Abstract
Introduction
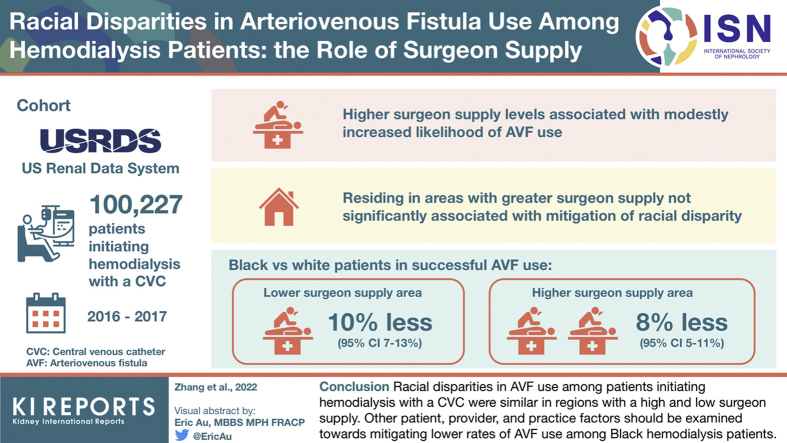
Factors contributing to racial disparities in arteriovenous fistula (AVF) use among hemodialysis (HD) patients remain poorly defined. We evaluated whether the Black/White race disparity in AVF use is affected by vascular access surgeon supply.
Methods
Using Consolidated Renal Operations in a Web-Enabled Network (CROWNWeb) and Medicare claims data from the US Renal Data System (USRDS), competing risk analyses of all US patients initiating HD with a central venous catheter (CVC) from 2016 to 2017 (n = 100,227) were performed. The likelihood of successful AVF use was compared between Black and White patients after adjusting for vascular access surgeon supply.
Results
Compared with the first (lowest) quartile of surgeon supply, higher supply levels were associated with modestly increased adjusted likelihoods of overall AVF use: 4% (95% CI 1.4%–7.2%), 4% (95% CI 1.4%–7.1%), and 3% (0.0%–6.1%) for second, third, and fourth quartiles, respectively. Although areas with lower surgeon supply had a higher proportion of Black patients, residing in areas with a greater surgeon supply was not significantly associated with a mitigation in racial disparity. Specifically, compared with White patients, Black patients were 10% (95% CI 7%–13%) and 8% (95% CI 5%–11%) less likely to have successful AVF use in lower and higher surgeon supply areas, respectively.
Conclusion
Regions with lower surgeon supply had a higher proportion of Black dialysis patients. However, racial disparities in AVF use among patients initiating HD with a CVC were similar in regions with a high and low surgeon supply. Other patient, provider, and practice factors should be evaluated toward mitigating lower rates of AVF use among Black HD patients.
Keywords: arteriovenous fistula, ESKD, hemodialysis, racial disparities, surgeon supply
Graphical abstract
Delivering HD thrice weekly to patients with end-stage kidney disease (ESKD) requires a reliable vascular access that can provide a sufficient blood flow into the extracorporeal dialysis circuit. Among the 3 types of vascular access, an AVF is preferred because its use is associated with better quality of life, fewer infections, and improved survival, compared with use of a CVC or an arteriovenous graft (AVG).1,2 AVF use has increased in the United States. HD patients increased from 24% in 1998 to 20003 to 66% in 2018.4 Despite these overall improvements, as compared with White patients, Black patients continue to lag in AVF use.5 In a cohort of older adults who initiated HD with a CVC, we previously reported lower rates of AVF placement, maturation, and primary patency among Black compared with White patients, after controlling for a range of patient and dialysis facility characteristics.6 The root causes contributing to persistent Black/White disparities in AVF use remain poorly defined.
A recent study reported substantial geographic variation in the supply of vascular access surgeons by Hospital Referral Region (HRR), calculated as the number of surgeons performing access procedures per 1000 prevalent patients with ESKD.7 Specifically, the supply of surgeons was lower in HRRs with a higher unemployment rate and a lower supply of primary care physicians and nephrologists. This observation suggests that vascular access surgeons are less likely to practice in socioeconomically disadvantaged areas. Previous publications have reported that area-level poverty was associated with both a lower likelihood of AVF use at the start of HD and a greater disparity in ESKD rates between Black and White individuals.8, 9, 10
The lower supply of surgeons in these geographic areas, a previously overlooked factor, may in part explain the lower frequency of AVF use among Black HD patients. To pursue this question, we investigated patients with ESKD in the United States who initiated HD with a CVC in 2016 and 2017 and assessed the likelihood that they would use an AVF in the ensuing 12 months. We evaluated the association between the availability of surgeons in the patients’ area of residence and AVF use 1 year after dialysis initiation. Finally, we assessed whether racial disparities in AVF use were attenuated in areas with higher surgeon supply.
Methods
Data Source
The primary data source for this study was the USRDS, a national registry of patients with ESKD.11 The following components of USRDS were used: (i) Centers for Medicare & Medicaid Services ESKD Medical Evidence Form 2728, completed by dialysis facilities on all incident dialysis patients, was used to obtain type of vascular access in use at the start of ESKD, including demographics, such as sex and race, and comorbid condition information. (ii) CROWNWeb data, submitted by dialysis facilities monthly, were used to extract and track the type of vascular access in use (during the last HD session of the month) for each outpatient dialysis month after onset of ESKD. Unlike Medicare claims data, CROWNWeb allowed us to include all outpatient dialysis patients in the United States, regardless of their use of Medicare as their primary payer. (iii) Medicare claims data, including inpatient, outpatient, and physician/supplier claims, were used to identify surgeons who performed vascular access procedures by their National Provider Identifier numbers. (iv) USRDS Patients File was used to obtain date of death and first kidney transplant. Furthermore, National Provider Identifier numbers of identified physicians were linked with Centers for Medicare & Medicaid Services National Provider Identifier files by crosswalk provided by USRDS to obtain physicians’ ZIP code, which was later linked with publicly available geographic boundary files from the Dartmouth Atlas of Health Care12 to obtain physicians’ HRR for calculation of surgeon supply at the HRR level. Finally, the Area Health Resources File was used to measure county-level sociodemographic factors.13,14 Institutional review board approval was exempt because all data were encrypted and deidentified.
Patient Population
For this retrospective cohort study, all patients with a first ESKD service date between January 1, 2016, and December 31, 2017, who started in-center HD with a CVC only and did not have a maturing vascular access in place were included. Patients were required to be 18 years and older, have had HD as their initial dialysis modality, and have survived the first 30 days of ESKD. We excluded patients whose primary cause of kidney failure was acute kidney injury, as many of these patients do not require long-term dialysis. We restricted the population to Black and White patients, regardless of ethnicity. Patients were followed up for 12 months starting from the study baseline (the patients’ second month of HD) until permanent use of AVF or AVG, switch to peritoneal dialysis, kidney transplantation, or death. The cascade of 100,227 patients included based on the study selection criteria is depicted in Figure 1.
Figure 1.
Flowchart of patient selection for study cohort. AVF, arteriovenous fistula; AVG, arteriovenous graft; CVC, central venous catheter; ESRD, end-stage renal disease; HRR, Hospital Referral Region.
Study Measures
The main independent variable of interest was the supply of surgeons, defined as the number of surgeons performing AVF or AVG placement per 1000 prevalent patients with ESKD, across 306 HRRs in 2016 and 2017.6 Physicians who performed AVF or AVG placement procedures were determined based on the National Provider Identifiers from all 2016 to 2017 Medicare claims for vascular access procedures and then aggregated for each HRR region. These claims included Current Procedural Terminology codes 36800, 36810, 36818, 36819, 36820, 36821, and 36825 for AVF placements and CPT code 36830 for AVG placements. Number of ESKD patients, as the denominator of surgeon supply measure, was calculated by adding all prevalent dialysis patients in 2016 to 2017 CROWNWeb data in each HRR region based on the ZIP code for patients’ residence.
Multivariable analyses were adjusted for patient, dialysis facility, and ZIP code-specific characteristics. Patient characteristics included age (<45, 45 to <65, 65 to <80, and 80+ years), sex, race (Black or White), primary cause of kidney failure (diabetes, hypertension, glomerulonephritis, and other), insurance type (Medicare, Medicaid, employer group coverage, Department of Veterans Affairs or other, and uninsured), pre-ESKD nephrology care, body mass index (<24.0, 24.0 to <28.3, 28.3 to <34.1, and ≥34.1 kg/m2), drug or alcohol dependence, functional status (amputation, inability to ambulate or transfer, needs assistance with daily activities), and institutionalization (e.g., in a nursing facility). The major comorbid conditions extracted were diabetes on insulin, hypertension, coronary artery disease including arteriosclerotic heart disease and myocardial infarction, congestive heart failure, peripheral vascular disease, and cerebrovascular disease. Facility characteristics evaluated included end-stage renal disease (ESRD) network (18 ESRD networks), ownership (major chain, minor chain, or independent), freestanding or hospital-based, profit status (profit or nonprofit), and facility size based on number of patients (<58, 58–86, 86–122, and ≥122; cutoffs were based on quartiles of the facility size distribution). Finally, we characterized the sociodemographic conditions corresponding with each patient’s residential county as percent of White population, percent of urban population, percent of adults with a high school diploma, percent of population living in poverty, unemployment rate, and per capita income (average income earned per person).
The primary study outcome was time (in months) to AVF use, defined as successful AVF use after removal of the CVC, within 12 months of dialysis initiation. Competing events (when CVC dialysis access would cease) included transition to AVG, switch to peritoneal dialysis, kidney transplantation, and death. Censoring events included administrative censoring (defined as 12 months after study baseline) and loss-to-follow up (defined as the second consecutive month with missing vascular access type).
Statistical Analysis
All analyses were performed at the patient-level after assigning HRR-level surgeon supply to individual patients. Counts and proportions were used to describe patient, facility, and community characteristics by surgeon supply level. Competing risk regression and survival analyses were used to estimate the association between surgeon supply and time to first AVF use. Because censoring on competing events precludes the occurrence of an event of interest, it can bias the estimates of the time to AVF use.15 We therefore adopted the subdistribution hazard model developed by Fine and Gray16 to account for the possible nonindependence of the censoring on competing events. Unadjusted and adjusted competing risk analysis of Fine and Gray16 was conducted with the event of interest (AVF use) being coded as 1. Using the algorithm developed by Zhang and Zhang17 for the subdistribution hazard model, a direct adjusted cumulative incidence function curve was produced by surgeon supply and patient race. All models were implemented in SAS, version 9.4 (SAS Institute, Inc., Cary, NC).
Results
A total of 100,227 patients were receiving HD with a CVC after surviving the first 30 days of ESKD (Figure 1). At the end of the 12-month study follow-up, 15.5% had died, 0.8% had a kidney transplant, 4.7% switched to peritoneal dialysis from HD, and 10.5% were censored owing to missing vascular access data for at least 2 consecutive months. Among the patients not censored owing to a competing event, 42.4% were using an AVF, 12.9% were using an AVG, and 13.5% were still using a CVC at 12 months.
Characteristics Across Surgeon Supply Levels
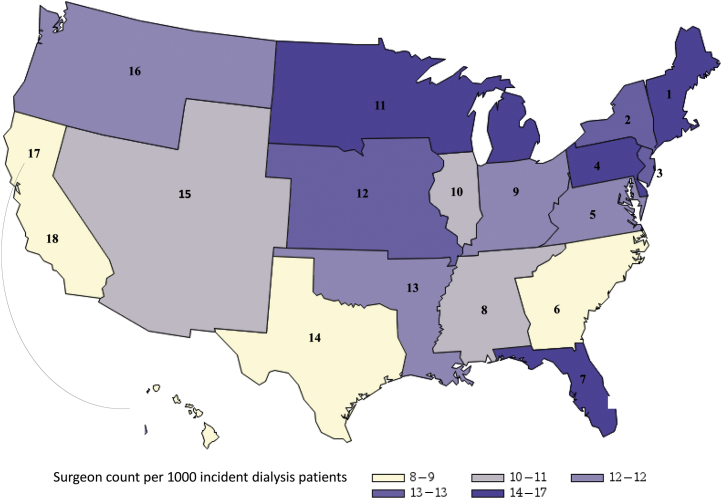
On average, there were 11.4 surgeons performing vascular access procedures in HRRs per 1000 US ESKD patients. These HRRs were divided into 4 quartiles, <8.6, 8.6–10.5, 10.6–13.5, and >13.6 per 1000 patients with ESKD, respectively. The median number of AVFs placed per surgeon in the 4 surgeon supply quartiles was inversely associated with the surgeon supply (Table 1). Patient age and sex distributions were similar across the 4 surgeon supply quartile areas. However, compared with the first quartile, the fourth quartile of surgeon supply included a lower proportion of Black patients; a higher prevalence of coronary artery disease, cerebrovascular disease, and peripheral vascular disease; a higher number of institutionalized patients; and a higher prevalence of predialysis nephrology care. The fourth quartile of surgeon supply also included a lower proportion of patients with markers of low socioeconomic status, including Medicaid insurance or lack of insurance at dialysis initiation. Patients in the fourth quartile of surgeon supply were also less likely to be dialyzing at a for-profit dialysis unit. The supply of vascular access surgeons seemed to be highest in the Northeast and lowest in the West regions (Table 1). Figure 2 illustrates the distribution of surgeon supply by individual ESRD network.
Table 1.
Differences in patient and dialysis facility characteristics between quartiles of vascular surgeon supply across Hospital Service Areas (N = 100,227)
| Variable | Quartiles of surgeon supply (number of surgeons/1000 patients) |
|||
|---|---|---|---|---|
| First (<8.6) | Second (8.6 to <10.6) | Third (10.6–<13.6) | Fourth (≥13.6) | |
| Median [IQR] number of AVF creations per surgeon | 11.3 [8.7–15.6] | 10.2 [9.2–12.0] | 8.0 [7.3–9.2] | 6.5 [5.5–7.8] |
| Age (yr) | ||||
| <45 | 11.6 | 12.3 | 11.2 | 10.6 |
| 45 to <65 | 39.7 | 39.5 | 36.9 | 34.8 |
| 65 to <80 | 36.4 | 35.9 | 37.7 | 39.7 |
| 80+ | 12.3 | 12.2 | 14.1 | 14.9 |
| Male sex | 56.2 | 56.8 | 56.4 | 57.8 |
| Black race | 31.6 | 30.0 | 31.9 | 24.1 |
| Primary cause of kidney failure | ||||
| Diabetes | 51.1 | 50.2 | 47.1 | 47.2 |
| Hypertension | 32.5 | 32.3 | 33.0 | 29.4 |
| Glomerulonephritis | 5.7 | 6.1 | 6.9 | 8.2 |
| Insurance | ||||
| Medicaid | 32.5 | 27.8 | 27.0 | 25.0 |
| Medicare | 45.8 | 47.5 | 51.9 | 54.8 |
| Private employer group health insurance | 9.8 | 10.8 | 10.2 | 10.8 |
| Uninsured | 5.7 | 7.4 | 5.0 | 3.2 |
| Predialysis nephrology care | 51.1 | 52.1 | 54.3 | 57.3 |
| BMI, mean (SD), kg/m2 | 29.9 (8.2) | 29.8 (8.1) | 29.9 (8.2) | 29.8 (8.2) |
| Coronary artery disease | 11.4 | 11.6 | 13.9 | 16.7 |
| Congestive heart failure | 32.5 | 30.3 | 33.7 | 33.7 |
| Diabetes mellitus | 44.5 | 44.2 | 43.1 | 43.8 |
| Hypertension | 88.0 | 87.9 | 87.9 | 87.1 |
| Transient ischemic attack | 8.9 | 8.4 | 9.8 | 9.8 |
| Peripheral vascular disease | 9.6 | 8.9 | 10.4 | 11.7 |
| Drug/alcohol use | 3.0 | 2.8 | 3.3 | 3.4 |
| Institutionalized | 9.2 | 8.6 | 11.1 | 11.7 |
| Needs assistance with daily activities | 15.2 | 14.6 | 16.2 | 16.0 |
| Dialysis chain affiliation | ||||
| DaVita | 36.2 | 35.8 | 34.1 | 32.1 |
| Fresenius | 34.6 | 40.1 | 39.1 | 34.9 |
| Midsized | 15.4 | 12.0 | 11.1 | 13.3 |
| Small/nonchain | 13.8 | 12.1 | 15.7 | 19.7 |
| Freestanding (nonhospital-based) facilities | 96.1 | 97.8 | 94.7 | 92.7 |
| For-profit facilities | 90.4 | 93.1 | 89.0 | 84.1 |
| Dialysis facility size (number of patient), mean (SD) | 108 (64) | 98 (57) | 94 (55) | 87 (51) |
| Geographic regiona | ||||
| Northeast (networks 1–5) | 9.9 | 9.4 | 29.3 | 33.9 |
| Southeast (networks 6–8, 13, 14) | 35.2 | 52.4 | 36.2 | 30.8 |
| Midwest (networks 9–12) | 23.0 | 15.9 | 25.4 | 26.6 |
| West (networks 15–18) | 31.9 | 22.2 | 9.1 | 8.7 |
BMI, body mass index; CO, Colorado; FL, Florida; IL, Illinois; IN, Indiana; IQR, interquartile range; MN, Minnesota; MO, Missouri; MS, Mississippi; NC, North Carolina; N-CA, North California; NJ, New Jersey; NY, New York; OK, Oklahoma; PA, Pennsylvania; S-CA, South California; TX, Texas; WA, Washington.
Northeast includes New England, NY, NJ Trans-Atlantic, PA, and Mid Atlantic. Southeast includes NC, FL, MS, OK, and TX. Midwest includes IN tristate, IL, MN, and MO. West includes CO, WA, N-CA, and S-CA.
Figure 2.
Average surgeon supply by ESRD network among study population (N = 100,227). ESRD, end-stage renal disease.
Table 2 illustrates the distribution of surgeon supply by HRR characteristics. The overall populations of HRRs with higher supply of surgeons were more likely to be White, have a high school diploma, be employed, and have a higher income (P < 0.001 for each population attribute).
Table 2.
Differences in community characteristics by quartiles of vascular surgeon supply
| Variable | Surgeon supply quartile (number of surgeons per 1000 patients with ESKD), mean (SD)a |
|||
|---|---|---|---|---|
| First (<8.6) | Second (8.6 to <10.6) | Third (10.6–<13.6) | Fourth (≥13.6) | |
| % White population, 2010 | 64.9 (17.0) | 67.2 (16.9) | 71.0 (17.6) | 77.7 (14.9) |
| % persons in poverty, 2017 | 16.3 (5.6) | 15.5 (5.4) | 14.5 (5.0) | 13.1 (4.7) |
| % Persons 25+ w/<HS diploma, 2013–2017 | 16.1 (6.3) | 16.0 (6.6) | 12.9 (4.6) | 11.2 (4.4) |
| Unemployment rate, age 16+, 2017 | 5.2 (1.4) | 4.8 (1.5) | 4.5 (1.1) | 4.4 (1.1) |
| Per capita personal income ($), 2017 | 45,801 (11,708) | 46,969 (11,273) | 50,686 (18,031) | 50,758 (14,237) |
| Per capita personal income ($), median (IQR) | 43,074 (38,816–50,197) | 45,642 (38,614–53,300) | 47,365 (41,202–55,859) | 46,829 (40,960–58,048) |
| % Urban population, 2010 | 81.6 (24.2) | 80.6 (26.4) | 79.8 (25.3) | 79.0 (24.8) |
ANOVA, analysis of variance; ESKD, end-stage kidney disease; HS, high school; IQR, interquartile range; w, with.
P < 0.001 by ANOVA analysis.
Association Between Surgeon Supply and Overall AVF Use
The unadjusted and adjusted hazard ratio estimates of AVF use derived from the multivariable competing risk models are found in Table 3. Compared with the first quartile of surgeon supply, the adjusted analysis revealed that higher supply quartiles were associated with modestly increased likelihoods of AVF use: 4% (95% CI 1.4%–7.2%), 4% (95% CI 1.4%–7.1%), and 3% (0.0%–6.1%) for second, third, and fourth quartiles, respectively. In unadjusted and adjusted models, Black race, older age, female sex, coronary artery disease, congestive heart failure, or cerebrovascular disease, drug or alcohol use, and being dependent in activities of daily living were each associated with a lower likelihood of AVF use within 1 year of HD initiation (Table 3). Higher body mass index and hypertension were associated with a higher likelihood of AVF use within 12 months of HD initiation. Private insurance at HD initiation, predialysis nephrology care, and treatment at a large-chain dialysis facility were also associated with increased likelihood of AVF use. In addition, AVF use varied by ESRD network (Table 3). A visual depiction of key findings from Table 3 can be found in Figure 3.
Table 3.
HR of AVF use based on univariate and multivariate competing risk analysis
| Covariatea | Level | Unadjusted HR (95% CI) | Adjusted HR (95% CI) |
|---|---|---|---|
| Primary exposure of interest | |||
| Surgeon supply group, by quartile | First (<8.6) | Reference | |
| Second (8.6 to <10.6) | 1.05 (1.02–1.07) | 1.04 (1.01–1.07) | |
| Third (10.6 to <13.6) | 0.98 (0.96–1.01) | 1.04 (1.01–1.07) | |
| Fourth (≥13.6) | 0.96 (0.94–0.99) | 1.03 (1.00–1.06) | |
| Race | Black | 0.93 (0.91–0.95) | 0.90 (0.88–0.92) |
| White | Reference | ||
| Patient sociodemographic and clinical factors at hemodialysis initiation | |||
| Age | <45 | Reference | |
| 45 to <65 | 1.01 (0.99–1.04) | 1.04 (1.01–1.07) | |
| 65 to <80 | 0.83 (0.81–0.86) | 0.92 (0.89–0.95) | |
| 80+ | 0.64 (0.61–0.66) | 0.74(0.71–0.77) | |
| Sex of patient | Male | 1.47 (1.44–1.50) | 1.46 (1.43–1.49) |
| Female | Reference | ||
| Insurance status at ESRD onset | Private employer group insurance | Reference | |
| Medicaid | 0.77 (0.75–0.80) | 0.92 (0.89–0.95) | |
| Medicare | 0.73 (0.71–0.76) | 0.93 (0.90–0.96) | |
| DVA or other | 0.86 (0.83–0.90) | 0.93 (0.89–0.97) | |
| Uninsured | 0.92 (0.88–0.96) | 0.95 (0.91–0.99) | |
| Primary cause of renal failure | Diabetes | 1.08 (1.06–1.11) | 1.05 (1.03–1.08) |
| Glomerulonephritis | 1.03 (0.99–1.07) | 0.94 (0.90–0.98) | |
| Hypertension | Reference | ||
| BMI (kg/m2) | <24.0 | Reference | |
| 24.0 to <28.3 | 1.18 (1.15–1.21) | 1.12 (1.09–1.15) | |
| 28.3 to <34.1 | 1.28 (1.24–1.31) | 1.19 (1.16–1.23) | |
| ≥34.1 | 1.25 (1.22–1.29) | 1.21 (1.17–1.24) | |
| Nephrology care before ESRD | Yes vs. no | 1.07 (1.04–1.09) | 1.08 (1.05–1.10) |
| Comorbid conditions | |||
| Coronary artery disease | Yes vs. no | 0.89 (0.86–0.91) | 0.97 (0.94–1.00) |
| Drug and alcohol use | Yes vs. no | 0.85 (0.81–0.90) | 0.82 (0.78–0.87) |
| Congestive heart failure | Yes vs. no | 0.83 (0.82–0.85) | 0.89 (0.88–0.91) |
| Need assistance with daily activities | Yes vs. no | 0.66 (0.64–0.68) | 0.82 (0.79–0.84) |
| Hypertension | Yes vs. no | 1.23 (1.19–1.27) | 1.19 (1.15–1.22) |
| Transient ischemic attack | Yes vs. no | 0.82 (0.79–0.85) | 0.91 (0.88–0.94) |
| Institutionalized | Yes vs. no | 0.57 (0.55–0.59) | 0.72 (0.69–0.75) |
| Dialysis facility characteristics and ESRD network | |||
| Dialysis chain affiliation | DaVita | 1.31 (1.27–1.35) | 1.27 (1.23–1.32) |
| Fresenius | 1.14 (1.11–1.17) | 1.08 (1.05–1.12) | |
| Midsized | 1.07 (1.03–1.11) | 1.05 (1.01–1.09) | |
| Small/nonchain | Reference | ||
| Hospital affiliation | Hospital based vs. Freestanding | 0.87 (0.83–0.91) | 0.97 (0.91–1.04) |
| Dialysis facility ownership | For profit vs. nonprofit | 1.11 (1.08–1.15) | 1.01 (0.97–1.06) |
| ESRD networkb | Southern California network | Reference | |
| (CT) network of New Englandb | 1.01 (0.95–1.07) | 1.11 (1.03–1.19) | |
| (NY) network of NYb | 0.91 (0.86–0.96) | 1.09 (1.03–1.16) | |
| (FL) ESRD network of FLc | 0.79 (0.76–0.83) | 0.82 (0.78–0.87) | |
| (IL) Renal network of ILc | 0.79 (0.74–0.83) | 0.87 (0.81–0.92) | |
| Community (county) characteristics | |||
| % With high school diploma | >12.9 (median) vs. ≤12.9 | 1.05 (1.03–1.07) | 1.06 (1.03–1.08) |
| Unemployment rate | >4.7% (median) vs. ≤4.7% | 0.97 (0.95–0.99) | 0.98 (0.96–1.00) |
| % Urban | >80.2 (median) vs. ≤80.2 | 1.01 (0.99–1.03) | 1.04 (1.01–1.06) |
| % White | >70.3 (median) vs. ≤70.3 | 1.01 (0.99–1.03) | 1.04 (1.01–1.06) |
AVF, arteriovenous fistula; BMI, body mass index; CT, Connecticut; ESRD, end-stage renal disease; FL, Florida; HR, hazard ratio; IL, Illinois; NY, New York.
All were adjusted in the multivariate analysis. Two additional patient-level covariates (diabetes on insulin and size of dialysis facility) and 3 area-level covariates (percent without insurance, median household income, and percent below poverty level) were found to be nonsignificant in both univariate and multivariate analyses.
Individual ESRD networks were adjusted in the model. Two ESRD networks with highest AVF use.
Two networks with lowest AVF use (18 networks).
Figure 3.
HRs of AVF use for selected covariates based on multivariate competing risk analysis. AVF, arteriovenous fistula; F, female; HR, hazard ratio; M, male; Ref., reference.
Surgeon Supply and Racial Disparities in AVF Use
We plotted both crude and direct adjusted cumulative incidence function curves for the incidence of AVF use to accommodate for competing risks, disaggregated by patient race (White vs. Black) and surgeon supply quartile (high [combined quartiles 3 and 4] vs. low [combined quartiles 1 and 2]) (Figure 4a and b). Residing in areas with a greater surgeon availability was not found to be associated with less racial disparity in likelihood of AVF use. Specifically, compared with White patients, Black patients were 10% (95% CI 7%–13%) and 8% (95% CI 5%–11%) less likely to have successful AVF use in low and high surgeon supply areas, respectively (Figure 2 illustrates the somewhat lower adjusted rates). These findings were consistent with the nonsignificant 2-way interaction between race and surgeon supply quartile groups (P = 0.235), indicating the relationship between race and AVF use was not modified by surgeon supply. In 1 sensitivity analysis, we included AVG as an event of interest, rather than a competing risk. The results were similar to the ones reported in the current study (data not found). In a second sensitivity analysis, we calculated surgeon supply using the dialysis facility’s ZIP code rather than the ZIP code corresponding to the patient’s residence. We repeated the analysis by restricting the study population to those who received pre-ESRD nephrology care. Again, the results were unchanged (data not found).
Figure 4.
Cumulative incidence of AVF use by SS and race. (a) Unadjusted; (b) adjusted. The weighted partial likelihood estimation directly assesses the intervention (SS) effects for the target event even in the presence of a competing and possibly informative relationship between multiple competing events. The racial disparities in AVF use are similar in areas with low and high SS. AVF, arteriovenous fistula; CVC, central venous catheter; HD, hemodialysis; HR, hazard ratio; SS, surgeon supply.
Discussion
The goal of the current study was to assess whether the surgeon supply was associated with the likelihood that a patient initiating HD with a CVC would use an AVF during follow-up and whether racial disparities in AVF use would be mitigated in areas with a higher surgeon supply. Using a large national database of dialysis patients, we had 3 major findings. First, patients living in areas with a higher surgeon supply had a modestly higher adjusted likelihood of using an AVF during follow-up. Second, Black HD patients were less likely to live in areas with a higher surgeon supply. Finally, the disparity in AVF use between Black and White HD patients was not attenuated in areas with a higher surgeon supply. Collectively, these observations suggest that racial disparities in AVF use among patients initiating HD with a CVC cannot be explained by variations in surgeon supply.
The lower rate of AVF use in Black, as compared with White, HD patients has been repeatedly demonstrated,18, 19, 20, 21 with Black patients having an absolute 10% lower rate of AVF use. However, the explanation for this racial disparity in AVF use remains poorly understood. Black and White HD patients have different demographic profiles, comorbid disease burden, access to medical care, income, and medical insurance. However, the lower AVF use among Black patients persists even after robust statistical adjustment for these factors.6 We recently reported significant differences in several AVF processes of care between older adult Black and White HD patients. Black patients starting HD with a CVC were less likely to have an AVF placed, less likely to have it mature following placement, and less likely to maintain AVF patency after its maturation.6 However, it is not clear what underlying factors account for these differences in AVF processes of care.
Potential contributors to lower AVF use among Black HD patients may be related to patient, provider, or system factors. The contribution of provider factors has been inadequately explored. A recent publication highlighted geographic differences in the supply of surgeons performing vascular access procedures.7 This study found lower surgeon supply in areas with greater proportions of Black residents, higher poverty rates, and lower availability of nephrologists, in agreement with the findings in the current study. In geographic areas with a lower surgeon supply, there could potentially be longer delays in achieving a mature AVF. Specifically, we hypothesized that in geographic areas with a low surgeon supply, racial disparities in AVF use would be augmented, whereas they would be attenuated in areas with a high surgeon supply. The current study observed similar disparities in AVF use between Black and White HD patients in regions with high and low surgeon supply, suggesting that the surgeon supply was not a major limiting factor in achieving a functional AVF. In other words, simply increasing the supply of vascular access surgeons is not likely to eliminate racial disparities in AVF use.
Theoretically, a higher surgeon supply would translate into a shorter waiting time for an HD patient to receive an AVF and to undergo subsequent interventions that may be needed if the AVF fails to mature. In contrast to this expectation, the likelihood of AVF use within 12 months of HD initiation with a CVC was only 4% greater in geographic regions with the highest versus lowest quartile of surgeon supply. What may account for such surprisingly small differences in AVF usage? One potential clue is that surgeons in the highest quartile of surgeon supply averaged a lower number of AVF placements, as compared with those in the lowest quartile. It is possible that the lower volume of procedures translates into less experienced surgeons with inferior AVF maturation outcomes.22 Unfortunately, the vascular access data from CROWNWeb are not sufficiently granular to permit reliable distinction between delays in the following 2 sequential AVF processes of care affecting AVF use: initial AVF placement versus subsequent interventions directed at salvaging AVFs that failed to mature after their placement.
The strengths of the current study include the very large national cohort of incident HD patients, which included >100,000 patients; the use of the CROWNWeb database to assess time to AVF use; and the inclusion of incident patients of all ages and different insurance status, rather than just older Medicare-eligible patients. Our study also has some limitations. First, we were not able to evaluate individual AVF processes of care that impact AVF use. Therefore, we were also not able to ascertain the clinical decision-making process of the surgeons regarding whether a given patient would undergo AVF creation. Second, we did not have information about preoperative vascular measurements that might affect the success of AVF maturation. Third, we could not assess HD patient beliefs or attitudes that may affect their willingness to have an AVF placed or revised. Fourth, we excluded from our analysis patients undergoing pre-ESKD AVF placement, so we cannot state whether the likelihood of AVF use differed between Black and White patients in this patient subset. However, there are substantial racial differences in health care insurance and access to medical care, which would greatly confound the analysis in such patients. In contrast, racial disparities in insurance and access to medical care are substantially diminished once maintenance dialysis has been initiated.6 Fifth, we were not able to determine whether the surgeon supply varied by surgical subspecialty (vascular surgery, general surgery, transplant surgery, or cardiac surgery), as this information was not available in the database we used. Finally, there may be residual confounding owing to factors not captured by the administrative data available from the USRDS database.
In summary, Black HD patients are more likely to live in areas with a low surgeon supply and are less likely to use an AVF. However, the disparity in AVF use between Black and White HD patients is not adequately explained by differences in surgeon supply. It is unlikely, therefore, that increasing the surgeon supply would improve racial disparities in AVF use. Further research is needed to explore other provider and health system factors that may have a greater impact on AVF use.
Disclosure
MA reports receiving personal fees as a consultant for CorMedix, outside of the submitted work. TL reports serving as a consultant for Boston Scientific, BD Bard, and Merck, outside of the submitted work. MT is a consultant for Proteon Therapeutics, Inc., and reports receiving a grant funded by Proteon Therapeutics, Inc., which was developing an investigational therapy for HD vascular access, outside of the submitted work. All the other authors declared no competing interests.
Acknowledgments
This paper was funded in part by a grant from the National Institute on Minority Health and Health Disparities, United States to MA (R01 MD013818).
References
- 1.Lee T., Barker J., Allon M. Tunneled catheters in hemodialysis patients: reasons and subsequent outcomes. Am J Kidney Dis. 2005;46:501–508. doi: 10.1053/j.ajkd.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 2.Xue J.L., Dahl D., Ebben J.P., Collins A.J. The association of initial hemodialysis access type with mortality outcomes in elderly Medicare ESRD patients. Am J Kidney Dis. 2003;42:1013–1019. doi: 10.1016/j.ajkd.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Pisoni R.L., Young E.W., Dykstra D.M., et al. Vascular access use in Europe and the United States: results from the DOPPS. Kidney Int. 2002;61:305–316. doi: 10.1046/j.1523-1755.2002.00117.x. [DOI] [PubMed] [Google Scholar]
- 4.Saran R., Robinson B., Abbott K.C., et al. US renal data system 2017 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2018;71(3 suppl 1):A7. doi: 10.1053/j.ajkd.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arya S., Melanson T.A., George E.L., et al. Racial and sex disparities in catheter use and dialysis access in the United States Medicare population. J Am Soc Nephrol. 2020;31:625–636. doi: 10.1681/ASN.2019030274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qian J., Lee T., Thamer M., Zhang Y., Crews D.C., Allon M. Racial disparities in the arteriovenous fistula care continuum in hemodialysis patients. Clin J Am Soc Nephrol. 2020;15:1796–1803. doi: 10.2215/CJN.03600320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee S.D., Xiang J., Kshirsagar A.V., Steffick D., Saran R., Wang V. Supply and distribution of vascular access physicians in the United States: a cross-sectional study. Kidney360. 2020;1:763–771. doi: 10.34067/kid.0002722020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McClellan W.M., Wasse H., McClellan A.C., Holt J., Krisher J., Waller L.A. Geographic concentration of poverty and arteriovenous fistula use among ESRD patients. J Am Soc Nephrol. 2010;21:1776–1782. doi: 10.1681/ASN.2009121235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nee R., Moon D.S., Jindal R.M., et al. Impact of poverty and health care insurance on arteriovenous fistula use among incident hemodialysis patients. Am J Nephrol. 2015;42:328–336. doi: 10.1159/000441804. [DOI] [PubMed] [Google Scholar]
- 10.Volkova N., McClellan W., Klein M., et al. Neighborhood poverty and racial differences in ESRD incidence. J Am Soc Nephrol. 2008;19:356–364. doi: 10.1681/ASN.2006080934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Port F.K., Held P.J. The US renal data system at 30 years: a historical perspective. Am J Kidney Dis. 2019;73:459–461. doi: 10.1053/j.ajkd.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Wennberg J.E. American Hospital Publishing; 2000. The Dartmouth Atlas of Health Care in the United States. [PubMed] [Google Scholar]
- 13.Wennberg J.E. American Hospital Publishing; 1996. The Dartmouth Atlas of Health Care in the United States. [PubMed] [Google Scholar]
- 14.Health Resources & Services Administration Area health resources files. https://data.hrsa.gov/topics/health-workforce/ahrf
- 15.Szychowski J.M., Roth D.L., Clay O.J., Mittelman M.S. Patient death as a censoring event or competing risk event in models of nursing home placement. Stat Med. 2010;29:371–381. doi: 10.1002/sim.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 17.Zhang X., Zhang M.J. SAS macros for estimation of direct adjusted cumulative incidence curves under proportional subdistribution hazards models. Comput Methods Programs Biomed. 2011;101:87–93. doi: 10.1016/j.cmpb.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pisoni R.L., Zepel L., Port F.K., Robinson B.M. Trends in vascular access use, patient preferences, and related practices: an update from the US DOPPS practice monitor with international comparisons. Am J Kidney Dis. 2015;65:905–915. doi: 10.1053/j.ajkd.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 19.Zarkowsky D.S., Arhuidese I.J., Hicks C.W., et al. Racial/ethnic disparities associated with initial hemodialysis access. JAMA Surg. 2015;150:529–536. doi: 10.1001/jamasurg.2015.0287. [DOI] [PubMed] [Google Scholar]
- 20.Patibandla B.K., Narra A., DeSilva R., Chawla V., Vin Y., Brown R.S. Disparities in arteriovenous fistula placement in older hemodialysis patients. Hemodial Int. 2014;18:118–126. doi: 10.1111/hdi.12099. [DOI] [PubMed] [Google Scholar]
- 21.Siracuse J.J., Gill H.L., Epelboym I., et al. Effect of race and insurance status on outcomes after vascular access placement for hemodialysis. Ann Vasc Surg. 2014;28:964–969. doi: 10.1016/j.avsg.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 22.Saran R., Elder S.J., Goodkin D.A., et al. Enhanced training in vascular access creation predicts arteriovenous fistula placement and patency in hemodialysis patients: results from the dialysis outcomes and practice patterns study. Ann Surg. 2008;247:885–891. doi: 10.1097/SLA.0b013e31816c4044. [DOI] [PubMed] [Google Scholar]