Abstract
Introduction
Earlier identification of individuals at high risk of chronic kidney disease (CKD) may facilitate improved risk factor mitigation.
Methods
We evaluated the association of novel plasma biomarkers with incident CKD using a case-cohort design in participants without diabetes and with baseline estimated glomerular filtration rate (eGFR) ≥ 60 ml/min per 1.73 m2 in the Multi-Ethnic Study of Atherosclerosis (MESA) and Reasons for Geographic and Racial Differences in Stroke (REGARDS) cohorts. Incident CKD was defined as development of eGFR < 60 ml/min per 1.73 m2 and ≥40% decline in eGFR from baseline. We measured plasma markers of inflammation/fibrosis—soluble tumor necrosis factor receptors (TNFRs) 1 and 2 (TNFR-1 and TNFR-2), monocyte chemotactic protein-1 (MCP-1), chitinase 3-like protein 1 (YKL-40), and soluble urokinase-type plasminogen activator receptor (suPAR)—and tubular injury (kidney injury molecule 1 [KIM-1]). Cox regression models weighted for the case-cohort design were used to estimate hazard ratios (HRs) of incident CKD after adjustment for CKD risk factors, eGFR, and albuminuria.
Results
In MESA (median follow-up of 9.2 years), there were 497 individuals in the random subcohort and 163 incident CKD cases. In REGARDS (median follow-up of 9.4 years), there were 497 individuals in the random subcohort and 497 incident CKD cases. Each 2-fold higher plasma KIM-1 (adjusted HR 1.38 [95% CI 1.05–1.81]), suPAR (1.96 [1.10–3.49]), TNFR-1 (1.65 [1.04–2.62]), TNFR-2 (2.02 [1.21–3.38]), and YKL-40 (1.38 [1.09–1.75]) concentrations were associated with incident CKD in MESA. In REGARDS, TNFR-1 (1.99 [1.43–2.76]) and TNFR-2 (1.76 [1.22–2.54]) were associated with incident CKD.
Conclusion
Plasma concentrations of soluble TNFR-1 and TNFR-2 are consistently associated with incident CKD in nondiabetic community-living individuals in MESA and REGARDS.
Keywords: chronic kidney disease, plasma biomarkers, risk factors for chronic kidney disease
Graphical abstract
CKD is an independent risk factor for cardiovascular disease (CVD) and mortality.1,2 Of those individuals with an eGFR of 44 to 59 ml/min per 1.73 m2, the lifetime risk of kidney failure is 7.5% in men and 3.2% in women.3 A much higher percentage of adults with CKD will develop CVD and incur significant costs, currently estimated at approximately $40 billion in the United States annually for persons with CKD and eGFR 30 to 60 ml/min per 1.73 m2 who use Medicare.4 Efforts to prevent CKD are therefore of great public health importance.
One of the major limiting factors in the prevention of CKD is the lack of sensitive biomarkers to quantify its risk, especially among persons without diabetes. Serum creatinine concentration, the current biomarker most often used to estimate GFR, has important limitations. These include variations with race, age, sex, muscle mass, and health status, which result in its being a relatively insensitive marker of early kidney damage. Furthermore, by the time serum creatinine level is elevated, most kidney biopsy results show considerable interstitial fibrosis and tubular atrophy.5 Thus, to truly prevent CKD, we need more sensitive early markers before scarring, and presumably, irreversible damage occurs. Although albuminuria may reflect glomerular or tubular damage, it is frequently in the normal or very low range in those who go on to develop CKD.6 Furthermore, although biomarkers that are associated with the progression of CKD have been identified,7 there are very few biomarkers that have been evaluated to help identify the risk of developing CKD, independent of albuminuria and eGFR.
A primary goal of the National Institute of Diabetes and Digestive and Kidney Diseases Biomarkers Consortium was to evaluate candidate biomarkers for the onset and progression of CKD. Accordingly, we evaluated the association of several novel biomarkers, including markers of inflammation/fibrosis—soluble TNFR-1 and TNFR-2, YKL-40, MCP-1,8, 9, 10, 11 and suPAR12—and tubular injury (KIM-1)13,14 for incident CKD. We compared the strength and consistency of the associations of these plasma proteins as risk factors for incident CKD in individuals without diabetes and with eGFR ≥ 60 ml/min per 1.73 m2 at baseline, because this is a specific population where albuminuria may be less prevalent and less useful for risk stratification of disease progression. We evaluated the associations of plasma biomarkers and incident CKD in 2 community-based samples—the MESA and the REGARDS studies.
Methods
Study Populations
MESA is a cohort study initiated by the National Heart, Lung, and Blood Institute to investigate the correlates, predictors, and progression of CVD in a diverse population-based sample of men and women, aged 45 to 84 years (mean 60 years) at entry (2000–2002), who had no evidence of clinical CVD at baseline. Overall, 6814 individuals were enrolled between July 2000 and August 2002 (28% Black, 38% White, 22% Hispanic, 12% Chinese, and 53% women). Details of the study design are published elsewhere.15
The REGARDS study is a population-based, cohort study designed to evaluate underlying causes for racial and regional differences in stroke mortality in the United States. Individuals aged ≥45 years were recruited (mean age 64 years). Overall, 30,239 individuals were enrolled between January 2003 and October 2007 (42% Black, 55% women). Details of the study design have been published elsewhere.16
Written informed consent was obtained from study participants from both MESA and REGARDS.
Study Design and Outcomes
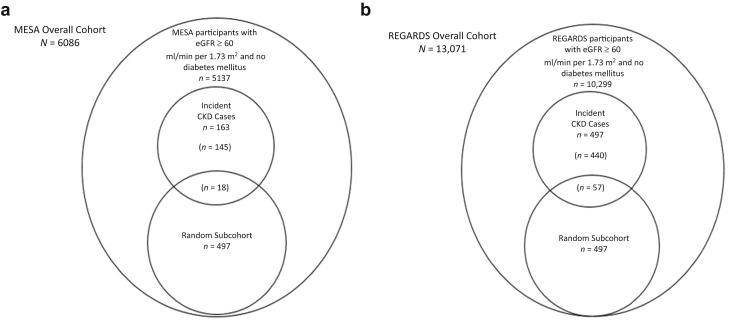
We used a case-cohort study design in both cohorts (Figure 1a and b). In MESA, cases included all individuals without diabetes and an eGFR ≥ 60 ml/min per 1.73 m2 at baseline who developed incident CKD, defined as the development of an eGFR < 60 ml/min per 1.73 m2 in follow-up and at least a 40% decline in eGFR from baseline to visits that occurred after a mean of 3.1, 4.7, and 9.4 years of follow-up (n = 163). Subcohort participants were randomly selected from the total cohort of individuals without diabetes and an eGFR ≥ 60 ml/min per 1.73 m2 at baseline (n = 497 of 5137 available). Because some individuals selected in the random subcohort met the case definition during follow-up (n = 18), the total sample size was 642. In REGARDS, cases included a subset of individuals without diabetes and an eGFR ≥ 60 ml/min per 1.73 m2 at baseline who developed incident CKD, defined as the development of an eGFR < 60 ml/min per 1.73 m2 and at least a 40% decline in eGFR from baseline to the first follow-up visit at a median of 9.4 years (n = 497). Subcohort participants were randomly selected from individuals without diabetes and an eGFR ≥ 60 ml/min per 1.73 m2 at baseline (n = 497 of 10,299 available). Because 57 cases occurred among individuals selected in the random subcohort, the total sample size was 937. Estimated GFR was determined from isotope dilution mass spectrometry-traceable serum creatinine concentration measurements using the CKD Epidemiology Collaboration equation.17 In MESA, creatinine was measured by rate reflectance spectrophotometry using thin-film adaptation of the creatinine amidinohydrolase method on the Vitros analyzer (Johnson & Johnson Clinical Diagnostics Inc., Raritan, NJ; www.orthoclinical.com) at the Collaborative Studies Clinical Laboratory at Fairview-University Medical Center (Minneapolis, MN) and calibrated to the Cleveland Clinic. In REGARDS, creatinine was also measured by a Vitros analyzer and calibrated to an international isotope dilution mass spectroscopic-traceable standard.
Figure 1.
Sampling of (a) MESA and (b) REGARDS cohorts per case-cohort design. (a) Among 6086 MESA participants, a total of 5137 had eGFR > 60 ml/min per 1.73 m2 and were nondiabetic at baseline, and a subcohort of 497 individuals was randomly selected from those participants. There were 163 cases of incident CKD, 18 of whom had also been selected into the subcohort and 145 cases arising outside the subcohort. (b) Among 13,071 REGARDS participants with 2 measures of serum creatinine, a total of 10,299 had eGFR > 60 ml/min per 1.73 m2 and were nondiabetic at baseline. A subcohort of 497 individuals was randomly selected from those participants. There were 497 cases of incident CKD, 57 of whom had also been selected into the subcohort and 440 cases arising outside the subcohort. CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; MESA, Multi-Ethnic Study of Atherosclerosis; REGARDS, Reasons for Geographic and Racial Differences in Stroke.
Exposure Variables
The primary exposures were plasma concentrations of TNFR-1, TNFR-2, YKL-40, MCP-1, suPAR, and KIM-1 sampled at participants’ baseline visits. Biomarkers were measured using samples stored at −80 °C after a single freeze-thaw using a multiplex assay on the Meso Scale Discovery platform (Meso Scale Discovery, Gaithersburg, MD) in the BioCon Central Laboratory at Brigham and Women’s Hospital. Each biomarker was measured in duplicate, and results were averaged to improve precision. All assays were performed blinded to clinical outcomes. Biomarker measurements were repeated on a participant’s plasma sample if ≥2 analytes had intra-assay coefficients of variation > 15%. The intra-assay and interassay coefficients of variation for all exposure variables were all <10%.
Covariates
Age, sex, race, smoking history, and educational attainment were determined by self-report in both cohorts. Body mass index was determined using measured height and weight. Blood pressure was defined as the average of 3 measures in MESA and 2 measures in REGARDS taken on seated participants after a 5-minute rest. The use of medications for hypertension was obtained by self-report. Urine albumin concentration was measured by the BNII ProSpec nephelometer (Siemens AG, Munich, Germany), and urine creatinine concentration was measured by the rate Jaffé method (Roche/Hitachi, Basel, Switzerland). The urine albumin-to-creatinine ratio (ACR) was expressed as mg/g.
Statistical Analysis
Descriptive statistics were used to compare participant characteristics in each of the 2 randomly selected subcohorts in MESA and REGARDS and by the presence of incident CKD. Age- and sex-adjusted Pearson pairwise correlations were used to evaluate associations of plasma biomarkers with each other and with eGFR and ACR. The risk of incident CKD was modeled using a time-to-event analysis with multivariable Cox regressions modified to account for the case-cohort design. We used the original pseudolikelihood method proposed by Prentice, weighted such that risk sets at event times consist of random subcohort members at risk whereas the cases outside the subcohort enter the risk sets only at their failure event times.18 Model 1 was adjusted for age, sex, race, education, body mass index, systolic blood pressure, hypertension medications, and smoking status. There was additional adjustment for prevalent CVD in REGARDS. Model 2 was adjusted additionally for baseline eGFR and ACR. In all models, plasma biomarkers were analyzed in quartiles, with the lowest quartile serving as the referent group, and on a continuous scale after log base 2 transformation (interpreted as per 2-fold higher concentration of each biomarker). Because our exposure variables were highly correlated, we used a data-driven regularization method to select the best set of biomarkers. The least absolute shrinkage and selection operator (Lasso) is a good method for automatic variable selection.19 This regression method penalizes the absolute size of the regression coefficients. A Lasso penalty with leave-one-out cross-validation was used to estimate the penalty parameters, and results were reported using the penalty that gave the best cross-validated fit. We used the implementation in the R glmnet package. The biomarkers selected from the Lasso method were then refitted in the Cox proportional hazards regression models. We evaluated effect modification by race and sex by testing the statistical significance of a multiplicative interaction term in fully adjusted models. We then performed a sensitivity analysis excluding those with ACR > 30 mg/g at baseline. Finally, we evaluated demographic and cardiovascular- and kidney-related factors as risk factors for incident CKD in a multivariable model. A 2-tailed P < 0.05 was considered statistically significant for all analyses, including interaction terms. Analyses were performed using IBM SPSS version 26 (IBM Corp., Armonk, NY) and R Core Team (2020) (R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria; URL: https://www.R-project.org/).
Results
Study Populations
Table 1 shows the baseline characteristics across the 2 randomly selected subcohorts of individuals in MESA and REGARDS and by incident CKD status. In the MESA subcohort, the mean age was 60 years, 47% were men, 24% were Black, mean eGFR was 91 ml/min per 1.73 m2, and median ACR was 5 mg/g. Those who developed incident CKD during the follow-up (cases) were older, more likely Black, and had higher blood pressure, body mass index, and ACR. In the REGARDS subcohort, the mean age was 62 years, 43% were men, 30% were Black, and mean eGFR was 90 ml/min per 1.73 m2 and median ACR 5.9 mg/g. Those who developed incident CKD during the follow-up were older and more likely to have hypertension. There were 15% and 13% of individuals in MESA and REGARDS who developed diabetes by the end of the follow-up, respectively.
Table 1.
Characteristics at baseline by subcohort and by incident CKD status
| Baseline characteristics | MESA |
REGARDS |
||||
|---|---|---|---|---|---|---|
| Subcohort | No CKD | Incident CKD | Subcohort | No CKD | Incident CKD | |
| N | 497 | 479 | 163 | 497 | 440 | 497 |
| Age (yr) | 60 (10) | 59 (10) | 66 (9) | 63 (8) | 62 (8) | 65 (9) |
| Men (%) | 47 | 48 | 33 | 42 | 43 | 33 |
| Black (%) | 24 | 24 | 34 | 30 | 30 | 32 |
| Education (%) | ||||||
| <High school | 15 | 14 | 22 | 8 | 9 | 8 |
| High school graduate | 17 | 17 | 20 | 22 | 21 | 27 |
| Some college | 25 | 25 | 34 | 23 | 23 | 25 |
| ≥College graduate | 44 | 44 | 24 | 47 | 48 | 41 |
| BMI (kg/m2) | 27.9 (5.1) | 27.9 (5.1) | 29.6 (6.2) | 28.4 (5.8) | 28.5 (5.7) | 28.6 (5.4) |
| HTN (%) | 36 | 35 | 65 | 44 | 43 | 51 |
| CAD (%) | 0 | 0 | 0 | 11 | 10 | 13 |
| Smoking status (%) | ||||||
| Never | 48 | 50 | 49 | 49 | 48 | 49 |
| Former | 37 | 37 | 37 | 43 | 43 | 39 |
| Current | 15 | 14 | 14 | 8 | 8 | 12 |
| SBP (mm Hg) | 123 (21) | 123 (21) | 136 (23) | 125 (16) | 125 (16) | 127 (16) |
| Antihypertensive medication (%) | 30 | 29 | 55 | 37 | 36 | 43 |
| eGFR (ml/min per 1.73 m2) | 91 (14) | 91 (14) | 85 (14) | 90 (14) | 90 (14) | 87 (13) |
| UACR (mg/g) | 5 (3–9) | 4.6 (3.1–8.8) | 8.8 (4.5–24.7) | 6.4 (4.4–11.1) | 5.9 (4.2–9.5) | 7.0 (4.8–13.1) |
| UACR > 30 mg/g | 7 | 6 | 21 | 8 | 7 | 11 |
BMI, body mass index; CAD, coronary artery disease; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HTN, hypertension; IQR, interquartile range; MESA, Multi-Ethnic Study of Atherosclerosis; REGARDS, Reasons for Geographic and Racial Differences in Stroke; SBP, systolic blood pressure; UACR, urine albumin-to-creatinine ratio.
Data are presented as mean (SD), median (IQR), or percentage.
Correlations
Table 2 shows the age- and sex-adjusted Pearson correlations among individual plasma biomarkers and eGFR and ACR in MESA. The strongest intercorrelations were among TNFR-1, TNFR-2, and suPAR, followed by moderate inverse correlations of TNFR-1 and TNFR-2 with eGFR. Similarly, in REGARDS, the strongest pairwise correlation was between TNFR-1 and TNFR-2, and each of those proteins had a moderate positive correlation with suPAR and a negative correlation with eGFR.
Table 2.
Partial Pearson correlations evaluating associations of plasma biomarkers with each other and with eGFR and UACR in MESA and REGARDS
| Plasma biomarkers | MESA |
REGARDS |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KIM-1 | MCP-1 | suPAR | TNFR-1 | TNFR-2 | YKL-40 | UACR | eGFR | KIM-1 | MCP-1 | suPAR | TNFR-1 | TNFR-2 | YKL-40 | UACR | eGFR | |
| KIM-1 | 1.000 | 0.169a | 0.184a | 0.243a | 0.147a | 0.268a | 0.267a | −0.157a | 1.000 | 0.154a | 0.234a | 0.272a | 0.163a | 0.217a | 0.150a | –0.215a |
| MCP-1 | 1.000 | 0.328a | 0.264a | 0.253a | 0.139a | 0.122a | –0.084 | 1.000 | 0.356a | 0.330a | 0.332a | 0.189a | 0.107a | –0.225a | ||
| suPAR | 1.000 | 0.603a | 0.547a | 0.285a | 0.090a | –0.184a | 1.000 | 0.553a | 0.551a | 0.244a | 0.034 | –0.261a | ||||
| TNFR-1 | 1.000 | 0.695a | 0.322a | 0.050 | –0.394a | 1.000 | 0.778a | 0.262a | –0.033 | –0.482a | ||||||
| TNFR-2 | 1.000 | 0.334a | 0.037 | –0.268a | 1.000 | 0.298a | –0.003 | –0.449a | ||||||||
| YKL-40 | 1.000 | 0.157a | –0.227a | 1.000 | 0.109a | –0.222a | ||||||||||
| UACR | 1.000 | –0.032 | 1.000 | 0.031 | ||||||||||||
| eGFR | 1.000 | 1.000 | ||||||||||||||
eGFR, estimated glomerular filtration rate; KIM-1, kidney injury molecule 1; MCP-1, monocyte chemotactic protein-1; MESA, Multi-Ethnic Study of Atherosclerosis; REGARDS, Reasons for Geographic and Racial Differences in Stroke; suPAR, soluble urokinase-type plasminogen activator receptor; TNFR, tumor necrosis factor receptor; YKL-40, chitinase 3-like protein 1; UACR, urine albumin-to-creatinine ratio.
All analyses are adjusted for age and sex.
P < 0.05.
Association of Plasma Biomarkers With Incident CKD
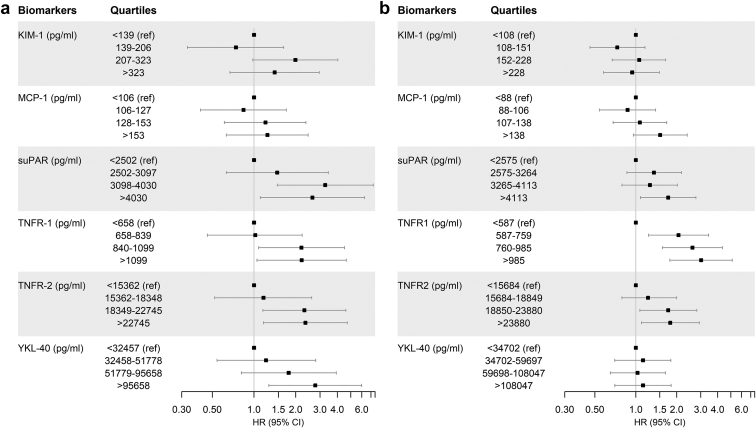
In MESA, during a median (interquartile range) follow-up of 9.2 (5.5–9.6) years, there were 163 incident CKD events. In the adjusted models, KIM-1, suPAR, TNFR-1, TNFR-2, and YKL-40 were individually associated with incident CKD. These results were consistent in continuous (Table 3) and quartile-based analyses (Figure 2a) and remained statistically significant after multivariable adjustment.
Table 3.
Association of plasma biomarkers with incident CKD in MESA and REGARDS
| Plasma biomarkers | HR (95% CI) of incident CKD in MESA |
HR (95% CI) of incident CKD in REGARDS |
||||
|---|---|---|---|---|---|---|
| Unadjusted | Model 1 | Model 2 | Unadjusted | Model 1a | Model 2 | |
| KIM-1 | 1.79 (1.46–2.20) | 1.49 (1.15–1.91) | 1.38 (1.05–1.81) | 1.14 (0.97–1.33) | 1.12 (0.95–1.31) | 1.11 (0.94–1.31) |
| MCP-1 | 1.59 (1.14–2.22) | 1.18 (0.79–1.77) | 1.17 (0.76–1.79) | 1.32 (1.05–1.66) | 1.24 (0.97–1.59) | 1.25 (0.98–1.59) |
| suPAR | 3.04 (2.08–4.45) | 2.19 (1.28–3.76) | 1.96 (1.10–3.49) | 1.33 (1.02–1.74) | 1.24 (0.93–1.67) | 1.28 (0.95–1.72) |
| TNFR-1 | 2.53 (1.80–3.55) | 1.82 (1.19–2.76) | 1.65 (1.04–2.62) | 1.57 (1.19–2.06) | 1.78 (1.31–2.41) | 1.99 (1.43–2.76) |
| TNFR-2 | 3.39 (2.25–5.12) | 2.37 (1.44–3.89) | 2.02 (1.21–3.38) | 1.52 (1.11–2.07) | 1.51 (1.06–2.13) | 1.76 (1.22–2.54) |
| YKL-40 | 1.90 (1.57–2.30) | 1.38 (1.10–1.73) | 1.38 (1.09–1.75) | 1.08 (0.95–1.24) | 1.05 (0.91–1.22) | 1.07 (0.92–1.24) |
ACR, albumin-to-creatinine ratio; BMI, body mass index; CAD, coronary artery disease; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HR, hazard ratio; KIM-1, kidney injury molecule 1; MCP-1, monocyte chemotactic protein-1; MESA, Multi-Ethnic Study of Atherosclerosis; REGARDS, Reasons for Geographic and Racial Differences in Stroke; suPAR, soluble urokinase-type plasminogen activator receptor; TNFR, tumor necrosis factor receptor; YKL-40, chitinase 3-like protein 1.
HR is per 2-fold higher level. Bold represents results that are significant at P < 0.05. Model 1 was adjusted for age, sex, race, education, BMI, systolic blood pressure, use of hypertension medications, and smoking status. Model 2 was model 1 with additional adjustment for baseline eGFR and ACR.
In REGARDS, model 1 was also additionally adjusted for CAD.
Figure 2.
(a) MESA: adjusted HR (95% CI) of incident CKD as a function of each biomarker in models adjusted for age, race, sex, education, body mass index, systolic blood pressure, use of antihypertensive medications, smoking status, urine albumin-to-creatinine ratio, and estimated glomerular filtration rate. All biomarkers were analyzed in quartiles. (b) REGARDS: adjusted HR (95% CI) of incident CKD as a function of each biomarker in models adjusted for age, race, sex, education, body mass index, systolic blood pressure, use of antihypertensive medications, smoking status, urine albumin-to-creatinine ratio, estimated glomerular filtration rate, and coronary artery disease. All biomarkers were analyzed in quartiles. CKD, chronic kidney disease; HR, hazard ratio; KIM-1, kidney injury molecule 1; MCP-1, monocyte chemotactic protein-1; MESA, Multi-Ethnic Study of Atherosclerosis; REGARDS, Reasons for Geographic and Racial Differences in Stroke; suPAR, soluble urokinase-type plasminogen activator receptor; TNFR, tumor necrosis factor receptor; YKL-40, chitinase 3-like protein 1.
In REGARDS, during a median (interquartile range) follow-up of 9.4 (8.6–9.9) years, there were 497 incident CKD events. In univariate (or demographic-adjusted) analyses, MCP-1, suPAR, TNFR-1, and TNFR-2 were associated with incident CKD. After full adjustment, however, several of these associations were attenuated, and only TNFR-1 and TNFR-2 remained significantly associated with incident CKD (Table 3 and Figure 2b). Comparing the highest versus lowest quartiles, the magnitude of association was largest for TNFR-1 with approximately a 3-fold difference in risk.
When evaluating biomarkers in parallel to identify the best set of markers using Lasso regression, KIM-1, TNFR-1, and YKL40 were chosen in both cohorts, with YKL-40 reaching statistical significance (HR of 1.29 per doubling [95% CI 1.02–1.64]) in MESA and TNFR-1 (HR 1.98 per doubling [95% CI 1.40–2.80]) reaching statistical significance in REGARDS. There were no interactions between the biomarkers and either race or sex for risk of incident CKD, with all P values for interactions > 0.05. Results were consistent when excluding those with ACR > 30 mg/g at baseline (data not shown). Finally, age, hypertensive medications, and ACR were independent risk factors for incident CKD in MESA, whereas female sex, high school graduates (in comparison to those who did not graduate from high school), and ACR were independent risk factors for incident CKD in REGARDS (Supplementary Table S1).
Discussion
The present investigation is one of the first studies to evaluate the relationships among a comprehensive set of novel plasma biomarkers of inflammation/fibrosis, tubular injury, and repair with incident CKD in nondiabetic individuals. In MESA, plasma KIM-1, suPAR, TNFR-1, TNFR-2, and YKL-40 were all associated with incident CKD independent of baseline eGFR, albuminuria, or CKD risk factors, whereas TNFR-1 and TNFR-2 were also associated with incident CKD in REGARDS. These findings suggest that novel biomarkers of inflammation/fibrosis and tubular injury can be used to identify risk of developing nondiabetic CKD. In this particular study, TNFR-1 and TNFR-2 were the strongest and most consistent across these 2 large cohorts.
Several prior and ongoing studies have evaluated the biomarkers that were evaluated in this analysis in participants with either diabetes or advanced CKD. For example, a recent study from the Biomarkers Consortium demonstrated that TNFR-1, TNFR-2, KIM-1, MCP-1, suPAR, and YKL-40 were all associated with progression of CKD in individuals with diabetes and eGFR < 60 ml/min per 1.73 m2 in the Chronic Renal Insufficiency Cohort Study.7 Similarly, in the Biomarkers Consortium, TNFR-1, TNFR-2, and KIM-1 were associated with progression to end-stage kidney disease in the Chronic Kidney Disease in Children Study.20 TNFR-1 and TNFR-2 were also associated with progression to end-stage kidney disease in individuals with a range of eGFR and type 2 diabetes.9
There are far fewer studies, however, that have evaluated incident CKD as an outcome, and most have focused on urine biomarkers21,22 or have been in individuals with diabetes.8 For example, urinary trefoil factor 3 was associated with incident CKD in the Atherosclerosis Risk and Communities Study21 and TNFR-1 and TNFR-2 were associated with incident CKD in type 1 diabetes.8 suPAR has been associated with incident CKD in a predominantly nondiabetic population,12 and in a recent study from MESA, TNFR-1 was also associated with faster declines in eGFR in a predominantly nondiabetic population.23 The latter studies are limited in that they included only 1 biomarker in a single cohort.
TNFR-1 and TNFR-2 reflect inflammatory pathway activation. The exact mechanism by which TNF receptors contribute to incident CKD is not clear, but strong associations with kidney outcomes in multiple cohorts of children and adults with and without CKD and with and without diabetes demonstrate an integral role in the pathway of GFR decline. MCP-1 is a chemokine that leads to the recruitment of monocytes, whereas circulating suPAR is released into the circulation during inflammation. YKL-40 is an indicator of tubular injury severity and may play a role in limiting tubular cell apoptosis during the repair phase of acute kidney injury.24 KIM-1 is a marker of proximal tubular injury and is detectable in the blood and urine after shedding of the KIM-1 ectodomain.13
In the current analyses, we noted that several biomarkers were associated with incident CKD in nondiabetic individuals in MESA; however, only TNFR-1 and TNFR-2 were associated with incident CKD in both studies. There are several potential reasons for these differences. First, MESA was selected to be free of CVD at baseline and had a lower percentage of Black patients in contrast with REGARDS. Second, in REGARDS, some of the traditional kidney-related risk factors were not risk factors for incident CKD, such as elevated blood pressure level and Black race. Third, the HRs for MCP-1 and suPAR were in the expected direction in REGARDS and significant in univariate analyses, so it is possible that there was insufficient statistical power to detect a moderate independent association. Last, in MESA, there were 3 follow-up visits over approximately 10 years, whereas in REGARDS, there was 1 visit 9.4 years after baseline.
There are several implications of our findings and those of others. First, we are able to identify novel plasma risk factors for incident nondiabetic CKD, although the relationships may not be as strong as have been observed for CKD progression. Second, TNF receptors are consistent risk factors throughout the spectrum of stages of CKD, including in individuals with diabetes and without diabetes and in children with CKD. Third, future studies should evaluate whether incorporation of TNF receptors may help with enrichment of trials with incident CKD as an outcome and to identify those in the general population who should be targeted for more aggressive traditional risk factor management to prevent the onset of CKD. Finally, these results may provide additional insights into the mechanisms of initiation of nondiabetic CKD.
Limitations of the study include its observational nature and, therefore, the potential for residual and unmeasured confounding. Biomarkers were only measured at one point in time, which may lead to measurement error. The latter however would have biased the results to the null. Strengths include incorporation of a precise and specific definition of incident CKD although we acknowledge the possibility of misclassification of the outcome given the requirement for only 1 follow-up measure to meet criteria for incident CKD and the lack of association of several traditional kidney risk factors with incident CKD in this study; an efficient study design that provided sufficient statistical power to detect associations in both MESA and REGARDS; 2 well-characterized prospective cohorts with a diversity of race/ethnicity and sex and detailed ascertainment of covariates; samples that were measured in duplicate; measurement of 6 biomarkers at the same time to compare interrelationship and strengths of association; and a high level of quality control in a laboratory that was developed as part of the CKD Biomarkers Consortium.
In conclusion, elevated TNFR-1 and TNFR-2 levels are independent risk markers for incident CKD in nondiabetic individuals in both MESA and REGARDS. Future studies are warranted to confirm these results and evaluate how the measurement of TNF receptors may be incorporated into the enrichment of future trials of incident CKD or whether they can be used to identify high-risk populations who may benefit from more intensive targeting of traditional CKD risk factors.
Disclosure
OMG discloses that he has received honoraria and grant support from Akebia and Amgen; has received honoraria from AstraZeneca, Reata, and Ardelyx; has received grant support from GlaxoSmithKline; and has served on a data monitoring committee for QED. MJS reports serving as a consultant for Cardurian. MGS discloses that he has received an honorarium and research support from Bayer Pharmaceuticals and consulting income from Cricket Health and Intercept Pharmaceuticals. JHI discloses that he is principal investigator of an investigator-initiated research grant supported by Baxter International; serves as a member of a data safety monitoring board for Sanifit Therapeutics; is a member of the scientific advisory board for Alpha Young; and has served on advisory boards for AstraZeneca and Ardelyx. SGC is a member of the scientific advisory board of Renalytix and owns equity in the same; and has received consulting fees from Renalytix, CHF Solutions, Vifor, Bayer, Boehringer Ingelheim, ProKidney, Axon Therapies, and Takeda Pharmaceuticals in the past 3 years. CRP is a member of the advisory board of and owns equity in Renalytix; and serves as a consultant for Genfit and Novartis. JVB is a consultant for Angion, Oisin, Praxis, Sarepta, Merck, and Janssen; is a co-inventor on KIM-1 patents assigned to Mass General Brigham; and has equity and is a consultant for Renalytix. PLK is an editor of the textbook Chronic Renal Disease and the monograph Psychosocial Aspects of Chronic Kidney Disease. All the other authors declared no competing interests.
Acknowledgments
The authors thank the other investigators, the staff, and the MESA and REGARDS study participants for their valuable contributions. A full list of investigators and institutions for REGARDS can be found at http://www.regardsstudy.org. This research project is supported by cooperative agreement U01 NS041588 co-funded by the National Institute of Neurological Disorders and Stroke (NINDS) and the National Institute on Aging (NIA), National Institutes of Health, Department of Health and Human Service; U01DK102730 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK); and N01-HC-95159 through N01-HC-95165 and by contract N01-HC-95169 from the National Heart, Lung, and Blood Institute (NHLBI). The opinions expressed in this article do not necessarily reflect those of the NINDS, NIA, NIDDK, NHLBI, NIH, the Department of Health and Humans Services, or the government of the United States. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the U.S. government. JVB was also supported by NIDDK and R37DK039773.
Footnotes
Table S1. Association of demographic and cardiovascular risk factors with incident CKD.
Supplementary Material
Table S1. Association of demographic and cardiovascular risk factors with incident CKD.
References
- 1.Sarnak M.J., Amann K., Bangalore S., et al. Chronic kidney disease and coronary artery disease: JACC state-of-the-art review. J Am Coll Cardiol. 2019;74:1823–1838. doi: 10.1016/j.jacc.2019.08.1017. [DOI] [PubMed] [Google Scholar]
- 2.Go A.S., Chertow G.M., Fan D., McCulloch C.E., Hsu C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization [published correction appears in N Engl J Med. 2008;18:4] N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 3.Turin T.C., Tonelli M., Manns B.J., et al. Lifetime risk of ESRD. J Am Soc Nephrol. 2012;23:1569–1578. doi: 10.1681/ASN.2012020164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Institute of Diabetes and Digestive and Kidney Diseases. 2020 USRDS Annual Data Report. 2020. Published 2020. Accessed October 12, 2021. https://adr.usrds.org/2020
- 5.Quinn G.Z., Abedini A., Liu H., et al. Renal histologic analysis provides complementary information to kidney function measurement for patients with early diabetic or hypertensive disease. J Am Soc Nephrol. 2021;32:2863–2876. doi: 10.1681/ASN.2021010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kramer H.J., Nguyen Q.D., Curhan G., Hsu C.Y. Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA. 2003;289:3273–3277. doi: 10.1001/jama.289.24.3273. [DOI] [PubMed] [Google Scholar]
- 7.Schrauben S.J., Shou H., Zhang X., et al. Association of multiple plasma biomarker concentrations with progression of prevalent diabetic kidney disease: findings from the chronic renal insufficiency cohort (CRIC) study. J Am Soc Nephrol. 2021;32:115–126. doi: 10.1681/ASN.2020040487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gohda T., Niewczas M.A., Ficociello L.H., et al. Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. J Am Soc Nephrol. 2012;23:516–524. doi: 10.1681/ASN.2011060628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niewczas M.A., Gohda T., Skupien J., et al. Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J Am Soc Nephrol. 2012;23:507–515. doi: 10.1681/ASN.2011060627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pavkov M.E., Nelson R.G., Knowler W.C., Cheng Y., Krolewski A.S., Niewczas M.A. Elevation of circulating TNF receptors 1 and 2 increases the risk of end-stage renal disease in American Indians with type 2 diabetes. Kidney Int. 2015;87:812–819. doi: 10.1038/ki.2014.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Titan S.M., Vieira J.M., Jr., Dominguez W.V., et al. Urinary MCP-1 and RBP: independent predictors of renal outcome in macroalbuminuric diabetic nephropathy. J Diabetes Complications. 2012;26:546–553. doi: 10.1016/j.jdiacomp.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Hayek S.S., Sever S., Ko Y.A., et al. Soluble urokinase receptor and chronic kidney disease. N Engl J Med. 2015;373:1916–1925. doi: 10.1056/NEJMoa1506362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabbisetti V.S., Waikar S.S., Antoine D.J., et al. Blood kidney injury molecule-1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. J Am Soc Nephrol. 2014;25:2177–2186. doi: 10.1681/ASN.2013070758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waikar S.S., Sabbisetti V., Arnlov J., et al. Relationship of proximal tubular injury to chronic kidney disease as assessed by urinary kidney injury molecule-1 in five cohort studies. Nephrol Dial Transplant. 2016;31:1460–1470. doi: 10.1093/ndt/gfw203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bild D.E., Bluemke D.A., Burke G.L., et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/ndt/gfw203. [DOI] [PubMed] [Google Scholar]
- 16.Howard V.J., Cushman M., Pulley L., et al. The Reasons for Geographic and Racial Differences in Stroke Study: objectives and design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 17.Levey A.S., Stevens L.A., Schmid C.H., et al. A new equation to estimate glomerular filtration rate [published correction appears in Ann Intern Med. 2011;155(6):408] Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prentice R.L. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika. 1986;73:1–11. [Google Scholar]
- 19.Tibshirani R. The lasso method for variable selection in the Cox model. Stat Med. 1997;16:385–395. doi: 10.1002/(sici)1097-0258(19970228)16:4<385::aid-sim380>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Greenberg J.H., Abraham A.G., Xu Y., et al. Plasma biomarkers of tubular injury and inflammation are associated with CKD progression in children. J Am Soc Nephrol. 2020;31:1067–1077. doi: 10.1681/ASN.2019070723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Astor B.C., Kottgen A., Hwang S.J., Bhavsar N., Fox C.S., Coresh J. Trefoil factor 3 predicts incident chronic kidney disease: a case-control study nested within the Atherosclerosis Risk in Communities (ARIC) study. Am J Nephrol. 2011;34:291–297. doi: 10.1159/000330699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peralta C.A., Katz R., Bonventre J.V., et al. Associations of urinary levels of kidney injury molecule 1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL) with kidney function decline in the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Kidney Dis. 2012;60:904–911. doi: 10.1053/j.ajkd.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhatraju P.K., Zelnick L.R., Shlipak M., Katz R., Kestenbaum B. Association of soluble TNFR-1 concentrations with long-term decline in kidney function: the multi-ethnic study of atherosclerosis. J Am Soc Nephrol. 2018;29:2713–2721. doi: 10.1681/ASN.2018070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt I.M., Hall I.E., Kale S., et al. Chitinase-like protein Brp-39/YKL-40 modulates the renal response to ischemic injury and predicts delayed allograft function. J Am Soc Nephrol. 2013;24:309–319. doi: 10.1681/ASN.2012060579. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.