 inhibitor treatment: the ABCD nationwide dapagliflozin audit. Prim Care Diabetes. 2018;12:45–50. doi: 10.1016/j.pcd.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 73.Engelhardt K., Ferguson M., Rosselli J.L. Prevention and management of genital mycotic infections in the setting of sodium-glucose cotransporter 2 inhibitors. Ann Pharmacother. 2021;55:543–548. doi: 10.1177/1060028020951928. [DOI] [PubMed] [Google Scholar]
- 74.Bersoff-Matcha S.J., Chamberlain C., Cao C., et al. Fournier gangrene associated with sodium-glucose cotransporter-2 inhibitors: a review of spontaneous postmarketing cases. Ann Intern Med. 2019;170:764–769. doi: 10.7326/M19-0085. [DOI] [PubMed] [Google Scholar]
- 75.Silverii G.A., Dicembrini I., Monami M., Mannucci E. Fournier’s gangrene and sodium-glucose co-transporter-2 inhibitors: a meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2020;22:272–275. doi: 10.1111/dom.13900. [DOI] [PubMed] [Google Scholar]
- 76.Geerlings S., Fonseca V., Castro-Diaz D., et al. Genital and urinary tract infections in diabetes: impact of pharmacologically induced glucosuria. Diabetes Res Clin Pract. 2014;103:373–381. doi: 10.1016/j.diabres.2013.12.052. [DOI] [PubMed] [Google Scholar]
- 77.Liu X.Y., Zhang N., Chen R., et al. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes: a meta-analysis of randomized controlled trials for 1 to 2years. J Diabetes Complications. 2015;29:1295–1303. doi: 10.1016/j.jdiacomp.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 78.Ryan P.B., Buse J.B., Schuemie M.J., et al. Comparative effectiveness of canagliflozin, SGLT2 inhibitors and non-SGLT2 inhibitors on the risk of hospitalization for heart failure and amputation in patients with type 2 diabetes mellitus: a real-world meta-analysis of 4 observational databases (OBSERVE-4D) Diabetes Obes Metab. 2018;20:2585–2597. doi: 10.1111/dom.13424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Inzucchi S.E., Iliev H., Pfarr E., Zinman B. Empagliflozin and assessment of lower-limb amputations in the EMPA-REG OUTCOME trial. Diabetes Care. 2018;41:e4–e5. doi: 10.2337/dc17-1551. [DOI] [PubMed] [Google Scholar]
- 80.Jabbour S., Seufert J., Scheen A., et al. Dapagliflozin in patients with type 2 diabetes mellitus: a pooled analysis of safety data from phase IIb/III clinical trials. Diabetes Obes Metab. 2018;20:620–628. doi: 10.1111/dom.13124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Watts N.B., Bilezikian J.P., Usiskin K., et al. Effects of canagliflozin on fracture risk in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2016;101:157–166. doi: 10.1210/jc.2015-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.de Liefde I.I., van der Klift M., de Laet C.E.D.H., et al. Bone mineral density and fracture risk in type-2 diabetes mellitus: the Rotterdam Study. Osteoporos Int. 2005;16:1713–1720. doi: 10.1007/s00198-005-1909-1. [DOI] [PubMed] [Google Scholar]
- 83.Yamamoto M., Yamaguchi T., Yamauchi M., et al. Diabetic patients have an increased risk of vertebral fractures independent of BMD or diabetic complications. J Bone Miner Res. 2009;24:702–709. doi: 10.1359/jbmr.081207. [DOI] [PubMed] [Google Scholar]
- 84.Erythropoulou-Kaltsidou A., Polychronopoulos G., Tziomalos K. Sodium-glucose co-transporter 2 inhibitors and fracture risk. Diabetes Ther. 2020;11:7–14. doi: 10.1007/s13300-019-00724-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Peters A.L., Henry R.R., Thakkar P., et al. Diabetic ketoacidosis with canagliflozin, a sodium-glucose cotransporter 2 inhibitor, in patients with type 1 diabetes. Diabetes Care. 2016;39:532–538. doi: 10.2337/dc15-1995. [DOI] [PubMed] [Google Scholar]
- 86.Dandona P., Mathieu C., Phillip M., et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes: the DEPICT-1 52-week study. Diabetes Care. 2018;41:2552–2559. doi: 10.2337/dc18-1087. [DOI] [PubMed] [Google Scholar]
- 87.Blau J.E., Tella S.H., Taylor S.I., Rother K.I. Ketoacidosis associated with SGLT2 inhibitor treatment: analysis of FAERS data. Diabetes Metab Res Rev. 2017;33:e2924. doi: 10.1002/dmrr.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Palmer B.F., Clegg D.J. Euglycemic ketoacidosis as a complication of SGLT2 inhibitor therapy. Clin J Am Soc Nephrol. 2021;16:1284–1291. doi: 10.2215/CJN.17621120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Heerspink H.J.L., Cherney D.Z.I. Clinical implications of an acute dip in eGFR after SGLT2 inhibitor initiation. Clin J Am Soc Nephrol. 2021;16:1278–1280. doi: 10.2215/CJN.02480221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sridhar V.S., Tuttle K.R., Cherney D.Z.I. We can finally stop worrying about SGLT2 inhibitors and acute kidney injury. Am J Kidney Dis. 2020;76:454–456. doi: 10.1053/j.ajkd.2020.05.014. [DOI] [PubMed] [Google Scholar]
- 91.Griffin M., Rao V.S., Ivey-Miranda J., et al. Empagliflozin in heart failure: diuretic and cardiorenal effects. Circulation. 2020;142:1028–1039. doi: 10.1161/CIRCULATIONAHA.120.045691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Verma A., Patel A.B., Waikar S.S. SGLT2 inhibitor: not a traditional diuretic for heart failure. Cell Metab. 2020;32:13–14. doi: 10.1016/j.cmet.2020.06.014. [DOI] [PubMed] [Google Scholar]
- 93.Mordi N.A., Mordi I.R., Singh J.S., et al. Renal and cardiovascular effects of SGLT2 inhibition in combination with loop diuretics in patients with Type 2 diabetes and chronic heart failure: the RECEDE-CHF trial. Circulation. 2020;142:1713–1724. doi: 10.1161/CIRCULATIONAHA.120.048739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Drucker D.J. The cardiovascular biology of glucagon-like peptide-1. Cell Metab. 2016;24:15–30. doi: 10.1016/j.cmet.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 95.Kodera R., Shikata K., Kataoka H.U., et al. Glucagon-like peptide-1 receptor agonist ameliorates renal injury through its anti-inflammatory action without lowering blood glucose level in a rat model of type 1 diabetes. Diabetologia. 2011;54:965–978. doi: 10.1007/s00125-010-2028-x. [DOI] [PubMed] [Google Scholar]
- 96.Thomas M.C. The potential and pitfalls of GLP-1 receptor agonists for renal protection in type 2 diabetes. Diabetes Metab. 2017;43(suppl 1):2S20–22S27. doi: 10.1016/S1262-3636(17)30069-1. [DOI] [PubMed] [Google Scholar]
- 97.Zelniker T.A., Wiviott S.D., Raz I., et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. 2019;139:2022–2031. doi: 10.1161/CIRCULATIONAHA.118.038868. [DOI] [PubMed] [Google Scholar]
- 98.Ludvik B., Frías J.P., Tinahones F.J., et al. Dulaglutide as add-on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD-10): a 24-week, randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2018;6:370–381. doi: 10.1016/S2213-8587(18)30023-8. [DOI] [PubMed] [Google Scholar]
- 99.Zinman B., Bhosekar V., Busch R., et al. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7:356–367. doi: 10.1016/S2213-8587(19)30066-X. [DOI] [PubMed] [Google Scholar]
- 100.Clegg L.E., Penland R.C., Bachina S., et al. Effects of exenatide and open-label SGLT2 inhibitor treatment, given in parallel or sequentially, on mortality and cardiovascular and renal outcomes in type 2 diabetes: insights from the EXSCEL trial. Cardiovasc Diabetol. 2019;18:138. doi: 10.1186/s12933-019-0942-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Provenzano M., Jongs N., Vart P., et al. The kidney protective effects of the sodium-glucose cotransporter-2 inhibitor, dapagliflozin, are present in patients with CKD treated with mineralocorticoid receptor antagonists. Kidney Int Rep. 2022;7:436–443. doi: 10.1016/j.ekir.2021.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Heerspink H.J.L., Parving H.H., Andress D.L., et al. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial. Lancet. 2019;393:1937–1947. doi: 10.1016/S0140-6736(19)30772-X. [DOI] [PubMed] [Google Scholar]
- 103.AlKindi F., Al-Omary H.L., Hussain Q., et al. Outcomes of SGLT2 inhibitors use in diabetic renal transplant patients. Transplant Proc. 2020;52:175–178. doi: 10.1016/j.transproceed.2019.11.007. [DOI] [PubMed] [Google Scholar]
- 104.Mahling M., Schork A., Nadalin S., et al. Sodium-glucose cotransporter 2 (SGLT2) inhibition in kidney transplant recipients with diabetes mellitus. Kidney Blood Press Res. 2019;44:984–992. doi: 10.1159/000501854. [DOI] [PubMed] [Google Scholar]
- 105.Hecking M., Jenssen T. Considerations for SGLT2 inhibitor use in post-transplantation diabetes. Nat Rev Nephrol. 2019;15:525–526. doi: 10.1038/s41581-019-0173-0. [DOI] [PubMed] [Google Scholar]
- 106.Bakris G.L., Agarwal R., Anker S.D., et al. Effect of Finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383:2219–2229. doi: 10.1056/NEJMoa2025845. [DOI] [PubMed] [Google Scholar]