Abstract
Introduction
Infantile oxalosis is the most severe form of primary hyperoxaluria type 1 (PH1), with onset of end-stage kidney disease (ESKD) during infancy. We aimed to analyze the outcome of these patients as our current understanding is limited owing to a paucity of reports.
Methods
A retrospective registry study was conducted using data from the OxalEurope registry. All PH1 patients with ESKD onset at age <1 year were analyzed.
Results
We identified 95 patients born between 1980 and 2018 with infantile oxalosis. Median (interquartile range [IQR]) age at ESKD was 0.4 (0.3–0.5) year. There were 4 patients diagnosed by family screening who developed ESKD despite early diagnosis. There were 11 patients who had biallelic missense mutations associated with vitamin B6 responsiveness. Of 89 patients, 27 (30%) died at a median age of 1.4 (0.6–2.0) years (5-year patient survival of 69%). Systemic oxalosis was described in 54 of 56 screened patients (96%). First transplantation was performed at a median age of 1.7 (1.3–2.9) years. In 42 cases, this procedure was a combined liver-kidney transplantation (LKTx), and in 23 cases, liver transplantations (LTx) was part of a sequential procedure. Survival rates of both strategies were similar. Patient survival was significantly higher in patients born after 2000. Intrafamilial phenotypic variability was present in 14 families of patients with infantile oxalosis.
Conclusion
Nearly all screened patients with infantile oxalosis developed systemic disease. Mortality is still high but has significantly improved over time and might further improve under new therapies. The intrafamilial phenotypic variability warrants further investigation.
Keywords: children, end-stage kidney disease, infant, infantile oxalosis, primary hyperoxaluria
Graphical abstract
PH1 is a rare genetic metabolic disease, characterized by endogenous oxalate overproduction owing to a deficiency of the peroxisomal liver enzyme alanine:glyoxylate aminotransferase (AGT).1 As oxalate is excreted by the kidney, oxalate overproduction leads to hyperoxaluria resulting in calcium oxalate deposits in the renal tubules and interstitial tissue.2,3 This may cause kidney stones, nephrocalcinosis, and, in many patients, ESKD. In the case of ESKD, renal oxalate excretion is severely compromised and high plasma oxalate levels ensue. Oxalate crystals form in the bones, eyes, heart, vessels, or other organs, causing systemic oxalosis, a multiorgan disease resulting in severe comorbidities, impaired quality of life, and ultimately death.2, 3, 4
Initial presenting features of patients with PH1 are highly variable, ranging from adults with a late-onset phenotype with an occasional stone passage to infants presenting with failure to thrive due to ESKD.4,5 When ESKD develops before the age of 1 year due to PH1, this is referred to as infantile oxalosis.6
The prognosis of infantile cases is considered to be poor. An earlier study found that 85% of patients with infantile oxalosis died within the first year of life, of whom two-thirds died before the age of 6 months.6 The management of children with infantile oxalosis is challenging. Intensive dialysis regimens, such as frequent hemodialysis (HD) combined with peritoneal dialysis (PD) or daily HD tailored in intensity based on plasma oxalate levels and patient’s tolerance are recommended as a bridge to orthotopic LTx with explant of native liver, which is still the only curative option.4 LTx should be performed at the earliest possible moment to stop oxalate accumulation, either simultaneously with KTx (LKTx) or as 2 sequential procedures (LTx followed by KTx).4 However, the patient’s size and generally poor clinical condition due to ESKD, systemic oxalosis, and organ availability can all be limiting factors in this process.
The assumption of an overall poor prognosis and the difficult and onerous management often pose a dilemma for physicians as to what extent treatment of these children is ethically acceptable. Reliable data on clinical outcome and optimal therapeutic strategy are limited. In addition, substantial clinical variability is reported within families with the same genotype; thus, as a result the prognosis of an individual patient with PH1 remains difficult to predict.7
This study in a large cohort of patients with infantile oxalosis aims to provide an overview of the course of the disease and treatment and outcome of these patients to improve our understanding of infantile oxalosis and facilitate recommendations regarding optimal therapeutic strategies in the future, especially in light of new promising therapies.8
Methods
We conducted a retrospective registry study using data from the OxalEurope Registry, a European database, which contains >900 patients with PH1. All data were kept on record by the treating physician and uploaded to the OxalEurope database, a procedure approved by the medical ethical committee of the Amsterdam UMC and other participating centers. Informed consent was obtained, if applicable.
We identified all cases of infantile oxalosis in the OxalEurope database, defined as ESKD before the age of 1 year and diagnosis of PH1. Confirmation of the PH1 diagnosis by genetic analysis, liver biopsy, or high clinical suspicion with both hyperoxaluria and raised levels of urinary glycolate was mandatory. ESKD was defined as an estimated glomerular filtration rate <15 ml/min per 1.73 m2 as calculated by the (modified) Schwartz formula9,10 or requirement of dialysis. Patients with biallelic c.508G>A, c.454T>A, or c.731T>C AGXT mutation were defined as vitamin B6 responsive.11, 12, 13 Systemic oxalosis was defined as calcium oxalate deposits in any organ other than the kidney or urinary tract. Intensive dialysis was defined as HD ≥6 times per week or HD combined with PD. If information on the age at diagnosis was not available, the age at ESKD was used for calculations.
Results are described as median with IQR and ranges for numerical data. Percentages and numbers are used for categorical data. We used the Mann-Whitney U test for testing independent numerical data within 2 groups and the Fisher exact test for testing categorical data with 2 groups. We calculated survival rates using Kaplan-Meier analyses. Patient survival was defined as the time from diagnosis to the time of last follow-up or death, and we marked patients lost to follow-up as alive at the date of last known follow-up for Kaplan-Meier analysis. We performed a sensitivity analysis marking patients who were lost to follow-up as death at last follow-up. For the post-transplant patient survival curve, we computed follow-up from the time of first transplantation to the moment of last follow-up or death and counted death as an event. For the death-censored kidney graft survival curve, we calculated follow-up from the moment of first KTx to the date of graft failure, death, or last follow-up with functioning kidney graft. Kidney graft failure was defined as return to dialysis or need for retransplantation. For the event-free survival curve, we computed follow-up time similarly to the post-transplant patient survival curve. However, if patients experienced liver or kidney graft failure, we defined the follow-up time as the moment of first transplantation to date of graft failure. We defined liver graft failure as the need for retransplantation or death due to failure of the liver. If patients experienced kidney graft failure, graft liver failure, or death, we counted this as an event. Retransplantations were excluded. We used the log-rank test to test for differences in survival rates between different groups. We calculated plasma oxalate outcomes using a baseline measurement obtained before transplantation and measurements performed after LTx. If patients received a second transplantation (KTx or retransplantation), measurements were censored from that time on. Considering interlaboratory differences of methods and reference values, plasma oxalate values are expressed as the individual reduction compared with the baseline value or as multiple of the local upper reference limit. We performed all tests 2-sided and considered a P < 0.05 statistically significant. We performed statistical analyses using SPSS version 26 (IBM Corp., Armonk, NY), RStudio version 1.4.1106 (RStudio PBC, Boston, MA) and GraphPad Prism version 8 (GraphPad Software, San Diego, CA).
Results
We identified 95 patients with infantile oxalosis, all born between 1980 and 2018, living in 12 European countries (Supplementary Table S1). The median (IQR) follow-up time was 3.6 (1.1–9.7) years. There were 6 patients (6%) lost to follow-up immediately following diagnosis and therefore no additional information was available. Detailed cohort characteristics are found in Table 1.
Table 1.
Clinical characteristics of children with infantile oxalosis
| Characteristic | Value |
|---|---|
| Number of patients, n | 95 |
| Male gender, n (%) | 59/95 (62) |
| Diagnosis | |
| Age at first symptoms, median (IQR) | 0.3 (0.2–0.4) |
| Age at diagnosis, median (IQR) | 0.4 (0.3–0.6) |
| Diagnosis through family screening, n (%) | 4/63 (6.3) |
Findings at diagnosis, n (%)
|
66/81 (82) 10/71 (14) 84/94 (89) |
| Age at ESKD, median (IQR) | 0.4 (0.3–0.5) |
| Follow-up | |
| Lost to follow-up, n (%) | 6 (6) |
| Follow-up time, median (IQR) | 3.6 (1.1–9.7) |
| Age at follow-up, median (IQR) | 4.2 (1.5–10) |
| Patients with systemic oxalosis, n (%) | 54/56 (96) |
| Patients with intensive dialysis, n (%) | 30/41 (71) |
| Transplanted patients (LKTx, LTx, or KTx), n (%) | 66/87 (76) |
| Age at first transplantation, median (IQR) | 1.7 (1.3–2.9) |
| Time between diagnosis and first transplantation, median (IQR) | 1.3 (0.9–2.1) |
| Deceased patients, n (%) | 27/89 (30) |
| Age at death, median (IQR) | 1.4 (0.6–2.0) |
ESKD, end-stage kidney disease; KTx, kidney transplantation; IQR, interquartile range; LKTx, liver-kidney transplantation; LTx, liver transplantation.
Values are either expressed as median with IQR or as n with percentage of (sub)group. Age or time is expressed in years.
Symptoms and Diagnosis
Patients were diagnosed at a median (IQR) age of 0.4 (0.3–0.6) year; 84 (89%) infants had already developed ESKD at that time and presented with symptoms associated with ESKD. Information on kidney function was unavailable for 1 patient. The remaining 10 patients not presenting in ESKD were diagnosed by family screening (n = 4) or presented with a variety of symptoms, notably failure to thrive. All developed ESKD within 7 months following diagnosis, leading to a median (IQR) age at ESKD of the total cohort of 0.4 (0.3–0.5) year.
The initial diagnosis was made by liver biopsy in 29 patients. All but 2 biopsies were performed before the year 2007. Genetic test results were obtained from 89 patients. Of 6 patients, no genetic information was available. Of these patients, 3 were diagnosed by genetic testing, but the genotype was missing in the database. In 2 children, the diagnosis of PH1 was based on the biochemical pattern of both hyperoxaluria and high urinary glycolate as no further diagnostics had been possible owing to early death. The last patient was diagnosed solely on liver biopsy results.
Genotype
In total, we found 49 different AGXT mutations in the infantile oxalosis cohort (Supplementary Table S2). The allelic frequency was highest for c.33dupC (19%), c.508G>A (13%), and c.731T>C (10%) (Figure 1). There were 11 patients who carried biallelic AGXT mutations associated with pyridoxine responsiveness. Genotypes in detail were as follow: c.508G>A; c.508G>A (n = 2), c.731T>C; c.713T>C (n = 8); and 1 patient was compound heterozygous c.508G>A; c.731T>C. Furthermore, 14 patients had the genotype c.33dupC; c.33dupC.
Figure 1.
Frequencies of AGXT mutations in children with infantile oxalosis. The 3 most common AGXT mutations are displayed. The 46 mutations defined as other all had a frequency ≤6 (equal to ≤3%). A complete list of all genotypes can be found in Supplementary Table S2.
Patient Survival
During follow-up, 27 of 89 patients (30%) died. Median (IQR) age of death was 1.4 (0.6–2.0) years, and time between diagnosis and death was 0.8 (0.2–1.6) years. The 5-year survival rate was 69%. There were 9 infants (10%) who died before the age of 1 year, owing to infection (n = 1), cardiac decompensation (n = 2), or unknown reasons (n = 6). In total, the cause of death was related to transplantation in 8 patients. In our cohort, 14 of the 26 patients (54%) born before the year 2000 died, in contrast to 13 of the 63 patients (21%) born in or after the year 2000. Kaplan-Meier analysis (Figure 2) found significantly higher survival rates of the cohort born after 2000 (log-rank test, P = 0.002), which was confirmed by sensitivity analysis (Supplementary Figure S1). No differences were found in main patient characteristics (sex, age at diagnosis, or age at ESKD). However, significantly more patients born after the year 2000 received a LTx (46% vs. 87%, P < 0.001).
Figure 2.
Kaplan-Meier analysis of patient survival of children with infantile oxalosis, stratified by year of birth. The 1-, 2- and 5-year survival rates were 69%, 60%, and 46% for patients born before 2000 and 89%, 80%, and 80% for patients born after 2000, respectively. This resulted in a significant difference in the survival rate between the 2 groups (log-rank test, P = 0.002).
Systemic Oxalosis
Of the 56 screened patients, systemic oxalosis was noted in 54 (96%). Oxalate deposits were most frequently found in the retina (n = 43), bones (n = 34), and heart (n = 11). More than half of the patients (n = 27) had oxalate deposits in both eyes and bones. Of these patients, 6 also presented with deposits in the heart. In 16 patients, oxalate deposits were solely found in the retina, in 4 solely in the bones, and in 2 solely in the heart. In total, 8 patients experienced pathologic fractures of the long bones. They all developed systemic oxalosis in multiple organs. Furthermore, 4 of 5 patients with multiple fractures were on dialysis for >5 years. In 1 patient, autopsy result revealed oxalate deposits in the myocardium, thymus, kidneys, and bone marrow.
Dialysis
Data regarding dialysis modality were available for 51 patients with infantile oxalosis. Most (n = 32; 63%) started with PD, 15 (29%) with HD, and 4 (8%) were treated with a combination of both HD and PD. Detailed follow-up information regarding dialysis modality and dose was available for only 41 patients. Of these, 29 (71%) underwent intensive dialysis.
Transplantation
Data on transplantation were available for 87 patients, of whom 66 (76%) underwent organ transplantation. Furthermore, 15 patients died before transplantation and 6 were awaiting transplantation at last follow-up. Median (IQR) age at first transplantation was 1.7 (1.3–2.9) years (Table 1). Of these patients, 42 (64%) received combined LKTx and 23 (35%) LTx as part of the sequential strategy (Table 2). LTx were performed at a significantly younger age if scheduled as part of a sequential transplantation procedure (median 1.3 [IQR 1.0–2.4] years) as opposed to combined LKTx (median 1.8 [IQR 1.5–2.8] years, P = 0.042), resulting in a shorter duration between diagnosis and transplantation (P = 0.021) (Table 2). Of the 23 patients scheduled for a sequential transplantation procedure, 12 underwent KTx at a median (IQR) time of 0.8 (0.6–2.3) years after LTx and 1 underwent LKTx after liver failure of the first liver graft. Furthermore, 3 patients died before receiving KTx and 7 patients were still awaiting KTx at last follow-up. Finally, 1 patient (2%) received isolated KTx, as the diagnosis of PH1 had not been established at time of transplantation. This kidney graft failed owing to thrombosis of the renal artery, and oxalate deposits were found in the graft. The patient died 1 year after isolated KTx.
Table 2.
Characteristics of infantile oxalosis patients undergoing LTx
| Characteristic | LKTx | LTx | P value |
|---|---|---|---|
| Number of patients, n | 42 | 23 | N/A |
| Age at transplantation, median (IQR) | 1.8 (1.5–2.8) | 1.3 (1.0–2.4) | 0.042a |
| Time between diagnosis and transplantation, median (IQR) | 1.4 (1.0–2.3) | 0.9 (0.7–1.5) | 0.021a |
| Time between LTx and KTx | N/A | 0.8 (0.7–2.3) | N/A |
| Deceased patients, n (%) | 8/42 (19) | 3/23 (13) | 0.733b |
| Age at death, median (range) | 1.6 (1.0–2.0) | 1.6 (1.3–5.6) | 0.838a |
| Follow-up time, median (IQR) | 6.1 (1.5–12) | 5.4 (2.0–9.5) | 0.426a |
IQR, interquartile range; KTx, kidney transplantation; LKTx, combined liver and kidney transplantation; LTx, isolated liver transplantation; N/A, not applicable.
Values are expressed as median with IQR or as n with percentage of (sub)group. Age or time is expressed in years.
Mann-Whitney U test.
Fisher exact test.
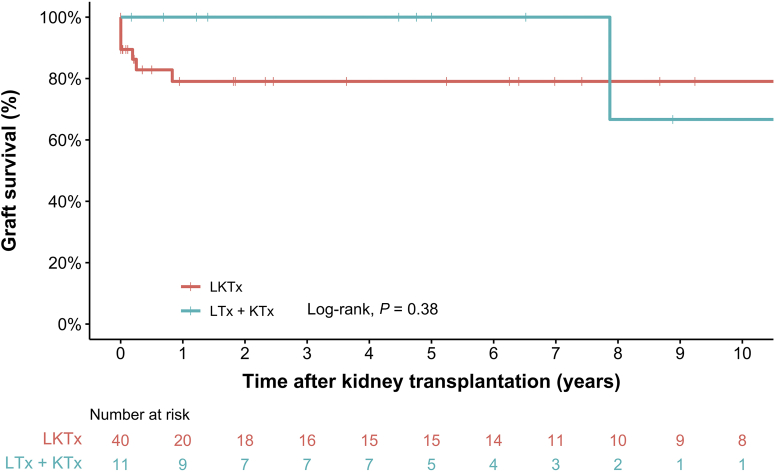
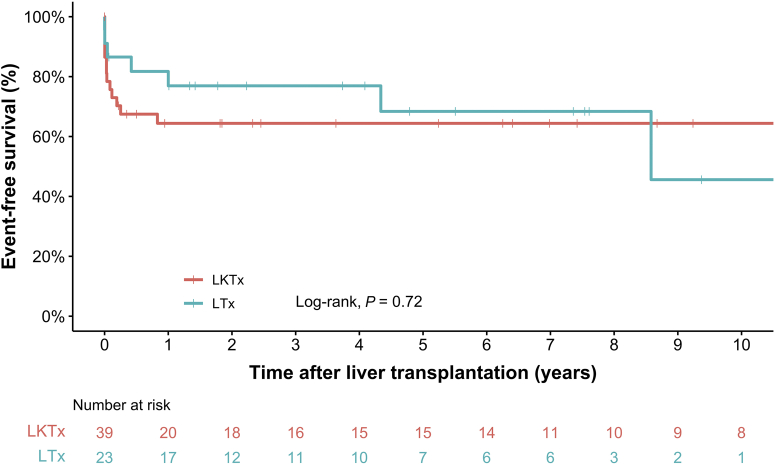
Kaplan-Meier analyses (Figure 3, Figure 4, Figure 5) revealed no difference in patient survival nor event-free survival after transplantation between patients receiving combined or sequential LKTx. In total, 11 patients had kidney graft failure after combined LKTx. Reasons for graft failure were thrombosis (n = 3), deposits of oxalate (n = 3), primary nonfunction of the kidney (n = 1), or unknown (n = 4). There were 4 patients who experienced liver graft failure, leading to death in 1 patient. The other 3 patients received a second liver graft; 1 had LKTx and 2 LTx. Of these patients, 1 died as a result of complications 2 months after the second LTx.
Figure 3.
Kaplan-Meier analysis of patient survival in children with infantile oxalosis after liver transplantation, stratified by transplantation procedure. Log-rank test analysis revealed no significant difference (P = 0.56) in patient survival between patients receiving a combined LKTx versus isolated LTx. LKTx, liver-kidney transplantation; LTx, liver transplantation.
Figure 4.
Kaplan-Meier analysis of death-censored kidney graft survival in children with infantile oxalosis after liver-kidney transplantation stratified by transplantation procedure. Log-rank test analysis revealed no significant difference (P = 0.38) in death-censored kidney graft survival between patients receiving a kidney graft as part of combined transplantation (LKTx) versus sequential transplantation (LTx + KTx). KTx, kidney transplantation; LKTx, liver-kidney transplantation; LTx, liver transplantation.
Figure 5.
Kaplan-Meier analysis of event-free survival in children with infantile oxalosis after liver transplantation, stratified by transplantation procedure. Log-rank test analysis revealed no significant difference (P = 0.72) in event-free survival between patients after combined LKTx and isolated LTx. LKTx, liver-kidney transplantation; LTx, liver transplantation.
Metabolites
The range of plasma oxalate values at diagnosis was 7.7 to 80 times the local upper reference limit, on the basis of samples of 28 patients who presented with ESKD. Figure 6 illustrates the course of plasma oxalate levels over time after LTx of 25 patients. LTx resulted in a median (IQR) reduction of plasma oxalate levels of 72% (58%–90%) within 1 week and 81% (77%–87%) within 6 months after combined LKTx and 48% (32%–54%) within 1 week and 59% (49%–68%) within 6 months after isolated LTx (differences between LKTx and LTx after 1 week: P = 0.04; after 6 months: P = 0.001; Table 3).
Figure 6.
The course of plasma oxalate values in children with infantile oxalosis after liver transplantation. In the left panel, POx levels of 12 patients after isolated LTx are displayed. In the right panel, POx levels of 13 patients after combined LKTx are displayed. LKTx, liver-kidney transplantation; LTx, liver transplantation; POx, plasma oxalate.
Table 3.
Reduction of POx levels in children with infantile oxalosis after LTx (combined with kidney or isolated)
| Time | Reduction (%) after LKTx, median (IQR) | n | Reduction (%) after LTx, median (IQR) | n | P value |
|---|---|---|---|---|---|
| <1 wk | 72 (58–90) | 8 | 48 (32–54) | 5 | 0.040a |
| <1 mo | 77 (68–87) | 10 | 51 (34–66) | 6 | 0.039a |
| <6 mo | 81 (77–87) | 10 | 59 (49–68) | 8 | 0.001a |
IQR, interquartile range; LKTx, combined liver and kidney transplantation; LTx, isolated liver transplantation; POx; plasma oxalate.
Mann-Whitney U test.
Associated Factors
Patients with biallelic AGXT mutations associated with vitamin B6 responsiveness did not have better survival rates (3 of 11 patients died) than patients with other mutations (24 of 78 patients died; P = 0.86); however, data regarding whether infants had been treated with vitamin B6 following diagnosis were incomplete. In total, 5 patients were confirmed to have received vitamin B6 (n = 4 patients with a homozygous c.731T>C mutation, n = 1 patient with a homozygous c.508G>A mutation). B6 responsiveness was tested in 1 homozygous c.731T>C patient. In none of the patients, clinical vitamin B6 responsiveness could be officially confirmed. All patients of whom information was available (n = 7) eventually received a liver transplant.
In our cohort, 4 patients were diagnosed with PH1 by family screening at a median (IQR) age of 0.04 (0.01–0.07) year. At time of diagnosis, 3 were found to already have nephrocalcinosis. All 4 patients developed ESKD before the age of 1 year, and 2 of these patients died (at the age of 1.3 and 7.8 years). At least 2 patients were followed up by a clinician regularly after diagnosis, and 1 of these patients was started on intensive conservative therapy 2 days after birth (e.g., hyperhydration, citrate, vitamin B6, and eventually pre-emptive PD). However, ESKD could not be prevented. Of the other 2 patients, no detailed information was available regarding follow-up between diagnosis and ESKD.
We evaluated the phenotypic variability in 16 families with a case of infantile oxalosis. There were 2 pairs of siblings who both presented with infantile oxalosis (families O and P; Table 4). They were therefore both included in the infantile oxalosis cohort. All other patients with infantile oxalosis had siblings with a milder clinical course, indicative of significant intrafamilial phenotypic variability in 14 pedigrees. In all but 1 family (family G; Table 4), at least 1 sibling had a later onset and diagnosis of PH1 than the infantile oxalosis case, affirming intrafamilial variability without interference of medical management.
Table 4.
Clinical and genetic characteristics of families with cases of infantile oxalosis
| Family | Gender | AGXT genotype 1 | AGXT genotype 2 | Index case | Family screening | Infantile oxalosis | Age diagnosis | Age ESKD | Age follow-up | |
|---|---|---|---|---|---|---|---|---|---|---|
| Families with 1 case of infantile oxalosis (displaying intrafamilial variability) | ||||||||||
| A1 | Female | c.1079G>A | c.1079G>A | - | - | Yes | 0.2 | 0.2 | 8.7 | |
| A2 | Female | c.1079G>A | c.1079G>A | Yes | - | - | 5.7 | 6.1a | 14 | |
| B1 | Male | c.731T>Cb | c.731T>Cb | Yes | - | Yes | 0.2 | 0.3 | 12 | |
| B2 | Female | c.731T>Cb | c.731T>Cb | - | Yes | - | 5.8 | N/A | 6.8 | |
| C1 | Male | c.364C>T | c.364C>T | - | - | Yes | 0.3 | 0.3 | 0.3c | |
| C2 | Female | c.364C>T | c.364C>T | Yes | - | - | 0.2 | N/A | 3.6 | |
| D1 | Male | c.595G>A | c.847-3C>G | Yes | - | Yes | 0.7 | 0.7 | 6.4 | |
| D2 | Female | c.595G>A | c.847-3C>G | - | Yes | - | 2.5 | N/A | 7.0 | |
| E1 | Male | c.33dupC | c.33dupC | Yes | - | Yes | 0.7 | 0.6 | 1.6c | |
| E2 | Female | c.33dupC | c.33dupC | - | Yes | - | 0.9 | N/A | 5.3 | |
| E3 | Male | c.33dupC | c.33dupC | - | Yes | - | 3.8 | N/A | 11 | |
| F1 | Male | c.508G>Ab | N/A | Yes | - | Yes | 0.5 | 0.5 | N/A | |
| F2 | Female | c.508G>Ab | N/A | - | N/A | - | 6.0 | N/A | N/A | |
| G1 | Female | c.33dupC | c.33dupC | Yes | - | Yes | 0.2 | 0.4 | 0.5c | |
| G2 | Female | c.33dupC | c.33dupC | - | Yes | - | 0.07 | N/A | 24 | |
| G3 | Female | c.33dupC | c.33dupC | - | Yes | - | 0.01 | N/A | 23 | |
| H1 | Male | c.508G>Ab | c.508G>Ab | Yes | - | Yes | 0.4 | 0.3 | 26 | |
| H2 | Male | c.508G>Ab | c.508G>Ab | - | Yes | - | 4.9 | N/A | 27 | |
| I1 | Male | c.244G>C | c.244G>C | Yesd | - | Yes | 3.7 | 0.6 | 3.9c | |
| I2 | Male | c.244G>C | c.244G>C | Yesd | - | - | 2.6 | N/A | 8.5 | |
| J1 | Male | c.424-2A>G | c.424-2A>G | Yes | - | Yes | 0.8 | 0.8 | 7.4 | |
| J2 | Male | c.424-2A>G | c.424-2A>G | - | Yes | - | 3.0 | N/A | 8.9 | |
| K1 | Male | c.508G>Ab | c.508G>Ab | Yes | - | Yes | 0.4 | 0.4 | 0.7c | |
| K2 | Female | c.508G>Ab | c.508G>Ab | - | Yes | - | 5.8 | N/A | 16 | |
| L1 | Male | c.508G>Ab | c.847-1G>C | Yes | - | Yes | 1.0 | 0.9 | 6.0 | |
| L2 | Male | c.508G>Ab | c.847-1G>C | - | Yes | - | 4.0 | N/A | 9.4 | |
| Families with 1 case of infantile oxalosis identified by family screening (displaying intrafamilial variability) | ||||||||||
| M1 | Male | c.33dupC | c.33dupC | - | Yes | Yes | 0.08 | 0.7 | 1.3c | |
| M2 | Male | c.33dupC | c.33dupC | Yes | - | - | 2.3 | 5.0a | 21 | |
| M3 | Female | c.33dupC | c.33dupC | - | Yes | - | 4.0 | 24a | 24c | |
| M4 | Female | c.33dupC | c.33dupC | - | Yes | - | 3.6 | N/A | 21 | |
| N1 | Female | Ex9_11del | Ex9_11del | - | Yes | Yes | 0.05 | 0.3 | 8.0 | |
| N2 | Female | Ex9_11del | Ex9_11del | Yes | - | - | 12 | 12a | 14 | |
| N3 | Male | Ex9_11del | Ex9_11del | - | Yes | - | 0.02 | N/A | 0.8 | |
| Families with recurrent infantile oxalosis (1 sibling identified by family screening) | ||||||||||
| O1 | Male | c.33dupC | c.33dupC | Yes | - | Yes | 0.2 | 0.2 | 0.3 | |
| O2 | Female | c.33dupC | c.33dupC | - | Yes | Yes | 0.01 | 0.4 | 3.2 | |
| P1 | Male | c.798-802 delinsACAATCTCAG | c.798-802 delinsACAATCTCAG | Yes | - | Yes | 0.4 | 0.3 | 4.5c | |
| P2 | Male | c.798-802 delinsACAATCTCAG | c.798-802 delinsACAATCTCAG | - | Yes | Yes | 0.02 | 0.3 | 7.8c | |
ESKD, end-stage kidney disease; N/A, not applicable or available; PH1, primary hyperoxaluria type 1.
Patients are labeled by family (letter) and number, starting with the infantile case followed by siblings with PH1. Age or time is expressed in years.
Received combined liver-kidney transplantation.
Vitamin B6-responsive AGXT mutation.
Patient deceased.
Diagnosed at the same time.
To illustrate this intrafamilial highly variable disease expressivity despite identical causative genotype, we describe the outcomes of a family homozygous for C.33dupC (family E). In this family, the index case E1 presented with infantile oxalosis and ESKD at the age of 7 months and died post-transplantation at an age of 1.6 years. Both siblings (E2 and E3) were started on conservative treatment (hyperhydration and citrate) right after diagnosis at the age of 0.9 and 3.8 years. Sibling E3 experienced kidney stones but had normal kidney function at last follow-up at the age of 11 years. Sibling E2 was found to have nephrocalcinosis at diagnosis but maintained normal kidney function until last follow-up at the age of 5.3 years albeit suffering recurrent urinary tract infections.
Discussion
In this study of 95 patients with infantile oxalosis, overall mortality was still high. Of the patients, 30% died at a median age of 1.4 years. However, survival rates have improved significantly in the past 2 decades. Transplantation of the liver and kidney was frequently performed but was linked to cause of death in approximately one-third of deceased patients. Combined and sequential LKTx procedures resulted in similar patient survival and event-free rates. Combined LKTx resulted in faster reductions in plasma oxalate levels. Nearly all screened patients were found to have systemic oxalosis. Finally, we found a significant intrafamilial phenotypic variability and that infantile oxalosis can occur in patients with genotypes associated with vitamin B6 responsiveness.
Previous studies on infantile oxalosis reported unfavorable outcomes.14,15 In a large cohort of 28 patients, Leumann et al.6 found in 1987 that 85% of subjects died before the age of 1 year. In contrast, only 10% of patients in our cohort died before this age. Moreover, in our cohort, we found significant higher survival rates of patients born after January 1, 2000. This finding of improved survival over time is supported by a more recent European study that found a survival rate of >60% and a recent small case series (n = 7) reporting no deaths.16,17 Both advanced transplantation and dialysis techniques, as overall PH1 management, may all have contributed to the improved survival.18,19 However, our outcomes may not be generalizable to developing countries because Cochat et al.20 found that survival rates of infants diagnosed with PH1 were significantly lower in developing countries than in developed countries.
Systemic oxalosis was present in almost all screened patients. Oxalate retinopathy was common, in line with earlier findings.21,22 Birtel et al.22 found that infantile patients were more prone to develop retinal oxalate depositions than later-onset patients. All patients with infantile oxalosis in their cohort (n = 12) had extensive retinal oxalate depositions, whereas only mild retinal alterations were found in 6 of the 56 noninfantile patients. This susceptibility might be explained by high plasma oxalate levels and/or immature blood-retinal barrier in infants resulting in increased oxalate diffusion and deposition. Of 5 patients with multiple pathologic fractures, 4 had been on dialysis for a relatively long period, suggesting this is an important factor causing systemic oxalate-related disease, particularly in the bones. Besides, it was remarkable that there was heterogeneity in manifestations of systemic deposits between patients. These outcomes underscore the need to thoroughly screen the retina, heart, and bones in patients with infantile oxalosis for oxalate deposits, especially when this finding has therapeutic consequences (e.g., timing of transplantation).
We found multiple patients with genotypes associated with vitamin B6 responsiveness in our cohort, revealing there is a large phenotypic spectrum of vitamin B6-responsive patients. This implies that early initiation of vitamin B6 therapy in patients with infantile oxalosis, even before the diagnosis is genetically confirmed, might be beneficial in some cases. The allelic frequency of AGXT mutation c.508G>A in the infantile oxalosis cohort (13%) is lower compared with the allelic frequency in the total OxalEurope PH1 registry (25%) (unpublished registry data). This is in contrast to the allelic frequency of AGXT mutation c.33dupC, which was higher among children with infantile oxalosis (19%), compared with the total PH1 registry (10%) (unpublished registry data). These findings suggest that certain genotypes may be associated with an earlier onset of the disease, which is in line with the findings of Williams et al.1 However, more research on the correlation between genotype with phenotype is required because we also found a remarkable difference in expressivity of the disease within 14 families in this study, which has previously been described.7,23,24 Possible explanations for this variability may be other yet to be discovered genetic modifiers or environmental factors (e.g., dehydration, spiking the intrarenal oxalate supersaturation) that might have provoked the severe clinical course in these infants. Insight and literature on genetic modifiers are currently lacking owing to the rarity of the disease, complicating research on this topic. Hence, more international genotype-phenotype studies would be helpful to gain better insights in the onset of this disease.
Although the numbers were small, a striking finding was that despite family screening and early diagnosis, rapid progression to ESKD could not be prevented in all patients. In our cohort, 4 infantile patients found by family screening nevertheless developed ESKD, and 2 of them died. It is unclear why ESKD could not be prevented in these patients. However, in at least 1 of these patients, intensive conservative management was started early after birth. A similar course of events was found in another PH1 cohort, where 6 of 45 patients developed ESKD despite diagnosis by family screening.25 It reveals how difficult prevention of kidney failure can be in PH1, even if the diagnosis is established early.
In our cohort, the combined and sequential transplantation strategy had similar patient and graft survival. We did, however, find small differences between both transplantation strategies that may be clinically relevant. For instance, LTx as part of a sequential procedure was performed at a younger age than combined LKTx. However, we found that plasma oxalate levels declined faster after LKTx than following LTx. This is explained by the lack of kidney function in the latter group as dialysis is not sufficient to adequately remove all oxalate from the plasma.26 Nevertheless, no firm conclusions can be drawn based on these data due to the retrospective character of the study. Therefore, we suggest that the most suitable transplantation strategy should be chosen case by case, based on the experience of the center, organ availability, and clinical features of the infant.
The future perspective of patients with infantile oxalosis might be hopeful, as new less-invasive RNA interference therapeutics have emerged, which were found to be highly effective in reducing oxalate production in patients with PH1.8 However, it remains to be found as to what extent they can reduce clinical disease and form a substitute for LTx.27
Our study has limitations, particularly those of a retrospective registry study. Some data were missing and follow-up and treatment differed from center to center. Consequently, not all patients underwent screening for systemic oxalosis routinely. Furthermore, these differences in therapeutic management caused heterogeneity within groups, possibly hindering identification of outcome differences between treatment modalities. In 2 patients, genetic testing for PH1 was not possible due to early death and the diagnosis was based on high urinary glycolate and oxalate levels.4 Furthermore, owing to the rarity of the disease, the numbers in this study are small. We used appropriate statistical analyses where possible, though this should be taken in consideration when interpreting results. Furthermore, there might be a small positive selection bias in our cohort as patients with infantile oxalosis might have died before a diagnosis of PH1 was established owing to the rarity of this disease.
In conclusion, we found a high mortality rate among children with infantile oxalosis and comorbidity due to systemic oxalosis was present in nearly all screened patients. We found significant intrafamilial phenotype variability. There were 4 patients who developed infantile oxalosis despite early diagnosis by family screening, illustrating how difficult prevention of ESKD in early established PH1 can be. Nevertheless, our study reveals that the survival has significantly improved in the last decades, presumably as a result of improved dialysis and transplantation techniques. With new therapeutics, the outcome of this most vulnerable subgroup of patients with PH1 might improve even further.
Disclosure
LJD, SFG, MJSO, and JWG have received an unconditional grant from both Alnylam Pharmaceuticals and Dicerna Pharmaceuticals, not related to this work. BH is the head of Global Medical Affairs at Dicerna Pharmaceuticals. SAH and PC have received consultation fees and travel grants from Dicerna Pharmaceuticals and Alnylam Pharmaceuticals. JB and GD have received consultation and/or speaking fees from Dicerna Pharmaceuticals, Alnylam Pharmaceuticals and Biocodex. JH, BBB, GM, and LC have received consultation fees from Alnylam Pharmaceuticals. All the other authors declared no competing interests.
Acknowledgments
The authors thank all patients, guardians, and treating clinicians for their contribution. The authors also thank all scientists of the OxalEurope Consortium for assisting with data collection. Several authors of this publication are members of the European Rare Kidney Disease Reference Network.
Footnotes
Figure S1. Sensitivity analysis of patient survival of children with infantile oxalosis, stratified by year of birth.
Table S1. Country of residency of infantile oxalosis patients.
Table S2. Complete list of genotypes in infantile oxalosis cohort.
STROBE Statement.
Supplementary Material
Figure S1. Sensitivity analysis of patient survival of children with infantile oxalosis, stratified by year of birth.
Table S1. Country of residency of infantile oxalosis patients.
Table S2. Complete list of genotypes in infantile oxalosis cohort.
STROBE Statement.
References
- 1.Williams E.L., Acquaviva C., Amoroso A., et al. Primary hyperoxaluria type 1: update and additional mutation analysis of the AGXT gene. Hum Mutat. 2009;30:910–917. doi: 10.1002/humu.21021. [DOI] [PubMed] [Google Scholar]
- 2.Cochat P., Rumsby G. Primary hyperoxaluria [published correction appears in N Engl J Med. 2013;369:2168] N Engl J Med. 2013;369:649–658. doi: 10.1056/NEJMra1301564. [DOI] [PubMed] [Google Scholar]
- 3.Hoppe B., Beck B.B., Milliner D.S. The primary hyperoxalurias. Kidney Int. 2009;75:1264–1271. doi: 10.1038/ki.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cochat P., Hulton S.A., Acquaviva C., et al. Primary hyperoxaluria type 1: indications for screening and guidance for diagnosis and treatment. Nephrol Dial Transplant. 2012;27:1729–1736. doi: 10.1093/ndt/gfs078. [DOI] [PubMed] [Google Scholar]
- 5.Cochat P., Liutkus A., Fargue S., et al. Primary hyperoxaluria type 1: still challenging. Pediatr Nephrol. 2006;21:1075–1081. doi: 10.1007/s00467-006-0124-4. [DOI] [PubMed] [Google Scholar]
- 6.Leumann E.P., Niederwieser A., Fanconi A. New aspects of infantile oxalosis. Pediatr Nephrol. 1987;1:531–535. doi: 10.1007/BF00849265. [DOI] [PubMed] [Google Scholar]
- 7.Frishberg Y., Rinat C., Shalata A., et al. Intra-familial clinical heterogeneity: absence of genotype-phenotype correlation in primary hyperoxaluria type 1 in Israel. Am J Nephrol. 2005;25:269–275. doi: 10.1159/000086357. [DOI] [PubMed] [Google Scholar]
- 8.Garrelfs S.F., Frishberg Y., Hulton S.A., et al. Lumasiran, an RNAi therapeutic for primary hyperoxaluria type 1. N Engl J Med. 2021;384:1216–1226. doi: 10.1056/NEJMoa2021712. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz G.J., Munoz A., Schneider M.F., et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz G.J., Haycock G.B., Edelmann C.M., Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58:259–263. [PubMed] [Google Scholar]
- 11.van Woerden C.S., Groothoff J.W., Wijburg F.A., et al. Clinical implications of mutation analysis in primary hyperoxaluria type 1. Kidney Int. 2004;66:746–752. doi: 10.1111/j.1523-1755.2004.00796.x. [DOI] [PubMed] [Google Scholar]
- 12.Mandrile G., Van Woerden C.S., Berchialla P., et al. Data from a large European study indicate that the outcome of primary hyperoxaluria type 1 correlates with the AGXT mutation type. Kidney Int. 2014;86:1197–1204. doi: 10.1038/ki.2014.222. [DOI] [PubMed] [Google Scholar]
- 13.Fargue S., Rumsby G., Danpure C.J. Multiple mechanisms of action of pyridoxine in primary hyperoxaluria type 1. Biochim Biophys Acta. 2013 doi: 10.1016/j.bbadis.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Shapiro R., Weismann I., Mandel H., et al. Primary hyperoxaluria type 1: improved outcome with timely liver transplantation: a single-center report of 36 children. Transplantation. 2001;72:428–432. doi: 10.1097/00007890-200108150-00012. [DOI] [PubMed] [Google Scholar]
- 15.Jellouli M., Ferjani M., Abidi K., et al. Primary hyperoxaluria in infants. Saudi J Kidney Dis Transpl. 2016;27:526–532. doi: 10.4103/1319-2442.182389. [DOI] [PubMed] [Google Scholar]
- 16.Harambat J., Fargue S., Acquaviva C., et al. Genotype-phenotype correlation in primary hyperoxaluria type 1: the p.Gly170Arg AGXT mutation is associated with a better outcome. Kidney Int. 2010;77:443–449. doi: 10.1038/ki.2009.435. [DOI] [PubMed] [Google Scholar]
- 17.Guillaume A., Chiodini B., Adams B., et al. The struggling odyssey of infantile primary hyperoxaluria. Front Pediatr. 2021;9:615183. doi: 10.3389/fped.2021.615183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carey W.A., Martz K.L., Warady B.A. Outcome of patients initiating chronic peritoneal dialysis during the first year of life. Pediatrics. 2015;136:e615–e622. doi: 10.1542/peds.2015-0980. [DOI] [PubMed] [Google Scholar]
- 19.Song A.T., Avelino-Silva V.I., Pecora R.A., et al. Liver transplantation: fifty years of experience. World J Gastroenterol. 2014;20:5363–5374. doi: 10.3748/wjg.v20.i18.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cochat P., Nogueira P.C.K., Mahmoud M.A., et al. Primary hyperoxaluria in infants: medical, ethical, and economic issues. J Pediatr. 1999;135:746–750. doi: 10.1016/s0022-3476(99)70095-8. [DOI] [PubMed] [Google Scholar]
- 21.Small K.W., Letson R., Scheinman J. Ocular findings in primary hyperoxaluria. Arch Ophthalmol. 1990;108:89–93. doi: 10.1001/archopht.1990.01070030095036. [DOI] [PubMed] [Google Scholar]
- 22.Birtel J., Herrmann P., Garrelfs S.F., et al. The ocular phenotype in primary hyperoxaluria type 1. Am J Ophthalmol. 2019;206:184–191. doi: 10.1016/j.ajo.2019.04.036. [DOI] [PubMed] [Google Scholar]
- 23.Hoppe B., Danpure C., Rumsby G., et al. A vertical (pseudodominant) pattern of inheritance in the autosomal recessive disease primary hyperoxaluria type 1: lack of relationship between genotype, enzymic phenotype, and disease severity. Am J Kidney Dis. 1997;29:36–44. doi: 10.1016/s0272-6386(97)90006-8. [DOI] [PubMed] [Google Scholar]
- 24.Alfadhel M., Alhasan K.A., Alotaibi M., Al Fakeeh K. Extreme intrafamilial variability of Saudi brothers with primary hyperoxaluria type 1. Ther Clin Risk Manag. 2012;8:373–376. doi: 10.2147/TCRM.S34954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sas D.J., Enders F.T., Mehta R.A., et al. Clinical features of genetically confirmed patients with primary hyperoxaluria identified by clinical indication versus familial screening. Kidney Int. 2020;97:786–792. doi: 10.1016/j.kint.2019.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Illies F., Bonzel K.E., Wingen A.M., et al. Clearance and removal of oxalate in children on intensified dialysis for primary hyperoxaluria type 1. Kidney Int. 2006;70:1642–1648. doi: 10.1038/sj.ki.5001806. [DOI] [PubMed] [Google Scholar]
- 27.Méaux M.N., Sellier-Leclerc A.L., Acquaviva-Bourdain C., et al. The effect of lumasiran therapy for primary hyperoxaluria type 1 in small infants. Pediatr Nephrol. 2022;37:907–911. doi: 10.1007/s00467-021-05393-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.