Abstract
Achieving parenthood can be an important priority for women and men with kidney failure. In recent decades, the paradigm has shifted toward greater support of parenthood, with advances in our understanding of risks related to pregnancy and improvements in obstetrical and perinatal care. This review, codesigned by people with personal experience of kidney disease, provides guidance for nephrologists on how to answer the questions most asked by patients when planning for parenthood. We focus on important issues that arise in preconception counseling for women receiving dialysis and postkidney transplant. We summarize recent studies reflecting pregnancy outcomes in the modern era of nephrology, obstetrical, and perinatal care in developed countries. We present visual aids to help clinicians and women navigate pregnancy planning and risk assessment. Key principles of pregnancy management are outlined. Finally, we explore outcomes of fatherhood in males with kidney failure.
Keywords: dialysis, kidney failure, parenthood, perinatal, pregnancy, transplantation
Parenthood is an important life goal, and parenthood planning is a core component of care for many people living with chronic kidney disease (CKD) and kidney failure. Among patients receiving kidney replacement therapy, 4% to 16% are aged 18 to 45 years,1,2 representing thousands of people who may consider parenthood. The first experiences with pregnancy in dialyzed3 and transplanted4 women were extraordinary events. With improvements in outcomes over the decades, historical attitudes have evolved toward shared decision-making that supports patient goals and autonomy.5, 6, 7, 8, 9 Although nephrologists should be prepared for questions about parenthood, nephrologist confidence and leadership in addressing women’s health issues remain suboptimal.10,11 The rarity of events and heterogeneity of data at all stages of kidney disease in pregnancy give rise to uncertainties that make counseling challenging.
In this review, we discuss the evidence and management recommendations on common questions that patients may ask (or be afraid to ask) about pregnancy with dialysis or a kidney transplant. The questions were codeveloped by our Parenthood in Kidney Disease Consumer Advisory Group and supported by personal perspectives from patient coauthors AW and BH (Table 1).
Table 1.
Personal perspective from patient co-authors
| Patient perspective 1 After living with dialysis (peritoneal dialysis then hemodialysis), my husband and I had hoped children were in our future. Having a family was a dream come true, even after a kidney transplant. We discussed pregnancy options with my Renal Specialist and made the decision to have my IUD removed. Amazingly, I fell pregnant naturally and chose a shared maternity care arrangement with my GP, Renal Specialist, and Obstetrician. I was closely monitored during my pregnancy … there were a lot of extra appointments, scans, and time off work. However, we were able to ask kidney and pregnancy questions throughout the process and developed relationships along the way which put us at ease. Things were smooth sailing until about 26 weeks, and I became sick with CMV. I had a steroid injection at 29 weeks to prepare for an early birth. Our beautiful girl Jade arrived at 30 weeks, because my kidney function levels were unstable and CMV made me unwell. My local hospital could not cater for a 30-week gestation baby, so I was rushed to a metropolitan hospital. This meant I did not have my usual Obstetrician or Renal Specialist when I had my cesarean section. This was not the birth plan I hoped for. I had complications during the birth and my transplanted kidney was slightly cut in the cesarean. It was a stressful birth; it took me mentally and physically a long time to recover. However, the joy of the beautiful baby my husband and I had always dreamt of made it all worth it. A partnership between Renal Specialist, GP, and Obstetrician creates a supportive environment for the patient during pregnancy. |
| Patient perspective 2 I never thought I wanted children, or that it was even possible with a transplant. But after meeting the right person, my mind started to change. Ten years after my kidney transplant, I was having a serious discussion with my nephrologist about falling pregnant and was told that I was high risk. It was important to me that my obstetrician has experience in caring for transplant patients and ideally has worked with my nephrologist previously. It took a while to fall pregnant, but I finally had a positive pregnancy test at the beginning of 2020, just when COVID hit Australia. This meant that my nephrology appointments were all conducted by telehealth; however, my obstetrical appointments were still face-to-face, which I was thankful for. I felt that I could manage my nephrology care via telehealth as I had my own scales, blood pressure machine, and thermometer at home. When I got to around 28 weeks, I started to get nervous because I really was hoping to make it to 30 weeks. I celebrated at 30 weeks when I was still pregnant with no signs of pre-eclampsia. At 37 weeks, it was decided that I would be induced as my creatinine was starting to creep up. I ended up requiring a cesarean section as the induction was not successful and my baby was born at 37 weeks and 3 days, full term! I felt very confident in my care because the obstetrician and my nephrologist were in contact discussing my results and plans going forward. My nephrologist always answered my many questions, which were mainly about my graft function during and after pregnancy. On reflection, the positive partnership between my Renal Specialist, Obstetrician, and my husband and I was critical in kidney prepregnancy and pregnancy conversations. If we can encourage these partnerships for kidney patients who are wanting a family, we will see better outcomes than those already possible. |
CMV, cytomegalovirus; COVID, coronavirus disease; IUD, intrauterine device; GP, general practitioner.
Women With Kidney Failure—Dialysis and Transplant Recipients
Should I Have a Baby?
Driven by major hemodynamic shifts, there is a significant alteration of renal physiology in pregnancy which results in increased renal perfusion and rise in glomerular filtration rate (GFR) by approximately 50%.12,13 Pregnancy confers significant kidney “stress” that may unmask or worsen underlying kidney conditions and challenge kidney reserve in women with established native renal impairment or allografts.
Nephrologist’s responses to the question “Should I have a baby?” will define the patient experience of parenthood planning. Table 27,8,14, 15, 16, 17 outlines our recommended approach to counseling women with kidney failure. Contraception should be in place while these discussions occur (reviewed by Sachdeva 202018). A dedicated counseling service is valuable to women with CKD.14,19 Shared decision-making is essential and must reflect the patients’ values and preferences, clinical context and complexity, the level of risk they are willing to accept, and the level of autonomy they desire.7,8,15,20 Input from other clinicians who may be part of the future antenatal care team, especially high-risk pregnancy obstetricians, is recommended to co-create a management plan.12,21 Peer support is also highly valued by women, if available.14
Table 2.
Recommended approach to pregnancy counseling in women with kidney failure
| Domain | Suggested approach |
|---|---|
| Timing | Raise potential motherhood as early as feasible to allow planning |
| Prospectively discuss the best window for pregnancy | |
| Allow sufficient time for evolution of discussions over multiple visits | |
| Review and revisit discussions at regular intervals | |
| Communication | Support the woman’s right to pursue pregnancy (or not) |
| Avoid making women defend their choices | |
| Avoid judgmental comments or “forbidding” pregnancy | |
| Explain risks without catastrophizing | |
| Provide hope where possible | |
| Identify and include any other key persons (partner, family) | |
| Provide reassurance that care will be given | |
| Patient values | Identify and acknowledge patient goals |
| Do not assume motherhood is desired by all | |
| Acknowledge grief related to limitations to motherhood | |
| Understand how fears are balanced with desire for parenthood | |
| Define external pressures, obligations and feelings of guilt | |
| Decision-making | Acknowledge the decisional burden |
| Identify how much decisional control women want | |
| Assess risk based on individual clinical context | |
| Understand how risks and decisions are rationalized | |
| Determine individual appetite for “risk” | |
| Facilitate autonomy and decisional ownership | |
| Adopt shared decision-making approaches | |
| Information | Identify how much information women want to have |
| Discuss maternal and fetal risks, long-term health impact, potential pregnancy outcomes, likely pregnancy management and progress | |
| Refer to other services (obstetrical, maternal-fetal medicine, genetic, reproductive medicine) for additional information and counseling | |
| Actively facilitate and address questions |
Whether to undertake a pregnancy posing potential hazards to maternal and infant health is a highly individualized decision.20 Women balance the desire for motherhood with fears of fetal harm, impact of pregnancy on future health, and parenting while juggling the demands of their condition (including potentially reduced survival). The emotional, social, and ethical challenges are heightened when clinicians catastrophize about pregnancy risk or if they “forbid” it.14,15 Women with kidney disease report feelings of guilt, failure, and grief, concerns about loss of control over decisions, and defending their choices to health professionals.15,16
The question “Should I have a baby?” raises ethical issues7,22 that may conflict with the clinician’s values, creating tensions between clinician duty to the patient, fetus, health system, and society. Navigating this requires shared decision-making and trusting relationships between clinician and patient. Advising women against pregnancy risks disengagement and significant distress through loss of reproductive rights and parenthood goals.15
Finally, we recognize that supporting pregnancy with comorbid kidney failure is especially challenging and possibly not feasible in low-income and medium-income settings where access to nephrology care and high-cost treatments are limited.23 This highlights the inequities in care globally for kidney disease, particularly affecting women.24
Can I Have a Baby?
Fertility and likelihood of pregnancy are closely linked to CKD stage. Uremia leads to dysregulation to the hypothalamic-pituitary-gonadal axis, hyperprolactinemia, and disrupted menstrual cycles.25,26 Sexual dysfunction is highly prevalent in dialyzed women,27,28 with few interventions available.29 Fertility is improved with kidney transplantation but not fully restored compared with women without kidney disease.30,31 The cosmetic and libido-reducing physiological effects of uremia, anemia, medications (particularly glucocorticoids and calcineurin inhibitors), dialysis access, and abdominal surgeries all may affect sexual desire and function.27,32
Anti-Mullerian hormone is a marker of ovarian function but requires cautious interpretation in kidney failure. Anti-Mullerian hormone levels, though globally lower in women with CKD, are dependent on age rather than GFR stage.33 Anti-Mullerian hormone levels have been found to be both lower and equivalent to healthy controls in women receiving hemodialysis, vary in dialyzed women with normal versus dysfunctional uterine bleeding, and do not normalize post-transplant.34, 35, 36 Anti-Mullerian hormone levels do not always predict the probability of conception, but may be helpful in predicting success of assisted reproduction and strategies for ovarian protection, such as gonadotropin-releasing hormone analog therapy in women receiving gamete-toxic therapy, for example cyclophosphamide.37
Fertility and Birth Rates in Dialyzed Women
Though secondary amenorrhea is common and pregnancy on dialysis is rare, kidney failure alone is not sufficiently contraceptive. Contraception should be addressed in predialysis preparation, although uptake remains low.38,39 At least 30% of dialyzed women menstruate and may have ovulatory cycles.40 Improvements in well-being after starting dialysis, including correction of hypertension, anemia, and uremia, may lead to improved fertility and unexpected ovulation. Most pregnancies occur in the first year after dialysis commencement41,42; the combination of low contraception use and improved health may facilitate unplanned pregnancy. Furthermore, the wider use of nocturnal home hemodialysis may have potential clinical benefits on female fertility. Improvements in prolactin and reproductive hormones have been observed in patients undergoing extended hours of dialysis.43 A nocturnal hemodialysis program in Canada observed a conception rate of 15.6% and improved fetal outcomes.44
Comparison of fertility data is difficult because of variability in definitions (conception, live birth, delivery) and heterogeneous data across studies. Nevertheless, pregnancy numbers are rising in hemodialysis populations across the world, although they remain significantly lower than transplanted women and nonkidney failure cohorts.41,42,45, 46, 47, 48, 49 Using the Australian and New Zealand Dialysis and Transplant registry linked to birth data sets from 1991 to 2013, we reported that the fertility rate (births/1000 women) for dialyzed women was 91% (95% CI: 87–93) lower than the nonkidney failure cohort and 73% (95% CI: 61–81) lower than transplanted women.41 Despite this, dialyzed women had a rise in both fertility rates and incident-rate ratio of births compared with women without kidney failure, whereas rates in transplanted women and the general nonkidney failure cohort remained stable. Oliverio et al.48 have also revealed that delivery rates increased from 2.1 to 3.6/1000 patient-years from 2002 to 2015 in women receiving hemodialysis in the United States Renal Data System registry. These data reflect the well-reported clinical paradigm shift toward supporting pregnancies on dialysis.6,21
Many registry and cohort studies have reported that pregnancy is exceedingly rare in peritoneal dialysis (PD) recipients and 40% to 50% lower compared with hemodialysis.41,42,46, 47, 48, 49, 50 The impact of i.p. fluid and residual renal function on ovulation in PD recipients also remains undefined. It is not clear whether fertility is reduced, early pregnancy loss is higher, or conversion to hemodialysis occurs, as recommended in recent guidelines.19
Fertility and Birth Rates Post-Kidney Transplant
Improvements in fertility occur early post-transplant, but awareness of this is poor and contraception use is low, even in women who do not desire pregnancy.39,51 Pregnancy rates in transplanted women are not normalized compared with the general population, although again early pregnancy loss is likely under-reported.41,52,53 Birth rates post-kidney transplantation increased in the 1980s and 1990s and have since stabilized.41,49,54 In Australia, we observed a fertility rate of 21.4 live births/1000 women/yr (95% CI: 18.6–24.6) in transplanted women from 1991 to 2013, with a minor drop in 2001 to 2005, potentially coinciding with the introduction of mycophenolate mofetil.41 Across both dialyzed and transplanted cohorts, lower pregnancy and birth rates are observed in women with diabetes, whereas White transplanted women have lower birth rates in the United States but significantly higher in Australia compared with other ethnicities.41,42,55
Assisted Reproduction and Preimplantation Genetic Testing
Robust fertility should not be assumed at any stage of CKD. Early pregnancy planning facilitates the opportunity for fertility assessments, fertility preservation, and assisted reproduction interventions if required.12 Little is known about assisted reproduction in the kidney failure cohort. Successful cases have been reported in transplanted women with no signal of increased adverse outcomes or excessive multiple births.56,57 In Australia, the Australian and New Zealand Dialysis and Transplant registry recently has commenced capturing assisted reproduction data, which may inform this field further.58
Access to genetic testing and genetic counseling for renal disorders has expanded substantially in recent years. It is estimated that 20% of patients aged <25 years with the most common causes of CKD for that age group will have a monogenic condition.59 Preimplantation genetic diagnosis and embryo selection for monogenic kidney diseases are available but not universally acceptable to all patients,60 nor may be affordable or covered by insurance. Patients with monogenic conditions should be made aware of this option and referred for genetic and fertility counseling if desired.61
When Is the Best Time for Me to Have a Baby?
Women value the opportunity to plan life decisions around the best timing of parenthood, based on their individual context. The optimal “window” for pregnancy reflects biological factors (age, CKD stage, likelihood and timing of transplantation, comorbidities, and potential fertility) and social factors and individual context. In established kidney failure, the likelihood of pregnancy and favorable outcomes is generally better in transplanted women compared with dialyzed women or advanced CKD.41,49,62, 63, 64 Advice to defer conception until post-transplantation must account for time-to-transplant, transplant-pregnancy interval, and the biological window for childbearing. Timing should be explicitly discussed to avoid expectations that pregnancy can occur immediately after transplantation.
In women established on dialysis who are unlikely to be transplanted within their childbearing years, the decision to proceed with a planned pregnancy on dialysis is complex and high risk, but increasingly the paradigm is shifting.6,9 Carefully planned pregnancies with extended-hour dialysis have had remarkably good outcomes44,65, 66, 67 but may not be feasible in all centers or all women. Recent UK data on women with advanced CKD (stages 3B–5) reassuringly suggest that the risk of commencing dialysis is <5% in pregnancy and 7% within a year postpartum.63
Meta-analyses of heterogeneous studies vary regarding the optimal transplant-pregnancy interval for graft and pregnancy outcomes55,68,69 and lack of data on early post-transplant pregnancy. The Australian and New Zealand Dialysis and Transplant registry reports that women have median transplant-to-pregnancy interval of 5 years, with very few pregnancies in the first year post-transplant and no impact of transplant-pregnancy interval on outcomes.53,70 Higher graft loss in women with pregnancies in the first 2 years post-transplantation compared has been reported.71 Guidelines currently recommend a minimum of 1 to 2 years post-transplant for lower risk of rejection and infection, switch to nonteratogenic drugs and optimization of overall health.21,72, 73, 74
What Happens If I Have an Unexpected Pregnancy?
Studies on pregnancy outcomes in kidney disease or transplant have rarely captured data on or stratified outcomes according to whether pregnancies were unplanned. An unexpected pregnancy without preconception planning presents challenging choices to women and their partners.7,8,75 Informing women that pregnancy will be safer and more successful if planned with their clinical team may provide compelling incentive to use contraception and optimize health. Decision-making around continuation of an unplanned pregnancy depends on the gestational age, potential teratogenic exposures, risks of miscarriage, risk of proceeding with pregnancy, and patient preferences. All unplanned pregnancies, regardless of whether they are continued or terminated, should be followed up with clear advice on safe and effective contraception options that suit their clinical context.18,76
Will Pregnancy Be Dangerous for Me or My Baby?
A consistent challenge with interpreting studies and syntheses of pregnancy outcomes in kidney failure is the substantial heterogeneity between studies—usually retrospective, often drawn from different eras and models of care, with varying clinical and outcomes data. Prospective studies will no doubt improve the quality of data for future research.77
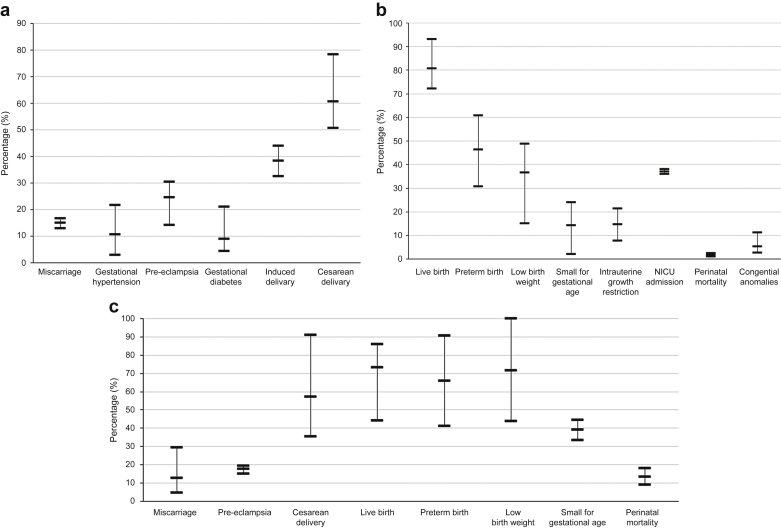
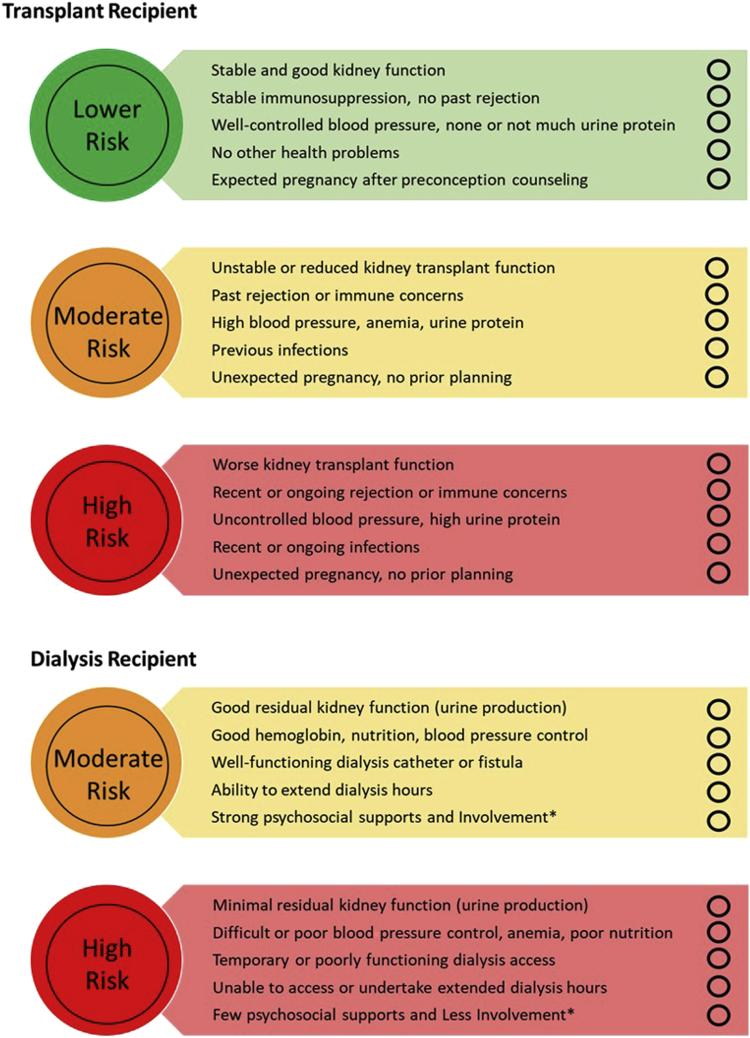
Quantifying and communicating pregnancy risk to women is an essential component of counseling.20 In Figure 1,42,48, 49, 50,65,68,78, 79, 80, 81, 82, 83, 84, 85 we provide a contemporaneous “snapshot” of key perinatal and obstetrical outcomes for dialyzed and transplanted women, based on selected recent, larger studies of cohorts in developed countries. In Figure 2, we provide a “traffic light system” co-designed by patients from our Pregnancy Advisory Group. Both figures may be used in discussions with patients about pregnancy risk.
Figure 1.
A snapshot of maternal and fetal outcomes for pregnancies in women with (a, b) a kidney transplant49,68,78, 79, 80, 81, 82, 83, 84 and (c) women receiving dialysis.42,48, 49, 50,65,82,85 Small for gestational age is defined as birth weight <10th percentile for gestational age. These studies were chosen based on large cohort size, contemporaneous cohorts from developed countries. A pooled average is illustrated with no weighting or other data manipulation or analysis to reveal the range of data reported for key outcomes. NICU, neonatal intensive care unit.
Figure 2.
A traffic light system to help women understand the potential risks of pregnancy with kidney failure. The text and graphics have been codeveloped with our Pregnancy Advisory Consumer Group, led by coauthors BH and AW. These are designed to be used as a shared decision-making tool to encourage open discussion between clinician and patient for determining level of risk based on a woman’s health, to assist informed discussions with women who are planning pregnancies while living with kidney failure. Women can use the circles for a “tick” system to determine their risk in conjunction with their nephrologists. Women can keep these traffic lights and use them to discuss with partners and families. ∗Involvement—The patient is an active partner in their own care and illustrates high level of self-management skills. This may include, however not limited to, the following: takes medications regularly, engages with health care staff, happy to ask questions, and turns up to regular appointments. Patient has strong family/friend support network.
Transplant recipient:
Green: Pregnancy outcome is likely to be very good, with some increased risks of adverse maternal and fetal outcomes. Close monitoring in pregnancy is required. Pregnancy is unlikely to affect kidney transplant function and transplant survival.
Yellow: Pregnancy has increased risks for mother and baby. Very close monitoring is required. There is some risk that the pregnancy will affect kidney transplant function and transplant survival.
Red: Very high-risk pregnancy for mother and baby. Very close monitoring in pregnancy required. Pregnancy is likely to affect the transplant temporarily or permanently.
Dialysis Recipient:
Yellow: Pregnancy has increased risks for mother and baby. Very close monitoring is required. Intensive (long hours and frequent) dialysis may not be required.
Red: Very high-risk pregnancy for mother and baby. Very close monitoring in pregnancy required. Requires intensive dialysis (long hours, more frequent) to improve health of mother and baby.
On the basis of systematic reviews of heterogeneous studies, women with kidney failure have 2- to 10-fold increased risk of adverse maternal-fetal outcomes, including early pregnancy loss, pregnancy-induced hypertension, including pre-eclampsia, preterm birth <37 weeks, and extreme preterm birth <28 weeks gestation, low birth weight <2500 g, and small for gestational age babies <10th centile, induced delivery, and cesarean section, requirement for neonatal intensive care, and perinatal death.47,55,68,69,86 Women should be made aware of the magnitude of risk, so they are prepared for these outcomes, but also reassured that maternal death with kidney failure or transplant is rarely found (if at all) in developed countries.42,55,64,70,78,87,88 Although these outcomes data underline the necessity for careful planning and specialized management, it is important to emphasize that successful pregnancy outcome is highly feasible with appropriate care—from the patient perspective, this means going home with a baby.
Outcomes in Dialyzed Women
The risk of pregnancy complications is highest in dialyzed women (Figure 142,48, 49, 50,65,68,78, 79, 80, 81, 82, 83, 84, 85). Live birth rates have improved greatly over time.41,42,46, 47, 48, 49, 50 Women with residual kidney function have better birth rates and perinatal outcomes,50,66,67 and dialysis dose may be titrated to maternal urea.66,89 In women without residual function, enhanced maternal urea clearances via intensive dialysis regimes (>36 hours per week) are associated with gains in live birth rate, gestational age, and fetal birth weight, less polyhydramnios, and superior hypertension control.47,65
Dialysis may be commenced in pregnancy for maternal symptoms, hypertension or fluid management, polyhydramnios, or other effects of uremia. Women who commence chronic dialysis during pregnancy have higher estimated GFR at dialysis start than women who conceive on dialysis, with >90% chance of live birth.50 The level of renal dysfunction at which dialysis commencement will optimize pregnancy outcomes remains uncertain with limited evidence base. Expert consensus has suggested commencing dialysis at urea 17 mmol/l,21,90 but this is not absolute; the individual context and gestational age should be considered.
PD may be initiated in pregnancy, but most often, women are switched from PD to hemodialysis. Peritonitis and catheter-related problems have been frequently reported,83 and along with concerns about tolerability of intraperitoneal fluid with a gravid uterus, may deter clinicians from continuing PD. Data are limited, but many cases of successful pregnancy outcome have been reported.47,91, 92, 93, 94 A recent systematic review found that gestational age was similar to the hemodialysis cohort; however, the rates of small for gestational age were significantly higher (67% vs. 31%).47 Hybrid therapy can also aid individualized care in selected women and ease hemodialysis burden.94
Outcomes in Transplanted Women
Transplanted women have an excellent live birth rate of 72% to 93%, similar or higher than that observed in the general population,52,55,62,78,79 but early pregnancy loss remains high (13%–17%). A recent meta-analysis of pregnancy outcomes in >4100 kidney transplant recipients globally highlighted the large variation in rates of adverse events, with substantial disparity between countries.55 Pregnancies in transplanted women have increased risk of pre-eclampsia, preterm birth, and low birth weight babies (Figure 142,48, 49, 50,65,68,78, 79, 80, 81, 82, 83, 84, 85), although drivers of these outcomes remain unclear. Risk factors for adverse pregnancy outcome in women with CKD including preconception estimated GFR, hypertension, diabetes, active primary disease, and proteinuria >1 g/l remain relevant post-transplant. Even women with “good” kidney function (serum creatinine 110–125 μmol/l) have increased risk of pregnancy complications.78,79,95 Majak et al.96 demonstrated chronic hypertension, previous pre-eclampsia, or preconception serum creatinine ≥125 μmol/l have cumulative risks, and women with all 3 factors had a 96% risk of pre-eclampsia.
Drivers of excess preterm birth in transplanted women remain undefined. Rates of preterm labor are not clearly increased.49 Higher gestational diabetes rates (5%–11%) have been reported in some studies but not consistently observed.55,68 Pregnancy-related hypertension and pre-eclampsia will precipitate early delivery but can be difficult to differentiate from declining kidney from other causes, including rejection.97 Calcineurin inhibitor toxicity maybe confused with pre-eclampsia as both cause graft dysfunction, thrombotic microangiopathy, and hypertension.79,98 Tacrolimus levels are difficult to interpret in pregnancy because of the change in the free versus bound tacrolimus ratio.99 Whole blood tacrolimus levels fall in the second trimester.79 Clinicians may escalate dosing in response, leading to third trimester blood pressure and creatinine creep which may be diagnosed as pre-eclampsia and precipitate early delivery, although high preterm delivery rates have been observed irrespective of calcineurin inhibitor use.98 Finally, clinician reluctance to progress with pregnancy in later gestation (where risks of continuing pregnancy outweigh risks of early delivery) may drive preterm birth. Regardless, women and clinicians should anticipate possible preterm delivery and undertake shared decision-making about timing of delivery.
Longer-Term Outcomes of Babies Born to Dialyzed and Transplanted Women
Maternal health affects babies via direct and epigenetic effects; therefore, it is reasonable to hypothesize that immunosuppression, placental dysfunction, uremia, and systemic comorbidity may have lasting impacts. Prematurity and restricted growth are linked to future kidney and cardiovascular disease in the general population.100,101 Owing to minimal follow-up studies, the long-term developmental, cognitive, and health outcomes of babies from mothers with CKD are uncertain.101 Children of 16 dialyzed mothers from Italy were found to have broadly normal development as reported by parents.102 Albuminuria was noted in 3 of 9 preterm children of dialyzed mothers in France (at 0.8–25 years follow-up), with normal blood pressure and kidney function.103
There are more data on children of transplanted mothers. By 2 years post-birth, “catch-up” growth to age-appropriate levels is reported.104,105 Immunosuppressive exposures from most often used drugs in transplantation have not been proven to increase congenital defects beyond the general population rate.55,106, 107, 108, 109 Babies of transplanted mothers have clearly altered immunologic cell profile at birth,110 particularly B-cell reduction, but normal immunoglobulin levels, preserved transfer of antibodies from maternal vaccination and normal vaccination responses.111, 112, 113 Neurocognitive development seems to be normal,105,108,114 but owing to small numbers and short duration of follow-up, cautious interpretation is required.
Overall, women should be advised of the paucity of evidence both suggesting or refuting long-term impacts on their children, and this should be incorporated into the risk-benefit matrix.
Will a Pregnancy Harm My Kidney Transplant?
Short-term and long-term impact on graft function is a key concern for transplanted women, who may fear gambling with this precious gift.15,16 Pregnancy has not been clearly found to accelerate graft failure. Matched cohorts with and without pregnancy have not demonstrated long-term impact on graft outcomes; however, these were studies in women with better clinical parameters or stable graft function.53,78,95 Pregnancy in women with worse renal function, hypertension, proteinuria >500 mg/d, and immunologic activity (recent or ongoing rejection) poses greatest risk. Temporary or permanent graft dysfunction is dependent on preconception graft function.69,78,79,98,115 Even in women with “good” graft function (serum creatinine <110 μmol/l), a proportion of women have decline in function antenatally and postpartum “stage-shift,”69,79; however, further studies have not linked this GFR drop to excessive graft failure.116 Late cessation of mycophenolic acid derivatives (<6 weeks prepregnancy) has been associated with increased risk of graft loss at 5 years,117 possibly reflecting unplanned pregnancy. Acute rejection during pregnancy has been reported in 1% to 10% of pregnancies.55,97 The impact of transplant-to-pregnancy interval on long-term graft outcomes is uncertain with conflicting data from various studies.53,69, 70, 71,78,95 Overall, the potential impact of pregnancy must be individualized to each patient and graft.
How Should I Prepare for Pregnancy?
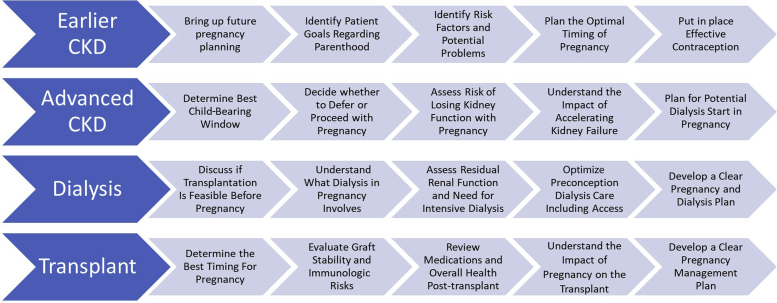
Figure 3 summarizes key elements to guide pregnancy preparation throughout the CKD stages. The ideal scenario is a planned pregnancy at the safest time, with a well-counseled patient, specialized multidisciplinary team, proximity to high-risk antenatal care, and contraception in place until ready for pregnancy.12,21 Referral to maternal-fetal medicine specialists ahead of pregnancy is ideal. Patients may also benefit from genetics, fertility, dietitian, and psychology input.12,21 Maternal health should be optimized across a range of domains: medication exposures, correction of anemia to >100 g/l, control of infection, smoking cessation, and updated vaccination. Blood pressure optimization is crucial, and a target blood pressure of <140/90 mmg should be achieved preconception. Continuation of angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker until conception should be determined on an individual basis, depending on risks of cessation, likelihood of time to conception, and benefits of antiproteinuric effect. Optimization of glycemic control in pre-existing diabetes reduces adverse maternal and fetal outcomes.118 Treating asymptomatic bacteriuria may reduce the risk of overt infection and pyelonephritis.119
Figure 3.
A summary of key decision points and elements for pregnancy planning and care through the spectrum of kidney disease. CKD, chronic kidney disease.
Preparing for a Pregnancy With Dialysis
Planned pregnancies in dialysis recipients are less common; more likely women will present with an unexpected or unplanned pregnancy. An individualized plan should include optimizing dialysis clearances, assessment of residual function, correcting anemia, and ensuring excellent control of hypertension. Women should be made aware of the increased dialysis burden during pregnancy. Vascular access options should be reviewed; arteriovenous fistulae or catheters are suitable for use in pregnancy. Extended-hour dialysis (>36 h/wk) for women with minimal residual kidney function is logistically challenging but can be achieved through daily or nocturnal dialysis44,65 and is recommended in clinical guidelines.21,120 For women established on PD, optimizing clearances may require changing to hemodialysis.
Preparing for Pregnancy With a Kidney Transplant
Ovulation and fertility may return rapidly; therefore, establishing contraception and pregnancy preparation (if desired) should occur well before transplantation. Stable graft function, stable and pregnancy-safe immunosuppression, low risk of rejection, and controlled infections are favorable conditions for planning for pregnancy. Screening for potential new-onset post-transplant diabetes should occur. Women should understand that there is fetal exposure to all immunosuppressants used in pregnancy, and robust data on safety are limited (reviewed in detail by Gonzalez Suarez et al.109). Prednisolone, azathioprine, and calcineurin inhibitors are the safer options and have been widely used, whereas all other immunosuppressants have very limited data to support common use in pregnancy. Although the minimum time for withdrawal of mycophenolate mofetil agents before conception is 6 weeks,117 our practice is to institute pregnancy-compatible immunosuppression at least 3 to 6 months preconception and allow a period of observation to ensure graft stability.12
There are little published data on the role of preconception kidney transplant biopsy, but this may be a highly informative step in women with suspicion of subclinical rejection after immunosuppression switch or any cause of unstable graft function. Women with significant allograft dysfunction should be prepared for decline in function and possible dialysis start during pregnancy.
Cytomegalovirus (CMV) infection in pregnancy can be a catastrophic event because of the teratogenicity of antiviral treatments, risk of systemic maternal infection, and fetal outcomes of congenital CMV.121 Women who have had CMV active viremia post-transplantation are at greatest risk of CMV in pregnancy, but data are very limited.109 In women who have received long-term antiviral prophylaxis for either past CMV viremia or seronegative status, our practice is to undertake a preconception trial of antiviral therapy cessation and observe whether CMV viremia emerges.
What Care Do I Need During Pregnancy?
Our detailed recommendations for management across several domains for dialyzed and transplanted women are presented in Table 3. A multidisciplinary team should be established before conception, where feasible, or soon after pregnancy diagnosis.21 A single point for coordination of care is preferred—likely the nephrologist. Both dialyzed and transplanted women require heightened maternal and fetal surveillance. Women should anticipate frequent clinic visits, investigations, and multiple care providers across disciplines. They should be warned of potential hospitalization during pregnancy and longer length of stay postpartum. In later gestation, women should remain in proximity to centers with access to high-risk pregnancy/perinatal and nephrology care. Women in regional centers may need to relocate for delivery depending on their clinical status.
Table 3.
Recommended medical management for pregnant women with kidney failure
| Dialysis recipient | Transplant recipient |
|---|---|
| Model of care | |
| |
| Medications | |
|
|
| Modality-specific management | |
|
|
| Blood pressure | |
|
|
| Anemia | |
| |
| Infection | |
|
|
| Nutrition | |
|
|
| Diabetes screening | |
| |
| Diabetes management | |
|
|
| Fetal monitoring | |
| |
| Delivery and early postpartum care | |
| |
| Postpregnancy care | |
|
|
AVF, arteriovenous fistula; BP, blood pressure; CMV, cytomegalovirus; CNI, calcineurin inhibitor; ESA, erythropoiesis-stimulating agent; Hb, hemoglobin; HD, hemodialysis; sFLT1, soluble fms-like tyrosine kinase 1; PD, peritoneal dialysis; PlGF, placental growth factor; UTI, urinary tract infection.
Can I Have a Normal Delivery?
The high-risk of preterm delivery and decisions about delivery timing, mode of delivery, and location of delivery should be discussed early in pregnancy. The mode of delivery should be based on obstetrical reasons. Vaginal delivery and trial of labor can be safely undertaken and may be associated with less perinatal morbidity122; therefore, they are recommended where feasible. However, cesarean section rates (particularly emergency section) and instrumental delivery are substantially higher in women receiving kidney replacement therapy and increasing over time.49,55,64,88,122 The use of analgesia for delivery is poorly defined in women with kidney failure—local practice and patient preference should guide choice. Transplant anatomy should be defined and documented early in pregnancy in case of emergency surgical delivery. We recommend early transplant imaging post−cesarean section; any reduction in urine output warrants immediate imaging.
Can I Breastfeed My Baby?
Mothers with kidney failure or transplants should not be discouraged from breastfeeding if they wish. True contraindications are extremely rare, and benefits outweigh the minimal risks; however, we recognize that there are practice variations across countries. Self-reported breastfeeding rates in transplanted women have risen.123 Medications used during pregnancy can be continued postpartum, and drug exposure is much higher in utero than through breast milk. Tacrolimus, prednisolone, and azathioprine all have minimal transfer into the breast milk.124, 125, 126, 127 Quinapril, enalapril, and captopril are suitable for lactating mothers requiring resumption of renin-angiotensin blockade.128
Men With Kidney Failure
The uremic environment effect has detrimental effects on male sexual hormones and erectile function, sperm quality, and fertility and is not improved by dialysis.129, 130, 131, 132, 133, 134 Disruption to the hypothalamic-pituitary-gonadal axis results in hyperprolactinemia and reduction in effective testosterone, with sexual function. Psychosocial stressors and body image issues are relevant to relationships and sexual activity, whereas many factors (vascular, endocrine, neurologic, and pharmacologic) can contribute to the high prevalence of erectile dysfunction observed.133, 134, 135 Nocturnal hemodialysis may improve the hormonal environment,43 but the impact on fertility is unknown. Proven treatments for sexual dysfunction in men with CKD are very limited.28 Although kidney transplantation restores the endocrine milieu, there may be incomplete resolution of erectile dysfunction and high prevalence of epididymal microcalcification, oligo/azoospermia, and morphologic abnormalities.134,136
Despite these challenges, men receiving kidney replacement therapy do successfully father children with excellent outcomes, although almost all data are for transplanted fathers with very few studies in dialyzed men.134,137, 138, 139 The largest study of fatherhood outcomes in dialyzed and transplanted men was from the Australian and New Zealand Dialysis and Transplant registry, analyzing 960 babies born to transplanted fathers and 333 babies of dialyzed fathers over several decades.140 Equivalent live birth rates, gestational age, and birth weight were observed for babies conceived by fathers treated with either modality, even after adjusting for paternal age, era of treatment, and comorbid conditions. Age-standardized paternity event rates for Australian fathers after transplantation have decreased in recent years, whereas the reported fatherhood rate for dialyzed men has remained stable (although rates are <50% of that in transplanted fathers).140 A likely reason is concern regarding the lack of safety data for mycophenolic acid derivatives on male reproduction and female exposure via semen, leading to recommendations for heightened contraception use by regulatory agencies in the mid-2010s. However, given several studies from different cohorts reporting excellent outcomes for children fathered by males exposed to mycophenolic acid, men may be counseled that there is no currently clear signal for increased fetal malformations.137,139, 140, 141, 142, 143, 144 Mammalian target of rapamycin inhibitors influence spermatogenesis and fertility rates in animal models and humans,145,146 but there is uncertainty about the effect of other immunosuppressants owing to rarity of cases. The impact of immunosuppression switch in male transplant recipients for the purpose of conception is also unknown. These uncertainties should be conveyed in preconception counseling.
Conclusions
Parenthood planning for men and women is an important part of nephrology care, but it may be complex and requires time, trusted relationships, and a robust understanding of the evidence base. Pregnancy should be raised early in the CKD journey, so patients understand the risks and challenges and can determine the safest window of opportunity for achieving this important life goal.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The authors are very grateful for insightful input from members of our Parenthood in Kidney Disease Consumer Advisory Group (Jane Boag, Dr. Shyam Muthuramalingam, Laura Heffernan, Adela Tolic, Kelli Owen, Dr Charmaine Green, Carolina Maistry), particularly for the framework of questions patients may ask and design of figures for patient counseling. EH is supported by the Better Evidence and Translation in Chronic Kidney Disease (BEAT-CKD) Program Grant (National Health and Medical Research Council, Australia, APP1092957) awarded to Prof. Stephen McDonald.
Author Contributions
SJ, BH, AW, and EH designed the manuscript concept. SJ and EH drafted the manuscript. AW and BH co-designed the question subheadings and figures and wrote the patient perspective pieces. All authors reviewed, contributed to, and approved the final version of the manuscript.
References
- 1.United States Renal Data System 2020 USRDS annual data report: Epidemiology of Kidney Disease in the United States. United States Renal Data System. https://adr.usrds.org/2020 Published 2020.
- 2.Australia and New Zealand Dialysis and Transplant Registry ANZDATA 43rd annual report 2020 (data to 2019). Australia and New Zealand Dialysis and Transplant Registry. https://www.anzdata.org.au/report/anzdata-43rd-annual-report-2020-data-to-2019/
- 3.Confortini P. Full term successful pregnancy and successful delivery in a patient on chronic haemodialysis. Proc Eur Dial Transpl Assoc Eur Dial Transpl Assoc. 1971:74–80. [Google Scholar]
- 4.Murray J.E., Reid D.E., Harrison J.H., Merrill J.P. Successful pregnancies after human renal transplantation. N Engl J Med. 1963;269:341–343. doi: 10.1056/NEJM196308152690704. [DOI] [PubMed] [Google Scholar]
- 5.Holley J.L., Reddy S.S. Pregnancy and CKD: lessons on communication from patients. Am J Kidney Dis. 2015;66:936–938. doi: 10.1053/j.ajkd.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Bramham K. Dialysis and pregnancy: no longer the impossible. Nephrol Dial Transplant. 2016;31:1763–1765. doi: 10.1093/ndt/gfw216. [DOI] [PubMed] [Google Scholar]
- 7.Hendren E., Hladunewich M.A., Lefkowitz A. Caring for pregnant patients with CKD---an ethical discussion of 5 cases. Kidney Int Rep. 2021;6:1273–1279. doi: 10.1016/j.ekir.2021.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jesudason S., Tong A. The patient experience of kidney disease and pregnancy. Best Pract Res Clin Obstet Gynaecol. 2019;57:77–88. doi: 10.1016/j.bpobgyn.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Piccoli G.B., Conijn A., Consiglio V., et al. Pregnancy in dialysis patients: is the evidence strong enough to lead us to change our counseling policy? Clin J Am Soc Nephrol. 2010;5:62–71. doi: 10.2215/CJN.05660809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hendren E.M., Reynolds M.L., Mariani L.H., et al. Confidence in women’s health: a cross border survey of adult nephrologists. J Clin Med. 2019;8:176. doi: 10.3390/jcm8020176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Ek G.F., Krouwel E.M., Nicolai M.P.J., et al. What is the role of nephrologists and nurses of the dialysis department in providing fertility care to CKD patients? A questionnaire study among care providers. Int Urol Nephrol. 2017;49:1273–1285. doi: 10.1007/s11255-017-1577-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fitzpatrick A., Mohammadi F., Jesudason S. Managing pregnancy in chronic kidney disease: improving outcomes for mother and baby. Int J Womens Health. 2016;8:273–285. doi: 10.2147/IJWH.S76819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Odutayo A., Hladunewich M. Obstetric nephrology: renal hemodynamic and metabolic physiology in normal pregnancy. Clin J Am Soc Nephrol. 2012;7:2073–2080. doi: 10.2215/CJN.00470112. [DOI] [PubMed] [Google Scholar]
- 14.van Buren M.C., Beck D.K., Lely A.T., van de Wetering J., Massey E.K. EXPloring attitudes and factors influencing reproductive Choices in kidney Transplant patients (The EXPECT-study) Clin Transplant. 2021;35:e14473. doi: 10.1111/ctr.14473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tong A., Brown M., Winkelmayer W., Craig J., Jesudason S. Perspectives on pregnancy in women with CKD: a semistructured interview study. Am J Kidney Dis. 2015;66:951–961. doi: 10.1053/j.ajkd.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 16.Tong A., Jesudason S., Craig J., Winkelmayer W. Perspectives on pregnancy in women with chronic kidney disease: systematic review of qualitative studies. Nephrol Dial Transplant. 2015;30:652–661. doi: 10.1093/ndt/gfu378. [DOI] [PubMed] [Google Scholar]
- 17.Schipper K., van der Borg W.E., de Jong-Camerik J., Abma T.A. Living with moderate to severe renal failure from the perspective of patients. BMC Nephrol. 2016;17:48. doi: 10.1186/s12882-016-0263-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sachdeva M. Contraception in kidney disease. Adv Chronic Kidney Dis. 2020;27:499–505. doi: 10.1053/j.ackd.2020.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Wiles K.S., Bramham K., Vais A., et al. Pre-pregnancy counselling for women with chronic kidney disease: a retrospective analysis of nine years’ experience. BMC Nephrol. 2015;16:28. doi: 10.1186/s12882-015-0024-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ralston E.R., Smith P., Chilcot J., Silverio S.A., Bramham K. Perceptions of risk in pregnancy with chronic disease: a systematic review and thematic synthesis. PLoS One. 2021;16 doi: 10.1371/journal.pone.0254956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiles K., Chappell L., Clark K., et al. Clinical practice guideline on pregnancy and renal disease. BMC Nephrol. 2019;20:401. doi: 10.1186/s12882-019-1560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross L.F. Ethical considerations related to pregnancy in transplant recipients. N Engl J Med. 2006;354:1313–1316. doi: 10.1056/NEJMsb041648. [DOI] [PubMed] [Google Scholar]
- 23.Bello A.K., Okpechi I.G., Jha V., Harris D.C.H., Levin A., Johnson D.W. Global variation in kidney care: national and regional differences in the care and management of patients with kidney failure. Kidney Int Suppl (2011) 2021;11:e1–e3. doi: 10.1016/j.kisu.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piccoli G.B., Alrukhaimi M., Liu Z.H., Zakharova E., Levin A., World Kidney Day Steering C. What we do and do not know about women and kidney diseases; questions unanswered and answers unquestioned: reflection on World Kidney Day and International Woman’s Day. Nephrology. 2018;23:199–209. doi: 10.1111/nep.13193. [DOI] [PubMed] [Google Scholar]
- 25.Holley J.L. The hypothalamic-pituitary axis in men and women with chronic kidney disease. Adv Chronic Kidney Dis. 2004;11:337–341. doi: 10.1053/j.ackd.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Palmer B.F., Clegg D.J. Gonadal dysfunction in chronic kidney disease. Rev Endocr Metab Disord. 2017;18:117–130. doi: 10.1007/s11154-016-9385-9. [DOI] [PubMed] [Google Scholar]
- 27.Navaneethan S.D., Vecchio M., Johnson D.W., et al. Prevalence and correlates of self-reported sexual dysfunction in CKD: a meta-analysis of observational studies. Am J Kidney Dis. 2010;56:670–685. doi: 10.1053/j.ajkd.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 28.Strippoli G.F., Collaborative Depression and Sexual Dysfunction (CDS) in Hemodialysis Working Group Sexual dysfunction in hemodialysis. Clin J Am Soc Nephrol. 2012;7:974–981. doi: 10.2215/CJN.12601211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vecchio M., Navaneethan S.D., Johnson D.W., et al. Interventions for treating sexual dysfunction in patients with chronic kidney disease. Cochrane Database Syst Rev. 2010;(12):CD007747. doi: 10.1002/14651858.CD007747.pub2. [DOI] [PubMed] [Google Scholar]
- 30.Josephson M.A., McKay D.B. Pregnancy and kidney transplantation. Semin Nephrol. 2011;31:100–110. doi: 10.1016/j.semnephrol.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 31.Josephson M.A., McKay D.B. Women and transplantation: fertility, sexuality, pregnancy, contraception. Adv Chronic Kidney Dis. 2013;20:433–440. doi: 10.1053/j.ackd.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Basok E.K., Atsu N., Rifaioglu M.M., Kantarci G., Yildirim A., Tokuc R. Assessment of female sexual function and quality of life in predialysis, peritoneal dialysis, hemodialysis, and renal transplant patients. Int Urol Nephrol. 2009;41:473–481. doi: 10.1007/s11255-008-9475-z. [DOI] [PubMed] [Google Scholar]
- 33.Wiles K., Anckaert E., Holden F., et al. Anti-Müllerian hormone concentrations in women with chronic kidney disease. Clin Kidney J. 2019;14:537–542. doi: 10.1093/ckj/sfz164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stoumpos S., Lees J., Welsh P., et al. The utility of anti-Müllerian hormone in women with chronic kidney disease, on haemodialysis and after kidney transplantation. Reprod Biomed Online. 2018;36:219–226. doi: 10.1016/j.rbmo.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 35.Fayed A., Soliman A., Naguib M., Soliman M., Salaheldin M. Ovarian reserve in an Egyptian cohort with end-stage kidney disease on hemodialysis and after successful kidney transplantation: a prospective study. Int Urol Nephrol. 2019;51:737–743. doi: 10.1007/s11255-019-02089-2. [DOI] [PubMed] [Google Scholar]
- 36.Sikora-Grabka E., Adamczak M., Kuczera P., Szotowska M., Madej P., Wiecek A. Serum anti-Müllerian hormone concentration in young women with chronic kidney disease on hemodialysis, and after successful kidney transplantation. Kidney Blood Press Res. 2016;41:552–560. doi: 10.1159/000443458. [DOI] [PubMed] [Google Scholar]
- 37.Lin C., Jing M., Zhu W., et al. The value of anti-Müllerian hormone in the prediction of spontaneous pregnancy: a systematic review and meta-analysis. Front Endocrinol. 2021;12:695157. doi: 10.3389/fendo.2021.695157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shah S., Christianson A.L., Thakar C.V., Kramer S., Meganathan K., Leonard A.C. Contraceptive use among women with end-stage kidney disease on dialysis in the United States. Kidney Med. 2020;2:707–715.e1. doi: 10.1016/j.xkme.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.French V.A., Davis J.S., Sayles H.S., Wu S.S. Contraception and fertility awareness among women with solid organ transplants. Obstet Gynecol. 2013;122:809–814. doi: 10.1097/AOG.0b013e3182a5eda9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chakhtoura Z., Meunier M., Caby J., et al. Gynecologic follow up of 129 women on dialysis and after kidney transplantation: a retrospective cohort study. Eur J Obstet Gynecol Reprod Biol. 2015;187:1–5. doi: 10.1016/j.ejogrb.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Hewawasam E, Davies CE, Gulyani A, et al. Factors influencing fertility rates in Australian women receiving kidney replacement therapy: analysis of linked ANZDATA registry and perinatal data over 22 years. Nephrol Dial Transplant. Published online April 12, 2021. https://doi.org/10.1093/ndt/gfab157 [DOI] [PubMed]
- 42.Shah S., Christianson A.L., Meganathan K., Leonard A.C., Schauer D.P., Thakar C.V. Racial differences and factors associated with pregnancy in ESKD patients on dialysis in the United States. J Am Soc Nephrol. 2019;30:2437–2448. doi: 10.1681/ASN.2019030234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Eps C., Hawley C., Jeffries J., et al. Changes in serum prolactin, sex hormones and thyroid function with alternate nightly nocturnal home haemodialysis. Nephrology (Carlton) 2012;17:42–47. doi: 10.1111/j.1440-1797.2011.01520.x. [DOI] [PubMed] [Google Scholar]
- 44.Barua M., Hladunewich M., Keunen J., et al. Successful pregnancies on nocturnal home hemodialysis. Clin J Am Soc Nephrol. 2008;3:392–396. doi: 10.2215/CJN.04110907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bagon J.A., Vernaeve H., De Muylder X., Lafontaine J.J., Martens J., Van Roost G. Pregnancy and dialysis. Am J Kidney Dis. 1998;31:756–765. doi: 10.1016/s0272-6386(98)70060-5. [DOI] [PubMed] [Google Scholar]
- 46.Okundaye I., Abrinko P., Hou S. Registry of pregnancy in dialysis patients. Am J Kidney Dis. 1998;31:766–773. doi: 10.1016/s0272-6386(98)70044-7. [DOI] [PubMed] [Google Scholar]
- 47.Piccoli G.B., Minelli F., Versino E., et al. Pregnancy in dialysis patients in the new millennium: a systematic review and meta-regression analysis correlating dialysis schedules and pregnancy outcomes. Nephrol Dial Transplant. 2016;31:1915–1934. doi: 10.1093/ndt/gfv395. [DOI] [PubMed] [Google Scholar]
- 48.Shahir A.K., Briggs N., Katsoulis J., Levidiotis V. An observational outcomes study from 1966–2008, examining pregnancy and neonatal outcomes from dialysed women using data from the ANZDATA Registry. Nephrology (Carlton) 2013;18:276–284. doi: 10.1111/nep.12044. [DOI] [PubMed] [Google Scholar]
- 49.Oliverio A.L., Bragg-Gresham J.L., Admon L.K., Wright Nunes J.A., Saran R., Heung M. Obstetric deliveries in US women with ESKD: 2002–2015. Am J Kidney Dis. 2020;75:762–771. doi: 10.1053/j.ajkd.2019.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jesudason S., Grace B.S., McDonald S.P. Pregnancy outcomes according to dialysis commencing before or after conception in women with ESRD. Clin J Am Soc Nephrol. 2014;9:143–149. doi: 10.2215/CJN.03560413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eide I.A., Rashidi F., Lønning K., et al. Contraceptive choices and counseling in Norwegian female renal transplant recipients. Transplant Proc. 2019;51:470–474. doi: 10.1016/j.transproceed.2019.01.068. [DOI] [PubMed] [Google Scholar]
- 52.Gill J.S., Zalunardo N., Rose C., Tonelli M. The pregnancy rate and live birth rate in kidney transplant recipients. Am J Transplant. 2009;9:1541–1549. doi: 10.1111/j.1600-6143.2009.02662.x. [DOI] [PubMed] [Google Scholar]
- 53.Levidiotis V., Chang S., McDonald S. Pregnancy and maternal outcomes among kidney transplant recipients. J Am Soc Nephrol. 2009;20:2433–2440. doi: 10.1681/ASN.2008121241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shah S., Christianson A.L., Verma P., et al. Racial disparities and factors associated with pregnancy in kidney transplant recipients in the United States. PLoS One. 2019;14 doi: 10.1371/journal.pone.0220916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shah S., Venkatesan R.L., Gupta A., et al. Pregnancy outcomes in women with kidney transplant: meta-analysis and systematic review. BMC Nephrol. 2019;20:24. doi: 10.1186/s12882-019-1213-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Norrman E., Bergh C., Wennerholm U.B. Pregnancy outcome and long-term follow-up after in vitro fertilization in women with renal transplantation. Hum Reprod. 2015;30:205–213. doi: 10.1093/humrep/deu293. [DOI] [PubMed] [Google Scholar]
- 57.Yaprak M., Doğru V., Sanhal C.Y., Özgür K., Erman M. In vitro fertilization after renal transplantation: a single-center experience. Transplant Proc. 2019;51:1089–1092. doi: 10.1016/j.transproceed.2019.01.105. [DOI] [PubMed] [Google Scholar]
- 58.ANZDATA Parenthood outcome form. https://www.anzdata.org.au/wp-content/uploads/2021/02/ParenthoodOutcomeForm.pdf
- 59.Vivante A., Hildebrandt F. Exploring the genetic basis of early-onset chronic kidney disease. Nat Rev Nephrol. 2016;12:133–146. doi: 10.1038/nrneph.2015.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Swift O., Vilar E., Rahman B., et al. Attitudes in patients with autosomal dominant polycystic kidney disease toward prenatal diagnosis and preimplantation genetic diagnosis. Genet Test Mol Biomarkers. 2016;20:741–746. doi: 10.1089/gtmb.2016.0050. [DOI] [PubMed] [Google Scholar]
- 61.Tchan M., Savige J., Patel C., et al. KHA-CARI autosomal dominant polycystic kidney disease guideline: genetic testing for diagnosis. Semin Nephrol. 2015;35:545–549.e2. doi: 10.1016/j.semnephrol.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 62.Chewcharat A., Kattah A.G., Thongprayoon C., et al. Comparison of hospitalization outcomes for delivery and resource utilization between pregnant women with kidney transplants and chronic kidney disease in the United States. Nephrology (Carlton) 2021;26:879–889. doi: 10.1111/nep.13938. [DOI] [PubMed] [Google Scholar]
- 63.Wiles K., Webster P., Seed P.T., et al. The impact of chronic kidney disease stages 3–5 on pregnancy outcomes. Nephrol Dial Transplant. 2020;36:2008–2017. doi: 10.1093/ndt/gfaa247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saliem S., Patenaude V., Abenhaim H. Pregnancy outcomes among renal transplant recipients and patients with end-stage renal disease on dialysis. J Perinat Med. 2016;44:321–327. doi: 10.1515/jpm-2014-0298. [DOI] [PubMed] [Google Scholar]
- 65.Hladunewich M.A., Hou S., Odutayo A., et al. Intensive hemodialysis associates with improved pregnancy outcomes: a Canadian and United States cohort comparison. J Am Soc Nephrol. 2014;25:1103–1109. doi: 10.1681/ASN.2013080825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Luders C., Titan S.M., Kahhale S., Francisco R.P., Zugaib M. Risk factors for adverse fetal outcome in hemodialysis pregnant women. Kidney Int Rep. 2018;3:1077–1088. doi: 10.1016/j.ekir.2018.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Luders C., Castro M.C., Titan S.M., et al. Obstetric outcome in pregnant women on long-term dialysis: a case series. Am J Kidney Dis. 2010;56:77–85. doi: 10.1053/j.ajkd.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 68.Deshpande N.A., James N.T., Kucirka L.M., et al. Pregnancy outcomes in kidney transplant recipients: a systematic review and meta-analysis. Am J Transplant. 2011;11:2388–2404. doi: 10.1111/j.1600-6143.2011.03656.x. [DOI] [PubMed] [Google Scholar]
- 69.van Buren M.C., Schellekens A., Groenhof T.K.J., et al. Long-term graft survival and graft function following pregnancy in kidney transplant recipients: a systematic review and meta-analysis. Transplantation. 2020;104:1675–1685. doi: 10.1097/TP.0000000000003026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wyld M.L., Clayton P.A., Jesudason S., Chadban S.J., Alexander S.I. Pregnancy outcomes for kidney transplant recipients. Am J Transplant. 2013;13:3173–3182. doi: 10.1111/ajt.12452. [DOI] [PubMed] [Google Scholar]
- 71.Rose C., Gill J., Zalunardo N., Johnston O., Mehrotra A., Gill J.S. Timing of pregnancy after kidney transplantation and risk of allograft failure. Am J Transplant. 2016;16:2360–2367. doi: 10.1111/ajt.13773. [DOI] [PubMed] [Google Scholar]
- 72.EBPG expert group on renal transplantation European best practice guidelines for renal transplantation. Section IV: long-term management of the transplant recipient. IV.10. Pregnancy in renal transplant recipients. Nephrol Dial Transplant. 2002;17(suppl 4):50–55. [PubMed] [Google Scholar]
- 73.Kidney disease: improving global outcomes transplant work G KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(suppl 3):S1–S155. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 74.McKay D.B., Josephson M.A., Armenti V.T., et al. Reproduction and transplantation: report on the AST Consensus Conference on Reproductive Issues and Transplantation. Am J Transplant. 2005;5:1592–1599. doi: 10.1111/j.1600-6143.2005.00969.x. [DOI] [PubMed] [Google Scholar]
- 75.Burgner A., Hladunewich M.A. Women’s reproductive health for the nephrologist. Am J Kidney Dis. 2019;74:675–681. doi: 10.1053/j.ajkd.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 76.Burgner A., Hladunewich M.A. Contraception and CKD. Clin J Am Soc Nephrol. 2020;15:563–565. doi: 10.2215/CJN.09770819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jesudason S. Pregnancy and kidney disease: it is time for the birth of prospective registries. Nephrology (Carlton) 2021;26:849–850. doi: 10.1111/nep.13971. [DOI] [PubMed] [Google Scholar]
- 78.Stoumpos S., McNeill S.H., Gorrie M., et al. Obstetric and long-term kidney outcomes in renal transplant recipients: a 40-yr single-center study. Clin Transplant. 2016;30:673–681. doi: 10.1111/ctr.12732. [DOI] [PubMed] [Google Scholar]
- 79.Mohammadi F., Borg M., Gulyani A., McDonald S., Jesudason S. Pregnancy outcomes and impact of pregnancy on graft function in women after kidney transplantation. Clin Transplant. 2017;31 doi: 10.1111/ctr.13089. [DOI] [PubMed] [Google Scholar]
- 80.Hebral A.L., Cointault O., Connan L., et al. Pregnancy after kidney transplantation: outcome and anti-human leucocyte antigen alloimmunization risk. Nephrol Dial Transplant. 2014;29:1786–1793. doi: 10.1093/ndt/gfu208. [DOI] [PubMed] [Google Scholar]
- 81.Egerup P., Carlson N., Bruun Oestergaard L., et al. Increased risk of neonatal complications and infections in children of kidney-transplanted women: a nationwide controlled cohort study. Am J Transplant. 2021;21:1171–1178. doi: 10.1111/ajt.16259. [DOI] [PubMed] [Google Scholar]
- 82.Piccoli G.B., Cabiddu G., Daidone G., et al. The children of dialysis: live-born babies from on-dialysis mothers in Italy--an epidemiological perspective comparing dialysis, kidney transplantation and the overall population. Nephrol Dial. 2014;29:1578–1586. doi: 10.1093/ndt/gfu092. [DOI] [PubMed] [Google Scholar]
- 83.Bramham K., Nelson-Piercy C., Gao H., et al. Pregnancy in renal transplant recipients: a UK national cohort study. Clin J Am Soc Nephrol. 2013;8:290–298. doi: 10.2215/CJN.06170612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Arab K., Oddy L., Patenaude V., Abenhaim H.A. Obstetrical and neonatal outcomes in renal transplant recipients. J Matern Fetal Neonatal Med. 2015;28:162–167. doi: 10.3109/14767058.2014.909804. [DOI] [PubMed] [Google Scholar]
- 85.Normand G., Xu X., Panaye M., et al. Pregnancy outcomes in French hemodialysis patients. Am J Nephrol. 2018;47:219–227. doi: 10.1159/000488286. [DOI] [PubMed] [Google Scholar]
- 86.Nevis I.F., Reitsma A., Dominic A., et al. Pregnancy outcomes in women with chronic kidney disease: a systematic review. Clin J Am Soc Nephrol. 2011;6:2587–2598. doi: 10.2215/CJN.10841210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sachdeva M., Barta V., Thakkar J., Sakhiya V., Miller I. Pregnancy outcomes in women on hemodialysis: a national survey. Clin Kidney J. 2017;10:276–281. doi: 10.1093/ckj/sfw130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kendrick J., Sharma S., Holmen J., Palit S., Nuccio E., Chonchol M. Kidney disease and maternal and fetal outcomes in pregnancy. Am J Kidney Dis. 2015;66:55–59. doi: 10.1053/j.ajkd.2014.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Asamiya Y., Otsubo S., Matsuda Y., et al. The importance of low blood urea nitrogen levels in pregnant patients undergoing hemodialysis to optimize birth weight and gestational age. Kidney Int. 2009;75:1217–1222. doi: 10.1038/ki.2009.48. [DOI] [PubMed] [Google Scholar]
- 90.Sato J.L., De Oliveira L., Kirsztajn G.M., Sass N. Chronic kidney disease in pregnancy requiring first-time dialysis. Int J Gynaecol Obstet. 2010;111:45–48. doi: 10.1016/j.ijgo.2010.04.029. [DOI] [PubMed] [Google Scholar]
- 91.Batarse R.R., Steiger R.M., Guest S. Peritoneal dialysis prescription during the third trimester of pregnancy. Perit Dial Int. 2015;35:128–134. doi: 10.3747/pdi.2013.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jefferys A., Wyburn K., Chow J., Cleland B., Hennessy A. Peritoneal dialysis in pregnancy: a case series. Nephrology (Carlton) 2008;13:380–383. doi: 10.1111/j.1440-1797.2008.00938.x. [DOI] [PubMed] [Google Scholar]
- 93.Asgari E., Bramham K., Shehata H., Makanjuola D. Successful pregnancy in a patient with end-stage renal failure secondary to HIV nephropathy on peritoneal dialysis. Nephrol Dial Transplant. 2007;22:3671. doi: 10.1093/ndt/gfm280. [DOI] [PubMed] [Google Scholar]
- 94.Ross L.E., Swift P.A., Newbold S.M., Bramham K., Hurley A., Gallagher H. An alternative approach to delivering intensive dialysis in pregnancy. Perit Dial Int. 2016;36:575–577. doi: 10.3747/pdi.2016.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Majak G.B., Reisæter A.V., Weedon-Fekjær H., Henriksen T., Michelsen T.M. The effect of pregnancy on the long-term risk of graft loss, cardiovascular disease, and death in kidney transplanted women in Norway: a retrospective cohort study. Transplantation. 2018;102:e391–e396. doi: 10.1097/TP.0000000000002167. [DOI] [PubMed] [Google Scholar]
- 96.Majak G.B., Reisaeter A.V., Zucknick M., et al. Preeclampsia in kidney transplanted women; outcomes and a simple prognostic risk score system. PLoS One. 2017;12 doi: 10.1371/journal.pone.0173420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yin O., Kallapur A., Coscia L., Constantinescu S., Moritz M., Afshar Y. Differentiating acute rejection from preeclampsia after kidney transplantation. Obstet Gynecol. 2021;137:1023–1031. doi: 10.1097/AOG.0000000000004389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Koenjer L.M., Meinderts J.R., van der Heijden O.W.H., et al. Comparison of pregnancy outcomes in Dutch kidney recipients with and without calcineurin inhibitor exposure: a retrospective study. Transpl Int. 2021;34:2669–2679. doi: 10.1111/tri.14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hebert M.F., Zheng S., Hays K., et al. Interpreting tacrolimus concentrations during pregnancy and postpartum. Transplantation. 2013;95:908–915. doi: 10.1097/TP.0b013e318278d367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Luyckx V.A., Chevalier R.L. Impact of early life development on later onset chronic kidney disease and hypertension and the role of evolutionary trade-offs. Exp Physiol. 2021 doi: 10.1113/EP089918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Haseler E., Melhem N., Sinha M.D. Renal disease in pregnancy: fetal, neonatal and long-term outcomes. Best Pract Res Clin Obstet Gynaecol. 2019;57:60–76. doi: 10.1016/j.bpobgyn.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 102.Piccoli G.B., Postorino V., Cabiddu G., et al. Children of a lesser god or miracles? An emotional and behavioural profile of children born to mothers on dialysis in Italy: a multicentre nationwide study 2000-12. Nephrol Dial Transplant. 2015;30:1193–1202. doi: 10.1093/ndt/gfv127. [DOI] [PubMed] [Google Scholar]
- 103.Abou-Jaoude P., Dubourg L., Bessenay L., et al. What about the renal function during childhood of children born from dialysed mothers? Nephrol Dial Transplant. 2012;27:2365–2369. doi: 10.1093/ndt/gfr617. [DOI] [PubMed] [Google Scholar]
- 104.Dinelli M.I.S., Ono E., Viana P.O., Dos Santos A.M.N., de Moraes-Pinto M.I. Growth of children born to renal transplanted women. Eur J Pediatr. 2017;176:1201–1207. doi: 10.1007/s00431-017-2965-1. [DOI] [PubMed] [Google Scholar]
- 105.Bachmann F., Budde K., Gerland M., et al. Pregnancy following kidney transplantation - impact on mother and graft function and focus on childrens’ longitudinal development. BMC Preg Childbirth. 2019;19:376. doi: 10.1186/s12884-019-2496-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bar Oz B., Hackman R., Einarson T., Koren G. Pregnancy outcome after cyclosporine therapy during pregnancy: a meta-analysis. Transplantation. 2001;71:1051–1055. doi: 10.1097/00007890-200104270-00006. [DOI] [PubMed] [Google Scholar]
- 107.Wyld M.L., Clayton P.A., Kennedy S., Alexander S.I., Chadban S.J. Pregnancy outcomes for kidney transplant recipients with transplantation as a child. JAMA Pediatr. 2015;169:1–6. doi: 10.1001/jamapediatrics.2014.3626. [DOI] [PubMed] [Google Scholar]
- 108.Boulay H., Mazaud-Guittot S., Supervielle J., et al. Maternal, foetal and child consequences of immunosuppressive drugs during pregnancy in women with organ transplant: a review. Clin Kidney J. 2021;14:1871–1878. doi: 10.1093/ckj/sfab049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gonzalez Suarez M.L., Parker A.S., Cheungpasitporn W. Pregnancy in kidney transplant recipients. Adv Chronic Kidney Dis. 2020;27:486–498. doi: 10.1053/j.ackd.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 110.Ono E., Dos Santos A.M., Viana P.O., et al. Immunophenotypic profile and increased risk of hospital admission for infection in infants born to female kidney transplant recipients. Am J Transplant. 2015;15:1654–1665. doi: 10.1111/ajt.13143. [DOI] [PubMed] [Google Scholar]
- 111.Dinelli M.I.S., Ono E., Viana P.O., et al. Response to immunization in children born to renal transplant recipients using immunosuppressive drugs during gestation. Vaccine. 2016;34:404–407. doi: 10.1016/j.vaccine.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 112.Drozdowska-Szymczak A., Kociszewska-Najman B., Schreiber-Zamora J., et al. Evaluation of selected markers of the immune system in children of renal transplant recipients. Transplant Proc. 2014;46:2703–2707. doi: 10.1016/j.transproceed.2014.09.062. [DOI] [PubMed] [Google Scholar]
- 113.Viana P.O., Ono E., Dinelli M.I., et al. Maternally acquired IgG immunity in neonates born to renal transplanted women. Vaccine. 2015;33:3104–3109. doi: 10.1016/j.vaccine.2015.04.104. [DOI] [PubMed] [Google Scholar]
- 114.Schreiber-Zamora J., Szpotanska-Sikorska M., Drozdowska-Szymczak A., et al. Neurological development of children born to mothers after kidney transplantation. J Matern Fetal Neonatal Med. 2019;32:1523–1527. doi: 10.1080/14767058.2017.1407754. [DOI] [PubMed] [Google Scholar]
- 115.Vannevel V., Claes K., Baud D., et al. Preeclampsia and long-term renal function in women who underwent kidney transplantation. Obstet Gynecol. 2018;131:57–62. doi: 10.1097/AOG.0000000000002404. [DOI] [PubMed] [Google Scholar]
- 116.Kattah A.G., Albadri S., Alexander M.P., et al. Impact of pregnancy on GFR decline and kidney histology in kidney transplant recipients. Kidney Int Rep. 2022;7:28–35. doi: 10.1016/j.ekir.2021.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.King R.W., Baca M.J., Armenti V.T., Kaplan B. Pregnancy outcomes related to mycophenolate exposure in female kidney transplant recipients. Am J Transplant. 2017;17:151–160. doi: 10.1111/ajt.13928. [DOI] [PubMed] [Google Scholar]
- 118.Davidson A.J.F., Park A.L., Berger H., et al. Association of improved periconception hemoglobin A1c with pregnancy outcomes in women with diabetes. JAMA Network Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.30207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Smaill F.M., Vazquez J.C. Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database Syst Rev. 2019 doi: 10.1002/14651858.CD000490.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.National Kidney Foundation KDOQI clinical practice guideline for hemodialysis adequacy: 2015 update. Am J Kidney Dis. 2015;66:884–930. doi: 10.1053/j.ajkd.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 121.Sebghati M., Khalil A. New evidence on prognostic features, prevention and treatment of congenital Cytomegalovirus infection. Curr Opin Obstet Gynecol. 2020;32:342–350. doi: 10.1097/GCO.0000000000000651. [DOI] [PubMed] [Google Scholar]
- 122.Yin O., Kallapur A., Coscia L., et al. Mode of obstetric delivery in kidney and liver transplant recipients and associated maternal, neonatal, and graft morbidity during 5 decades of clinical practice. JAMA Network Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.27378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Constantinescu S., Pai A., Coscia L., Davison J., Moritz M., Armenti V. Breast-feeding after transplantation. Best Pract Res Clin Obstet Gynaecol. 2014;28:1163–1173. doi: 10.1016/j.bpobgyn.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 124.Bramham K., Chusney G., Lee J., Lightstone L., Nelson-Piercy C. Breastfeeding and tacrolimus: serial monitoring in breast-fed and bottle-fed infants. Clin J Am Soc Nephrol. 2013;8:563–567. doi: 10.2215/CJN.06400612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Armenti V.T., Moritz M.J., Davison J.M. Breastfeeding and tacrolimus: is it a reasonable approach? Expert Rev Clin Immunol. 2013;9:623–626. doi: 10.1586/1744666X.2013.811042. [DOI] [PubMed] [Google Scholar]
- 126.Ryu R.J., Easterling T.R., Caritis S.N., et al. Prednisone pharmacokinetics during pregnancy and lactation. J Clin Pharmacol. 2018;58:1223–1232. doi: 10.1002/jcph.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Christensen L.A., Dahlerup J.F., Nielsen M.J., Fallingborg J.F., Schmiegelow K. Azathioprine treatment during lactation. Aliment Pharmacol Ther. 2008;28:1209–1213. doi: 10.1111/j.1365-2036.2008.03843.x. [DOI] [PubMed] [Google Scholar]
- 128.Shannon M.E., Malecha S.E., Cha A.J. Angiotensin converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs) and lactation: an update. J Hum Lact. 2000;16:152–155. doi: 10.1177/089033440001600213. [DOI] [PubMed] [Google Scholar]
- 129.Fugl-Meyer K.S., Nilsson M., Hylander B., Lehtihet M. Sexual function and testosterone level in men with conservatively treated chronic kidney disease. Am J Mens Health. 2017;11:1069–1076. doi: 10.1177/1557988317703207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lehtihet M., Hylander B. Semen quality in men with chronic kidney disease and its correlation with chronic kidney disease stages. Andrologia. 2015;47:1103–1108. doi: 10.1111/and.12388. [DOI] [PubMed] [Google Scholar]
- 131.Prem A.R., Punekar S.V., Kalpana M., Kelkar A.R., Acharya V.N. Male reproductive function in uraemia: efficacy of haemodialysis and renal transplantation. Br J Urol. 1996;78:635–638. doi: 10.1046/j.1464-410x.1996.14624.x. [DOI] [PubMed] [Google Scholar]
- 132.Chou F.F., Lee C.H., Lee C.T., Huang F.J., Hsu K.L. Spermatogenesis after parathyroidectomy in patients with symptomatic secondary hyperparathyroidism. J Am Coll Surg. 2003;196:854–858. doi: 10.1016/S1072-7515(03)00129-7. [DOI] [PubMed] [Google Scholar]
- 133.Edey M.M. Male sexual dysfunction and chronic kidney disease. Front Med (Lausanne) 2017;4:32. doi: 10.3389/fmed.2017.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lundy S.D., Vij S.C. Male infertility in renal failure and transplantation. Transl Androl Urol. 2019;8:173–181. doi: 10.21037/tau.2018.07.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Collaborative Depression and Sexual Dysfunction in Hemodialysis Working Group. Vecchio M., Palmer S., et al. Prevalence and correlates of erectile dysfunction in men on chronic haemodialysis: a multinational cross-sectional study. Nephrol Dial Transplant. 2012;27(6):2479–2488. doi: 10.1093/ndt/gfr635. [DOI] [PubMed] [Google Scholar]
- 136.Bozzini G., Lunelli L., Berlingheri M., Groppali E., Carmignani L. Epididymis microlithiasis and semen abnormalities in young adult kidney transplant recipients. Andrologia. 2013;45:357–360. doi: 10.1111/and.12036. [DOI] [PubMed] [Google Scholar]
- 137.Xu L.G., Yang Y.R., Wang H.W., et al. Characteristics of male fertility after renal transplantation. Andrologia. 2011;43:203–207. doi: 10.1111/j.1439-0272.2010.01052.x. [DOI] [PubMed] [Google Scholar]
- 138.Cohen A.D., Gower P.E., Rogers K.L., Pegrum G.D., De Wardener H.E. Reproductive potential in males treated by chronic haemodialysis. Proc Eur Dial Transpl Assoc Eur Dial Transpl Assoc. 1973;10:282–288. [PubMed] [Google Scholar]
- 139.Morken N.H., Diaz-Garcia C., Reisaeter A.V., et al. Obstetric and neonatal outcome of pregnancies fathered by males on immunosuppression after solid organ transplantation. Am J Transplant. 2015;15:1666–1673. doi: 10.1111/ajt.13159. [DOI] [PubMed] [Google Scholar]
- 140.Jesudason S., Fitzpatrick A., Gulyani A., et al. Fatherhood and kidney replacement therapy: analysis of the Australian and New Zealand dialysis and transplant (ANZDATA) registry. Am J Kidney Dis. 2020;76:444–446. doi: 10.1053/j.ajkd.2020.03.020. [DOI] [PubMed] [Google Scholar]
- 141.Martin-Moreno P.L., Sánchez-Fructuoso A.I., Mazuecos A., et al. Paternal safety of the use of mycophenolic acid in kidney transplant recipients. Results of the EMVARON study. Clin Transplant. 2021;35:e14256. doi: 10.1111/ctr.14256. [DOI] [PubMed] [Google Scholar]
- 142.Xu L., Han S., Liu Y., et al. The influence of immunosuppressants on the fertility of males who undergo renal transplantation and on the immune function of their offspring. Transpl Immunol. 2009;22:28–31. doi: 10.1016/j.trim.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 143.Jones A., Clary M.J., McDermott E., et al. Outcomes of pregnancies fathered by solid-organ transplant recipients exposed to mycophenolic acid products. Prog Transplant. 2013;23:153–157. doi: 10.7182/pit2013636. [DOI] [PubMed] [Google Scholar]
- 144.Midtvedt K., Bergan S., Reisaeter A.V., Vikse B.E., Asberg A. Exposure to mycophenolate and fatherhood. Transplantation. 2017;101:e214–e217. doi: 10.1097/TP.0000000000001747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zuber J., Anglicheau D., Elie C., et al. Sirolimus may reduce fertility in male renal transplant recipients. Am J Transplant. 2008;8:1471–1479. doi: 10.1111/j.1600-6143.2008.02267.x. [DOI] [PubMed] [Google Scholar]
- 146.Huyghe E., Zairi A., Nohra J., Kamar N., Plante P., Rostaing L. Gonadal impact of target of rapamycin inhibitors (sirolimus and everolimus) in male patients: an overview. Transpl Int. 2007;20:305–311. doi: 10.1111/j.1432-2277.2006.00423.x. [DOI] [PubMed] [Google Scholar]