Abstract
Introduction
The diagnosis of antibody-mediated vascular rejection (AM-VR) should be reliable and accurate. We hypothesized that arterial C4d (C4dart) immunoperoxidase deposition represents endothelial interaction with antibody.
Methods
From 3309 consecutive, kidney transplant biopsies from a single center, 100 vascular rejection (VR) cases were compared against rejection without arteritis (n = 540) and normal controls (n = 1108). The clinical utility of C4dart for diagnosis and classification of AM-VR was evaluated against an independent reference test.
Results
C4dart occurred in 20.4% of acute, 11.0% of subclinical, and 46% of VR episodes. Semiquantitative C4dart score significantly correlated with immunodominant donor-specific antibodies (DSAs) (rho = 0.500, P < 0.001), peritubular capillary C4d (C4dptc), microvascular inflammation, and Banff v scores. Banff v3 arteritis suggested AM-VR. Addition of C4dart to Banff antibody-mediated rejection (AMR) schema increased diagnostic sensitivity for AM-VR from 57.9% to 93.0%, accuracy 74.0% to 92.0%, and specificity 95.4% to 90.2% versus Banff 2019 (using C4dptc). Death-censored graft failure was associated with C4dart AM-VR criteria using Cox regression (adjusted hazard ratio [HR] 4.310, 95% CI 1.322–14.052, P = 0.015). VR was then etiologically classified into AM-VR (n = 57, including 36 mixed VR) or “pure” (TCM-VR, n = 43). AM-VR occurred within all post-transplant periods, characterized by greater total, interstitial, and microvascular inflammation, arterial and peritubular C4d, DSA levels, and graft failure rates compared with TCM-VR. Mixed VR kidneys had the greatest inflammatory burden and graft loss (P < 0.001).
Conclusion
C4dart is a suggestive biomarker of the humoral alloresponse toward muscular arteries. Inclusion of C4dart into the Banff schema improved its diagnostic performance for detection of AM-VR and etiologic classification of arteritis.
Keywords: antibody, C4d, kidney transplantation, vascular rejection
Graphical abstract
AMR is an important cause of alloimmune injury in kidney transplantation, characterized by DSA, microvascular inflammation (MVI), and C4d deposition in the peritubular capillaries (C4dptc).1, 2, 3, 4, 5 Early AMR from pre-existing DSA is contrasted with late rejection from de novo DSA with transplant glomerulopathy and chronic arteriolopathy.6, 7, 8 Active AMR also presents as inflammation of the transplanted muscular arteries, variably accompanied by MVI, C4dptc, and DSA (“acute/active AMR with intimal/transmural arteritis,” Banff v ≥1),4,9, 10, 11, 12, 13 as distinct from T-cell–mediated vascular rejection (TCM-VR) driven by CD4 and CD8 T lymphocytes.14 Lefaucheur et al.13 categorized rejection by arteritis and DSA, segregating cases into 4 immunologic phenotypes. Acute arteritis with DSA (DSA+VR) comprised 21% of rejections which presented early with severe dysfunction, steroid resistance, and inferior graft survival compared with TCM-VR and AMR without arteritis.13 C4dptc occurred in 56.2% (using Banff C4d2/3 threshold), and interestingly, arterial C4d (C4dart) immunoperoxidase staining occurred in 42.1% indicating local DSA binding and suggesting causal AM-VR.13 C4dart is a novel biomarker that warrants further scientific exploration.
C4d is the “footprint” of classical complement activation which localizes vascular DSA deposition in transplantation.15,16 In nephrology, glomerular C4d signifies complement activation in immune-complex glomerulonephritis (e.g., membranous or lupus nephritis).17 Cleaved C4b exposes reactive sulfhydryl groups that covalently bind to adjacent tissue amino and carbohydrate groups, including endothelial cells, underling intimal proteins, basement membrane, and tissue collagen. Stable C4d remains detectable after proteolytic inactivation5 in muscular arteries and peritubular and glomerular capillaries in AMR, where intensity varies over time.18 C4d is integral to the histologic diagnosis of AMR. When C4d was incorporated into the Banff schema in 2001, peritubular capillaries were explicitly designated and “not deposition in glomerular capillaries, arteries or arterioles” using immunofluorescence because the arteries are constitutionally positive from elastin and collagen autofluorescence.19 Underdiagnosis of “C4d-negative” AMR was first highlighted by abnormal endothelial transcripts (molecular AMR) or microvascular inflammation (histologic AMR)18,20 using conservative diffuse C4dptc3 threshold (50%), where molecular AMR occurred in 46% of “isolated arteritis” classified as TCM-VR.21 C4dptc3 threshold was reduced to C4d2 (10%–50%) for immunofluorescence and C4d1 (1%–9%) for immunoperoxidase; however, C4dptc remains insensitive20 with “negative” AMR cases revealing arterial or glomerular staining using C4d immunoperoxidase, where nonspecific arterial autofluorescence is absent. The modern literature lacks a detailed clinical and histologic description of AM-VR.
We hypothesized the following: that (i) endothelial and intimal C4dart immunoperoxidase staining constitutes a humoral biomarker of endothelial interaction with antibody in muscular arteries; and (ii) its incorporation into Banff schema would improve diagnostic sensitivity for AM-VR. We correlated C4dart and Banff v scores against authenticated AMR markers including DSA, C4dptc, and histologic MVI in a well-characterized cohort of 3309 consecutive adequate biopsy samples. VR was etiologically classified using the 3-tier Banff diagnostic criteria using DSA, C4dptc and/or C4dart and MVI, and arteritis into AM-VR or TCM-VR. The performance of this expanded definition of AM-VR (incorporating C4dart and C4dptc) to correctly classify AMR was compared with Banff 2019 schema (using C4dptc) against an independent diagnostic reference standard. The resultant clinical and pathologic phenotypes, immediate and long-term outcomes of AM-VR and TCM-VR are described.
Methods
Study Design and Principal Histologic Categories
Consecutive, kidney transplant indication and protocol biopsy specimens with sufficient evaluable vascular and cortical tissues from May 2012 to December 2020 were screened. Study biopsy specimens were derived from acute and chronic rejection and normal/near-normal controls containing at least 1 evaluable muscular artery (“minimal” Banff vascular adequacy). Samples without an artery, non-alloimmune diseases (including recurrent glomerulitis, diabetic nephropathy, BK virus nephropathy without VR, nonspecific moderate-to-severe interstitial fibrosis / tubular atrophy) and complement-dependent pathology, including ABO-incompatible and thrombotic microangiopathy (TMA)/atypical hemolytic uremic syndrome patients were excluded (Figure 1).
Figure 1.

Study flow diagram. Numbers are biopsy samples, except for graft outcome which used the first biopsy in a unique kidney for actuarial survival (i.e., not total kidney number). Absent C4d was treated as indeterminant. ABOi, ABO-incompatible; aHUS, (atypical) hemolytic uremic syndrome; NIL, no significant histology; TMA, thrombotic microangiopathy; VR, vascular rejection; v0REJ, rejection without arteritis.
Histologic Evaluation and AM-VR Definition
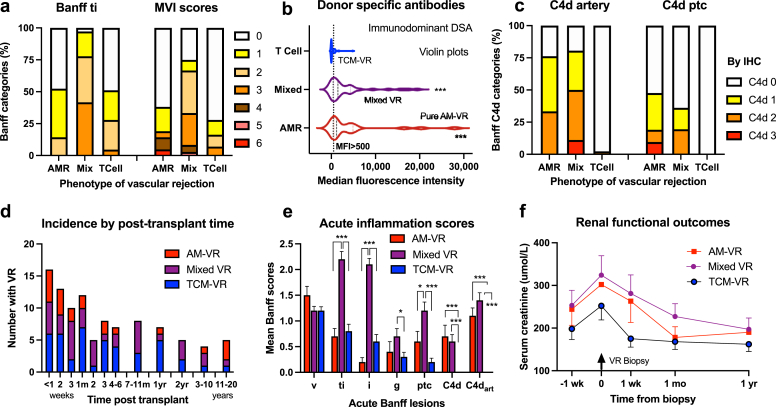
Histology was contemporaneously scored by 5 specialized nephropathologists with diagnostic categories retrospectively reclassified using Banff 2019.22 All samples were tested for C4d using immunoperoxidase in formalin-fixed, paraffin-embedded tissue with a concurrent positive control (rabbit polyclonal anti-C4d antibody, Cell Marque, CA). C4dart in endothelial and intimal layers (including internal elastic lamina [IEL]) was semiquantitatively scored as follows: C4dart 1, patchy noncircumferential staining in at least 1 artery (worst affected, Figure 2b and c); C4dart 2, circumferential staining and/or incomplete staining in the worst affected of several arteries (≤50%, Figure 2d–f); C4dart 3, moderate circumferential and/or diffuse staining involving multiple (>50%) arteries (Figure 2g and h).
Figure 2.
Arterial C4d in arteries. (a) Normal artery without C4d staining (C4dart 0). (b and c) Incomplete noncircumferential C4d in endothelial/subendothelial and intimal layers of muscular arteries with variable involvement of the IEL (C4dart 1). (d, e, f) Small, medium, and large muscular arteries with circumferential arterial C4d staining (C4dart 2) of moderate intensity. (h) Antibody-mediated VR with fibrinoid necrosis (Banff v3 lesion) (g,h) with circumferential C4d staining of multiple arteries (C4dart 3, arrowed g) at post-transplant day 8 with strong class 2 donor-specific antibody (MFI 20,360). Immunoperoxidase from formalin-fixed paraffin-embedded tissue with rabbit polyclonal anti-C4d antibody and hematoxylin and eosin stains are illustrated. C4dart, C4d staining of arterial endothelium and intima; IEL, internal elastic lamina; MFI, median fluorescence intensity; VR, vascular rejection.
C4dart of preimplantation donor kidney (n = 140 samples) and native diabetic and vascular disease (n = 21) was assessed for nonspecific staining by a single nephropathologist (CHP).
The 3 mutually exclusive diagnostic categories were as follows:
-
1.
Acute “active” VR as the principal study group, subdivided into TCM-VR, AM-VR, or mixed VR.
-
2.
Acute and/or chronic rejection without arteritis (v0REJ) was the positive comparator.
-
3.
Normal or near-normal samples were negative controls (NIL).
Clinical Utility of Arterial C4d and Test Reference Standard
The test reference for etiologic VR was the composite clinicopathologic diagnosis using clinical, antibody, MVI, C4dptc, and C4dart, supplemented by detailed arterial evaluation (excluding AM-VR diagnosis to avoid incorporation bias) by a single, blinded pathologist (MS) and clinician (BJN). Arterial cellular morphology, CD3 T cell lymphocytic infiltration (rabbit monoclonal anti-CD3, Ventana clone 2GV6, Tuscon, AZ), and C4dart aided the diagnosis. Canonical TCM-VR was diagnosed by pure or dominant lymphocytic infiltrate using CD3 immunoperoxidase without C4d, MVI, or DSA (using Banff classification). AM-VR was histologically diagnosed by C4dart and/or C4dptc, MVI, and DSA. Clinical risk factors (median fluorescence intensity [MFI], sensitization, regraft, prior and subsequent DSA, AMR, and extent of C4dart) contributed to the final, blinded probabilistic categorization.
Anti-human leukocyte antigen specific IgG DSA used specific class I and/or class II solid-phase assays (LABScreen Single Antigen Bead, Luminex, One Lambda, CA). DSA positivity was defined as MFI ≥ 500. HLA class I (A, B, C) and class II (DRB1/3/4/5, DQα/β, DPα/β) alleles were defined by 2-field sequence-based typing (Applied Biosystems, Thermo Fisher Scientific, CA) after 2017, replacing single-field molecular HLA typing by sequence-specific oligonucleotides (LABType SSO, One Lambda).
Statistical Analysis
The research design was a retrospective, single-center, observational cohort, with a nested case-control study. It was investigator initiated, independent, and undertaken without external funding. Institutional ethics was HREC LNR/12/WMEAD/114. A STARD checklist is included (Supplementary Table S1).
An unpaired Student's t test or Wilcoxon rank sum tested parametric and nonparametric nominal data, respectively, a conditional binomial exact test or χ2 test for categorical data and Pearson’s (r) or Spearman’s (rho) for correlations. Multivariable models were constructed following backward elimination, adjusted for confounding factors if collinearity was present. Analysis of repeated samples used generalized estimating equation. Banff score results were confirmed using ordinal logistic regression. Principal component analysis illustrated covariance associations of C4dart against Banff histology. Internal validation of classification models was performed using k-fold cross-validation (k = 10) with the “caret” package in R.
Survival analyses used the first Banff v lesion (only) from each patient as index case (or first biopsy in v0REJ and NIL groups) from a unique kidney to avoid double-counting of repeat samples. v0REJ and NIL kidneys that developed VR were excluded to avoid group cross-contamination. Time-to-event or last follow-up was calculated from index biopsy until death-censored graft survival and patient mortality. Kaplan-Meier actuarial survival (logrank test) was used for binary predictors and Cox regression for multivariable factors. P values were 2-sided, and probability below 0.05 was considered significant. Missing data were excluded. Results were expressed as mean ± SD.
Results
Population Screening and Study Exclusions
From 3715 consecutive biopsy specimens screened, 3309 remained from ABO-compatible kidneys for prevalence estimates and C4dart relationship after exclusions for unsatisfactory tissue (n = 64), absent artery (n = 264), ABO-incompatible, atypical hemolytic uremic syndrome, or TMA (n = 55). From 3256 evaluable samples with C4d results, cases with arteritis (Banff v ≥1, n = 100) were selected to form the principal study group (VR, n = 100) and compared against negative controls (NIL, n = 1108) and rejection biopsies without arteritis (v0REJ, n = 540), totaling 1748 study samples (Figure 1). Biopsy prevalence of VR was 3.0% (100/3309) and 15.9% of acute rejection cases (100/629).
Study Group Recipient Demographics
The mean (±SD) age was 46.8 ± 12.9 years, 62.3% male, 7.1% retransplanted, 75.9% deceased donor, and 30.7% kidney-pancreas recipients. HLA mismatch was 3.9 ± 2.1 (of 6). Induction was basiliximab in 81.2%, antithymocyte globulin 7.8%, desensitization 1.0%, nil 9.1%, and unknown 0.2% (Supplementary Table S2). Prior to each biopsy (n = 1748), rates of antecedent early (≤3 months) acute interstitial, vascular, and C4d-positive antibody rejection were 19.0%, 3.8% and 5.6%, respectively; and 12.0% received dialysis for delayed graft function. Previous rejection before biopsy was treated with methylprednisolone in 37.4% and antithymocyte globulin in 8.9% of the cases.
Banff v Score and Antibody Markers
Kidneys with VR more frequently displayed peritubular capillaritis (P < 0.05), glomerulitis (P < 0.001), MVI (P < 0.001), and C4dart (P < 0.001) compared with v0REJ, although C4dptc (P = nonsignificant; Supplemental Figure S1 and Supplementary Table S3) and DSA results were comparable for v0REJ. Acute and chronic Banff scores markedly increased in VR kidneys versus normal.
Analyses of VR (n = 100) revealed that Banff v score correlated with C4dptc (rho = 0.211, P = 0.036) and C4dart scores (rho = 0.217, P = 0.031) and inversely with post-transplant time (rho = −0.211, P = 0.036). Banff v2/v3 presented earlier compared with v1. Moderate v2 (16%) and severe v3 (5%) arteritis comprised 21% of VR with mild Banff v1 in 79%. Banff v2 and v3 had C4dart (65.1% and 60.0% vs. 41.8% for v1), although DSA detection was similar (56.2% and 80% vs. 56.9% for v1, Supplementary Figures S1E and F, Supplementary Table S3). Univariable ordinal regression found that Banff v score predicted arterial C4d score (P < 0.001, Supplementary Table S4A). Banff v score increased with early (≤1 mo) VR, delayed function, total inflammation, glomerulitis, and arterial C4d scores using multivariable ordinal regression adjusted for sample size (Supplementary Table S4B).
Of 5 Banff v3 cases, 4 were classified as AM-VR with DSA (MFI 5763 ± 9738) and 3 displaying 2 or more antibody markers (Banff ptc, C4dptc, C4dart, MVI). The only v3 case (postoperative day 4) classified as TCM-VR without C4dptc or detectable DSA at biopsy had anti-HLA C∗07:04 at transplantation (MFI 4469) and was reclassified to AM-VR by reference pathologist using C4dart (2 of 8 arteries including v3 lesion). Subsequent DSA testing and 1-year biopsy excluded AMR. Our retrospective analysis concluded that all v3 cases involved antibody.
Arterial C4d Correlated With AMR Markers
The overall biopsy prevalence (n = 3256) of C4dart was 5.8% (189 with C4dart results) and predominantly mild (n = 151), whereas 32 were moderate and 6 were diffuse/severe (C4d unavailable in 53/3256). C4dart occurred in 20.4% of acute rejection (54/264), 11.0% of subclinical rejection (23/207), and 14.5% of total rejection episodes (90/622, including chronic rejection) and 46% of all VR cases. The Cohen’s kappa for C4dart occurrence was 0.867 (95%CI 0.69–1.00, 93.5% agreement between 2 pathologists, n = 31, 41.9% prevalence). C4dart score correlated with immunodominant DSA MFI (rho = 0.500, P < 0.001), Banff g (rho = 0.172, P = 0.088), ptc (rho = 0.294, P = 0.003), v (rho = 0.217, P = 0.031), MVI (rho = 0.284, P = 0.031), and C4dptc scores (rho = 0.391, P < 0.001, Supplementary Figure S2A and B).
Arterial C4d was independently associated with DSA (MFI > 500), C4dptc, arteritis severity (Banff v), interstitial inflammation (Banff i), and peritubular capillaritis (Banff ptc), using multivariable binomial generalized estimating equation (Table 1). Glomerulitis score, time post-transplant, and MFI lost significance. Determinants of C4dart were confirmed using multivariable ordinal regression, which treats C4dart scores as ordinal categorical values (P < 0.001, Supplementary Table S4C). C4dart colocalized with histologic AMR markers (Banff g, ptc, MVI, C4dptc) and T-cell–mediated rejection (TCMR) features (Banff ti, t, i) using principal component analysis (Supplementary Figure S2C).
Table 1.
Determinants of arterial C4d staining
| Parameters | OR | 95% CI | P value |
|---|---|---|---|
| Banff v score | 5.155 | 3.285–8.089 | <0.001 |
| Banff ptc score | 1.734 | 1.188–2.531 | 0.004 |
| Banff i score | 1.722 | 1.392–2.131 | <0.001 |
| Banff C4dptc score | 1.572 | 1.015–2.434 | 0.043 |
| Any DSA (≥500 MFI) | 1.546 | 1.095–2.182 | 0.013 |
C4dptc, C4d staining of peritubular capillaries; DSA, donor-specific antibody; IEL, internal elastic lamina; OR, odds ratio; MFI, median fluorescence intensity.
Binomial generalized estimating equation for predictors of any arterial C4d staining in the endothelial and intimal/IEL layers of the muscular artery (n = 3309 samples, 1079 kidneys).
Kaplan-Meier graft survival was reduced in VR kidneys with C4dart (logrank 3.651, P = 0.056, Figure 3a). Graft loss was associated with C4dart (HR 1.827, 95% CI 1.123–2.972, P = 0.015) and Banff ci score (HR 2.180, 95% CI 1.294–3.672, P = 0.003), but not v score using multivariable Cox regression restricted to histologic variables (Supplementary Table S5). Hence, C4dart is an independent marker of antibody-endothelial interaction displaying a positive, graded relationship against circulating DSA and histologic microvascular inflammation.
Figure 3.
Graft survival by AM-VR classifier. (a) Kaplan-Meier death-censored graft survival of VR (from first index biopsy) of 91 unique kidney transplants dichotomized by presence of C4dart reported by routine pathologists (P = 0.056 vs. negative). (b) Actuarial graft loss of AM-VR (including mixed AM-VR) classified by Banff 2019 (using C4dptc, P = 0.068) or expanded Banff 2019 (using both C4dptc and C4dart, P = 0.037) versus TCM-VR assessed by routine pathologists. (c) Actuarial graft loss of AM-VR classified by expanded Banff 2019 with re-evaluation of C4dart by the reference pathologist (P = 0.011 vs. pure TCM-VR). AM-VR, antibody-mediated vascular rejection; C4dart, C4d staining of arterial endothelium and intima; C4dglom, endothelial C4d staining of glomerular capillaries; C4dptc, C4d staining of peritubular capillaries; TCM-VR, T-cell–mediated vascular rejection; VR, vascular rejection.
Reclassification of VR Episodes Using Arterial C4d
Incorporation of C4dart for endothelial-antibody interaction into the Banff schema increased AM-VR diagnosis from 35 (Banff 2019 using C4dptc) to 57 cases (using C4dart and/or C4dptc, which also substituted for absent/missing DSA in 11, detailed in Supplementary Table S6C). All 22 reclassified C4dart-positive cases were C4dptc negative, and 72.7% were mixed VR involving TCMR. DSA was detected in 72.7% (MFI, 4416 ± 6548, class II in 87.5%), glomerular C4d in 31.8%, and MVI ≥ 2 in 27.3%, whereas glomerulitis was uncommon (12.5%). All MVI ≥ 2 cases (n = 6) contained interstitial inflammation where absent glomerulitis disqualified AMR diagnosis in 66.7%. Six DSA-negative AM-VR with C4dart were additionally diagnosed (MVI ≥ 2 occurred in 4, Supplementary Table S7).
Diagnostic Test Performance for AM-VR Using Arterial C4d
Addition of arterial C4dart improved the test performance of Banff 2019 to detect AMR (including mixed AM-VR) against reference test diagnosis, itself verified by comprehensive clinical and pathologic data and externally validated with the highest HR for graft loss of 8.362 (vs. 2.752 Banff 2019, Supplementary Table S5, models 3 vs. 1) using Cox regression. The sensitivity of Banff AMR criteria increased from 57.9% to 93.0% by including C4dart, and accuracy improved from 74.0% to 92.0% from Banff 2019 (using only C4dptc; Table 2). Using 10-fold cross-validation, the model’s classification accuracy was 77.9% (k = 0.599) for Banff 2019 and 96.0% for AMR with C4dart (k = 0.921).
Table 2.
Diagnostic performance of Banff AM-VR criteria with use of arterial C4d
| Parameters | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| AM-VR test criteria | ||||
| Banff 2019 (C4dptc only), % | 57.9 | 95.4 | 94.3 | 63.1 |
| 95% CI | 44.1–70.9 | 84.2–99.4 | 80.7–98.5 | 55.6–70.0 |
| Expanded Banff criteria (C4dptc and C4dart), % | 93.0 | 90.2 | 92.9 | 90.7 |
| 95% CI | 83.0–98.1 | 77.8–97.4 | 3.9–97.1 | 79.0–96.2 |
AM-VR, antibody-mediated vascular rejection; C4dptc, C4d staining of peritubular capillaries; C4dart, C4d staining of arterial endothelium and intima; PPV, positive predictive value; NPV, negative predictive value.
The test performances of the Banff 2019 AM-VR criteria using conventional C4dptc and expanded diagnostic criteria allowing C4dart (using immunoperoxidase) as evidence endothelial-antibody interaction against an independent clinical-pathologic reference standard are derived from all VR cases (n = 100 biopsies).
Results are percentages, 95% CI below.
Diagnostic classification of AM-VR using C4dart was independently supported by its association with graft failure (logrank 4.370, P = 0.037) versus Banff 2019 (logrank 3.342, P = 0.068) using Kaplan-Meier actuarial survival (n = 91 kidneys, Figure 3b and c). The graft failure HR using C4dart AM-VR criteria was 4.310 (95% CI 1.322–14.052, P = 0.015) and 2.752 (95% CI 0.957–7.911, P = 0.060) using Banff 2019 (Supplementary Table S5), when controlled for under-immunosuppression and chronic fibrosis by multivariable Cox regression. The reference diagnostic standard AM-VR HR was 8.362 (95% CI 1.850–37.799, P = 0.006).
Heterogeneity of Arterial C4d Within Transplant Kidneys
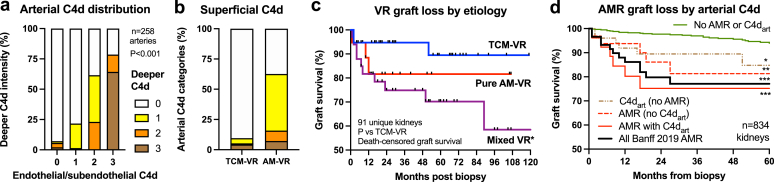
Variability of C4dart staining was assessed in 65 VR kidneys containing 258 arterial cross-sections (3.0 ± 1.8 per biopsy, range 1–9). C4dart occurred in 83.8% in AM-VR arteries (134/160) and 2.0% (2/98) of TCM-VR (P < 0.001). In samples containing 2 or more arteries, C4dart occurred in 75.0% of AM-VR kidneys (24/32), where 50% were diffusely C4dart positive in most arteries, 25% had focal C4dart (12.5% to 75.5%), and 25% were negative. Mean proportion with C4dart was 63.8% (±43.3%) in AM-VR. Endothelial or subendothelial C4d correlated with deeper intimal/IEL C4dart using semiquantitative visual intensity (χ2 = 198.9, P < 0.001) and AM-VR classification (Figure 4a and b). MFI correlated with superficial C4dart (r = 0.271, P < 0.001) and intimal/IEL C4dart scores (r = 0.298, P < 0.001).
Figure 4.
Arterial wall C4d staining in VR and outcomes. (a) Superficial endothelial/subendothelial C4d staining correlated with C4d in intimal layers extending to IEL, P < 0.001, n = 258 muscular arteries) in VR samples (n = 65) of mixed etiologies. (b) Endothelial/subendothelial C4d was common in AM-VR compared with T-cell–mediated vascular rejection (TCM-VR, P < 0.001). (c) Death-censored graft survival of VR (from first index biopsy only) of 91 unique kidney transplants etiologically into TCM-VR (n = 38), pure AM-VR (n = 19), and mixed VR (AM-VR with TCMR, n = 34). Key: mean ± SEM. Key: ∗P < 0.05 versus TCM-VR. (d) Death-censored graft survival of 834 first biopsies from unique kidney transplants classified by Banff 2019 AMR with and without C4dart (text for numbers) versus a negative control group without AMR or C4dart. Key: ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 for each combination. AM-VR, antibody-mediated vascular rejection; C4dart, C4d staining of arterial endothelium and intima; IEL, internal elastic lamina; MVI, microvascular inflammation; TCMR, T-cell–mediated rejection; TCM-VR, T-cell–mediated vascular rejection; VR, vascular rejection.
Normal Arterial C4d Immunoperoxidase Background Staining
C4dart from 140 preimplantation donor kidneys evaluated nonspecific staining (Supplementary Table S8). Background C4dart occurred in 3.5% (restricted to IEL of larger arteries with fibroelastosis, not endothelium) correlating with cv scores (χ2 = 17.61, P = 0.024). Arteriolar staining occurred in 5.0%. C4dart was observed in 1 medium artery (4.7% prevalence) from 21 native diabetic and hypertensive vascular kidney samples.
From the excluded samples, C4dart occurred in 9.1% of TMA/atypical hemolytic uremic syndrome, 83.3% of ABOi AM-VR episodes, and 10.9% of other ABOi samples. When classified by dominant pathologic diagnosis of included study groups, C4dart was positive in 3.3% for NIL, 4.3% acute tubular injury, 3.8% interstitial fibrosis / tubular atrophy, and 5.0% calcineurin inhibitor nephrotoxicity. Mild arteriolar C4d staining occurred in 10.8% (351 of 3256) and trace mesangial uptake in 3.5% (115 of 3261).
VR Phenotypes by Pathophysiological Diagnoses
Using C4dart AM-VR diagnostic criteria, VR (n = 100) was subclassified into “pure” AM-VR (n = 21), mixed VR with associated TCMR (Banff i/t ≥ 1, n = 36), and “pure” TCM-VR (n = 43, Table 3 and Supplementary Table S9). The presentation of AM-VR and relative etiologic case-mix of VR were not time dependent and occurred within all post-transplant periods (Figure 5d). Early AM-VR within the first month associated with presensitization, whereas late VR followed under-immunosuppression in 71.4%. Isolated VR (n = 34, defined as arteritis and Banff i < 1/t < 1) was mediated by AM-VR in 10 (29.4%) and TCM-VR in 24 episodes (70.6%, P < 0.001). Mixed AM-VR (not pure AM-VR) had significantly greater total, interstitial, and microvascular inflammation versus TCM-VR. In AM-VR (both pure and mixed VR), C4dart, C4dptc, DSA prevalence (MFI ≥ 500, 82.1% vs. 34.3%, P < 0.001) and immunodominant MFI (4091 ± 6095 vs. 348 ± 827, P < 0.001) were increased compared with TCM-VR (Supplementary Table S9).
Table 3.
Summary histopathology by etiologic diagnosis of VR
| Category | AM-VR | Mixed VR | TCM-VR |
|---|---|---|---|
| Biopsies (n) | 21 | 36 | 43 |
| Time (mo) | 34.8 ± 77.3 | 14.4 ± 39.7 | 8.8 ± 26.5a |
| Indication, n (%) | 14 (66.7) | 28 (77.8) | 20 (46.5)b |
| Serum creatinine (μmol/l) | 302 ± 225 | 324 ± 274 | 252 ± 219 |
| Mean MFI (nil DSA = 0) | 4776 ± 7494 | 3680 ± 5157 | 348 ± 827b,c |
| Banff v score | 1.5 ± 0.8 | 1.2 ± 0.5 | 1.2 ± 0.4 |
| Banff ti score | 0.7 ± 0.7 | 2.2 ± 0.9c | 0.8 ± 0.9d |
| Banff i score | 0.2 ± 0.4 | 2.1 ± 0.7c | 0.6 ± 0.9d |
| Banff t score | 0.5 ± 0.6 | 1.8 ± 0.7c | 0.9 ± 0.8d |
| Banff ptc score | 0.6 ± 0.9 | 1.2 ± 1.0a | 0.2 ± 0.5d |
| Banff g score | 0.4 ± 0.9 | 0.7 ± 0.9 | 0.3 ± 0.6e |
| C4dptc score | 0.7 ± 1.0 | 0.6 ± 0.8 | 0 ± 0b,c |
| C4dglom score | 0.7 ± 0.9 | 0.5 ± 0.9 | 0.2 ± 0.4a,b |
| C4dart score | 1.1 ± 0.7 | 1.4 ± 0.9 | 0.0 ± 0.3c,d |
| Recurrent rejection, n (%)f | 7 (33.3) | 12 (33.3) | 15 (34.9) |
| Graft failure, n (%)f | 5 (23.8) | 10 (27.8) | 3 (7.0)e |
AM-VR, antibody-mediated vascular rejection; C4dart, C4d staining of arterial endothelium and intima; C4dglom, endothelial C4d staining of glomerular capillaries; C4dptc, C4d staining of peritubular capillaries; MFI, median fluorescence intensity; TCM-VR, T-cell–mediated vascular rejection; VR, vascular rejection.
Detailed histopathology of VR classified by pathophysiology. Mean ± SD, n (%).
Key:
P < 0.05; versus AM-VR (pure).
P < 0.01; versus mixed VR.
P < 0.001 versus AM-VR (pure).
P < 0.001 versus mixed VR.
P < 0.05; versus mixed VR.
Graft failure and recurrent rejection denominator is per biopsy (including repeat biopsies).
Figure 5.
VR classified by pathophysiology. VR was etiologically classified into AM-VR, TCM-VR, or mixed VR and evaluated against histologic and clinical markers. (a) Prevalence of total inflammation (Banff ti category) and MVI by etiology. (b) Median fluorescence intensity of the immunodominant DSA was greater in AM-VR and mixed VR compared with TCM-VR. (c) Prevalence of arterial and peritubular capillary C4d against etiologic phenotypes. (d) Incidence of VR by time post-transplant and etiologic case-mix. (e) Acute histologic inflammation scores by etiologic VR diagnosis. (f) Renal function by VR phenotypes. Mean ± SEM. Key: ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. AM-VR, antibody-mediated vascular rejection; C4dart, C4d staining of arterial endothelium and intima; DSA, donor-specific antibody; IEL, internal elastic lamina; MVI, microvascular inflammation; TCM-VR, T-cell–mediated vascular rejection; VR, vascular rejection.
Mixed VR samples (36%, n = 36) contained the greatest inflammatory burden involving all compartments, acute renal impairment, slower functional recovery (Figure 5), and allograft failure (27.8%, P < 0.001) versus pure AM-VR (23.8%) and TCM-VR (7.0%, Supplementary Table S9, Figure 4c). Principal component analysis found mixed VR diagnosis colocalized with cellular interstitial inflammation and C4dart (Supplementary Figure S2C).
Comparative Graft Survivals of Study Group Categories
Death-censored graft survival with data linked to primary study groups (n = 834 first biopsy, median 52 months follow-up, IQR 24–84) resulted in 91 VR (9 repeat samples were excluded), 309 v0REJ (Banff 2019 AMR prevalence 31.7%), and 433 NIL unique kidneys. Kaplan-Meier survival was reduced by VR (Banff v ≥ 1, logrank 27.638, P < 0.001), DSA detection (logrank 10.772, P < 0.001), and Banff 2019 AMR diagnosis (logrank 22.322, P < 0.001).
The 5-year Kaplan-Meier graft survival (n = 834 kidneys) was reduced in cases meeting Banff 2019 AMR criteria to 77.1% (n = 74, logrank 23.322, P < 0.0010), Banff 2019 AMR with C4dart of 74.9% (n = 83, logrank 12.610, P < 0.001), Banff 2019 AMR without C4dart of 81.3% (logrank 7.190, P = 0.007), in C4dart cases without AMR of 84.7% (logrank 4.835, P = 0.028) versus 93.9% for cases without C4dart or AMR (n = 586, logrank 24.213, P < 0.001, Figure 4d).
Graft loss was associated with VR (HR 4.030, 95% CI 2.296–7.074, P < 0.001), C4dart score (HR 1.835, 95% CI 1.331–2.530, P < 0.001) and C4dptc score (HR 1.814, 95% CI 1.335–2.466, P < 0.001), Banff ptc, g, ci, cg scores, late occurrence (≥1 month), and suboptimal serum creatinine using univariable Cox regression (Supplementary Table S10). The HR for dichotomous C4dart was 2.714 (95% CI 1.449–5.082, P = 0.002). Independent predictors of graft failure were VR (any arteritis), renal dysfunction, C4dptc, and transplant glomerulopathy and interstitial fibrosis Banff scores using multivariable Cox regression (Table 4).
Table 4.
Histologic and functional multivariable determinants of graft failure
| Parameters | HR | 95% CI | P value |
|---|---|---|---|
| Any VR (Banff v > 0) | 2.103 | 1.134–3.898 | 0.018 |
| Banff ci score | 2.078 | 1.596–2.704 | <0.001 |
| Banff cg score | 1.785 | 1.233–2.585 | 0.002 |
| C4dptc score | 1.365 | 0.993–1.877 | 0.056 |
| S Creatinine (μmol/l) | 1.002 | 1.000–1.003 | 0.033 |
HR, hazard ratio; C4dptc, C4d staining of peritubular capillaries.
Parsimonious Cox regression predictors of death-censored allograft failure (n = 834 unique kidneys, −2LogL 588.493). DSA, MVI, and C4dart were not retained in the multivariable model being subsumed by cg and C4dptc scores which reflect biological effect of antibody on tissue. Time post-transplant was lost to Banff ci score and serum creatinine concentration, reflecting late rejection associated with chronic damage and graft dysfunction.
Discussion
An accurate and reliable etiologic diagnosis of VR cases is important for clinical management of rejection. The traditional Banff schema specifies linear C4d staining of peritubular capillaries or medullary vasa recta as evidence of antibody binding to endothelium and does not include muscular arteries or glomerular capillaries based on immunofluorescence. Our hypothesis that arterial C4d represents AMR was supported by the following: correlations with circulating DSA, histologic C4dptc, and MVI; colocalization of C4dart with AMR marker using principal component analysis; and biological plausibility. When Banff AMR ruleset included C4dart, the diagnostic sensitivity against the verified AM-VR reference test was increased from 57.9% to 93.0% and accuracy from 74.0% to 92.0%, compared with Banff 2019 (using C4dptc). Reclassification of C4dptc-negative AM-VR by positive C4dart staining included 72.7% with DSA (mean MFI 4416, class II in 87.5%) where histologic antibody markers were insufficient or disqualified from diagnosis. Arteritis with DSA and C4dart (without C4dptc) could then be recognized as AM-VR rather than inappropriately defaulting to TCM-VR. Reclassified AM-VR had equally poor graft survival to Banff AMR cases.
Our study recognized arteritis with C4dart and DSA within the diverse AM-VR spectrum, including cases without C4dptc and/or “countable” MVI (MVI is disqualified with Banff i ≥1, unless glomerulitis). Under inflammatory stress, larger caliber arteries may differentially express endothelial antigens (relative to microcirculation), influencing DSA binding, C4d activation, and the phenotypical expression of AMR.23 C4dart occurred in 46% of VR. Staining was often patchy, nonlinear, and variably circumferential in arteries of all calibers. Some AM-VR with strong DSA had diffuse uptake in multiple arterial sections, including non-inflamed arteries (which we score). C4dart was associated with inferior graft survival in VR, Banff 2019 AMR, and cases without AMR. We propose immunoperoxidase C4dart be included as a criterion for antibody-endothelial interaction to avoid artifactual false-negative reporting (i.e., mislabeling DSA-driven positive C4dart as “C4d negative” simply by its absence in peritubular capillaries).
Many transplant pathology laboratories use automated immunoperoxidase of formalin-fixed, paraffin-embedded tissue for C4d detection, which allows easy visualization of arteries and peritubular and glomerular capillaries against clear anatomical landmarks within a larger tissue sample. Many North American laboratories detect C4dptc by immunofluorescence in a small separate core of unfixed frozen cortex, which often will not contain an artery. The mild endogenous endothelial positivity and stronger autofluorescence of arterial IEL elastin are absent using immunoperoxidase visualization.24 Our C4d immunoperoxidase displayed clean backgrounds using indirect, biotin-free detection (Ultra DAB, Ventana BenchMark, Tucson, AZ), except for occasional arteriolar staining (containing C3 and nondiagnostic)3,9,24 and mild nonspecific in IEL of larger arteries with fibroelastosis from extended criteria donors (3.5%). The C4dart interrater kappa was exceptional at 0.867. Nonspecific staining in preimplantation donor kidney and native diabetic and hypertensive vascular kidney samples was virtually absent indicating relative specificity (for complement). We deliberately excluded diseases involving complement activation such as TMA and atypical hemolytic uremic syndrome (C4dart prevalence 9.1%) and ABOi (C4dart 83.3% with AM-VR) which form the differential diagnosis of C4dart positive cases. Rarely, AMR manifests as TMA characterized by neutrophilic glomerulitis and peritubular capillaritis, mesangiolysis, segmental glomerular thrombi, and intimal arteritis with C4dptc.25,26 We regard C4dart of endothelial/subendothelial layers and/or intima/IEL as pathologic and recommend C4d immunoperoxidase for optimal AMR assessment.
Banff does not specify a distinct category of AM-VR but includes arteritis as a marker of tissue injury within generic active AMR category as “acute/active AMR with intimal/transmural arteritis.” Lefaucheur categorized rejection by arteritis and DSA (2-tier), where DSA+VR (corresponding to AM-VR) was segregated into a distinct histologic and immunologic phenotype with inferior outcomes using unsupervised principal component analysis and hierarchical clustering of pathology scores.13 DSA+VR displayed MVI (88.0%), interstitial inflammation (71.8%, indicating mixed VR), and C4dptc (66%). Interestingly, 75% of DSA+VR expressing C4dptc also had C4dart immunoperoxidase deposition, comparable with our study. We classified VR by immunopathophysiology using 3-tier AMR criteria4 accepted by Banff consensus comprising: (i) DSA (or C4d surrogate); (ii) C4dart and/or C4dptc or conditional MVI; and (iii) and arteritis defining acute injury. AM-VR cases unsurprisingly expressed higher MVI, C4dart, and C4dptc scores, but also displayed greater total and interstitial inflammation compared with TCM-VR. AM-VR occurred within all post-transplant periods, from early post-transplant weeks in sensitized recipients until years later after underimmunosuppression. Acute rejection is conventionally categorized into TCMR or AMR subtypes; however, mixed phenotypes were common comprising 36% of VR and 63.2% of AM-VR. Mixed VR colocalized with cellular inflammation using principal component analysis. These kidneys had the greatest inflammatory burden, renal dysfunction, and graft losses.
How should we diagnose AM-VR? We consider arteritis accompanied by DSA and C4dart (and/or C4dptc) as diagnostic of AM-VR. Generic microvascular antibody markers including C4dptc and moderate MVI were less sensitive for AM-VR diagnosis, especially when admixed with TCMR. Moderate MVI is a conditional criterion for diagnosis (concomitant TCMR requires glomerulitis, which was infrequent and insensitive in our study); this precondition disqualified 6 mixed AM-VR cases incorrectly classified as TCM-VR by Banff 2019. Counting C4dart antibody-endothelial interaction improved the sensitivity and diagnostic accuracy for Banff AMR schema, especially when scrutinized by our reference pathologist. Fibrinoid necrosis is another suggestive marker of AM-VR, where myocytes are replaced by acellular eosinophilic material associated with fragmented elastica.2,4,27, 28, 29 C4dart was not observed in necrotic areas but occurred within adjacent vascular walls (Figure 2g). Anti-class I antibody correlated with fibrinoid arteritis, fibrin thrombi, and infarction in older studies.27,29 Forensic evaluation using modern diagnostics concluded that all our v3 cases were AM-VR. We regard fibrinoid necrosis as pathognomonic of antibody in sensitized recipients with early VR.4,30
Most arterial infiltrates are mixed phenotypes with T cells, intimal macrophages denoting chronicity, and neutrophils, which bind endothelial antibody via Fc receptors and adhere to peritubular capillaries and/or arteries.2,4,27,30 CD15+ neutrophils comprised 27.8% of arterial immunocytes within v3 lesions in explanted C4dptc-positive AMR kidneys.30 Although CD68+ glomerulitis and peritubular capillaritis constitute indirect evidence of microcirculation AMR,31, 32, 33, 34 CD3+/CD68+ arterial VR ratios failed to distinguish C4dptc.32 We regard dominant lymphocytic infiltration without C4d, MVI, or DSA as diagnostic of TCM-VR. Combining molecular diagnostics (transcriptomics) with histologic markers of antibody may help separate AM-VR from TCM-VR.21 Some AM-VR lacked detectable DSA,35 explicable by graft absorption or technical factors (prozone, absent or undenatured antigen).22 DSA results require clinical interpretation by class, onset (de novo from pre-existing), and semiquantitative MFI.6 Our data suggest that some DSA preferentially bind arteries in VR.
Study strengths include our large, well-annotated cohort containing universal C4d and Banff scores using current thresholds, DSA detected by Luminex SAB, and complete longitudinal follow-up. Our retrospective, single-center study used immunoperoxidase in formalin-fixed, paraffin-embedded tissue only, and results cannot be extrapolated to immunofluorescence which display intrinsic arterial C4d autofluorescence of elastin and collagen, rendering it unsuitable for macrovascular evaluation.24 Further collaborative research using preimplantation controls for background staining and archetypal AM-VR with DSA to assess individual laboratory sensitivity and specificity and C4dart standardization is needed. Although our C4dart results confirm the DSA+VR study of Lefaucheur et al.,13 further multicenter collaborative research is required before adoption of this novel biomarker.
In summary, arterial C4d immunoperoxidase deposition is an indicative biomarker of endothelial interaction with DSA in VR, correlating with circulating DSA, multiple histologic markers of AMR, and adverse graft outcomes. Its addition to the Banff schema improved the diagnostic sensitivity for AM-VR detection and etiologic classification of arteritis and was associated with reduced graft survival. We consider arteritis with DSA and arterial C4d as diagnostic of AM-VR and advocate for its inclusion into diagnostic schema to improve accuracy and minimize underdiagnosis of active AMR involving muscular arteries.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This study was investigator initiated, independent, and undertaken without external funding.
Data Sharing
Extensive summary data and analysis are presented within the supplementary material, which contains 10 highly detailed tables of deidentified summated clinical data with their univariable and multivariable statistical analyses to allow for open scientific scrutiny. Federal privacy laws and local institutional ethics forbid the placement of confidential individual patient information onto any public data-sharing website nor allow for its unauthorized sharing. Specific questions of clinical science may be directed to the corresponding author.
Author Contributions
All authors participated in manuscript review and histologic definitions. MS was responsible for blinded reference histology; CHP for C4d background; BJN for research design, and BJN and AS for data analysis.
Footnotes
Figure S1. VR severity and antibody markers. VR, vascular rejection.
Figure S2. Correlations of arterial wall C4d in acute VR. VR, vascular rejection.
Table S1. STARD guidelines for diagnostic studies: checklist.
Table S2. Detailed demographic and pathologic of principal study groups.
Table S3. Severity of arteritis and demographic features.
Table S4A. Confirmatory analysis: Determinants of Banff v scores using ordinal regression against clinical and histologic risk factors.
Table S4B. Arterial C4d score determinants by univariable ordinal regression.
Table S4C. Confirmatory analysis: Determinants of arterial C4d score by multivariable univariable ordinal regression.
Table S5. Confirmatory analysis: Graft failure by Banff AM-VR diagnosis.
Table S6 Comparative VR phenotypes classified by that presence of antibody-mediation versus not.
Table S7. Reclassified samples by pathophysiology of VR. Detailed histopathology and clinical data.
Table S8. Nonspecific background staining for C4dart in normal tissue.
Table S9. Detailed histopathology and clinical phenotypes of vascular rejection classified by pathophysiology (AM-VR, mixed, TCM-VR).
Table S10. Univariable and multivariable determinants of death-censored allograft failure using Cox regression.
Supplementary Material
Figure S1. VR severity and antibody markers. VR, vascular rejection.
Figure S2. Correlations of arterial wall C4d in acute VR. VR, vascular rejection.
Table S1. STARD guidelines for diagnostic studies: checklist.
Table S2. Detailed demographic and pathologic of principal study groups.
Table S3. Severity of arteritis and demographic features.
Table S4A. Confirmatory analysis: Determinants of Banff v scores using ordinal regression against clinical and histologic risk factors.
Table S4B. Arterial C4d score determinants by univariable ordinal regression.
Table S4C. Confirmatory analysis: Determinants of arterial C4d score by multivariable univariable ordinal regression.
Table S5. Confirmatory analysis: Graft failure by Banff AM-VR diagnosis.
Table S6 Comparative VR phenotypes classified by that presence of antibody-mediation versus not.
Table S7. Reclassified samples by pathophysiology of VR. Detailed histopathology and clinical data.
Table S8. Nonspecific background staining for C4dart in normal tissue.
Table S9. Detailed histopathology and clinical phenotypes of vascular rejection classified by pathophysiology (AM-VR, mixed, TCM-VR).
Table S10. Univariable and multivariable determinants of death-censored allograft failure using Cox regression.
References
- 1.Bishop G.A., Waugh J.A., Landers D.V., et al. Microvascular destruction in renal transplant rejection. Transplantation. 1989;48:408–414. doi: 10.1097/00007890-198909000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Halloran P.F., Wadgymar A., Ritchie S., et al. The significance of the anti-class I antibody response. I. Clinical and pathologic features of anti-class I-mediated rejection. Transplantation. 1990;49:85–91. doi: 10.1097/00007890-199001000-00019. [DOI] [PubMed] [Google Scholar]
- 3.Feucht H.E., Schneeberger H., Hillebrand G., et al. Capillary deposition of C4d complement fragment and early renal graft loss. Kidney Int. 1993;43:1333–1338. doi: 10.1038/ki.1993.187. [DOI] [PubMed] [Google Scholar]
- 4.Mauiyyedi S., Crespo M., Collins A.B., et al. Acute humoral rejection in kidney transplantation: II. Morphology, immunopathology, and pathologic classification. J Am Soc Nephrol. 2002;13:779–787. doi: 10.1681/ASN.V133779. [DOI] [PubMed] [Google Scholar]
- 5.Regele H., Bohmig G.A., Habicht A., et al. Capillary deposition of complement split product C4d in renal allografts is associated with basement membrane injury in peritubular and glomerular capillaries: a contribution of humoral immunity to chronic allograft rejection. J Am Soc Nephrol. 2002;13:2371–2380. doi: 10.1097/01.asn.0000025780.03790.0f. [DOI] [PubMed] [Google Scholar]
- 6.Loupy A., Lefaucheur C. Antibody-mediated rejection of solid-organ allografts. N Engl J Med. 2018;379:1150–1160. doi: 10.1056/NEJMra1802677. [DOI] [PubMed] [Google Scholar]
- 7.Nankivell B.J., Alexander S.I. Rejection of the kidney allograft. N Engl J Med. 2010;363:1451–1462. doi: 10.1056/NEJMra0902927. [DOI] [PubMed] [Google Scholar]
- 8.Sellares J., de Freitas D.G., Mengel M., et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012;12:388–399. doi: 10.1111/j.1600-6143.2011.03840.x. [DOI] [PubMed] [Google Scholar]
- 9.Colvin R.B. Antibody-mediated renal allograft rejection: diagnosis and pathogenesis. J Am Soc Nephrol. 2007;18:1046–1056. doi: 10.1681/ASN.2007010073. [DOI] [PubMed] [Google Scholar]
- 10.Mengel M., Sis B., Haas M., et al. Banff 2011 meeting report: new concepts in antibody-mediated rejection. Am J Transplant. 2012;12:563–570. doi: 10.1111/j.1600-6143.2011.03926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimizu T., Ishida H., Shirakawa H., et al. Clinicopathological analysis of acute vascular rejection cases after renal transplantation. Clin Transpl. 2010;24(suppl 22):22–26. doi: 10.1111/j.1399-0012.2010.01277.x. [DOI] [PubMed] [Google Scholar]
- 12.Sis B., Mengel M., Haas M., et al. Banff ‘09 meeting report: antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant. 2010;10:464–471. doi: 10.1111/j.1600-6143.2009.02987.x. [DOI] [PubMed] [Google Scholar]
- 13.Lefaucheur C., Loupy A., Vernerey D., et al. Antibody-mediated vascular rejection of kidney allografts: a population-based study. Lancet. 2013;381:313–319. doi: 10.1016/S0140-6736(12)61265-3. [DOI] [PubMed] [Google Scholar]
- 14.Alpers C.E., Gordon D., Gown A.M. Immunophenotype of vascular rejection in renal transplants. Mod Pathol. 1990;3:198–203. [PubMed] [Google Scholar]
- 15.Feucht H.E. Complement C4d in graft capillaries -- the missing link in the recognition of humoral alloreactivity. Am J Transplant. 2003;3:646–652. doi: 10.1034/j.1600-6143.2003.00171.x. [DOI] [PubMed] [Google Scholar]
- 16.Feucht H.E., Felber E., Gokel M.J., et al. Vascular deposition of complement-split products in kidney allografts with cell-mediated rejection. Clin Exp Immunol. 1991;86:464–470. doi: 10.1111/j.1365-2249.1991.tb02954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drachenberg C.B., Papadimitriou J.C., Chandra P., et al. Epidemiology and pathophysiology of glomerular C4d staining in native kidney biopsies. Kidney Int Rep. 2019;4:1555–1567. doi: 10.1016/j.ekir.2019.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loupy A., Hill G.S., Suberbielle C., et al. Significance of C4d Banff scores in early protocol biopsies of kidney transplant recipients with preformed donor-specific antibodies (DSA) Am J Transplant. 2011;11:56–65. doi: 10.1111/j.1600-6143.2010.03364.x. [DOI] [PubMed] [Google Scholar]
- 19.Croce A.C., Bottiroli G. Autofluorescence spectroscopy and imaging: a tool for biomedical research and diagnosis. Eur J Histochem. 2014;58:2461. doi: 10.4081/ejh.2014.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sis B., Jhangri G.S., Bunnag S., et al. Endothelial gene expression in kidney transplants with alloantibody indicates antibody-mediated damage despite lack of C4d staining. Am J Transplant. 2009;9:2312–2323. doi: 10.1111/j.1600-6143.2009.02761.x. [DOI] [PubMed] [Google Scholar]
- 21.Salazar I.D., Merino Lopez M., Chang J., Halloran P.F. Reassessing the significance of intimal arteritis in kidney transplant biopsy specimens. J Am Soc Nephrol. 2015;26:3190–3198. doi: 10.1681/ASN.2014111064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loupy A., Haas M., Roufosse C., et al. The Banff 2019 kidney meeting report (I): updates on and clarification of criteria for T cell- and antibody-mediated rejection. Am J Transplant. 2020;20:2318–2331. doi: 10.1111/ajt.15898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paul L.C., Baldwin W.M., 3rd, van Es L.A. Vascular endothelial alloantigens in renal transplantation. Transplantation. 1985;40:117–123. doi: 10.1097/00007890-198508000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Nickeleit V., Mengel M., Colvin R.B. In: Heptinstall’s Pathology of the Kidney. 7th ed. Jennette J.C., D’Agati V.D., Olson J.L., Silva F.G., editors. Wolters Kluwer; Philadelphia, PA: 2015. Renal transplant pathology; pp. 1321–1459. [Google Scholar]
- 25.Meehan S.M., Kremer J., Ali F.N., et al. Thrombotic microangiopathy and peritubular capillary C4d expression in renal allograft biopsies. Clin J Am Soc Nephrol. 2011;6:395–403. doi: 10.2215/CJN.05870710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kikic Z., Regele H., Nordmeyer V., et al. Significance of peritubular capillary, glomerular, and arteriolar C4d staining patterns in paraffin sections of early kidney transplant biopsies. Transplantation. 2011;91:440–446. doi: 10.1097/TP.0b013e3182052be8. [DOI] [PubMed] [Google Scholar]
- 27.Colvin R.B., Cohen A.H., Saiontz C., et al. Evaluation of pathologic criteria for acute renal allograft rejection: reproducibility, sensitivity, and clinical correlation. J Am Soc Nephrol. 1997;8:1930–1941. doi: 10.1681/ASN.V8121930. [DOI] [PubMed] [Google Scholar]
- 28.Nickeleit V., Vamvakas E.C., Pascual M., et al. The prognostic significance of specific arterial lesions in acute renal allograft rejection. J Am Soc Nephrol. 1998;9:1301–1308. doi: 10.1681/ASN.V971301. [DOI] [PubMed] [Google Scholar]
- 29.Trpkov K., Campbell P., Pazderka F., et al. Pathologic features of acute renal allograft rejection associated with donor-specific antibody, analysis using the Banff grading schema. Transplantation. 1996;61:1586–1592. doi: 10.1097/00007890-199606150-00007. [DOI] [PubMed] [Google Scholar]
- 30.Sun H.J., Zhou T., Wang Y., et al. Macrophages and T lymphocytes are the predominant cells in intimal arteritis of resected renal allografts undergoing acute rejection. Transpl Immunol. 2011;25:42–48. doi: 10.1016/j.trim.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Desai H.S., Parasuraman R.K., Samarpungavan D., et al. Glomerulitis during acute cellular rejection may be a surrogate marker of vasculitis in renal allografts--better index for diagnosis of vasculitis. Transplant Proc. 2011;43:1629–1633. doi: 10.1016/j.transproceed.2011.01.187. [DOI] [PubMed] [Google Scholar]
- 32.Kozakowski N., Bohmig G.A., Exner M., et al. Monocytes/macrophages in kidney allograft intimal arteritis: no association with markers of humoral rejection or with inferior outcome. Nephrol Dial Transplant. 2009;24:1979–1986. doi: 10.1093/ndt/gfp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nair R., Fraer M., Agrawal N., Suneja M. Acute transplant glomerulopathy is associated with antibody-mediated rejection and poor graft outcome. Transplant Proc. 2010;42:3507–3512. doi: 10.1016/j.transproceed.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 34.Papadimitriou J.C., Drachenberg C.B., Munivenkatappa R., et al. Glomerular inflammation in renal allografts biopsies after the first year: cell types and relationship with antibody-mediated rejection and graft outcome. Transplantation. 2010;90:1478–1485. doi: 10.1097/TP.0b013e3181ff87f5. [DOI] [PubMed] [Google Scholar]
- 35.Filippone E.J., Farber J.L. Histologic antibody-mediated kidney allograft rejection in the absence of donor specific HLA antibodies. Transplantation. 2021;105:e181–e190. doi: 10.1097/TP.0000000000003797. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.