Introduction
In clinical and epidemiologic practice, estimated glomerular filtration rate (eGFR) is most often calculated using serum creatinine-based equations, such as the Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) equation.1,2 There is considerable global variability in the accuracy of these equations, which have largely been developed and validated in high-income settings. Some of the variability can be explained by genetic differences in renal function, dietary habits, body composition, and age distribution of the population, among other factors.3 Importantly, the common practice of including race/ethnicity coefficients in eGFR equations has finally been questioned owing to the lack of a biological basis and for obscuring the social determinants leading to inequity in kidney health.4
Another potentially important cause of systematic bias in GFR estimates is the use of body surface area (BSA) indexing. Because GFR is strongly associated with metabolic rate and body size, GFR-estimating equations have most often incorporated BSA adjustments to allow for GFR comparisons between individuals. This practice began in the field of comparative animal physiology and was subsequently adopted into human nephrology, where it allows for clinicians to meaningfully compare GFR estimates between males and females and across a range of body sizes.5 An implicit assumption of this approach is that BSA indexing largely controls for the association between GFR and is linear across a broad range of metabolic rates and body sizes. Although this is generally correct, this assumption does not hold true at the extremes of the weight and height distributions.6 BSA indexing of both creatinine-based and cystatin c-based equations can underestimate GFR in very obese individuals and overestimate GFR in individuals with sarcopenia with chronic illness.6,7
Less well appreciated is how BSA indexing may affect GFR estimates in low-resource settings, where endemic undernutrition affects adult body proportions. Guatemala has one of the highest prevalence of child stunting (low height-for-age) worldwide, which persists into adulthood as a fixed height deficit. Indeed, Guatemalan women are among the shortest adult populations in the world, and a rising prevalence of obesity leads to the so-called “double burden of malnutrition” (overweight and short stature). At the extremes of the anthropometric distribution, some have advocated for “deindexing” of eGFR by correcting BSA adjustments for individually calculated BSA.8 In this brief research report, we assessed the impact of deindexing BSA on eGFR estimates obtained with the CKD-EPI equation in a population-representative sample from rural Guatemala.9
Results
Anthropometric and serum creatinine data were analyzed from a sample of 807 adults living in rural Guatemala, 793 with creatinine and 785 with anthropometric data. Full details of the sample have previously been published, and additional analysis is included in the Supplementary Methods.9
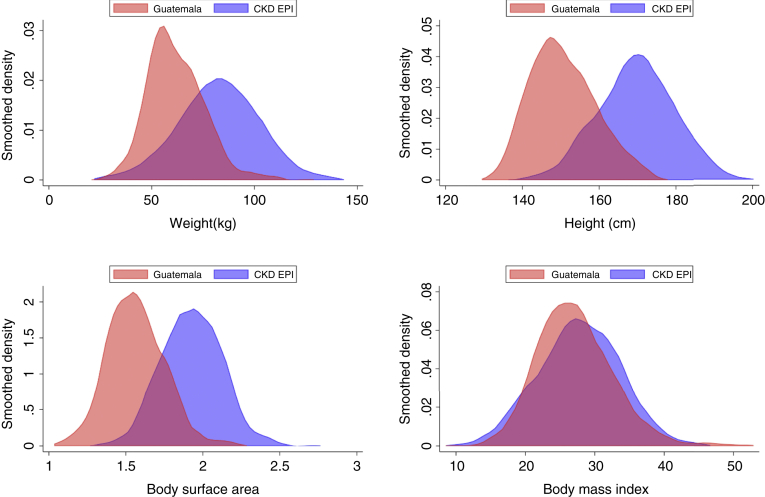
We first compared the distribution of height, weight, body mass index, and BSA data from our sample as compared with the original CKD-EPI development cohort.1 There were marked differences in baseline anthropometrics. For example, the mean height from our sample was 150.7 ± 8.7 cm, compared with 170 ± 10.0 cm for the CKD-EPI cohort. Similarly, mean weight was 61.8 ± 13.9 kg compared with 82.0 ± 20.0 kg, and BSA was 1.57 ± 0.19 m2 compared with 1.93 ± 0.20 m2. In practical terms, this means that the BSA of 42% of our sample falls below −2 Z-scores and 80% below −1 Z-scores for the CKD-EPI development cohort (Figure 1).
Figure 1.
Kernel density estimates for weight, height, body surface area, and body mass index for a rural Guatemalan population survey as compared with the CKD-EPI development cohort. CKD-EPI, chronic kidney disease-epidemiology collaboration.
We next assessed the impact of deindexing eGFR from BSA by multiplying the eGFR obtained from CKD-EPI by the calculated BSA divided by 1.73 m2. Because of the scarcity of established CKD in our data set, in a first analysis, we excluded those with eGFR <60 ml/min per 1.73 m2, n = 26, and compared eGFR with deindexed eGFR. In this analysis, the deindexed eGFR was significantly lower than the eGFR, except at the highest height and weight quintiles. These differences were similar whether using the original CKD-EPI equation or the 2021 revised equation with recalculated coefficients after removal of the race coefficient. Mean eGFR was higher using the 2021 CKD-EPI equation (115.1 ± 15.6 ml/min per 1.73 m2 vs. 105.8 ± 18.2 ml/min per 1.73 m2). In the lowest height quintile, the mean difference between deindexed eGFR and eGFR was −20.0 (95% CI −21.4 to −18.6) ml/min using the original CKD-EPI equation and −21.7 (95% CI −23.2 to −20.3) ml/min using the revised 2021 equation. In the lowest weight quintile, the mean difference was −24.9 (95% CI −25.9 to −23.8) ml/min using the original CKD-EPI equation and −26.6 (95% CI −27.7 to −25.5) ml/min using the revised 2021 equation. These findings are represented in Figure 2 for both the highest and lowest height and weight quintiles, disaggregated by sex.
Figure 2.
Effect of deindexing eGFR estimates obtained using the CKD-EPI equation for body surface area in a population sample from rural Guatemala. Difference between deindexed eGFR and eGFR are given for the highest and lowest weight and height quintiles disaggregated by sex for both the original (blue, red) and revised (orange, green) 2021 CKD-EPI equations. CKD-EPI, chronic kidney disease-epidemiology collaboration; eGFR, estimated glomerular filtration rate.
Next, we evaluated the impact of deindexing on the Kidney Disease Improving Global Outcomes kidney disease risk map including both individuals with and without established CKD, comparing the percentage of individuals falling into each risk category (Supplementary Figure S1).9 For the original CKD-EPI equation, 3.3% of individuals were classified as stage G3a or higher and 19.5% as stage G2. After deindexing for BSA, these proportions increased to 6.6% and 33.5%, respectively (Supplementary Figure 1A). For the revised 2021 CKD-EPI equation, 2.1% of the individuals were classified as stage G3a or higher and 7% as stage G2. After deindexing for BSA, these proportions increased to 3.8% and 19.8%, respectively (Supplementary Figure 1B).
Discussion
To determine the burden of CKD in low-resource settings worldwide, accurate GFR estimates are required. The practice of adjusting GFR estimates for race/ethnicity coefficients is fortunately falling out of favor, because it may perpetuate racism and lacks a biological basis. However, multiple genetic and environmental factors do play an important role in determining population-level variation in GFR.
In this analysis, we use data from Guatemala to highlight the marked anthropometric deviations found in a low-resource setting from the reference norms used to validate GFR-estimating equations. Although the implications of BSA indexing of eGFR in obese and populations with sarcopenia in high-resource settings is most often discussed, we are unaware of any similar discussion of the implications of BSA indexing on constitutionally small adult populations, where low weight and height may be primarily driven by a pervasive endemic of chronic fetal and postnatal undernutrition.
In summary, we find that except at the upper end of the BSA distribution, deindexing eGFR for BSA produces GFR estimates that are significantly lower than standard CKD-EPI estimates for both women and men. In addition, deindexing results in classifying more individuals as Kidney Disease Improving Global Outcomes stage G3a or higher and reclassifies a substantial number of individuals from stage G1 to G2. These findings were observed with both the original and revised 2021 CKD-EPI equations and suggest that, in constitutionally small populations, CKD-EPI may systematically overestimate GFR, leading to underdetection of CKD.
This analysis has several limitations. First, and most importantly, we do not have measured GFR values to confirm these findings. Therefore, our analysis remains exploratory. Second, the proportion of women in our sample (65%) is significantly higher than the CKD-EPI development cohort (43%) which skews the anthropometric distributions presented in Figure 1. However, the patterns observed for deindexed eGFR are similar when stratified by sex (Figure 2).
Our findings stress the urgency of obtaining measured GFR values from diverse global populations, especially those with chronic undernutrition, which have to date been under-represented in rigorous studies of global CKD burden.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The authors thank Alvaro Danilo Sir López, Glenda Gómez Hernández, Héctor Gomez Hernández, Irma Yolanda Raquec Teleguario, and Mérida Isabel Coj Sajvin for recruitment and data collection efforts. The authors thank Dr. Sushrut Waikar for helpful comments and insights. The study was funded by the Fogarty International Center of the National Institutes of Health R21 TW010831-02.
Footnotes
Supplementary Methods.
Figure S1. Effect on KDIGO kidney disease risk map of de-indexing eGFR estimates obtained using the CKD-EPI equation for body surface area in a population sample from rural Guatemala. Proportions before and after deindexing are indicated by arrows for both the original CKD-EPI equation (A) and the revised 2021 equation (B).
Supplementary Material
Supplementary Methods.
Figure S1. Effect on KDIGO kidney disease risk map of de-indexing eGFR estimates obtained using the CKD-EPI equation for body surface area in a population sample from rural Guatemala. Proportions before and after deindexing are indicated by arrows for both the original CKD-EPI equation (A) and the revised 2021 equation (B).
References
- 1.Levey A.S., Stevens L.A., Schmid C.H., et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inker L.A., Eneanya N.D., Coresh J., et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021;385:1737–1749. doi: 10.1056/NEJMoa2102953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glassock R.J., Warnock D.G., Delanaye P. The global burden of chronic kidney disease: estimates, variability and pitfalls. Nat Rev Nephrol. 2017;13:104–114. doi: 10.1038/nrneph.2016.163. [DOI] [PubMed] [Google Scholar]
- 4.Eneanya N.D., Yang W., Reese P.P. Reconsidering the consequences of using race to estimate kidney function. JAMA. 2019;322:113–114. doi: 10.1001/jama.2019.5774. [DOI] [PubMed] [Google Scholar]
- 5.Singer M.A. Of mice and men and elephants: metabolic rate sets glomerular filtration rate. Am J Kidney Dis. 2001;37:164–178. doi: 10.1016/s0272-6386(01)80073-1. [DOI] [PubMed] [Google Scholar]
- 6.Delanaye P., Krzesinski J.M. Indexing of renal function parameters by body surface area: intelligence or folly? Nephron Clin Pract. 2011;119:c289–292. doi: 10.1159/000330276. [DOI] [PubMed] [Google Scholar]
- 7.López-Martínez M., Luis-Lima S., Morales E., et al. The estimation of GFR and the adjustment for BSA in overweight and obesity: a dreadful combination of two errors. Int J Obes (Lond) 2020;44:1129–1140. doi: 10.1038/s41366-019-0476-z. [DOI] [PubMed] [Google Scholar]
- 8.Wuerzner G., Pruijm M., Maillard M., et al. Marked association between obesity and glomerular hyperfiltration: a cross-sectional study in an African population. Am J Kidney Dis. 2010;56:303–312. doi: 10.1053/j.ajkd.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 9.Miller A.C., Tuiz E., Shaw L., et al. Population estimates of GFR and risk factors for CKD in Guatemala. Kidney Int Rep. 2021;6:796–805. doi: 10.1016/j.ekir.2020.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.